Abstract
Hypertension (HTN) is among the leading global preventable risk factors for cardiovascular disease and premature mortality. Early detection and effective management of HTN have demonstrated significant reductions in mortality, morbidity rate, and health care costs. Furthermore, screening for HTN by nonphysician health care providers improves detection rates and medical management. As physical therapist practice advances to a more independent care model, physical therapists may serve as the first point of contact into the health care system, thereby necessitating a need for routine blood pressure (BP) monitoring. This is especially relevant in the outpatient physical therapist practice setting, where there is evidence for elevated BP measures among patients, yet omission of routine screening in this setting is well documented. Leading physical therapy professional organizations include statements in their guidelines that suggest that physical therapists have a duty to provide a standard of care that protects the safety and optimizes the overall health of patients under their care. Therefore, it is imperative not only that physical therapists include BP examination into routine practice protocols but that the knowledge and skills to accurately measure and interpret BP at rest and during exercise be integrated into the standard of care. The authors suggest that the profession of physical therapy proactively embrace their potential to address the national and worldwide HTN epidemic through routine assessment of BP, appropriate referral for elevated BP measures, and exploration of HTN management by physical therapists.
Despite improved medical management, cardiovascular disease (CVD) remains the leading cause of mortality for both men and women in the United States1 and worldwide,2 with other vascular-related diseases such as stroke, diabetes, and chronic kidney disease ranking in the top 10.1 Hypertension (HTN), an elevation in blood pressure (BP) meeting a cut point for medical diagnosis, is a modifiable leading risk factor for CVD and is present in approximately 34% of the US population.1,3 Independently, HTN is a leading cause of mortality and is also found as a coexisting diagnosis in 16% of deaths in the United States.1 The rate of HTN-associated mortality has increased by 23% while the combined mortality rate for all other causes has decreased by 21%.1 These alarming figures may be partially due to HTN being asymptomatic even at critical values (>180/120 mmHg).4 The asymptomatic nature of HTN causes awareness, treatment, and effective control to be significant challenges facing the medical system.5,6 In fact, the Center for Disease Control and Prevention reports that among individuals who are aware of their HTN diagnosis and who receive treatment, slightly under 50% are effectively controlled,4 and the rate of effective control has decreased between 2013 to 2016.1,7 It has been suggested that this may be due to significant issues regarding medication adherence in patients with HTN, as approximately one-half of patients cease to take their medications within 1 year.7 Even patients who remain adherent with medication consumption and demonstrate controlled HTN at rest may still demonstrate exaggerated BP responses to exercise,8 which is associated with increased risk of major cardiovascular events and mortality.1,9,10 There are also several other confounding factors to effective HTN screening and management such as white coat HTN, masked HTN, and measurement error. The prevalence of HTN is of concern to practicing physical therapists across all settings, including outpatient practice, due to the intersectional relationships between lifestyle factors, functional limitations, and disability. Individuals with disabilities demonstrate higher rates of HTN compared with those without disabilities, especially those possessing mobility limitations.11 HTN and the associated risk factors of physical inactivity, obesity, diabetes, and smoking are frequently observed in the most common clinical populations managed by outpatient orthopedic physical therapists, including low back pain, hip and knee osteoarthritis, and even certain tendinopathies.12–15 A study by Severin et al demonstrated that over 75% of outpatient orthopedic physical therapists report that at least 25% of their current case load included patients either with diagnosed CVD or at moderate or greater risk for CVD16; over 50% reported that at least one-half of their current caseload included such patients.16 The same study also demonstrated that 69% of outpatient orthopedic physical therapists encounter a new patient either diagnosed with CVD or at moderate risk or greater for CVD at least twice per week and that 30% encounter such patients daily.16
Early detection and effective management of HTN is important and can result in significant reductions in mortality, morbidity rate, and health care costs.3,17,18 Every 10% increase in effective HTN treatment could prevent an additional 14,000 deaths per year in the adult population.19 However, as most medical organizations advocate for early detection and management of HTN, it continues to be a considerable challenge within the health care industry. Studies have demonstrated that HTN screening by nonphysician health care providers can improve detection rates and medical management of HTN.18,20,21
Given the prevalence of individuals at an increased risk of developing HTN or with a confirmed diagnosis of HTN who are under the care of a physical therapist, the measurement of BP should be included in the routine examination of all new patients. This is especially true in outpatient settings where physical therapists may lack access to more advanced physiological monitoring tools. Additionally, with varied forms of direct access available throughout the United States and other countries worldwide, physical therapists may serve as the initial entry point to the health care system for patients. Examining findings from BP measurements during patient encounters are essential to informed clinical decision-making, differential diagnosis, appropriate referral, and timely medical management.
Despite the prevalence of patients with HTN or at risk for HTN encountered in outpatient physical therapist practice, resting measures of BP are not routinely performed.16,22–24 Only 10% to 15% of outpatient physical therapists report measuring resting BP on all new patients.16,23 These low rates of screening are present despite outpatient physical therapists reporting that they possess adequate access to necessary equipment and competency in performing resting BP measurement.16,23 Additionally, the most often reported rationale for omitting BP as a component of routine screening among outpatient physical therapists includes a lack of perceived importance, lack of clinic policy, and time constraints.16,23,24 Outpatient physical therapists who screen most often report that they perceive BP measurement to be important and practice in clinics with a policy aimed towards routine BP screening.16 These barriers and facilitators suggest that transforming the paradigm of BP omissions may involve changing perceptions regarding the importance of BP screening if improved screening rates are to be achieved. Furthermore, providing clinicians with evidence-based resources that inform decision-making in combination with institutional policies regarding BP screening may be necessary to bring about change in current practice habits. The purpose of this article is a call to action for routine BP screening by outpatient physical therapists. This article will also establish the importance of screening, provide evidence-based recommendations on HTN screening and interpretation in outpatient physical therapist practice, and discuss potential future directions regarding HTN screening and clinical management by physical therapists.
Ethical Duty to Screen
Physical therapists have a significant role in addressing the epidemic rates of HTN by including routine measurement and assessment of BP as well as medical referral when necessary. BP screening by nonphysician health care providers in outpatient clinics has been shown to be an effective strategy for early detection and appropriate medical referral.18 A study by Engstrom et al investigated the effects of routine HTN screening by dentists using a cutoff of either 160 mmHg systolic blood pressure (SBP) or 90 mmHg diastolic pressure (DBP) for physician referral for HTN.18 In their sample of 1149 patients without a prior diagnosis of HTN, 237 patients had BP readings that exceed the cutoff values to screen positive for medical referral. 32% of those referred ultimately received a diagnosis of HTN.18 The sensitivity of BP screening was 79.1%, and specificity was 84.8% with a number needed to screen of 18 patients.18 Multiple studies across different physical therapist practice settings and patient populations have demonstrated a high prevalence of HTN and other CVD risk factors among patients receiving physical therapy services.16,23,25,26 With the advent of direct access, outpatient physical therapists are more likely to encounter patients without prior physician referral. Therefore, it is reasonable that similar benefits may be observed with routine BP screening of patients receiving outpatient physical therapy services. Several prominent physical therapy professional organizations, such as the American Physical Therapy Association (APTA)27 and Chartered Society of Physiotherapists,28 include statements in their practice guidelines that suggest that physical therapists have a duty to provide a standard of care that protects the safety and optimizes the overall health of patients under their care. Therefore, given the prevalence of HTN in patients encountered in outpatient physical therapist practice; the effects of HTN on morbidity, disability, and mortality; and the associated beneficial effects of early HTN detection, the implementation of routine BP screening and appropriate medical referral is not only plausible in outpatient physical therapist practice but an ethical duty of care.
Recommendations for BP Screening in Outpatient Physical Therapy
Processes to develop and disseminate best practice recommendations are ongoing within the physical therapy profession. Since Frese et al29 published recommendations for BP measurement by physical therapists in 2011, additional evidence in physical therapist practice has emerged, and several new BP measurement devices have been approved for clinical use. Therefore, current evidence exists within the health care literature that provides a solid framework from which physical therapists can draw best practice recommendations. These will be discussed in more detail within the ensuing sections.
Equipment Requirements
Although standardized recommendations regarding the amount and types of BP measuring equipment are not yet available and may be clinic or practice setting specific, previous research has indicated the following for outpatient physical therapist practice settings: 1 stethoscope per therapist, 1 standard aneroid BP cuff for every 2 therapists, 1 small and 1 large BP cuff per clinic, and 1 child cuff for clinics providing care to patients younger than 12 years.23 Outpatient clinics serving a large number of patients who are obese may need to consider additional large cuffs to accommodate patient volumes. Additionally, 1 thigh cuff would be warranted if lower extremity measures need to be performed. Considerations for other practice settings would include having enough equipment to assure no wait time for therapists to conduct a BP measure and that sizing of equipment is available for all patient body types that may be encountered.
Equipment Function and Calibration
Routine BP equipment maintenance and calibration checks are essential to obtaining an accurate reading. Prior studies have reported 22% of aneroid gauges used in physical therapist practice are not adequately calibrated.30,31 Additionally, these studies identified equipment in use that had bulb malfunction, tubing tears, and excessive wear, all of which impact the physical therapist’s ability to obtain an accurate BP reading. Regular calibration and inspection of equipment at regular intervals ranging from 6 months to 2 years is essential to assure BP measures are not under- or overestimated.32,33 The European Society of Hypertension recommended a standardized protocol that can be followed to conduct the gauge calibration checks.34 In this procedure, a Y tube connector allows for synchronized comparison of the mercury and aneroid gauges at 50-mmHg increments to assure workability throughout the range of possible readings. A video tutorial on this setup is available on the APTA-Cardiovascular and Pulmonary Section website.35
Because the use of mercury in physical therapy and other health care practice settings has been significantly reduced due to potential neurotoxic risk,36 an acceptable alternative to mercury sphygmomanometers is the use of digital pressure gauges to conduct these regular inspections.
However, this device may be cost-prohibitive to some practice settings, and consultation with a bioengineer may be warranted. Many health systems have biosafety departments that can provide regular equipment inspection, but free-standing clinics may need to contract with companies possessing both the skill and equipment to conduct regular rehabilitation equipment checks (eg, electrical stimulation or ultrasound units) to assure the validity of BP equipment.
Instrumentation
There are several options available to measure BP in the clinic. The 2 most popular are aneroid devices, which are used for manual auscultatory measurement, and oscillometric devices used for automatic measurement. Both devices have been shown to measure BP accurately.32,34,37–39 However, they utilize slightly different methods.
Manual auscultatory BP measurement is based on the identification of sounds resulting from the return of arterial blood flow following a period of temporary occlusion, which are known as Korotkoff sounds. As the cuff deflates below peak pressure, blood returns into the artery in a turbulent fashion, causing vibration along the arterial wall. This vibration creates a tapping sound that can be appreciated on auscultation. Once the cuff pressure falls below the diastolic pressure, laminar blood flow is restored in the artery. At this point, the arterial wall no longer vibrates, the tapping sound disappears, and silence is appreciated on auscultation. The pressure at the emergence of sound is used as the SBP and pressure at the subsequent disappearance of sound is used as the DBP.
Automatic oscillometric devices operate on the principle that vibrations in the arterial wall during cuff inflation and deflation can be detected and transduced into electrical signals. These devices inflate the cuff to a pressure about 20 mmHg above the patient’s SBP. As the cuff deflates, the vibrations during deflation are transferred from the arterial wall through the air inside the cuff into a transducer in the monitor. The point of maximal oscillation (vibration) is identified by the device, which corresponds to the mean intra-arterial pressure. SBP and DBP are then estimated based on the maximal point of oscillation by the device.
Both devices possess unique advantages and disadvantages for BP measurement. The use of automatic oscillometric devices has demonstrated a reduction in the white coat response compared with manual measurement.39,40 This is because BP measurement using an oscillometric device can be taken without the clinician present in the room. Automatic oscillometric devices are also less susceptible to auscultatory gap (a period of diminished or absent Korotkoff, which causes SBP to be underestimated) than manual measurement.38 However, because automatic oscillometric devices measure BP by detecting vibrations, they cannot accurately measure BP during exercise,37,38,40,41 whereas the manual method using an aneroid device can be used during exercise.
Wrist monitors have emerged as a potential option for BP measurement, especially for home monitoring.42 These devices may be particularly useful for obese patients where appropriate cuff-sizing options may be limited,43 because wrist diameter is often not affected by obesity. However, these devices have demonstrated issues with accuracy with a tendency to overestimate BP.37,42,43
Automatic auscultatory devices have been proposed as an alternative to manual and oscillometric devices.44,45 These devices combine the best features of manual auscultatory readings and those of automated devices. They can also be used to measure BP during exercise with comparable accuracy to manual measurement.44 These devices are often used in clinical exercise testing laboratories. However, they may be cost-prohibitive for routine outpatient physical therapist practice.
BP Cuff Size Selection
Miscuffing is a common BP measurement error.37 It has been reported that undercuffing large arms is a common miscuffing error and may have a substantial clinical effect.46 Therefore, it is recommended that practice settings in which physical therapists provide patient care have varied cuff sizes to accommodate the variety of arm sizes encountered in their clinic. This may also include thigh and pediatric cuffs if applicable. It is generally recommended the cuff should have a bladder length that is 80% and a width that is at least 40% of the arm circumference.37 The bladder is not to be confused with the entire BP cuff; rather, the bladder refers to the plastic inflatable piece inside the outer cuff wrap. Two alternatives to the aforementioned sizing method include determining the radius of the BP measurement site or using the index line and reference range printed on the BP cuff. In the latter method, the clinician wraps the cuff around the measurement site to assure the index line falls within the reference range. If it does not, a larger or smaller cuff is required as is indicated on the inner markings of the cuff. These techniques are detailed in a video titled “Selection of Blood Pressure Cuff Size” available on the APTA Cardiovascular Pulmonary Section #VitalsAreVITAL website.35
When measuring at a site other than the upper arm, this same cuffing technique should be employed to assure an accurate fit. Furthermore, there are situations for which a patient’s body type and the associated geometry may warrant relocating the measurement site due to improper cuff fit. For example, some individuals may have significant upper arm girth but a short upper arm length (eg, obesity or significant muscle hypertrophy). In these situations, the cuff sizes needed to fit around the upper arm could also have excessive length for the extremity and extend to the axilla or below the antecubital fossa. In these examples, forearm BP measurement could be considered.
Standardized Measurement Technique
Standardizing a patient’s position when obtaining a BP measurement provides improved inter- and intrarater reliability of the measurement obtained.47 The standard measurement position includes having the individual in a seating and resting position for 5 minutes prior to obtaining the measurement.29,32 Feet should be flat on the floor and uncrossed with back supported. Considerations for seating options available in each practice setting will vary; however, sitting on the edge of a plinth with feet dangling would not be an optimal choice because variations of up to 8 mmHg have been reported with a nonstandardized positioning.48–50
The Kortokoff sounds should be auscultated at a measurement location level with the right atrium, which can be externally located at the midpoint of the sternum or the fourth intercostal space to assure standardization of the measurement.37 The measured arm is typically positioned with the shoulder in a mid-point of flexion and the elbow fully extended and pronated. If the measurement site is below the level of the right atrium, the readings will be too high. If the arm is above the heart level, the readings will be too low.37 These differences can be attributed to the effects of hydrostatic pressure and may contribute to 2 mmHg for every inch above or below the heart level.51,52 The patient’s arm should also be supported to minimize muscle activity during measurement.37 Although the nondominant arm is often recommended for measurement, it is not required and either arm can be used unless it is contraindicated.37 During initial patient encounters, BP measurement of both arms is recommended and the arm with the higher reading should be used for subsequent readings.32 Lane et al have identified clinically significant inter-arm differences of greater than 10 mmHg in SBP at rates of 20% and DBP at rates of 11%.48
Additional items that may alter the BP measurement and should be controlled for include speaking, sneezing, coughing, and isometric contraction of the surrounding musculature during measurement.9 Furthermore, pain, room temperature, full urinary bladder, overinflated BP cuff bladder, and the speed of cuff deflation (faster than 2–3 mm/s) can impact the reading obtained.37,53 The Table provides an overview of the impact of positioning error on the associated BP reading.
Table.
BP Reading Error Related to Positioning Techniquea
| Positioning Technical Error | Associated BP Reading Error |
|---|---|
| Full bladder | Elevated 10–15 mmg |
| Unsupported back | Elevated 5–10 mmHg |
| Unsupported feet | Elevated 5–10 mmHg |
| Crossed legs | Elevated 2–8 mmHg |
| Cuff over clothing | Elevated 10–40 mmHg |
| Unsupported arm | Elevated 10 mmHg |
| Patient talking | 10–15 mmHg |
Forearm, Thigh, and Calf BP Measurement
Although measurement in the upper arm is generally the first choice for obtaining a BP measure, there are situations for which this may not be possible or indicated. For example, an upper arm with an increased circumference and decreased length may be unable to accommodate the parameters of any cuff size; therefore, a forearm measurement may be indicated. Additionally, BP measurement at an upper extremity measurement location may not be recommended due to medical precautions. An example of this would be individuals who have undergone lymph node resection during a double breast mastectomy.54 In this case, a calf or thigh measurement would be indicated. Furthermore, if a physical therapist is screening for peripheral vascular disease utilizing an ankle brachial index, a ratio of the upper arm to calf SBP measurements would be utilized. Considerations for pain location, extremity fractures, and wounds may also indicate modification to measurement location.
In general, measurement at the alternate locations follows the standard measurement procedures and positions previously described with a few distinct exceptions. First, auscultation of the Kortokoff sounds would take place at the artery below the site of cuff inflation. Additionally, a supine position would be the best option to limit muscular contraction when obtaining a lower extremity measure. It is also notable that the bony structures of the ankle or wrist may make sound transmission through the stethoscope more challenging; therefore, manual palpation or use of a Doppler to obtain the SBP may be indicated. The APTA Cardiovascular and Pulmonary Section has BP video tutorials available that include an overview on the use of a Doppler for BP measurement.35 Finally, the examiner should recognize that the diagnostic and categorical values established for BP classification in the literature, for example 140 mmHg SBP and/or 90 mmHg DBP indicative of a HTN measure, are determined from an upper arm measurement and variability may exist in different parts of the arterial tree.37 Specifically, SBP may increase in more distal arteries whereas the DBP may decrease.37 Variability can also exist between the sides of the body at which the measure is taken.37 Therefore, regular measurement at each physical therapy encounter is recommended to establish each individual BP measurement and ranges.
Clinical Decision-Making and Referral
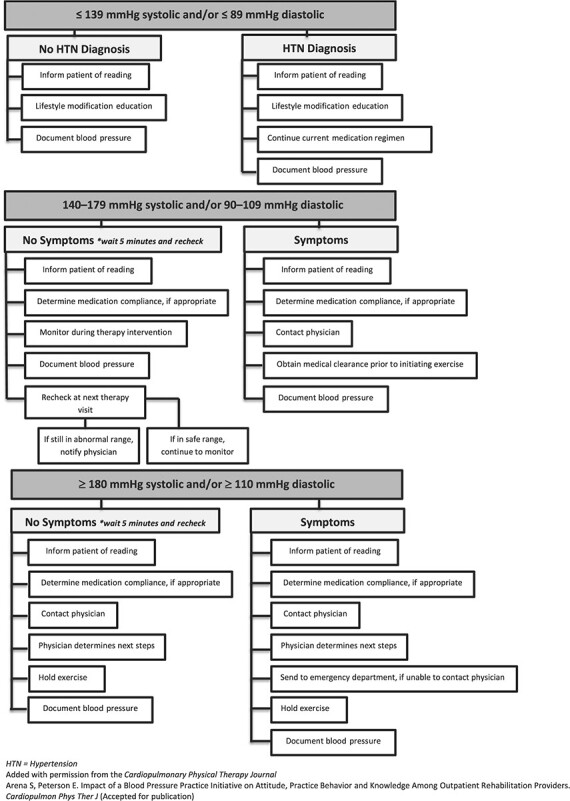
Appendix 1 provides a decision-making algorithm for elevated BP from a recent publication in Cardiopulmonary Physical Therapy Journal. The algorithm was developed as an amalgamation of recommendations and evidence from multiple resources53,55–58 and in response to a need for the unique considerations of rehabilitation providers. Given the paucity of available evidence and lack of established clinical guidelines, a decision-making algorithm for hypotension was not provided.
Hypotension is defined as a resting BP below 90 mmHg SBP, 60 mmHg DBP, 60 mmHg mean, or a decrease ≥40 mmHg from baseline.59 Patients demonstrating hypotension at rest should be further assessed for symptoms and potential contributors in collaboration with the patient’s physician. Individuals identified to be asymptomatic may be safe to continue with the planned intervention; however, close physiological monitoring with attention to findings suggesting further medical assessment is warranted.
Standing BP and Response to Position Change
Measurement of BP in positions other than sitting may be necessary during the course of a patient examination or intervention, including to screen for orthostatic hypotension or to measure BP during standing exercise. Some elevation in BP is expected when moving from the more dependent position of supine to a sitting and then to a standing posture. For example, DBP in sitting can be up to 5 mmHg higher than in supine.60 A video demonstration overviewing methodology is available on the APTA Cardiovascular and Pulmonary Section #VitalsAREVITAL website.35
An orthostatic response to position change would be considered abnormal if the SBP decreases ≥20 mmHg or the DBP decreases ≥10 mmHg as the individual moves to a more upright position. These measures are recommended at a 1- and/or 3-minute time frame after the position change occurs.61 Additionally, a condition known as initial orthostatic hypotension is defined as a decrease in SBP of 40 mmHg and/or a decrease in DBP of >20 mmHg presenting within the first 15 seconds of standing and correcting within 30 to 60 seconds.62 However, in most clinical settings, the equipment functionality limits the physical therapist’s ability to capture these fast adaptations within the vascular system.
Should a physical therapist identify an abnormal response to position change, responsive actions and intervention include notifying the physician, especially if there is a new finding; patient education on fall prevention and counter pressure maneuvers; and a referral to a medication management team or pharmacist as available in each practice setting.
Additional Considerations
Although safety, and ultimately a patient’s health, can be impacted by a sustained elevated BP, prior evidence suggests perceived and actual barriers to a physical therapist’s performing routine BP screening.16,23 For example, it is understandable that time constraints in a clinical setting may present barriers to having a patient rest 5 minutes prior to BP measurement. Therefore, practice patterns including obtaining the measurement in a waiting room area or performing immediately after a seated time used for a subjective interview may be most efficient. Other practical solutions to limiting the time barrier could include training of patients, technicians, or other support staff to perform the initial BP screening for physical therapy interpretation. This could be accomplished using an automated BP machine with follow-up manual assessment if indicated.
Diligent attention to the recommended measurement protocol is essential to identify an abnormal BP reading and to confirm a standardized measurement technique that is in congruence with other health care provider methodology. Additionally, a follow-up communication by the physical therapist with the patient’s physicians or associated advanced care practitioners can be beneficial to develop a relationship of trust. Specifically, this communication should include an assurance that the BP measurement methods, and any associated recommendations, are being initiated by the physical therapist only after careful evidence-based BP evaluation has been employed.
Blood Pressure Response to Exercise
Although much attention has been placed on resting BP measurement, emerging evidence indicates that the measurement of BP during and after exercise may provide a more robust assessment of a patient’s hemodynamic stability and clinical prognosis.63–66
The expected physiological response of the cardiovascular system to dynamic exercise is an increase in cardiac output (CO) to match the increase in metabolic demand of the working muscles. The 2 determinants of CO are stroke volume and heart rate (HR), both of which increase during exercise.
However, at moderate-intensity exercise levels (50–60% maximal oxygen uptake [VO2Max] or HR reserve), stroke volume reaches a plateau (except in elite athletes) and the increase in CO is driven solely by increases in HR.67 Arterial BP is the product of CO and total peripheral resistance (TPR). The BP response to dynamic exercise is an increase in SBP and no change or a slight drop in DBP. A failure of an individual’s SBP to increase with increased workloads indicates inadequate CO and thus cardiac function, while a rapid increase in SBP with minimal workload or minimal increase in CO would indicate high TPR and thus impaired vascular function (eg, metabolic autoregulation or arterial stiffness).10
During exercise, there is a rapid increase in CO to match the metabolic demand and a drop in TPR due to functional sympatholysis and metabolic autoregulation that lead to vasodilation within working muscles. However, the gain in CO is greater than the decrease in TPR and thus BP begins to rise.
An increase of 8 to 12 ± 2 mmHg of SBP per metabolic equivalent, with a plateau at peak exercise, is to be expected.55 The normal increase in SBP from rest to maximal exercise is generally from 55 to 65 mmHg in men and from 45 to 60 mmHg in women; however, these values vary considerably across the lifespan.68 For DBP, women tend to show greater changes from rest to peak exercise than men, with these values also increasing along the lifespan.68
BP Screening During Exercise
A cornerstone of physical therapist intervention is prescribing a tailored exercise program with specific intensity. To prescribe a tailored exercise program, clinicians need to perform a maximal or submaximal exercise test to determine baseline cardiorespiratory fitness and hemodynamic responses. Although the purpose of this article is not to discuss clinical exercise testing, we recognize the importance of these tests69 and will discuss the clinical importance and relevance of monitoring the BP response during exercise.
The physiological stress induced by exercise may help uncover early-stage pathology in individuals free from overt CVD and thus serve as a valuable screening tool. It is imperative for physical therapists to monitor BP responses to exercise during treatment sessions for safety reasons; however, the BP response to exercise has also been found to serve as a valuable prognostic marker for future cardiovascular events, independent of resting BP levels.9 Physical therapists are uniquely positioned to provide this level of screening. However, 12% of outpatient physical therapists report utilizing measures of BP during exercise and only 35% report measuring static BP following exercise.16 If a patient is not referred to cardiopulmonary exercise testing by their medical doctor, their BP response to exercise may go unscreened. It was previously estimated that 15 to 30% of adults with office BP readings <140/80 mmHg do in fact record elevated BP readings when measured using ambulatory BP measurements,70,71 a condition known as masked HTN (MH). MH is associated with a 2-fold increase in risk of CVD compared with normotensive individuals.72 The prevalence of MH ranges between 10% and 26% in population-based surveys and between 14% and 30% in normotensive clinic populations.58 The improved ability of ambulatory BP measurements over routine office readings to detect MH may be attributed to an exaggerated BP response to exercise and physical activity.70 Indeed, the prevalence of MH among individuals with an exaggerated BP response to exercise has been found to be 41%.73 This BP response to exercise and physical activity is not usually measured during routine physician-initiated resting BP readings. The most recent guidelines by the American Heart Association placed an emphasis on the importance of improved detection of MH in the clinic.32 Additionally, individuals who show an exaggerated BP response to exercise, also known as exercise HTN, have been shown to possess a 1.4- to 3.0-fold higher relative risk for cardiovascular events compared with individuals with a normal BP response to exercise.74 An exaggerated BP response to exercise has been defined as a reading ≥90th percentile from relative normative data.65,68 Because exercise is typically an integral component of physical therapy treatment sessions, physical therapists are uniquely positioned to capture BP responses to exercise, which may improve the detection of exaggerated BP responses to exercise and MH. As such, measurement of exercise BP can and should be considered during routine physical therapy treatment sessions. Sabbahi et al have published updated normative peak exercising BP data across the age span for both sexes in a predominantly white North American cohort.68 A table of normative peak BP data has been included in Appendix 2.
Furthermore, an abnormally low BP response to exercise has been demonstrated to be a strong prognosticator of cardiovascular events and all-cause mortality independent of clinical presentation or exercise intensity.64 In fact, when accompanied by any evidence of ischemia, a persistent decrease in SBP ≥ 10 mmHg with increased workload is an absolute indication to terminate an exercise test.55,67 A decrease in BP during exercise or an increase >20 to 30 mmHg can indicate multiple underlying pathologies, including severe left ventricular dysfunction, aortic outflow tract obstruction, or myocardial ischemia. β-Blocker medication may also cause this response. Some individuals without any clinically significant cardiac disease may exhibit exercise-induced hypotension due to factors such as dehydration, prolonged strenuous exercise, or antihypertensive medication.67
To serve as a prognostic indicator, patients do not necessarily need to be subjected to maximal exercise to assess the BP response. In a cohort of individuals with an exaggerated BP response to exercise, a single bout of light-intensity exercise at 60% to 70% of age-predicted maximal HR was able to unmask BP regularities associated with MH.10,75 We presume this level of submaximal exercise intensity to be routinely achieved during physical therapy treatment sessions, and an abnormal BP response may serve as an accessible screening tool to warrant further clinical testing.
Exercise Management for HTN
Exercise training (ET) for HTN is an effective and integral component of nonpharmacological interventions for BP control. In 2010, HTN was determined to be the number 1 overall risk factor for disease76 at a prevalence of 34% of US adults.77 Therefore, considerable attention must be given to the effective management of this disease. ET has been shown to be as effective as pharmacologic therapy in terms of treatment effectiveness for HTN across many etiologies.78,79 In patients who have had a stroke, ET was found to be even more effective than pharmacological interventions in terms of mortality reduction.79,80 In patients with heart failure, ET was only surpassed by diuretic drug therapies.79,80 Furthermore, in patients with stable coronary heart disease, a 12-month ET program resulted in higher event-free survival rates than a standard percutaneous coronary intervention and at nearly one-half the cost.79,81 In the 2017 American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, ET for the treatment of HTN received a class I recommendation, with evidence level A.58 Although well-established evidence for the effectives of ET on BP is present, widely accepted, and continuously growing, only 15% of US adults with HTN have been reported to meet ET recommendations.82
Exercise interventions are an integral component of physical therapy, and physical therapists are in a unique position to prescribe and implement these treatment programs. An aerobic exercise program for 90 to 150 minutes per week at 65% to 75% HR reserve can result in a SBP reduction of about 5 to 8 mmHg in individuals with HTN and 2 to 4 mmHg in individuals with normotensive BP.58,83 Reductions of this magnitude have been associated with a decrease in the risk of stroke by 14%, coronary artery disease by 9%, and total mortality by 7%.79,84 These well-established effects of ET for BP reduction and its widespread usage by physical therapists present a promising opportunity for the physical therapy profession to address the growing HTN crisis. Future advocacy efforts by professional organizations within the physical therapy profession should be directed towards exercise-based management of HTN by physical therapists.
Measurement of BP During Exercise
In the absence of automated BP devices specifically validated for exercising BP, clinicians should rely on manual auscultatory methods. Evidence for accurate BP measures using the manual auscultatory methods have been reported for low- to moderate-intensity exercise.10 However, during maximal exercise, BP measurements may become increasingly difficult to auscultate due to movement and noise artifact, and DBP becomes less reliable due to difficulty differentiating between Korotkoff phase IV (muffling) and phase V (disappearing of sound).10,85 All BP measurement considerations discussed above (eg, cuff size and placement) are relevant and should be considered during exercise BP measurement.
Furthermore, BP should be measured in the exercising posture at rest, during exercise, and post exercise. If BP measurement during exercise is not possible, measuring BP immediately after exercise in the exercising posture is suggested; however, this measurement would not be referred to as the “exercise BP” but should instead be referred to as the “post-exercise BP.” Additionally, clinicians can expect SBP to return to pre-exercise levels within 6 minutes55 and a post-exercise hypotensive response (reduction in BP relative to pre-exercise values) may be present.79
Conclusion
Growing evidence supports the need for outpatient physical therapists to regularly screen and assess BP at rest and with exercises. Although this body of knowledge is continually expanding, significant evidence exists related to a standardized BP technique and methodology across the health care industry. Because physical therapists are identified as health care providers with a vision to “transform society by optimizing movement to improve the human experience,”86 it is within our professional scope and part of good clinical practice to encompass assessment of cardiovascular responses to movement to assure safe movement experiences even outside of a strictly CVD clinical practice setting. Additionally, the physical therapy profession is uniquely positioned to provide exercise-based solutions to the HTN epidemic.
Therefore, the authors suggest that physical therapists proactively embrace their potential to curtail the national and worldwide HTN epidemic through routine assessment of BP, appropriate referral for elevated BP measures, and exploration of HTN management by physical therapists.
Appendix 1.
Rehabilitation Provider BP Decision-Making Algorithm
|
|---|
Appendix 2.
Sex-Specific Percentiles for BP Values (mmHg) From Treadmill Exercise Tests Obtained From FRIEND Cohort
| Age Group (y) | 5th | 10th | 25th | 50th | 75th | 90th | 95th | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP |
| 20-29 | 147 | 58 | 150 | 62 | 160 | 70 | 176 | 80 | 187 | 84 | 202 | 90 | 212 | 96 |
| 30-39 | 147 | 60 | 154 | 64 | 168 | 70 | 178 | 80 | 190 | 88 | 206 | 96 | 214 | 100 |
| 40-49 | 150 | 65 | 158 | 70 | 169 | 76 | 182 | 82 | 198 | 90 | 212 | 98 | 224 | 100 |
| 50-59 | 154 | 68 | 162 | 70 | 178 | 78 | 190 | 84 | 206 | 92 | 220 | 98 | 234 | 105 |
| 60-69 | 152 | 64 | 162 | 70 | 172 | 76 | 190 | 82 | 206 | 94 | 220 | 100 | 238 | 110 |
| 70-79 | 146 | 66 | 156 | 66 | 168 | 71 | 190 | 80 | 210 | 86 | 224 | 96 | 240 | 100 |
| Women | SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP |
| 20-29 | 132 | 58 | 136 | 62 | 144 | 68 | 154 | 74 | 165 | 80 | 175 | 88 | 180 | 90 |
| 30-39 | 130 | 62 | 138 | 66 | 148 | 70 | 160 | 78 | 170 | 84 | 182 | 92 | 194 | 96 |
| 40-49 | 134 | 64 | 140 | 68 | 152 | 72 | 166 | 80 | 180 | 86 | 196 | 94 | 208 | 98 |
| 50-59 | 138 | 64 | 144 | 70 | 156 | 74 | 172 | 80 | 190 | 90 | 204 | 96 | 216 | 100 |
| 60-69 | 144 | 58 | 150 | 64 | 166 | 74 | 184 | 83 | 204 | 91 | 224 | 100 | 235 | 110 |
| 70-79 | 128 | 60 | 144 | 64 | 165 | 72 | 203 | 90 | 216 | 102 | 231 | 110 | 234 | 112 |
Contributor Information
Richard Severin, University of Illinois, Chicago, 1919 West Taylor St, Room 506G, MC 898, Chicago, IL (USA); Doctor of Physical Therapy Program, Robbins College of Health and Human Sciences, Baylor University, Waco, Texas.
Ahmad Sabbahi, Department of Physical Therapy, College of Applied Health Sciences, University of Illinois, Chicago.
Ali Albarrati, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia.
Shane A Phillips, Department of Physical Therapy, College of Applied Health Sciences, University of Illinois, Chicago.
Sara Arena, Physical Therapy Program, School of Health Science, Oakland University, Rochester, Michigan.
References
- 1. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics–2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm hg, 1990–2015. JAMA. 2017;317:165. [DOI] [PubMed] [Google Scholar]
- 3. Bromfield S. High blood pressure: the leading global burden of disease risk factor and the need for worldwide prevention programs. Curr Hypertens Rep. 2014;15:134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kessler CS, Joudeh Y. Evaluation and treatment of severe asymptomatic hypertension. Am Fam Physician. 2010;81:470–476. [PubMed] [Google Scholar]
- 5. Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017;(289):1–8. [PubMed] [Google Scholar]
- 6. Olives C, Myerson R, Mokdad AH, Murray CJL, Lim SS. Prevalence, awareness, treatment, and 13 control of hypertension in United States counties, 2001-2009. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vrijens B, Antoniou S, Burnier M, Sierra A, Volpe M. Current situation of medication adherence in hypertension. Front Pharmacol. 2017;8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chant B, Bakali M, Hinton T, et al. Antihypertensive treatment fails to control blood pressure during exercise. Hypertension. 2018;72:102–109. [DOI] [PubMed] [Google Scholar]
- 9. Schultz MG, Otahal P, Cleland VJ, Blizzard L, Marwick TH, Sharman JE. Exercise-induced hypertension, cardiovascular events, and mortality in patients undergoing exercise stress testing: a systematic review and meta-analysis. Am J Hypertens. 2013;26:357–366. [DOI] [PubMed] [Google Scholar]
- 10. Sharman JE, LaGerche A. Exercise blood pressure: clinical relevance and correct measurement. J Hum Hypertens. 2015;29:351–358. [DOI] [PubMed] [Google Scholar]
- 11. Stevens A, Courtney-Long E, Gillespie C, Armour BS. Hypertension among US adults by disability status and type, National Health and Nutrition Examination Survey, 2001–2010. Prev Chronic Dis. 2014;11:E139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernandes GS, Valdes AM. Cardiovascular disease and osteoarthritis: common pathways and 7 patient outcomes. Eur J Clin Invest. 2015;45:405–414. [DOI] [PubMed] [Google Scholar]
- 13. Leino-Arjas P, Solovieva S, Kirjonen J, Reunanen A, Riihimäki H. Cardiovascular risk factors and low-back pain in a long-term follow-up of industrial employees. Scand J Work Environ Heal. 2006;32:12–19. [DOI] [PubMed] [Google Scholar]
- 14. Ranger TA, Wong AMY, Cook JL, Gaida JE. Is there an association between tendinopathy and diabetes mellitus? A systematic review with meta-analysis. Br J Sport Med. 2016;50:982–989. [DOI] [PubMed] [Google Scholar]
- 15. Singh G, Miller JD, Lee FH, Pettitt D, Russell MW. Prevalence of cardiovascular disease risk factors among US adults with self reported osteoarthritis. Am J Manag Care. 2002;8:383–391. [PubMed] [Google Scholar]
- 16. Severin R, Wang E, Wielechowski A, Phillips SA. Outpatient physical therapist attitudes toward and behaviors in cardiovascular disease screening: a national survey. Phys Ther. 2019;99:833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Danaei G, Ding EL, Mozaffarian D, et al. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med. 2009;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Engström S, Berne C, Gahnberg L, Svärdsudd K. Efficacy of screening for high blood pressure in 1 dental health care. BMC Public Health. 2011;11:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farley TA, Dalal MA, Mostashari F, Frieden TR. Deaths preventable in the U.S. by improvements in use of clinical preventive services. Am J Prev Med. 2010;38:600–609. [DOI] [PubMed] [Google Scholar]
- 20. Fleming S, Atherton H, Mccartney D, et al. Self-screening and non-physician screening for hypertension in communities: a systematic review. Am J Hypertens. 2015;28:1316–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Community Preventive Services Task Force . Cardiovascular disease prevention and control: team-based care to improve blood pressure control. 2016. Available from: https://www.thecommunityguide.org/findings/cardiovascular-disease-team-based-care-improve-blood-pressure-control. Accessed May 14, 2020.
- 22. Frese EM, Richter RR, Burlis TV. Self-reported measurement of heart rate and blood pressure in 10 patients by physical therapy clinical instructors. Phys Ther. 2002;82:1192–1200. [PubMed] [Google Scholar]
- 23. Arena SK, Reyes A, Rolf M, Schlagel N, Peterson E. Blood pressure attitudes, practice behaviors, and knowledge of outpatient physical therapists. Cardiopulm Phys Ther J. 2018;29:3–12. [Google Scholar]
- 24. Albarrati AM. Outpatient physical therapy cardiovascular assessment: physical therapist perspective and experience. Physiother Theory Pract. 2018;29:1–8. [DOI] [PubMed] [Google Scholar]
- 25. Arena SK, Drouin JS, Thompson KA, et al. Prevalence of pre-hypertension and hypertension blood pressure readings among individuals managed by physical therapists in the home care setting : a descriptive study. Cardiopulm Phys Ther J. 2014;25:18–23. [Google Scholar]
- 26. Arena S, LaBelle L, Larsen J, Palomino L, Hew-Butler TPE. Description and comparison of pre and post season blood pressure measures among collegiate athletes: a prospective observational study. Cardiopulm Phys Ther J. 2018. doi. 10.1097/CPT.0000000000000085. [DOI] [Google Scholar]
- 27. APTA (The American Physical Therapy Association) . Guide to physical therapist practice. Phys Ther. 2014;81:9–746. [PubMed] [Google Scholar]
- 28. Chartered Society of Physiotherapists Duty of Care. Information Paper. 2013. Available from: https://www.csp.org.uk/publications/duty-care.Accessed January 6, 2020.
- 29. Frese EM, Fick A, Sadowsky HS. Blood pressure measurement guidelines for physical therapists. Cardiopulm Phys Ther J. 2011;22:5–12. [PMC free article] [PubMed] [Google Scholar]
- 30. Arena SK, Simon L, Peterson EL. Aneroid blood pressure manometer calibration rates in physical therapy curricula: a descriptive study. Cardiopulm Phys Ther J. 2016;27:56–61. [Google Scholar]
- 31. Arena SK, Bacyinski A, Simon L, Peterson EL. Aneroid blood pressure manometer calibration rates of devices used in home health. Home Healthc Now. 2016;34:23–82. [DOI] [PubMed] [Google Scholar]
- 32. Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Turner MJ, Irwig L, Bune AJ, Kam PC, Baker AB. Lack of sphygmomanometer calibration causes over- and under-detection of hypertension: a computer simulation study. J Hypertens. 2006;24:1931–1938. [DOI] [PubMed] [Google Scholar]
- 34. O’Brien E, Asmar R, Beilin L, et al. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–848. [DOI] [PubMed] [Google Scholar]
- 35. Severin R, Arena SK. #Vitals Are VITAL Resource Page. Cardiovascular & Pulmonary Section-American Physical Therapy Association. http://www.cardiopt.org/vitalsarevital/. Accessed January 6, 2020.
- 36. EPA (The Environmental Protection Agency) . Mercury Study Report to Congress Volume I: Executive Summary; EPA-452/R-97-003. 1998.
- 37. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals. Part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American Heart Association Council on high blood pressure research. Hypertension. 2005;45:142–161. [DOI] [PubMed] [Google Scholar]
- 38. Ogedegbe G, Pickering T. Principles and techniques of blood pressure measurement. Cardiol Clin. 2010;28:571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roerecke M, Kaczorowski J, Myers MG. Comparing automated office blood pressure readings with other methods of blood pressure measurement for identifying patients with possible hypertension: a systematic review and meta-analysis. JAMA Intern Med. 2019;179:351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Myers MG, McInnis NH, Fodor GJ, Leenen FHH. Comparison between an automated and manual sphygmomanometer in a population survey. Am J Hypertens. 2008;21:280–283. [DOI] [PubMed] [Google Scholar]
- 41. Shinohara T, Tsuchida N, Seki K, et al. Can blood pressure be measured during exercise with an automated sphygmomanometer based on an oscillometric method? J Phys Ther Sci. 2017;29:1006–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stergiou GS, Christodoulakis GR, Nasothimiou EG, Giovas PP, Kalogeropoulos PG. Can validated wrist devices with position sensors replace arm devices for self-home blood pressure monitoring? A randomized crossover trial using ambulatory monitoring as reference. Am J Hypertens. 2008;21:753–758. [DOI] [PubMed] [Google Scholar]
- 43. De Senarclens O, Feihl F, Giusti V, et al. Brachial or wrist blood pressure in obese patients: which is the best? Blood Pressure Monitoring. 2008;13:149–151. [DOI] [PubMed] [Google Scholar]
- 44. Cameron JD, Stevenson I, Reed E, McGrath BP, Dart AM, Kingwell BA. Accuracy of automated auscultatory blood pressure measurement during supine exercise and treadmill stress electrocardiogram-testing. Blood Press Monit. 2004;9:269–275. [DOI] [PubMed] [Google Scholar]
- 45. Parati G, Ochoa JE. Automated-auscultatory (hybrid) sphygmomanometers for clinic blood pressure measurement: a suitable substitute to mercury sphygmomanometer as reference standard. J Hum Hypertens. 2012;26:211–213. [DOI] [PubMed] [Google Scholar]
- 46. Bovet P, Hungerbuhler P, Quilindo J, Grettve ML, Waeber B, Burnand B. Systematic difference between blood pressure readings caused by cuff type. Hypertension. 1994;24:786–792. [DOI] [PubMed] [Google Scholar]
- 47. Ostchega Y, Prineas RJ, Paulose-Ram R, Grim CM, Willard G, Collins D. National Health and 11 nutrition examination survey 1999–2000: effect of observer training and protocol standardization on reducing blood pressure measurement error. J Clin Epidemiol. 2003;57:652. [DOI] [PubMed] [Google Scholar]
- 48. Lane D, Beevers M, Barnes N, et al. Inter-arm differences in blood pressure: when are they clinically significant? J Hypertens. 2002;20:1089–1095. [DOI] [PubMed] [Google Scholar]
- 49. Cushman WC, Cooper KM, Horne RA, Meydrech EF. Effect of back support and stethoscope head on seated blood pressure determinations. Am J Hypertens. 1990;3:240–241. [DOI] [PubMed] [Google Scholar]
- 50. Peters GL, Binder SK, Campbell NR. The effect of crossing legs on blood pressure: a randomized single-blind cross-over study. Blood Press Monit. 1999;4:97–101. [PubMed] [Google Scholar]
- 51. Mitchell P, Parlin R, Blackburn H. Effect of vertical displacement of the arm on indirect blood- pressure measurement. N Engl J Med. 1964;271:72–74. [DOI] [PubMed] [Google Scholar]
- 52. Mourad A, Carney SL, Gillies A, Jones B, Nanra R, Trevillian P. Arm position and blood pressure: a risk factor for hypertension? J Hum Hypertens. 2003;17:389–395. [DOI] [PubMed] [Google Scholar]
- 53. James PA, Oparil S, Carter BL, et al. Evidence-based guideline for the management of high blood pressure in adults. JAMA. 2013;1097:1–14. [DOI] [PubMed] [Google Scholar]
- 54. Bryant JR, Hajjar RT, Lumley C, Chaiyasate K. Clinical question: in women who have undergone breast cancer surgery, including lymph node removal, do blood pressure measurements taken in the ipsilateral arm increase the risk of lymphedema? J Okla State Med Assoc. 2016;109:474–479. [PubMed] [Google Scholar]
- 55. Pescatello LS, Arena R, Riebe D, Thompson PD. ACSM’s Guidelines for Exercise Testing and Prescription. 9th ed. Philadelphia, PA, USA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2014. [Google Scholar]
- 56. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. JAMA. 2003;289:2560. [DOI] [PubMed] [Google Scholar]
- 57. Torres J. Measure up pressure down. Health Promot Pract. 2016;17:317–319. [Google Scholar]
- 58. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. [DOI] [PubMed] [Google Scholar]
- 59. Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–538. [DOI] [PubMed] [Google Scholar]
- 60. Netea RT, Lenders JWM, Smits P, Thien T. Both body and arm position significantly influence blood pressure measurement. J Hum Hypertens. 2003;17:459–462. [DOI] [PubMed] [Google Scholar]
- 61. Centers for Disease Control and Prevention . Stopping elderly accidents deaths and injuries (STEADI): measuring orthostatic blood pressure .2017. Available fromhttps://www.cdc.gov/steadi/pdf/STEADI-Assessment-Measuring BP-508.pdf. Accessed January 6, 2020. [Google Scholar]
- 62. McJunkin B, Rose B, Amin O, et al. Detecting initial orthostatic hypotension: a novel approach. J Am Soc Hypertens. 2015;9:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schultz MG, Otahal P, Picone DS, Sharman JE. Clinical relevance of exaggerated exercise blood pressure. J Am Coll Cardiol. 2015;66:1843–1845. [DOI] [PubMed] [Google Scholar]
- 64. Barlow PA, Otahal P, Schultz MG, Shing CM, Sharman JE. Low exercise blood pressure and risk of cardiovascular events and all-cause mortality: systematic review and meta-analysis. Atherosclerosis. 2014;237:13–22. [DOI] [PubMed] [Google Scholar]
- 65. Schultz MG, Sharman JE. Exercise hypertension. Pulse. 2013;1:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weiss SA, Blumenthal RS, Sharrett AR, Redberg RF, Mora S. Exercise blood pressure and future cardiovascular death in asymptomatic individuals. Circulation. 2010;121:2109–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. [DOI] [PubMed] [Google Scholar]
- 68. Sabbahi A, Arena R, Kaminsky LA, Myers J, Phillips SA. Peak blood pressure responses during maximum cardiopulmonary exercise testing. Hypertension. 2018;71:229–236. [DOI] [PubMed] [Google Scholar]
- 69. Ross R, Blair SN, Arena R, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134:e653–e699. [DOI] [PubMed] [Google Scholar]
- 70. Peacock J, Diaz KM, Viera AJ, Schwartz JE, Shimbo D. Unmasking masked hypertension: prevalence, clinical implications, diagnosis, correlates and future directions. J Hum Hypertens. 2014;28:521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bromfield SG, Shimbo D, Booth JN, et al. Cardiovascular risk factors and masked hypertension novelty and significance. Hypertension. 2016;68:1475–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193–2198. [DOI] [PubMed] [Google Scholar]
- 73. Kayrak M, Bacaksiz A, Vatankulu MA, et al. Exaggerated blood pressure response to exercise: a new portent of masked hypertension. Clin Exp Hypertens. 2010;32:560–568. [DOI] [PubMed] [Google Scholar]
- 74. Keller K, Stelzer K, Ostad MA, Post F. Impact of exaggerated blood pressure response in normotensive individuals on future hypertension and prognosis: systematic review according to PRISMA guideline. Adv Med Sci. 2017;62:317–329. [DOI] [PubMed] [Google Scholar]
- 75. Schultz MG, Hare JL, Marwick TH, Stowasser M, Sharman JE. Masked hypertension is “unmasked” by low-intensity exercise blood pressure. Blood Press. 2011;20:284–289. [DOI] [PubMed] [Google Scholar]
- 76. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380:2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 78. Green DJ. Exercise training as vascular medicine: direct impacts on the vasculature in humans. Exerc Sport Sci Rev. 2009;37:196–202. [DOI] [PubMed] [Google Scholar]
- 79. Sabbahi A, Arena R, Elokda A, Phillips SA. Exercise and hypertension: uncovering the mechanisms of vascular control. Prog Cardiovasc Dis. 2016;59:226–234. [DOI] [PubMed] [Google Scholar]
- 80. Naci H, Ioannidis JPA. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hambrecht R, Walther C, Möbius-Winkler S, et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: a randomized trial. Circulation. 2004;109:1371–1378. [DOI] [PubMed] [Google Scholar]
- 82. Mu L, Cohen AJ, Mukamal KJ. Prevalence and predictors of resistance and aerobic exercise among hypertensive adults in the United States. J Hum Hypertens. 2015;29:394–395. [DOI] [PubMed] [Google Scholar]
- 83. Pescatello LS, Buchner DM, Jakicic JM, et al. Physical activity to prevent and treat hypertension. Med Sci Sport Exerc. 2019;51:1314–1323. [DOI] [PubMed] [Google Scholar]
- 84. Whelton PK, He J, Appel LJ, et al. Primary prevention of hypertension: clinical and public health advisory from the National High Blood Pressure Education program. JAMA. 2002;288:1882–1888. [DOI] [PubMed] [Google Scholar]
- 85. Sagiv M, Ben-Sira D, Goldhammer E. Direct vs. indirect blood pressure measurement at peak anaerobic exercise. Int J Sports Med. 1999;20:275–278. [DOI] [PubMed] [Google Scholar]
- 86. APTA (The American Physical Therapy Association) . Vision statement for the physical therapy profession. http://www.apta.org/Vision/. Accessed January 6, 2020.


