Abstract
A clinical practice guideline on total knee arthroplasty was developed by an American Physical Therapy (APTA) volunteer guideline development group that consisted of physical therapists, an orthopedic surgeon, a nurse, and a consumer. The guideline was based on systematic reviews of current scientific and clinical information and accepted approaches to management of total knee arthroplasty.
Keywords: Knee Arthroplasty, Knee, Knee Injuries
This clinical practice guideline (CPG) is based on a systematic review of published studies with regard to the physical therapist management of patients undergoing total knee arthroplasty. In addition to providing practice recommendations, this guideline also highlights limitations in the literature, areas that require future research, intentional vagueness, and quality improvement activities.
This guideline is intended to be used by all qualified and appropriately trained physical therapists involved in the management of patients undergoing total knee arthroplasty (TKA). It is also intended to serve as an information resource for decision makers and developers of practice guidelines and recommendations.
Overview
Goals and Rationale
The purpose of this CPG is to help improve treatment based on the current best evidence. Current evidence-based medicine standards demand that clinicians use the best available evidence in their clinical decision making, incorporate clinical expertise, and consider the patient’s values. To assist clinicians, this guideline contains a systematic review of the available literature regarding the management of patients undergoing TKA. The systematic review detailed herein was conducted on studies published between 1995 and 2018 and demonstrates where there is good evidence, where evidence is lacking, and the topics that future research must target in order to improve the management of patients undergoing TKA.
Musculoskeletal care is provided in many different settings by many different providers. This guideline is an educational tool to guide qualified clinicians through a series of treatment decisions in an effort to improve quality and efficiency and reduce unwarranted variation of care. Recommendations are part of evidence-based practice, and the patient’s wants and needs must be considered in the clinical decision-making process. This guideline should not be construed as including all proper methods of care or excluding methods of care reasonably directed to obtaining the same results. The ultimate judgment regarding any specific procedure or treatment must be made in light of all circumstances presented by the patient, including preferences, safety, and postoperative time period, as well as the needs and resources particular to the locality or institution.
Intended Users
This guideline is intended to be used by physical therapists for the management of patients who will undergo or have undergone TKA. Physical therapists are health care professionals who help individuals maintain, restore, and improve movement, activity, and functioning, thereby enabling optimal performance and enhancing health, well-being, and quality of life. Typically, the physical therapist is a graduate of a physical therapist education program accredited by the Commission on Accreditation in Physical Therapy Education and is licensed to practice physical therapy. Orthopedic surgeons, adult primary care clinicians, geriatricians, hospital-based adult medicine specialists, physiatrists, occupational therapists, nurse practitioners, physician assistants, emergency clinicians, and other health care professionals who routinely see this type of patient in various practice settings may also benefit from this guideline. This guideline is not intended for use as a benefits determination document.
Care for individuals undergoing TKA is based on decisions made by them in consultation with their health care team, which may comprise physicians, surgeons, nurses, physical therapists, and occupational therapists. Care includes conservative management approaches and consideration of CPGs such as the American Academy of Orthopaedic Surgeons’ (AAOS) “Evidence-Based Clinical Practice Guideline on the Surgical Management of Osteoarthritis of the Knee.”1
Once the individual (or advocate) has been informed of the nature of the available therapies and their rationale, duration, benefits, and risks and has discussed the options with their health care provider, an informed and shared decision can be made.
Patient Population
This guideline addresses the management of adult patients with knee osteoarthritis undergoing primary TKA. It is not intended to address management of revision or partial knee arthroplasty, pediatric patients, or patients with rheumatoid arthritis. In addition, this guideline is not intended to address nonoperative management of patients with osteoarthritis.
Burden of Disease
Chronic knee pain is a leading cause of musculoskeletal disability in the United States. This condition often leads to TKA (also known as total knee replacement), which is the most commonly performed orthopedic surgery in the lower extremity. In 2013, 662,545 TKAs were performed, a steady increase in the number of procedures since 1992.2 Although the length of stay has declined during the same time period—from 8.9 days to 3.4 days (67%)—hospital charges have steadily increased.2 In 2013, the total hospital charges for TKA were $36.64 billion.2 Additionally, the number of TKAs performed annually in the United States is expected to increase by 855% between 2012 and 2050, equating to 2854 procedures per 100,000 US citizens over 40 years of age.3 In 2010, the prevalence of knee osteoarthritis in North America was 3.1%, and globally the prevalence was 3.8%. Prevalence was higher in women and peaked at around 50 years of age. Globally, hip and knee osteoarthritis was ranked as the 11th highest contributor to disability among almost 300 health conditions.4
Etiology
TKA consists of resection of the diseased or degenerative articular surfaces of the knee, replacing the surface with metal and polyethylene prosthetic components. The disease or degeneration is a result of destruction of the joint cartilage from osteoarthritis, rheumatoid arthritis, posttraumatic degenerative joint disease, or other pathologic conditions accounting for more than 95% of TKA surgeries.5
Risk Factors
Both treatable or modifiable risk factors and nonmodifiable risk factors will impact outcomes after TKA.
An understanding and appreciation of the risk factors helps inform care and determine prognosis. The guideline development group (GDG; also “work group”) (Appendix) identified aspects of the relationship between risk factors and outcomes in this patient population and made specific searches and recommendations. Refer to the specific recommendations below for details. A summary of recommendations is provided in Table 1.
Table 1.
Summary of Recommendations for Total Knee Arthroplasty (TKA)
| Interventions | Rating | Practice Recommendations |
|---|---|---|
| Preoperative exercise program | ♦♦♦◊ | Physical therapists should design preoperative exercise programs and teach patients undergoing total knee arthroplasty (TKA) to implement strengthening and flexibility exercises. |
| Preoperative education | ♦◊◊◊ | It is the consensus of the work group that physical therapists or other team members should provide preoperative education for patients undergoing TKA, including, at a minimum: patient expectations during hospitalization and factors influencing discharge planning and disposition, the postoperative rehabilitation program, safe transferring techniques, use of assistive devices, and fall prevention. |
| Continuous passive motion (CPM) device use for mobilization | ♦♦♦◊ | Physical therapists should NOT use CPMs for patients who have undergone primary, uncomplicated TKA. |
| Cryotherapy | ♦♦♦◊ | Physical therapists should teach patients and other care givers use of cryotherapy and encourage its use for early postoperative pain management for patients who have undergone TKA. |
| Physical activity | ♦◊◊◊ | It is the consensus of the work group that physical therapists should develop an early mobility plan and teach patients who have undergone TKA regarding the importance of early mobility and appropriate progression of physical activity, based on safety, functional tolerance, and physiological response. |
| Motor function training (balance, walking, movement, symmetry) | ♦♦♦♦ | Physical therapists should include motor function training (eg, balance, walking, movement symmetry) for patients who have undergone TKA. |
| Postoperative knee range-of-motion (ROM) exercise | ♦◊◊◊ | It is the consensus of the work group that physical therapists should teach and encourage patients to implement passive, active assistive, and active ROM exercises for the involved knee following TKA. |
| Immediate postoperative knee flexion during rest for blood loss and swelling | ♦♦◊◊ | To reduce immediate postoperative blood loss and swelling in the first 7 days after surgery, physical therapists or other team members may teach patients to position the operated knee in some degree of flexion (30°-90°) while resting. |
| Neuromuscular electrical stimulation (NMES) | ♦♦♦◊ | Physical therapists should use NMES for patients who have undergone TKA to improve quadriceps muscle strength, gait performance, performance-based outcomes, and patient-reported outcomes. |
| Resistance and intensity of strengthening exercise | ♦♦♦◊ | Physical therapists should design, implement, teach, and progress patients who have undergone TKA in high-intensity strength training and exercise programs during the early postacute period (ie, within 7 days after surgery) to improve function, strength, and ROM. |
| Prognostic factors: body mass index (BMI), depression, preoperative ROM, physical function and strength, age, diabetes, number of comorbidities, and sex | ♦♦♦◊ | Physical therapist management should take into consideration the following factors when determining prognosis, providing treatment, and engaging in informed decision making and expectation setting with patients undergoing TKA: |
| Higher BMI is associated with more postoperative complications and worse postoperative outcomes. | ||
| Depression is associated with worse postoperative outcomes. | ||
| Preoperative ROM is positively associated with postoperative ROM but has minimal, if any, effect on physical function and quality of life. | ||
| Preoperative physical function is positively associated with postoperative physical function. | ||
| Preoperative strength is positively associated with postoperative physical function. | ||
| Age is associated with mixed patient-reported, performance-based, and impairment-based outcomes. | ||
| Diabetes is not associated with worse functional outcomes. | ||
| A greater degree of comorbidity is associated with worse patient-reported outcomes. | ||
| Sex is associated with both positive and negative effects on postoperative outcomes. | ||
| Prognostic factors: tobacco and patient support | ♦◊◊◊ | It is the consensus of the work group that active tobacco use and lack of patient support (eg, environmental factors including, but not limited to, support and relationships) should be considered as prognostic/risk factors associated with less than optimal functional outcomes. |
| Postoperative physical therapy supervision | ♦♦♦◊ | Supervised physical therapist management should be provided for patients who have undergone TKA. The optimal setting should be determined by patient safety, mobility, and environmental and personal factors. |
| Group-based vs individual-based therapy | ♦♦◊◊ | Physical therapists may use group-based or individual-based physical therapy sessions for patients who have undergone TKA. |
| Physical therapy postoperative timing | ♦♦♦◊ | Physical therapist management should start within 24 hours of surgery and prior to discharge for patients who have undergone TKA. |
| Physical therapy discharge planning | ♦♦♦◊ | It is the consensus of the work group that physical therapists should provide guidance to the care team and to the patient on safe and objective discharge planning, patient functional status, assistance equipment, and services needed to support a safe discharge from the acute care setting. |
| Outcomes assessment | ♦◊◊◊ | It is the consensus of the work group that physical therapists should collect data using the Knee Injury Osteoarthritis Outcomes Survey Joint Replacement (KOOS JR) as a patient-reported outcome measure and both the 30-Second Sit-to-Stand and Timed “Up and Go” (TUG) tests as performance-based outcomes to demonstrate the effectiveness of care provided. At a minimum, these measures should be collected at the first visit and upon conclusion of care from each setting. |
Figure.
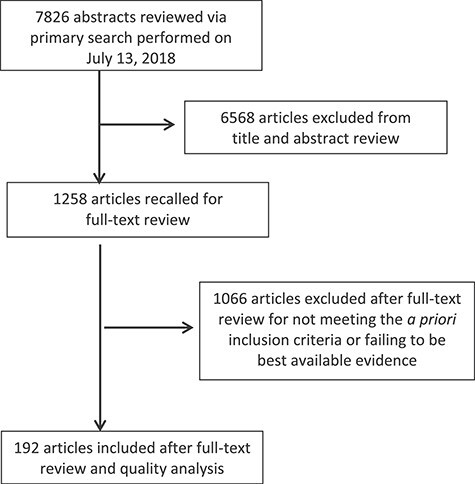
Study attrition flowchart.
Potential Benefits, Risks, Harms, and Costs
The potential benefits, risks, harms, and costs are provided for each recommendation within this document.
Future Research
Consideration for future research is provided for each recommendation within this document.
Methods
The methods used to create this CPG were intended to minimize bias and enhance transparency in the selection, appraisal, and analysis of the available evidence. These processes are vital to the development of reliable, transparent, and accurate clinical recommendations for management of patients undergoing TKA.6 Methods from the APTA Clinical Practice Guideline Process Manual6 and AAOS Clinical Practice Guideline Methodology7 were used in the development of this CPG.
This CPG evaluates the effectiveness of approaches in the management of patients undergoing TKA. APTA sought out the expertise of the AAOS Evidence-Based Medicine Unit as paid consultants to assist in the creation of this CPG. The GDG consisted of members from APTA and its representative sections, AAOS, the National Association of Orthopaedic Nurses, and a patient safety activist from Consumers United for Evidence-Based Healthcare. All GDG members, APTA staff, and methodologists were free of potential conflicts of interest relevant to the topic under study, as recommended by CPG development experts.8
This CPG was prepared by the APTA GDG with the assistance of APTA staff and the AAOS Clinical Quality and Value Department (staff evidence-based medicine methodologists). To develop this guideline, the GDG held an introductory meeting on September 22, 2017, to establish the scope of the CPG. The GDG defined the scope by creating PICO(T) questions (population, intervention, comparison, outcome, and time) that directed the literature search. The medical librarian from AAOS created and executed the searches. Supplementary Appendix 1 contains the search strategies used. AAOS chose the included studies (Figure; Supplementary Appendix 2), performed quality assessments, and wrote initial recommendations based on the published guideline methodology. The GDG performed final reviews of recommendations, provided rationale in the context of physical therapist practice, and adjusted the strength of the recommendations depending on the magnitude of benefit, risk, harm, and cost.
Additional background on the people and processes involved in the creation of this guideline are provided in Supplementary Appendix 1.
Best-Evidence Synthesis
The guideline includes only the best available evidence for any given outcome addressing a recommendation. Accordingly, the highest-quality evidence for any given outcome is included first, if it was available. In the absence of 2 or more occurrences of an outcome based on the highest-quality evidence, outcomes based on the next level of quality were considered until at least 2 or more occurrences of an outcome had been acquired. For example, if there were 2 “moderate”-quality occurrences of an outcome that addressed a recommendation, the recommendation does not include “low”-quality occurrences of evidence for this outcome. A summary of excluded articles can be viewed in Supplementary Appendix 1, and the data findings for each recommendation can be viewed in Supplementary Appendix 3.
Literature Searches
The medical librarian conducted a comprehensive search of MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials based on key terms and concepts from the PICO(T) questions. Retrospective noncomparative case series, medical records review, meeting abstracts, meta-analyses, systematic reviews, historical articles, editorials, letters, and commentaries were excluded. Bibliographies of relevant systematic reviews were hand searched for additional references. All databases were last searched on July 13, 2018, and searches were limited to publication dates from 1995 to 2018 and publications in the English language.
Defining the Strength of the Recommendations
Judging the strength of evidence is only a steppingstone toward arriving at the strength of a CPG recommendation. The operational definitions for the quality of evidence are listed in Table 2, and rating of magnitude of benefits versus risk, harms, and cost is provided in Table 3. The strength of recommendation, listed in Table 4, takes into account the quality, quantity, and trade-off among the benefits and harms of a treatment, the magnitude of a treatment’s effect, and whether there are data on critical outcomes. Table 5 addresses how to link the assigned grade with the level of obligation of each recommendation.
Table 2.
Rating Quality of Evidence
| Rating of Overall Quality of Evidence | Definition |
|---|---|
| High | Preponderance of Level 1 or 2 evidence with at least 1 Level I study. Indicates a high level of certainty that further research is not likely to change outcomes of the combined evidence. |
| Moderate | Preponderance of Level 2 evidence. Indicates a moderate level of certainty that further research is not likely to change the outcomes direction of the combined evidence; however, further evidence may impact the magnitude of the outcome. |
| Low | A moderate level of certainty of slight benefit, harm, or cost, or a low level of certainty for moderate-to-substantial benefit, harm, or cost. Based on Level II thru V evidence. Indicates that there is some but not enough evidence to be confident of the true outcomes of the study and that future research may change the direction of the outcome and/or impact magnitude of the outcome. |
| Insufficient | Based on Level II thru V evidence. Indicates minimal or conflicting evidence to support the true direction and/or magnitude of the outcome. Future research may inform the recommendation. |
Table 3.
Magnitude of Benefit, Risk, Harm, and Cost
| Rating of Magnitude | Definition |
|---|---|
| Substantial | The balance of the benefits versus risk, harms, or cost overwhelmingly supports a specified direction. |
| Moderate | The balance of the benefits versus risk, harms, or cost supports a specified direction. |
| Slight | The balance of the benefits versus risk, harms, or cost demonstrates a small support in a specified direction. |
Table 4.
Strength of Recommendations
| Strength | Strength Visual | Definition |
|---|---|---|
| Strong | ♦♦♦♦ | A high level of certainty of moderate-to-substantial benefit, harm, or cost, or a moderate level of certainty for substantial benefit, harm, or cost (based on a preponderance of Level 1 or 2 evidence with at least 1 Level 1 study). |
| Moderate | ♦♦♦◊ | A high level of certainty of slight-to-moderate benefit, harm, or cost, or a moderate level of certainty for a moderate level of benefit, harm, or cost (based on a preponderance of Level 2 evidence, or a single high-quality RCT). |
| Weak | ♦♦◊◊ | A moderate level of certainty of slight benefit, harm, or cost, or a low level of certainty for moderate-to-substantial benefit, harm, or cost (based on Level 2 through 5 evidence). |
| Theoretical/ foundational | ♦◊◊◊ | A preponderance of evidence from animal or cadaver studies, from conceptual/theoretical models/principles, or from basic science/bench research; or published expert opinion in peer-reviewed journals that supports the recommendation. |
| Best Practice | ♦◊◊◊ | Recommended practice based on current clinical practice norms; exceptional situations in which validating studies have not or cannot be performed yet there is a clear benefit, harm, or cost; or expert opinion. |
| Research | An absence of research on the topic or disagreement among conclusions from higher-quality studies on the topic. |
Table 5.
Linking Strength of Recommendation, Quality of Evidence, Rating of Magnitude, and Preponderance of Risk Versus Harm to the Language of Obligation
| Recommendation Strength | Quality of Evidence and Rating of Magnitude | Preponderance of Benefit or Risk, Harm, or Cost | Level of Obligation to Follow the Recommendation |
|---|---|---|---|
| Strong | High quality and moderate-to-substantial magnitude or | Benefit | Must or Should |
| Moderate quality and substantial magnitude | Risk, harms, or cost | Must not or Should not | |
| Moderate | High quality and slight-to-moderate magnitude or | Benefit | Should |
| Moderate quality and moderate magnitude | Risk, harms, or cost | Should not | |
| Weak | Moderate quality and slight magnitude or | Benefit | May |
| Low quality and moderate-to-substantial magnitude | Risk, harms, or cost | May not | |
| Theoretical/ foundational | N/A | Benefit | May |
| Risk, harms, or cost | May not | ||
| Best Practice | Insufficient quality and clear magnitude | Benefit | Should or May |
| Risk, harms, or cost | Should not or May not | ||
| Research | Insufficient quality and unclear magnitude or Conflicting high-to-moderate quality and conflicting magnitude |
Varies | N/A |
Voting on the Recommendations
GDG members agreed upon the strength of every recommendation. When changes were made to the strength of a recommendation based on the magnitude of benefit or potential risk, harm, or cost, the GDG voted in person, via phone, or email and provided an explanation in the rationale.
Role of the Funding Source
The American Physical Therapy Association, which funded the volunteer GDG, provided coordination and played no role in the design, conduct, and reporting of the recommendations.
Peer Review and Public Commentary
Following the formation of a final draft, the CPG review draft was subjected to a 4-week peer review for additional input from external content experts and stakeholders. More than 350 comments (Supplementary Appendix 4) were collected via an electronic structured review form. All peer reviewers were required to disclose any potential conflicts of interest, which were recorded and, as necessary, addressed.
After modifying the draft in response to peer review, the CPG was subjected to a 2-week public comment period. Commenters consisted of members of the APTA Board of Directors (Board), the APTA Scientific and Practice Affairs Committee (SPAC), all relevant APTA sections, stakeholder organizations, and the physical therapy community at large. More than 194 public comments were received. Revisions to the draft were made in response to relevant comments.
Recommendations
Preoperative Exercise Program ♦♦♦◊
Physical therapists should design preoperative exercise programs and teach patients undergoing total knee arthroplasty (TKA) to implement strengthening and flexibility exercises. Evidence Quality: High; Recommendation Strength: Moderate.
Action Statement Profile
Aggregate evidence quality: 6 high-quality studies9–14 and 3 moderate-quality studies.15–17
Rationale
Four high-quality studies9,11,13, 14 and 2 moderate-quality studies15,16 support the use of preoperative physical therapy training/exercise programs for patients undergoing TKA and are associated with better postoperative functional outcomes. Summary of the outcomes measured and length of follow-up:
Total Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), subscales including function, pain and stiffness, and the Medical Outcomes Study 36-Item Short Form Survey (SF-36) physical function scores improved at 1 and 3 months postsurgery with preoperative training.9
Modified WOMAC, subscales including function and stiffness, and pain using the visual analog scale (VAS) improved at 1 and 3 months postsurgery with preoperative quadriceps muscle exercise.16
SF-36 physical function component score improved at 12 weeks postsurgery with preoperative lower extremity exercise.14
Knee Injury Osteoarthritis Outcomes Survey (KOOS) activities of daily living (ADL) improved at 6 weeks and 3 months postsurgery with preoperative physical therapy.15
KOOS ADL, KOOS pain score, and EuroQol Five Dimensions Questionnaire and visual analog scale (EQ5D –VAS) (generic health status instrument) improved at 6 weeks postsurgery with preoperative neuromuscular exercise program and standard education.17
Iowa Level of Assistance Scale total score improved at 3 days postsurgery with preoperative physical therapy.11
Hospital for Special Surgery Knee Rating improved at 12 weeks postsurgery with preoperative cardiovascular conditioning.10
Length of inpatient stay was reduced with preoperative training.9
Stair Test improved at 1 and 3 months postsurgery with preoperative training.9
Timed “Up and Go” (TUG) Test improved at 1 and 3 months postsurgery with preoperative training.9
Biodex overall stability index score improved at 6 weeks postsurgery with preoperative training.12
Knee flexion range of motion (ROM) improved at 3 months postoperative with preoperative training.9
Knee extension ROM improved at 1 and 3 months postsurgery with preoperative training.9
Quadriceps strength improved at 1 and 3 months postsurgery with preoperative quadriceps training.16
Isometric hip abduction strength improved at 1 and 3 months postsurgery with preoperative training.9
Isometric knee flexion strength improved at 1 and 3 months postsurgery with preoperative training.9
Isometric knee extension strength improved at 3 months postsurgery with preoperative training.9
Fewer postoperative days were required to reach 90 degrees of knee flexion with preoperative exercise.13
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Improved activities
Decreased pain
Improved balance
Improved knee flexion ROM
Improved knee extension ROM
Improved isometric knee and hip strength
Improved report of quality of life (eg, as measured by SF-36)
Reduced length of stay of inpatient stay
Risk, harm, and/or cost are as follows:
No reported harms were associated with implementing this recommendation.
Team members should be aware of potential complications after TKA that may affect exercise including incision healing, thromboembolism, and joint stiffness/arthrofibrosis. While costs were not reported in studies, there may be an expected associated expense.
Benefit-harm assessment: With no reported risk or harm in the studies, there is a preponderance of evidence-supported benefit for this recommendation.
Future research
Additional research on the effects of preoperative exercise programs is required. This research should examine specific regimens or recommendations for type, frequency, duration, and progression and should consider patient preferences. Outcomes related to length of stay, discharge to home, patient satisfaction, and return to activities and participation should be included.
Value judgments
None were identified.
Intentional vagueness
Specific exercises are identified based on the individual patient. The preoperative examination and evaluation guide the discussions for appropriate interventions included in the plan of care.
Exclusions
None were identified.
Quality improvement
Organizations may use the completion of a preoperative visit to physical therapy that includes preoperative strengthening and flexibility instruction as a performance indicator.
Implementation and audit
Organizations may audit the rate of occurrence of preoperative physical therapy visits that includes preoperative strengthening and flexibility instructions for patients who receive a TKA.
Preoperative Education ♦◊◊◊
It is the consensus of the work group that physical therapists or other team members should provide preoperative education of patients undergoing TKA, including, at a minimum, patient expectations during hospitalization and factors influencing discharge planning and disposition, postoperative rehabilitation program, safe transferring techniques, use of assistive devices, and fall prevention. Evidence Quality: Insufficient; Recommendation Strength: Best Practice.
Action Statement Profile
Aggregate evidence quality: There was 1 study of moderate quality18 that supported the use of preoperative education to shorten inpatient length of stay and decrease medical expenses.
Rationale
In light of limited evidence, the GDG believed that preoperative education supports best practice and was in consensus with this recommendation. Patient education is an essential part of patient care in all settings, particularly in an increasingly patient-centered health care environment. Ingadottir et al19 reported that patients undergoing TKA experienced a significant difference in the knowledge they expected to have preoperatively as compared with the information they were given by the time of hospital discharge. The authors found that the closer the match between expectations and information given, the more satisfied patients were with their care; however, they suggested that preoperative education did not completely fulfill patients’ expectations and that postoperative education was also important.
Soeters et al20 implemented preoperative physical therapy education for patients with total joint arthroplasty (hip and knee) that included a one-time, one-on-one educational session with a physical therapist and gave patients access to a microsite providing additional information about postoperative complications, precautions, and mobility. They found that patients reached the criteria for readiness for discharge from physical therapy services in the inpatient setting faster if they had the educational intervention. They also found that all patients accessed the microsite at least once, suggesting a desire for knowledge. In a systematic review of preoperative education for patients with total hip or knee replacement in 2014, the evidence suggested that preoperative education might be a useful treatment adjunct, particularly in people with depression, anxiety, or unrealistic expectations.21 The review also noted the low risks associated with the intervention.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Improved patient adherence
Decreased postsurgical complication
Shortened inpatient length of stay
Risk, harm, and/or cost are as follows:
No expected risk or harms were associated with this recommendation.
There may be an expected associated expense for the visit.
Benefit-harm assessment. There is a preponderance of benefit for this recommendation.
Future research
Additional research on patient preoperative education led by a physical therapist or other team member is required. This research should evaluate the method and frequency of the education delivered. In addition, outcomes such as patient anxiety, health literacy, and satisfaction should be considered when evaluating the benefits of preoperative education.
Value judgments
Although there was not a preponderance of high-quality evidence, the GDG felt compelled to make a recommendation to support the use of preoperative education for patients undergoing TKA. Preoperative education was believed to be associated with better postoperative functional outcomes.
Intentional vagueness
Not applicable.
Exclusions
None were identified.
Quality improvement
Organizations may use the completion of a preoperative visit to physical therapy that includes education as a performance indicator.
Implementation and audit
Organizations may audit the rate of occurrence of preoperative physical therapy visits that include education for patients who have undergone TKA and its association with postoperative complication, length of stay, and patient satisfaction.
Continuous Passive Movement Device (CPM) Use for Mobilization ♦♦♦◊
Physical therapists should NOT use CPMs for patients who have undergone primary, uncomplicated TKA. Evidence Quality: High; Recommendation Strength: Moderate.
Action Statement Profile
Aggregate evidence quality: 4 high-quality studies,22–25 6 moderate-quality studies,26–31 and 2 low-quality studies.32,33
Rationale
Four high-quality studies,22–25 6 moderate-quality studies,26–31 and 2 low-quality studies32,33 examined the effect of CPM use. Findings from 1 moderate-quality study31 and 2 low-quality studies32,33 reported some significant statistical effects; however, these findings were contradicted by nonsignificant statistical findings in higher-quality studies.22–25 The outcomes measured included knee flexion and extension ROM as well as need for manipulation under anesthesia. Additionally, meta-analyses for the outcomes of function (standardized mean difference [SMD] = 0.14 [−1.10 to 0.39])23,24,28 and hospital length of stay (weighted mean difference [WMD] = −0.15 [−0.60 to 0.30])24,28,29 showed nonsignificant results.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Results for outcomes in function were nonsignificant.
Results for hospital length of stay were nonsignificant.
Risk, harm, and/or cost are as follows:
Bed rest may be prolonged with CPM use.
There is an inconvenience of use.
Although costs were not reported in studies, there is an expected associated expense.
Benefit-harm assessment: There is a preponderance of evidence to support that there is increased risk, harm, and/or cost related to use of CPM for uncomplicated TKA.
Future research
Some subpopulations may benefit from CPM, and this could be explored with studies large enough to allow subgroup analyses or by narrowing inclusion criteria. Examples may be those with TKA revisions or those with particularly poor preoperative ROM.
Value judgments
None were identified.
Intentional vagueness
The nature of an uncomplicated TKA is not explicit in most studies. Only 1 study implied a definition of uncomplicated by exclusions of patients with “concurrent intervention during surgery that could interfere with outcomes (eg, collateral ligament repair), infection of the affected knee, and any major health complication during the hospital stay (eg, pulmonary embolism, heart attack, problems with scar healing).”20
Exclusions
None were identified.
Quality improvement
When CPM is used, there should be documented complications associated with TKA that justify its use.
Implementation and audit
Organizations may audit the use of CPM after TKA and discourage its use unless justified by documented complications associated with the procedure.
Cryotherapy ♦♦♦◊
Physical therapists should teach and encourage use of cryotherapy for early postoperative pain management for patients who have undergone TKA. Evidence Quality: High; Recommendation Strength: Moderate.
Action Statement Profile
Aggregate evidence quality: 6 high-quality studies34–39 and 4 moderate-quality studies.40–43
Rationale
Six high-quality studies34–39 and 4 moderate-quality studies40–43 examined the use of cryotherapy after TKA. One high-quality study37 and 1 low-quality study44 favored cryotherapy over control for pain management, and 1 high-quality study found no difference. Findings from 1 high-quality study37 found improvement in pain management (VAS pain) with the use of ethyl chloride spray (applied during exercise for about 40 seconds at a distance of about 10 cm) versus controls 4 weeks after TKA, and 1 high-quality study35 found no difference at 30 days after TKA in pain management (VAS pain) comparing 45 °F versus 75 °F cryotherapy. One low-quality study44 found improved pain (VAS pain) at 30 days after surgery with the use of a continuous-flow cooling device versus no cooling device. One high-quality study35 and 1 low-quality study44 found no increased complications with cryotherapy versus controls. Findings from 2 high-quality studies34,39 and 2 moderate-quality studies40,43 found no increased complications between cryotherapy modalities. A meta-analysis of 3 studies36,38,43 evaluated cryotherapy devices versus standard cold packs for pain management and found no statistically significant difference.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Improvement in pain management.
Low cost and relatively easy application in all settings.
Risk, harm, and/or cost are as follows:
There were no differences in the rate of adverse events reported between a group receiving cryotherapy and a control group.
There is potential risk of skin irritation, burns, and frostbite; however, risk or harms are not expected when prescribed and monitored appropriately. Appropriately prescribing includes ensuring intact sensation.
Benefit-harm assessment: A preponderance of evidence supports the use of cryotherapy for pain management. There were no reported risks or harms to patients using cryotherapy. As with all treatments, it is advised that the patient be instructed on the use of the delivery system (eg, cooling devices, ethyl chloride spray, ice packs). Furthermore, the therapist and patient should discuss any barriers to using cryotherapy (eg, cost, lack of adequate storage, physical disability) in choosing the appropriate delivery system.
Future research
Future research focused on frequency of use and the length of time cryotherapy is used postsurgery would further inform use.
Value judgments
None were identified.
Intentional vagueness
Although no one application method was shown to be superior, using cryotherapy is supported in managing postoperative pain. There was not sufficient evidence to provide a prescriptive time frame for the application after surgery. In addition, there was insufficient evidence to identify how many days postsurgery cryotherapy should be continued.
Exclusions
None were identified.
Quality improvement
Organizations may use documentation of the use of cryotherapy after TKA as a performance indicator.
Implementation and audit
Organizations may audit occurrence of documentation of use of cryotherapy after TKA to assist in the management of pain.
Physical Activity ♦◊◊◊
It is the consensus of the work group that physical therapists should develop an early mobility plan and teach patients who have undergone TKA regarding the importance of early mobility and appropriate progression of physical activity, based on safety, functional tolerance, and physiological response. Evidence Quality: Insufficient; Recommendation Strength: Best Practice.
Action Statement Profile
Aggregate evidence quality: No high-quality studies related to physical activity for patients undergoing TKA.
Rationale
The GDG members were in consensus with this recommendation that physical activity is an important aspect in recovery and the progression of activities and participation. The second edition of the US Department of Health and Human Services Physical Activity Guidelines provides evidence that routine physical activity, including moderate-to-vigorous aerobic and muscle-strengthening exercises, results in substantial health benefits.45 The recommendations include weight-bearing exercises for bone health, balance activities, and flexibility activities. Furthermore, the guidelines note that people with chronic conditions or disabilities (eg, osteoarthritis of the knee or TKA) benefit from engaging in physical activity to the extent they are able. There is a long list of known health benefits of physical activity, including lowering risk of all-cause mortality, heart disease and its risk factors, and certain types of cancer. According to the guidelines, benefits of physical activity generally outweigh the risk of injuries.
One study of physical activity 1 year following TKA reported that 42% of participants did not meet recommendations for levels of physical activity that promote health.46 An observational study noted that women who were inactive prior to TKA had increased odds of having mobility limitations and dying by age 85.47 Studies of specific types of exercise regimens (aquatic exercise,48,49 Pilates,50 tai chi chuan51) following TKA have shown positive effects on a variety of outcomes, including health-related quality of life, walking distance, balance, and physical function. One study examining a resistance exercise regimen 4 years following TKA demonstrated benefits of increased strength as well as increased walking speed and physical function.52 Amount of physical activity has also been shown to be positively associated with improvements in gait function following TKA.53,54 Furthermore, a dose-response relationship between exercise intensity and gait function has been demonstrated following TKA.55
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Improved gait function, walking distance, balance, physical function, and health-related quality of life.
Improved activities and participation (eg, mobility, self-care, domestic life).
Risk, harm, and/or cost are as follows:
No expected risk or harms are expected when progression is monitored and prescribed appropriately.
Team members should be aware of potential complications after TKA that may affect exercise including incision healing, thromboembolism, and joint stiffness/arthrofibrosis.
Benefit-harm assessment: There is a preponderance of benefit for this recommendation.
Future research
Additional research on the effects of progressive physical activity is required. This research should examine specific regimens or recommendations for physical activity type, frequency, duration, and progression and report patient preferences and safety. Outcomes related to functions of cardiovascular, neurological, and musculoskeletal systems as well as patient’s activities and participation should be included.
Value judgments
Expert opinion and low-quality evidence support the use of progressive physical activity for patients who have undergone TKA for better postoperative functional outcomes. The individualization of physical activity progression with both land-based and aquatic options to match the patient’s goals, abilities, and physiological response should include documentation of objective baseline data, the patient’s goals, and plan of care (interventions, dosage, frequency, and duration) as well as appropriate outcomes to demonstrate patients’ response to the specific approach.
Intentional vagueness
Not applicable.
Exclusions
None were identified.
Quality improvement
Organizations may use the documentation of plan of care and progression of physical activity that include items such as patient preferences, safety, functional tolerance, and physiological response as a performance indicator.
Implementation and audit
Organizations may audit occurrence of documentation of plan of care and progression of physical activity during the physical therapy visits for patients who undergo TKA.
Motor Function Training (Balance, Walking, Movement Symmetry) ♦♦♦♦
Physical therapists should include motor function training (eg, balance, walking, movement symmetry) for patients who have undergone TKA. Evidence Quality: High; Recommendation Strength: Strong.
Action Statement Profile
Aggregate evidence quality: 5 high quality studies56–60 and 1 moderate quality study.61
Rationale
Five high-quality studies56–60 and 1 moderate-quality study61 addressed different aspects of movement retraining after TKA. These studies varied in the types of interventions but included dynamic balance training,56,59 robot-assisted gait retraining,61 movement training with visual biofeedback to promote weight-bearing symmetry,50 or motor functional training.58
The studies that included balance training56,59 found that the balance interventions improved walking function as measured by gait speed, stair-climbing time, and the TUG test 32 weeks after training59 and by the Six-Minute Walk Test 9 months after training.56 Self-reported function was also better in the balance groups on the self-efficacy and sports and recreation subscales of the KOOS56 and physical function subscale on the WOMAC.59 Liao also found that balance training improved reaching and single-leg standing tests of balance.59
The single study that evaluated 2-week robot-assisted gait training61 found better outcomes in the experimental group for balance using the Berg Balance Scale and walking ability measured with the Six-Minute Walk Test. Knee proprioception was also better than that of the control group.
The single study that evaluated feedback on weight-bearing symmetry61 found that subjects in the experimental group had better sagittal plane knee moments 26 weeks after surgery and better times for the Five Times Sit-to-Stand Test 6 and 26 weeks after surgery, but no other differences.
The single study that evaluated functional training,60 warm-up exercise, chair rise, walking, and leg lifts while standing did not find any benefit to the functional training protocol, but the retraining was performed in an unsupervised home setting, and there was a large loss to follow-up (>50%), and the authors concluded they were underpowered to detect potential differences.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Improvement in balance.
Improvement in walking function.
Improvement in activities and participation (eg, getting in and out of car, shopping, household duties).
Risk, harm, and/or cost are as follows:
No expected risk or harms are associated with this recommendation.
Team members should be aware of potential complications after TKA that may affect exercise including incision healing, thromboembolism, and joint stiffness/arthrofibrosis. Some of the more advanced training programs that include weight-bearing biofeedback or robot-assisted gait training may be cost- and resource-prohibitive for most clinical settings.
Benefit-harm assessment: A preponderance of evidence supports including motor function training. The individualization of progression to match the patient’s goals, abilities, and physiological response should include documentation of objective baseline data, the patient’s goals, and plan of care (interventions, dosage, frequency, and duration). This includes the use of appropriate outcomes to demonstrate patient response to the specific approach.
Future research
The long-term impact of normalizing movement patterns or improving balance after TKA remains unknown. Future research should determine whether improving movement symmetry reduces long-term sequelae on the surgical and nonsurgical limbs and whether improving balance after TKA reduces fall prevalence and long-term morbidity. As technology improves, the use of biofeedback-based movement interventions may become more applicable for this patient population. Future research is warranted to determine the feasibility of such systems and long-term impact.
Value judgments
None were identified.
Intentional vagueness
Given the varied nature of the study interventions, the work group cannot recommend a single postoperative movement training program. However, exercises that promote dynamic balance and movement symmetry appear to be appropriate.
Exclusions
None were identified.
Quality improvement
Organizations may use documentation of the use of motor function training (balance, gait, posture) after TKA as a performance indicator.
Implementation and audit
Organizations may audit occurrence of documentation of use of motor function training after a TKA to assist in the management of pain.
Postoperative Knee ROM Exercise ♦◊◊◊
It is the consensus of the work group that physical therapists should engage and teach patients to implement passive, active assistive, and active ROM exercises for the involved knee following TKA. Evidence Quality: Insufficient; Recommendation Strength: Best Practice.
Action Statement Profile
Aggregate evidence quality: Because ROM exercises are considered a standard of care, there have been no studies comparing patients who received ROM exercises to those who did not.
Rationale
Patients with TKA may have restricted knee ROM preoperatively associated with loss of elasticity of the extensor mechanism and capsular structures. Preoperative knee ROM is positively associated with postoperative knee flexion,62–64 and patients with severe-to-moderate knee flexion contractures preoperatively may have a greater risk of postoperative knee flexion contracture.65 Inadequate knee ROM postoperatively may be associated with worse pain and reduction in Knee Society Score (KSS), walking score, and stair climbing 3 to 5 years after surgery.66 Physical therapists have the skills to work with patients to encourage movement, including knee ROM to enhance patients’ potential to reach full functional mobility. The GDG, therefore, were in consensus with this recommendation.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Improved ROM of the knee.
Decreased postsurgical complication.
Improved functional outcomes.
Risk, harm, and/or cost are as follows:
No expected risk or harms were associated with this recommendation.
Team members should be aware of potential complications after TKA that may affect exercise including incision healing, thromboembolism, and joint stiffness/arthrofibrosis.
Benefit-harm assessment: There is a preponderance of benefit for this recommendation.
Future research
Additional research is not anticipated given that a true control group without ROM exercise is unlikely to be approved in any trial.
Value judgments
Despite a lack of high-quality evidence, the GDG felt compelled to make a strong recommendation to support the use of ROM exercises. However, other factors besides ROM substantially contribute to successful postoperative outcomes; therefore, strategies targeting ROM should be complemented with other interventions.
Intentional vagueness
Not applicable.
Exclusions
None were identified.
Quality improvement
Organizations may use the application of postoperative ROM exercises as a quality indicator.
Implementation and audit
Organizations may audit the rate of occurrence of postoperative physical therapy visits that include ROM exercises for patients that receive a TKA.
Immediate Postoperative Knee Flexion During Rest for Blood Loss and Swelling ♦♦◊◊
To reduce immediate postoperative blood loss and swelling in the first 7 days after surgery, physical therapists or other team members may teach patients to position the operated knee in some degree of flexion (30°–90°) while resting. Evidence Quality: High; Recommendation Strength: Weak.
Action Statement Profile
Aggregate evidence quality: 4 high-quality studies67–70 and 1 moderate-quality71 study.
Rationale
Four high-quality studies67–70 and 1 moderate-quality71 study evaluated knee positioning during the immediate postoperative period and its effect on blood loss, swelling, edema management, and ROM. One high-quality study68 found decreased knee blood loss and knee circumference and improved knee flexion ROM when comparing the resting position of 30 degrees of hip flexion and 30 degrees of knee flexion with 30 degrees of hip flexion and full knee extension at 3 and 7 days after TKA. The study did not indicate if ROM was measured passively or actively, and extension ROM was not measured. There was no difference between groups in flexion ROM at 6 weeks.
One high-quality study67 found decreased knee blood loss and circumference when comparing mild flexion (leg elevated 25 cm at the ankle over a backing pad, with a 20-cm backing pad set behind the upper calf to bend the knee mildly) with extension (leg elevated 25 cm at the ankle over a backing pad with full extension of the knee) at 7 days after surgery. The knee positioned in mild flexion, from postoperative days 1 through 7, had greater venous return, less postoperative blood loss and knee swelling, and greater knee flexion ROM. There were no differences in knee flexion ROM at 6 weeks’ follow-up. Extension ROM was not measured.
One high-quality study69 found decreased blood loss and knee circumference at 7 days after surgery in a group of patients with the knee positioned in 45 degrees of hip flexion and 90 degrees of knee flexion when compared with a second group with the knee positioned in full extension. These positions were maintained for the first 6 hours postoperatively. There were no statistical differences in knee flexion ROM at 7 days between groups. Extension ROM was not measured.
One high-quality study70 compared patients with the knee positioned in 60 degrees of hip flexion and 60 degrees of knee flexion with a group of patients positioned in full knee extension. These positions were maintained for the first 48 hours after surgery. The results showed decreased blood loss, shorter hospital length of stay by 1.9 days, decreased knee circumference, and greater flexion ROM at 6 weeks postsurgery (105 degrees vs 98 degrees) in the group with the knee resting in flexion. Overall length of stay for both groups averaged over 10 days in these Chinese hospitals. There were no differences in knee flexion ROM at 6 months follow-up.
One moderate-quality study71 found no difference in knee blood loss, circumference, or flexion ROM when comparing high flexion (70 degrees) to mild flexion (30 degrees) at 7 days after surgery. The mean length of stay in this Italian hospital was 8 days.
These findings further support the meta-analysis by Jiang et al72 that assessed the impact of flexion versus extension of knee position on outcomes after TKA. This later study concluded that positioning the knee in flexion in the early postoperative stage was associated with significantly less total calculated blood loss, less hidden blood loss, decreased requirement for blood transfusion, and better ROM at least in the early postoperative period, which may contribute to early rehabilitation. Importantly, no significant difference was found in ROM at 6 weeks.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Decrease in blood loss associated with TKA surgery.
Decrease in swelling in the first 7 days postsurgery.
Improvement in short term flexion ROM.
Risk, harm, and/or cost are as follows: There is a potential risk of developing limited extension ROM with this recommendation. Knee extension ROM was not measured in these studies. Limited knee extension could be a risk factor with patients being placed in a knee flexion resting position postoperatively.
Benefit-harm assessment: There is benefit in reducing blood loss and swelling in the first 7 days postsurgery. Improved flexion ROM is not long term, with only 1 study showing improvement after 6 weeks and none after 6 months. The impact on extension ROM is not known. Most of these studies had a length of stay greater than 7 days or the length of stay was unreported. There is a question about the generalizability of the results of these studies to postoperative care due to the length of stay. For these reasons, the strength of this recommendation is weak.
Future research
Continued comparative studies that have larger sample sizes and compare positioning the knee at different degrees of flexion during the immediate postoperative period after TKA may further clarify the use of this approach to minimize swelling and edema. Furthermore, the optimal degrees of flexion are still to be determined, as is the minimal timing required to obtain the reported effect. Future studies should include outcomes related to knee extension rom.
Value judgments
Given the potential for short term reduced blood loss and swelling, the work group recommend knee flexion during rest immediately postsurgery.
Intentional vagueness
Given the varied nature of the study interventions, the work group cannot recommend a specific length of time or degree of flexion after the surgery; however, most studies looked at a time frame of 7 days and knee flexion between 30 and 90 degrees. It was unclear the amount of time per day that the knee was in flexion.
Exclusions
None were identified.
Quality improvement
Organizations may use documentation of patient and/or caregiver education for patient resting knee flexion for the immediate postoperative period after TKA as a performance indicator of reduced blood loss and swelling.
Implementation and audit
Organizations may audit occurrence of documentation of patient and/or caregiver education for patient resting knee flexion for the immediate postoperative period after a TKA to assist in the management of blood loss and swelling.
Neuromuscular Electrical Stimulation (NMES) ♦♦♦◊
Physical therapists should use NMES for patients who have undergone TKA to improve quadriceps strength, gait performance, performance-based outcomes, and patient-reported outcomes. Evidence Quality: High; Recommendation Strength: Moderate.
Action Statement Profile
Aggregate evidence quality: 4 high-quality studies73–76 and 1 moderate-quality77 study.
Rationale
Four high-quality studies73–76 and 1 moderate-quality77 study compared the use of neuromuscular electrical stimulation (NMES) with no NMES use in the treatment of patients after TKA. Two high quality studies75,76 found that NMES improved quadriceps and hamstring muscle maximum voluntary isometric contractions from 2 to 52 weeks after TKA. Four high-quality studies73–76 reported greater improvement in walking, stair-climbing performance, and patient-reported outcomes with NMES use compared with no NMES from 2 to 52 weeks after TKA. Postoperative ROM with NMES use was not different from no NMES use from 2 to 52 weeks after TKA.75–77 Earlier NMES (as early as postoperative day 2) and more frequent (5–7 times daily) application with longer cumulative time at the maximal intensity tolerated by patients improved outcomes.73–76 Patients after TKA who would most likely benefit are those with quadriceps muscle activation deficits, often measured in terms of a quadriceps extensor lag or quadriceps activation battery. NMES should be applied for at least a minimum of 3 weeks.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Improvement in quadriceps and hamstrings maximum voluntary isometric contractions from 2 to 52 weeks after TKA.
Improvement in walking, stair-climbing performance, and patient-reported outcomes.
Risk, harm, and/or cost are as follows:
The financial cost of using NMES and its availability to patients may be prohibitive for patients.
Pain/discomfort with use.
Decreased tolerance.
Benefit-Harm Assessment: There is a preponderance of benefit for this recommendation.
Future research
Although current evidence supports the use of NMES after TKA, additional research might continue to refine NMES benefits by understanding the best patient factors for NMES use, optimal dosage, stimulation parameters, application with and without concurrent muscle contraction, mechanisms explaining NMES efficacy, adjuncts to NMES (eg, nutritional supplementation), and when to discontinue NMES.
Value judgments
Independent application (placement) of electrodes and inappropriate implementation of the parameters of NMES by the patient may lead to less optimal outcomes; however, preoperative education improves the quality of implementation.
Intentional vagueness
Given the varied nature of the study interventions, the work group cannot recommend a specific setting for NMES; however, studies consistently used parameters that allowed for tetanic quadriceps muscle contractions with stronger contractions leading to greater quadriceps strength.
Exclusions
None were identified.
Quality improvement
Organizations may use documentation of use of NMES after TKA as a performance indicator.
Implementation and audit
Audits of occurrence of documentation of use of NMES after a TKA to assist with isometric contractions of the quadriceps and hamstrings may be used.
Resistance and Intensity of Strengthening Exercise ♦♦♦◊
Physical therapists should design, implement, teach, and progress patients who have undergone TKA in high-intensity strength training and exercise programs during the early postacute period (ie, within 7 days after surgery) to improve function, strength, and ROM. Evidence Quality: High; Recommendation Strength: Moderate.
Action Statement Profile
Aggregate evidence quality: 3 high-quality studies11,78,79 and 1 moderate-quality study.80
Rationale
Three high-quality studies11,78,79 and 1 moderate-quality study80 support the benefits of land-based, high-intensity resistance training based on patient tolerance, muscle function, functional performance, and balance. Evgeniadis11 found that postoperative resistance training (8 weeks) resulted in higher levels of functional mobility and better knee extension ROM.
One additional high-quality study evaluated the safety of early high-intensity resistance training using specified progression criteria78 on knee ROM and adverse events and found that early high-intensity resistance training is as safe as low-intensity resistance training. Knee ROM (flexion or extension) was not compromised with early high-intensity resistance training initiated 72 hours after TKA. The study did not find improvements in muscle strength or physical function, but both groups demonstrated substantially better outcomes than have been previously reported. In particular, the control group also exceeded historical controls in ROM and function and may not have provided a true low-intensity comparison.
Effectiveness of high-intensity resistance training may be limited by arthrogenic muscular inhibition of the quadriceps (muscle activation deficits) in the early postoperative period.78 Patients with large muscle activation deficits may not experience sufficient muscle overload with resistance training to achieve comparable strength gains as those of patients without muscle activation deficits.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Improvement in muscle strength.
Improvement in activities related to mobility (eg, getting into and out of a chair).
Improvement in balance.
Improvement in knee extension.
Risk, harm, and/or cost are as follows:
Early postoperative high intensity resistance training after TKA, does not have harms or risks when the therapist follows appropriate progression criteria (eg, avoiding excessive swelling, pain, or prolonged soreness following intervention) and educates the patient accordingly.78
In the absence of appropriate criteria, overly aggressive progression can exacerbate pain and swelling.
Team members should be aware of potential complications after TKA that may affect exercise including incision healing, thromboembolism, and joint stiffness/arthrofibrosis.
Benefit-harm assessment: There is a preponderance of benefit for this recommendation.
Future research
Future studies should evaluate the impact of muscle activation deficits on the effectiveness of early progressive resistance exercise in terms of muscle strength gains and functional outcomes. Additional work should focus on the optimal timing of resistance training, potentially targeting later postoperative recovery when muscle activation deficits have resolved.
Value judgments
None were identified.
Intentional vagueness
High-intensity strength training should include the use of progression criteria such as described in Bade et al78 to tailor speed of progression with individual responses to exercise (eg, excessive swelling, pain, or prolonged soreness following intervention). Given the varied nature of the study interventions, the work group cannot recommend a specific parameter for resistance and intensity; however, Molla et al79 found that 6 weeks of progressive resistance training 3 times per week starting the day after surgery resulted in better balance outcomes on the Sharpened Romberg, Star Excursion, and Berg Balance Scale tests. Husby et al80 found that resistance training 3 times per week for 8 weeks initiated 8 days after TKA resulted in better muscle strength and that gains persisted through 12 months after TKA.
Exclusions
None were identified.
Quality improvement
Organizations may use documentation of use of high-intensity strength training and exercise programs with appropriate progression criteria after TKA as a performance indicator.
Implementation and audit
Organizations may audit occurrence of use of high-intensity strength training and exercise programs with appropriate progression criteria after a TKA to optimize function.
Prognostic Factors: Body Mass Index (BMI), Depression, Preoperative ROM, Physical Function and Strength, Age, Diabetes, Comorbidities, and Sex ♦♦♦◊
Physical therapist management should take into consideration the following factors when determining prognosis, treatment, and informed decision making and expectation setting with patients undergoing TKA:
(a) Higher BMI is associated with more postoperative complications and worse postoperative outcomes.
(b) Depression is associated with worse postoperative outcomes.
(c) Preoperative ROM is positively associated with postoperative ROM but has minimal, if any, effect on physical function and quality of life.
(d) Preoperative physical function is positively associated with postoperative physical function.
(e) Preoperative strength is positively associated with postoperative physical function.
(f) Age is associated with mixed patient-reported, performance-based, and impairment-based outcomes.
(g) Diabetes is not associated with worse functional outcomes.
(h) A greater degree of comorbidity is associated with worse patient-reported outcomes.
(i) Sex is associated with both positive and negative effects on postoperative outcomes.
Evidence Quality: High; Recommendation Strength: Moderate.
Action Statement Profile
Aggregate evidence quality was not performed for all prognostic factors in relation to each other; however, it is noted with each prognostic factor.
Rationale
The evidence for each prognostic factor is as follows:
(a) BMI: Five high-quality62,81–84 and 7 moderate-quality studies85–91 were included. Five of the high- and moderate-quality studies82,84–87 supported higher BMI as a factor related to worse patient outcomes, including patient-reported outcomes on the Oxford Knee Score,82,84 12-Item Short-Form Health Survey (SF-12),84 WOMAC composite score,85 and KSS function84 and impairment-based outcomes for range of knee flexion.85,86 Physical therapists should consider higher BMI as a risk factor for worse patient-reported and impairment-based outcomes of TKA. Adverse events that were associated with increased BMI included deep-vein thrombosis, joint infection, surgical complications, unplanned readmissions, and wound complications.
(b) Depression: One high-quality83 and 2 moderate-quality92,93 studies were included. The 2 moderate-quality studies reported depression as a factor related to worse patient-reported outcomes on the Oxford Knee Score.92,93 The high-quality study83 reported nonsignificant results for patient-reported outcomes on the WOMAC.
(c) Preoperative ROM: Four high-quality studies were included.62–64,94 They generally suggested that preoperative ROM predicts postoperative ROM62–64 but that preoperative ROM does not predict functional outcomes of the TUG test and Stair-Climbing Test.94 One high-quality study indicated that patients with flexion contractures before surgery do just as well after surgery as do patients without flexion contractures.63 Four studies provided moderate-quality evidence,85,86,95,96 with some limitations; Holm95 measured knee ROM only at hospital discharge, and Park96 found only a weak correlation between postoperative maximum flexion and the pain, function, and quality of life 12 months after TKA. The latter study did not investigate how preoperative ROM predicts postoperative ROM. The most important factors that influenced knee ROM after arthroplasty were preoperative range of flexion and body weight of the patient.
(d) Preoperative function: Although there is mixed evidence in this area, most studies found that higher levels of preoperative function were associated with higher levels of postoperative function. Five high-quality studies62,64,83,94,97 and 11 moderate-quality studies85–89,91,93,96,98–100 examined correlations between preoperative function and postoperative outcomes. One high-quality study97 found a higher preoperative SF-12 to be associated with higher postoperative step count. A moderate-quality study85 found a higher preoperative WOMAC to be associated with better knee flexion postoperative. A moderate-quality study91 found better results on preoperative TUG test, 6-Minute Walk Test, Stair-Climbing Test, and SF-36 physical component score to be associated with better results in these same tests at 1, 3, and 6 months postoperatively. Another moderate-quality study100 found preoperative scores below reference value significantly decreased the possibility of achieving the level of health-related quality of life of the general population at a 1-year follow-up. Jones et al99 found that greater preoperative WOMAC function and walking distance were significantly associated with better 6-month postoperative WOMAC function scores. Another moderate-quality study93 found that a higher preoperative Oxford Knee Score was associated with a higher postoperative score. Four high-quality studies62,64,83,94 and 4 moderate-quality studies86,89,96,98 did not find significant correlations with preoperative function and postoperative outcomes.
(e) Preoperative strength: One moderate-quality study95 found that a decrease in knee extension strength of 0.5% was associated with a 1% decrease in 10-m fast-speed walking. This finding suggests that greater preoperative strength is related to better walk speed postoperatively. One moderate-quality study suggested no relationship between preoperative strength and postoperative SF-36 physical function score.89
(f) Age: Eight high-quality62,64,81–83,94,101,102 and 9 moderate-quality86,88–90,95,96,98–100 studies investigated the association of age with outcome. Of the 17 studies, 3 reported older age as a factor related to worse patient outcomes—including patient-reported outcomes from SF-12,81 KSS function,81 WOMAC function,81,83 and health-related quality of life100—and 2 reported negative effects on impairment-level outcomes of gait speed101 and extension lag.81 One high-quality study83 reported nonsignificant differences for WOMAC. The remainder of studies reported nonsignificant results for a variety of outcomes. One moderate-quality study reported a greater fall rate with older age.90
(g) Diabetes: One moderate-quality study was included.93 Five studies were of low quality.87,93,98–100 Study results were nonsignificant for effects of diabetes on Oxford Knee Score.
(h) Comorbid conditions: Two high-quality83,102 and 6 moderate-quality87,93,98–100,103 studies were included. Of the included studies, 5 reported greater degree of comorbidity as a factor related to worse patient-reported outcomes on WOMAC function,83,99,100 Oxford Knee Score,93 SF-12,103 KSS,103 and quality of life.100 One high-quality study reported a higher total complication rate with greater degree of comorbidity.102
(i) Sex: Five high-quality62,64,81–83 and 11 moderate-quality86,88–90,92,95,96,98–100,104 studies were included. Male and female sex may or may not be related either positively or negatively to recovery after TKA, depending on the outcome measures studied; therefore, a general statement of the impact of sex on recovery cannot be made. Three high-quality studies62,64,81 and 8 moderate-quality studies86,88,89,92,95,96,99,104 reported no effect of sex on a variety of outcomes including ROM, walking speed, knee extension strength, and measures of function. One high-quality study reported that women had worse scores than men on the Oxford Knee Score and that men had worse terminal knee extension.82 Another high-quality study reported that woman had more knee pain than men at 6 weeks following TKA.83 One moderate-quality study reported that men had less improvement in self-reported function than women.100 Another moderate-quality study reported that women had longer rehabilitation hospital stays (25 days) than men (23 days).98
Potential benefits, risks, and harms of implementing this recommendation
Benefits are that patients and practitioners can analyze and discuss the potential effects of these factors on recovery after TKA.
Risk, harm, and/or cost: No expected risk or harms are associated with this recommendation.
Benefit-harm assessment: There is a preponderance of benefit for this recommendation.
Future research
Age, sex, and diabetes require more research regarding subgroups.
Value judgments
Recognition of prognostic factors may modify decisions about dosage, rate of progression, and duration of interventions.
Intentional vagueness
The impact of multiple, concomitant factors is not discussed. Additional prognostic factors that have not been described may also influence outcomes.
Exclusions
None were identified.
Quality improvement
Organizations may use documentation of risk factors with patient before TKA as a performance indicator.
Implementation and audit
Organizations may audit occurrence of risk factors with a patient prior to patient undergoing a TKA.
Prognostic Factors: Tobacco Use and Patient Support ♦◊◊◊
It is the consensus of the work group that active tobacco use and lack of patient support (eg, environmental factors including, but not limited to, support and relationships) should be considered as prognostic/risk factors associated with less than optimal functional outcomes. Evidence Quality: Insufficient; Recommendation Strength: Best Practice.
Action Statement Profile
Aggregate evidence quality is as follows: There were no studies of sufficient quality related to the association of tobacco use or lack of patient support associated with functional outcomes for patients undergoing TKA.
Rationale
Without strong evidence in research, it is the consensus of the group that tobacco use and lack of patient support are important aspects in recovery and functional outcomes associated with TKA. Support includes social structures, people, and relationships that help a person manage recovery. Although there is not specific evidence to support the impact of tobacco use and patient support related to risk factors associated with TKA, there is significant evidence to support the impact on overall health.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are that patients can make informed decisions about use of tobacco and analyze their availability of support systems in understanding the potential effects of these factors on recovery after TKA.
Risk, harm, and/or cost are as follows: No expected risk or harms are associated with this recommendation.
Benefit-harm assessment: There is a preponderance of benefit for this recommendation.
Future research
Additional research needed on the use of tobacco and the use of available support related to outcomes for patients undergoing TKA. Additionally, the GDG felt it is important to also study the impact of level of education and socioeconomic status as prognostic/risk factors for patients undergoing TKA.
Value judgments
Tobacco use affects healing, which is an important component of TKA, leading to complications. Patients who undergo TKA should be tobacco-free or engaged in a tobacco cessation program prior to surgery. Optimally, patients benefit from support of family, friends, and/or community after TKA, especially on return to home, work, or community participation.
Intentional vagueness
Not applicable.
Exclusions
None were identified.
Quality improvement
Organizations may use documentation of prognostic factors with patient before TKA as a performance indicator.
Implementation and audit
Organizations may audit occurrence of prognostic factors prior to patients undergoing TKA.
Postoperative Physical Therapy Supervision ♦♦♦◊
Supervised physical therapist management should be provided for patients who have undergone TKA. The optimal setting should be determined by patient safety, mobility, environmental, and personal factors. Evidence Quality: Moderate; Recommendation Strength: Moderate.
Action Statement Profile
Aggregate evidence quality: 1 high-quality study105 and 1 moderate-quality study,106 which examined various aspects of the supervised versus less supervised physical therapy after TKA.
Rationale
This recommendation indicates there was limited supporting evidence for supervised physical therapy after TKA compared with home exercise-based approaches. One high-quality study105 allocated 40 subjects to either a home-based program or a supervised program and found that the supervised program had better quality-of-life scores 3 and/or 6 months after surgery. One moderate-quality study106 randomized 40 individuals to either a supervised program that met 2 times a week for 1 month or a supervised program that met twice a month and performed home exercises. The more frequently supervised group had better self-reported outcomes, mobility scores, and balance at the end of the intervention compared with the less-supervised group.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Approaches that include supervised physical therapist management may produce better outcomes than approaches with less supervision from a physical therapist.
Given that most patients progress after TKA with respect to function, strength, and ROM, supervised physical therapy may allow for more appropriate and safe exercise progression.
Risk, harm, and/or cost are as follows: There were no reported risk or harms associated with providing supervised care from the physical therapist.
Benefit-harm assessment: There is a preponderance of benefit that supervised physical therapist management should be provided after TKA to address physical impairments and functional limitations that are commonplace after surgery.
Future research
Existing studies on this topic varied widely in their scope and methodology. Although there was limited evidence supporting supervised versus unsupervised physical therapy programs, there is a dearth of literature on this topic, perhaps due to ethical issues related to withholding supervised physical therapy after surgery. Studies that compare supervised physical therapy with a true nonactive control or home exercise program are warranted. Future work should include (1) randomized trials that evaluate patient outcomes after allocation and (2) prognostic studies that identify patient characteristics that make an individual better suited for less supervision after discharge from the hospital.
Value judgments
Physical therapist supervision is warranted after TKA; however, additional research may identify the individuals who may succeed with less structured supervised rehabilitation after discharge from the hospital.
Intentional vagueness
Given the lack of experimental studies, the work group cannot recommend a specific level or amount of supervision for postoperative physical therapy. The choice of a singular ideal level is further complicated by heterogeneity in patient goals, needs, comorbidities, risk factors, and level of function in this population.
Exclusions
None were identified.
Quality improvement
Organizations may use documentation of justification of supervision level for postoperative TKA physical therapist management.
Implementation and audit
Organizations may audit occurrence of documentation of supervision level for justification for postoperative physical therapist management of patients who undergo TKA.
Group-Based Versus Individual-Based Therapy ♦♦◊◊
Physical therapists may use group-based or individual-based physical therapy sessions for patients who have undergone TKA. Evidence Quality: Moderate; Recommendation Strength: Weak.
Action Statement Profile
Aggregate evidence quality: 1 high-quality study107 and 2 moderate-quality studies,108,109 which examined group-based versus individual-based therapy after TKA.
Rationale
There is limited evidence to support the benefit of individualized physical therapist management over group-based sessions. One high-quality,107 2 moderate-quality,108,109 and 1 low-quality110 studies reported individualized therapy was neither superior nor inferior to group rehabilitation. Various patient functional outcomes improved over the course of the studies, regardless of participation in individual or group physical therapy sessions.
The evidence was limited given differences in study populations, the timing of the programs postsurgery and differences in doses of the intervention between and within groups. In the only high-quality study,107 8-week group-based physical therapy was compared with usual care, in which 15% of patients in usual care did not receive physical therapy at all. In the Kauppila et al 2010 study,109 10-day group-based therapy was implemented 2 to 4 months after surgery and compared to usual care, which resulted in a larger exposure in the group-based arm of the study. Artz et al108 reported a preliminary study and only included self-reported outcomes in 46 subjects. The low-quality study110 evaluated only the benefit of 2 additional group therapy sessions in the acute care environment. All studies compared the group-based management to usual care, which differs greatly among geographic locales and health care delivery systems.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Individualized physical therapist management allows for a tailored plan of care for patients based on their physical and psychosocial needs.
Group-based therapy may be less costly than individually based therapy.
Risk, harm, and/or cost are as follows:
Group therapy may fail to provide enough progression of therapy for more advanced patients or to provide adequate engagement for patients with lower abilities or significant impairments.
Group-based physical therapist management after TKA will require careful selection of patients based on physical therapist examination, and patients’ progress should be monitored throughout their course of care.
Benefit-harm assessment: There is a preponderance of benefit for the use of group-based or individual-based physical therapy sessions after TKA.
Future research
Given the lack of evidence that directly compares balanced dosage of group-based physical therapist management with individualized sessions, additional comparative studies are warranted before a stronger recommendation can be made.
Value judgments
Limitations in the existing research reduced the ability to draw a conclusion about the superiority of either treatment approach, and the decision to use either treatment may be based on a variety of institutional-, therapist-, and patient-related factors.
Intentional vagueness
The lack of a clear, high-quality, comparative study that included dose-equivalent group and individual-based treatment plans precluded a preferential recommendation for either approach.
Exclusions
None were identified.
Quality improvement
Organizations may use documentation of individual versus group therapy for patients with TKA to allow for future research in this area.
Implementation and audit
Organizations may audit occurrence of documentation of the use of individual versus group therapy for patients with TKA to support future research.
Physical Therapy Postoperative Timing ♦♦♦◊
Physical therapist management should start within 24 hours of surgery and prior to discharge for patients who have undergone TKA. Evidence Quality; Low, Recommendation Strength: Moderate.
Action Statement Profile
Aggregate evidence quality: 2 low-quality studies.111,112
Rationale
Two low-quality studies111,112 examined postoperative timing to receiving physical therapist management after TKA and support the use of starting inpatient physical therapy earlier rather than later in hospital settings. One low-quality study compared a group that initiated rehabilitation within the first 24 hours postsurgery with a group that received the same rehabilitation protocol but did not start rehabilitation until 48 to 72 hours postsurgery.111 The study had issues with randomization, incomplete outcome reporting, and confounding variables. The group that started earlier rehabilitation had a shorter hospital stay, reduced pain, and improved physical function (gait, balance, ROM, and strength). The other low-quality study, a retrospective study, compared a group that began ambulation 1 day postsurgery with a group that began ambulation 2 days postsurgery.112 The early ambulation group had a shorter hospital stay, lower hospital costs, higher odds of achieving 90 degrees of knee flexion, and higher odds of requiring a walking aid with a smaller base of support (eg, straight cane vs walker).
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Shortened inpatient hospital stay.
Reduced pain.
Improved physical function.
Risk, harm, and/or cost are as follows: No expected harms are expected with implementing this recommendation. The retrospective study reported no difference in 90-day readmission rate between the early-ambulation group and the later-ambulation group.
Benefit-harm assessment: There is a preponderance of moderate-to-substantial benefit for this recommendation that led the group to upgrade the recommendation strength in the presence of low evidence quality.
Future research
As evolving management emphasizes shorter lengths of hospital stays, including discharge within 24 hours after surgery and surgery on an outpatient basis for some patients, additional high-quality research is needed to investigate the optimal timing of TKA rehabilitation for these management models.
Value judgments
None were identified.
Intentional vagueness
Given the limitations of the current evidence, the optimal timeframe within the first 24 hours to start rehabilitation is not yet known.
Exclusions
None were identified.
Quality improvement
Organizations may use documentation of the timing of postoperative therapy for TKA patients as a performance indicator.
Implementation and audit
Organizations may audit occurrence of documentation of the timing of postoperative therapy for TKA patients.
Physical Therapy Discharge Planning ♦♦♦◊
It is the consensus of the work group that physical therapists should provide guidance to the care team and patient on safe and objective discharge planning, patient functional status, assistance equipment, and services needed to support a safe discharge from the acute care setting. Evidence Quality: Low; Recommendation Strength: Moderate.
Action Statement Profile
Aggregate evidence quality is as follows: No quality studies were found specific to the effectiveness of physical therapists being involved in objective (using standardized measures) discharge planning for the patient following TKA. After review of the available literature, the work group decided to include information from studies related to services available to patients as a result of discharge planning that were not included in other sections of this guideline.
Rationale
One study113 compared usual care postdischarge to usual care (home and outpatient physical therapy) with health coaching and a financial incentive to improve physical activity. The health coaching consisted of phone calls to the patient focused on motivational interviewing. The financial incentive rewarded patients for increased physical activity measured by steps/day as recorded by a Fitbit (Fitbit Inc, San Francisco, CA, USA). At 6-month follow-up, steps per day and physical activity increased to a greater extent among those receiving both health coaching and Fitbit as compared wtih an attention control group. A second study114 found no difference in WOMAC scores between groups receiving usual care and those receiving usual care and “Care Navigation,” which was described as up to 10 phone calls by the “Navigator” trained in motivational interviewing to improve the patient’s functional status.
McLawhorn et al115 analyzed over 100,000 patients with TKA from the National Surgical Quality Improvement Data Base. Approximately 30% were discharged to skilled facilities. At 30 days postdischarge, those who went to the skilled facilities compared with those who went directly home had higher complications and readmission rates. Patients discharged to skilled facilities were older, had more comorbidities, and were more likely to have been nonindependent presurgery, suggesting a selection bias for those discharged to skilled care versus home. The authors of that study recommended that patients who have undergone TKA be discharged directly home when possible. Padget et al116 concluded that patients can be safely discharged home after TKA. They also reported that referral to inpatient rehabilitation facilities did not influence 6-month reductions of complications, nor did it lead to functional differences at 2 years when compared with those discharged directly home. Tribe et al117 found significant functional improvements in patients with TKA who were discharged home or were treated in an inpatient rehab facility. Physical therapy visits were similar in both groups, and there were no differences in functional improvements.
The studies reviewed found inpatient rehabilitation for TKA to be no better than discharge directly home. In addition, Brennan et al118 found that fewer days from hospital discharge to start of outpatient therapy predicted improvement in patient-reported pain and outcome in an outpatient setting. Patients in this study were covered by commercial insurance and were relatively young (mean age = 56.1 years).
Predicting discharge disposition to avoid ineffective services could save costs. Smith et al119 found that physical therapists make accurate and appropriate discharge recommendations. When physical therapists’ recommendations matched actual discharge setting, there was a lower probability of hospital readmissions than when they did not match. Similarly, Falvey et al120 identified the contributions of physical therapists to the discharge planning team when preparing for care transitions and the potential to mitigate readmissions. Two studies have shown a measure of function, using a standardized tool administered on the first therapy visit, to have predictive value in discharge disposition from acute settings.121,122 These findings suggest benefits of the inclusion of physical therapy with interdisciplinary discharge planning early in the hospitalization. Jette et al121 evaluated all types of diagnoses and Menendez et al122 looked specifically at the discharge of patients with joint replacement including TKA.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Physical therapists can provide the care team with valuable information to assure the most appropriate discharge setting.
Involving physical therapists in discharge planning can prepare the patient for a safe and independent transition to the home environment.
Health coaching and financial incentives can improve patient functional performance.
Inpatient rehabilitation may not be more beneficial than discharge directly home.
Risk, harm, and/or cost are as follows: No expected risks or harm are associated to implementing the recommendation.
Benefit-harm assessment: There is a preponderance of benefit for this recommendation that led the work group to upgrade the recommendation strength in the presence of low evidence quality ( Tab. 4 ).
Future research
From the current research regarding care navigator/discharge planner and social support, the work group was left with far more questions than answers. It is unclear whether the care navigator/discharge planner was present from the beginning of the decision process. Similarly unclear is whether the care navigator went home with the patient and assisted in personal care, safety, and home physical therapy. The interventions provided by the care navigator regarding home safety assessment, developing a personal home care plan, securing assistive devices, and supporting pain management strategies were not indicated by the researchers. Likewise, the study of social support was narrowly focused on contact from provider to patient related to function. More research using a broader application of different types of social support from within patients’ larger communities is needed to assess the impact on patient safety in postoperative discharge to home, on pain levels, and on return to function.
Related to future research in the area of postoperative setting (or location), no high-quality studies were evaluated on this topic. There is a lack of studies evaluating measures or standard processes for objective discharge decision making. The current Medicare joint replacement bundle includes all costs incurred up to 90 days postsurgery. Studies evaluating the outcome and cost of the timing and setting of postacute care will become more and more important. Studies are needed that evaluate the cost and outcome of patients discharged directly to an outpatient setting compared to a skilled nursing or home care setting prior to outpatient care.
Value judgments
Patients who participate in discharge planning have greater confidence in their ability to manage rehabilitation at home. Less stress in a safe, supportive environment creates a positive patient outlook and encouragement to fully participate in their own rehabilitation.
Intentional vagueness
Given the need for more research using broader models of patient caregiver support, the recommendation is based on anticipated positive impact of coordinated, intentional discharge planning.
Exclusions
None were identified.
Quality improvement
Organizations may use documentation of discharge planning with the patient and health services team as a performance indicator.
Implementation and audit
Organizations may audit occurrence of documentation discharge planning with the patient and health services team.
Outcomes Assessment ♦◊◊◊
It is the consensus of the work group that physical therapists should collect data from the Knee Injury Osteoarthritis Outcomes Survey Joint Replacement (KOOS JR) as a patient-reported outcome measure and from both the 30-Second Sit-to-Stand and TUG tests as performance-based outcomes to demonstrate the effectiveness of care provided. At a minimum, these measures should be collected at the first visit and upon conclusion of care from each setting. Evidence Quality: Insufficient; Recommendation Strength: Best Practice.
Action Statement Profile
Aggregate evidence quality: No PICO(T) questions were created to specifically look at outcomes assessment, and, as such, the search returned no studies addressing this topic.
Rationale
The GDG discussed the psychometric properties, clinical utility, and importance to the patient of multiple tests and measures that have been used when describing outcomes for patients undergoing TKA. The group was in consensus with this recommendation. There is evidence for the reliability and validity of the use of the tools in TKA.123–127 Lyman et al124 found that internal consistency for the KOOS JR was high and that correlation with other validated knee surveys was excellent. Responsiveness was high, and floor and ceiling effects were reasonable. Alghadir et al123,126,127 reported excellent interrater and intrarater reliability for the TUG test, and Yuksel et al127 reported similarly excellent test-retest reliability. Unver et al126 reported excellent test-retest reliability for the 30-Second Sit-to-Stand test. Evidence for validity of the 30-Second Sit-to-Stand test has not been established for patients after TKA. The TUG test has been shown to be moderately correlated with the functional dimension of the Postoperative Quality of Recovery Scale and the Readiness for Hospital Discharge Scale and highly correlated with the Iowa Level of Assistance Scale.128 These findings provide some evidence of the TUG test’s validity for use in patients with TKA. The new short knee PROM, the KOOS JR, provides a single score representing “knee health” as it combines pain, symptoms, and functional limitations in a single score. This short-form PROM is patient-relevant and efficient.
Potential benefits, risks, and harms of implementing this recommendation
Benefits are as follows:
Documentation of actual results of implementing the plan of care.
Consistent measures can standardize communication across the continuum of care.
Minimal time to administer assessments.
Utilization of information that is understood by different health care providers for patients undergoing TKA.
Risk, harm, and/or cost are as follows:
No expected risk or harms are associated with this recommendation.
There is an increase in time to perform these outcome measures.
Those using published assessment measures should contact publishers and/or authors regarding costs and limitations.
Benefit-harm assessment: There is a preponderance of benefit for this recommendation.
Future research
Additional research is needed to identify appropriate outcome measures with adequate psychometrics for patients who undergo TKA. This research should include validity and reliability studies.
Value judgments
Expert opinion supports the use of identified outcome assessments. In addition, there are value-based incentive payment programs (eg, Comprehensive Care for Joint Replacement Model) and regulatory mandate’s for Joint Commission Advanced Certification that require the use of KOOS JR assessment tool. There is need for consistency of outcomes to improve understanding of the effectiveness of care provided for patients undergoing TKA.
Intentional vagueness
Not applicable.
Exclusions
None were identified.
Quality improvement
Organizations may use the completion of identified outcome measures at the initial visit and conclusion of care visit as a performance indicator.
Implementation and audit
Organizations may audit completion of identified outcome measures at the initial visit and conclusion of physical therapy visits for patients that receive a TKA.
Dissemination Plans
The primary purpose of this CPG is to provide interested readers with full documentation of the best available evidence for various procedures associated with TKA. Publication of this guideline will be announced by press release and published in PTJ (Physical Therapy), the journal of the American Physical Therapy Association.
Education and awareness about this CPG will be disseminated via online resources, such as webinars and continuing education courses, at professional annual meetings, and via social media. Pocket Guides (www.GuidelineCentral.com/TKA) developed by Guideline Central have been created as implementation tools to aid in the dissemination of the CPG.
Revision and Reaffirmation Plans
This CPG represents a cross-sectional view of current treatment and may become outdated as new evidence becomes available. It will be reviewed in 5 years and will be updated in accordance with new evidence, changing practice, rapidly emerging treatment options, and new technology; reaffirmed; or withdrawn.
Supplementary Material
Appendix. Development Group Roster
Voting Members
Stephen J. Hunter PT, DPT, OCS, FAPTA—Co-chair. American Physical Therapy Association. Area representing: Chair Clinical.
Diane U. Jette, PT, DPT, DSc, FAPTA—Co-Chair. American Physical Therapy Association. Area representing: Chair Research.
Lynn Burkett, MBA, BSN, RN, ONC. National Association of Orthopaedic Nurses. Bud Langham, PT, MBA. American Physical Therapy Association. Area representing: Home Health.
David Logerstedt, PT, PhD, MPT. American Physical Therapy Association. Area representing: Orthopaedics.
Nicolas Piuzzi, MD. American Academy of Orthopaedic Surgeons. Noreen Poirier, PT, DPT. American Physical Therapy Association. Area representing: Acute Care.
Linda J.L. Radach. Consumers United for Evidence-Based Healthcare. Jennifer E. Ritter, PT. American Physical Therapy Association. Area representing: Acute Care.
David Scalzitti, PT, PhD, OCS. American Physical Therapy Association. Area representing: Orthopaedics.
Jennifer Stevens-Lapsley, PT, MPT, PhD. American Physical Therapy Association. Area representing: Geriatrics.
James Tompkins, PT, DPT. American Physical Therapy Association. Area representing: Acute Care.
Joseph Zeni, Jr. PT, PhD. American Physical Therapy Association. Area representing: Orthopaedics.
Non-voting Members Matt Elrod, PT, DPT, MEd, NCS. American Physical Therapy Association. Jayson Murray, MA. American Academy of Orthopaedic Surgeons. Danielle Schulte, MS. American Academy of Orthopaedic Surgeons. Anita Bemis-Dougherty, PT, DPT, MAS. American Physical Therapy Association. William Boissonnault, PT, DHSc. American Physical Therapy Association. Heidi Kosakowski, PT, DPT. American Physical Therapy Association.
Contributor Information
Diane U Jette, FAPTA, MGH, Institute of Health Professions, Boston, Massachusetts.
Stephen J Hunter, FAPTA, MGH, Institute of Health Professions, Boston, Massachusetts.
Lynn Burkett, ONC, National Association of Orthopaedic Nurses (NAON), Wyomissing, Pennsylvania.
Bud Langham, Home Health and Hospice Services, Encompass Health, Birmingham, Alabama.
David S Logerstedt, Department of Physical Therapy, University of the Sciences, Philadelphia, Pennsylvania.
Nicolas S Piuzzi, Department of Orthopaedic Surgery, Cleveland Clinic, Cleveland, Ohio.
Noreen M Poirier, Department of Orthopedics and Rehabilitation, University of Wisconsin (UW) Health, Madison, Wisconsin.
Linda J L Radach, Consumers United for Evidence Based Healthcare, Lake Forest Park, Washington.
Jennifer E Ritter, Department of Rehabilitation Services/Physical Therapy, University of Pittsburgh Medical Center (UPMC) St Margaret Hospital/Catholic Relief Services, Pittsburgh, Pennsylvania.
David A Scalzitti, OCS, School of Medicine and Health Sciences, George Washington University, Washington, DC.
Jennifer E Stevens-Lapsley, Department of Physical Medicine and Rehabilitation, University of Colorado at Denver & Health Sciences Center, Denver, Colorado.
James Tompkins, Department of Physical Medicine and Rehabilitation, Mayo Clinic, Scottsdale, Arizona.
Joseph Zeni Jr, Department of Physical Therapy, University of Delaware, Newark, Delaware.
Author Contributions
Concept/idea/research design: S. Hunter, L. Burkett, N. Piuzzi, N. Poirier, J. Ritter, J. Stevens-Lapsley, J. Zeni Jr.
Writing: D.U. Jette, S. Hunter, D. Logerstedt, N. Piuzzi, N. Poirier, L.J.L. Radach, J. Ritter, D. Scalzitti, J. Stevens-Lapsley, J. Tompkins, J. Zeni Jr.
Data collection: N. Piuzzi, N. Poirier, J. Ritter, D. Scalzitti.
Data analysis: D.U. Jette, S. Hunter, B. Langham, D. Logerstedt, N. Piuzzi, N. Poirier, L.J.L. Radach, D. Scalzitti, J. Tompkins, J. Zeni Jr.
Project management: H. Kosakowski, S. Hunter, A. Bemis-Dougherty, N. Piuzzi.
Providing participants: J. Ritter.
Consultation (including review of manuscript before submitting): D.U. Jette, S. Hunter, N. Piuzzi, N. Poirier, L.J.L. Radach, J. Ritter, D. Scalzitti, J. Stevens-Lapsley, J. Tompkins, J. Zeni Jr.
Funding
This clinical practice guideline (CPG) was funded exclusively by the American Physical Therapy Association (APTA), which received no funding from outside commercial sources to support the guideline’s development. The views of the funding body have not influenced the content of the guideline.
Disclaimer
This CPG was developed by an American Physical Therapy (APTA) volunteer guideline development group that consisted of physical therapists, an orthopedic surgeon, a nurse, and a consumer and was based on systematic reviews of current scientific and clinical information and accepted approaches to management of total knee arthroplasty. This guideline is not intended to be a fixed protocol, as some patients may require more or less treatment or different means of diagnosis. Clinical patients may not necessarily be the same as those found in a clinical trial. Patient care and treatment always should be based on a clinician’s independent medical judgment, given the individual patient’s clinical circumstances.
Disclosures
In accordance with APTA policy, all individuals whose names appear as authors of or contributors to this CPG filed a disclosure statement as part of the submission process. All panel members provided full disclosure of potential conflicts of interest prior to voting on the recommendations contained within this CPG. In addition, prior to the development of this CPG, development group members disclosed conflicts of interest in writing to APTA via a private online reporting database and also verbally at the recommendation approval meeting. Any potential conflicts of interest have been recorded and addressed.
Non-voting GDG members Heidi Kosakowski, Matthew Elrod, Anita Bemis-Dougherty, and Bill Boissonnault were employed by the American Physical Therapy Association during the development and review of this CPG.
APTA reimbursed travel expenses for GDG voting members D.U. Jette, S. Hunter, L. Burkett, D. Logerstedt, N. Poirier, L. Radach, J. Ritter, and J. Zeni Jr in the development of this CPG.
References
- 1. Surgical management of osteoarthritis of the knee . Evidence-based clinical practice guideline. AAOS. https://www.aaos.org/globalassets/quality-and-practice-resources/surgical-management-knee/smoak-cpg_4.22.2016.pdf. 2016. Accessed June 10, 2020. [DOI] [PubMed]
- 2. Hochberg MC, Watkins-Castillo SIC. Joint pain and joint replacement. United States Bone and Joint Initiative: the Burden of Musculoskeletal Diseases in the United States (BMUS). 4th ed. 2018. https://www.boneandjointburden.org/fourth-edition/iiib70/joint-pain-and-joint-replacement. Accessed January 13, 2020.
- 3. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States - an alternative projection model. Osteoarthritis Cartilage. 2017;25:1797–1803. [DOI] [PubMed] [Google Scholar]
- 4. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. [DOI] [PubMed] [Google Scholar]
- 5. Helmick CG, Watkins-Castillo SI. Knee replacement procedures. United States Bone and Joint Initiative: the Burden of Musculoskeletal Diseases In the United States (BMUS). 3rd ed. 2018. https://www.boneandjointburden.org/2014-report/ive1/knee-replacement-procedures. Accessed January 13, 2020.
- 6.APTA. APTA Clinical Practice Guideline Process Manual. Alexandria, VA, USA: American Physical Therapy Association; 2018: http://www.apta.org/uploadedFiles/APTAorg/Practice_and_Patient_Care/Evidence_and_Research/Evidence_Tools/Components_of_EBP/CPGs/APTACPGManual2018.pdf. Accessed January 13, 2020. [Google Scholar]
- 7. AAOS. AAOS Clinical Practice Guideline Methodology, v 4.0 and v 3.0. https://www.aaos.org/globalassets/quality-and-practice-resources/methodology/cpg-methodology.pdf. Rosemont, IL, USA: American Academy of Orthopaedic Surgeons; 2018. [Google Scholar]
- 8. Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E. Clinical Practice Guidelines We Can Trust. Washington DC, USA: National Academies Press; 2011. https://www.ncbi.nlm.nih.gov/books/NBK209539/. Accessed January 13, 2020. [PubMed]
- 9. Calatayud J, Casana J, Ezzatvar Y, Jakobsen MD, Sundstrup E, Andersen LL. High-intensity preoperative training improves physical and functional recovery in the early post-operative periods after total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25:2864–2872. [DOI] [PubMed] [Google Scholar]
- 10. D'Lima DD, Colwell CW Jr, Morris BA, Hardwick ME, Kozin F. The effect of preoperative exercise on total knee replacement outcomes. Clin Orthop Relat Res. 1996;326:174–182. [DOI] [PubMed] [Google Scholar]
- 11. Evgeniadis G, Beneka A, Malliou P, Mavromoustakos S, Godolias G. Effects of pre- or postoperative therapeutic exercise on the quality of life, before and after total knee arthroplasty for osteoarthritis. J Back Musculoskel Rehabil. 2008;21:161–169. [Google Scholar]
- 12. Gstoettner M, Raschner C, Dirnberger E, Leimser H, Krismer M. Preoperative proprioceptive training in patients with total knee arthroplasty. Knee. 2011;18:265–270. [DOI] [PubMed] [Google Scholar]
- 13. Matassi F, Duerinckx J, Vandenneucker H, Bellemans J. Range of motion after total knee arthroplasty: the effect of a preoperative home exercise program. Knee Surg Sports Traumatol Arthrosc. 2014;22:703–709. [DOI] [PubMed] [Google Scholar]
- 14. McKay C, Prapavessis H, Doherty T. The effect of a prehabilitation exercise program on quadriceps strength for patients undergoing total knee arthroplasty: a randomized controlled pilot study. PM&R. 2012;4:647–656. [DOI] [PubMed] [Google Scholar]
- 15. Mat Eil Ismail MS, Sharifudin MA, Shokri AA, Ab Rahman S. Preoperative physiotherapy and short-term functional outcomes of primary total knee arthroplasty. Singapore Med J. 2016;57:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tungtrongjit Y, Weingkum P, Saunkool P. The effect of preoperative quadriceps exercise on functional outcome after total knee arthroplasty. J Med Assoc Thai. 2012;95 Suppl 10:S58–66. [PubMed] [Google Scholar]
- 17. Villadsen A, Overgaard S, Holsgaard-Larsen A, Christensen R, Roos EM. Postoperative effects of neuromuscular exercise prior to hip or knee arthroplasty: a randomised controlled trial. Ann Rheum Dis. 2014;73:1130–1137. [DOI] [PubMed] [Google Scholar]
- 18. Huang SW, Chen PH, Chou YH. Effects of a preoperative simplified home rehabilitation education program on length of stay of total knee arthroplasty patients. Orthopaedics & Traumatology: surgery & Research. 2012;98:259–264. [DOI] [PubMed] [Google Scholar]
- 19. Ingadottir B, Johansson Stark A, Leino-Kilpi H, Sigurdardottir AK, Valkeapaa K, Unosson M. The fulfilment of knowledge expectations during the perioperative period of patients undergoing knee arthroplasty–a Nordic perspective. J Clin Nurs. 2014;23:2896–2908. [DOI] [PubMed] [Google Scholar]
- 20. Soeters R, White PB, Murray-Weir M, Koltsov JCB, Alexiades MM, Ranawat AS. Preoperative physical therapy education reduces time to meet functional milestones after total joint arthroplasty. Clin Orthop Relat Res. 2018;476:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McDonald S, Page MJ, Beringer K, Wasiak J, Sprowson A. Preoperative education for hip or knee replacement. Cochrane Database Syst Rev. 2015:cd003526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alkire MR, Swank ML. Use of inpatient continuous passive motion versus no CPM in computer-assisted total knee arthroplasty. Orthop Nurs. 2010;29:36–40. [DOI] [PubMed] [Google Scholar]
- 23. Beaupre LA, Davies DM, Jones CA, Cinats JG. Exercise combined with continuous passive motion or slider board therapy compared with exercise only: a randomized controlled trial of patients following total knee arthroplasty. Phys Ther. 2001;81:1029–1037. [PubMed] [Google Scholar]
- 24. Denis M, Moffet H, Caron F, Ouellet D, Paquet J, Nolet L. Effectiveness of continuous passive motion and conventional physical therapy after total knee arthroplasty: a randomized clinical trial. Phys Ther. 2006;86:174–185. [PubMed] [Google Scholar]
- 25. Lenssen TA, van Steyn MJ, Crijns YH, et al. Effectiveness of prolonged use of continuous passive motion (CPM), as an adjunct to physiotherapy, after total knee arthroplasty. BMC Musculoskelet Disord. 2008;9:1471–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Can F, Alpaslan M. Continuous passive motion on pain management in patients with total knee arthroplasty. The Pain Clinic. 2003;15:479–485. [Google Scholar]
- 27. Chiarello CM, Gundersen L, O'Halloran T. The effect of continuous passive motion duration and increment on range of motion in total knee arthroplasty patients. J Orthop Sports Phys Ther. 1997;25:119–127. [DOI] [PubMed] [Google Scholar]
- 28. Herbold JA, Bonistall K, Blackburn M, et al. Randomized controlled trial of the effectiveness of continuous passive motion after total knee replacement. Arch Phys Med Rehabil. 2014;95:1240–1245. [DOI] [PubMed] [Google Scholar]
- 29. MacDonald SJ, Bourne RB, Rorabeck CH, McCalden RW, Kramer J, Vaz M. Prospective randomized clinical trial of continuous passive motion after total knee arthroplasty. Clin Orthop Relat Res. 2000;380:30–35. [DOI] [PubMed] [Google Scholar]
- 30. Montgomery F, Eliasson M. Continuous passive motion compared to active physical therapy after knee arthroplasty: similar hospitalization times in a randomized study of 68 patients. Acta Orthop Scand. 1996;67:7–9. [DOI] [PubMed] [Google Scholar]
- 31. Pope RO, Corcoran S, McCaul K, Howie DW. Continuous passive motion after primary total knee arthroplasty. Does it offer any benefits? J Bone Joint Surg Br. 1997;79:914–917. [DOI] [PubMed] [Google Scholar]
- 32. Ververeli PA, Sutton DC, Hearn SL, Booth RE Jr, Hozack WJ, Rothman RR. Continuous passive motion after total knee arthroplasty. Analysis of cost and benefits. Clin Orthop Relat Res. 1995;321:208–215. [PubMed] [Google Scholar]
- 33. Worland RL, Arredondo J, Angles F, Lopez-Jimenez F, Jessup DE. Home continuous passive motion machine versus professional physical therapy following total knee replacement. J Arthroplasty. 1998;13:784–787. [DOI] [PubMed] [Google Scholar]
- 34. Desteli EE, Imren Y, Aydin N. Effect of both preoperative andpostoperative cryoceutical treatment on hemostasis and postoperative pain following total knee arthroplasty. Int J Clin Exp Med. 2015;8:19150–19155. [PMC free article] [PubMed] [Google Scholar]
- 35. Radkowski CA, Pietrobon R, Vail TP, Nunley JA 2nd, Jain NB, Easley ME. Cryotherapy temperature differences after total knee arthroplasty: a prospective randomized trial. J Surg Orthop Adv. 2007;16:67–72. [PubMed] [Google Scholar]
- 36. Ruffilli A, Castagnini F, Traina F, et al. Temperature-controlled continuous cold flow device after total knee arthroplasty: a randomized controlled trial study. J Knee Surg. 2017;30:675–681. [DOI] [PubMed] [Google Scholar]
- 37. Rui W, Long G, Li G, Yang Y, Hengjin L, Zhenhu W. Effects of ethyl chloride spray on early recovery after total knee arthroplasty: a prospective study. J Orthop Sci. 2017;22: 89–93. [DOI] [PubMed] [Google Scholar]
- 38. Schinsky MF, McCune C, Bonomi J. Multifaceted comparison of two cryotherapy devices used after total knee arthroplasty: cryotherapy device comparison. Orthop Nurs. 2016;35:309–316. [DOI] [PubMed] [Google Scholar]
- 39. Su EP, Perna M, Boettner F, et al. A prospective, multi-center, randomised trial to evaluate the efficacy of a cryopneumatic device on total knee arthroplasty recovery. J Bone Joint Surg Br. 2012;94:153–156. [DOI] [PubMed] [Google Scholar]
- 40. Demoulin C, Brouwers M, Darot S, Gillet P, Crielaard JM, Vanderthommen M. Comparison of gaseous cryotherapy with more traditional forms of cryotherapy following total knee arthroplasty. Ann Phys Rehabil Med. 2012;55:229–240. [DOI] [PubMed] [Google Scholar]
- 41. Gibbons CE, Solan MC, Ricketts DM, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: a prospective randomized trial. Int Orthop. 2001;25:250–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pan L, Hou D, Liang W, Fei J, Hong Z. Comparison the effects of pressurized salt ice packs with water ice packs on patients following total knee arthroplasty. Int J Clin Exp Med. 2015;8:18179–18184. [PMC free article] [PubMed] [Google Scholar]
- 43. Thienpont E. Does advanced cryotherapy reduce pain and narcotic consumption after knee arthroplasty? Clin Orthop Relat Res. 2014;472:3417–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Morsi E. Continuous-flow cold therapy after total knee arthroplasty. J Arthroplasty. 2002;17:718–722. [DOI] [PubMed] [Google Scholar]
- 45. Physical Activity Guidelines for Americans . Office of Disease Prevention and Health Promotion. 2018. https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf. Accessed November 23, 2019.
- 46. Groen JW, Stevens M, Kersten RF, Reininga IH, van den Akker-Scheek I. After total knee arthroplasty, many people are not active enough to maintain their health and fitness: an observational study. J Physiother. 2012;58:113–116. [DOI] [PubMed] [Google Scholar]
- 47. Shadyab AH, Eaton CB, Li W, LaCroix AZ. Association of Physical Activity with late-life mobility limitation among women with total joint replacement for knee or hip osteoarthritis. J Rheumatol. 2018;45:1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Valtonen A, Poyhonen T, Sipila S, Heinonen A. Effects of aquatic resistance training on mobility limitation and lower-limb impairments after knee replacement. Arch Phys Med Rehabil. 2010;91:833–839. [DOI] [PubMed] [Google Scholar]
- 49. Valtonen A, Poyhonen T, Sipila S, Heinonen A. Maintenance of aquatic training-induced benefits on mobility and lower-extremity muscles among persons with unilateral knee replacement. Arch Phys Med Rehabil. 2011;92:1944–1950. [DOI] [PubMed] [Google Scholar]
- 50. Karaman A, Yuksel I, Kinikli GI, Caglar O. Do Pilates-based exercises following total knee arthroplasty improve postural control and quality of life? Physiother Theory Pract. 2017;33:289–295. [DOI] [PubMed] [Google Scholar]
- 51. Li L, Cheng S, Wang G, Duan G, Zhang Y. Tai chi chuan exercises improve functional outcomes and quality of life in patients with primary total knee arthroplasty due to knee osteoarthritis. Complement Ther Clin Pract. 2019;35:121–125. [DOI] [PubMed] [Google Scholar]
- 52. Unver B, Bakirhan S, Karatosun V. Does a weight-training exercise programme given to patients four or more years after total knee arthroplasty improve mobility: a randomized controlled trial. Arch Gerontol Geriatr. 2016;64:45–50. [DOI] [PubMed] [Google Scholar]
- 53. Arnold JB, Mackintosh S, Olds TS, Jones S, Thewlis D. Improvements in knee biomechanics during walking are associated with increased physical activity after total knee arthroplasty. J Orthop Res. 2015;33:1818–1825. [DOI] [PubMed] [Google Scholar]
- 54. Taniguchi M, Sawano S, Kugo M, Maegawa S, Kawasaki T, Ichihashi N. Physical activity promotes gait improvement in patients with total knee arthroplasty. J Arthroplasty. 2016;31: 984–988. [DOI] [PubMed] [Google Scholar]
- 55. Piva SR, Farrokhi S, Almeida G, Fitzgerald GK, Levison TJ, DiGioia AM. Dose-associated changes in gait parameters in response to exercise programs after total knee arthroplasty: secondary analysis of two randomized studies. Int J Phys Med Rehabil. 2015;3:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bruun-Olsen V, Heiberg KE, Wahl AK, Mengshoel AM. The immediate and long-term effects of a walking-skill program compared to usual physiotherapy care in patients who have undergone total knee arthroplasty (TKA): a randomized controlled trial. Disabil Rehabil. 2013;35:2008–2015. [DOI] [PubMed] [Google Scholar]
- 57. Christiansen CL, Bade MJ, Davidson BS, Dayton MR, Stevens-Lapsley JE. Effects of weight-bearing biofeedback training on functional movement patterns following total knee arthroplasty: a randomized controlled trial. J Orthop Sports Phys Ther. 2015;45:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Frost H, Lamb SE, Robertson S. A randomized controlled trial of exercise to improve mobility and function after elective knee arthroplasty. Feasibility, results and methodological difficulties. Clin Rehabil. 2002;16:200–209. [DOI] [PubMed] [Google Scholar]
- 59. Liao CD, Lin LF, Huang YC, Huang SW, Chou LC, Liou TH. Functional outcomes of outpatient balance training following total knee replacement in patients with knee osteoarthritis: a randomized controlled trial. Clin Rehabil. 2015;29:855–867. [DOI] [PubMed] [Google Scholar]
- 60. Moffet H, Collet JP, Shapiro SH, Paradis G, Marquis F, Roy L. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: a single-blind randomized controlled trial. Arch Phys Med Rehabil. 2004;85:546–556. [DOI] [PubMed] [Google Scholar]
- 61. Li J, Wu T, Xu Z, Gu X. A pilot study of post-total knee replacement gait rehabilitation using lower limbs robot-assisted training system. Eur J Orthop Surg Traumatol. 2014;24:203–208. [DOI] [PubMed] [Google Scholar]
- 62. Bin SI, Nam TS. Early results of high-flex total knee arthroplasty: comparison study at 1 year after surgery. Knee Surg Sports Traumatol Arthrosc. 2007;15:350–355. [DOI] [PubMed] [Google Scholar]
- 63. Cheng K, Ridley D, Bird J, McLeod G. Patients with fixed flexion deformity after total knee arthroplasty do just as well as those without: ten-year prospective data. Int Orthop. 2010;34:663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gatha NM, Clarke HD, Fuchs R, Scuderi GR, Insall JN. Factors affecting postoperative range of motion after total knee arthroplasty. J Knee Surg. 2004;17:196–202. [DOI] [PubMed] [Google Scholar]
- 65. Ritter MA, Lutgring JD, Davis KE, Berend ME, Pierson JL, Meneghini RM. The role of flexion contracture on outcomes in primary total knee arthroplasty. J Arthroplasty. 2007;22: 1092–1096. [DOI] [PubMed] [Google Scholar]
- 66. Ritter MA, Lutgring JD, Davis KE, Berend ME. The effect of postoperative range of motion on functional activities after posterior cruciate-retaining total knee arthroplasty. J Bone Joint Surg Am. 2008;90:777–784. [DOI] [PubMed] [Google Scholar]
- 67. Li B, Wang G, Wang Y, Bai L. Effect of two limb positions on venous hemodynamics and hidden blood loss following total knee arthroplasty. J Knee Surg. 2017;30:70–74. [DOI] [PubMed] [Google Scholar]
- 68. Li B, Wen Y, Liu D, Tian L. The effect of knee position on blood loss and range of motion following total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20:594–599. [DOI] [PubMed] [Google Scholar]
- 69. Panni AS, Cerciello S, Vasso M, Del Regno C. Knee flexion after total knee arthroplasty reduces blood loss. Knee Surg Sports Traumatol Arthrosc. 2014;22:1859–1864. [DOI] [PubMed] [Google Scholar]
- 70. Yang Y, Yong-Ming L, Pei-jian D, Jia L, Ying-ze Z. Leg position influences early blood loss and functional recovery following total knee arthroplasty: a randomized study. Int J Surg . 2015;23(Pt A):82–86. [DOI] [PubMed] [Google Scholar]
- 71. De Fine M, Traina F, Giavaresi G, et al. Effect of different postoperative flexion regimes on the outcomes of total knee arthroplasty: randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25:2972–2977. [DOI] [PubMed] [Google Scholar]
- 72. Jiang C, Lou J, Qian W, Ye C, Zhu S. Impact of flexion versus extension of knee position on outcomes after total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2017;137:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Avramidis K, Karachalios T, Popotonasios K, Sacorafas D, Papathanasiades AA, Malizos KN. Does electric stimulation of the vastus medialis muscle influence rehabilitation after total knee replacement? Orthopedics. 2011;34:175. [DOI] [PubMed] [Google Scholar]
- 74. Avramidis K, Strike PW, Taylor PN, Swain ID. Effectiveness of electric stimulation of the vastus medialis muscle in the rehabilitation of patients after total knee arthroplasty. Arch Phys Med Rehabil. 2003;84:1850–1853. [DOI] [PubMed] [Google Scholar]
- 75. Stevens-Lapsley JE, Balter JE, Wolfe P, Eckhoff DG, Kohrt WM. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther. 2012;92:210–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yoshida Y, Ikuno K, Shomoto K. Comparison of the effect of sensory-level and conventional motor-level neuromuscular electrical stimulations on quadriceps strength after total knee arthroplasty: a prospective randomized single-blind trial. Arch Phys Med Rehabil. 2017;98:2364–2370. [DOI] [PubMed] [Google Scholar]
- 77. Petterson SC, Mizner RL, Stevens JE, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum. 2009;61:174–183. [DOI] [PubMed] [Google Scholar]
- 78. Bade MJ, Struessel T, Dayton M, et al. Early high-intensity versus low-intensity rehabilitation after total knee arthroplasty: a randomized controlled trial. Arthritis Care Res (Hoboken). 2017;69:1360–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Molla RY, Sadeghi H, Kahlaee AH. The effect of early progressive resistive exercise therapy on balance control of patients with total knee arthroplasty: a randomized controlled trial. Topics in Geriatric Rehabilitation. 2017; 33:286–294. [Google Scholar]
- 80. Husby VS, Foss OA, Husby OS, Winther SB. Randomized controlled trial of maximal strength training vs. standard rehabilitation following total knee arthroplasty. Eur J Phys Rehabil Med. 2018;54:371–379. [DOI] [PubMed] [Google Scholar]
- 81. Lizaur-Utrilla A, Martinez-Mendez D, Miralles-Munoz FA, Marco-Gomez L, Lopez-Prats FA. Comparable outcomes after total knee arthroplasty in patients under 55 years than in older patients: a matched prospective study with minimum follow-up of 10 years. Knee Surg Sports Traumatol Arthrosc. 2017;25:3396–3402. [DOI] [PubMed] [Google Scholar]
- 82. Naylor JM, Yeo AE, Mittal R, Ko VW, Harris IA. Improvements in knee range and symptomatic and functional behavior after knee arthroplasty based on preoperative restriction in range. J Arthroplasty. 2012;27:1100–1105. [DOI] [PubMed] [Google Scholar]
- 83. Sullivan M, Tanzer M, Stanish W, et al. Psychological determinants of problematic outcomes following total knee arthroplasty. Pain. 2009;143:123–129. [DOI] [PubMed] [Google Scholar]
- 84. Xu S, Chen JY, Lo NN, et al. The influence of obesity on functional outcome and quality of life after total knee arthroplasty: a ten-year follow-up study. Bone Joint J. 2018;100-b:579–583. [DOI] [PubMed] [Google Scholar]
- 85. Liao CD, Huang YC, Lin LF, et al. Continuous passive motion and its effects on knee flexion after total knee arthroplasty in patients with knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2016;24:2578–2586. [DOI] [PubMed] [Google Scholar]
- 86. Lizaur A, Marco L, Cebrian R. Preoperative factors influencing the range of movement after total knee arthroplasty for severe osteoarthritis. J Bone Joint Surg Br. 1997;79:626–629. [DOI] [PubMed] [Google Scholar]
- 87. Lizaur-Utrilla A, Miralles-Munoz FA, Sanz-Reig J, Collados-Maestre I. Cementless total knee arthroplasty in obese patients: a prospective matched study with follow-up of 5-10 years. J Arthroplasty. 2014;29:1192–1196. [DOI] [PubMed] [Google Scholar]
- 88. Rossi MD, Eberle T, Roche M, et al. Use of a squatting movement as a clinical marker of function after total knee arthroplasty. Am J Phys Med Rehabil. 2013;92:53–60. [DOI] [PubMed] [Google Scholar]
- 89. Sharma L, Sinacore J, Daugherty C, et al. Prognostic factors for functional outcome of total knee replacement: a prospective study. J Gerontol A Biol Sci Med Sci. 1996;51: M152–M157. [DOI] [PubMed] [Google Scholar]
- 90. Si HB, Zeng Y, Zhong J, et al. The effect of primary total knee arthroplasty on the incidence of falls and balance-related functions in patients with osteoarthritis. Sci Rep. 2017;7:16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stevens-Lapsley JE, Petterson SC, Mizner RL, Snyder-Mackler L. Impact of body mass index on functional performance after total knee arthroplasty. J Arthroplasty. 2010;25: 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Clement ND, Jenkins PJ, MacDonald D, et al. Socioeconomic status affects the Oxford knee score and short-form 12 score following total knee replacement. Bone Joint J. 2013;95-b: 52–58. [DOI] [PubMed] [Google Scholar]
- 93. Clement ND, MacDonald D, Simpson AH, Burnett R. Total knee replacement in patients with concomitant back pain results in a worse functional outcome and a lower rate of satisfaction. Bone Joint J. 2013;95-b:1632–1639. [DOI] [PubMed] [Google Scholar]
- 94. Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder-Mackler L. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol. 2005;32:1533–1539. [PubMed] [Google Scholar]
- 95. Holm B, Kristensen MT, Bencke J, Husted H, Kehlet H, Bandholm T. Loss of knee-extension strength is related to knee swelling after total knee arthroplasty. Arch Phys Med Rehabil. 2010;91:1770–1776. [DOI] [PubMed] [Google Scholar]
- 96. Park KK, Chang CB, Kang YG, Seong SC, Kim TK. Correlation of maximum flexion with clinical outcome after total knee replacement in Asian patients. J Bone Joint Surg Br. 2007;89:604–608. [DOI] [PubMed] [Google Scholar]
- 97. Twiggs J, Salmon L, Kolos E, Bogue E, Miles B, Roe J. Measurement of physical activity in the pre- and early post-operative period after total knee arthroplasty for osteoarthritis using a Fitbit flex device. Med Eng Phys. 2018;51:31–40. [DOI] [PubMed] [Google Scholar]
- 98. Dauty M, Smitt X, Menu P, Dubois C. Which factors affect the duration of inpatient rehabilitation after total knee arthroplasty in the absence of complications? Ann Phys Rehabil Med. 2009;52:234–245. [DOI] [PubMed] [Google Scholar]
- 99. Jones CA, Voaklander DC, Suarez-Alma ME. Determinants of function after total knee arthroplasty. Phys Ther. 2003;83:696–706. [PubMed] [Google Scholar]
- 100. Kauppila AM, Kyllonen E, Ohtonen P, Leppilahti J, Sintonen H, Arokoski JP. Outcomes of primary total knee arthroplasty: the impact of patient-relevant factors on self-reported function and quality of life. Disabil Rehabil. 2011;33:1659–1667. [DOI] [PubMed] [Google Scholar]
- 101. Kramers-de Quervain IA, Kampfen S, Munzinger U, Mannion AF. Prospective study of gait function before and 2 years after total knee arthroplasty. Knee. 2012;19:622–627. [DOI] [PubMed] [Google Scholar]
- 102. Tay KS, Cher EWL, Zhang K, Tan SB, Howe TS, Koh JSB. Comorbidities have a greater impact than age alone in the outcomes of octogenarian total knee arthroplasty. J Arthroplasty. 2017;32:3373–3378. [DOI] [PubMed] [Google Scholar]
- 103. Collados-Maestre I, Lizaur-Utrilla A, Martinez-Mendez D, Marco-Gomez L, Lopez-Prats FA. Concomitant low back pain impairs outcomes after primary total knee arthroplasty in patients over 65 years: a prospective, matched cohort study. Arch Orthop Trauma Surg. 2016;136:1767–1771. [DOI] [PubMed] [Google Scholar]
- 104. Lin J, Yang B, Weng XS, Jin J, Zhao Q, Qiu GX. Effect of osteoarthritis patients' gender on rehabilitation after total knee arthroplasty. Chin Med Sci J. 2009;24:102–106. [DOI] [PubMed] [Google Scholar]
- 105. Hudakova Z, Zieba HR, Lizis P, et al. Evaluation of the effects of a physiotherapy program on quality of life in females after unilateral total knee arthroplasty: a prospective study. J Phys Ther Sci. 2016;28:1412–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Akbaba YA, Yeldan I, Guney N, Ozdincler AR. Intensive supervision of rehabilitation programme improves balance and functionality in the short term after bilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2016; 24:26–33. [DOI] [PubMed] [Google Scholar]
- 107. Fransen M, Nairn L, Bridgett L, et al. Post-acute rehabilitation after total knee replacement: a multicenter randomized clinical trial comparing long-term outcomes. Arthritis Care Res (Hoboken). 2017;69:192–200. [DOI] [PubMed] [Google Scholar]
- 108. Artz N, Dixon S, Wylde V, et al. Comparison of group-based outpatient physiotherapy with usual care after total knee replacement: a feasibility study for a randomized controlled trial. Clin Rehabil. 2017;31:487–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kauppila AM, Kyllonen E, Ohtonen P, et al. Multidisciplinary rehabilitation after primary total knee arthroplasty: a randomized controlled study of its effects on functional capacity and quality of life. Clin Rehabil. 2010;24:398–411. [DOI] [PubMed] [Google Scholar]
- 110. Hiyama Y, Kamitani T, Wada O, Mizuno K, Yamada M. Effects of group-based exercise on range of motion, muscle strength, functional ability, and pain during the acute phase after total knee arthroplasty: a controlled clinical trial. J Orthop Sports Phys Ther. 2016;46:742–748. [DOI] [PubMed] [Google Scholar]
- 111. Labraca NS, Castro-Sanchez AM, Mataran-Penarrocha GA, Arroyo-Morales M, Sanchez-Joya Mdel M, Moreno-Lorenzo C. Benefits of starting rehabilitation within 24 hours of primary total knee arthroplasty: randomized clinical trial. Clin Rehabil. 2011;25:557–566. [DOI] [PubMed] [Google Scholar]
- 112. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93: 962–970. [DOI] [PubMed] [Google Scholar]
- 113. Losina E, Collins JE, Deshpande BR, et al. Financial incentives and health coaching to improve physical activity following total knee replacement: a randomized controlled trial. Arthritis Care Res (Hoboken). 2018;70: 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Losina E, Collins JE, Wright J, et al. Postoperative care navigation for total knee arthroplasty patients: a randomized controlled trial. Arthritis Care Res (Hoboken). 2016;68: 1252–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. McLawhorn AS, Fu MC, Schairer WW, Sculco PK, MacLean CH, Padgett DE. Continued inpatient care after primary total knee arthroplasty increases 30-day post-discharge complications: a propensity score-adjusted analysis. J Arthroplasty. 2017;32:S113–s118. [DOI] [PubMed] [Google Scholar]
- 116. Padgett DE, Christ AB, Joseph AD, Lee YY, Haas SB, Lyman S. Discharge to inpatient rehab does not result in improved functional outcomes following primary total knee arthroplasty. J Arthroplasty. 2018;33:1663–1667. [DOI] [PubMed] [Google Scholar]
- 117. Tribe KL, Lapsley HM, Cross MJ, Courtenay BG, Brooks PM, March LM. Selection of patients for inpatient rehabilitation or direct home discharge following total joint replacement surgery: a comparison of health status and out-of-pocket expenditure of patients undergoing hip and knee arthroplasty for osteoarthritis. Chronic Illn. 2005;1:289–302. [DOI] [PubMed] [Google Scholar]
- 118. Brennan GP, Fritz JM, Houck LT, Hunter SJ. Outpatient rehabilitation care process factors and clinical outcomes among patients discharged home following unilateral total knee arthroplasty. J Arthroplasty. 2015;30:885–890. [DOI] [PubMed] [Google Scholar]
- 119. Smith BA, Fields CJ, Fernandez N. Physical therapists make accurate and appropriate discharge recommendations for patients who are acutely ill. Phys Ther. 2010;90:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Falvey JR, Burke RE, Malone D, Ridgeway KJ, McManus BM, Stevens-Lapsley JE. Role of physical therapists in reducing hospital readmissions: optimizing outcomes for older adults during care transitions from hospital to community. Phys Ther. 2016;96:1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jette DU, Stilphen M, Ranganathan VK, Passek SD, Frost FS, Jette AM. AM-PAC "6-clicks" functional assessment scores predict acute care hospital discharge destination. Phys Ther. 2014;94:1252–1261. [DOI] [PubMed] [Google Scholar]
- 122. Menendez ME, Schumacher CS, Ring D, Freiberg AA, Rubash HE, Kwon Y-M. Does “6-clicks” day 1 postoperative mobility score predict discharge disposition after total hip and knee arthroplasties? The Journal of Arthroplasty. 2016;31: 1916–1920. [DOI] [PubMed] [Google Scholar]
- 123. Alghadir A, Anwer S, Brismee JM. The reliability and minimal detectable change of timed up and go test in individuals with grade 1-3 knee osteoarthritis. BMC Musculoskelet Disord. 2015;16:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lyman S, Lee YY, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin Orthop Relat Res. 2016;474: 1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Peer MA, Lane J. The knee injury and osteoarthritis outcome score (KOOS): a review of its psychometric properties in people undergoing total knee arthroplasty. J Orthop Sports Phys Ther. 2013;43:20–28. [DOI] [PubMed] [Google Scholar]
- 126. Unver B, Kalkan S, Yuksel E, Kahraman T, Karatosun V. Reliability of the 50-foot walk test and 30-sec chair stand test in total knee arthroplasty. Acta Ortop Bras. 2015;23:184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Yuksel E, Kalkan S, Cekmece S, Unver B, Karatosun V. Assessing minimal detectable changes and test-retest reliability of the timed up and go test and the 2-minute walk test in patients with total knee arthroplasty. J Arthroplasty. 2017;32:426–430. [DOI] [PubMed] [Google Scholar]
- 128. Poitras S, Wood KS, Savard J, Dervin GF, Beaulé PE. Assessing functional recovery shortly after knee or hip arthroplasty: a comparison of the clinimetric properties of four tools. BMC Musculoskelet Disord. 2016;17:478–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


