Abstract
Background
Although previous studies have shown that opioid agonist therapy (OAT) is linked to reductions in illicit opioid use, less is known about how OAT impacts the use of other psychoactive substances. We aimed to examine the changes in use of different substances by comparing patterns before and after initiating OAT.
Methods
Data for this study was derived from three ongoing prospective cohorts involving people who use drugs in Vancouver, Canada from 1996 to 2018. We assessed use patterns for heroin, illicit prescription opioid, cocaine, crack cocaine, crystal methamphetamine, cannabis, daily alcohol use, and benzodiazepines. Segmented regression was conducted to compare the trends of substance use between pre-treatment and post-treatment periods.
Results
The study included 1107 participants. After OAT engagement, we observed an immediate decline in the proportion as well as a decreasing trend for heroin (Adjusted Odds Ratio (AOR): 0.80, 95% confidence interval (CI): 0.77, 0.83), illicit prescription opioid (AOR: 0.87, 95% CI: 0.83, 0.90), and benzodiazepines (AOR: 0.73, 95% CI: 0.67, 0.80). There was no significant difference comparing the pre-treatment and post-treatment trends for cocaine, crack cocaine, crystal methamphetamine, and cannabis. However, higher growth slope was noted during the post-treatment period for daily alcohol use (P = 0.016).
Conclusions
We observed significant reduction in illicit opioids use following OAT initiation, but not for stimulant and cannabis. The increasing problematic use of alcohol may pose challenges to the safety and effectiveness of OAT. Development of comprehensive and tailored treatment strategies is needed for poly-substance users accessing OAT.
Keywords: Opioid agonist therapy, poly-substance use, segmented regression, longitudinal studies
1. Introduction
In the context of the current opioid crisis in the United States and Canada, opioid agonist therapies (OAT), either methadone or buprenorphine/naloxone, have proven effective in reducing opioid use (Schuckit, 2016). Furthermore, OAT has been found to help protect against a range of opioid-related harms (Degenhardt et al., 2011; MacArthur et al., 2012; Nolan et al., 2015; Platt et al., 2018), reduce criminal activity (Krebs et al., 2014; Russolillo et al., 2018), and improve quality of life (Krebs et al., 2016).
It has been shown that ongoing use of illicit opioids and other substances is common in a wide variety of OAT treatment settings (Kidorf and Stitzer, 1993; Saunders et al., 2012; Heikman et al., 2017). Such information has great clinical implication since concomitant use of other substances while on OAT has been found to be associated with negative treatment retention and outcomes (Williamson et al., 2006; Sullivan et al., 2011; Wang et al., 2017), as well as high rates of health risk behaviours such as binge drinking, unprotected sex, needles and syringes sharing (Meredith et al., 2017; Lorvick et al., 2018; Tavitian-Exley et al., 2018).
Despite the benefit of OAT on reducing opioid use, there have been mixed findings regarding how OAT impacts the use of other substances. Several studies observed an overall decline in other substances among people on opioid treatment programs. Findings from the Treatment Outcome Prospective Study in the United States suggested that methadone programs could indirectly reduce the use of other substances including cocaine, amphetamines, and cannabis (Dunteman et al., 1992; Fairbank et al., 1993). Similarly, an Australian study involving heroin users reported that a decline in heroin use was associated with less frequent use of cocaine, amphetamine, cannabis, benzodiazepines, and other opioids (Darke et al., 2006).
However, there is also a common concern that during opioid treatment, patients could substitute opioid with other types of substances, resulting in an increased use of other drugs. From the National Treatment Outcome Research Study in the United Kingdom, researchers found that frequencies for crack cocaine and alcohol use increased significantly from 1 year post-treatment to 4-5 years post-treatment (Gossop et al., 2000; Gossop et al., 2003). Herdener et al reported that among patients on OAT in Switzerland, there were significant declines in the frequent use of heroin and cocaine, while frequent alcohol use increased (Herdener et al., 2017). A few studies used a life course perspective and focused on differential clinical response among subpopulations. Grella et al examined trajectories of heroin, alcohol and other drug use over 30 years among participants receiving methadone treatment in California, and found that among those who reduced heroin use rapidly, over half increased their use of alcohol and other drugs (particularly amphetamines) concurrently (Grella and Lovinger, 2011). Eastwood et al pointed out that if opioid treatment does not suppress opioid use to any clinically meaningful extent, approximately 40% of the patients will use alcohol or crack cocaine at a consistently high or increasing level (Eastwood et al., 2019).
In the context of these mixed findings, it should be noted that previous studies have mostly examined the substance use patterns after engaging in treatment programs. However, pretreatment substance use patterns have received substantially less consideration. It may be that the observed difference in the frequency of substance use after engaging in treatment could be partially explained by a pre-existing trend. For example, suppose that an individual has already attempted to reduce cocaine use before initiating opioid treatment, one could incorrectly attribute the reduction in cocaine use to treatment engagement, which would have been observed even in the absence of the treatment, and only due to the pre-treatment trend. Further, to our knowledge there is no study examining to what extent OAT impacts on other substance use in a Canadian setting using community recruited cohorts of people who use illicit drugs. Therefore, the present study extends previous research by comparing long-term substance use patterns before and after engaging in OAT. The study utilizes data from longitudinal cohort studies involving people who use illicit drugs in Vancouver, Canada. It is hoped that the findings from this study may serve to inform the development of comprehensive treatment strategies tailored for poly-substance users.
2. Methods
2.1. Study population and setting
Data from this study were drawn from three ongoing open prospective cohort studies of people who use drugs in Vancouver: the Vancouver Injection Drug Users Study (VIDUS), AIDS Care Cohort to evaluate Exposure to Survival Services (ACCESS), and the At-Risk Youth Study (ARYS). These cohorts have been described in detail previously (Strathdee et al., 1998; Wood et al., 2009; Cheng et al., 2018). Briefly, recruitment for all cohorts uses extensive snowball sampling, self-referral, and street outreach. VIDUS includes participants who were at least 18 years old, HIV-seronegative, and had used injection drugs in the month prior to baseline interview. ACCESS includes participants who were at least 18 years old, HIV-seropositive, and reported using an illicit drug other than or in addition to cannabis in the month preceding enrolment. To be eligible for ARYS, participants had to be aged 14-26 years, have used illegal drugs other than or in addition to cannabis in the month preceding enrolment, and be street-involved. Youth who were homeless or using services for homeless youth were considered street-involved in this study. For all cohorts, participants had to reside in the Greater Vancouver area and provide written informed consent.
Recruitment and follow-up procedures are harmonized among these cohorts to facilitate analyses of merged data. Specifically, at enrolment and on a semi-annual basis, participants complete an interviewer-administered questionnaire. Nurses also assess participants for various health conditions, and obtain blood samples for HIV and HCV serologic testing, and HIV disease monitoring, as appropriate. At each study visit, participants are provided with a stipend of $40 (CAD) for their time. These studies have been approved by the University of British Columbia/Providence Health Care Research Ethics Board.
For the present analysis, participants were included if they were enrolled between May 1996 and May 2018. The sample was further restricted to those who started OAT during study follow-up and completed at least one study visit before starting OAT and one study visit after OAT. To understand the change of different types of substance use, we included individual observations from 5 years prior to OAT engagement and up to 5 years after.
2.2. Measurements
We assessed eight types of substance use, each of which was used as dichotomous outcome (yes vs. no) and collected through self-report. First, we evaluated opioid use including any injection or non-injection heroin use and illicit prescription opioid use. Illicit prescription opioid use was defined as selecting any prescription opiates for the survey question “In the last 6 months, when you were using, which of the following prescription opiates did you use when they were not prescribed for you or that you took only for the experience or feeling they caused”. Next, we examined different types of stimulant use, including any injection or non-injection cocaine use, non-injection crack cocaine use, and injection or non-injection crystal methamphetamine use. Furthermore, we included non-injection cannabis use, daily alcohol use, and injection or noninjection benzodiazepines use. All these substance use variables were referred to the behaviours in the previous six months from study visit.
We considered a number of explanatory variables that could be associated with different types of substance use. The following socio-demographic variables were included: age (per year); sex (male vs. female); ethnicity (white vs. others); and current housing status (unstable housing vs. stable housing). Behavioural and socio-structural factors hypothesized to be associated with substance use included: employment, defined as having a regular job, temporary job or self-employed (yes vs. no); incarceration, defined as being in detention, jail, or prison (yes vs. no); sex work involvement, defined as exchanging sex for money, drugs, gifts, food, clothes, shelter or favours (yes vs. no); and drug dealing, defined as selling drugs as a source of income (yes us. no). We also included a variable indicating engagement in any other addiction treatment or services except for OAT (yes vs. no). When examining the time trend of substance use after OAT enrolment, we included a variable indicating OAT adherence, defined as reporting methadone/methadose or buprenorphine/naloxone from the question “In the last 6 months, have you been in any kind of alcohol or drug treatment” (yes vs. no). As the data of the study were collected over an extended time period, year of study enrolment was also included. All behavioural variables were time-updated and referred to the period beginning six months before each study visit unless otherwise specified.
2.3. Statistical analysis
We used segmented regression to assess the extent to which OAT engagement impacted on the levels and trends of different types of substance use. OAT enrolment information was collected through self-reported engagement in maintenance treatment using methadone/methadose or buprenorphine/naloxone. The observations before and after the first report of OAT engagement during follow-up period constituted the two segments of our regression models. Under the assumption that the existing trend in the outcome would have remained unchanged absent the treatment, the observed trend during pre-treatment period served as the counterfactual scenario. A segmented regression differs from a non-segmented regression as it controls for pre-existing secular trend, and allows one to assess the difference of slope (i.e., change in time trend) between pre-treatment and post-treatment periods.
As a first step, we calculated the proportions of the different substance use variables for each six-month period, and used segmented regression on this aggregate data to describe the overall substance use trend before and after OAT engagement. Residual autocorrelation was tested using the Durbin-Watson test (Durbin and Watson, 1950), and autocorrelation orders were included in the model if necessary.
Next, to further account for heterogeneity at the individual level, segmented regression was fit using participant-level data. Since the outcomes were binary variables, the logit link function was used. This model allows one to include a two-piece linear function of time corresponding to the pre-treatment and post-treatment segments, and at the same time accounts for the correlation inherent in repeated measures observed from individuals over time by assuming exchangeable working correlation structure. By comparing the growth slope between pre-treatment and posttreatment segments, we were able to assess whether OAT engagement had an enduring impact on substance use patterns. All hypothesized explanatory variables were adjusted in the multivariable models.
As an exploratory secondary analysis, we used injection opioid use and non-injection opioid use as outcomes after combining heroin and illicit prescription opioid use information. Similarly, the outcomes of injection stimulant use and non-injection stimulant use were generated including cocaine, crack cocaine, and crystal methamphetamine use. Segmented regression was then applied to assess whether OAT engagement had an impact on overall injection and non-injection drug use trend.
As the first sensitivity analysis, we identified participants who dropped out of the study after OAT enrolment, and conducted the segmented regression on the sample excluding the dropouts. As the second sensitivity analysis, for participants who were alive but dropped out of the study, we imputed their substance use and covariate information for the missing follow-up visits using the last observation carried forward method. For participants who were dead due to overdose, we imputed their substance use information as being actively using for the missing follow-up visits. Then we conducted the segmented regression using the entire study sample with the imputed data. Information on death was obtained through a confidential data linkage with the British Columbia Vital Statistics Agency. All analyses were performed using SAS 9.4, and the segmented regression on participant-level data was conducted using the GENMOD procedure (SAS Institute, USA). All P values were two-sided.
3. Results
3.1. General characteristics
There were 4693 participants enrolled in the three cohort studies between May 1996 and May 2018. Among them, 1253 participants were not on OAT at study enrolment and later initiated OAT during study follow-ups. 146 participants were excluded from the analysis due to not completing at least one study visit during pre-treatment segment and one study visit during posttreatment segment. Therefore, a total of 1107 participants were included in the present analysis. Compared to participants in the analytical sample, the 146 excluded participants were younger (median age: 31.2 years; Wilcoxon rank-sum test P = 0.018), but there was no significant difference regarding sex (chi-squared test P = 0.286), ethnicity (chi-squared test P = 0.385), heroin use (chi-squared test P = 0.583) and illicit prescription opioid use (chi-squared test P = 0.086).
Among included participants, the median age at OAT initiation was 35.5 years (quartile 1 – quartile 3: 26.8 – 43.6), 669 (60.4%) were male, 621 (56.1%) self-reported white ethnicity. There were in total 798 VIDUS participants, 145 ACCESS participants, and 164 ARYS participants. The median age at OAT initiation was 24.0 years for ARYS participants, which was significantly younger than the other two cohorts (VIDUS: 37.2 years; ACCESS: 41.4 years; Kruskal-Wallis test P < 0.001). Besides, higher proportion of ARYS participants self-reported white ethnicity (VIDUS: 55.1%; ACCESS: 45.5%; ARYS: 70.1%; chi-squared testf P < 0.001), but there was no significant difference regarding sex (chi-squared test P = 0.154). At each time point before OAT engagement, among people who used stimulants, including cocaine, crack cocaine, and methamphetamine, around 74.3% to 91.4% of them reported using opioids. Characteristics of participants at OAT initiation are presented in Table 1. The median number of study visit per participant was 11 (quartile 1 – quartile 3: 8 – 14). In the pre-treatment segment, the average number of observations at each time point was 442 (range 218 – 814). In the post-treatment segment, the average number of observations at each time point was 729 (range 488 – 1107). The distribution of participants and responses of the eight types of substances at each time point are presented in Table 2. In the post-treatment segment, the OAT adherence rate for each six-month period was 71.1% on average, ranging from 65.8% to 100%. All explanatory variables had less than 0.5% missing value, and a total of 140 (1.1%) observations were excluded from the multivariable analysis.
Table 1.
Characteristics of participants at opioid agonist therapy initiation among 1107 people who use illicit drugs in Vancouver, British Columbia, Canada.
| Characteristics | Frequency | Proportion (%) |
|---|---|---|
| Sociodemographic factors | ||
| Age in years, median (quartile 1 – quartile 3) | 35.5 | 26.8–43.6 |
| Male | 669 | 60.4 |
| White ethnicity | 621 | 56.1 |
| Employment status (regular/temporary job; self-employed) a | 192 | 17.3 |
| Unstable housing | 710 | 64.1 |
| Substance use a | ||
| Heroin | 855 | 77.2 |
| Injection | 815 | 73.6 |
| Non-injection | 160 | 14.5 |
| Illicit prescription opioid | 350 | 31.6 |
| Injection | 240 | 21.7 |
| Non-injection | 169 | 15.3 |
| Cocaine | 559 | 50.5 |
| Injection | 505 | 45.6 |
| Non-injection | 138 | 12.5 |
| Crack cocaine | 548 | 49.5 |
| Crystal methamphetamine | 226 | 20.4 |
| Injection | 179 | 16.2 |
| Non-injection | 107 | 9.7 |
| Cannabis | 577 | 52.1 |
| Daily alcohol | 92 | 8.3 |
| Benzodiazepines | 93 | 8.4 |
| Treatment experience a | ||
| Any other addiction treatment or services | 0 | 0.0 |
| Behavioural risk factor a | ||
| Incarceration | 271 | 24.5 |
| Sex work involvement | 191 | 17.3 |
| Drug dealing | 224 | 20.2 |
| Other factor | ||
| Year of study enrolment | 2005 | 1997-2008 |
Denotes behaviours in the previous six months from the time of interview.
Table 2.
Distribution of participants and responses of the eight types of substances at each time point among 1107 people who use illicit drugs in Vancouver, British Columbia, Canada.
| Frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time point | Total | Heroin | Illicit prescription opioid | Cocaine | Crack cocaine | Crystal methamphetamine | Cannabis | Daily alcohol | Benzodiazepines |
| Years before OAT | |||||||||
| −5.0 | 218 | 218 | 218 | 218 | 218 | 218 | 218 | 218 | 218 |
| −4.5 | 247 | 246 | 246 | 246 | 247 | 246 | 247 | 247 | 247 |
| −4.0 | 284 | 284 | 284 | 284 | 284 | 284 | 284 | 282 | 284 |
| −3.5 | 342 | 341 | 341 | 341 | 341 | 341 | 341 | 341 | 342 |
| −3.0 | 388 | 388 | 388 | 388 | 388 | 388 | 388 | 388 | 388 |
| −2.5 | 454 | 454 | 454 | 453 | 454 | 454 | 453 | 454 | 454 |
| −2.0 | 529 | 529 | 529 | 529 | 529 | 529 | 529 | 528 | 529 |
| −1.5 | 653 | 653 | 650 | 652 | 652 | 650 | 653 | 651 | 653 |
| −1.0 | 814 | 811 | 810 | 811 | 813 | 810 | 813 | 812 | 814 |
| −0.5 | 495 | 495 | 494 | 495 | 495 | 495 | 495 | 493 | 495 |
| Years after OAT | |||||||||
| 0.0 | 1107 | 1105 | 1106 | 1106 | 1107 | 1106 | 1106 | 1105 | 1107 |
| 0.5 | 488 | 488 | 487 | 488 | 488 | 487 | 488 | 485 | 488 |
| 1.0 | 903 | 900 | 899 | 899 | 902 | 899 | 903 | 898 | 903 |
| 1.5 | 839 | 836 | 836 | 836 | 839 | 836 | 839 | 837 | 839 |
| 2.0 | 791 | 788 | 788 | 788 | 791 | 789 | 791 | 788 | 791 |
| 2.5 | 750 | 750 | 750 | 750 | 750 | 750 | 750 | 746 | 750 |
| 3.0 | 706 | 704 | 704 | 703 | 706 | 704 | 706 | 701 | 706 |
| 3.5 | 659 | 659 | 659 | 659 | 659 | 659 | 659 | 654 | 659 |
| 4.0 | 624 | 622 | 622 | 621 | 624 | 621 | 623 | 624 | 624 |
| 4.5 | 609 | 608 | 608 | 608 | 609 | 608 | 609 | 606 | 609 |
| 5.0 | 546 | 542 | 543 | 543 | 545 | 543 | 545 | 545 | 546 |
OAT opioid agonist therapy.
There were in total 303 participants dropped out of the study after OAT enrolment. 63 participants dropped out after engaging in OAT for one year, 87 participants dropped out after the second year, 73 participants dropped out after the third year, and 80 participants dropped out after the fourth year. Compared to the 804 participants who remained in the study, the 303 participants were younger at OAT enrolment (median age 32.4 vs. 36.6 years; Wilcoxon rank-sum test P < 0.001), but there was no significant difference regarding sex (chi-squared test P = 0.231), ethnicity (chi-squared test P = 0.236), heroin use (chi-squared test P = 0.237) and illicit prescription opioid use (chi-squared test P = 0.459).
3.2. Heroin and illicit prescription opioid use
Results of segmented regression on proportion of substance use over time are summarized in Table 3, and results of bivariable and multivariable segmented regression on participant-level data are presented in Table 4 and Table 5. As shown in Figure 1, the estimated proportion of heroin use started at 64.8% (95% confidence interval (CI): 61.3%, 68.1%) and increased to 84.2% (95% CI: 82.5%, 85.8%) six-month before OAT initiation. After OAT engagement, we observed a significant drop in the proportion (70.6%, 95% CI: 68.9%, 72.3%). In the pre-treatment segment, with one year increase, there was about 19.0% increase in the odds of heroin use (adjusted odds ratio (AOR): 1.19, 95% CI: 1.12, 1.27). However, in the post-treatment segment, we observed a strong decreasing trend over time (AOR: 0.80, 95% CI: 0.77, 0.83).
Table 3.
Segmented regression analysis of the impact of opioid agonist therapy on different types of substance use among 1107 people who use illicit drugs in Vancouver, British Columbia, Canada.
| Estimated prevalence, % (95% confidence interval) | ||||
|---|---|---|---|---|
| Substance type | Baseline a | Before OAT initiation | After OAT initiation | End of study b |
| Heroin | 64.8 (61.3, 68.1) | 84.2 (82.5, 85.8) | 70.6 (68.9, 72.3) | 43.4 (41.2, 45.6) |
| Illicit prescription opioid | 29.6 (26.7, 32.8) | 39.7 (37.3, 42.1) | 28.7 (26.9, 30.5) | 18.9 (17.3, 20.6) |
| Cocaine | 61.2 (57.8, 64.4) | 55.3 (52.8, 57.7) | 49.4 (47.4, 51.3) | 30.1 (28.2, 32.1) |
| Crack cocaine | 51.9 (48.5, 55.2) | 47.4 (45.0, 49.9) | 48.9 (46.9, 50.8) | 54.8 (52.6, 56.9) |
| Crystal methamphetamine | 13.6 (11.5, 16.0) | 20.0 (18.1, 22.1) | 19.0 (17.5, 20.6) | 14.8 (13.4, 16.4) |
| Cannabis | 57.0 (53.6, 60.3) | 52.1 (49.7, 54.6) | 51.3 (49.4, 53.2) | 45.7 (43.5, 47.9) |
| Daily alcohol | 15.1 (12.7, 17.8) | 9.6 (8.3, 11.1) | 8.1 (7.2, 9.3) | 9.3 (8.1, 10.7) |
| Benzodiazepines | 15.8 (13.4, 18.5) | 13.2 (11.7, 14.9) | 8.4 (7.3, 9.6) | 2.4 (1.9, 3.0) |
OAT opioid agonist therapy.
The first observation upon joining cohort.
At five years post opioid agonist therapy enrolment.
Table 4.
Bivariable segmented regression analysis accessing different substance use time trend before and after opioid agonist therapy initiation among 1107 people who use illicit drugs in Vancouver, British Columbia, Canada.
| Substance type | Pre-treatment trend, per year increase | Post-treatment trend, per year increase | Compare trends | ||
|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P | Odds Ratio (95% CI) | P | P | |
| Heroin | 1.17 (1.10, 1.24) | <0.001 | 0.80 (0.77, 0.82) | <0.001 | <0.001 |
| Illicit prescription opioid | 1.05 (1.00, 1.10) | 0.036 | 0.89 (0.85, 0.92) | <0.001 | <0.001 |
| Cocaine | 0.87 (0.83, 0.92) | <0.001 | 0.83 (0.80, 0.85) | <0.001 | 0.107 |
| Crack cocaine | 1.00 (0.94, 1.06) | 0.980 | 1.02 (0.98, 1.05) | 0.311 | 0.637 |
| Crystal methamphetamine | 1.11 (1.05, 1.18) | <0.001 | 1.02 (0.99, 1.06) | 0.132 | 0.018 |
| Cannabis | 0.95 (0.92, 0.99) | 0.017 | 0.97 (0.94, 0.99) | 0.013 | 0.520 |
| Daily alcohol | 0.91 (0.85, 0.99) | 0.020 | 1.04 (0.98, 1.10) | 0.185 | 0.011 |
| Benzodiazepines | 0.85 (0.80, 0.90) | <0.001 | 0.74 (0.68, 0.80) | <0.001 | 0.008 |
CI confidence interval.
Table 5.
Multivariable segmented regression analysis accessing different substance use time trend before and after opioid agonist therapy initiation among 1107 people who use illicit drugs in Vancouver, British Columbia, Canada.
| Substance type | Pre-treatment trend, per year increase | Post-treatment trend, per year increase | Compare trends | ||
|---|---|---|---|---|---|
| Adjusted Odds Ratio (95% CI) a | P | Adjusted Odds Ratio (95% CI) b | P | P | |
| Heroin | 1.19 (1.12, 1.27) | <0.001 | 0.80 (0.77, 0.83) | <0.001 | <0.001 |
| Illicit prescription opioid | 1.04 (0.99, 1.09) | 0.149 | 0.87 (0.83, 0.90) | <0.001 | <0.001 |
| Cocaine | 0.85 (0.81, 0.90) | <0.001 | 0.83 (0.80, 0.85) | <0.001 | 0.297 |
| Crack cocaine | 0.96 (0.91, 1.03) | 0.246 | 1.01 (0.98, 1.05) | 0.546 | 0.207 |
| Crystal methamphetamine | 1.17 (1.08, 1.27) | <0.001 | 1.13 (1.08, 1.19) | <0.001 | 0.478 |
| Cannabis | 0.97 (0.93, 1.01) | 0.142 | 1.00 (0.97, 1.03) | 0.933 | 0.200 |
| Daily alcohol | 0.91 (0.84, 0.98) | 0.016 | 1.03 (0.97, 1.09) | 0.311 | 0.016 |
| Benzodiazepines | 0.84 (0.78, 0.90) | <0.001 | 0.73 (0.67, 0.80) | <0.001 | 0.026 |
CI confidence interval.
Multivariable model adjusted for age, sex, ethnicity, unstable housing, employment status, incarceration, sex work involvement, drug dealing, and any other addiction treatment or services except for opioid agonist therapy, and year of study enrolment.
Multivariable model adjusted for age, sex, ethnicity, unstable housing, employment status, incarceration, sex work involvement, drug dealing, any other addiction treatment or services except for opioid agonist therapy, year of study enrolment, and adherence to opioid agonist therapy.
Figure 1.
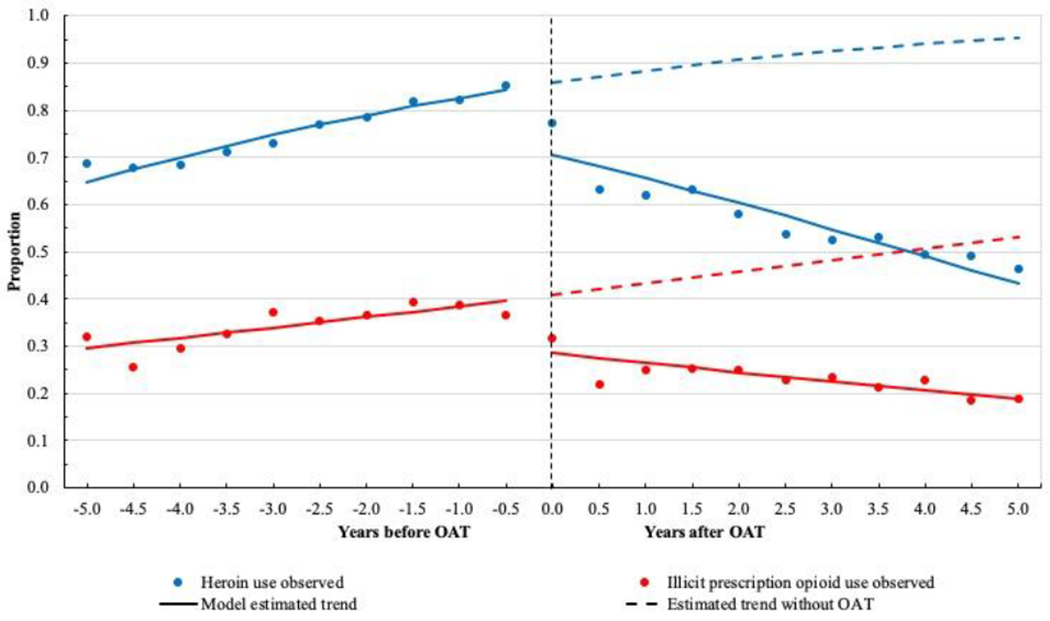
Proportion of heroin and illicit prescription opioid use before and after opioid agonist therapy initiation among 1107 people who use illicit drugs in Vancouver, British Columbia, Canada.
Similar to heroin use, the proportion of illicit prescription opioid use also indicated an upward trend before OAT (Figure 1). There was an immediate reduction in illicit prescription opioid use after initiating OAT, resulting in an estimated proportion of 18.9% (95% CI: 17.3%, 20.6%) five years after initiating OAT, which was significantly lower than the expected proportion of 53.1% if these participants did not initiate OAT. In the post-treatment segment, there was a significant declining trend for illicit prescription opioid use (AOR: 0.87, 95% CI: 0.83, 0.90).
Results for the exploratory secondary analysis are presented in the Supplementary Table S1. There was an increasing trend for overall injection opioid use in the pre-treatment segment (AOR: 1.17, 95% CI: 1.10, 1.24). Similarly, a significant decreasing trend was observed in the post-treatment segment (AOR: 0.80, 95% CI: 0.77, 0.83). For non-injection opioid use, there was no apparent trend in the pre-treatment segment, however, the use decreased over time as well in the post-treatment segment (AOR: 0.89, 95% CI: 0.85, 0.92).
3.3. Stimulant use
During the study period, an overall decreasing trend of cocaine use was noted (Figure 2). The estimated proportion of cocaine use was 55.3% (95% CI: 52.8%, 57.7%) before engaging in OAT, and dropped immediately to 49.4% (95% CI: 47.4%, 51.3%) after OAT engagement. In adjusted model, a downward trend of cocaine use was observed during both the pre-treatment segment (AOR: 0.85, 95% CI: 0.81, 0.90) and the post-treatment segment (AOR: 0.83, 95% CI: 0.80, 0.85), however, the difference was not significant (P = 0.297).
Figure 2.
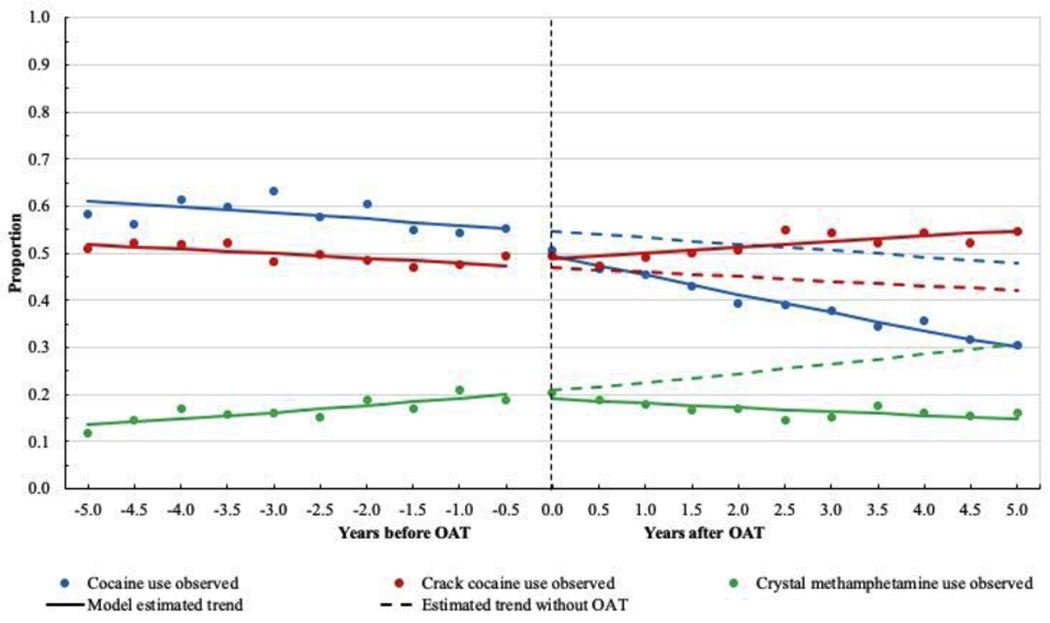
Proportion of cocaine, crack cocaine, and crystal methamphetamine use before and after opioid agonist therapy initiation among 1107 people who use illicit drugs in Vancouver, British Columbia, Canada.
The proportion of crack cocaine use was constantly high across the study period (Figure 2). We did not observe a significant change of the proportion after OAT initiation (47.4% vs. 48.9%). In addition, there was no apparent upward or downward trend in the pre-treatment segment (AOR: 0.96, 95% CI: 0.91, 1.03) and the post-treatment segment (AOR: 1.01, 95% CI: 0.98, 1.05).
For crystal methamphetamine use, the estimated proportion started at 13.6% and reached 20.0% before OAT initiation (Figure 2). Time was positively associated with odds of methamphetamine use during the pre-treatment segment (AOR: 1.17, 95% CI: 1.08, 1.27). We did not observe an apparent change of the proportion right after OAT initiation (20.0% vs. 19.0%). Even though in the post-treatment segment, time was associated with increased odds of methamphetamine use (AOR: 1.13, 95% CI: 1.08, 1.19), it was not significantly different compared to the trend in pretreatment segment (P = 0.478).
Looking at overall injection stimulant use, we observed decreasing trend in both pre-treatment segment (AOR: 0.92, 95% CI: 0.87, 0.97) and post-treatment segment (AOR: 0.86, 95% CI: 0.83, 0.89). However, the decreasing trend of non-injection stimulant use significantly weakened in the post-treatment segment (AOR: 0.99, 95% CI: 0.96, 1.02) compared to the pre-treatment segment (AOR: 0.92, 95% CI: 0.87, 0.97; P = 0.030).
3.4. Cannabis, daily alcohol, and benzodiazepines use
Cannabis use was relatively common among these participants, with the estimated proportion remaining above 50.0% prior to OAT initiation (Figure 3). There was no apparent upward or downward trend in the pre-treatment segment (AOR: 0.97, 95% CI: 0.93, 1.01) and the posttreatment segment (AOR: 1.00, 95% CI: 0.97, 1.03).
Figure 3.
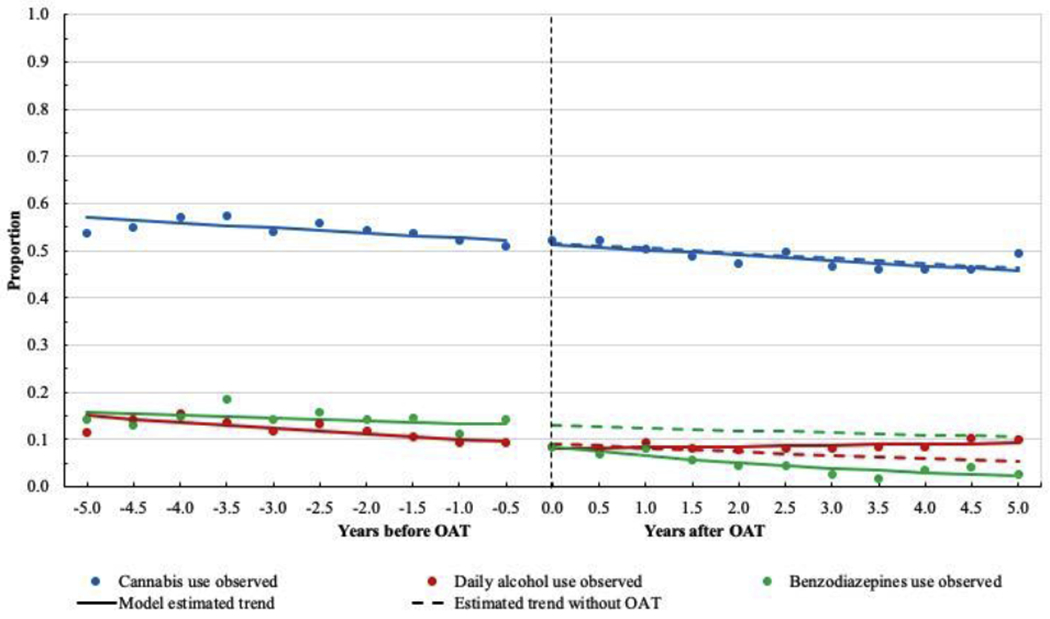
Proportion of cannabis, daily alcohol, and benzodiazepines use before and after opioid agonist therapy initiation among 1107 people who use illicit drugs in Vancouver, British Columbia, Canada.
For daily alcohol use, there was a slightly decreasing trend during the pre-treatment segment (Figure 3). The estimated proportion started at 15.1% (95% CI: 12.7%, 17.8%) and decreased to 9.6% (95% CI: 8.3%, 11.1%) before OAT initiation. However, this decreasing trend weakened during post-treatment segment (AOR: 1.03, 95% CI: 0.97, 1.09), which represented a significantly higher growth slope than that of the pre-treatment segment (P = 0.016).
As shown in Figure 3, the estimated proportion of benzodiazepines use before OAT initiation was 13.2% (95% CI: 11.7%, 14.9%), and we observed a significant drop in the proportion after OAT initiation (8.4%, 95% CI: 7.3%, 9.6%). There was a decreasing trend in the pre-treatment segment (AOR: 0.84, 95% CI: 0.78, 0.90), and this decreasing trend became significantly stronger during post-treatment segment (AOR: 0.73, 95% CI: 0.67, 0.80).
3.5. Sensitivity analyses
After excluding the 303 dropouts, the first sensitivity analysis result is presented in Supplementary Table S2. We still observed a strong decreasing trend over time in the posttreatment segment for heroin (AOR: 0.80, 95% CI: 0.77, 0.83) and illicit prescription opioid use (AOR: 0.87, 95% CI: 0.83, 0.91), which was consistent with the primary analysis result. Similarly, there was an increased odds of daily alcohol use during the post-treatment segment (AOR: 1.04, 95% CI: 0.98, 1.11), resulting in a significantly higher growth slope during the posttreatment segment (P = 0.037). There was still a slightly stronger decreasing trend for benzodiazepines use during the post-treatment segment, however, compared to the pre-treatment segment, it was not statistically significant (P = 0.135).
In the second sensitivity analysis, we identified 84 deaths among the 303 participants who dropped out of the study. Therefore, for the 219 participants with unknown reasons, we imputed their missing follow-up visits with the last observation carried forward. Among the 84 deaths, 24 were due to drug overdose, whose missing substance use information was imputed by assuming these individuals were actively using. The result for the second sensitivity analysis is presented in Supplementary Table S3. Similar to the primary analysis result, we observed apparent decreasing trend for heroin (AOR: 0.83, 95% CI: 0.80, 0.86) and illicit prescription opioid use (AOR: 0.93, 95% CI: 0.89, 0.96), but higher growth slope for daily alcohol use (P < 0.001) during the post-treatment segment.
4. Discussion
This study examined patterns of use of eight types of substances before and after engaging OAT. Compared with the pre-treatment segment, significant declines in use after engaging in OAT were noted for heroin and illicit prescription opioid use. For cocaine and crystal methamphetamine use, there was an existing trend prior to OAT initiation, and this trend continued during the post-treatment segment. We did not observe a change in stimulant and cannabis use when comparing the time trend before and after engaging in OAT. There was a stronger decreasing trend during the post-treatment segment for benzodiazepines use. By contrast, an increasing trend was observed for daily alcohol use.
Our results demonstrated a significant reduction in the proportion of heroin use and illicit opioid use immediately after OAT engagement. Moreover, the upward trend during pre-treatment segment was reversed after initiating OAT. This observed trend remained so when examining overall injection and non-injection opioid use. These findings reinforce the benefit of OAT on reducing opioid use. However, by the end of five years after initiating OAT, the prevalence remained around 43.4% for heroin use and 18.9% for illicit opioid use among these participants. This observation is in line with previous research demonstrating the chronic relapsing nature of opioid use disorder (Hser et al., 2015). Therefore, adopting long-term care strategies for the treatment of opioid use disorder are needed (Taha and Broker, 2018).
Over half of the participants concomitantly used opioid and cocaine at the time of OAT initiation. Even though OAT is not specifically intended for treating cocaine use disorder, there was a significant immediate reduction in the proportion of cocaine use after initiating OAT. We also observed a decline in cocaine use over time after engaging in OAT, which is consistent with previous findings using a longitudinal design (Fairbank et al., 1993; Darke et al., 2006; Bravo et al., 2010; Herdener et al., 2017). Additionally, when we compared it with the declining trend in the pre-treatment segment, we failed to find a significant difference. This observation indicates that the observed decline in cocaine use could be a continuation of the pre-treatment trend. The proportion of crack cocaine use remained consistently high throughout the study period. In an adjusted segmented regression analysis, the odds of crack cocaine use in the post-treatment period was not significantly different from that of the pre-treatment period. However, when looking at overall non-injection stimulant use, the decreasing trend in the pre-treatment period weakened in the post-treatment segment. Considering the high rates of concomitant use of cocaine as well as crack cocaine among these opioid-dependent individuals, it is critical for clinicians and caregivers to be aware of the poly-substance use patterns in order to provide the most appropriate and effective treatment. In a meta-analysis including 3029 participants who used both heroin and cocaine, it has been found that adjunctive interventions, including indirect dopaminergic agonists and contingency management focusing on cocaine abstinence, could be used in combination with OAT to improve rates of sustained cocaine abstinence (Castells et al., 2009). A recent review also found that among methadone-maintained patients, psychostimulants could improve cocaine abstinence compared to placebo (Castells et al., 2016).
We did not observe a significant change in the proportion of cannabis use before and after engaging in OAT. While outside the scope of this paper, it is interesting to note the growing body of research supporting cannabis-based interventions in addressing the opioid crisis (Lucas, 2017; Vyas et al., 2018). Particularly, a small number of studies have suggested that cannabis may be an efficacious tool to decrease opioid withdrawal symptoms, reduce craving, as well as increase opioid treatment retention rate (Scavone et al., 2013; Hurd et al., 2015; Wiese and Wilson-Poe, 2018). However, cannabis use disorder has received increasing attention, with treatment options such as motivational enhancement therapy, cognitive behavioral therapy, and contingency management showing modest benefits (Sherman and McRae-Clark, 2016).
Concomitant use of benzodiazepines has shown to increase the risk of overdose significantly among people with opioid use disorder (Kandel et al., 2017). Individuals who are on OAT could potentially increase the use of benzodiazepines as a coping strategy to treat their elevated anxiety and sleep disorder after stopping the use of opiates. However, in our analysis, we observed a decline in benzodiazepine use over time before initiating OAT, and this decreasing trend continued and became stronger after engaging in OAT.
Compared with the pre-treatment segment, daily alcohol use significantly increased during the post-treatment segment, which is consistent with findings from previous studies (Gossop et al., 2003; Soyka, 2015; Herdener et al., 2017). This presents additional clinical challenges to the treatment of opioid use disorder, given that alcohol use has been found to be associated with increased risk of overdose and mortality (Gossop et al., 2002; Coffin et al., 2003), relapse into illicit drug use (Stenbacka et al., 2007), and other health problems (Degenhardt and Hall, 2003; Senbanjo et al., 2007) among people who receive treatment for opioid use disorder. Unfortunately, clinical interventions or treatment strategies to reduce alcohol consumption for people who are on OAT have not been fully investigated (Soyka, 2015). Our finding suggests the need for early detection and ongoing monitoring, as well as interventions for unhealthy alcohol use during the opioid treatment process. However, for patients diagnosed with co-occurring alcohol use disorder and opioid use disorder, established treatment strategies for alcohol use disorder, including acamprosate and extended-release naltrexone, should be explored (British Columbia Centre on Substance Use, 2019).
The strengths of this study include a large number of community-recruited participants over an extended period of time (i.e., over 10 years). Furthermore, with a time series of outcome measurements before and after OAT initiation, the impact of OAT on different types of substance use was evaluated after controlling for the underlying secular trend. This approach could reduce bias that might be present in a simple two time points before-and-after design or analysis using only baseline and measurements after treatment (Torgerson and Torgerson, 2008). Some limitations in the current study are also acknowledged: first, because of using self-reported data, there could be recall and social-desirability bias, especially for socially stigmatized and criminalized behaviours (e.g., illicit substance use). However, self-reported data have been widely used in the field of substance use and found to be valid (Darke, 1998; Langendam et al., 1999). Besides, we observed that the time trends and changes in trends differed by substance type, which suggested that these results could not be simply explained by recall and social-desirability bias. Second, this study utilized a community-based sample recruited through snowball sampling, self-referral, and street outreach approach. The participants were people who use drugs in Vancouver, Canada from 1996 to 2018, where the evolving social-structural conditions, such as harm reduction strategies and drug availability, could be different from other study settings. Besides, the included participants were mostly intravenous drug users, and the median age at OAT initiation was 35.5 years, which may not be representative of individuals who receive OAT in other settings. Therefore, all these factors could limit the generalizability of the findings. Third, research has indicated that the use of other substances during treatment may relate to inadequate doses of OAT (Heikman et al., 2017). However, due to data limitations, we were not able to evaluate whether the observed trends were consistent among different OAT dosage levels. Besides, we treated each participant as adhering to OAT if the participant reported having methadone/methadose or buprenorphine/naloxone in the last 6 months. However, if the information of OAT dosage is available, a better metric to reflect OAT adherence could be the number of days that participants missed their dose. Fourth, our study instrument did not allow for diagnosis and severity of alcohol use disorder. Although we included daily alcohol use as a proxy for unhealthy alcohol use, tailored interventions are needed for people with alcohol use disorder or at risk of transitioning from daily alcohol use to alcohol use disorder while on OAT. Fifth, the dichotomous variables of drug use (yes vs. no) were included for the primary analysis, therefore, the observed changes in drug use trends could not reflect the variability in quantity and frequency of drug use. Similarly, for the secondary analysis, in the absence of more precise frequency information, we could not adequately characterize the proportion of injection and noninjection drug use events among participants who reported using both modes of consumption. In addition, due to the nature of observational study design as well as the analytical approaches used in the study, the relationships between OAT engagement and patterns of substance use do not imply causality. More specifically, in the absence of an external control group (i.e., ideally people who were identical to the study sample, followed over the same time period, but who were not on opioid agonist treatment), we cannot exclude the possibility that the observed changes were the result of other interventions that may have influenced drug use patterns and occurred simultaneously with engaging in OAT. However, we are unaware of such events. There could also be potential bias due to adjustment for time-dependent confounding when the time-varying covariates were also impacted by OAT engagement (Keogh et al., 2017). Finally, there could be bias due to loss to follow-up. It is expected that participants who were lost to follow-up might have poorer treatment outcomes, therefore the prevalence of heroin use and illicit opioid use during post-treatment period could be underestimated. To reduce the bias associated with comparisons between pre-treatment and post-treatment periods, the sample was restricted to participants who completed at least one study visit before starting OAT and one study visit after OAT. Furthermore, in sensitivity analysis after excluding the dropouts, the results were consistent with the primary findings.
4.1. Conclusions
In summary, our study investigated substance use patterns before and after OAT engagement over a 10-year period and revealed a marked reduction in use of heroin and illicit opioid, which further supports the well-described efficacy of OAT. Findings from this study also underscore the challenges of addressing poly-substance use among people enrolled in OAT, especially given the high prevalent concomitant use of cocaine and crack cocaine, as well as the increasing problematic use of alcohol after treatment initiation. Therefore, there is an urge to develop comprehensive and tailored treatment strategies in order to enhance the safety and effectiveness of OAT for poly-substance users.
Supplementary Material
Highlights.
Use of illicit opioids is significantly reduced with opioid agonist therapy
Concomitant use of cocaine and crack cocaine is highly prevalent
Problematic use of alcohol increases after initiating opioid agonist therapy
Tailored strategies are needed for poly-substance users accessing opioid treatment
Acknowledgements
The authors thank the study participants for their contribution to the research, as well as current and past researchers and staff.
Declarations of interest:
Dr. M-J Milloy is supported in part by the United States National Institutes of Health [U01-DA021525], a New Investigator Award from the CIHR, and a Scholar Award from the MSFHR. The University of British Columbia has received an arms’ length gift from NG Biomed, Ltd., a private firm seeking a license to produce cannabis, to support Dr. M-J Milloy. He is the Canopy Growth professor of cannabis science, a position at the University of British Columbia created by arms’ length gifts from Canopy Growth, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions.
Role of Funding Source
This work was supported by the US National Institutes on Drug Abuse at the US National Institutes of Health [U01-DA038886, U01-DA021525]. Huiru Dong is supported through a Canadian Institutes of Health Research (CIHR) Doctoral Award. Dr. Thomas Kerr is supported by a foundation grant from the CIHR [20R74326], Dr. Evan Wood receives support through a Tier 1 Canada Research Chair in Addiction Medicine. Dr. Kanna Hayashi is supported by a CIHR New Investigator Award [MSH-141971], a Michael Smith Foundation for Health Research (MSFHR) Scholar Award, and the St. Paul’s Foundation. Dr. Kora DeBeck is supported by a MSFHR/ St. Paul’s Hospital Foundation-Providence Health Care Career Scholar Award and a CIHR New Investigator Award. Dr. M-J Milloy is supported in part by the United States National Institutes of Health [U01-DA021525], a New Investigator Award from the CIHR, and a Scholar Award from the MSFHR. The University of British Columbia has received an arms’ length gift from NG Biomed, Ltd., a private firm seeking a license to produce cannabis, to support Dr. M-J Milloy. He is the Canopy Growth professor of cannabis science, a position at the University of British Columbia created by arms’ length gifts from Canopy Growth, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions. Funding sources had no role in the design of this study; collection, analysis, and interpretation of the data; writing of the report; or the decision to submit the paper for publication.
Conflict of Interest
The University of British Columbia has received an unstructured gift from NG Biomed Ltd., a private firm seeking a license to produce medical cannabis, to support Dr. M-J Milloy. He is the Canopy Growth professor of cannabis science at the University of British Columbia, a position created using unstructured gifts to the university from Canopy Growth, a licensed producer of cannabis, and the Government of British Columbia’s Ministry of Mental Health and Addictions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi.
References
- Bravo MJ, Llorens N, Barrio G, Brugal MT, Santos S, Sordo L, De la Fuente L, Group IP, 2010. Methadone maintenance treatment: a protective factor for cocaine injection in a street-recruited cohort of heroin users. Drug Alcohol Depend. 112, 62–68. [DOI] [PubMed] [Google Scholar]
- British Columbia Centre on Substance Use, 2019. Provincial guideline for the clinical management of high-risk drinking and alcohol use disorder. Accessed on January 28, 2020 https://www.bccsu.ca/wp-content/uploads/2019/12/AUD-Guideline.pdf.
- Castells X, Cunill R, Perez-Mana C, Vidal X, Capella D, 2016. Psychostimulant drugs for cocaine dependence. Cochrane Database Syst. Rev 9, CD007380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells X, Kosten TR, Capella D, Vidal X, Colom J, Casas M, 2009. Efficacy of opiate maintenance therapy and adjunctive interventions for opioid dependence with comorbid cocaine use disorders: A systematic review and meta-analysis of controlled clinical trials. Am. J. Drug Alcohol Abuse 35, 339–349. [DOI] [PubMed] [Google Scholar]
- Cheng T, Small W, Dong H, Nosova E, Hayashi K, DeBeck K, 2018. An age-based analysis of nonmedical prescription opioid use among people who use illegal drugs in Vancouver, Canada. Subst. Abuse Treat. Prev. Policy 13, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PO, Galea S, Ahern J, Leon AC, Vlahov D, Tardiff K, 2003. Opiates, cocaine and alcohol combinations in accidental drug overdose deaths in New York City, 1990-98. Addiction 98, 739–747. [DOI] [PubMed] [Google Scholar]
- Darke S, 1998. Self-report among injecting drug users: a review. Drug Alcohol Depend. 51, 253–263. [DOI] [PubMed] [Google Scholar]
- Darke S, Williamson A, Ross J, Teesson M, 2006. Reductions in Heroin use are Not Associated with Increases in Other Drug use: 2-Year Findings from the Australian Treatment Outcome Study. Drug Alcohol Depend. 84, 201–205. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, 2003. Patterns of co-morbidity between alcohol use and other substance use in the Australian population. Drug Alcohol Rev. 22, 7–13. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J, 2011Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction 106, 32–51. [DOI] [PubMed] [Google Scholar]
- Dunteman GH, Condelli WS, Fairbank JA, 1992. Predicting cocaine use among methadone patients: analysis of findings from a national study. Hosp. Community Psychiatry 43, 608–611. [DOI] [PubMed] [Google Scholar]
- Durbin J, Watson GS, 1950. Testing for serial correlation in least squares regression. I. Biometrika 37, 409–428. [PubMed] [Google Scholar]
- Eastwood B, Strang J, Marsden J, 2019. Change in alcohol and other drug use during five years of continuous opioid substitution treatment. Drug Alcohol Depend. 194, 438–446. [DOI] [PubMed] [Google Scholar]
- Fairbank JA, Dunteman GH, Condelli WS, 1993. Do methadone patients substitute other drugs for heroin? Predicting substance use at 1-year follow-up. Am. J. Drug Alcohol Abuse 19, 465–474. [DOI] [PubMed] [Google Scholar]
- Gossop M, Marsden J, Stewart D, 2000. Treatment outcomes of stimulant misusers: one year follow-up results from the national treatment outcome research study (NTORS). Addict. Behav 25, 509–522. [PubMed] [Google Scholar]
- Gossop M, Marsden J, Stewart D, Kidd T, 2003. The National Treatment Outcome Research Study (NTORS): 4-5 year follow-up results. Addiction 98, 291–303. [DOI] [PubMed] [Google Scholar]
- Gossop M, Stewart D, Treacy S, Marsden J, 2002. A prospective study of mortality among drug misusers during a 4-year period after seeking treatment. Addiction 97, 39–47. [DOI] [PubMed] [Google Scholar]
- Grella CE, Lovinger K, 2011. 30-year trajectories of heroin and other drug use among men and women sampled from methadone treatment in California. Drug Alcohol Depend. 118, 251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikman PK, Muhonen LH, Ojanpera IA, 2017. Polydrug abuse among opioid maintenance treatment patients is related to inadequate dose of maintenance treatment medicine. BMC Psychiatry 17, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdener M, Dursteler KM, Seifritz E, Nordt C, 2017. Changes in substance use in patients receiving opioid substitution therapy and resulting clinical challenges: a 17-year treatment case register analysis. Lancet Psychiatry 4, 302–309. [DOI] [PubMed] [Google Scholar]
- Hser Y, Evans E, Grella C, Ling W, 2015. Long-Term Course of Opioid Addiction. Harv. Rev. Psychiatry 23, 76–89. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Yoon M, Manini AF, Hernandez S, Olmedo R, Ostman M, Jutras-Aswad D, 2015. Early Phase in the Development of Cannabidiol as a Treatment for Addiction: Opioid Relapse Takes Initial Center Stage. Neurotherapeutics 12, 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel DB, Hu MC, Griesler P, Wall M, 2017. Increasing from 2002 to 2015 in prescription opioid overdose deaths in combination with other substances. Drug Alcohol Depend. 178, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh RH, Daniel RM, VanderWeele TJ, Vansteelandt S, 2017. Analysis of longitudinal studies with repeated outcome measures: adjusting for time-dependent confounding using conventional methods. Am. J. Epidemiol 187, 1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidorf M, Stitzer ML, 1993. Descriptive analysis of cocaine use of methadone patients. Drug Alcohol Depend. 32, 267–275. [DOI] [PubMed] [Google Scholar]
- Krebs E, Kerr T, Montaner J, Wood E, Nosyk B, 2014. Dynamics in the costs of criminality among opioid dependent individuals. Drug Alcohol Depend. 144, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs E, Kerr T, Wood E, Nosyk B, 2016. Characterizing long-term health related quality of life trajectories of individuals with opioid use disorder. J. Subst. Abuse Treat 67, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langendam MW, van Haastrecht HJ, van Ameijden EJ, 1999. The validity of drug users’ selfreports in a non-treatment setting: prevalence and predictors of incorrect reporting methadone treatment modalities. Int. J. Epidemiol 28, 514–520. [DOI] [PubMed] [Google Scholar]
- Lorvick J, Browne EN, Lambdin BH, Comfort M, 2018. Polydrug use patterns, risk behavior and unmet healthcare need in a community-based sample of women who use cocaine, heroin or methamphetamine. Addict. Behav 85, 94–99. [DOI] [PubMed] [Google Scholar]
- Lucas P, 2017. Rationale for cannabis-based interventions in the opioid overdose crisis. Harm Reduct. J 14, 58–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, Degenhardt L, Hickman M, 2012. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 345, e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith SE, Rash CJ, Petry NM, 2017. Alcohol use disorders are associated with increased HIV risk behaviors in cocaine-dependent methadone patients. J. Subst. Abuse Treat 83, 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan S, Hayashi K, Milloy M, Kerr T, Dong H, Lima VD, Lappalainen L, Montaner J, Wood E, 2015. The impact of low-threshold methadone maintenance treatment on mortality in a Canadian setting. Drug Alcohol Depend. 156, 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt L, Minozzi S, Reed J, Vickerman P, 2018. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction 113, 545–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russolillo A, Moniruzzaman A, McCandless LC, Patterson M, Somers JM, 2018. Associations between methadone maintenance treatment and crime: a 17-year longitudinal cohort study of Canadian provincial offenders. Addiction 113, 656–667. [DOI] [PubMed] [Google Scholar]
- Saunders KW, Von Korff M, Campbell CI, Banta-Green CJ, Sullivan MD, Merrill JO, Weisner C, 2012. Concurrent use of alcohol and sedatives among persons prescribed chronic opioid therapy: prevalence and risk factors. J. Pain 13, 266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scavone JL, Sterling RC, Van Bockstaele EJ, 2013. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience 248, 637–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, 2016. Treatment of Opioid-Use Disorders. N. Engl. J. Med 375, 357–368. [DOI] [PubMed] [Google Scholar]
- Senbanjo R, Wolff K, Marshall J, 2007. Excessive alcohol consumption is associated with reduced quality of life among methadone patients. Addiction 102, 257–263. [DOI] [PubMed] [Google Scholar]
- Sherman BJ, McRae-Clark AL, 2016. Treatment of cannabis use disorder: current science and future outlook. Pharmacotherapy 36, 511–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyka M, 2015. Alcohol use disorders in opioid maintenance therapy: prevalence, clinical correlates and treatment. Eur. Addict. Res 21, 78–87. [DOI] [PubMed] [Google Scholar]
- Stenbacka M, Beck O, Leifman A, Romelsjo A, Helander A, 2007. Problem drinking in relation to treatment outcome among opiate addicts in methadone maintenance treatment. Drug Alcohol Rev. 26, 55–63. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Palepu A, Cornelisse PG, Yip B, O’Shaughnessy MV, Montaner JS,Schechter MT, Hogg RS, 1998. Barriers to use of free antiretroviral therapy in injection drug users. JAMA 280, 547–549. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Botsko M, Cunningham CO, O’Connor PG, Hersh D, Mitty J, Lum PJ,Schottenfeld RS, Fiellin DA, BHIVES Collaborative, 2011. The impact of cocaine use on outcomes in HIV-infected patients receiving buprenorphine/naloxone. J. Acquir. Immune Defic. Syndr 56 Suppl 1, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha S, Broker K, 2018. Best Practices Across the Continuum of Care for the Treatment of Opioid use Disorder. Ottawa, Ont.: Canadian Centre on Substance Use and Addiction. [Google Scholar]
- Tavitian-Exley I, Boily MC, Heimer R, Uuskula A, Levina O, Maheu-Giroux M, 2018. Polydrug Use and Heterogeneity in HIV Risk Among People Who Inject Drugs in Estonia and Russia: A Latent Class Analysis. AIDS Behav. 22, 1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson DJ, Torgerson CJ, 2008. The limitations of before and after designs, in: Designing Randomised Trials in Health, Education and the Social Sciences. Palgrave Macmillan, London, pp. 9–16. [Google Scholar]
- Vyas MB, LeBaron VT, Gilson AM, 2018. The use of cannabis in response to the opioid crisis: A review of the literature. Nurs. Outlook 66, 56–65. [DOI] [PubMed] [Google Scholar]
- Wang L, Min JE, Krebs E, Evans E, Huang D, Liu L, Hser Y, Nosyk B, 2017. Polydrug use and its association with drug treatment outcomes among primary heroin, methamphetamine, and cocaine users. Int. J. Drug Policy 49, 32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese B, Wilson-Poe AR, 2018. Emerging Evidence for Cannabis’ Role in Opioid Use Disorder. Cannabis Cannabinoid Res. 3, 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A, Darke S, Ross J, Teesson M, 2006. The association between cocaine use and short-term outcomes for the treatment of heroin dependence: findings from the Australian Treatment Outcome Study (ATOS). Drug Alcohol Rev. 25, 141–148. [DOI] [PubMed] [Google Scholar]
- Wood E, Kerr T, Marshall BD, Li K, Zhang R, Hogg RS, Harrigan PR, Montaner JS, 2009. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ 338, b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


