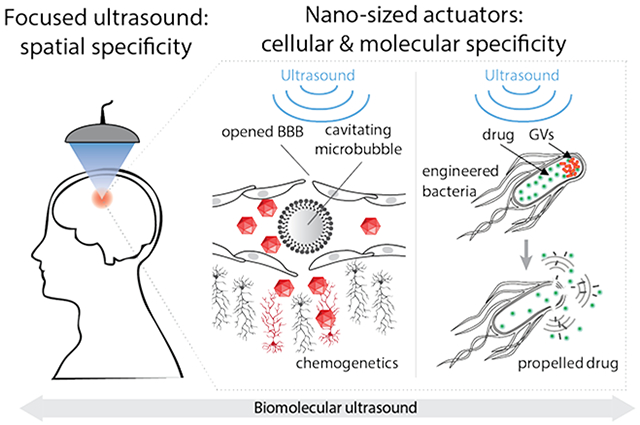
Abstract
The precise targeting of cells in deep tissues is one of the primary goals of nanomedicine. However, targeting a specific cellular population within an entire organism is challenging due to off-target effects and the need for deep tissue delivery. Focused ultrasound can reduce off-targeted effects by spatially restricting the delivery or action of molecular constructs to specific anatomical sites. Ultrasound can also increase the efficiency of nanotherapeutic delivery into deep tissues by enhancing the permeability of tissue boundaries, promoting convection, or depositing energy to actuate cellular activity. In this review we focus on the interface between biomolecular engineering and focused ultrasound, and describe the applications of this intersection in neuroscience, oncology, and synthetic biology. Ultrasound can be used to trigger the transport of therapeutic payloads into a range of tissues, including specific regions of the brain, where it can be targeted with millimeter precision through intact skull. Locally delivered molecular constructs can then control specific cells and molecular pathways within the targeted region. When combined with viral vectors and engineered neural receptors, this technique enables non-invasive control of specific circuits and behaviors. The penetrant energy of ultrasound can also be used to more directly actuate micro- and nanotherapeutic constructs, including microbubbles, vaporizable nanodroplets and polymeric nanocups, which nucleate cavitation upon ultrasound exposure, leading to local mechanical effects. In addition, it was recently discovered that a unique class of acoustic biomolecules – genetically encodable nanoscale protein structures called gas vesicles – can be acoustically “detonated” as sources of inertial cavitation. This enables the targeted disruption of selected cells within the area of insonation by gas vesicles that are engineered to bind cell surface receptors. It also facilitates ultrasound-triggered release of molecular payloads from engineered therapeutic cells heterologously expressing intracellular gas vesicles. Finally, focused ultrasound energy can be used to locally elevate tissue temperature and activate temperature-sensitive proteins and pathways. The elevation of temperature allows non-invasive control of gene expression in vivo in cells engineered to express thermal bioswitches. Overall, the intersection of biomolecular engineering, nanomaterials and focused ultrasound can provide unparalleled specificity in controlling, modulating and treating physiological processes in deep tissues.
Graphical Abstract
Main text
One of the key goals of nanomedicine is to enable more selective treatment of diseased cells without invasive surgery. Attempts to achieve such selectivity often rely on targeting therapeutics to molecular markers over-represented on the target cell population or taking advantage of tissue accumulation mechanisms such as enhanced permeability and retention in tumors. However, the complexity of living organisms makes it difficult to achieve perfect specificity and avoid off-target effects. In particular, molecular targeting is often insufficient to direct systemically administered nanomedicines to desired anatomical locations such as tumors or specific regions of the brain. In this perspective, we discuss how anatomical specificity can be improved by combining nanomedicines with ultrasound – a versatile form of physical energy that can be applied and focused at depth in a variety of tissues with millimeter precision. Ultrasound enables the spatial targeting of therapy through diverse mechanisms that include localized ultrasound-enhanced transport, the activation of local mechanical events such as inertial cavitation, the elevation of temperature at the ultrasound focus, and via direct interactions with mechanosensitive components of tissue 1, 2.
On its own, focused ultrasound is already a clinical tool used to treat diseases raging from prostate cancer to essential tremor, owing to the ability of modern ultrasound instruments to focus high intensity sound waves on millimeter-sized regions of tissue and deliver ablative heat, often under the guidance of magnetic resonance imaging (MRI). In the approaches described in this perspective, the same ultrasound technology is used with pulse parameters that do not on their own result in tissue ablation. The goal of these approaches is to combine nanoscale and genetically encoded materials with focused ultrasound to enable more selective biological perturbation and disease treatment.
Ultrasound enhanced and triggered transport into the central nervous system
The use of ultrasound to enhance or target the delivery of nano-sized therapeutic compounds into tissues relies on its ability to open biological barriers, trigger physical changes in nanoscale drug delivery vehicles, or propel materials via convective transport. These capabilities have been used for site-specific delivery of small molecules, nanoparticles and viral vectors to tissues such as tumors3-5, the gastrointestinal tract6, the eye7, muscle8 and the brain9. Several recent reviews have covered ultrasound-enhanced delivery to these areas of the body10,11. In this review, we focus specifically on delivery to the brain as an example target.
Brain delivery and targeting represent a particularly challenging problem. The brain is composed of anatomically defined regions containing a multitude of different cell types, including neurons, that cannot be easily distinguished by their molecular markers but perform vastly different functions. For example, nearly identical neuron types can control movement, register sensory inputs or perform complex reasoning depending on where they are in the brain. In addition, the entry of most molecules into the central nervous system (CNS) is restricted by a specialized endothelial structure called the blood brain barrier (BBB), making it difficult to deliver nanomaterials to the brain by systemic administration. Even if the BBB can be crossed at a specific anatomical site, additional selectivity is needed at that site to target the correct subset of the multiple cell types present at that location12-14.
These challenges can be addressed by combining nanomaterials with focused ultrasound BBB opening (FUS-BBBO). In this combination, low intensity focused ultrasound interacts with systemically administered, intravascular microbubble contrast agents – micron-scale bubbles of gas typically stabilized by a lipid shell – which are also used for clinical diagnostic ultrasound. When insonated, the microbubbles oscillate in size and exert mechanical forces on the endothelium, resulting in the temporary opening of the tight junctions comprising the BBB. FUS-BBBO allows for the delivery of small molecules9, proteins15, viral vectors16, and nanoparticles17-19 to brain sites defined by the ultrasound focus (Fig. 1A). Larger molecules typically require higher pressures of ultrasound for efficient delivery20. Typically, after several hours the BBB closes21 leaving little to no damage at the site of insonation22. The use of FUS-BBBO is safe even after multiple exposures22,23 and has been successfully used in humans24,25 (Fig. 1B). Pioneering applications of this technology include the treatment of brain cancer24 and neurodegenerative diseases25-27.
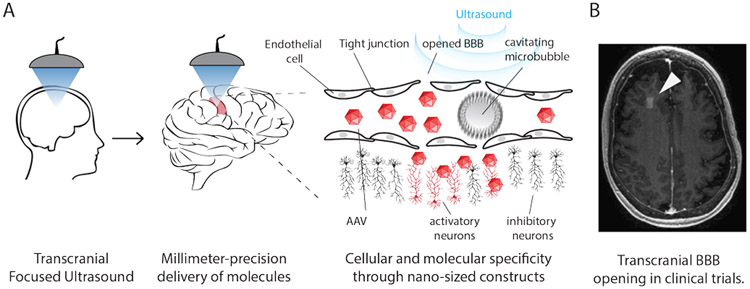
Figure 1. Ultrasound enhanced and triggered transport into the brain.
A) The human body contains thousands of molecules in different tissues. Restricting the region of delivery to a small subset of cells by focused ultrasound-enhanced delivery reduces off-target effects in non-targeted tissues. By combining ultrasound specificity with molecular engineering it is possible to both target the specific sites within the body and specific cells within the targeted site. Such specificity can be achieved by localized delivery of molecular constructs (AAV viral vectors, nanoparticles, proteins, small molecules) through the BBB into the brain. When microbubbles are injected into the bloodstream and insonated, they begin to oscillate (cavitate) and loosen tight junctions in the BBB, transiently, locally, and safely, improving transport from blood into the brain tissue. B) Example of ultrasound-enhanced molecule delivery to the brain. Arrowhead points to the area of the BBB opened with ultrasound to allow passage of small molecule MRI contrast-agent. Reproduced with permission from ref. 25. Copyright (2018) Nature-Springer.
To combine the spatial precision of ultrasound with the molecular, cell type and temporal control provided by genetically engineered therapeutics, we recently developed an approach to non-invasive control of neural circuits called acoustically targeted chemogenetics, or ATAC28 (Fig. 2A-B). This technology uses FUS-BBBO to deliver adeno-associated viral (AAV) vectors into specific brain regions (Fig. 2C). These vectors transfect neurons and cause genetically defined neuronal subtypes to express engineered receptors, which provide control over the activity of these neurons using an otherwise inert brain-permeable drug29. With dimensions of approximately 20 nm, AAVs are small enough to efficiently enter the brain after non-damaging FUS-BBBO, and can be delivered in sufficient dose to achieve more than 50% transfection in certain brain regions (Fig. 2D). Cellular specificity is achieved by engineering the DNA contained inside the AAV to express the desired protein under a promoter that is only active in select cell types30, for example excitatory neurons or neurons that use the neurotransmitter dopamine. In this case, the protein payload comprises a “chemogenetic” G-protein coupled receptor that has been engineered to no longer respond to endogenous neurotransmitters, and instead become activated by an otherwise inert, systemically bioavailable drug. Several such receptor-drug combinations are available, allowing metabotropic or ionotropic excitation or inhibition of neurons29. ATAC comprises a brief, non-invasive FUS-BBBO treatment and a several-week period to attain robust expression of the chemogenetic receptor that lasts for at least several months28. Subsequently, the transfected neurons are controlled on-demand via the simple systemic administration of the chemogenetic ligand.
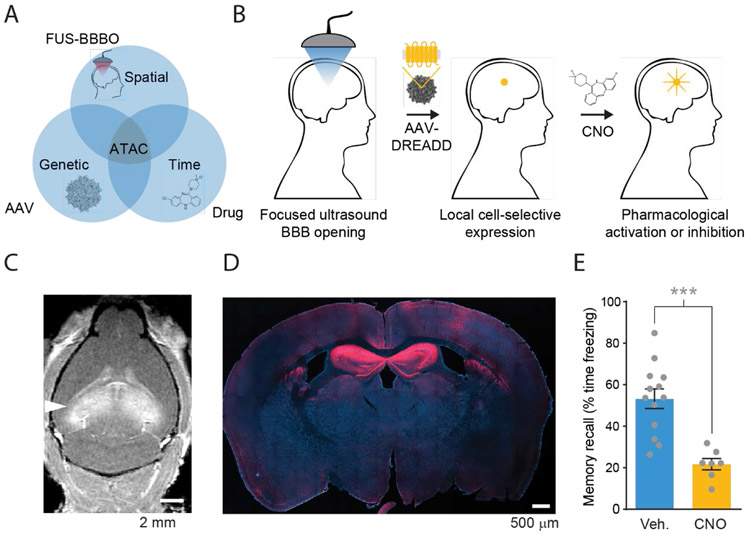
Figure 2. Acoustically targeted chemogenetics (ATAC).
A) ATAC combines FUS-BBBO, viral vector gene delivery, and chemogenetics to achieve fully noninvasive spatially, genetically, and temporally specific control cells in the brain. B) In ATAC process MRI-guided focused ultrasound reversibly opens the BBB to deliver viral vectors carrying chemogenetic receptors that can be activated specifically by a BBB-permeable ligand. (C) Safe and noninvasive opening of the BBB with FUS in hippocampus which was used to deliver viral vectors carrying DNA with a cell specific promoter and a chemogenetic receptor. BBB opening is visualized by extravasation of gadolinium contrast agent in a T1-weighted MRI. (D) Gene expression of engineered chemogenetic receptors that respond to a specific BBB-permeable drug, as visualized by immunostaining (red). (E) The expression of engineered receptors allows subsequent pharmacological control of specific neurons and resulting behavior, such as memory recall. Adapted with permission from ref. 28. Copyright (2018) Nature-Springer.
In a proof-of-concept study28, we used ATAC to non-invasively inactivate the mouse hippocampus (Fig. 2D) and inhibit the formation of associative memories (Fig. 2E). The effect was highly specific; we did not observe off-target effects on untargeted neurons or untargeted behaviors. The unprecedented combination of targeting based on spatial focusing, genetic specificity, and the molecular precision of chemogenetics creates opportunities for more specific therapies and neuroscience experiments. Importantly, all three components of ATAC – FUS-BBBO, viral vectors, and chemogenetics – have either been used in clinical trials25,31 or in non-human primates32, increasing the feasibility of clinical translation.
In some scenarios, a shorter-term approach to neuromodulation not involving gene therapy is beneficial, for example in clinical research studies and in piloting potential site-specific interventions in patients. In these cases, direct delivery of therapeutics would be beneficial (Fig. 3). One such approach is based on nanodroplets that are superheated liquid droplets with typical diameters of 200 to 300 nm33 made of perfluorocarbons or halocarbons, covered by a stabilizing shell made of albumin or lipids34. In their liquid state, nanodroplets can circulate in the blood for several hours35. Once delivered to the desired location, nanodroplets can be activated using ultrasound to phase-transition into gas bubbles in a process called acoustic droplet vaporization. This results in unstable gas bubbles that are 3-5.5 times larger and can be used as either contrast agents for imaging, or mechanical actuators35. Recently, a method of transient localized delivery of neuroactive therapeutics was developed by loading the local anesthetic Propofol into perfluorocarbon nanodroplets, then activating the local release of this drug from the droplets in the neurovasculature using focused ultrasound36. While the mechanism by which the drug is released is still uncertain in this particular case, the flow rate of the relevant neurovasculature and the kinetics of Propofol uptake into the brain parenchyma allows the inhibition of neural activity to be localized within the insonated region37. This approach is conceptually related to previous and ongoing work on the localized delivery of therapeutics to various organs of the body38. For example, doxorubicin has been delivered to tumors using temperature-sensitive liposomes, which release their drug contents at the ultrasound focus as a result of temperature elevation above their phase transition threshold39,40, typically below 42°C. Another application is the delivery of tissue-plasminogen activator using echogenic liposomes41,42.
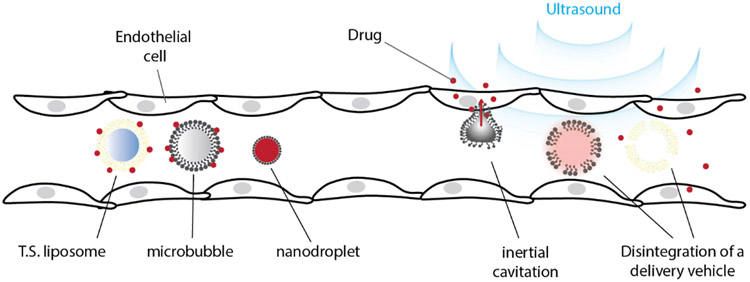
Figure 3. Site-specific delivery of drugs for control of cellular functions.
Site specific molecular delivery is enabled through transcranially focused ultrasound, which can target brain regions with millimeter precision. Multiple approaches can be used to control cellular activity in the brain, including focused-ultrasound BBB opening with intravenous co-administration of viral vectors or nanoparticles. Delivery of molecules to all sites within the body can be achieved with ultrasound-responsive delivery vectors, such as nanodroplets, microbubbles, and temperature-sensitive liposomes (T.S. liposomes). Molecules can be incorporated within the core or shell of these delivery vehicles, or can be attached to the exterior. Upon insonation these vehicles cavitate (in case of gas-containing bubbles) and/or disintegrate, releasing their cargo.
Ultrasound-actuated nanomechanical therapeutics
In addition to facilitating the delivery of nanoscale and molecular therapeutics into targeted regions of the body, focused ultrasound can produce more immediate mechanical and thermal effects in the target tissue2. For example, low frequency ultrasound in combination with cavitation nuclei can produce a range of mechanical effects via stable and inertial bubble cavitation. The latter phenomenon, occurring at relatively high acoustic pressure, involved the unstable expansion and violent collapse of bubbles43. In comparison to the gentle opening of tight junctions in the BBB and microstreaming achieved by stable cavitation, inertial cavitation can lyse cells or enhance drug delivery by creating pores in their membranes43.
Targeted cavitation treatments are often facilitated by the use of untargeted or, less frequently, targeted micron-sized bubbles as cavitation nuclei40. However, microbubbles have several characteristics limiting their biological specificity. First, bubbles are fundamentally unstable, making it difficult for them to undergo extended wash-in and clearance protocols to achieve specific biological targeting. Second, micron-sized bubbles cannot exit the vascular system, and thus cannot interact with the extracellular matrix or cells located deep within tissues of interest44 (Fig. 4A, B). For ultrasound-activated therapeutics to reach such targets, the cavitation nuclei will either need to become nanoscale to enable extravasation, or they will need to be genetically encoded within the cells themselves. To overcome these limitations, several acoustically active nanomaterials have recently been developed.
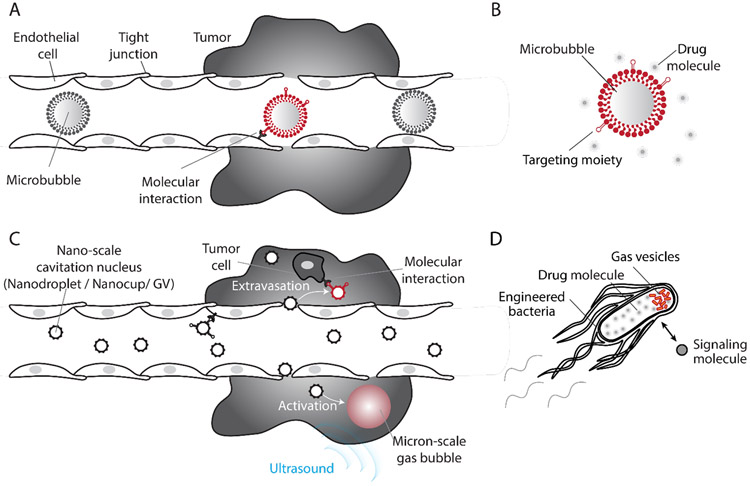
Figure 4. Nanoscale and genetically encodable nuclei for molecularly-targeted cavitation.
A) Due to their micron scale, microbubbles cannot exit the vasculature through leaky tumor blood vessels and reach the cancerous tissue. B) Therefore, they primarily engage in molecular interactions with endothelial cells. C) Nano-scale cavitation nuclei can exit the vasculature and interact with cells and other targets in the tissue in addition to endovascular targets. Their activation inside the tumor microenvironment enables selective generation of strong mechanical forces within the tumor core. D) Engineered cells expressing gas vesicles can be triggered with ultrasound to nucleate cavitation, producing potent mechanical effects and releasing therapeutics they produce in-situ.
Among synthetic materials, perfluorocabon nanodroplets were among the first nanoscale cavitation nuclei shown to extravasate through leaky tumor vessels (Fig. 4C), which then can facilitate drug delivery into tumors through an ultrasound-triggered phase transition4. In addition to liquid droplets, another recent approach has focused on seeding bubble nucleation using polymeric “nanocups” 45. These polymeric structures with diameter of 480 ± 24 nm44 have a cup shape that holds an air nanobubble stabilized by an inner hydrophobic cavity. Upon insonation, the nanobubble grows and then detaches from the nanocup to nucleate free bubble cavitation activity. In vivo studies showed that the resulting cavitation can propel drug models deeper into cancerous tissue44. Moreover, the stable hydrophobic cavity can assist in nucleating additional cavitation even after the release of the initial bubble.
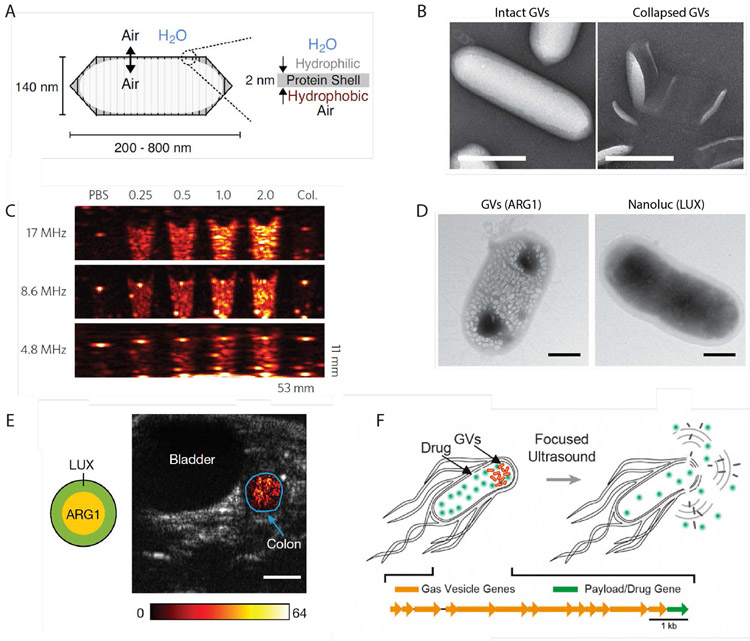
To expand the potential of ultrasound-targeted therapy to specific cells and biomolecular targets, we recently introduced the first genetically encodable acoustic biomolecules (Fig. 4D). Gas vesicles (GVs) are genetically encoded all-protein nanostructures that in nature are used by photosynthetic bacteria to regulate their flotation46. GVs are composed of gas-filled protein shell with a hydrophobic interior and hydrophilic exterior. Thus, while enabling the free exchange of gas, GVs exclude water from their interior, and are instead filled with gas that partitions into them from surrounding media (Fig. 5A). Wild type GVs have dimensions on the order of 45-800 nm (depending on their genotype) and indefinite physical stability. In the last few years, GVs have been shown to produce robust contrast in ultrasound46-49, MRI50,51 and optical imaging52. Furthermore, gene cassettes have been engineered for heterologous expression of GVs as acoustic reporter genes in bacteria47 (Fig. 5C) and mammalian cells53, enabling deep tissue imaging of cell location and activity (Fig. 5D-E). The GV shell can be collapsed hydrostatically or acoustically54 (Fig 5B), releasing the enclosed air and producing background-less differential images47,51.
Figure 5. Gas vesicles as genetically encoded nuclei for imaging and therapy.
A) An illustration of a gas vesicle (GV) structure. Reproduced with permission from ref. 62. Copyright (2018) Elsevier. B) Transmission electron micrographs of purified GVs from Anabaena flos-aquae. GVs can be collapsed either hydrostatically or acoustically, releasing the encapsulated air bubble. Scale bars, 200 nm. C) Purified gas vesicles produce robust acoustic contrast with concentration-dependent signal. B-C reproduced with permission from ref. 46. Copyright (2014) Nature-Springer. D) Transmission electron micrographs of an E. Coli cell transformed with the acoustic reporter gene (ARG1), and chemically induced to produce GVs. An E. Coli cells express nanoluc luciferase is presented for comparison. Scale bars, 500 nm. E) Ultrasound abdominal scan of a mouse showing ARG1-expressing E. coli cells arranged in the colon as indicated in the diagram. Functional GV contrast is overlaid in color on top of a grayscale anatomical scan. D-E reproduced with permission from ref. 47. Copyright (2018) Nature-Springer. F) GVs used as genetically encoded cavitation nuclei that can lyse the host cell and release co-expressed payload.
The ability to collapse GVs and break their protein shell also provides a new mechanism for non-invasively producing local mechanical forces55. While typical diagnostic ultrasound frequencies in the range of several MHz can be safely used to visualize GVs, we found that low-frequency ultrasound pulses can drive the growth and cavitation of air nanobubbles released following GV collapse55. The ability of purified GVs to nucleate cavitation was demonstrated using passive cavitation recording, which detects the acoustic signature of cavitation, and using ultra-high frame rate optical microscopy, which provided direct images of GV collapse and subsequent formation of cavitating bubbles. Based on the insights obtained from these in vitro experiments, in vivo GVs cavitation inside disease-relevant tissue was shown with a subcutaneous tumor model55.
In addition to being genetically encodable, GVs have a range of unique characteristics that make them exceptional contrast agents and actuators. In comparison to microbubbles, GVs’ nanoscale size is compatible with their assembly inside bacterial47 and mammalian cells53, and potentially with passing through leaky tumor vessels. In addition, the constituent proteins comprising GVs can be engineered to provide new acoustic, structural, surface, and functional properties54. These changes can enable, highly specific ultrasound imaging of GVs based on nonlinear acoustic output48,49, tailored collapse pressure, selective attachment of GV to particular cells, and fluorescent GVs54. For example, the fusion of GVs’ external shell protein GvpC with a C-terminal RGD peptide enables targeting to αVβ3 integrin receptors, which are overexpressed in certain tumors54. When insonated with focused ultrasound waves, GVs attached to U87 glioblastoma tumor cells nucleated cavitation activity, opening the membrane of these cells55. This sonoporation resulted in an influx of a cell-impermeable dye, functioning as a drug model55. Cavitation of GVs attached to U87 cells was also documented using high frame rate microscopy55.
An even wider range of therapeutic effects can be achieved by expressing GVs in engineered cells. In previous studies, engineered bacteria were shown to selectively home to and colonize tumors, monitor the microenvironment and produce anti-tumor therapy in situ56,57. GV cavitation complements these capabilities by providing a new mechanism to deliver mechanical effects and externally trigger the release of intracellular therapeutic proteins with high spatial and temporal precision. In our recent study, GV cavitation was shown to facilitate ultrasound-triggered lysis of engineered bacteria and selective release of co-expressed luminescent protein Nanoluc, which served as a payload model55 (Fig 5F). In addition, GV cavitation provides these engineered cells with a mechanism for producing strong mechanical forces that can potentially be used to propel drugs deeper into adjacent tissue. The recent mammalian expression of GVs53 could extend these capabilities to a broader range of therapeutic cell types.
The ability of GVs to serve as imaging as well as therapeutic agents is expected to enable their use in theranostics – an emerging therapeutic paradigm in which molecular imaging modalities are used to guide and control targeted therapeutic activity. In particular, it is possible to use non-destructive imaging modes to visualize GV biodistribution in vivo before applying focused ultrasound pulses to collapse and cavitate the GVs and resulting bubbles for therapeutic purposes, then using imaging to confirm that GVs at the targeted location have been destroyed.
Ultrasound-actuated thermal bioswitches
In addition to the mechanical effects mediating the ultrasound uses described above, focused ultrasound can also be used to locally elevate temperature. This can be performed under real-time MRI guidance, allowing the delivered temperature to be within a desired target range. Focused ultrasound heating to ablative temperatures is used clinically for focal ablation. However, it can also be used in combination with temperature-sensitive biomolecules to achieve control over cellular functions such as gene expression using thermal pulses within the well-tolerated range of 37–42°C. This has been accomplished in mammalian cells using their endogenous heat shock promoter machinery, allowing FUS to drive the expression of genes driven by a heat shock promoter (HSP) genes58-60. In bacteria, endogenous HSPs were found to provide poor switching responses within the temperatue range compatible with mammalian physiology, prompting us to develop new classes of orthogonal transcriptional bioswitches with tunable temperature set-points61. These bioswitches enable gene expression in engineered microbes to be controlled with more than 300-fold switching induction.
Summary.
The combination of molecular engineering and ultrasound allows for more specific targeting of cell populations in deep tissues. The idea of a silver bullet - a single molecule that specifically binds to a single target – is challenging to achieve in vivo due to the sheer number of molecular interactions throughout the body. However, restricting the area of interest to the sites specified by an ultrasound beam will simplify the problem of specific targeting. The millimeter-sized volume of a tissue exposed to focused ultrasound has fewer cells and fewer off-target binding partners than the entirety of a human body. Thus, the simple use of ultrasound and its application to nanomedicine provides an added layer of specificity that would be difficult to achieve with molecular engineering alone.
Acknowledgements
The authors are grateful to members of the Shapiro laboratory for helpful discussions. Related research was supported by the National Institutes of Health (R01EB018975 to MGS) and the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 792866 (A.B.-Z.).
Biography
Jerzy O. Szablowski is an incoming Assistant Professor of Bioengineering at Rice University, where he is establishing the Laboratory for Noninvasive Neuroengineering. He received his B.Sc. in Biological Engineering from MIT in 2009, where he worked on engineering protein contrast agents for MRI in the laboratories of Alan Jasanoff and Robert Langer. He then received his Ph.D. in Bioengineering from Caltech in 2015 for his work on programmable therapeutics for modulating gene expression in animal models of cancer in the laboratory of Peter Dervan. As a postdoctoral fellow in the Shapiro laboratory at Caltech, he developed the acoustically targeted chemogenetics technology for non-invasive control of neural circuits. More information about the Laboratory for Noninvasive Neuroengineering can be found online at szablowskilab.org.
Avinoam Bar-Zion is a Marie Skłodowska-Curie postdoctoral fellow at the Shapiro laboratory at Caltech. He received his B.Sc. degree Summa Cum Laude in biomedical engineering from the Technion in 2010. His PhD, also received from the Technion in 2016, was focused on imaging of tumor angiogenesis using contrast-enhanced ultrasound. During his PhD, he also completed a year of research at the University of Toronto, as a part of a collaboration between the Technion and with the Medical Biophysics Department, University of Toronto. His research interests include contrast-enhanced ultrasound imaging and therapy, medical signal and image processing, synthetic biology, and computer-aided diagnosis.
Mikhail G. Shapiro is a Professor of Chemical Engineering and an Investigator of the Heritage Medical Research Institute at the California Institute of Technology. The Shapiro laboratory develops biomolecular technologies allowing cells to be imaged and controlled inside the body using sound waves and magnetic fields to enable the study of biological function in vivo and the development of cell-based diagnostic and therapeutic agents. Mikhail received his PhD in Biological Engineering from MIT and his BSc in Neuroscience from Brown University, and conducted post-doctoral research at the University of Chicago and the University of California, Berkeley, where he was a Miller Fellow. More information about the Shapiro Lab can be found online at shapirolab.caltech.edu.
References
- (1).Piraner DI; Farhadi A; Davis HC; Wu D; Maresca D; Szablowski JO; Shapiro MG Going Deeper: Biomolecular Tools for Acoustic and Magnetic Imaging and Control of Cellular Function. Biochemistry 2017, 56, 5202–5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Maresca D; Lakshmanan A; Abedi M; Bar-Zion A; Farhadi A; Lu GJ; Szablowski JO; Wu D; Yoo S; Shapiro MG Biomolecular Ultrasound and Sonogenetics. Annu. Rev. Chem. Biomol. Eng 2018, 9, 229–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Dromi S; Frenkel V; Luk A; Traughber B; Angstadt M; Bur M; Poff J; Xie J; Libutti SK; Li KCP; Wood BJ Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin. Cancer Res 2007, 13, 2722–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Rapoport NY; Kennedy AM; Shea JE; Scaife CL; Nam K-H Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J. Control. Release : official journal of the Controlled Release Society 2009, 138, 268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Nelson JL; Roeder BL; Carmen JC; Roloff F; Pitt WG Ultrasonically Activated Chemotherapeutic Drug Delivery in a Rat Model. Cancer Res. 2002, 62, 7280. [PubMed] [Google Scholar]
- (6).Schoellhammer CM; Schroeder A; Maa R; Lauwers GY; Swiston A; Zervas M; Barman R; DiCiccio AM; Brugge WR; Anderson DG; Blankschtein D; Langer R; Traverso G Ultrasound-mediated gastrointestinal drug delivery. Sci. Transl. Med 2015, 7, 310ra168–310ra168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zderic V; Vaezy S; Martin RW; Clark JI Ocular drug delivery using 20-kHz ultrasound. Ultrasound. Med. Biol 2002, 28, 823–829. [DOI] [PubMed] [Google Scholar]
- (8).Dayton P; Klibanov A; Brandenburger G; Ferrara K Acoustic radiation force in vivo: a mechanism to assist targeting of microbubbles. Ultrasound. Med. Biol 1999, 25, 1195–1201. [DOI] [PubMed] [Google Scholar]
- (9).Hynynen K; McDannold N; Vykhodtseva N; Jolesz FA Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001, 220, 640–646. [DOI] [PubMed] [Google Scholar]
- (10).Escoffre J-M; Bouakaz A: Therapeutic ultrasound; Springer, 2015; Vol. 880. [Google Scholar]
- (11).Mullick Chowdhury S; Lee T; Willmann JK Ultrasound-guided drug delivery in cancer. Ultrasonography 2017, 36, 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lochhead JJ; Thorne RG Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev 2012, 64, 614–628. [DOI] [PubMed] [Google Scholar]
- (13).Patel T; Zhou J; Piepmeier JM; Saltzman WM Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev 2012, 64, 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Curtis C; Zhang M; Liao R; Wood T; Nance E Systems-level thinking for nanoparticle-mediated therapeutic delivery to neurological diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2017, 9. [DOI] [PubMed] [Google Scholar]
- (15).Kinoshita M; McDannold N; Jolesz FA; Hynynen K Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc. Natl. Acad. Sci. U S A 2006, 103, 11719–11723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Thévenot E; Jordão JF; O'Reilly MA; Markham K; Weng Y-Q; Foust KD; Kaspar BK; Hynynen K; Aubert I Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum. Gene Ther 2012, 23, 1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Nance E; Timbie K; Miller GW; Song J; Louttit C; Klibanov AL; Shih T-Y; Swaminathan G; Tamargo RJ; Woodworth GF; Hanes J; Price RJ Non-invasive delivery of stealth, brain-penetrating nanoparticles across the blood-brain barrier using MRI-guided focused ultrasound. J. Control. Release 2014, 189, 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Timbie KF; Afzal U; Date A; Zhang C; Song J; Wilson Miller G; Suk JS; Hanes J; Price RJ MR image-guided delivery of cisplatin-loaded brain-penetrating nanoparticles to invasive glioma with focused ultrasound. J. Control. Release 2017, 263, 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Timbie KF; Mead BP; Price RJ Drug and gene delivery across the blood-brain barrier with focused ultrasound. J. Control. Release 2015, 219, 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Chen H; Konofagou EE The size of blood-brain barrier opening induced by focused ultrasound is dictated by the acoustic pressure. J. Cereb. Blood. Flow Metab 2014, 34, 1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Samiotaki G; Konofagou EE Dependence of the reversibility of focused- ultrasound-induced blood-brain barrier opening on pressure and pulse length in vivo. IEEE Trans. Sonics Ultrason 2013, 60, 2257–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Kobus T; Vykhodtseva N; Pilatou M; Zhang Y; McDannold N Safety Validation of Repeated Blood-Brain Barrier Disruption Using Focused Ultrasound. Ultrasound Med. Biol 2016, 42, 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Downs ME; Buch A; Sierra C; Karakatsani ME; Teichert T; Chen S; Konofagou EE; Ferrera VP Long-Term Safety of Repeated Blood-Brain Barrier Opening via Focused Ultrasound with Microbubbles in Non-Human Primates Performing a Cognitive Task. PLoS One 2015, 10, e0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Carpentier A; Canney M; Vignot A; Reina V; Beccaria K; Horodyckid C; Karachi C; Leclercq D; Lafon C; Chapelon JY; Capelle L; Cornu P; Sanson M; Hoang-Xuan K; Delattre JY; Idbaih A Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med 2016, 8, 343re342. [DOI] [PubMed] [Google Scholar]
- (25).Lipsman N; Meng Y; Bethune AJ; Huang Y; Lam B; Masellis M; Herrmann N; Heyn C; Aubert I; Boutet A; Smith GS; Hynynen K; Black SE Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Comm 2018, 9, 2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Burgess A; Dubey S; Nhan T; Aubert I; Hynynen K Therapeutic effects of focused ultrasound-mediated blood-brain barrier opening in a mouse model of Alzheimer’s disease. J. Ther. Ultrasound 2015, 3, O16–O16. [Google Scholar]
- (27).Baseri B; Choi JJ; Deffieux T; Samiotaki G; Tung Y-S; Olumolade O; Small SA; Morrison B; Konofagou EE Activation of signaling pathways following localized delivery of systemically administered neurotrophic factors across the blood-brain barrier using focused ultrasound and microbubbles. Phys. Med. Biol 2012, 57, N65–N81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Szablowski JO; Lee-Gosselin A; Lue B; Malounda D; Shapiro MG Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng 2018, 2, 475. [DOI] [PubMed] [Google Scholar]
- (29).Sternson SM; Roth BL Chemogenetic Tools to Interrogate Brain Functions. Annu. Rev. Neurosci 2014, 37, 387–407. [DOI] [PubMed] [Google Scholar]
- (30).Dittgen T; Nimmerjahn A; Komai S; Licznerski P; Waters J; Margrie TW; Helmchen F; Denk W; Brecht M; Osten P Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc. Natl. Acad. Sci. U S A 2004, 101, 18206–18211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Ginn SL; Amaya AK; Alexander IE; Edelstein M; Abedi MR Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med 2018, 20, e3015. [DOI] [PubMed] [Google Scholar]
- (32).Eldridge MAG; Lerchner W; Saunders RC; Kaneko H; Krausz KW; Gonzalez FJ; Ji B; Higuchi M; Minamimoto T; Richmond BJ Chemogenetic disconnection of monkey orbitofrontal and rhinal cortex reversibly disrupts reward value. Nat, Neurosci. 2016, 19, 37–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Sheeran PS; Matsunaga TO; Dayton PA Phase-transition thresholds and vaporization phenomena for ultrasound phase-change nanoemulsions assessed via high-speed optical microscopy. Phys. Med. Biol 2013, 58, 4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kripfgans OD; Fowlkes JB; Miller DL; Eldevik OP; Carson PL Acoustic droplet vaporization for therapeutic and diagnostic applications. Ultrasound Med. Biol 2000, 26, 1177–1189. [DOI] [PubMed] [Google Scholar]
- (35).Sheeran PS; Dayton PA Phase-change contrast agents for imaging and therapy. Curr. Pharm. Des 2012, 18, 2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Airan RD; Meyer RA; Ellens NPK; Rhodes KR; Farahani K; Pomper MG; Kadam SD; Green JJ Noninvasive Targeted Transcranial Neuromodulation via Focused Ultrasound Gated Drug Release from Nanoemulsions. Nano Lett. 2017, 17, 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Wang JB; Aryal M; Zhong Q; Vyas DB; Airan RD Noninvasive Ultrasonic Drug Uncaging Maps Whole-Brain Functional Networks. Neuron 2018, 100, 728–738.e727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Sirsi SR; Borden MA State-of-the-art materials for ultrasound-triggered drug delivery. Adv. Drug Delivery Rev 2014, 72, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Needham D; Anyarambhatla G; Kong G; Dewhirst MW A New Temperature-sensitive Liposome for Use with Mild Hyperthermia: Characterization and Testing in a Human Tumor Xenograft Model. Cancer Res. 2000, 60, 1197. [PubMed] [Google Scholar]
- (40).Ferrara KW; Borden MA; Zhang H Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery. Acc. Chem. Res 2009, 42, 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Smith DA; Vaidya SS; Kopechek JA; Huang SL; Klegerman ME; McPherson DD; Holland CK Ultrasound-triggered release of recombinant tissue-type plasminogen activator from echogenic liposomes. Ultrasound Med. Biol 2010, 36, 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Kandadai MA; Meunier JM; Hart K; Holland CK; Shaw GJ Plasmin-loaded echogenic liposomes for ultrasound-mediated thrombolysis. Transl. Stroke Res 2015, 6, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Ferrara K; Pollard R; Borden M Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng 2007, 9, 415–447. [DOI] [PubMed] [Google Scholar]
- (44).Kwan JJ; Myers R; Coviello CM; Graham SM; Shah AR; Stride E; Carlisle RC; Coussios CC Ultrasound-Propelled Nanocups for Drug Delivery. Small 2015, 11, 5305–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Kwan J; Graham S; Myers R; Carlisle R; Stride E; Coussios C Ultrasound-Induced Inertial Cavitation from Gas-Stabilizing Nanoparticles. Phys. Rev. E 2015, 92, 023019. [DOI] [PubMed] [Google Scholar]
- (46).Shapiro MG; Goodwill PW; Neogy A; Yin M; Foster FS; Schaffer DV; Conolly SM Biogenic Gas Nanostructures as Ultrasonic Molecular Reporters. Nat. Nanotechnol 2014, 9, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Bourdeau RW; Lee-Gosselin A; Lakshmanan A; Farhadi A; Kumar SR; Nety SP; Shapiro MG Acoustic Reporter Genes for Noninvasive Imaging of Microorganisms in Mammalian Hosts. Nature 2018, 553, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Maresca D; Lakshmanan A; Lee-Gosselin A; Melis JM; Ni Y-L; Bourdeau RW; Kochmann DM; Shapiro MG Nonlinear Ultrasound Imaging of Nanoscale Acoustic Biomolecules. Appl. Phys. Lett 2017, 110, 073704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Maresca D; Sawyer DP; Renaud G; Lee-Gosselin A; Shapiro MG Nonlinear X-Wave Ultrasound Imaging of Acoustic Biomolecules. Phys. Rev. X 2018, 8, 041002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Shapiro MG; Ramirez RM; Sperling LJ; Sun G; Sun J; Pines A; Schaffer DV; Bajaj VS Genetically Encoded Reporters for Hyperpolarized Xenon Magnetic Resonance Imaging. Nat. Chem 2014, 6, 629. [DOI] [PubMed] [Google Scholar]
- (51).Lu GJ; Farhadi A; Szablowski JO; Lee-Gosselin A; Barnes SR; Lakshmanan A; Bourdeau RW; Shapiro MG Acoustically Modulated Magnetic Resonance Imaging of Gas-Filled Protein Nanostructures. Nat. Mater 2018, 17, 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Lu GJ; Chou L.-d.; Malounda D; Patel AK; Welsbie DS; Chao DL; Ramalingam T; Shapiro MG Biomolecular Contrast Agents for Optical Coherence Tomography. bioRxiv 2019, 595157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Farhadi A; Ho GH; Sawyer DP; Bourdeau RW; Shapiro MG Ultrasound Imaging of Gene Expression in Mammalian Cells. bioRxiv 2019, 580647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Lakshmanan A; Farhadi A; Nety SP; Lee-Gosselin A; Bourdeau RW; Maresca D; Shapiro MG Molecular Engineering of Acoustic Protein Nanostructures. ACS nano 2016, 10, 7314–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Bar-Zion A; Nourmahnad A; Mittelstein D; Yoo S; Malounda D; Abedi M; Lee-Gosselin A; Maresca D; Shapiro MG Acoustically Detonated Biomolecules for Genetically Encodable Inertial Cavitation. bioRxiv 2019, 620567. [Google Scholar]
- (56).Danino T; Prindle A; Kwong GA; Skalak M; Li H; Allen K; Hasty J; Bhatia SN Programmable Probiotics for Detection of Cancer in Urine. Sci. Transl. Med 2015, 7, 289ra284–289ra284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Din MO; Danino T; Prindle A; Skalak M; Selimkhanov J; Allen K; Julio E; Atolia E; Tsimring LS; Bhatia SN Synchronized Cycles of Bacterial Lysis for in Vivo Delivery. Nature 2016, 536, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Madio DP; van Gelderen P; DesPres D; Olson AW; de Zwart JA; Fawcett TW; Holbrook NJ; Mandel M; Moonen CT On the Feasibility of Mri-Guided Focused Ultrasound for Local Induction of Gene Expression. J Magn. Reson. Imaging 1998, 8, 101–104. [DOI] [PubMed] [Google Scholar]
- (59).Deckers R; Quesson B; Arsaut J; Eimer S; Couillaud F; Moonen CT Image-Guided, Noninvasive, Spatiotemporal Control of Gene Expression. Proc. Natl. Acad. Sci. U.S.A 2009, 106, 1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Guilhon E; Voisin P; de Zwart JA; Quesson B; Salomir R; Maurange C; Bouchaud V; Smirnov P; de Verneuil H; Vekris A; Canioni P; Moonen CT Spatial and Temporal Control of Transgene Expression in Vivo Using a Heat-Sensitive Promoter and Mri-Guided Focused Ultrasound. J Gene Med. 2003, 5, 333–342. [DOI] [PubMed] [Google Scholar]
- (61).Piraner DI; Abedi MH; Moser BA; Lee-Gosselin A; Shapiro MG Tunable Thermal Bioswitches for in Vivo Control of Microbial Therapeutics. Nat. Chem. Biol 2017, 13, 75. [DOI] [PubMed] [Google Scholar]
- (62).Lu GJ; Farhadi A; Mukherjee A; Shapiro MG Proteins, Air and Water: Reporter Genes for Ultrasound and Magnetic Resonance Imaging. Curr. Opin. Chem. Biol 2018, 45, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]