Abstract
Background
Immunotherapy with immune checkpoint inhibitor (ICI) is a promising treatment for unresectable hepatocellular carcinoma (HCC). However, whether ICIs would have the risk of hepatitis B virus (HBV) reactivation and the necessary of nucleos(t)ide analogs (NUCs) prophylaxis are still unclear. We aimed to investigate the role of NUCs prophylaxis in HBV-infected patients who underwent ICIs treatment.
Methods
The study was a retrospective prospective design to review and follow-up consecutive 62 patients with chronic hepatitis B or resolved HBV infection who had received ICIs treatment for the unresectable HCC. Of them, 60 patients with documented baseline serum HBV DNA value were classified into three categories according to the baseline HBV viral load and the status of antiviral therapy before ICI treatment. The clinical status, including tumor response, viral kinetics and liver function, was recorded and investigated.
Results
No HBV reactivation occurred in the 35 patients with HBV DNA ≤100 IU/mL on NUCs therapy. Of the 19 patients with HBV DNA >100 IU/mL who started NUCs simultaneously with ICI treatment, none encountered HBV reactivation during the immunotherapy. Of the six HBV patients without NUCs treatment, three had a greater than 1 log decrease in HBV viral load, and one maintained his serum HBV DNA in undetectable status during ICI treatment. Eventually, one out of six experienced HBV reactivation after 9 weeks of ICI treatment.
Conclusion
No patients on antiviral therapy developed HBV reactivation, and one out of six not receiving antiviral therapy had HBV reactivation. HBV viral load higher than 100 IU/mL is safe and not a contraindication for ICI treatment for HCC, if NUCs can be coadministrated.
Keywords: liver neoplasms, immunotherapy
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second-leading cause of cancer-related death worldwide that constitutes 854 000 new cases and 810 000 deaths per year.1 Hepatitis B virus (HBV) infection is common across the world, and globally approximate 54% of HCCs are attributed to chronic HBV infection.2 In Asian-Pacific region, chronic hepatitis B is endemic and plays much more important role in the development of HCC and its complications.3 4 Despite the improvement in surveillance and treatment of viral hepatitis, many patients still present with or progress to unresectable or advanced disease and require systemic therapy.2 Immunotherapies with immune checkpoint inhibitors (ICIs), such as nivolumab and pembrolizumab, the antiprogrammed cell death-1 (PD-1) antibodies, are recently emerged, effective immunotherapeutic agents for HCC.5–7
HBV reactivation is defined as the abrupt reappearance or rise of HBV DNA in the serum of a patient with resolved or inactive chronic HBV infection.8 This event can be triggered by the administration of either anticancer agents, immunosuppressive or biological therapies.8–11 CD8 T cell exhaustion due to PD-1 upregulation is characterized in chronic viral infection, including chronic hepatitis B (CHB).12 13 Previous studies suggested that blockade of the axis of PD-1 and its ligand (PD-L1) could restore anti-HBV T cell responses, which could enhance the control of HBV.14–16 In CheckMate-040 trial, 3 of 51 HBV-HCC patients (6%) presented a 1 log decline of HBV surface antigen (HBsAg) level during nivolumab therapy.5 However, two case reports described HBV reactivation in patients received ICI treatment for lung cancer.17 18 Currently, most of the clinical trials, including CheckMate-040 and Keynote-224, request CHB patients should be on nucleos(t)ide analogs (NUCs) treatment and had a HBV viral load <100 IU/mL before receiving their first dose of ICI treatment.5 6 So far, whether immunotherapy with ICIs would have the risk of HBV reactivation and the necessary of NUCs prophylaxis are still unclear.2 11 19 In this study, we aimed to investigate the risk of HBV reactivation and the role of NUCs treatment in HCC patients with CHB or resolved HBV infection undergoing ICIs treatment.
Materials and methods
Patients
The study was a retrospective prospective design to review and follow-up consecutive 62 patients with CHB or resolved HBV infection who had received nivolumab or pembrolizumab treatment for the unresectable HCC in Taipei Veterans General Hospital from May 2017 to September 2019. Of them, 60 patients with documented baseline serum HBV DNA level and evaluable image studies following the immunotherapy were finally enrolled in this study. Thirty-three patients who underwent immunotherapy before the end of June 2019 were retrospective reviewed of medical records. Since July 2019, the rest of the patients were recruited in an immunotherapy biomarker study and had prospectively observational monitoring.
The diagnosis of HCC was according to the AASLD treatment guidelines.20 ICI treatment was administered for HCC patients with intermediate stage after locoregional treatment failure by transarterial chemoembolization (TACE), or advanced stage with intolerable adverse events or treatment failure to sorafenib, or deteriorated liver reserves beyond Child-Pugh class A so that target therapy was not approved based on the reimbursement criteria of National Health Insurance (NHI) in Taiwan.21 22 All the enrolled patients did not receive other locoregional therapies, including TACE, during the ICI treatment. According to their baseline HBV viral load and the status of antiviral therapy before ICI treatment, the enrolled patients were classified into three categories, including (1) patients with HBV DNA ≤100 IU/mL on NUCs therapy (fulfilled CheckMate-040 and Keynote-224 HBV criteria),5 6 (2) patients with HBV DNA >100 IU/mL on NUCs therapy and (3) patients with HBV but without receiving NUCs therapy throughout the ICI treatment because of not fulfilling the NUCs reimbursement criteria for CHB in NHI, Taiwan.
Treatment and outcome assessment
ICIs were prescribed according to the recommended dosage and safety information (2–3 mg/kg, every 2 weeks for nivolumab and every 3 weeks for pembrolizumab).5 6 National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; V.5.0) was applied for the assessment of therapeutic safety. Clinical evaluation with Child-Pugh class, albumin-bilirubin (ALBI) grade, hemogram, serum chemistry and alpha-fetoprotein (AFP) level were performed every 2–3 weeks during the treatment. Clinical tumor response was assessed by RECIST V.1.1 based on contrast-enhanced abdominal CT scan or MRI.23 The image examinations were carried out every 6–8 weeks during ICIs treatment.22 The study complied with Taiwan NHI regulation; HBV viral load monitoring was done every 6 months while on NUCs treatment. For patients on ICI treatment without NUCs, HBV viral loads were monitored every 2–3 months in this study. Additional HBV DNA test was performed if a serum alanine aminotransferase (ALT) level exceeded 100 U/L with suspicion of HBV reactivation.24 25 According to this guidance, 45 had follow-up HBV DNA values.
Definitions
HBV reactivation was defined as a 10-fold increase in HBV DNA from baseline, or reappearance of HBsAg in HBsAg-negative case, or HBV DNA from undetectable to higher than 1000 IU/mL.11 26 Hepatitis related to HBV reactivation was defined as a threefold or greater increase in serum ALT that exceeds 100 U/L.24 25 The upper normal limit of ALT was 40 U/L. The ALT flare was defined as a rise in ALT>5 fold upper limit of normal (ULN), which is classified as grade III hepatic toxicity by CTCAE 5.0 grading system. The ALBI score was calculated using the formula: (log10 bilirubin x 0.66) + (albumin x 0.085), where bilirubin is in umol/L and albumin in g/L; and the cut points of the ALBI grade were based on previous report.27
Virological and biochemical tests
Serum HBV DNA level was measured by quantitative PCR method (Roche COBAS 6800 HBV test) with the lower detection limit of 10 IU/mL. Serum biochemistry tests were measured by systemic multiauto-analyzer (Technicon SMAC, Technicon Instruments, Tarrytown, New York, USA). Serum AFP level was measured by chemiluminescent microparticle immunoassay (Elecsys AFP Assay, Roche Diagnostics, Mannheim, Germany) with clinically reportable range from 0.908 to 1 998 000 ng/mL.
Statistical analysis
Continuous variables were expressed as median (range), while categorical variables were analyzed as frequency and percentages. The Pearson X2 analysis or Fisher’s exact test was used to compare categorical variables, while the Student t-test or Mann-Whitney U test was applied for continuous variables. For all analyzes, p<0.05 was considered statistically significant. All statistical analyzes were performed using the Statistical Package for Social Sciences (SPSS V.17.0 for Windows, SPSS).
Results
Demographic characteristics of the study cohort
The patients were classified into three categories, including HBV DNA ≤100 IU/mL on NUCs therapy, HBV DNA >100 IU/mL on NUCs therapy and HBV without NUCs therapy. For the six patients without NUCs treatment, four had HBV viral load less than 2000 IU/mL; the other two had elevated HBV viral loads, but their ALT levels were less than 2 folds of ULN; which were not fulfilled the regulation of Taiwan NHI for NUCs treatment. No significant differences of liver reserves could be identified between these three groups. The patients with HBV DNA ≤100 IU/mL on antiviral therapy were significantly older (median age: 63.4 vs 58.0 and 56.5 years old, p=0.019) compared with the others. Besides, these patients had smaller tumor size (4.0 vs 8.7 and 8.8 cm, p<0.001), lower serum level of AFP (373.2 vs 1992.6 and 3082.2 ng/mL, p=0.028); and more of them had experienced surgical resection for HCC before ICI treatment (62.9% vs 21.1% and 50.0%, p=0.013). Generally, most of the patients (80%) were at BCLC stage C. The majority (73.3%) of them was within Child-Pugh class A; but 65.0% was classified beyond ALBI grade 1. Two patients presented with positive anti-HCV antibody. Both their HCV viral loads were undetectable throughout the ICI treatment. The median cycles and duration of ICI treatment were five cycles (1–35) and 2.1 months (0.5–24.5) for patients with HBV DNA ≤100 IU/mL on NUCs; five cycles (1 – 23) and 2.3 months (0.2–15.0) for HBV DNA >100 IU/mL on NUCs; nine cycles (4 – 19) and 5.1 months (1.6–13.6) for patients not on NUCs, respectively. In addition, the follow-up period of the three patient groups was 5.4 months (0.5–25.7), 3.9 months (0.2–17.9) and 10.4 months (4.0–24.8), respectively. The detailed baseline characteristics were presented in table 1.
Table 1.
Characteristics of 60 HBV-HCC patients treated with ICIs
| Characteristics | HBV DNA ≤100 IU/mL on NUCs | HBV DNA >100 IU/mL on NUCs | Patient with HBV without NUCs |
P value |
| (n=35) | (n=19) | (n=6) | ||
| Age, year | 63.4 (45.6–78.8) | 58.0 (40.8–77.7) | 56.5 (40.1–66.5) | 0.019 |
| Sex (male), n (%) | 31 (88.6) | 15 (78.9) | 4 (66.7) | 0.341 |
| NUCs, ETV/TDF/TAF, n (%) | 24/8/3 (68.6/22.9/8.6) | 15/1/3 (78.9/5.3/15.8) | – | – |
| Anti-HCV positive, n (%) | 1 (2.9) | 0 (0) | 1 (16.7) | 0.136 |
| Max. tumor size, cm | 4.0 (1.0–12.1) | 8.7 (3.3–17.0) | 8.8 (1.6–14.4) | <0.001 |
| Tumor >50% liver volume, n (%) | 6 (17.1) | 10 (52.6) | 4 (66.7) | 0.006 |
| Multiple tumors, n (%) | 32 (91.4) | 19 (100.0) | 6 (100.0) | 0.324 |
| Extrahepatic metastasis, n (%) | 19 (54.3) | 8 (42.1) | 2 (33.3) | 0.514 |
| Portal vein invasion, n (%) | 16 (45.7) | 4 (73.7) | 5 (83.3) | 0.058 |
| AFP, ng/mL | 373.2 (1.8–272 689.4) | 1992.6 (13.7–785 992.2) | 3082.2 (1076.2–1 148 415.7) | 0.028 |
| BCLC stage B/C, n (%) | 8/27 (22.9/77.1) | 4/15 (21.1/78.9) | 0/6 (0/100.0) | 0.429 |
| Prothrombin time, INR | 1.16 (0.90–1.47) | 1.10 (1.00–3.12) | 1.09 (0.97–1.42) | 0.600 |
| Platelet count, K/cumm | 122 (43–360) | 182 (71–553) | 148 (128–367) | 0.057 |
| ALT, U/L | 35 (11–254) | 47 (17–213) | 39 (22–64) | 0.448 |
| AST, U/L | 42 (16–366) | 90 (27–480) | 61 (29–140) | 0.106 |
| Total bilirubin, mg/dL | 0.69 (0.22–2.41) | 1.10 (0.25–10.08) | 0.97 (0.29–1.44) | 0.854 |
| Albumin, g/dL | 3.8 (2.7–4.9) | 3.6 (2.3–4.4) | 3.5 (3.2–4.0) | 0.106 |
| Neutrophil, /cumm | 4800 (2300–11900) | 5550 (2500–12300) | 5500 (3900–11600) | 0.258 |
| Neutrophil-lymphocyte ratio | 3.89 (1.46–11.01) | 3.90 (2.52–15.13) | 4.45 (2.03–10.68) | 0.684 |
| Presence of ascites, n (%) | 11 (31.4) | 9 (47.4) | 4 (66.7) | 0.194 |
| Child-Pugh score | 6 (5–9) | 6 (5–12) | 6 (5–7) | 0.368 |
| Child-Pugh class A/B/C, n (%) | 28/7/0 (80.0/20.0/0) | 12/6/1 (63.2/31.6/5.3) | 4/2/0 (66.7/33.3/0) | 0.475 |
| ALBI grade 1/2/3, n (%) | 15/18/2 (42.9/51.4/5.7) | 4/12/3 (21.1/63.2/15.8) | 2/4/0 (33.3/66.7/14.3) | 0.386 |
| ICI treatment cycle | 5 (1–35) | 5 (1–23) | 9 (4–19) | 0.232 |
| ICI treatment duration, months | 2.1 (0.5–24.5) | 2.3 (0.2–15.0) | 5.1 (1.6–13.6) | 0.257 |
| Prior therapy to ICI, n (%) | ||||
| Surgical resection | 22 (62.9) | 4 (21.1) | 3 (50.0) | 0.013 |
| RFA | 16 (45.7) | 3 (15.8) | 2 (33.3) | 0.088 |
| TACE | 22 (62.9) | 8 (42.1) | 5 (83.3) | 0.143 |
| Sorafenib | 19 (54.3) | 10 (52.6) | 5 (83.3) | 0.378 |
| Combined ICI with TKI, n (%) | 9 (25.7) | 2 (10.5) | 2 (33.3) | 0.605 |
| Immune-related AEs, n (%) | 6 (17.2) | 3 (15.8) | 0 (0) | 0.550 |
| Follow-up period, months | 5.4 (0.5–25.7) | 3.9 (0.2–17.9) | 10.4 (4.0–24.8) | 0.199 |
Continuous variables are expressed as median (range).
AEs, adverse events; AFP, alpha-fetoprotein; AL(S)T, alanine (aspartate) aminotransferase; ETV, entecavir; ALBI grade, albumin-bilirubin grade; HBV, hepatitis B virus; HCV, hepatitis C virus; ICI, immune checkpoint inhibitor; INR, international normalized ratio; NUCs, nucleos(t)ide analogs; RFA, radiofrequency ablation; BCLC stage, Barcelona Clinic Liver Cancer stage; TACE, transarterial chemoembolization; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; TKI, tyrosine kinase inhibitor.
Viral kinetics of HBV during ICI treatment
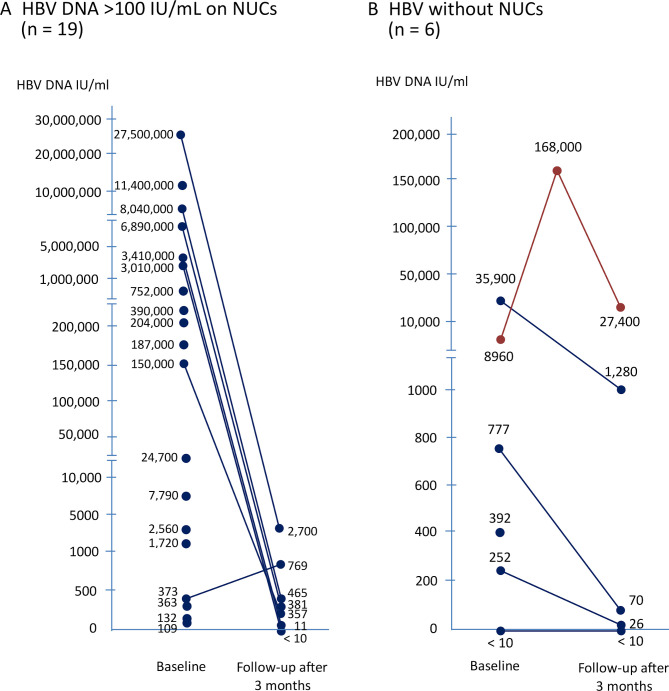
In patients with HBV DNA ≤100 IU/mL on NUCs therapy, no case experienced HBV reactivation during the ICIs treatment. Of the 19 patients with HBV DNA >100 IU/mL who started antiviral therapy simultaneously with ICI treatment, seven cases had longer survival to monitor their HBV DNA after NUCs treatment (figure 1A). Only one experienced suboptimal suppression by tenofovir with low HBV viremia but without ALT elevation or adverse events (details were presented in figure 2A); the others had a substantial decrease in HBV viral load during the follow-up period (table 2).
Figure 1.
Kinetics of HBV DNA during ICI treatment. Kinetics of HBV DNA during ICI treatment in (A) patients with HBV DNA >100 IU/mL on NUCs, and (B) patients with HBV not on NUCs. Of 19 patients with HBV DNA >100 IU/mL on antiviral therapy, nine developed early tumor progression and short survival (<3 months), and three did not have significant ALT elevation during ICI treatment so that their followed data of HBV viral load were unavailable. ALT, alanine aminotransferase; HBV, hepatitis B virus; ICI, immune checkpoint inhibitor; NUCs, nucleos(t)ide analogs.
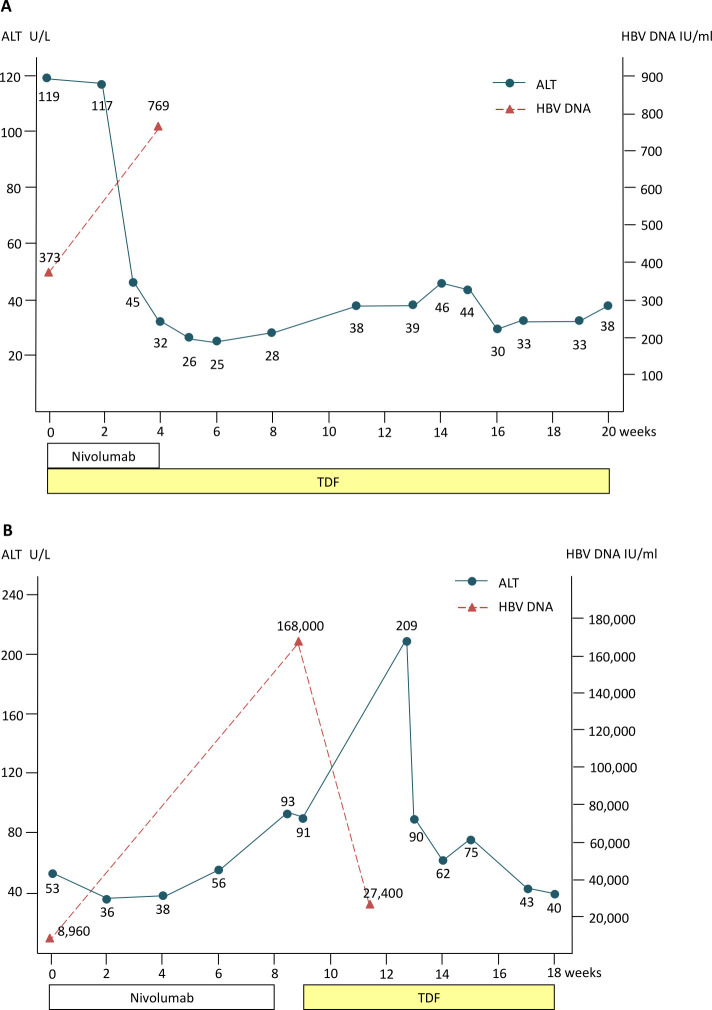
Figure 2.
Kinetics of serum alanine aminotransferase (ALT) of two patients with hepatitis B virus (HBV) DNA elevation Kinetics of serum ALT of patients with HBV DNA elevation during ICI treatment who was classified in patients with HBV DNA >100 IU/mL on NUCs (A), and patients with HBV not on NUCs (B), respectively. ICI, immune checkpoint inhibitor; NUCs, nucleos(t)ide analogs; TDF, tenofovir disoproxil fumarate.
Table 2.
HBV status during immune checkpoint inhibitors therapy
| N (%) | HBV DNA ≤100 IU/mL on NUCs (n=35) | HBV DNA >100 IU/mL on NUCs (n=19) | Patients with HBV without NUCs (n=6) |
| Baseline HBV DNA | |||
| Undetectable | 31 (88.6) | 0 | 1 (16.7) |
| Median (range) for detectable cases, IU/mL | 41 (12–82) | 187 000 (109 –27 500 000) | 777 (252–35 900) |
| HBV reactivation | 0 | 0 | 1 |
| HBV DNA during ICI treatment | |||
| ≥1 log10 elevation | 0 | 0 | 1 (16.7) |
| ≥2 log10 elevation | 0 | 0 | 0 |
| Undetectable to detectable | 3 (8.6) | 0 | 0 |
| Undetectable to >1000 IU/mL | 0 | 0 | 0 |
| Peak HBV DNA during ICI, IU/mL. median (range) | <10 (<10–1130) | 381 (<10–2700) | 70 (<10–1 68 000) |
| Hepatitis flare | |||
| ALT >100 U/L | 10 (28.6) | 11 (57.9) | 2 (33.3) |
| ALT >5X ULN | 5 (14.3) | 4 (21.1) | 1 (16.7) |
| ALT >10X ULN | 2 (5.7) | 2 (10.5) | 0 (0) |
| Icteric flare* | 5 (14.3) | 6 (31.6) | 2 (33.3) |
| HBV DNA elevation and ALT >100 U/L | 0 | 0 | 1 (16.7) |
| iRAE hepatitis | 1 (2.9) | 1 (5.3) | 0 (0) |
*Icteric flare is defined as serum ALT raised >3X ULN together with serum total bilirubin >2X ULN.
ALT, alanine aminotransferase; HBV, hepatitis B virus; ICI, immune checkpoint inhibitor; iRAE, immunotherapy related adverse event; NUCs, nucleos(t)ide analogs; ULN, upper limit of normal.
Of the six patients without antiviral treatment, five had detectable HBV viral load at baseline. Interestingly, three patients developed a notable decrease in HBV viral load during ICI treatment; and one maintained his serum HBV DNA in undetectable level during ICI treatment. Eventually, one patient experienced HBV reactivation with a >10 fold increase in HBV DNA level and ALT flare after 9 weeks of ICI treatment (figure 1B). After HBV reactivation, his HBV was controlled by tenofovir treatment and did not lead to HBV-related liver failure (table 3 and figure 2B). On the other hand, case N6 died of rapid tumor progression, and we could not get the followed data of HBV viral load.
Table 3.
Clinical characteristics of the six patients without pre-emptive nucleot(s)ide treatment
| Patient no | Age (years) |
Sex | Baseline HBsAg (IU/mL) | Baseline HBV DNA (IU/mL) |
Baseline AFP (ng/mL) |
ICI ±TKI therapy |
FU HBsAg (IU/mL) | FU HBV DNA (IU/mL) |
FU AFP at BR (ng/mL) |
Peak ALT (U/L) |
Peak t.bili (mg/dL) |
Rescue NUCs | Death caused by HBV | Best Tumor response to ICI |
| N1 | 40.9 | M | 26.4 | 35 900 | 2719.2 | Nivolumab | 2.01 | 1280 | 2799.9 | 85 | 0.63 | – | None | PD |
| N2 | 66.5 | F | 27.8 | 252 | 1859.3 | Nivolumab | 58.2 | 26 | 4974.5 | 202 | 5.23 | – | None | PD |
| N3 | 49.2 | M | 559.8 | 8960 | 1148415.8 | Nivolumab | Missing | 168 000 | 544 176.1 | 174 | 19.75 | TDF | None | PD |
| N4 | 40.1 | F | 1856.77 | 777 | 1076.2 | Nivolumab Lenvatinib |
Missing | 70 | 179.2 | 46 | 0.52 | – | None | CR |
| N5 | 63.9 | M | 557.88 | <20 | 3445.2 | Nivolumab Regorafenib |
Missing | <20 | 8905.3 | 37 | 2.77 | – | None | PD |
| N6 | 64.1 | M | 0 | 392 | 129 258.1 | Nivolumab | Missing | Missing | 76 472.3 | 26 | 9.49 | – | None | PD |
AFP, alpha-fetoprotein; ALT, alanine aminotransferase; BR, best response; F, female; HBsAg, HBV surface antigen; HBV, hepatitis B virus; ICI, immune checkpoint inhibitor; M, male; NUCs, nucleos(t)ide analogs; T.bili, total bilirubin; TDF, tenofovir disoproxil fumarate; TKI, tyrosine kinase inhibitor.
Status of hepatitis during ICI treatment
Twenty-three patients (38.3%) experienced ALT>100 U/L during the follow-up period. Of them, 10 patients had ALT flare (>5 fold ULN), but only four had more than 10-fold ULN ALT increase (5.7% and 10.5% in HBV DNA ≤100 IU/mL and HBV DNA >100 IU/mL on NUCs patients) (table 2). As presented in table 4, tumor progression is the main cause of ALT elevation in each group of patients. Two patients on NUCs therapy (one with baseline HBV DNA ≤100 IU/mL and the other one>100 IU/mL) had developed immune-related adverse event (iRAE) hepatitis. The incidence of iRAE hepatitis was not significantly different among the three groups. Of the six HBV patients without NUCs treatment, two developed ALT elevation >100 U/L during ICI therapy. One was resulted from HBV reactivation, and the other one was attributed to tumor progression.
Table 4.
Causes of ALT >100 U/L in HBV-HCC patients on ICI treatment
| N (%) | HBV DNA ≤100 IU/mL on NUCs (n=10) | HBV DNA >100 IU/mL on NUCs (n=11) | Patients with HBV without NUCs (n=2) |
| Tumor progression | 9 (90.0) | 10 (90.9) | 1 (50.0) |
| HBV reactivation | 0 | 0 | 1 (50.0) |
| iRAE hepatitis | 1 (10.0) | 1 (9.1) | 0 (0) |
ALT, alanine aminotransferase; HBV, hepatitis B virus; ICI, immune checkpoint inhibitor; iRAE, immunotherapy related adverse event; NUCs, nucleos(t)ide analogs.
Discussion
This is the largest real-world cohort from Asian patients with HBV-related HCC treated by ICIs till now. In our cohort, HBV reactivation developed in only one patient who did not receive antiviral agent during ICI therapy; and was controlled by tenofovir treatment that did not lead to HBV-related liver failure. Otherwise, no patients with ongoing NUCs suffered from noteworthy HBV reactivation during ICI treatment; even their baseline HBV DNA levels were higher than 100 IU/mL.
For patients with HBV-related HCC, HBV reactivation and its subsequent morbidity were identified as poor prognostic factors of the overall survival.28 According to previous studies, the incidence of HBV reactivation ranges from 4% to 67% in patients with HBV-related HCC undergoing chemotherapy; and the contributed mortality rate was up to 18%.29 30 Apart from the direct cause of death, the scheduled treatment to HCC would be delayed or premature terminated due to deteriorated liver function by HBV reactivation that would also have impacts on the prognosis.31 32 For patients who received hepatectomy or loco-regional therapies for HCC, HBV reactivation was also reported and identified in relation to the prognosis.33–35 However, the data regarding to the kinetics of HBV viral loads during ICI therapy for HCC were still limited.
In patients with chronic hepatitis B, the upregulated inhibitory receptors on the CD8 T cells limit their defensive function and lead to the exhausted phenotype.13 PD-1 is the most expressed inhibitory receptor, especially on the intrahepatic HBV-specific T cells.36 37 Blockage of PD-1/PD-L1 pathway could restore not only antitumor but also antiviral T cell function then help to suppress HBV viral load.12 37 According to the recent phase 1b study, a single dose of nivolumab at 0.3 mg/kg could lead to HBsAg decline in HBeAg-negative chronic hepatitis B.38 Despite the lack of data to support HBsAg decline in our study, we still demonstrated three out of six cases had substantial decline in HBV DNA and additional one case maintained his HBV viral load in undetectable status during ICI therapy among patients who did not receive NUCs treatment. These findings may imply the potential role of ICI in the treatment of chronic hepatitis B. However, even under NUCs treatment, a more than 1 log increase in HBV DNA during nivolumab treatment was reported in 11% (5/47) of HBV-related HCC patients in the Asian cohort of checkmate 040.39 In spite of that, their peak HBV viral load were all less than 2000 IU/mL and no HBV-related adverse events were reported. In our cohort, presence of low HBV viremia was observed in one patient during tenofovir treatment which did not lead to ALT elevation or adverse events. According to AASLD (American Association for the Study of Liver Diseases) guideline, change NUCs is not required as it did not meet the definition of antiviral drug resistance (1 log increase in HBV DNA).11 In addition to the previous case reports,17 18 40 a recent Chinese study demonstrated that 6 of 114 (5.3%) HBsAg-positive patients with various cancers developed HBV reactivation during anti-PD-1/PD-L1 treatment, but no HBV-related fatal events occurred.41 Our real-world data also demonstrated that HBV reactivation would occur in one out of six patients who did not receive pre-emptive anti-viral treatment. Accordingly, we still have to keep awareness of HBV reactivation during ICI treatment for HBV-HCC.42
In checkmate-040 and Keynote-224 trials, patients were required to receive effective anti‐viral therapy and had a viral load <100 IU/mL before initiating ICI treatment.5 6 In the real life, however, ICIs would be delivered to more complex populations of patients than those of clinical trials that raise a question to the necessity of keeping low viral load before ICI therapy. Our data suggested that HBV viral load higher than 100 IU/mL is safe and not a contraindication for ICI treatment for HCC, if NUCs can be coadministrated. By this way, none developed HBV viral load increase during the treatment course.
Although our data supported the feasibility of administering anti-PD-1 therapy in patients with HCC and HBV, the optimal use of NUCs remains uncertain. Whether patients at low risk of HBV reactivation may be monitored rather than receive prophylaxis in this setting, and an optimal threshold of HBV viral load that ICIs can be safely initiated without NUCs required further studies to determine.
There are several limitations in this study. First, this study only enrolled patients from a single hospital. However, our hospital is the main leading tertiary medical center in Taiwan. The information bias would be ameliorated by regular clinical assessment. Besides, it is so far the largest real-world HBV-HCC cohort that investigated the viral kinetics of HBV during ICI treatment. Second, the serum level of HBsAg was not frequently rechecked in our patients during ICI treatment according to the reimbursement criteria of NHI in Taiwan. Third, no ideal control arm could be designed in this study. For sorafenib-failed HCC patients, sorafenib on treatment group could not serve as control. For patients who received ICIs as first-line therapy, sorafenib on treatment patients were also not suitable as a control group because the pharmacologic difference between sorafenib and ICIs. The ideal control group should be HCC patients with serum HBV DNA >100 IU/mL who received ICI treatment after sorafenib failure or intolerance but was prohibited to use NUCs throughout the treatment. However, it is not ethical for this design. Forth, the risk of HBV reactivation might be underestimated because some patients only received very few cycles of ICIs and experienced early tumor progression and mortality. In our study, 15 did not have follow-up HBV DNA values; including nine patients did not experience ALT elevation during ICIs treatment; and the other six patients met early tumor progression. The risk of HBV reactivation might be underestimated in these cases. Finally, the antiviral agents prescribed to our patients were not consistent. Most of the patients (72.2%) on antiviral therapy were prescribed with entecavir; and the others used tenofovir disoproxil fumarate or tenofovir alafenamide. However, all these NUCs belong to high potency antiviral agents that could suppress high HBV viral loads.43 44
In conclusion, no patients on antiviral therapy (regardless of HBV viral load at baseline) developed HBV reactivation, and one out of six not receiving NUCs had HBV reactivation. Patients with HBV viral load higher than 100 IU/mL could safely receive ICI treatment under the protection of NUCs treatment.
Footnotes
Contributors: Study concept and design: Y-HH; Acquisition of data: P-CL, YC, M-HC and K-HL; Analysis and interpretation of data: P-CL and M-CH; Drafting of manuscript: P-CL and Y-HH; Critical revision: M-CH and YC; Study supervision: YC and Y-HH.
Funding: The work was partly supported by the Taipei Veterans General Hospital, Taipei, Taiwan (grant numbers V109C-048); and Ministry of Science and Technology, Taiwan (grant numbers MOST108-2628-B-075-006).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: This study was approved by the Institutional Review Board of the Taipei Veterans General Hospital (IRB numbers: 2017-09-007CC, 2019-07-007AC and 2019-08-006B).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplementary information. The data of this study were obtained from Taipei Veterans General Hospital, Taiwan which were stored in a computer in the office that required a password to enter in. Only the principal investigator and coprincipal investigator had the password to get the data for investigation.
References
- 1.Global Burden of Disease Liver Cancer Collaboration, Akinyemiju T, Abera S, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the global burden of disease study 2015. JAMA Oncol 2017;3:1683–91. 10.1001/jamaoncol.2017.3055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2018;69:182–236. 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 3.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98. 10.1007/s12072-015-9675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Omata M, Cheng A-L, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317–70. 10.1007/s12072-017-9799-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–502. 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–52. 10.1016/S1470-2045(18)30351-6 [DOI] [PubMed] [Google Scholar]
- 7.Finn RS, Ryoo B-Y, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol 2020;38:193–202. 10.1200/JCO.19.01307 [DOI] [PubMed] [Google Scholar]
- 8.Hoofnagle JH. Reactivation of hepatitis B. Hepatology 2009;49:S156–65. 10.1002/hep.22945 [DOI] [PubMed] [Google Scholar]
- 9.Huang Y-H, Hsiao L-T, Hong Y-C, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol 2013;31:2765–72. 10.1200/JCO.2012.48.5938 [DOI] [PubMed] [Google Scholar]
- 10.Chen M-H, Chen M-H, Liu C-Y, et al. Hepatitis B virus reactivation in rheumatoid arthritis patients undergoing biologics treatment. J Infect Dis 2017;215:566–73. 10.1093/infdis/jiw606 [DOI] [PubMed] [Google Scholar]
- 11.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–99. 10.1002/hep.29800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682–7. 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- 13.Bertoletti A, Ferrari C. Adaptive immunity in HBV infection. J Hepatol 2016;64:S71–83. 10.1016/j.jhep.2016.01.026 [DOI] [PubMed] [Google Scholar]
- 14.Maier H, Isogawa M, Freeman GJ, et al. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J Immunol 2007;178:2714–20. 10.4049/jimmunol.178.5.2714 [DOI] [PubMed] [Google Scholar]
- 15.Zhang E, Zhang X, Liu J, et al. The expression of PD-1 ligands and their involvement in regulation of T cell functions in acute and chronic woodchuck hepatitis virus infection. PLoS One 2011;6:e26196. 10.1371/journal.pone.0026196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisicaro P, Valdatta C, Massari M, et al. Combined blockade of programmed death-1 and activation of CD137 increase responses of human liver T cells against HBV, but not HCV. Gastroenterology 2012;143:1576–85. 10.1053/j.gastro.2012.08.041 [DOI] [PubMed] [Google Scholar]
- 17.Lake AC. Hepatitis B reactivation in a long-term nonprogressor due to nivolumab therapy. AIDS 2017;31:2115–8. 10.1097/QAD.0000000000001599 [DOI] [PubMed] [Google Scholar]
- 18.Pandey A, Ezemenari S, Liaukovich M, et al. A rare case of Pembrolizumab-Induced reactivation of hepatitis B. Case Rep Oncol Med 2018;2018:5985131. 10.1155/2018/5985131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy KR, Beavers KL, Hammond SP, et al. American gastroenterological association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:215–9. quiz e216-217. 10.1053/j.gastro.2014.10.039 [DOI] [PubMed] [Google Scholar]
- 20.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358–80. 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 21.Lee P-C, Chen Y-T, Chao Y, et al. Validation of the albumin-bilirubin grade-based integrated model as a predictor for sorafenib-failed hepatocellular carcinoma. Liver Int 2018;38:321–30. 10.1111/liv.13527 [DOI] [PubMed] [Google Scholar]
- 22.Lee P-C, Chao Y, Chen M-H, et al. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers 2020;12:E182. 10.3390/cancers12010182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 24.Cheng A-L, Hsiung CA, Su I-J, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology 2003;37:1320–8. 10.1053/jhep.2003.50220 [DOI] [PubMed] [Google Scholar]
- 25.Hsu C, Hsiung CA, Su I-J, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin's lymphoma: a randomized trial. Hepatology 2008;47:844–53. 10.1002/hep.22106 [DOI] [PubMed] [Google Scholar]
- 26.Hwang JP, Somerfield MR, Alston-Johnson DE, et al. Hepatitis B virus screening for patients with cancer before therapy: American Society of clinical oncology provisional clinical opinion update. J Clin Oncol 2015;33:2212–20. 10.1200/JCO.2015.61.3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 2015;33:550–8. 10.1200/JCO.2014.57.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang JW. Hepatitis B virus reactivation in patients with hepatocellular carcinoma undergoing anti-cancer therapy. World J Gastroenterol 2014;20:7675–85. 10.3748/wjg.v20.i24.7675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeo W, Chan PK, Zhong S, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol 2000;62:299–307. [DOI] [PubMed] [Google Scholar]
- 30.Yeo W, Johnson PJ. Diagnosis, prevention and management of hepatitis B virus reactivation during anticancer therapy. Hepatology 2006;43:209–20. 10.1002/hep.21051 [DOI] [PubMed] [Google Scholar]
- 31.Yeo W, Lam KC, Zee B, et al. Hepatitis B reactivation in patients with hepatocellular carcinoma undergoing systemic chemotherapy. Ann Oncol 2004;15:1661–6. 10.1093/annonc/mdh430 [DOI] [PubMed] [Google Scholar]
- 32.Jang JW, Choi JY, Bae SH, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology 2006;43:233–40. 10.1002/hep.21024 [DOI] [PubMed] [Google Scholar]
- 33.Huang L, Li J, Lau WY, et al. Perioperative reactivation of hepatitis B virus replication in patients undergoing partial hepatectomy for hepatocellular carcinoma. J Gastroenterol Hepatol 2012;27:158–64. 10.1111/j.1440-1746.2011.06888.x [DOI] [PubMed] [Google Scholar]
- 34.Lao X-M, Luo G, Ye L-T, et al. Effects of antiviral therapy on hepatitis B virus reactivation and liver function after resection or chemoembolization for hepatocellular carcinoma. Liver Int 2013;33:595–604. 10.1111/liv.12112 [DOI] [PubMed] [Google Scholar]
- 35.Huang G, Lai ECH, Lau WY, et al. Posthepatectomy HBV reactivation in hepatitis B-related hepatocellular carcinoma influences postoperative survival in patients with preoperative low HBV-DNA levels. Ann Surg 2013;257:490–505. 10.1097/SLA.0b013e318262b218 [DOI] [PubMed] [Google Scholar]
- 36.Boni C, Fisicaro P, Valdatta C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol 2007;81:4215–25. 10.1128/JVI.02844-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fisicaro P, Valdatta C, Massari M, et al. Antiviral intrahepatic T-cell responses can be restored by blocking programmed death-1 pathway in chronic hepatitis B. Gastroenterology 2010;138:682–93. 693.e1-4. 10.1053/j.gastro.2009.09.052 [DOI] [PubMed] [Google Scholar]
- 38.Gane E, Verdon DJ, Brooks AE, et al. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: a pilot study. J Hepatol 2019;71:900–7. 10.1016/j.jhep.2019.06.028 [DOI] [PubMed] [Google Scholar]
- 39.Yau T, Hsu C, Kim T-Y, et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J Hepatol 2019;71:543–52. 10.1016/j.jhep.2019.05.014 [DOI] [PubMed] [Google Scholar]
- 40.Koksal AS, Toka B, Eminler AT, et al. HBV-related acute hepatitis due to immune checkpoint inhibitors in a patient with malignant melanoma. Ann Oncol 2017;28:3103–4. 10.1093/annonc/mdx502 [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Zhou Y, Chen C, et al. Hepatitis B virus reactivation in cancer patients with positive hepatitis B surface antigen undergoing PD-1 inhibition. J Immunother Cancer 2019;7:322. 10.1186/s40425-019-0808-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang JP, Feld JJ, Hammond SP, et al. Hepatitis B virus screening and management for patients with cancer prior to therapy: ASCO provisional clinical opinion update. J Clin Oncol 2020:JCO.20.01757. 10.1200/JCO.20.01757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu I-T, Hu T-H, Hung C-H, et al. Comparison of the efficacy and safety of entecavir and tenofovir in nucleos(t)ide analogue-naive chronic hepatitis B patients with high viraemia: a retrospective cohort study. Clin Microbiol Infect 2017;23:464–9. 10.1016/j.cmi.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 44.Kim SU, Seo YS, Lee HA, et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea. J Hepatol 2019;71:456–64. 10.1016/j.jhep.2019.03.028 [DOI] [PubMed] [Google Scholar]