Abstract
Progressive supranuclear palsy (PSP) is a complex clinicopathologic disease with no current cure or disease modulating therapies that can only be definitively confirmed at autopsy. Growing understanding of the phenotypic diversity of PSP has led to expanded clinical criteria and new insights into etiopathogenesis that coupled with improved in vivo biomarkers makes increased access to current clinical trials possible. Current standard-of-care treatment of PSP is multidisciplinary, supportive and symptomatic, and several trials of potentially disease modulating agents have already been completed with disappointing results. Current ongoing clinical trials target the abnormal aggregation of tau through a variety of mechanisms including immunotherapy and gene therapy offer a more direct method of treatment. Here we review PSP clinicopathologic correlations, in vivo biomarkers including MRI, PET, and CSF biomarkers. We additionally review current pharmacologic and non-pharmacologic methods of treatment, prior and ongoing clinical trials in PSP. Newly expanded clinical criteria and improved specific biomarkers will aid in identifying patients with PSP earlier and more accurately and expand access to these potentially beneficial clinical trials.
Keywords: Tauopathy, progressive supranuclear palsy, treatment, immunotherapy, gene therapy
Introduction
The clinicopathological syndrome of Progressive supranuclear palsy (PSP) was originally described in 1964 by Drs. Steele, Richardson, and Olszewski where they detailed a series of patients with postural instability, ocular motor abnormalities, facial and cervical dystonia, dementia, and other features [1]. This seminal work also detailed the initial neuropathologic findings including argyrophyllic globose and flame-shaped inclusions in both the gray and white matter throughout brainstem, subcortical and neocortical regions and accompanying neuronal loss and white matter degeneration. Further study using immunohistochemistry ultimately revealed that these inclusions were accumulations of the tau protein affecting neurons, astrocytes, and glia in a variety of morphologies [2]. Tau is a microtubule associated protein which contributes to the stability of the axonal cytoskeleton. Due to alternative splicing of the of microtubule binding site domains E2, E3, and E10, six tau isoforms exist, either with 4 repeated domains (4R, which includes the E10 region), or 3 repeated domains (3R, which excludes E10) [3]. In Alzheimer’s disease, tau pathology exists in a fairly even 3R/4R mix in paired helical filament conformation [4–6] which occurs in combination with amyloid-β plaque deposits. In contrast, PSP is a ‘pure tauopathy’, where tau accumulations composed primarily of 4R tau species are the pathogenic lesions. For this reason, PSP is often targeted for trials for anti-tau therapeutics. PSP and corticobasal degeneration (CBD), are both 4R tauopathies where tau species are arranged in straight filament conformations [7, 8]. The differences in conformations are due to differing orientations of the C-shaped tau subunits that compose them, with 3R/4R tau subunits being directed towards each other resulting in the helical conformation, whereas in 4R tauopathies, the C-shaped subunits are positioned back to back [9]. While PSP and CBD are both 4R tauopathies, the pathological features of PSP are typically tufted astrocytes and globose neurofibrillary tangles, whereas CBD has astrocytic plaques and ballooned pale neurons with thready neuronal tau inclusions that affect cortical regions more severely than subcortical regions and in a distinct conformation from what is seen in PSP [10, 11].
The prevalence of the classic Richardson syndrome presentation of PSP is approximately 6/100,000, with average age of onset in the mid-60s and disease duration of approximately 6 years [12–15]. However, it has become increasingly recognized that multiple clinical phenotypes aside from the originally described Richardson syndrome phenotype may result from PSP pathology. PSP pathology may be found in patients with parkinsonism mimicking Parkinson’s disease (PSP-P), frontotemporal dementia (PSP-F), and corticobasal syndrome (PSP-CBS), and others [16–18]. The growing recognition of the clinical spectrum of PSP pathology has resulted in an expanded research criteria for the diagnosis of PSP which incorporates these clinical phenotypes [19]. Consequently, more recent age-adjusted prevalence estimates in Europe have increased to 8.8–10.8/100,000 patients [13, 20], and in Yonogo Japan, PSP age-adjusted prevalence increased from 5.8/100,000 patients in 1999 to 17/100,000 patients in 2010 [21, 22]. This recognition has also increased the need for specific biomarkers to diagnose patients early during life; advancements have progressed in MRI, PET, and biofluid biomarkers in PSP. Despite these advances, current therapies for PSP remain symptomatic and disease-modulating medications remain elusive despite extensive efforts. Current strategies are focused on targeting the tau protein by different mechanisms including immunotherapy and gene therapy. Here, we review the clinicopathological complexity of PSP, etiopathogenesis, and emerging biomarkers and well as a review of past and current clinical trials.
Clinicopathologic Complexity of PSP
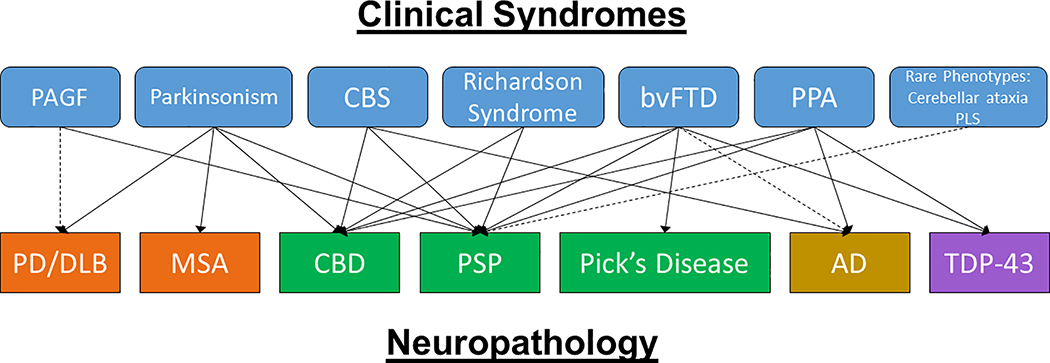
While several early clinical criteria exist [23–26] the first criteria based on a large autopsy-confirmed series and consensus of experts was performed by Litvan et al. in 1996 (the NINDS-SPSP criteria) which outlined the core clinical features of what is currently known as the PSP-Richardson syndrome (PSP-RS) of a gradual progressive disorder affecting patients over 40 years old with a vertical supranuclear gaze palsy or postural instability and falls within the first year of onset [27]. Having both a vertical supranuclear palsy and postural instability is indicative of clinically probable PSP whereas having either an isolated vertical gaze palsy or having slowed saccades with postural instability is indicative of clinically possible PSP [27]. Pathologic confirmation remains necessary for a ‘definite’ diagnosis of PSP. The clinical entity of PSP-RS has a high probability of demonstrating neuropathologic PSP at autopsy, but many other clinical phenotypes may harbor PSP pathology at autopsy (Figure 1) [17, 27, 28]. Up to one third of PSP patients have a presentation initially marked by parkinsonism (PSP-P), which can have asymmetric akinetic rigid features, resting tremor, and even moderate levodopa responsiveness early in the disease with more classic Richardson syndrome developing only years later [15, 17, 18, 29–31]. Patients with PSP-P have a significantly longer survival compared patients with a classic PSP-RS presentation [17, 18, 31, 32] and progression. Other patients may present with frontotemporal dementia features of prominent apathy and/or disinhibition (PSP-F) [33–35], or a progressive non-fluent aphasia with apraxia of speech (PSP-SL) [36–38]. Up to one third of patients with a corticobasal syndrome presentation with limb apraxia, cortical sensory deficits, alien limb phenomena, dystonia and myoclonus, may harbor PSP pathology at autopsy rather than corticobasal degeneration (PSP-CBS) [10, 39–41]. Other, more rare presentations of PSP have also been described (PSP-PGF: PSP with progressive gait freezing [42, 43], PSP-OM: PSP with exclusive ocular motor dysfunction [17, 27], PSP-PLS: PSP with primary lateral sclerosis [44, 45], PSP-C: PSP with a cerebellar ataxia phenotype [46, 47]). As patients progress, they are more likely to begin to exhibit core features of PSP-RS including supranuclear gaze palsy and postural instability, but these features may be delayed by several years making early and accurate diagnosis a continued challenge [17]. The International Parkinson and Movement Disorder’s Society has published recent diagnostic criteria (MDS-PSP) which acknowledges the wide array of clinical symptoms and signs that may be associated with PSP pathology [19]. These criteria are more sensitive but less specific than the NINDS-SPSP criteria and can lead to diagnosing multiple overlapping phenotypes in the same subject [48, 49]. While the MDS-PSP criteria includes a new definition of the PSP-P phenotype, this criteria needs refinement in view of inclusion of PSP-RS in addition to PSP-P[50]. Thus, subsequent rules (Multiple Allocation eXtinction: MAX rules) were recently developed to improve the assignment of individual phenotypes in clinical practice and research settings [49]. Using MAX rules has improved diagnostic overlap of PSP-RS and PSP-P, but unfortunately may still fail to disentangle 40% of these patients [50].
Figure 1: Clinicopathologic Complexity of PSP.
PSP neuropathology may be associated with a number of different clinical phenotypes (shown in light blue boxes with rounded edges). Other neuropathologies that may also present with these clinical phenotypes are shown in the lower row. Green: Tau, Orange: alpha-synuclein, Yellow: Alzheimer’s disease, Purple: TDP-43. Abbreviations: PAGF: pure akinesia freezing of gait, CBS: corticobasal syndrome, bvFTD: behavioral variant FTD, PPA: primary progressive aphasia, PLS: primary lateral sclerosis, PD/DLB: Parkinson’s Disease/dementia with Lewy bodies, MSA: multiple systems atrophy, CBD: corticobasal degeneration, PSP: progressive supranuclear palsy, AD: Alzheimer’s disease, TDP-43: TAR DNA binding protein 43.
The cardinal neuropathologic features of PSP at autopsy include abnormal accumulations of tau in the forms of tufted astrocytes [51] and globose neurofibrillary tangles in grey matter and coiled bodies in oligodendrocytes in white matter [52]. As stated previously, in PSP the accumulated tau is mostly of a 4R variety and is typically phosphorylated [53, 54], often acetylated [55], and thioflavin-S positive [56]. The globus pallidus, subthalamic nucleus, substantia nigra, and dentate nucleus of the cerebellum are core regions affected [2]. Frontal, temporal, and parietal cortices may also show disease [57] and patients who exhibit more cortically localizing clinical signs have been described to have higher burdens of corresponding cortical tau pathology [58, 59] (figure 2). Pathological subtypes based on the relative distribution of tau pathology and its relation to clinical phenotypes have been proposed [60]. As opposed to corticobasal degeneration (CBD) which is also a 4R tauopathy marked by astrocytic plaque pathology where tau accumulates in distal astrocytic processes which, the tufted astrocytes of PSP have a radial distribution of tau which affects astrocyte cell bodies and more proximal processes (Figure 2) [60].
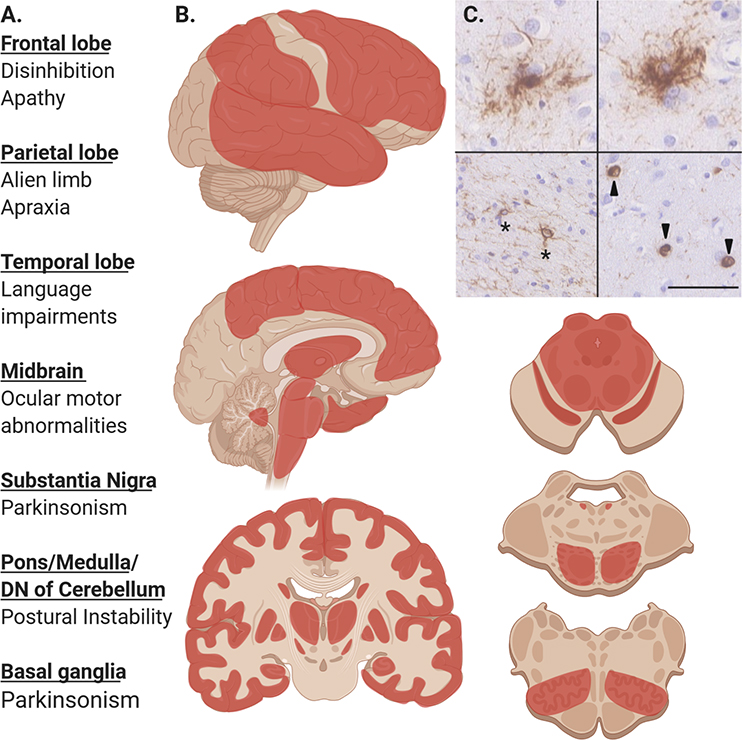
Figure 2: Pathologic Distribution and Clinical Correlations in PSP.
A. Common clinical features of PSP associated with pathology in these affected regions.
B. Regions in red commonly affected by PSP tau pathology and gliosis including, frontal, temporal, parietal lobes, globus pallidus, putamen, caudate, subthalamic nucleus, hippocampus, midbrain tectum and tegmentum, substantia nigra, pontine base and locus ceruleus, inferior olivary nucleus. Darker areas or red are more commonly affected. Certain phenotypes are more likely to exhibit pathology in specific areas (i.e. PSP-SL and temporal lobe pathology and PSP-CBS with parietal lobe pathology)
C. Characteristic microscopic lesions seen in PSP after immunohistochemical staining for phospho-tau with AT8 antibody. Upper row shows two tufted astrocytes, bottom left showing coiled bodies (asterisk), bottom right showing globose neurofibrillary tangles. Scale bar is 50 μm.
Etiopathogenesis
Environmental Factors
While no single cause of PSP has been identified, a number of environmental and genetic associations have been investigated. The ENGENE-PSP study found that lower educational attainment, exposure to well water and industrial wastes, and firearm use was related to higher risk of developing PSP [61, 62]. A cluster of PSP patients was observed in northern France in an area of high industrial waste contamination that also contained heavy metals and an independent study also documented that occupational exposure to heavy metals was associated with risk of developing PSP [61, 63]. Consumption of high levels of annonacin, a mitochondrial complex 1 inhibitor found in the tropical fruit pawpaw was associated with developing PSP or other atypical parkinsonism in studies in Guadeloupe in the Caribbean [64, 65]. There may be a slight male predominance within PSP patients [18, 24], and one study documented that increased estrogen exposure in women may be associated with lower likelihood of developing PSP [66].
Genetics
Genetic mutations in the MAPT gene have been described leading to PSP [67–69] as well as frontotemporal dementia, FTLD with parkinsonism, primary progressive aphasia and CBD [70]. The H1 haplotype specifically elevates 5.6 times the risk for developing PSP, which is comparable to the ApoE ε3/ε4 risk for developing Alzheimer disease [19, 71]. Interestingly, the H1/H1 haplotype is more common in PSP-RS than PSP-P [72]. A genome wide association study in a large pathologically-validated study confirmed the MAPT variants and the H1 haplotype as associated with PSP, and also identified other gene loci including MOBP, STX6, and EIF2AK3 [73]. MOBP, which encodes for myelin basic protein, is also implicated in CBD and highlights to potential importance of white matter in these conditions [74]. STX6 encodes for a SNARE protein implicated in fusing vesicles in the Golgi network and endosomal structures [75]. EIF2AK3 encodes for a protein responsible for inhibiting protein synthesis in the face of excess endoplasmic reticulum stress [76, 77]. These have been validated in a second GWAS study which also identified SLCO1A2, which is involved in ion transport, and DUSP10, which is involved in tau trafficking, as other genes of interest requiring further study [78].
Oxidative Stress, Mitochondrial Dysfunction, and Inflammation
In PSP, as with other neurodegenerative diseases, mitochondrial dysfunction and oxidative stress has been demonstrated in vitro models and in human tissue [79–82]. Mitochondrial enzymatic activity is decreased and lipid peroxidation is increased in PSP which leads to excessive oxidative stress [81–84]. Interestingly, ATP production is decreased in muscle tissue in PSP patients as well as PSP cybrids [79, 85–87]. Elevated oxidative stress, mitochondrial dysfunction and neurodegeneration, leads to inflammation and in PSP, higher levels of the inflammatory cytokine, IL1β is seen in PSP affected brain regions and correlates with levels of microglial activation [88]. Other studies have reported that microglial activation correlates with tau deposition [89] and higher levels of pro-inflammatory cytokines can be demonstrated in the CSF of PSP patients when compared to Parkinson’s disease patients [90]. Superoxide dismutase and glutathione, essential antioxidants, are often seen to be elevated in PSP brain tissue, likely sources of defense [80, 91]. Activated microglia can be visualized using positron emission tomography in PSP which will be discussed below [92]. Such observations have led to disease modifying trials using anti-inflammatory or anti-oxidant agents. CoQ10 showed improvements in cerebral metabolism as well as improvements in the total PSP-Rating Scale scores and frontal assessment battery in a small six week trial[93]. The short duration of this trial made differentiating symptomatic relief from disease modification difficult. A follow up year-long study of CoQ10 showed a nearly significant difference in the progression of the PSP Rating scale compared to placebo (p=0.07), but also had a high drop-out rate of patients with more severe disease making interpretation of results difficult [94]. Regrettably, trials of riluzole and rasagiline have failed to show meaningful benefit in large trials [95, 96].
Prion-like Spread of Tau
Of note, more recent data suggests that abnormally fibrillated tau is capable of acting as a template to further induce misfolding of normal monomeric tau leading to increasing disease spread in a ‘prion-like’ manner. In vivo animal studies using preformed fibrils [97, 98], human diseased brain homogenates [99], and other techniques [100, 101] have shown distal spread of tau pathology via trans-synaptic spread [102, 103]. There may be specific ‘strains’ of tau capable of seeding unique pathologies [51, 100, 104]. In fact, the recent use of cryo-electron microscopy technology to study several tauopathies shows that the molecular structure of tau varies throughout tauopathies which may in part confer strain-specific properties in these conditions [4, 105, 106]. The molecular structure of CBD related tau has been identified [11] but similar studies for PSP related tau have not been published.
Biomarkers
Magnetic Resonance Imaging
Structural neuroimaging biomarkers in PSP include the well described ‘hummingbird sign’ [107], ‘morning glory sign’ [108], or Mickey-Mouse sign [109] all of which result from midbrain atrophy. One study where features of ante mortem MRIs from 48 pathologically-confirmed PSP and MSA cases were compared, 16/22 (73%) of PSP cases could be correctly identified by a radiologist subjectively identifying the ‘hummingbird sign’, with 100% specificity but only 69% sensitivity [109]. Studies that have relied on the qualitative identification of atrophy patterns document high specificity with lower sensitivity, likely because these changes are visually more apparent in late disease[107–109]. Studies making use of quantitative measurements of the midbrain, superior cerebellar peduncles and other brainstem structures, have shown much higher sensitivity and specificity [110–114]. Perhaps the best studied quantitative MRI signature is the magnetic resonance parkinsonism index (MRPI), which has documented >80% sensitivity and specificity differentiating PSP from non-PSP patients and predicting the occurrence of a supranuclear gaze palsy in PSP-P patients [111, 112, 115]. A subsequent version has been reported to predict clinical evolution of PSP-P in patients initially diagnosed with PD, raising the possibility of its utility to differentiate PD from PSP in early disease when clinical diagnosis is often most ambiguous [116, 117].
Positron Emission Tomography
Several tracers are under development that bind to the tau protein including 18F-5105, 18F-FDDNP, 18F-THK523, 11C-PBB3, and others [118]. 18F-Flortaucipir (formerly AV-1451 and T807) is the most researched tau tracer to date and binds to paired helical filaments in 3R/4R tauopathies such as AD [119] and exhibits expected retention patterns in both amnestic AD [120, 121] and non-amnestic variants [122, 123]. However, retention appears to be less robust in 4R tauopathies [119, 124, 125]. While group-wise differences can still be demonstrated differentiating PSP from healthy controls largely due to increased retention in the basal ganglia and nigral regions [120, 126–128], individual patient-level distinctions remain difficult because of significant overlap and the ligand is not expected to be of use diagnosis PSP subjects in early stages of the disease. PET tracers targeting activated microglia (11C-(R) PK11195) may offer methods to assess inflammation associated with neurodegeneration in PSP and other related diseases [92, 129]
Biofluids
CSF biomarkers for PSP are still under development. Tau fragments, including measures of total tau (t-tau) and phosphorylated tau (p-tau) have been more extensively studied in Alzheimer’s disease [130], but these tau species tend not to be reliably elevated in PSP [7, 131, 132]. One study reported that a ratio of certain tau fragments may aid in distinguishing PSP from healthy controls and different neurodegenerative diseases [133] but the findings were not easily replicated [134]. CSF neurofilament light chain (NfL), an intermediate filament and non-specific measure of neuronal injury [135], shows elevation in PSP and other atypical parkinsonian syndromes [132, 136–139]. New single molecular arrays (SIMoA) are capable of detecting NfL on the ng/L levels, making blood based assays possible. Serum NfL correlates tightly with CSF NfL concentrations [140] and higher baseline levels of serum NfL has been associated with worse clinical and radiologic outcomes in PSP [141]. Thus, serum NfL could be used as secondary outcome measures in future therapeutic trials. Real time quaking induced conversion (RT-QuIC), which was originally pioneered in Creutzfeldt-Jakob disease, makes use of the ability of abnormally aggregated proteins to act as a template to seed further aggregation of monomeric proteins [142]. RT-QuIC are very promising in the diagnosis of synucleinopathies (Parkinson’s disease, dementia with Lewy bodies, multiple systems atrophy) [143–145] and is also expanding into tauopathies including PSP [146, 147].
Neurophysiologic Markers
The slowness of vertical eye movements that is a clinical hallmark of PSP can be demonstrated through electro-oculogram, a version of electromyogram [148] and more recently small studies of non-invasive computerized eye tracking software can reliably differentiate PSP from non-PSP cases using a variety of eye movement features [149, 150]. Spontaneous blink rate is decreased in PSP as expected, and in comparative neurophysiologic studies, blink rate is much lower in PSP than PD or other disorders [151]. Early recovery and enhanced excitability of the blink reflex has been shown as well [152]. Auditory startle reflex is severely decreased in PSP, likely related to damage to the reticulospinal system [153, 154]. Abnormal autogenic inhibition of spinal interneuron circuits has been described as well [155].
Current Therapies
Symptomatic Pharmacologic Therapies
Current pharmacologic therapies for PSP are symptomatic, and tend to show mild to moderate efficacy. Levodopa preparations may be used to treat functionally-impairing bradykinesia and rigidity. In one retrospective study of pathologically confirmed PSP patients, 32% of cases showed a >30% improvement in the Unified Parkinson’s Disease Rating Scale and 4% of cases showed levodopa induced dyskinesias [18]. Other studies have documented similar response rates[156–159]; however, levodopa responses are often milder than what is seen in PD and require higher doses of levodopa to achieve and can often wane over time [26, 160, 161]. It is recommend titrating up to 1.0 gm/day and continuing this dose for at least a month to attempt to achieve benefits before weaning. Marked and prolonged improvement with levodopa therapy is considered an exclusionary criteria for a diagnosis of PSP and makes a diagnosis of Parkinson’s disease more likely. Dopamine agonists have also been trialed in PSP but are generally less effective than levodopa and carry a greater likelihood of causing side effects [157, 162, 163]. A few studies have documented improvement in features of bradykinesia, rigidity, and dystonia with the use of amantadine in PSP but side effects have been also been reported in nearly half of patients treated with this medication, ranging in severity from leg edema and livedo reticularis to hallucinations and worsened cognitive impairments [157, 164–166]. If dose-limiting side effects do occur, we recommend a slow wean of medications, removing 100 mg per week, as a withdrawal syndrome with delirium from abrupt cessation of amantadine has been described [167, 168]. Zolpidem was reported to offer mild improvement of ocular motor deficits and saccadic speed, but this has not been confirmed in other studies [169–171]. Ophthalmic lubricants are useful to treat dry eyes. Sunglasses are also useful for the photosensitivity. Prism glasses may be employed to improve the double vision due to decreased convergence, but when not useful, alternating eye patches may be employed. Problems with sleep initiation and sleep maintenance are common in PSP and there have been no major studies of pharmacologic interventions, but treatment can be attempted with melatonin, clonazepam or trazodone [172–174]. Constipation may be managed with dietary changes, agents that accelerate bowel movements, gentle laxatives, or by increasing fluid secretion. For depression, tricyclic antidepressants, selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors may be employed. However, they do not improve the apathy that in PSP is often prevalent. Amitriptyline, a TCA, in particular has been reported to improve depression in PSP patients and has improved motor parkinsonism in case reports [157, 175, 176]. For cognition, while donepezil may show mild selective benefits on cognition in PSP, currently this medication is not recommended due to potential deleterious effects on gait and dysphagia [177]. Botulinum toxin for blepharospasm, eyelid opening apraxia, retrocollis, or sialorrhea may be considered but must be weighed against the potential to cause side effects including worsening dysphagia [178–181]. Sublingual administration of atropine 1% drops can be considered but carries a significant risk of causing cognitive and urinary side effects if not dosed carefully [182]. Urinary urgency and frequency is a common symptom in PSP and can be treated with medications which are less likely to cross the blood brain barrier including mirabegron and solifenacin. Pseudobulbar affect, abrupt crying or laughing episodes that are not always mood-congruous or proportional to stimuli, can be treated effectively with dextromethorphan-quinidine or antidepressants [183]. Agitation may occur in PSP. Seroquel or clozapine could be used as pharmacologic treatments to augment non-pharmacological interventions. However, there are no therapeutic trials showing beneficial effects in PSP, but clozapine as well as pimavanserin have been shown to improve psychosis in PD, although they are rarely used in PSP. Clozapine therapy must be accompanied by frequent blood monitoring to avoid the rare but life-threatening side effect of agranulocytosis. Other neuroleptics and dopamine blocking anti-emetics should be avoided in PSP given the possibility of worsening parkinsonism. There are no formal studies of medical marijuana or cannabidiols in PSP, but studies in other conditions have shown improvements in sleep, pain, anxiety, and spasticity which are common problems in PSP, so we await formal studies in PSP to guide utility [184–187].
Non-pharmacologic therapies
Patients with PSP benefit from multidisciplinary non-pharmacological care. Physical therapy improves function and decreases falls [188, 189]. Use of weighted walkers are helpful to improve patient’s safe gait and independence. Speech therapy may help with coping strategies for vocal changes, techniques for communication, and safe eating and drinking. When difficulties with swallowing liquids are identified, a modified barium swallow evaluations can diagnose the extent of the problem and help identify proper compensation techniques or diets. Occupational therapists can do safety inspection of the homes. Social workers and palliative care consultants can aid in the management of the PSP patients and families including stress, nursing home placement, end of life care and decision making which positively affects patients and families quality of life [190]. Therefore, PSP patients and families benefit from having a multidisciplinary team knowledgeable in the management of these patients.
Clinical Trials
Drug development for PSP disease modulating trials has focused on inhibiting post translational modifications of tau, enhancing immune mediated clearing, stabilizing microtubules, or reducing levels of expression through gene therapies.
Tau post-translational modifications: Phosphorylation and Acetylation, and Others
In PSP aggregated tau is hyper-phosphorylated [191]; therefore, kinases which phosphorylate tau, including GSK 3β, have been examined as potential therapeutic targets [192]. Both valproic acid and lithium are GSK 3β inhibitors which showed some promise in animal models [193–195]. Valproic acid did not result in significant improvements in human trials and the lithium trial was stopped early because of poor tolerability (NCT00385710, NCT00703677) [196]. Tideglusib is a small molecule inhibitor of GSK 3β which failed to show significant clinical differences in the PSP-Rating Scale between treated patients and patients receiving placebo in a multicenter, randomized, double-blind, placebo-controlled trial [197]. However, in a subset of patients with MRIs, less brain atrophy was observed in treated patients than controls in parietal and occipital cortical regions [198]. These areas are not typically affected by tau pathology in PSP as are the frontal lobes, basal ganglia, or brainstem structures [197, 198]. Possible explanations for this observation include better quality of volumetric MRI data from cortical areas versus subcortical and brainstem regions, variable expression of GSK 3β throughout the brain, or selection bias of the included MRI subgroup [198].
CDK5 is another kinase which phosphorylates tau and inhibitor molecules are in development (NCT04253132). Salsalate inhibits tau acetylation which may alleviate tau pathology as well [199] and is undergoing a phase I pilot study in PSP (NCT02422485). O-GlcNAc modification, which is involved in intracellular tau trafficking, and caspase-mediated cleavage are other potential therapeutic targets [200–202]. Most recently, the company Retrotope has obtained orphan drug designation for a stabilized fatty acid compound to prevent lipid peroxidation for investigation in PSP although no trials in humans have been conducted yet [203].
Microtubule-stabilizing agents
A neuropeptide with neuroprotective and microtubule stabilizing properties, davunetide, which looked promising in animal models [204] was studied in a multicenter phase IIb/III trial of over 300 patients with PSP but regrettably failed to show clinical efficacy on all endpoints [205]. TPI-287 is a taxane derivative that stabilizes microtubules, is able to cross the blood brain barrier, and may decrease cell proliferation in cancer [206]. A preliminary study resulted in some anaphylactoid reactions at the higher dosing arm without significant clinical improvements and drug development on this agent is likely stopped [207]. A separate compound, epothilone D, which also stabilizes microtubules may be of interest, but is still early in development [208].
Tau Immunotherapy
Recent in vitro and in vivo experiments have shown that abnormally folded proteins may be capable of inducing misfolding in normal native proteins and creating spread of pathology through ‘prion-like’ templating [99, 103, 209, 210]. Brain homogenates from human PSP and CBD induce tau inclusions in mice that spread well beyond the injection sites [99, 211]. These aggregations may be transported within the cellular structure and be transferred from cell to cell in a variety of mechanisms [212, 213]. Such experiments have also suggested different therapeutic strategies in PSP including tau-directed immunotherapy to promote clearance of aggregates before further toxicity can occur.
Again, a number of studies in animal models using different immunization strategies, showed reduction of tau pathology with favorable safety profiles [214–219]. Several tau directed passive immunotherapy trials in Alzheimer’s disease have been performed with limited success (for a recent review see [220]). In PSP, Biogen’s antibody product BIIB092 (Gosuranemab), directed against N-terminal fragments of extracellular tau [221], showed a favorable safety profile in a phase I trial and lowered CSF tau levels as a measure of target engagement [222] [223]. However, a phase II study (PASSPORT NCT03068468) was halted as the trial failed to show differences in the primary and secondary endpoints [224]. Abbvie also tested a monoclonal antibody product, ABBV-8E12 with favorable phase I safety results [225] and adequate, dose ascending CSF penetration [226]; however, a phase II study in PSP was recently stopped after failing a futility analysis. Other antibody products are in development (NCT04185415).
A number of challenges and questions remain in the field of immunotherapy and neurodegeneration. Selecting the right epitope target may be challenging as antibodies can be raised to target different regions of tau [227, 228], tau species with specific post-translational modifications [229, 230], or oligomers or specific conformations [120, 228, 231–234]. Ensuring adequate penetration of the blood brain barrier of peripherally administered products is a challenge and several different strategies including viral vector delivery, ultrasound with microbubbles, or antibodies tagged to small molecules are being explored [120]. Development of these and other strategies are critical given that brain penetration of intravenously administered IgG antibodies is typically cited as 0.1–1% [223, 235–239]. Most of the products to date have targeted predominantly extracellular tau, which likely constitutes ghost pathology from deceased cells but may be beneficial in intercepting the transmission of misfolded toxic species from cell to cell. Intracellular tau is more difficult to target with polarized antibody products and it is not certain whether the attendant immune response would be prohibitively damaging [240]. Targeting other tau epitopes may provide different responses compared to the agents that have been tested to date.
Gene Therapy
Tau reduction through genetic strategies may prove beneficial in PSP. In mouse models, there are conflicting reports of whether tau knockouts have preserved function [241, 242] or if they exhibit cognitive or motor symptoms [243–246]. Anti-sense oligonucleotides (ASOs) reduce protein expression by binding to mRNA where it can be degraded by mRNA-ase H to prevent translation. Specific ASOs that are capable of lowering total tau expression or shifting expression from 4R to more 3R tau [247] have been tested in animal models [248–250]. Small interfering RNA (siRNA) also prevent protein expression by binding to mRNA prior to translation and have been examined in mouse models [251]. Similar to antibody therapies, drug delivery to ensure adequate brain penetration is a consideration. Again strategies include tagging molecules to more lipophilic compounds [252] or other small molecules [253, 254], intrathecal injections [255], intraparenchymal or intraventricular injections [256], and the use of viral vectors [257, 258].
Conclusion
Progressive supranuclear palsy is a complex clinicopathologic entity with diverse clinical manifestations which can led to delays in diagnosis. While the Richardson syndrome is the most indicative of underlying PSP pathology at autopsy, growing understanding of the diverse clinical phenotypes resulting from PSP pathology has led to a significant expansion of the PSP diagnostic criteria aimed at increasing sensitivity of diagnosis. Clinical treatment of PSP is supportive and there is mild to moderate efficacy of symptomatic therapies to treat a myriad of associated symptoms that can occur with the disease; however, disease modulating treatments have remained elusive. New generation immunotherapies rationally designed small molecules, and genetic therapies directed against the tau protein are under development. Multiple challenges remain in the drive to establish disease modulating therapies in PSP. The use of better imaging and biofluid biomarkers to promote early and accurate diagnosis continues to be essential to aid in ensuring the enrollment of appropriate patients who are likely to more clearly show beneficial effects into clinical trials. Furthermore, the establishment of sensitive and specific markers to track disease progression in PSP to augment clinical scales is crucial for evaluation of therapies. Lastly, many treatments have failed to make the leap from animal models to human therapies. Underlying differences in physiology, the appropriateness of the original model systems, and the challenges of drug delivery in humans are major considerations as development continues in the search for disease modifying therapies in PSP, but several antibody products, small molecules, and gene therapy strategies are still under development.
Table 1:
Mechanistic Based Trials for PSP
| Pathogenic Mechanism | Therapeutic Mechanism | Drug Name | Trials/References | Phase | Main Outcomes | Results | Ongoing |
|---|---|---|---|---|---|---|---|
| Inflammation | Anti-inflammatory/antioxidants | CoQ10 | [93] [94] | II, II | PSP-RS, ADL, MMSE, PDQ-39 | No benefit | Completed |
| Riluzole | [95] | III | Survival, H/Y, SEADL, MMSE | No benefit | Completed | ||
| Rasagiline | [96] | II | PSP-RS | No benefit | Completed | ||
| Lipid Peroxidation | Lipid Stabilization | RT-001 | [203] | Pre-Clinical | |||
| Tau | GSK-3β Inhibitors | Valproic Acid | NCT00385710 | II | PSP-RS | No benefit | Completed |
| Phosphorylation | [196] | ||||||
| Lithium | NCT00703677 | I (PSP) | Tolerability, PSP-RS, PSP-QOL | Not tolerated | Completed | ||
| No benefit | |||||||
| Tideglusib | NCT01350362 | II | PSP-RS, DRS, SEADL, | No benefit | Completed | ||
| [197] [198] | EuroQol | ||||||
| CDK5 inhibitors | Tolfenamic Acid | NCT04253132 | IIa | Tolerability | Not yet recruiting | ||
| Tau Acetylation | Acetylation Inhibitors | Salsalate | NCT02422485 | I | Tolerability, PSP-RS | Not posted | Active, not recruiting |
| O-GlcNAc | O-GlcNAc Inhibitors | [200] [201] | Pre-clinical | ||||
| Tau Assembly | Microtubule Stabilizing Agents | Davunetide | NCT01056965 | II/III | PSP-RS, SEADL, RBANS | No benefit | Completed |
| [205] | |||||||
| TPI-287 | NCT02133846 | I | Tolerability | Not tolerated | Active not recruiting | ||
| [207] | |||||||
| Epothilone D | [208] | Pre-clinical | |||||
| Tau | Passive | ABBV-8E12 | NCT02985879 | II | PSP-RS, Tolerability | No benefit | Completed |
| Accumulation/Spread | Immunotherapy | NCT03391765 | II Extension | PSP-RS | No benefit | Completed | |
| BIIB092 | NCT03068468 | II | PSP-RS, Tolerability | No benefit | Completed | ||
| [224] | |||||||
| UCB0107 | NCT04185415 | Ib | Tolerability | Recruiting | |||
| Active Immunotherapy | No agents in PSP | ||||||
| Tau Production | ASO | [251] | Pre-clinical | ||||
| siRNA | [251] | Pre-clinical | |||||
| Neurotrophic | Neurotrophic Inducer | AZP2006 | NCT04008355 | II | Not yet recruiting | ||
| Inducer | |||||||
Abbreviations: PSP-RS: Progressive Supranuclear Palsy Rating Scale, ADL: activities of daily living, MMSE: minimental status exam, PDQ-39: Parkinson's disease questionnaire-39, H/Y: Hoehn and Yahr scale, SEADL: Schwab and England activities of daily living, PSPQOL: PSP quality of life scale, EuroQol: European quality of life scale, RBANS: repeatable battery for the assessment of neuropsychological status, ASO: antisense oligonucleotides, siRNA: small interfering RNA.
Highlights.
New PSP diagnostic criteria recognizes diverse phenotypes, but refinement is needed
Multiple genetic and environmental factors increase the risk for developing PSP
Improving biofluid and imaging biomarkers will aid in early and accurate diagnoses
Symptomatic treatments with a multidisciplinary approach are standard of care
PSP is an ideal disease for novel therapeutic approaches targeting the tau protein
Acknowledgments
Funding: David Coughlin MD is supported by a Clinical Research Training Scholarship in Parkinson’s disease grant from the American Academy of Neurology/American Brain Foundation/Parkinson’s Foundation (2059) and P30 AG062429. Dr. Litvan is supported by the National Institutes of Health grants: 5P50AG005131-33, 2R01AG038791-06A, U01NS090259, 1U54 NS 092089, U01NS100610, U01NS80818, R25NS098999, P20GM109025; Parkinson Study Group, Michael J Fox Foundation, Lewy Body Association, AVID Pharmaceuticals, Abbvie, Biogen and Roche. She was member of a Lundbeck Advisory Board and participated in a symposium organized by Sunovion. She receives her salary from the University of California San Diego. She is Chief Editor of Frontiers in Neurology
Footnotes
Appendix: none
Declarations: none
References
- [1].Steele JC, Richardson JC, Olszewski J, PROGRESSIVE SUPRANUCLEAR PALSY. A HETEROGENEOUS DEGENERATION INVOLVING THE BRAIN STEM, BASAL GANGLIA AND CEREBELLUM WITH VERTICAL GAZE AND PSEUDOBULBAR PALSY, NUCHAL DYSTONIA AND DEMENTIA, Arch. Neurol. 10 (1964) 333–59. [DOI] [PubMed] [Google Scholar]
- [2].Hauw J-J, Daniel S, Dickson D, Horoupian D, Jellinger K, Lantos P, McKee A, Tabaton M, Litvan I, Preliminary NINDS neuropathologic criteria for Steele‐Richardson‐Olszewski syndrome (progressive supranuclear palsy), Neurology 44(11) (1994) 2015–2015. [DOI] [PubMed] [Google Scholar]
- [3].Goedert M, Jakes R, Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization, The EMBO journal 9(13) (1990) 4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Goedert M, Spillantini M, Cairns N, Crowther R, Tau proteins of Alzheimer paired helical filaments: abnormal phosphorylation of all six brain isoforms, Neuron 8(1) (1992) 159–168. [DOI] [PubMed] [Google Scholar]
- [5].Greenberg S, Davies P, Schein J, Binder L, Hydrofluoric acid-treated tau PHF proteins display the same biochemical properties as normal tau, J. Biol. Chem. 267(1) (1992) 564–569. [PubMed] [Google Scholar]
- [6].Iseki E, Togo T, Suzuki K, Katsuse O, Marui W, de Silva R, Lees A, Yamamoto T, Kosaka K, Dementia with Lewy bodies from the perspective of tauopathy, Acta Neuropathol. 105(3) (2003) 265–270. [DOI] [PubMed] [Google Scholar]
- [7].Arai T, Ikeda K, Akiyama H, Shikamoto Y, Tsuchiya K, Yagishita S, Beach T, Rogers J, Schwab C, McGeer PL, Distinct isoforms of tau aggregated in neurons and glial cells in brains of patients with Pick’s disease, corticobasal degeneration and progressive supranuclear palsy, Acta Neuropathol. 101(2) (2001) 167–173. [DOI] [PubMed] [Google Scholar]
- [8].Sergeant N, Wattez A, Delacourte A, Neurofibrillary degeneration in progressive supranuclear palsy and corticobasal degeneration: tau pathologies with exclusively “exon 10” isoforms, J. Neurochem. 72(3) (1999) 1243–1249. [DOI] [PubMed] [Google Scholar]
- [9].Crowther RA, Goedert M, Abnormal tau-containing filaments in neurodegenerative diseases, J. Struct. Biol. 130(2–3) (2000) 271–9. [DOI] [PubMed] [Google Scholar]
- [10].Kouri N, Whitwell JL, Josephs KA, Rademakers R, Dickson DW, Corticobasal degeneration: a pathologically distinct 4R tauopathy, Nat. Rev. Neurol. 7(5) (2011) 263–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang W, Tarutani A, Newell KL, Murzin AG, Matsubara T, Falcon B, Vidal R, Garringer HJ, Shi Y, Ikeuchi T, Murayama S, Ghetti B, Hasegawa M, Goedert M, Scheres SHW, Novel tau filament fold in corticobasal degeneration, a four-repeat tauopathy, bioRxiv (2019) 811703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nath U, Ben-Shlomo Y, Thomson R, Morris HR, Wood N, Lees A, Burn D, The prevalence of progressive supranuclear palsy (Steele–Richardson–Olszewski syndrome) in the UK, Brain 124(7) (2001) 1438–1449. [DOI] [PubMed] [Google Scholar]
- [13].Coyle-Gilchrist IT, Dick KM, Patterson K, Rodríquez PV, Wehmann E, Wilcox A, Lansdall CJ, Dawson KE, Wiggins J, Mead S, Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes, Neurology 86(18) (2016) 1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schrag A, Ben-Shlomo Y, Quinn N, Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study, The Lancet 354(9192) (1999) 1771–1775. [DOI] [PubMed] [Google Scholar]
- [15].Respondek G, Kurz C, Arzberger T, Compta Y, Englund E, Ferguson LW, Gelpi E, Giese A, Irwin DJ, Meissner WG, Which ante mortem clinical features predict progressive supranuclear palsy pathology?, Mov. Disord. 32(7) (2017) 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Williams DR, Lees AJ, Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges, The Lancet Neurology 8(3) (2009) 270–279. [DOI] [PubMed] [Google Scholar]
- [17].Respondek G, Stamelou M, Kurz C, Ferguson LW, Rajput A, Chiu WZ, van Swieten JC, Troakes C, Al Sarraj S, Gelpi E, Gaig C, Tolosa E, Oertel WH, Giese A, Roeber S, Arzberger T, Wagenpfeil S, Hoglinger GU, The phenotypic spectrum of progressive supranuclear palsy: a retrospective multicenter study of 100 definite cases, Mov. Disord. 29(14) (2014) 1758–66. [DOI] [PubMed] [Google Scholar]
- [18].Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, Holton JL, Revesz T, Lees AJ, Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism, Brain 128(Pt 6) (2005) 1247–58. [DOI] [PubMed] [Google Scholar]
- [19].Höglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Müller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol J-C, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I, P.S.P.S.G. for the Movement Disorder Society–endorsed, Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria, Mov. Disord. (2017) n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fleury V, Brindel P, Nicastro N, Burkhard PR, Descriptive epidemiology of parkinsonism in the Canton of Geneva, Switzerland, Parkinsonism Relat. Disord. 54 (2018) 30–39. [DOI] [PubMed] [Google Scholar]
- [21].Kawashima M, Miyake M, Kusumi M, Adachi Y, Nakashima K, Prevalence of progressive supranuclear palsy in Yonago, Japan, Mov. Disord. 19(10) (2004) 1239–1240. [DOI] [PubMed] [Google Scholar]
- [22].Takigawa H, Ikeuchi T, Aiba I, Morita M, Onodera O, Shimohata T, Tokuda T, Murayama S, Nakashima K, Japanese Longitudinal Biomarker Study in PSP and CBD (JALPAC): A prospective multicenter PSP/CBD cohort study in Japan, Parkinsonism Relat. Disord. 22 (2016) e120–e121. [Google Scholar]
- [23].Maher E, Lees A, The clinical features and natural history of the Steele‐Richardson‐Olszewski syndrome (progressive supranuclear palsy), Neurology 36(7) (1986) 1005–1005. [DOI] [PubMed] [Google Scholar]
- [24].Golbe LI, Davis PH, Schoenberg BS, Duvoisin RC, Prevalence and natural history of progressive supranuclear palsy, Neurology 38(7) (1988) 1031–1031. [DOI] [PubMed] [Google Scholar]
- [25].Litvan I, Agid Y, Progressive supranuclear palsy: clinical and research approaches, Oxford: University Press, USA; 1992. [Google Scholar]
- [26].Collins S, Ahlskog J, Parisi JE, Maraganore D, Progressive supranuclear palsy: neuropathologically based diagnostic clinical criteria, J. Neurol. Neurosurg. Psychiatry 58(2) (1995) 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS, Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop, Neurology 47(1) (1996) 1–9. [DOI] [PubMed] [Google Scholar]
- [28].Respondek G, Roeber S, Kretzschmar H, Troakes C, Al-Sarraj S, Gelpi E, Gaig C, Chiu WZ, van Swieten JC, Oertel WH, Hoglinger GU, Accuracy of the National Institute for Neurological Disorders and Stroke/Society for Progressive Supranuclear Palsy and neuroprotection and natural history in Parkinson plus syndromes criteria for the diagnosis of progressive supranuclear palsy, Mov. Disord. 28(4) (2013) 504–9. [DOI] [PubMed] [Google Scholar]
- [29].Hughes AJ, Daniel SE, Kilford L, Lees AJ, Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases, J. Neurol. Neurosurg. Psychiatry 55(3) (1992) 181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ, The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service, Brain 125(Pt 4) (2002) 861–70. [DOI] [PubMed] [Google Scholar]
- [31].Williams DR, Lees AJ, What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)?, Mov. Disord. 25(3) (2010) 357–62. [DOI] [PubMed] [Google Scholar]
- [32].Shoeibi A, Litvan I, Tolosa E, del Ser T, Lee E, Investigators T, Progression of two Progressive Supranuclear Palsy phenotypes with comparable initial disability, Parkinsonism Relat. Disord. (2019). [DOI] [PubMed] [Google Scholar]
- [33].Donker Kaat L, Boon AJ, Kamphorst W, Ravid R, Duivenvoorden HJ, van Swieten JC, Frontal presentation in progressive supranuclear palsy, Neurology 69(8) (2007) 723–9. [DOI] [PubMed] [Google Scholar]
- [34].Han HJ, Kim H, Park JH, Shin HW, Kim GU, Kim DS, Lee EJ, Oh HE, Park SH, Kim YJ, Behavioral changes as the earliest clinical manifestation of progressive supranuclear palsy, J. Clin. Neurol. 6(3) (2010) 148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hassan A, Parisi JE, Josephs KA, Autopsy-proven progressive supranuclear palsy presenting as behavioral variant frontotemporal dementia, Neurocase 18(6) (2012) 478–88. [DOI] [PubMed] [Google Scholar]
- [36].Boeve B, Dickson D, Duffy J, Bartleson J, Trenerry M, Petersen R, Progressive nonfluent aphasia and subsequent aphasic dementia associated with atypical progressive supranuclear palsy pathology, Eur. Neurol. 49(2) (2003) 72–8. [DOI] [PubMed] [Google Scholar]
- [37].Mochizuki A, Ueda Y, Komatsuzaki Y, Tsuchiya K, Arai T, Shoji S, Progressive supranuclear palsy presenting with primary progressive aphasia--clinicopathological report of an autopsy case, Acta Neuropathol. 105(6) (2003) 610–4. [DOI] [PubMed] [Google Scholar]
- [38].Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, Dickson DW, Jack CR Jr., Petersen RC, Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech, Brain 129(Pt 6) (2006) 1385–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ling H, O’Sullivan SS, Holton JL, Revesz T, Massey LA, Williams DR, Paviour DC, Lees AJ, Does corticobasal degeneration exist? A clinicopathological re-evaluation, Brain 133(7) (2010) 2045–2057. [DOI] [PubMed] [Google Scholar]
- [40].Lee SE, Rabinovici GD, Mayo MC, Wilson SM, Seeley WW, DeArmond SJ, Huang EJ, Trojanowski JQ, Growdon ME, Jang JY, Sidhu M, See TM, Karydas AM, Gorno-Tempini ML, Boxer AL, Weiner MW, Geschwind MD, Rankin KP, Miller BL, Clinicopathological correlations in corticobasal degeneration, Ann. Neurol. 70(2) (2011) 327–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, Parisi JE, Dickson DW, Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP, Neurology 66(1) (2006) 41–8. [DOI] [PubMed] [Google Scholar]
- [42].Compta Y, Valldeoriola F, Tolosa E, Rey MJ, Martí MJ, Valls‐Solé J, Long lasting pure freezing of gait preceding progressive supranuclear palsy: a clinicopathological study, Mov. Disord. 22(13) (2007) 1954–1958. [DOI] [PubMed] [Google Scholar]
- [43].Williams DR, Holton JL, Strand K, Revesz T, Lees AJ, Pure akinesia with gait freezing: a third clinical phenotype of progressive supranuclear palsy, Mov. Disord. 22(15) (2007) 2235–2241. [DOI] [PubMed] [Google Scholar]
- [44].Josephs KA, Katsuse O, Beccano-Kelly DA, Lin W-L, Uitti RJ, Fujino Y, Boeve BF, Hutton ML, Baker MC, Dickson DW, Atypical progressive supranuclear palsy with corticospinal tract degeneration, J. Neuropathol. Exp. Neurol. 65(4) (2006) 396–405. [DOI] [PubMed] [Google Scholar]
- [45].Nagao S, Yokota O, Nanba R, Takata H, Haraguchi T, Ishizu H, Ikeda C, Takeda N, Oshima E, Sakane K, Progressive supranuclear palsy presenting as primary lateral sclerosis but lacking parkinsonism, gaze palsy, aphasia, or dementia, J. Neurol. Sci. 323(1–2) (2012) 147–153. [DOI] [PubMed] [Google Scholar]
- [46].Kanazawa M, Tada M, Onodera O, Takahashi H, Nishizawa M, Shimohata T, Early clinical features of patients with progressive supranuclear palsy with predominant cerebellar ataxia, Parkinsonism Relat. Disord. 19(12) (2013) 1149–1151. [DOI] [PubMed] [Google Scholar]
- [47].Koga S, Josephs KA, Ogaki K, Labbé C, Uitti RJ, Graff‐Radford N, Van Gerpen JA, Cheshire WP, Aoki N, Rademakers R, Cerebellar ataxia in progressive supranuclear palsy: An autopsy study of PSP‐C, Mov. Disord. 31(5) (2016) 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ali F, Martin PR, Botha H, Ahlskog JE, Bower JH, Masumoto JY, Maraganore D, Hassan A, Eggers S, Boeve BF, Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy, Mov. Disord. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ali F, Botha H, Whitwell JL, Josephs KA, Utility of the Movement Disorders Society Criteria for Progressive Supranuclear Palsy in Clinical Practice, Movement Disorders Clinical Practice 6(6) (2019) 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shoeibi A, Litvan I, Juncos JL, Bordelon Y, Riley D, Standaert D, Reich SG, Shprecher D, Hall D, Marras C, Are the International Parkinson disease and Movement Disorder Society progressive supranuclear palsy (IPMDS-PSP) diagnostic criteria accurate enough to differentiate common PSP phenotypes?, Parkinsonism Relat. Disord. 69 (2019) 34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yamada T, McGeer P, McGeer E, Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue, Neurosci. Lett. 135(1) (1992) 99–102. [DOI] [PubMed] [Google Scholar]
- [52].Dickson DW, Kouri N, Murray ME, Josephs KA, Neuropathology of Frontotemporal Lobar Degeneration-Tau (FTLD-Tau), J. Mol. Neurosci. 45(3) (2011) 384–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Hanger DP, Anderton BH, Noble W, Tau phosphorylation: the therapeutic challenge for neurodegenerative disease, Trends Mol. Med. 15(3) (2009) 112–119. [DOI] [PubMed] [Google Scholar]
- [54].Wray S, Saxton M, Anderton BH, Hanger DP, Direct analysis of tau from PSP brain identifies new phosphorylation sites and a major fragment of N‐terminally cleaved tau containing four microtubule‐binding repeats, J. Neurochem. 105(6) (2008) 2343–2352. [DOI] [PubMed] [Google Scholar]
- [55].Irwin DJ, Cohen TJ, Grossman M, Arnold SE, Xie SX, Lee VM-Y, Trojanowski JQ, Acetylated tau, a novel pathological signature in Alzheimer’s disease and other tauopathies, Brain 135(3) (2012) 807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Schmidt ML, Schuck T, Sheridan S, Kung M-P, Kung H, Zhuang Z-P, Bergeron C, Lamarche JS, Skovronsky D, Giasson BI, The fluorescent Congo red derivative,(trans, trans)− 1-bromo-2, 5-Bis-(3-hydroxycarbonyl-4-hydroxy) styrylbenzene (BSB), labels diverse β-pleated sheet structures in postmortem human neurodegenerative disease brains, The American journal of pathology 159(3) (2001) 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, Parisi JE, Jack CR Jr., Voxel-based morphometry in autopsy proven PSP and CBD, Neurobiol. Aging 29(2) (2008) 280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA, Neuropathology of variants of progressive supranuclear palsy, Curr. Opin. Neurol. 23(4) (2010) 394–400. [DOI] [PubMed] [Google Scholar]
- [59].Williams DR, Holton JL, Strand C, Pittman A, de Silva R, Lees AJ, Revesz T, Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome, Brain 130(6) (2007) 1566–1576. [DOI] [PubMed] [Google Scholar]
- [60].Yoshida M, Astrocytic inclusions in progressive supranuclear palsy and corticobasal degeneration, Neuropathology 34(6) (2014) 555–570. [DOI] [PubMed] [Google Scholar]
- [61].Litvan I, Lees PS, Cunningham CR, Rai SN, Cambon AC, Standaert DG, Marras C, Juncos J, Riley D, Reich S, Hall D, Kluger B, Bordelon Y, Shprecher DR, Environmental and occupational risk factors for progressive supranuclear palsy: Case-control study, Mov. Disord. 31(5) (2016) 644–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Kelley KD, Checkoway H, Hall DA, Reich SG, Cunningham C, Litvan I, Traumatic Brain Injury and Firearm Use and Risk of Progressive Supranuclear Palsy Among Veterans, Front. Neurol 9 (2018) 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Caparros-Lefebvre D, Golbe LI, Deramecourt V, Maurage CA, Huin V, Buee-Scherrer V, Obriot H, Sablonniere B, Caparros F, Buee L, Lees AJ, A geographical cluster of progressive supranuclear palsy in northern France, Neurology 85(15) (2015) 1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Caparros-Lefebvre D, Sergeant N, Lees A, Camuzat A, Daniel S, Lannuzel A, Brice A, Tolosa E, Delacourte A, Duyckaerts C, Guadeloupean parkinsonism: a cluster of progressive supranuclear palsy-like tauopathy, Brain 125(Pt 4) (2002) 801–11. [DOI] [PubMed] [Google Scholar]
- [65].Lannuzel A, Ruberg M, Michel PP, Atypical parkinsonism in the Caribbean island of Guadeloupe: etiological role of the mitochondrial complex I inhibitor annonacin, Mov. Disord. 23(15) (2008) 2122–2128. [DOI] [PubMed] [Google Scholar]
- [66].Park HK, Ilango S, Charriez CM, Checkoway H, Riley D, Standaert DG, Bordelon Y, Shprecher DR, Reich SG, Hall D, Lifetime exposure to estrogen and progressive supranuclear palsy: Environmental and Genetic PSP study, Mov. Disord. 33(3) (2018) 468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Kouri N, Ross OA, Dombroski B, Younkin CS, Serie DJ, Soto-Ortolaza A, Baker M, Finch NCA, Yoon H, Kim J, Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy, Nature communications 6 (2015) 7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Rohrer JD, Paviour D, Vandrovcova J, Hodges J, De Silva R, Rossor MN, Novel L284R MAPT mutation in a family with an autosomal dominant progressive supranuclear palsy syndrome, Neurodegenerative Diseases 8(3) (2011) 149–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ogaki K, Li Y, Takanashi M, Ishikawa K-I, Kobayashi T, Nonaka T, Hasegawa M, Kishi M, Yoshino H, Funayama M, Analyses of the MAPT, PGRN, and C9orf72 mutations in Japanese patients with FTLD, PSP, and CBS, Parkinsonism Relat. Disord. 19(1) (2013) 15–20. [DOI] [PubMed] [Google Scholar]
- [70].Boeve BF, Hutton M, Refining frontotemporal dementia with parkinsonism linked to chromosome 17: introducing FTDP-17 (MAPT) and FTDP-17 (PGRN), Arch. Neurol. 65(4) (2008) 460–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Baker M, Litvan I, Houlden H, Adamson J, Dickson D, Perez-Tur J, Hardy J, Lynch T, Bigio E, Hutton M, Association of an extended haplotype in the tau gene with progressive supranuclear palsy, Hum. Mol. Genet. 8(4) (1999) 711–715. [DOI] [PubMed] [Google Scholar]
- [72].Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, Holton JL, Revesz T, Lees AJ, Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism, Brain 128(6) (2005) 1247–1258. [DOI] [PubMed] [Google Scholar]
- [73].Höglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang L-S, Klei L, Rademakers R, De Silva R, Litvan I, Riley DE, Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy, Nat. Genet. 43(7) (2011) 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Yokoyama JS, Karch CM, Fan CC, Bonham LW, Kouri N, Ross OA, Rademakers R, Kim J, Wang Y, Höglinger GU, Shared genetic risk between corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia, Acta Neuropathol. 133(5) (2017) 825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Wendler F, Tooze S, Syntaxin 6: the promiscuous behaviour of a SNARE protein, Traffic 2(9) (2001) 606–611. [DOI] [PubMed] [Google Scholar]
- [76].Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D, Perk is essential for translational regulation and cell survival during the unfolded protein response, Mol. Cell 5(5) (2000) 897–904. [DOI] [PubMed] [Google Scholar]
- [77].Yuan SH, Hiramatsu N, Liu Q, Sun XV, Lenh D, Chan P, Chiang K, Koo EH, Kao AW, Litvan I, Tauopathy-associated PERK alleles are functional hypomorphs that increase neuronal vulnerability to ER stress, Hum. Mol. Genet. 27(22) (2018) 3951–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sanchez-Contreras MY, Kouri N, Cook CN, Serie DJ, Heckman MG, Finch NA, Caselli RJ, Uitti RJ, Wszolek ZK, Graff-Radford N, Replication of progressive supranuclear palsy genome-wide association study identifies SLCO1A2 and DUSP10 as new susceptibility loci, Mol. Neurodegener 13(1) (2018) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Chirichigno JW, Manfredi G, Beal MF, Albers DS, Stress-induced mitochondrial depolarization and oxidative damage in PSP cybrids, Brain Res. 951(1) (2002) 31–35. [DOI] [PubMed] [Google Scholar]
- [80].Cantuti-Castelvetri I, Keller-McGandy CE, Albers DS, Beal MF, Vonsattel J-P, Standaert DG, Augood SJ, Expression and activity of antioxidants in the brain in progressive supranuclear palsy, Brain Res. 930(1–2) (2002) 170–181. [DOI] [PubMed] [Google Scholar]
- [81].Park LC, Albers DS, Xu H, Lindsay JG, Beal MF, Gibson GE, Mitochondrial impairment in the cerebellum of the patients with progressive supranuclear palsy, J. Neurosci. Res. 66(5) (2001) 1028–1034. [DOI] [PubMed] [Google Scholar]
- [82].Albers DS, Swerdlow RH, Manfredi G, Gajewski C, Yang L, Parker WD Jr, Beal MF, Further evidence for mitochondrial dysfunction in progressive supranuclear palsy, Exp. Neurol. 168(1) (2001) 196–198. [DOI] [PubMed] [Google Scholar]
- [83].Albers DS, Augood SJ, Martin DM, Standaert DG, Vonsattel JPG, Beal MF, Evidence for oxidative stress in the subthalamic nucleus in progressive supranuclear palsy, J. Neurochem. 73(2) (1999) 881–884. [DOI] [PubMed] [Google Scholar]
- [84].Albers DS, Augood SJ, Park LC, Browne SE, Martin DM, Adamson J, Hutton M, Standaert DG, Vonsattel JPG, Gibson GE, Frontal lobe dysfunction in progressive supranuclear palsy: evidence for oxidative stress and mitochondrial impairment, J. Neurochem. 74(2) (2000) 878–881. [DOI] [PubMed] [Google Scholar]
- [85].Albers DS, Beal MF, Mitochondrial dysfunction in progressive supranuclear palsy, Neurochem. Int. 40(6) (2002) 559–564. [DOI] [PubMed] [Google Scholar]
- [86].Martinelli P, Scaglione C, Lodi R, Iotti S, Barbiroli B, Deficit of brain and skeletal muscle bioenergetics in progressive supranuclear palsy shown in vivo by phosphorus magnetic resonance spectroscopy, Mov. Disord. 15(5) (2000) 889–893. [DOI] [PubMed] [Google Scholar]
- [87].Swerdlow R, Golbe L, Parks J, Cassarino D, Binder D, Grawey A, Litvan I, Bennett J Jr, Wooten G, Parker W, Mitochondrial dysfunction in cybrid lines expressing mitochondrial genes from patients with progressive supranuclear palsy, J. Neurochem. 75(4) (2000) 1681–1684. [DOI] [PubMed] [Google Scholar]
- [88].Fernandez-Botran R, Ahmed Z, Crespo FA, Gatenbee C, Gonzalez J, Dickson DW, Litvan I, Cytokine expression and microglial activation in progressive supranuclear palsy, Parkinsonism Relat. Disord. 17(9) (2011) 683–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ishizawa K, Dickson DW, Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration, J. Neuropathol. Exp. Neurol. 60(6) (2001) 647–57. [DOI] [PubMed] [Google Scholar]
- [90].Starhof C, Winge K, Heegaard NHH, Skogstrand K, Friis S, Hejl A, Cerebrospinal fluid pro-inflammatory cytokines differentiate parkinsonian syndromes, J. Neuroinflammation 15(1) (2018) 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy‐Agid F, Jenner P, Marsden CD, Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia, Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 36(3) (1994) 348–355. [DOI] [PubMed] [Google Scholar]
- [92].Gerhard A, Trender‐Gerhard I, Turkheimer F, Quinn NP, Bhatia KP, Brooks DJ, In vivo imaging of microglial activation with [11C](R)‐PK11195 PET in progressive supranuclear palsy, Mov. Disord. 21(1) (2006) 89–93. [DOI] [PubMed] [Google Scholar]
- [93].Stamelou M, Reuss A, Pilatus U, Magerkurth J, Niklowitz P, Eggert KM, Krisp A, Menke T, Schade-Brittinger C, Oertel WH, Hoglinger GU, Short-term effects of coenzyme Q10 in progressive supranuclear palsy: a randomized, placebo-controlled trial, Mov. Disord. 23(7) (2008) 942–9. [DOI] [PubMed] [Google Scholar]
- [94].Apetauerova D, Scala SA, Hamill RW, Simon DK, Pathak S, Ruthazer R, Standaert DG, Yacoubian TA, CoQ10 in progressive supranuclear palsy: A randomized, placebo-controlled, double-blind trial, Neurology(R) neuroimmunology & neuroinflammation 3(5) (2016) e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bensimon G, Ludolph A, Agid Y, Vidailhet M, Payan C, Leigh PN, Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study, Brain 132(Pt 1) (2009) 156–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Nuebling G, Hensler M, Paul S, Zwergal A, Crispin A, Lorenzl S, PROSPERA: a randomized, controlled trial evaluating rasagiline in progressive supranuclear palsy, J. Neurol. 263(8) (2016) 1565–1574. [DOI] [PubMed] [Google Scholar]
- [97].Iba M, Guo JL, McBride JD, Zhang B, Trojanowski JQ, Lee VM-Y, Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy, J. Neurosci. 33(3) (2013) 1024–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Clavaguera F, Lavenir I, Falcon B, Frank S, Goedert M, Tolnay M, “Prion‐like” templated misfolding in tauopathies, Brain Pathol. 23(3) (2013) 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, Brain homogenates from human tauopathies induce tau inclusions in mouse brain, Proceedings of the National Academy of Sciences 110(23) (2013) 9535–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sanders DW, Kaufman SK, DeVos SL, Sharma AM, Mirbaha H, Li A, Barker SJ, Foley AC, Thorpe JR, Serpell LC, Distinct tau prion strains propagate in cells and mice and define different tauopathies, Neuron 82(6) (2014) 1271–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Probst A, Götz J, Wiederhold K, Tolnay M, Mistl C, Jaton A, Hong M, Ishihara T, Lee V-Y, Trojanowski J, Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein, Acta Neuropathol. 99(5) (2000) 469–481. [DOI] [PubMed] [Google Scholar]
- [102].Dujardin K, Defebvre L, Duhamel A, Lecouffe P, Rogelet P, Steinling M, Destee A, Cognitive and SPECT characteristics predict progression of Parkinson’s disease in newly diagnosed patients, J. Neurol 251(11) (2004) 1383–92. [DOI] [PubMed] [Google Scholar]
- [103].Clavaguera F, Hench J, Lavenir I, Schweighauser G, Frank S, Goedert M, Tolnay M, Peripheral administration of tau aggregates triggers intracerebral tauopathy in transgenic mice, Acta Neuropathol. 127(2) (2014) 299–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nishimura M, Namba Y, Ikeda K, Oda M, Glial fibrillary tangles with straight tubules in the brains of patients with progressive supranuclear palsy, Neurosci. Lett. 143(1–2) (1992) 35–38. [DOI] [PubMed] [Google Scholar]
- [105].Goedert M, Tau filaments in neurodegenerative diseases, FEBS Lett. 592(14) (2018) 2383–2391. [DOI] [PubMed] [Google Scholar]
- [106].Fitzpatrick AW, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, Crowther RA, Ghetti B, Goedert M, Scheres SH, Cryo-EM structures of tau filaments from Alzheimer’s disease, Nature 547(7662) (2017) 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kato N, Arai K, Hattori T, Study of the rostral midbrain atrophy in progressive supranuclear palsy, J. Neurol. Sci. 210(1–2) (2003) 57–60. [DOI] [PubMed] [Google Scholar]
- [108].Adachi M, KAWANAMI T, OHSHIMA H, Sugai Y, Hosoya T, Morning glory sign: a particular MR finding in progressive supranuclear palsy, Magn. Reson. Med. Sci 3(3) (2004) 125–132. [DOI] [PubMed] [Google Scholar]
- [109].Massey LA, Micallef C, Paviour DC, O’Sullivan SS, Ling H, Williams DR, Kallis C, Holton JL, Revesz T, Burn DJ, Yousry T, Lees AJ, Fox NC, Jager HR, Conventional magnetic resonance imaging in confirmed progressive supranuclear palsy and multiple system atrophy, Mov. Disord. 27(14) (2012) 1754–62. [DOI] [PubMed] [Google Scholar]
- [110].Massey LA, Jager HR, Paviour DC, O’Sullivan SS, Ling H, Williams DR, Kallis C, Holton J, Revesz T, Burn DJ, Yousry T, Lees AJ, Fox NC, Micallef C, The midbrain to pons ratio: a simple and specific MRI sign of progressive supranuclear palsy, Neurology 80(20) (2013) 1856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Quattrone A, Nicoletti G, Messina D, Fera F, Condino F, Pugliese P, Lanza P, Barone P, Morgante L, Zappia M, Aguglia U, Gallo O, MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy, Radiology 246(1) (2008) 214–21. [DOI] [PubMed] [Google Scholar]
- [112].Nigro S, Arabia G, Antonini A, Weis L, Marcante A, Tessitore A, Cirillo M, Tedeschi G, Zanigni S, Calandra-Buonaura G, Tonon C, Pezzoli G, Cilia R, Zappia M, Nicoletti A, Cicero CE, Tinazzi M, Tocco P, Cardobi N, Quattrone A, Magnetic Resonance Parkinsonism Index: diagnostic accuracy of a fully automated algorithm in comparison with the manual measurement in a large Italian multicentre study in patients with progressive supranuclear palsy, Eur. Radiol 27(6) (2017) 2665–2675. [DOI] [PubMed] [Google Scholar]
- [113].Hussl A, Mahlknecht P, Scherfler C, Esterhammer R, Schocke M, Poewe W, Seppi K, Diagnostic accuracy of the magnetic resonance Parkinsonism index and the midbrain-to-pontine area ratio to differentiate progressive supranuclear palsy from Parkinson’s disease and the Parkinson variant of multiple system atrophy, Mov. Disord. 25(14) (2010) 2444–9. [DOI] [PubMed] [Google Scholar]
- [114].Morelli M, Arabia G, Novellino F, Salsone M, Giofre L, Condino F, Messina D, Quattrone A, MRI measurements predict PSP in unclassifiable parkinsonisms: a cohort study, Neurology 77(11) (2011) 1042–1047. [DOI] [PubMed] [Google Scholar]
- [115].Quattrone A, Morelli M, Williams DR, Vescio B, Arabia G, Nigro S, Nicoletti G, Salsone M, Novellino F, Nisticò R, MR parkinsonism index predicts vertical supranuclear gaze palsy in patients with PSP–parkinsonism, Neurology 87(12) (2016) 1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Quattrone A, Morelli M, Nigro S, Quattrone A, Vescio B, Arabia G, Nicoletti G, Nisticò R, Salsone M, Novellino F, A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson’s disease, Parkinsonism Relat. Disord. 54 (2018) 3–8. [DOI] [PubMed] [Google Scholar]
- [117].Quattrone A, Morelli M, Vescio B, Nigro S, Le Piane E, Sabatini U, Caracciolo M, Vescio V, Quattrone A, Barbagallo G, Refining initial diagnosis of Parkinson’s disease after follow‐up: A 4‐year prospective clinical and magnetic resonance imaging study, Mov. Disord. 34(4) (2019) 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Villemagne VL, Fodero-Tavoletti MT, Masters CL, Rowe CC, Tau imaging: early progress and future directions, Lancet Neurol 14(1) (2015) 114–124. [DOI] [PubMed] [Google Scholar]
- [119].Marquié M, Normandin MD, Vanderburg CR, Costantino IM, Bien EA, Rycyna LG, Klunk WE, Mathis CA, Ikonomovic MD, Debnath ML, Validating novel tau positron emission tomography tracer [F‐18]‐AV‐1451 (T807) on postmortem brain tissue, Ann. Neurol. 78(5) (2015) 787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Passamonti L, Vazquez Rodriguez P, Hong YT, Allinson KS, Williamson D, Borchert RJ, Sami S, Cope TE, Bevan-Jones WR, Jones PS, Arnold R, Surendranathan A, Mak E, Su L, Fryer TD, Aigbirhio FI, O’Brien JT, Rowe JB, 18F-AV-1451 positron emission tomography in Alzheimer’s disease and progressive supranuclear palsy, Brain 140(3) (2017) 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Pontecorvo MJ, Devous MD Sr, Navitsky M, Lu M, Salloway S, Schaerf FW, Jennings D, Arora AK, McGeehan A, Lim NC, Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition, Brain 140(3) (2017) 748–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Nasrallah IM, Chen YJ, Hsieh M-K, Phillips JS, Ternes K, Stockbower GE, Sheline Y, McMillan CT, Grossman M, Wolk DA, 18F-Flortaucipir PET/MRI correlations in nonamnestic and amnestic variants of Alzheimer disease, J. Nucl. Med. 59(2) (2018) 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O’Neil JP, Janabi M, Lazaris A, Cantwell A, Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease, Brain 139(5) (2016) 1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, Kantarci K, Boeve BF, Pandey MK, Bruinsma T, Knopman DS, Jones DT, Petrucelli L, Cook CN, Graff-Radford NR, Dickson DW, Petersen RC, Jack CR Jr., Murray ME, An autoradiographic evaluation of AV-1451 Tau PET in dementia, Acta neuropathologica communications 4(1) (2016) 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Bevan Jones WR, Cope TE, Passamonti L, Fryer TD, Hong YT, Aigbirhio F, Kril JJ, Forrest SL, Allinson K, Coles JP, Simon Jones P, Spillantini MG, Hodges JR, O’Brien JT, Rowe JB, [18F]AV-1451 PET in behavioral variant frontotemporal dementia due to MAPT mutation, Annals of clinical and translational neurology 3(12) (2016) 940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Cho H, Choi JY, Hwang MS, Lee SH, Ryu YH, Lee MS, Lyoo CH, Subcortical 18 F-AV-1451 binding patterns in progressive supranuclear palsy, Mov. Disord. 32(1) (2017) 134–140. [DOI] [PubMed] [Google Scholar]
- [127].Smith R, Schain M, Nilsson C, Strandberg O, Olsson T, Hagerstrom D, Jogi J, Borroni E, Scholl M, Honer M, Hansson O, Increased basal ganglia binding of 18 F-AV-1451 in patients with progressive supranuclear palsy, Mov. Disord. 32(1) (2017) 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Whitwell JL, Lowe VJ, Tosakulwong N, Weigand SD, Senjem ML, Schwarz CG, Spychalla AJ, Petersen RC, Jack CR Jr., Josephs KA, [18 F]AV-1451 tau positron emission tomography in progressive supranuclear palsy, Mov. Disord. 32(1) (2017) 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Gerhard A, Watts J, Trender‐Gerhard I, Turkheimer F, Banati RB, Bhatia K, Brooks DJ, In vivo imaging of microglial activation with [11C](R)‐PK11195 PET in corticobasal degeneration, Mov. Disord. 19(10) (2004) 1221–1226. [DOI] [PubMed] [Google Scholar]
- [130].Shaw LM, Vanderstichele H, Knapik‐Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects, Ann. Neurol. 65(4) (2009) 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Urakami K, Wada K, Arai H, Sasaki H, Kanai M, Shoji M, Ishizu H, Kashihara K, Yamamoto M, Tsuchiya-Ikemoto K, Diagnostic significance of tau protein in cerebrospinal fluid from patients with corticobasal degeneration or progressive supranuclear palsy, J. Neurol. Sci. 183(1) (2001) 95–98. [DOI] [PubMed] [Google Scholar]
- [132].Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, Nilsson C, Widner H, Decraemer H, Nägga K, Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders, Arch. Neurol. 69(11) (2012) 1445–1452. [DOI] [PubMed] [Google Scholar]
- [133].Borroni B, Malinverno M, Gardoni F, Alberici A, Parnetti L, Premi E, Bonuccelli U, Grassi M, Perani D, Calabresi P, Tau forms in CSF as a reliable biomarker for progressive supranuclear palsy, Neurology 71(22) (2008) 1796–1803. [DOI] [PubMed] [Google Scholar]
- [134].Kuiperij HB, Borroni B, Verbeek MM, Gardoni F, Malinverno M, Padovani A, Di Luca M, <span hwp:id=“article-title-1” class=“article-title”>Tau forms in CSF as a reliable biomarker for progressive supranuclear palsy<span hwp:id=“article-title-2” class=“sub-article-title”/><span hwp:id=“article-title-3” class=“sub-article-title”/>, Neurology 76(16) (2011) 1443–1443.21502610 [Google Scholar]
- [135].Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Neurofilaments as biomarkers in neurological disorders, Nature Reviews Neurology (2018) 1. [DOI] [PubMed] [Google Scholar]
- [136].Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, Linder J, Forsgren L, Constantinescu R, Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder, Neurology 88(10) (2017) 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Holmberg B, Rosengren L, Karlsson JE, Johnels B, Increased cerebrospinal fluid levels of neurofilament protein in progressive supranuclear palsy and multiple‐system atrophy compared with Parkinson’s disease, Mov. Disord. 13(1) (1998) 70–77. [DOI] [PubMed] [Google Scholar]
- [138].Marques TM, van Rumund A, Oeckl P, Kuiperij HB, Esselink RA, Bloem BR, Otto M, Verbeek MM, Serum NFL discriminates Parkinson disease from atypical parkinsonisms, Neurology 92(13) (2019) e1479–e1486. [DOI] [PubMed] [Google Scholar]
- [139].Sako W, Murakami N, Izumi Y, Kaji R, Neurofilament light chain level in cerebrospinal fluid can differentiate Parkinson’s disease from atypical parkinsonism: evidence from a meta-analysis, J. Neurol. Sci. 352(1–2) (2015) 84–87. [DOI] [PubMed] [Google Scholar]
- [140].Kuhle J, Barro C, Andreasson U, Derfuss T, Lindberg R, Sandelius A, Liman V, Norgren N, Blennow K, Zetterberg H, Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa, Clin. Chem. Lab. Med. 54(10) (2016) 1655–61. [DOI] [PubMed] [Google Scholar]
- [141].Rojas JC, Karydas A, Bang J, Tsai RM, Blennow K, Liman V, Kramer JH, Rosen H, Miller BL, Zetterberg H, Plasma neurofilament light chain predicts progression in progressive supranuclear palsy, Annals of clinical and translational neurology 3(3) (2016) 216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Orrú CD, Groveman BR, Hughson AG, Zanusso G, Coulthart MB, Caughey B, Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid, MBio 6(1) (2015) e02451–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Groveman BR, Orrù CD, Hughson AG, Raymond LD, Zanusso G, Ghetti B, Campbell KJ, Safar J, Galasko D, Caughey B, Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC, Acta neuropathologica communications 6(1) (2018) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, Joachim C, Esiri M, Evetts SG, Rolinski M, Alpha-synuclein RT-Qu IC in the CSF of patients with alpha-synucleinopathies, Annals of clinical and translational neurology 3(10) (2016) 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].van Rumund A, Green AJ, Fairfoul G, Esselink RA, Bloem BR, Verbeek MM, Alpha‐synuclein RT‐QuIC in the CSF of uncertain cases of parkinsonism, Ann. Neurol. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kraus A, Saijo E, Metrick MA, Newell K, Sigurdson CJ, Zanusso G, Ghetti B, Caughey B, Seeding selectivity and ultrasensitive detection of tau aggregate conformers of Alzheimer disease, Acta Neuropathol. 137(4) (2019) 585–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Saijo E, Metrick MA, Koga S, Parchi P, Litvan I, Spina S, Boxer A, Rojas JC, Galasko D, Kraus A, 4-repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration, Acta Neuropathol. 139(1) (2020) 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Vidailhet M, Rivaud S, Gouider‐Khouja N, Pillon B, Bonnet AM, Gaymard B, Agid Y, Pierrot‐Deseilligny C, Eye movements in parkinsonian syndromes, Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 35(4) (1994) 420–426. [DOI] [PubMed] [Google Scholar]
- [149].Marx S, Respondek G, Stamelou M, Dowiasch S, Stoll J, Bremmer F, Oertel WH, Höglinger GU, Einhauser W, Validation of mobile eye-tracking as novel and efficient means for differentiating progressive supranuclear palsy from Parkinson’s disease, Front. Behav. Neurosci. 6 (2012) 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Otero-Millan J, Serra A, Leigh RJ, Troncoso XG, Macknik SL, Martinez-Conde S, Distinctive features of saccadic intrusions and microsaccades in progressive supranuclear palsy, J. Neurosci. 31(12) (2011) 4379–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Karson CN, Burns RS, LeWitt PA, Foster NL, Newman RP, Blink rates and disorders of movement, Neurology 34(5) (1984) 677–677. [DOI] [PubMed] [Google Scholar]
- [152].Kimura J, Disorder of interneurons in parkinsonism: the orbicularis oculi reflex to paired stimuli, Brain 96(1) (1973) 87–96. [DOI] [PubMed] [Google Scholar]
- [153].Vidailhet M, Rothwelll J, Thompson P, Lees A, Marsden C, The auditory startle response in the Steele-Richardson-Olszewski syndrome and Parkinson’s disease, Brain 115(4) (1992) 1181–1192. [DOI] [PubMed] [Google Scholar]
- [154].Valldeoriola F, Valls-Solé J, Tolosa E, Ventura P, Nobbe F, Marti M, Effects of a startling acoustic stimulus on reaction time in different parkinsonian syndromes, Neurology 51(5) (1998) 1315–1320. [DOI] [PubMed] [Google Scholar]
- [155].Fine EJ, Hallett M, Litvan I, Tresser N, Katz D, Dysfunction of Ib (autogenic) spinal inhibition in patients with progressive supranuclear palsy, Mov. Disord. 13(4) (1998) 668–672. [DOI] [PubMed] [Google Scholar]
- [156].Richardson J, Steele J, Olszewski J, SUPRANUCLEAR OPHTHALMOPLEGIA, PSEUDOBULBAR PALSY, NUCHAL DYSTONIA AND DEMENTIA. A CLINICAL REPORT ON EIGHT CASES OF” HETEROGENOUS SYSTEM DEGENERATION”, Trans. Am. Neurol. Assoc. 88 (1963) 25. [PubMed] [Google Scholar]
- [157].Nieforth KA, Golbe LI, Retrospective study of drug response in 87 patients with progressive supranuclear palsy, Clin. Neuropharmacol. 16(4) (1993) 338–46. [DOI] [PubMed] [Google Scholar]
- [158].Tan E, Chan L, Wong M, Levodopa-induced oromandibular dystonia in progressive supranuclear palsy, Clin. Neurol. Neurosurg. 105(2) (2003) 132–134. [DOI] [PubMed] [Google Scholar]
- [159].Lang AE, Treatment of progressive supranuclear palsy and corticobasal degeneration, Mov. Disord. 20(S12) (2005) S83–S91. [DOI] [PubMed] [Google Scholar]
- [160].Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, Quinn N, Sethi K, Shults C, Wenning G, Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for parkinsonian disorders, Mov. Disord. 18(5) (2003) 467–486. [DOI] [PubMed] [Google Scholar]
- [161].Litvan I, Mangone CA, McKee A, Verny M, Parsa A, Jellinger K, D’Olhaberriague L, Chaudhuri KR, Pearce RK, Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: a clinicopathological study, J. Neurol. Neurosurg. Psychiatry 60(6) (1996) 615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Jackson JA, Jankovic J, Ford J, Progressive supranuclear palsy: clinical features and response to treatment in 16 patients, Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 13(3) (1983) 273–278. [DOI] [PubMed] [Google Scholar]
- [163].Stowe R, Ives N, Clarke CE, Ferreira J, Hawker RJ, Shah L, Wheatley K, Gray R, Dopamine agonist therapy in early Parkinson’s disease, Cochrane Database Syst. Rev (2) (2008). [DOI] [PubMed] [Google Scholar]
- [164].Rajrut A, Uitti R, Fenton M, George D, Amantadine effectiveness in multiple system atrophy and progressive supranuclear palsy, Parkinsonism Relat. Disord. 3(4) (1997) 211–214. [DOI] [PubMed] [Google Scholar]
- [165].Silver DE, Sahs AL, Double blind study using amantadine hydrochloride in the therapy of Parkinson’s disease, Trans. Am. Neurol. Assoc. 96 (1971) 307–8. [PubMed] [Google Scholar]
- [166].Crosby NJ, Deane K, Clarke CE, Amantadine in Parkinson’s disease, Cochrane Database Syst. Rev (1) (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Simpson DM, Davis GC, Case report of neuroleptic malignant syndrome associated with withdrawal from amantadine, The American journal of psychiatry (1984). [DOI] [PubMed] [Google Scholar]
- [168].Factor SA, Molho ES, Brown DL, Acute delirium after withdrawal of amantadine in Parkinson’s disease, Neurology 50(5) (1998) 1456–1458. [DOI] [PubMed] [Google Scholar]
- [169].Daniele A, Moro E, Bentivoglio AR, Zolpidem in progressive supranuclear palsy, N. Engl. J. Med. 341(7) (1999) 543–544. [DOI] [PubMed] [Google Scholar]
- [170].Cotter C, Armytage T, Crimmins D, The use of zolpidem in the treatment of progressive supranuclear palsy, J. Clin. Neurosci 17(3) (2010) 385–386. [DOI] [PubMed] [Google Scholar]
- [171].Mayr BJ, Bonelli RM, Niederwieser G, Költringer P, Reisecker F, Zolpidem in progressive supranuclear palsy, Eur. J. Neurol. 9(2) (2002) 184–185. [DOI] [PubMed] [Google Scholar]
- [172].Bhatt MH, Podder N, Chokroverty S, Sleep and neurodegenerative diseases, Semin. Neurol, Copyright© 2005 by Thieme Medical Publishers, Inc., 333 Seventh Avenue, New; …, 2005, pp. 39–51. [DOI] [PubMed] [Google Scholar]
- [173].Radicati FG, Martinez Martin P, Fossati C, Chaudhuri KR, Torti M, Rodriguez Blazquez C, Vacca L, Stocchi F, Non motor symptoms in progressive supranuclear palsy: prevalence and severity, NPJ Parkinsons Dis 3 (2017) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Aldrich MS, Foster NL, White RF, Bluemlein L, Ba GP, Sleep abnormalities in progressive supranuclear palsy, Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society 25(6) (1989) 577–581. [DOI] [PubMed] [Google Scholar]
- [175].Kompoliti K, Goetz C, Litvan I, Jellinger K, Verny M, Pharmacological therapy in progressive supranuclear palsy, Arch. Neurol. 55(8) (1998) 1099–1102. [DOI] [PubMed] [Google Scholar]
- [176].Engel PA, Treatment of progressive supranuclear palsy with amitriptyline: therapeutic and toxic effects, J. Am. Geriatr. Soc. 44(9) (1996) 1072–1074. [DOI] [PubMed] [Google Scholar]
- [177].Litvan I, Phipps M, Pharr VL, Hallett M, Grafman J, Salazar A, Randomized placebo-controlled trial of donepezil in patients with progressive supranuclear palsy, Neurology 57(3) (2001) 467–73. [DOI] [PubMed] [Google Scholar]
- [178].Müller J, Wenning G, Wissel J, Seppi K, Poewe W, Botulinum toxin treatment in atypical parkinsonian disorders associated with disabling focal dystonia, J. Neurol. 249(3) (2002) 300–304. [DOI] [PubMed] [Google Scholar]
- [179].Polo KB, Jabbari B, Botulinum toxin-A improves the rigidity of progressive supranuclear palsy, Ann. Neurol. 35(2) (1994) 237–9. [DOI] [PubMed] [Google Scholar]
- [180].Gómez-Caravaca MT, Cáceres-Redondo MT, Huertas-Fernández I, Vargas-González L, Carrillo F, Carballo M, Mir P, The use of botulinum toxin in the treatment of sialorrhea in parkinsonian disorders, Neurol. Sci 36(2) (2015) 275–279. [DOI] [PubMed] [Google Scholar]
- [181].Piccione F, Mancini E, Tonin P, Bizzarini M, Botulinum toxin treatment of apraxia of eyelid opening in progressive supranuclear palsy: report of two cases, Arch. Phys. Med. Rehabil. 78(5) (1997) 525–529. [DOI] [PubMed] [Google Scholar]
- [182].Hyson HC, Johnson AM, Jog MS, Sublingual atropine for sialorrhea secondary to parkinsonism: a pilot study, Mov. Disord. 17(6) (2002) 1318–1320. [DOI] [PubMed] [Google Scholar]
- [183].Pattee GL, Wymer JP, Lomen-Hoerth C, Appel SH, Formella AE, Pope LE, An open-label multicenter study to assess the safety of dextromethorphan/quinidine in patients with pseudobulbar affect associated with a range of underlying neurological conditions, Curr. Med. Res. Opin. 30(11) (2014) 2255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Corey-Bloom J, Wolfson T, Gamst A, Jin S, Marcotte TD, Bentley H, Gouaux B, Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial, CMAJ 184(10) (2012) 1143–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D, Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology, Neurology 82(17) (2014) 1556–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Hill KP, Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review, JAMA 313(24) (2015) 2474–2483. [DOI] [PubMed] [Google Scholar]
- [187].Schierenbeck T, Riemann D, Berger M, Hornyak M, Effect of illicit recreational drugs upon sleep: cocaine, ecstasy and marijuana, Sleep Med. Rev. 12(5) (2008) 381–389. [DOI] [PubMed] [Google Scholar]
- [188].Clerici I, Ferrazzoli D, Maestri R, Bossio F, Zivi I, Canesi M, Pezzoli G, Frazzitta G, Rehabilitation in progressive supranuclear palsy: Effectiveness of two multidisciplinary treatments, PLoS One 12(2) (2017) e0170927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Zampieri C, Di Fabio RP, Balance and eye movement training to improve gait in people with progressive supranuclear palsy: quasi-randomized clinical trial, Phys. Ther. 88(12) (2008) 1460–1473. [DOI] [PubMed] [Google Scholar]
- [190].Wiblin L, Lee M, Burn D, Palliative care and its emerging role in multiple system atrophy and progressive supranuclear palsy, Parkinsonism Relat. Disord. 34 (2017) 7–14. [DOI] [PubMed] [Google Scholar]
- [191].Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR, Tau protein isoforms, phosphorylation and role in neurodegenerative disorders, Brain Res. Rev. 33(1) (2000) 95–130. [DOI] [PubMed] [Google Scholar]
- [192].Ferrer I, Barrachina M, Puig B, Glycogen synthase kinase-3 is associated with neuronal and glial hyperphosphorylated tau deposits in Alzheimer’s disease, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration, Acta Neuropathol. 104(6) (2002) 583–91. [DOI] [PubMed] [Google Scholar]
- [193].Long ZM, Zhao L, Jiang R, Wang KJ, Luo SF, Zheng M, Li XF, He GQ, Valproic Acid Modifies Synaptic Structure and Accelerates Neurite Outgrowth Via the Glycogen Synthase Kinase-3beta Signaling Pathway in an Alzheimer’s Disease Model, CNS Neurosci. Ther 21(11) (2015) 887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Nakashima H, Ishihara T, Suguimoto P, Yokota O, Oshima E, Kugo A, Terada S, Hamamura T, Trojanowski JQ, Lee VM, Kuroda S, Chronic lithium treatment decreases tau lesions by promoting ubiquitination in a mouse model of tauopathies, Acta Neuropathol. 110(6) (2005) 547–56. [DOI] [PubMed] [Google Scholar]
- [195].Noble W, Planel E, Zehr C, Olm V, Meyerson J, Suleman F, Gaynor K, Wang L, LaFrancois J, Feinstein B, Burns M, Krishnamurthy P, Wen Y, Bhat R, Lewis J, Dickson D, Duff K, Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo, Proc. Natl. Acad. Sci. U. S. A. 102(19) (2005) 6990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Leclair-Visonneau L, Rouaud T, Debilly B, Durif F, Houeto JL, Kreisler A, Defebvre L, Lamy E, Volteau C, Nguyen JM, Dily SL, Damier P, Boutoleau-Bretonniere C, Lejeune P, Derkinderen P, Randomized placebo-controlled trial of sodium valproate in progressive supranuclear palsy, Clin. Neurol. Neurosurg. 146 (2016) 35–9. [DOI] [PubMed] [Google Scholar]
- [197].Tolosa E, Litvan I, Hoglinger GU, Burn D, Lees A, Andres MV, Gomez-Carrillo B, Leon T, Del Ser T, A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy, Mov. Disord. 29(4) (2014) 470–8. [DOI] [PubMed] [Google Scholar]
- [198].Hoglinger GU, Huppertz HJ, Wagenpfeil S, Andres MV, Belloch V, Leon T, Del Ser T, Tideglusib reduces progression of brain atrophy in progressive supranuclear palsy in a randomized trial, Mov. Disord. 29(4) (2014) 479–87. [DOI] [PubMed] [Google Scholar]
- [199].Min S-W, Chen X, Tracy TE, Li Y, Zhou Y, Wang C, Shirakawa K, Minami SS, Defensor E, Mok SA, Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits, Nat. Med. 21(10) (2015) 1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Yuzwa SA, Shan X, Macauley MS, Clark T, Skorobogatko Y, Vosseller K, Vocadlo DJ, Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation, Nat. Chem. Biol. 8(4) (2012) 393–9. [DOI] [PubMed] [Google Scholar]
- [201].Wang AC, Jensen EH, Rexach JE, Vinters HV, Hsieh-Wilson LC, Loss of O-GlcNAc glycosylation in forebrain excitatory neurons induces neurodegeneration, Proc. Natl. Acad. Sci. U. S. A. 113(52) (2016) 15120–15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Shoeibi A, Olfati N, Litvan I, Frontrunner in Translation: Progressive Supranuclear Palsy, Front. Neurol 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Beaudoin-Chabot C, Wang L, Smarun AV, Vidović D, Shchepinov MS, Thibault G, Deuterated polyunsaturated fatty acids reduce oxidative stress and extend the lifespan of C. elegans, Front. Physiol 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Magen I, Ostritsky R, Richter F, Zhu C, Fleming SM, Lemesre V, Stewart AJ, Morimoto BH, Gozes I, Chesselet MF, Intranasal NAP (davunetide) decreases tau hyperphosphorylation and moderately improves behavioral deficits in mice overexpressing alpha-synuclein, Pharmacology research & perspectives 2(5) (2014) e00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Boxer AL, Lang AE, Grossman M, Knopman DS, Miller BL, Schneider LS, Doody RS, Lees A, Golbe LI, Williams DR, Corvol JC, Ludolph A, Burn D, Lorenzl S, Litvan I, Roberson ED, Hoglinger GU, Koestler M, Jack CR Jr., Van Deerlin V, Randolph C, Lobach IV, Heuer HW, Gozes I, Parker L, Whitaker S, Hirman J, Stewart AJ, Gold M, Morimoto BH, Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial, Lancet Neurol. 13(7) (2014) 676–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Fitzgerald DP, Emerson DL, Qian Y, Anwar T, Liewehr DJ, Steinberg SM, Silberman S, Palmieri D, Steeg PS, TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells, Mol. Cancer Ther. 11(9) (2012) 1959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Boxer A MZ, Tsai R, Koestler M, Rojas J, Ljubenkov P, Rosen H, Rabinovici G, Fagan-Niven A, Cobigo Y, Jung J, Luong P, Chuu E, Powers R, Mumford P, Miller B, Roberson E, A PHASE 1B, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED, SEQUENTIAL COHORT, DOSE-RANGING STUDY OF THE SAFETY, TOLERABILITY, PHARMACOKINETICS, PHARMACODYNAMICS, AND PRELIMINARY EFFICACY OF TPI 287 (ABEOTAXANE) IN PATIENTS WITH PRIMARY FOUR REPEAT TAUOPATHIES: CORTICOBASAL SYNDROME OR PROGRESSIVE SUPRANUCLEAR PALSY; OR THE SECONDARY TAUOPATHY, ALZHEIMER’S DISEASE., J Prev Alz Dis 4(4) (2017) 282–428. [Google Scholar]
- [208].Zhang B, Carroll J, Trojanowski JQ, Yao Y, Iba M, Potuzak JS, Hogan AM, Xie SX, Ballatore C, Smith AB 3rd, Lee VM, Brunden KR, The microtubule-stabilizing agent, epothilone D, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice, J. Neurosci. 32(11) (2012) 3601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Peeraer E, Bottelbergs A, Van Kolen K, Stancu I-C, Vasconcelos B, Mahieu M, Duytschaever H, Ver Donck L, Torremans A, Sluydts E, Intracerebral injection of preformed synthetic tau fibrils initiates widespread tauopathy and neuronal loss in the brains of tau transgenic mice, Neurobiol. Dis. 73 (2015) 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Guo JL, Narasimhan S, Changolkar L, He Z, Stieber A, Zhang B, Gathagan RJ, Iba M, McBride JD, Trojanowski JQ, Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice, J. Exp. Med. 213(12) (2016) 2635–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Ahmed Z, Cooper J, Murray TK, Garn K, McNaughton E, Clarke H, Parhizkar S, Ward MA, Cavallini A, Jackson S, A novel in vivo model of tau propagation with rapid and progressive neurofibrillary tangle pathology: the pattern of spread is determined by connectivity, not proximity, Acta Neuropathol. 127(5) (2014) 667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI, Trans-cellular propagation of Tau aggregation by fibrillar species, J. Biol. Chem. 287(23) (2012) 19440–19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Lee S-J, Desplats P, Sigurdson C, Tsigelny I, Masliah E, Cell-to-cell transmission of non-prion protein aggregates, Nature Reviews Neurology 6(12) (2010) 702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM, Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements, J. Neurosci. 27(34) (2007) 9115–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H, Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice, Exp. Neurol. 224(2) (2010) 472–85. [DOI] [PubMed] [Google Scholar]
- [216].Bi M, Ittner A, Ke YD, Gotz J, Ittner LM, Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice, PLoS One 6(12) (2011) e26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Rozenstein-Tsalkovich L, Grigoriadis N, Lourbopoulos A, Nousiopoulou E, Kassis I, Abramsky O, Karussis D, Rosenmann H, Repeated immunization of mice with phosphorylated-tau peptides causes neuroinflammation, Exp. Neurol. 248 (2013) 451–6. [DOI] [PubMed] [Google Scholar]
- [218].Selenica ML, Davtyan H, Housley SB, Blair LJ, Gillies A, Nordhues BA, Zhang B, Liu J, Gestwicki JE, Lee DC, Gordon MN, Morgan D, Dickey CA, Epitope analysis following active immunization with tau proteins reveals immunogens implicated in tau pathogenesis, J. Neuroinflammation 11 (2014) 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Theunis C, Crespo-Biel N, Gafner V, Pihlgren M, Lopez-Deber MP, Reis P, Hickman DT, Adolfsson O, Chuard N, Ndao DM, Borghgraef P, Devijver H, Van Leuven F, Pfeifer A, Muhs A, Efficacy and safety of a liposome-based vaccine against protein Tau, assessed in tau.P301L mice that model tauopathy, PLoS One 8(8) (2013) e72301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Congdon EE, Sigurdsson EM, Tau-targeting therapies for Alzheimer disease, Nature Reviews Neurology 14(7) (2018) 399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Bright J, Hussain S, Dang V, Wright S, Cooper B, Byun T, Ramos C, Singh A, Parry G, Stagliano N, Human secreted tau increases amyloid-beta production, Neurobiol. Aging 36(2) (2015) 693–709. [DOI] [PubMed] [Google Scholar]
- [222].Qureshi IA, Tirucherai G, Ahlijanian MK, Kolaitis G, Bechtold C, Grundman M, A randomized, single ascending dose study of intravenous BIIB092 in healthy participants, Alzheimer’s & Dementia: Translational Research & Clinical Interventions 4 (2018) 746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Boxer AL, Qureshi I, Ahlijanian M, Grundman M, Golbe LI, Litvan I, Honig LS, Tuite P, McFarland NR, O’Suilleabhain P, Xie T, Tirucherai GS, Bechtold C, Bordelon Y, Geldmacher DS, Grossman M, Isaacson S, Zesiewicz T, Olsson T, Muralidharan KK, Graham DL, O’Gorman J, Haeberlein SB, Dam T, Safety of the tau-directed monoclonal antibody BIIB092 in progressive supranuclear palsy: a randomised, placebo-controlled, multiple ascending dose phase 1b trial, Lancet Neurol. 18(6) (2019) 549–558. [DOI] [PubMed] [Google Scholar]
- [224].Dam T, Boxer A, Golbe LI, Höglinger G, Morris HR, Litvan I, Corvol J-C, Lang A, Yuasa T, Bechtold C, Efficacy and Safety of BIIB092 in Patients with Progressive Supranuclear Palsy: PASSPORT Phase 2 Study Design (P6. 073), AAN Enterprises, 2018. [Google Scholar]
- [225].Budur K, West T, Braunstein JB, Fogelman I, Bordelon YM, Litvan I, Roberson ED, Hu H, Verghese PB, Bateman RJ, Results of a phase 1, single ascending dose, placebo-controlled study of ABBV-8E12 in patients with Progressive Supranuclear Palsy and phase 2 study design in early Alzheimer’s disease, Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 13(7) (2017) P599–P600. [Google Scholar]
- [226].West T, Hu Y, Verghese P, Bateman R, Braunstein J, Fogelman I, Budur K, Florian H, Mendonca N, Holtzman D, Preclinical and clinical development of ABBV-8E12, a humanized anti-tau antibody, for treatment of Alzheimer’s disease and other tauopathies, J Prev Alzheimers Dis 4(04) (2017) 236–241. [DOI] [PubMed] [Google Scholar]
- [227].Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM, Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain, J. Neurochem. 118(4) (2011) 658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O’Neill MJ, Hutton ML, Citron M, Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression, J. Biol. Chem. 286(39) (2011) 34457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Walls KC, Ager RR, Vasilevko V, Cheng D, Medeiros R, LaFerla FM, p-Tau immunotherapy reduces soluble and insoluble tau in aged 3xTg-AD mice, Neurosci. Lett. 575 (2014) 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Collin L, Bohrmann B, Göpfert U, Oroszlan-Szovik K, Ozmen L, Grüninger F, Neuronal uptake of tau/pS422 antibody and reduced progression of tau pathology in a mouse model of Alzheimer’s disease, Brain 137(10) (2014) 2834–2846. [DOI] [PubMed] [Google Scholar]
- [231].Sankaranarayanan S, Barten DM, Vana L, Devidze N, Yang L, Cadelina G, Hoque N, DeCarr L, Keenan S, Lin A, Cao Y, Snyder B, Zhang B, Nitla M, Hirschfeld G, Barrezueta N, Polson C, Wes P, Rangan VS, Cacace A, Albright CF, Meredith J Jr., Trojanowski JQ, Lee VM, Brunden KR, Ahlijanian M, Passive immunization with phospho-tau antibodies reduces tau pathology and functional deficits in two distinct mouse tauopathy models, PLoS One 10(5) (2015) e0125614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Ittner A, Bertz J, Suh LS, Stevens CH, Gotz J, Ittner LM, Tau-targeting passive immunization modulates aspects of pathology in tau transgenic mice, J. Neurochem. 132(1) (2015) 135–45. [DOI] [PubMed] [Google Scholar]
- [233].Castillo-Carranza DL, Gerson JE, Sengupta U, Guerrero-Muñoz MJ, Lasagna-Reeves CA, Kayed R, Specific targeting of tau oligomers in htau mice prevents cognitive impairment and tau toxicity following injection with brain-derived tau oligomeric seeds, J. Alzheimers Dis. 40(s1) (2014) S97–S111. [DOI] [PubMed] [Google Scholar]
- [234].Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, Guerrero-Munoz MJ, Kiritoshi T, Neugebauer V, Jackson GR, Kayed R, Alzheimer brain-derived tau oligomers propagate pathology from endogenous tau, Sci. Rep. 2 (2012) 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Chen N, Wang W, Fauty S, Fang Y, Hamuro L, Hussain A, Prueksaritanont T, The effect of the neonatal Fc receptor on human IgG biodistribution in mice, MAbs, Taylor & Francis, 2014, pp. 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Mavoungou C, Schindowski K, Immunotherapy with anti-abeta monoclonal antibodies in Alzheimer’s disease: A critical review on the molecules in the pipelines with regulatory considerations, Front Clin Drug Res Alzheimer Disord 1 (2013) 3–85. [Google Scholar]
- [237].Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease, Nature 537(7618) (2016) 50–56. [DOI] [PubMed] [Google Scholar]
- [238].Poduslo JF, Curran GL, Berg CT, Macromolecular permeability across the blood-nerve and blood-brain barriers, Proceedings of the National Academy of Sciences 91(12) (1994) 5705–5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Felgenhauer K, Protein size and cerebrospinal fluid composition, Klin. Wochenschr 52(24) (1974) 1158–1164. [DOI] [PubMed] [Google Scholar]
- [240].Igawa T, Tsunoda H, Kuramochi T, Sampei Z, Ishii S, Hattori K, Engineering the variable region of therapeutic IgG antibodies, MAbs 3(3) (2011) 243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP, Inhibition of neuronal maturation in primary hippocampal neurons from τ deficient mice, J. Cell Sci. 114(6) (2001) 1179–1187. [DOI] [PubMed] [Google Scholar]
- [242].Morris M, Hamto P, Adame A, Devidze N, Masliah E, Mucke L, Age-appropriate cognition and subtle dopamine-independent motor deficits in aged tau knockout mice, Neurobiol. Aging 34(6) (2013) 1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, Bush AI, Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export, Nat. Med. 18(2) (2012) 291–5. [DOI] [PubMed] [Google Scholar]
- [244].Lei P, Ayton S, Moon S, Zhang Q, Volitakis I, Finkelstein DI, Bush AI, Motor and cognitive deficits in aged tau knockout mice in two background strains, Mol. Neurodegener 9 (2014) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Ma QL, Zuo X, Yang F, Ubeda OJ, Gant DJ, Alaverdyan M, Kiosea NC, Nazari S, Chen PP, Nothias F, Chan P, Teng E, Frautschy SA, Cole GM, Loss of MAP function leads to hippocampal synapse loss and deficits in the Morris Water Maze with aging, J. Neurosci. 34(21) (2014) 7124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Ahmed T, Van der Jeugd A, Blum D, Galas M-C, D’Hooge R, Buee L, Balschun D, Cognition and hippocampal synaptic plasticity in mice with a homozygous tau deletion, Neurobiol. Aging 35(11) (2014) 2474–2478. [DOI] [PubMed] [Google Scholar]
- [247].Peacey E, Rodriguez L, Liu Y, Wolfe MS, Targeting a pre-mRNA structure with bipartite antisense molecules modulates tau alternative splicing, Nucleic Acids Res. (2012) gks710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].DeVos SL, Miller TM, Antisense oligonucleotides: treating neurodegeneration at the level of RNA, Neurotherapeutics 10(3) (2013) 486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].DeVos SL, Miller RL, Schoch KM, Holmes BB, Kebodeaux CS, Wegener AJ, Chen G, Shen T, Tran H, Nichols B, Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy, Sci. Transl. Med. 9(374) (2017) eaag0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Schoch KM, DeVos SL, Miller RL, Chun SJ, Norrbom M, Wozniak DF, Dawson HN, Bennett CF, Rigo F, Miller TM, Increased 4R-tau induces pathological changes in a human-tau mouse model, Neuron 90(5) (2016) 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Xu H, W Rosler T, Carlsson T, de Andrade A, Fiala O, Hollerhage M, H Oertel W, Goedert M, Aigner A, U Hoglinger G, Tau silencing by siRNA in the P301S mouse model of tauopathy, Curr. Gene Ther. 14(5) (2014) 343–351. [DOI] [PubMed] [Google Scholar]
- [252].Gomes MJ, Dreier J, Brewer J, Martins S, Brandl M, Sarmento B, A new approach for a blood-brain barrier model based on phospholipid vesicles: Membrane development and siRNA-loaded nanoparticles permeability, Journal of Membrane Science 503 (2016) 8–15. [Google Scholar]
- [253].Gao X, Qian J, Zheng S, Changyi Y, Zhang J, Ju S, Zhu J, Li C, Overcoming the blood-brain barrier for delivering drugs into the brain by using adenosine receptor nanoagonist, ACS nano 8(4) (2014) 3678–89. [DOI] [PubMed] [Google Scholar]
- [254].Scoles DR, Minikel EV, Pulst SM, Antisense oligonucleotides: a primer, Neurology Genetics 5(2) (2019) e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Darras BT, Farrar MA, Mercuri E, Finkel RS, Foster R, Hughes SG, Bhan I, Farwell W, Gheuens S, An Integrated Safety Analysis of Infants and Children with Symptomatic Spinal Muscular Atrophy (SMA) Treated with Nusinersen in Seven Clinical Trials, CNS drugs (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Grondin R, Kaytor MD, Ai Y, Nelson PT, Thakker DR, Heisel J, Weatherspoon MR, Blum JL, Burright EN, Zhang Z, Kaemmerer WF, Six-month partial suppression of Huntingtin is well tolerated in the adult rhesus striatum, Brain 135(Pt 4) (2012) 1197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Franich NR, Fitzsimons HL, Fong DM, Klugmann M, During MJ, Young D, AAV Vector–mediated RNAi of mutant Huntingtin expression is neuroprotective in a novel genetic rat model of Huntington’s disease, Mol. Ther. 16(5) (2008) 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Raoul C, Abbas-Terki T, Bensadoun J-C, Guillot S, Haase G, Szulc J, Henderson CE, Aebischer P, Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS, Nat. Med. 11(4) (2005) 423–428. [DOI] [PubMed] [Google Scholar]