Abstract
The B-cell coreceptor, CD19 is a transmembrane protein expressed throughout B-cell ontogeny from pro–B cell to plasmablast. It plays an important role in B-cell development and function and is an attractive target for antibody-directed immunotherapies against B-cell malignancies, including acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL), and non-Hodgkin lymphoma (B-NHL) in humans. With the rapid development of next-generation immunotherapies aimed at improving therapeutic efficacy, there is a pressing need for a clinically relevant, immune-competent, spontaneous animal model to derisk these new approaches and inform human immunotherapy clinical trials. Pet dogs develop spontaneous B-cell malignancies, including B-NHL and leukemias that share comparable oncogenic pathways and similar immunosuppressive features to human B-cell malignancies. Despite treatment with multiagent chemotherapy, durable remissions in canine B-NHL are rare and most dogs succumb to their disease within 1 year of diagnosis. Here we report the development and validation of an anti-canine CD19-targeting monoclonal antibody and its single-chain derivatives, which enable next-generation CD19-targeted immunotherapies to be developed and evaluated in client-owned dogs with spontaneous B-NHL. These future in vivo studies aim to provide important information regarding the safety and therapeutic efficacy of CD19-targeted mono- and combination therapies and identify correlative biomarkers of response that will help to inform human clinical trial design. In addition, development of canine CD19-targeted immunotherapies aims to provide better therapeutic options for pet dogs diagnosed with B-cell malignancies.
Keywords: B-cell lymphoma, canine, CD19, large animal model, monoclonal antibody, single-chain variable fragment, immunotherapy
Many Food and Drug Administration (FDA)–approved cancer immunotherapies in humans are monoclonal antibodies (mAb) or are based on mAb derivatives and include single-chain variable fragments (scFvs), bispecific antibodies, bispecific T-cell engagers (BiTEs), and chimeric antigen receptor (CAR) T cells. Target antigens for antibody-directed therapy include those that are expressed exclusively by malignant cells or the malignant cell of origin, or those that exhibit quantitative or qualitative differences in surface expression enabling selective targeting of malignant cells. In humans, the B-cell surface antigen CD19 is a 95-kDa type I transmembrane glycoprotein belonging to the immunoglobulin (Ig) superfamily.40 It is expressed exclusively on B cells from pro–B cell to early plasmablast and on follicular dendritic cells. It plays an important role in B-cell development and, when complexed with CD21, functions as a B-cell coreceptor, lowering the threshold for immunoglobulin-associated B-cell activation.7,47 Due to its restricted lineage expression and persistence throughout B-cell ontogeny, it has emerged as an attractive target for immunotherapies used to treat B-cell malignancies, autoimmune diseases, and inflammatory disorders.16
Blinatumomab, a BiTE consisting of an anti-CD19 scFv linked to an anti-CD3 scFv, was the first human CD19-targeting agent to received FDA approval for the treatment of relapsed or refractory B-acute lymphocytic leukemia (B-ALL). More recently, CD19-targeting CAR T cells (CART19; Kymriah and Yescarta) have received FDA approval for the treatment of B-ALL and/or B-cell non-Hodgkin lymphoma (B-NHL).2,6,35 While the clinical responses of human patients with B-cell malignancies have been impressive with CD19-targeted therapies, many challenges still exist for the field. For CAR T cells, these include generation of effective CAR T-cell products from patients with advanced disease,10,30 target-antigen escape and adaptive resistance leading to clinical relapse,12,21,34 trafficking to tumor sites and failure of CAR T-cell persistence, functional inhibition in the immune suppressive tumor microenvironment (TME),43 and morbidity associated with cytokine release syndrome and central nervous system (CNS) toxicity.14 To overcome some of these barriers, advanced next-generation CAR technologies that include allogeneic and universal CAR T cells,41,51 armored CARs,42,50 and CAR T cells redirected for universal cytokine killing (TRUCKs)8 are now being developed and combination approaches using checkpoint inhibitors and TME modifiers are being investigated.9,25 Evaluation of these next-generation and combination therapies in a spontaneous, immunologically intact, large animal “model” system that accurately recapitulates the immunological barriers to therapeutic success and the intact cytokine networks that contribute to toxicity may more accurately inform future human CAR T-cell trials when compared to many current murine model systems.
There has been a surge of interest in integrating pet dogs with spontaneous cancer into the preclinical drug development paradigm with special emphasis in the immuno-oncology space.31,32,46 Immunologically intact pet dogs spontaneously develop cancers that have escaped immune recognition and present a hostile TME that contributes to T-cell exclusion and exhaustion.29,48 Thus, canine cancer patients pose challenges to effective immunotherapy similar to human patients, and given their comparable physiology to humans as well as intact immunological networks, they are also likely to develop similar therapy-related side effects.4 B-NHL is the most common canine hematopoietic cancer that occurs with an incidence of 13 to 24 of 100 000 dogs per year.44 Standard of care consists of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)–based chemotherapy, and although 85% of canine patients achieve clinical remission with this regime, the majority will relapse and succumb to their disease within 1 year of diagnosis.45 Treatment is much less effective for diffuse large B-cell lymphoma (DLBCL) in dogs than in humans, a fact that is thought to be associated with an intrinsically more aggressive disease, the use of lower doses of chemotherapy to maintain good quality of life, and the lack of a mAb targeting CD20 (eg, rituximab), which is routinely employed in combination with CHOP in human patients. As such, novel approaches that induce durable remissions in canine lymphoma patients are needed. CD19 is a clinically relevant target for B-NHL and B-cell leukemias in human patients and is likely to be an important target in the treatment of these diseases in dogs. Therefore, we have developed and validated a murine anti-canine CD19 mAb and its scFv derivative that can be used for diagnostic and therapeutic purposes in dogs with the aim to benefit both human and canine health.
Materials and Methods
Mice and Immunization Protocol
BALB/c mice were immunized subcutaneously (SQ) with 50 μg C-terminal, HIS-tagged recombinant canine CD19 (cCD19 extracellular domain [ECD] 21–292 aa) in complete Freund’s adjuvant. The nucleotide sequence used to generate the protein was taken from the NCBI reference sequence XM_005621381.3 and generated by GenScript. Briefly, the DNA sequence was synthesized with the C-terminal His tag and cloned into the pET expression vector. Escherichia coli BL21 (DE3) was transformed, and a single colony was inoculated into medium containing kanamycin. Protein production was induced using isopropyl β-D-1-thiogalactopyranoside (IPTG). Protein was harvested from inclusion bodies and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot, using a mouse anti-His mAb. Protein concentration was determined by Bicinchoninic Acid (BCA) protein assay. Mice received 2 sub-cutaneous booster immunizations with 25 μg cCD19 ECD in incomplete Freund’s adjuvant at 14-day intervals. Test bleeds were performed 1 week after the third immunization. Mice received a final booster immunization with 25 μg cCD19 ECD intravenously 50 days after the first immunization. Four days after the final boost, mice were sacrificed and splenocytes were harvested to generate hybridomas.
Cells and Cell Lines
Peripheral blood mononuclear cells (PBMCs) were obtained from a heparinized healthy donor dog blood by discontinuous density centrifugation over Ficoll-Paque PLUS (GE Healthcare, Chicago, IL). Cells were washed twice in complete (c)RPMI medium (RPMI 1640 containing 2 mM L-glutamine; Mediatech, Manassas, VA), 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Flowery Branch, GA), 10 mM HEPES (Gibco, Grand Island, NY), and 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco) prior to their use in flow cytometry or Western blot. The canine B-cell lymphoma cell line, CLBL-1 line (a gift from Dr. Rutgen, University of Vienna, Austria), was grown in cRPMI. All K562 cell lines were cultured in cRPMI supplemented with 1 mM sodium pyruvate (Mediatech) and 30 μg/mL gentamicin (Gibco).
Generation of a CD19-expressing target cell line
To generate a canine CD19 (cCD19)–expressing target cell line for antibody validation, the full-length cCD19 was amplified from complementary DNA (cDNA) derived from CLBL-1 cells by reverse transcription polymerase chain reaction (RT-PCR), and the resulting cDNA was cloned into a bicistronic, self-inactivating pCLPS lentiviral vector 5’ to an internal ribosomal entry site-green fluorescent protein (IRES-GFP), where transgene expression was under the control of the cytomegalovirus (CMV) promoter. Lentivirus was generated using a split genome approach as previously described28 and used to stably transduce the human erythroleukemic K562 cell line to generate KT.cCD19 target cells as previously described.39 K562 cells transduced with lentivirus containing canine OX40L IRES-GFP were used as cCD19-negative, control target cells. Target cells were single cell sorted based on GFP expression and clones were expanded to produce KT.cCD19 or KT.cOX40L.
Evaluation of CD19 messenger RNA in canine PBMCs and CLBL-1 cells
Total RNA was extracted from canine PBMCs and CLBL-1 cells using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA). Reverse transcription was performed using random hexamers and Superscript III reverse transcriptase as per the manufacturer’s instructions (ThermoFisher Scientific, Waltham, MA), followed by a RNAse H digestion to remove any remaining RNA. Primers to amplify the extracellular, N-terminal domain of canine CD19 were designed using Primer3 software (http://frodo.wi.mit.edu/primer3/). Primer sequences were as follows:cCD19 forward:5’-CACTGCAGGTGGAGGCTAAA-3’, cCD19 reverse: 5’TTGAGGCTCTGGTTCAGAGTGC-3’. Primers for the housekeeping gene GAPDH were as follows: cGAPDH forward: 5’-GGCAAATTCCACGGCACAGTCAAGGC-3’ cGAPDH reverse: 5’CAGAGGGGCCGTCCACGGTCTTCTGGGTGG-3’. Primers were synthesized by Sigma-Aldrich (St. Louis, MO). A conventional PCR with the following thermal cycling program was used: 95°C for 5minutes; 30 cycles of 94°C for 15seconds, 65°C for 30seconds, and 72°C for 1 minutes; and 72°C for 10minutes and cooling to 4°C. Water was used in place of cDNA as a negative control. Expected band size for the amplified extracellular domain of cCD19 was 475 bp.
Hybridoma Generation and Screening
Mouse splenocytes were fused with the multiple myeloma fusion partner SP2/0. Hybridoma cells were selected in hypoxanthine aminopterin thymidine (HAT) media and supernatants were screened for cCD19 specificity by indirect enzyme-linked immunosorbent assay (ELISA). Briefly, ELISA plates were coated with 1 μg/ml of either canine CD19 ECD or an irrelevant HIS-tagged protein in 1× phosphate-buffered saline (PBS). Serial dilutions of supernatants were incubated with plate-bound antigens for 2 hours at 37°C. Plates were washed and incubated with peroxidase-AffiniPure goat anti-mouse IgG (Fcγ fragment specific) and developed using a horseradish peroxidase (HRP) substrate (Suppl. Fig. S1). Selected hybridomas were subcloned and expanded in DMEM (10% FBS + 90% DMEM). Monoclonal antibodies were purified from selected supernatants over an Ultra Protein A Resin column (GenScript, Piscataway, NJ). Antibody isotype was determined using the SBA Clonotyping System–HRP (Southern Biotech, Birmingham, AL).
Flow Cytometry
Canine and feline PBMCs and the CLBL-1 and Nalm6 cell lines were washed twice in flow cytometry staining (FACS) buffer (1% heat-inactivated FBS in 1× PBS with calcium and magnesium). Canine cells were blocked with 10 μg canine IgG for 10 minutes at room temperature prior to cell surface labeling with mouse sera (1:100 dilution), hybridoma supernatant (undiluted), or 250 ng mAb. Specificity of mAb binding was evaluated in blocking experiments where 250 ng mAb was preincubated with 1.25 μg cCD19 ECD peptide for 1 hour at room temperature (RT) prior to incubation with target cells. Cells were washed in FACS buffer and incubated with either unconjugated mAb or unconjugated antibody preincubated with soluble cCD19. After washing, antibody binding was detected using polyclonal BV421-conjugated goat antimouse IgG (Bio-Legend, San Diego, CA) and the viability dye 7-AAD (Bio-Legend). Following surface labeling, cells were washed twice in FACS buffer and fixed in 1% paraformaldehyde (Thermo Fisher Scientific). For cell surface labeling using M13 phage-expressing scFv, target cells and phage were preincubated in 5% milk (w/v) in PBS for 1 hour at 4°C. Cells were incubated with 1010 to 1011 phage particles for 30 minutes at room temperature and then washed twice in FACS buffer. Bound phage was detected using PE-conjugated anti-M13 phage antibody (BioRad, Hercules, CA). Cells were washed and fixed in 1% paraformaldehyde prior to acquisition. Where intracellular labeling was performed, cells were permeabilized with 0.1% saponin (Millipore Sigma, Burlington, MA) after paraformaldehyde fixation and incubated with the cross-reactive APC-labeled mouse antihuman CD79a antibody (clone HM57; BD Biosciences, San Jose, CA). Cells were washed once in 0.1% saponin and resuspended in FACS buffer prior to acquisition and analysis. Acquisition was performed on a FACS Canto II flow cytometer (BD Biosciences) and data were analyzed using FlowJo software version X (Treestar, Ashland, OR). All plots shown were gated on 7-AAD–negative cells.
Western Blot
Whole-cell lysates from canine peripheral blood lymphocytes and the canine B-cell lymphoma cell line CLBL-1 were prepared using 1% NP-40 lysis buffer containing 50 mM Tris-HCl (pH 7.4) and 150 mM NaCl, and they were supplemented with 2.5 mM EDTA, 100 mM Na3VO4, 1 mM NaF, and 1 mM PMSF. After cell lysis, samples were centrifuged at 1300 rpm for 10 minutes at 4°C and supernatants were collected. Protein concentration was determined by Pierce BCA Protein Assay (Thermo Fisher Scientific). Then, 100 mM DTT was added to 20 μg of each protein sample, and samples were then boiled at 99°C for 5 minutes and resolved by 10% SDS-PAGE. Proteins were transferred onto a Polyvinylidene fluoride (PVDF) membrane, blocked for 2 hours at RT with 5% bovine serum albumin (BSA) in TRIS buffered saline-Tween (TBS-T), and then incubated with mAb overnight at 4°C. Membranes were probed with polyclonal HRP-labeled rabbit antimouse IgG secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at RT, and protein bands were detected using the Supersignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific).
Immunohistochemistry
Formalin-fixed and paraffin-embedded retropharyngeal lymph nodes from 2 healthy dogs were retrieved from the archive of the anatomic pathology diagnostic service at the School of Veterinary Medicine at the University of Pennsylvania. Then, 5-μm-thick paraffin sections mounted on Fisherbrand ProbeOn slides (Thermo Fisher Scientific) were prepared from these tissues. For studies on other tissue types, a canine tissue microarray (TMA) containing 30 normal canine tissues arranged in a 40-spot TMA format was used. Tissues included on the TMA were bone marrow, thymus, tonsil, lymph node, adrenal gland, thyroid, salivary gland, esophagus, stomach, small intestine, colon, liver, pancreas, heart, lung, cerebrum, cerebellum, brainstem, spinal cord, skeletal muscle, kidney (medulla and cortex), urinary bladder, adipose, skin, mammary gland, ovary, prostate, epididymis, and testis. The Leica BOND RXm automated platform combined with the Polymer Refine Detection kit (Leica Biosystems, Wetzlar, Germany) was used to perform the immunohistochemistry (IHC) workup. After dewaxing and rehydration of paraffin sections, sequential titers of the mouse anti-CD19 mAb were tested in combination with diverse pre-treatment options to identify the optimal performance conditions. These included epitope retrieval performed using BOND ER2 high pH buffer (EDTA based, pH 9.0; 20 minutes, 95°C), endogenous peroxidase inactivated with 3% H2O2 (10 minutes, RT), nonspecific protein-protein interactions blocked with Leica PowerVision IHC/ISH Super Blocking solution (Leica Biosystems) (30 minutes, RT), incubation of primary antibody diluted 1:1500 (1 μm/mL) in the same solution used for the blocking step above (45 minutes, RT), and incubation with the biotin-free polymeric IHC detection system Power Vision HRP-conjugated antimouse IgG (Leica Biosystems) (25 minutes, RT). Immunoreactivity was revealed with the diaminobenzidine (DAB) chromogen reaction. Slides were finally counterstained in hematoxylin, dehydrated in an ethanol series, cleared in xylene, and permanently mounted with a resinous mounting medium (ClearVue; Thermo Fisher Scientific). Specificity of the IHC assay was further confirmed via preincubation of the working mouse anti-CD19 mAb solution with the canine CD19 ECD protein at 5 μm/ml.
scFv Generation and Expression in M13 Phage
The nucleotide sequences of the cloned variable heavy (VH) and variable light (VL) domains of the anticanine CD19 antibody (clone 4E9) were amplified from hybridoma cDNA using the Mouse IgG Library Primer Set (Progen, Heidelberg, Germany). The variable domains were synthesized into scFv formats in the VH-VL and VL-VH orientation using the Whitlow peptide linker49 and cloned into the pComb3X phagemid vector (a kind gift from Dr. Donald Siegel). XL-1 BLUE electrocompetent cells (Agilent Technologies, Santa Clara, CA) were transformed and cultured in the presence of carbenicillin to select for transformed colonies. Phagemid was rescued with the addition of M13 helper phage and further selected using kanamycin. Resulting phage was precipitated with 5× PEG/NaCl and resuspended in 1% BSA in PBS. The titer of each phage preparation was determined using a plaqueforming assay as previously described.3
Surface Plasmon Resonance Binding Assays
A Biacore T200 with version 2.0 and HC30 M (Xantec Bioanalytics, Duesseldorf, Germany) sensor chips were used to determine the binding affinity of anticanine CD19 antibody to soluble cCD19 ECD. The anti-CD19 antibody was immobilized on the surface of the HC30 M chip via standard amine coupling. Briefly, EDC/NHS was applied to the surface of the chip for 7 minutes. Monoclonal antibodies were diluted to 10 μg/ml in 20 mM acetate at a pH of 5.0 and injected into the experimental flow cell over 70 seconds, achieving 3000 response units (RU). Any remaining activated sites were blocked with 1 M ethanolamine (pH 8.5) for 10 minutes. Then, 15 × 1:2 serial dilutions of cCD19 ECD were prepared in duplicate in running buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, and 0.01% Tween20). The highest concentration of cCD19 ECD tested was 500 nM. The flow rate across the chip was 30 μL/min, and the contact time of the cCD19 ECD sample with the chip surface was 300 seconds and then dissociation was monitored for 900 seconds. Report points were recorded before and after each injection and the amount of antigen binding for each analysis cycle was reported in relative RU. Plots of response vs antigen concentration were generated using the Biacore wizard program. The chip surface was regenerated with 20 mM glycine at pH 2.0 for 60 seconds after the RU for each concentration of analyte was recorded. The total runtime for samples was 26 hours. All experiments were carried out at 25°C. Assay data were processed using Biacore Evaluation Software, version 2.0 to obtain kinetic values and reported as Kon, Koff, and Kd.
Results
Generation of a Canine CD19-Specific Monoclonal Antibody
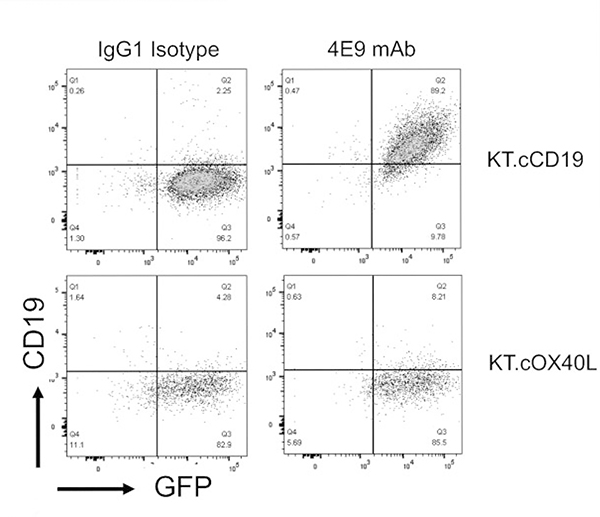
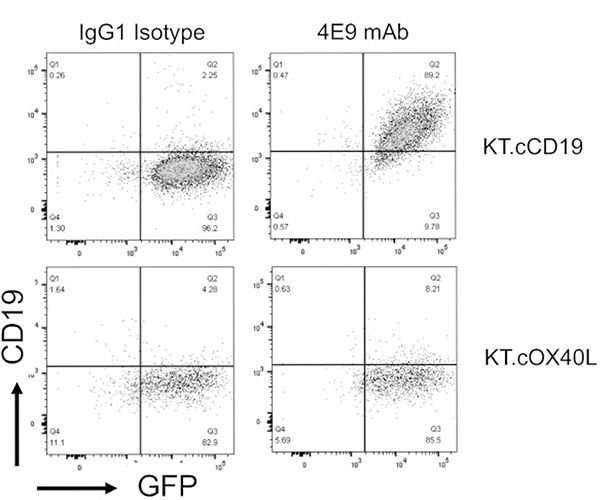
Soluble protein comprising the extracellular domain (21–292 aa) of canine CD19 (XM_005621381) (cCD19 ECD) was used to immunize mice for hybridoma generation (as described in Materials and Methods). Supernatants produced by 2 subclones of the generated murine hybridoma cell line (clone 4E9) contained IgG that specifically bound to plate-bound soluble cCD19 ECD and not to an irrelevant His-tagged protein (Suppl. Fig. S1). Supernatant from clone 4E9 (subclone 1) was purified using a protein A column and concentrated to 1 mg/ml. The isotype of the 4E9 mAb was confirmed as IgG1 κ. To determine whether 4E9 mAb specifically bound to cCD19 expressed on the cell surface, the canine B-cell lymphoma cell line (CLBL-1) that expresses CD19 messenger RNA (mRNA) (Suppl. Fig. S2) was incubated with 4E9 mAb either alone or following preincubation of the mAb with recombinant cCD19 ECD peptide (used for mouse immunization) to block the antigen binding sites and evaluated by flow cytometry (Fig. 1). 4E9 bound to CD79a+CLBL-1 cells, which confirmed its ability bind to CD19 in its natural conformation. Blocking of 4E9 with soluble cCD19 ECD peptide abolished the signal against CLBL-1 cells, confirming CD19 specificity. To further confirm binding specificity, 4E9 was incubated with K562 cells genetically engineered to express canine CD19 (KT.cCD19) or canine OX40L (KT.cOX40L; negative control). 4E9 bound to KT.cCD19 cells but not to KT.cOX40L cells, further confirming that 4E9 binds specifically to canine CD19 (Fig. 2).
Figure 1.
Monoclonal antibody 4E9 binds specifically to cCD19 expressed on the cell surface. Flow cytometry plots of the canine B-cell lymphoma cell line CLBL-1 labeled with the cross-reactive intracellular CD79a antibody only, 4E9 (anti-canine CD19) antibody only, CD79a and 4E9, and CD79a and 4E9 that had been preblocked with cCD19 peptide.
Figure 2.
4E9 binds to scaffold cells engineered to express cCD19. Flow cytometry plots of 4E9 binding to K562 cells genetically engineered to express cCD19 (KT.cCD19). In contrast, 4E9 does not bind to K562 cells genetically engineered to express cOX40L (KT.cOX40L; negative control). An antibody of IgG1 isotype was used as a negative control. In both cases, green fluorescent protein (GFP) expression confirms transduction of K562 cells with either cCD19 or cOX40L.
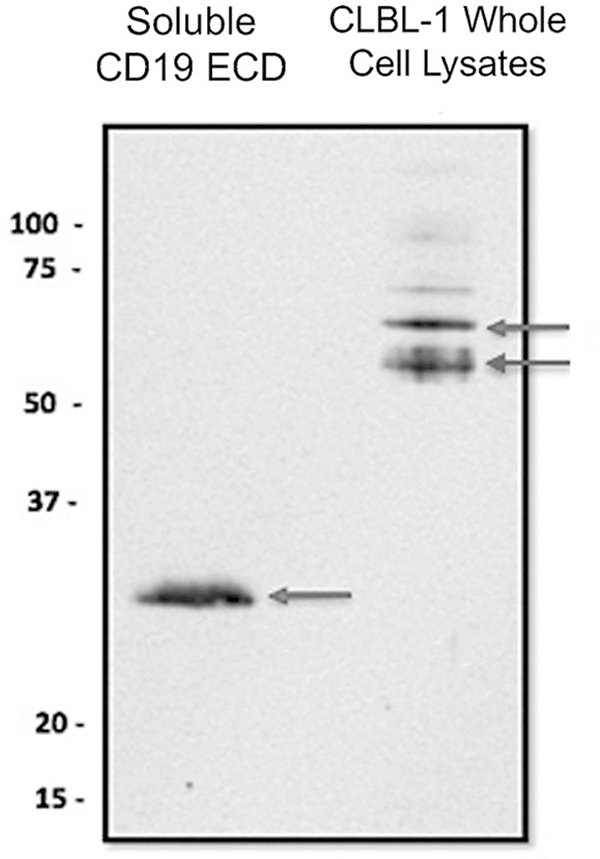
Western blot analysis was performed using cell lysates from CLBL-1 cells and soluble cCD19 ECD protein as a positive control (Fig. 3). A single band at ~30 kDa corresponding to the molecular weight of soluble cCD19 ECD (29.93 kDa) was identified in the positive control lane. Two bands were identified at ~58 and 61 kDa against CLBL-1 lysates. Although smaller than human CD19, these bands correspond to 3 mRNA transcript variants predicted for cCD19 (variant X1 XM_014114516.2, 61.5 kDa; variant X2 XM_005621381.3, 60.9 kDa; variant X3 XM_022419914.1, 58.1 kDa). Taken together, these data confirm the specificity of 4E9 mAb for cCD19.
Figure 3.
Western blot analysis of 4E9 binding to soluble cCD19 extracellular domain (ECD) and CLBL-1 whole-cell lysates. The arrow points to a single band at 30 kDa for the soluble cCD19 ECD and to bands at ~58 and 61 kDa that correspond to the size of the predicted canine CD19 protein encoded by transcriptional variants.
4E9 Demonstrates a High Binding Affinity for Soluble cCD19
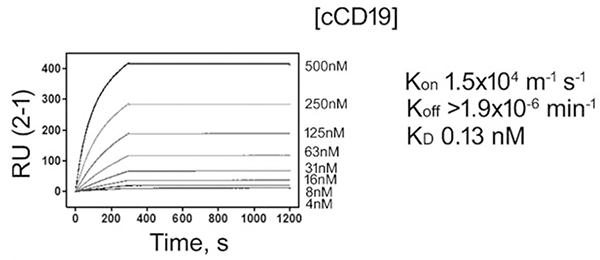
The affinity of 4E9 for cCD19 ECD was evaluated to determine whether 4E9 may have therapeutic value. Using surface plasmon resonance (SPR), the on and off rates of 4E9 against cCD19 were determined (Fig. 4). The kon rate was 1.5 × 104 M–1 s–1. The Koff rate was very slow at >1.9 × 10–6 min–1. The dissociation constant (KD) of 4E9 was found to be in the subnanomolar range (KD 0.13 nM), indicating high binding affinity consistent with affinity maturation. These values suggest that 4E9 has sufficient affinity to cCD19 to potentially serve as a therapeutic targeting agent in the future.
Figure 4.
Binding affinity of 4E9 to canine CD19 extracellular domain (ECD) peptide. 4E9 was immobilized on the Biacore sensor surface and serial dilutions of CD19 analyte were evaluated for binding kinetics. The amount of antigen binding for each analysis cycle is reported in relative response units (RU) and kinetic response curves are shown for different concentrations of analyte. Kinetic values are reported as Kon, Koff, and KD.
4E9 Detects Canine B Cells in Peripheral Blood and Lymph Node Aspirates
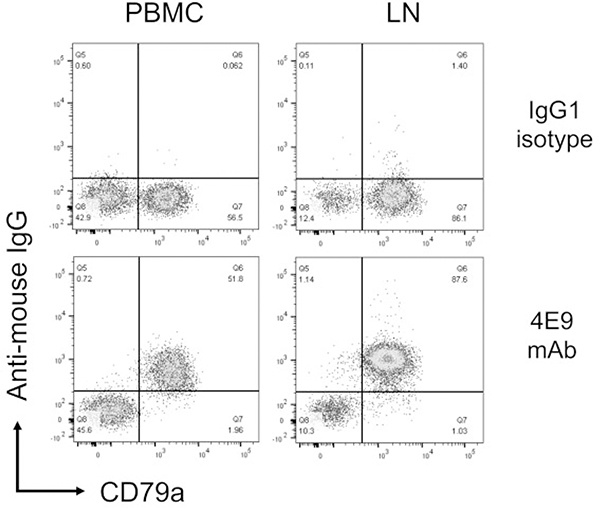
To confirm that 4E9 binds to cCD19 expressed by peripheral blood B cells and B cells within lymph node aspirates, cells from the peripheral blood of a healthy donor dog and lymph nodes of a dog with DLBCL were labeled with 4E9 and the B-cell marker CD79a and analyzed by flow cytometry (Fig. 5). 4E9 labeled CD79a+ cells exclusively, both in the peripheral blood and the lymph node. This indicates specificity of 4E9 in clinically relevant samples.
Figure 5.
4E9 binds specifically to CD79a+ B cells in peripheral blood and lymph nodes. Flow cytometry plots of peripheral blood mononuclear cells (PBMCs, left column) and a lymph node aspirate from a dog with B-cell lymphoma (LN, right column) labeled with CD79a and either an IgG1 isotype control or 4E9 mAb. 4E9 binds to CD79a+ B cells in PBMCs and LN.
4E9 Shows Antigen-Specific Binding in Immunohistochemistry
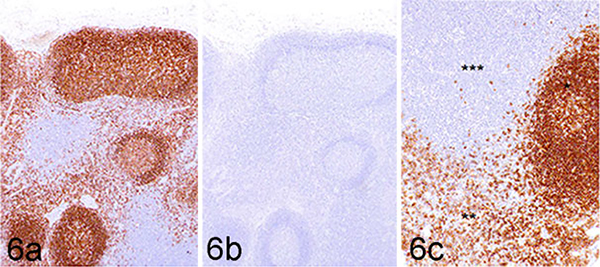
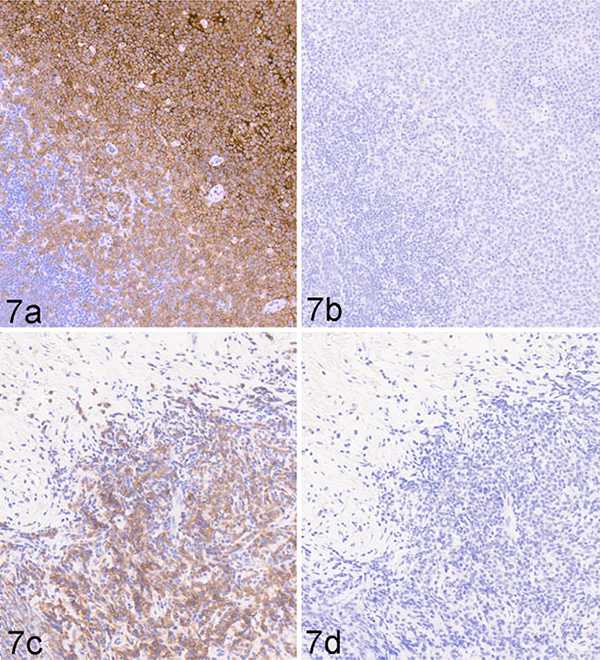
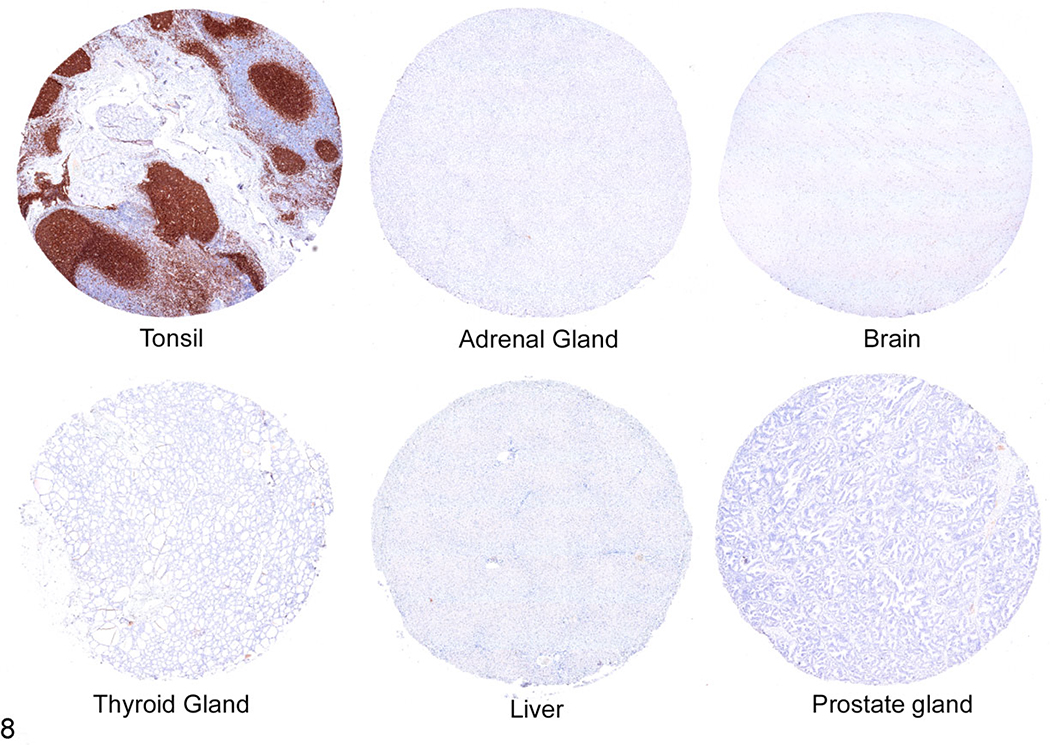
To determine whether 4E9 can specifically identify cCD19-expressing cells in formalin-fixed, paraffin-embedded (FFPE) tissues by immunohistochemistry, FFPE sections of normal canine lymph node and a canine TMA containing 30 different normal canine tissues were immunolabeled with 4E9 as described in the Materials and Methods. To confirm binding specificity, 4E9 was blocked with recombinant cCD19 ECD protein prior to use in the labeling protocol. Unblocked 4E9 produced highly specific and well-defined labeling of B cells within the lymphoid follicles and maturing plasma cells populating the medullary cords (Fig. 6a). No signal was observed in the paracortical T-cell population, nodal stroma, or extranodal connective tissues. Blocking of 4E9 with recombinant cCD19 ECD prior to its use in IHC abolished all labeling, confirming specificity for CD19 (Fig. 6b). At higher magnification, immunolabeling with 4E9 was membranous in germinal center cells, consistent with the cell surface location of CD19 (Fig. 6c). To confirm that 4E9 can be used in IHC to identify B-cell malignancies, tissue sections of a malignant popliteal lymph node (Fig. 7a,b) and a malignant mesenteric lymph node (Fig. 7c,d) from 2 different dogs were immunolabeled with 4E9 alone (Fig. 7a,c) or 4E9 preblocked with cCD19 ECD prior to its use in IHC. Clear membranous immunolabeling was noted in both samples, which was abolished when 4E9 was preblocked with the peptide, confirming specific binding of 4E9 binding to malignant B lymphocytes in IHC. Evaluation of immunolabeling against a variety of tissues included in a comprehensive canine TMA showed specific and intense membranous B-cell labeling within the follicular zones of primary and secondary lymphoid tissues. Nonspecific labeling of interstitial mast cell granules that did not resolve with preblocking of 4E9 was consistently observed as previously described following immunoperoxidase labeling.36 No specific cellular immunolabeling was observed within any other tissues, including the adrenal gland, liver, and prostate, although very weak fibrillary staining of the pericapillary space that was not clearly associated with any cellular elements was seen in the cerebrum, cerebellum, and brainstem (Fig. 8).
Figure 6.
Normal retropharyngeal lymph node, dog. Immunohistochemistry using the 4E9 mAb. (a) Strong labeling is detected within lymphoid follicles (consistent with B cells) and cells populating medullary cords (consistent with maturing plasma cells). Immunohistochemistry (IHC) for 4E9 mAb without CD19 extracellular domain blocking. (b) No labeling is detected in sections where 4E9 mAb was preincubated with the CD19 immunizing peptide (blocking peptide) prior to its use in IHC. (c) Higher magnification image of panel a. Lymphoid follicle (*), medullary cords (**), and paracortical zone (***).
Figure 7.
B-cell lymphoma, lymph nodes, dog. Immunohistochemistry using the 4E9 mAb. Popliteal lymph node (a, b) and mesenteric lymph node (c, d). Strong membranous labeling is detected in malignant B cells using 4E9 (a, c). No labeling is detected in sections (b, d) where the 4E9 mAb has been preblocked with cCD19 peptide prior to its use in immunohistochemistry.
Figure 8.
Tissue microarray including normal tonsil, adrenal gland, brain, thyroid gland, liver, and prostate gland; dog. Immunohistochemistry using the 4E9 mAb. There is clear labeling of follicles in tonsillar tissue (consistent with B cells), but no labeling is detected in other canine tissues within the tissue microarray.
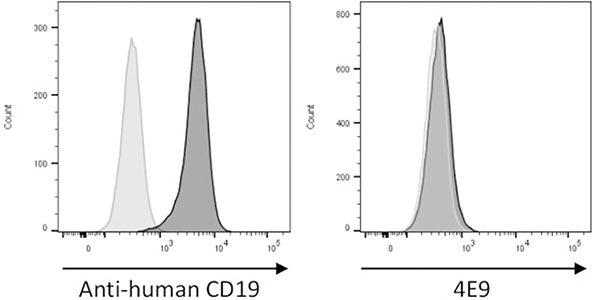
4E9 Does Not Cross-React With Human or Feline CD19
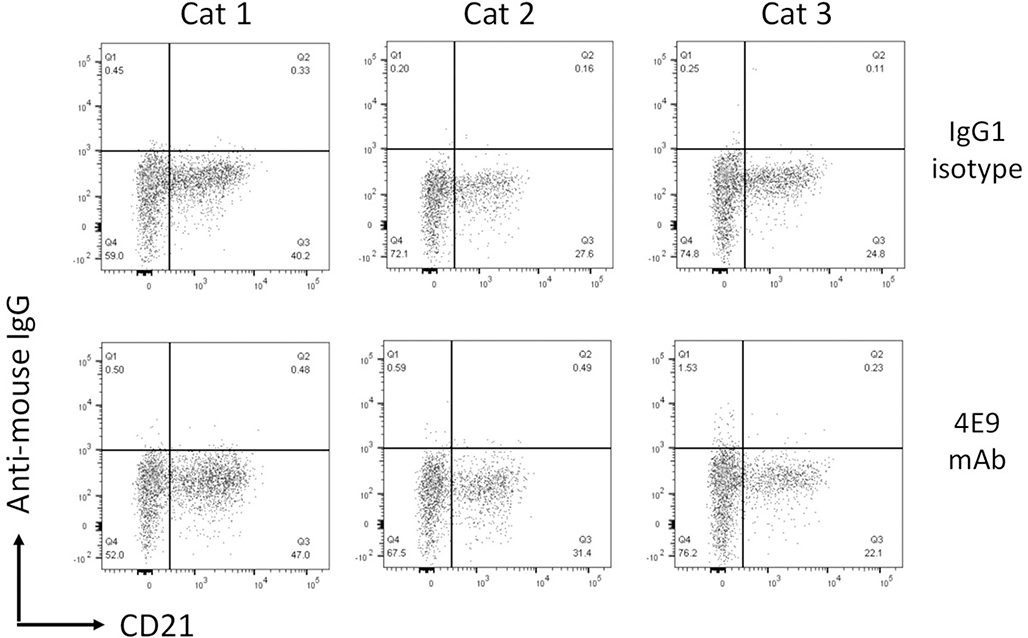
Due to the relatively low sequence identity in the extracellular domain of CD19 across species, antibodies raised against CD19 are commonly species specific. To determine whether 4E9 cross-reacts with CD19 from other species, feline PBMCs were labeled with CD21 to identify peripheral B cells and then colabeled with 4E9 (Fig. 9). In addition, the human B-cell leukemia line Nalm-6 was labeled with either antihuman CD19 or 4E9 (Fig. 10). No positive immunolabeling was observed in either species with 4E9, indicating that 4E9 does not cross-react with human or feline CD19 and thus cannot be employed in diagnostic or therapeutic applications for these species.
Figure 9.
4E9 does not cross-react with feline CD19. Flow cytometry plots of feline peripheral blood mononuclear cells labeled with an anti-CD21 mAb and either a murine IgG1 isotype or 4E9 mAb. No labeling was detected in any of the 3 cats tested.
Figure 10.
4E9 does not cross-react with human CD19. The human B-cell line Nalm6 was labeled with an antihuman CD19 antibody (positive control, left plot, dark gray histogram) or 4E9 (right plot, dark gray histogram). The light gray histogram represents unstained cells, which is used as a negative control. Clear labeling is observed with an antihuman CD19 antibody, but no labeling is seen with 4E9.
Single-Chain Variable Fragments Derived From 4E9 Maintain CD19 Binding Capacity
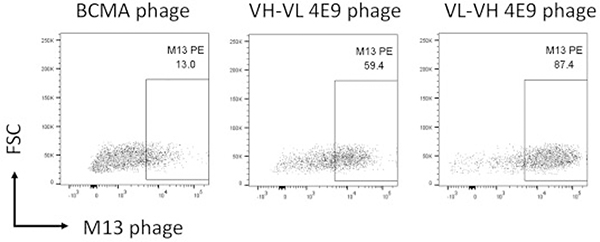
CD19 binding in BiTEs and in CART19 cells is mediated via a CD19-specific scFv. Single-chain variable fragments are the smallest antibody fragments that maintain antigen-binding specificity. They are composed of the VH and VL chains of a monoclonal antibody joined together by a flexible linker. The orientation of the VH and VL fragments of the scFv influences its ability to bind its target. Therefore, to determine whether scFv derived from 4E9 would maintain antigen-binding specificity and which orientation of variable sequences produces the most optimal binding, VH and VL domains of 4E9 were amplified from 4E9 hybridoma cDNA and sequences were linked in both VH-VL and VL-VH orientations with the Whitlow peptide49 and cloned into the pComb3X phagemid vector. Bacteria were transformed with vector, and phagemids were rescued with M13 helper phage. The antigen-binding specificity of both scFvs was determined against CLBL-1 cells by flow cytometry. Both VH-VL and VL-VH scFvs bound to CLBL-1 cells; however, the VL-VH orientation showed enhanced binding when compared to the VH-VL orientation. Minor background binding was observed using a control M13 phage expressing the irrelevant anti-murine B-cell maturation antigen (BCMA) scFv (Fig. 11).
Figure 11.
Single-chain variable fragments (scFvs) derived from 4E9 mAb maintain antigen-binding specificity. Flow cytometry plots of CLBL-1 cells labeled with an irrelevant anti-murine B-cell maturation antigen (BCMA)–specific scFv phage (negative control), a variable light (VL)–variable heavy (VH) 4E9 scFv phage, or a VH-VL 4E9 scFv phage, as indicated. Bound phage was detected with an anti-M13 mAb. Minor background labeling is observed with the negative control BCMA scFv. Both orientations of the 4E9 scFv label CLBL-1 cells, with the VL-VH scFv phage showing greatest binding.
Discussion
The resurgence of interest in cancer immunotherapy over the past decade has brought with it the recognition that many preclinical mouse models do not accurately predict the safety or effectiveness of immunotherapeutics.27 This has led to efforts to identify more accurate modeling systems that include animal models that are immunocompetent and develop spontaneous malignancies that present similar challenges to successful immunotherapy.26 Spontaneous malignancies that occur in immunocompetent pet dogs display remarkable clinical, biological, and genetic similarities to human cancers. Furthermore, these cancers frequently exhibit a hostile tumor microenvironment where regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs) reside and suppress antitumor immune responses through, for example, checkpoint inhibition and metabolic alterations via indoleamine-2, 3-dioxygenase (IDO) and arginase production.11,22,23,29,37 Therefore, pet dogs with cancer are increasingly recognized as important parallel patient populations that can be used to assess safety of novel immunotherapies or combination approaches, as well as help to inform human clinical trial design.33 Furthermore, the same work provides new avenues of therapeutic exploration in the veterinary arena, where there is an increasing demand for effective immunotherapies to treat cancer and other common clinical disorders. The concept of using pet dogs to study cancer biology, immunology, and immunotherapy has been reinforced in the literature by field leaders,15,20 at the US National Academy of Medicine’s National Cancer Policy Forum,17 and by the inclusion of canine cancer immunotherapy clinical trials and correlative studies in the Cancer Moonshot Initiative announced in 2017. Taken together, spontaneous canine tumors provide a unique and valuable platform to gain insight into the future development of immunotherapeutics in both the human and veterinary clinics.
CD19-targeted therapeutics have dramatically improved the standard of care for B-cell malignancies in human patients. Currently, there are 2 FDA-approved CD19-targeted immunotherapies (blinatumomab and CART19) for the treatment of ALL, and CART19 is also approved for treatment of B-NHL in human patients. Other CD19-targeted antibodybased therapeutics are being explored for their use in human B-cell malignancies in phase I and II clinical trials.18 Monoclonal CD19-targeted antibodies exert their target antigen-specific effects through antibody-dependent cellular cytotoxicity, direct cytotoxicity, and antibody-dependent cellular phagocytosis.1 CD19-targeted therapies that lead to B-cell depletion may also be beneficial in the treatment of patients with autoimmune, infectious, and inflammatory diseases and may contribute to successful allotransplant.19 One of the main impediments to full enablement of the canine patient to inform human drug development and clinical trial design is the lack of canine-specific immunoreagents for diagnostic and therapeutic use. Here we have developed and validated a canine CD19-specific mouse mAb and its scFv derivatives, which may be employed in diagnostic or therapeutic enterprises to advance human and veterinary health. For example, it is anticipated that the availability of an antibody that recognizes the extracellular portion of CD19, which is expressed at earlier stages of ontogeny than CD20, will expand our flow cytometric arsenal to identify B cells and may enable more accurate classification of canine leukemias. Furthermore, it will be necessary to confirm CD19 expression on target cells to fulfill entry criteria for clinical trials evaluating CD19-targeted therapeutics. Therapeutically, CD19-targeted therapeutics aim to provide value in the treatment of both human and canine patients that have been rendered resistant to CD20-targeted therapeutics through CD20 epitope splicing or CD20 downregulation. In addition, generation of a canine CD19-targeting CAR may allow exploration of allogeneic or universal CART19 technology in canine cancer patients to inform next-generation human CART development. This approach would address the considerable difficulty of generating “fit” autologous CAR T-cell products capable of inducing complete remissions from many advanced, heavily pretreated human patients with cancer. Furthermore, development of canine CD19 CAR T cells may allow rapid evaluation of combination therapies (eg, CART19 with checkpoint blockade and/or biological response modifiers such as IDO inhibitors to improve clinical responses of patients with bulky nodal disease in B-NHL). As 4E9 is a mouse monoclonal antibody, its use in vivo is likely to induce canine anti-mouse antibodies (CAMAs), reducing the effectiveness of repeat administrations. Nevertheless, B-cell targeting mAbs that rapidly and efficiently deplete B cells may be somewhat protected from CAMA formation as the B cells that mediate this response will be eliminated. Indeed, rituximab is a chimeric mouse-human CD20 targeting antibody, and the FMC63-based scFv expressed on the commercial CART19 products is murine in origin.24 T-cell responses against the foreign protein may still be induced, however, and 4E9 VH and VL sequences might be used for complementarity determine region (CDR) grafting and caninization to improve pharmacokinetics if they were found to be unfavorable.
Serious adverse events, including cytokine release syndrome and neurotoxicity, have been reported in human patients receiving CD19-targeted therapies.5,13 These events were not predicted in immunocompromised preclinical mouse models most likely because of the lack of intact cytokine networks necessary to mediate these effects.38 The development of novel cCD19-specific antibodies and CARs provides the potential to study CART-related neurotoxicity in an immune-competent large animal patient with spontaneous disease. Although it is not known if dogs and humans will respond to anti-CD19– targeting therapies in the same way, their shared biology and comparable immune systems provide a high level of expectation that responses will be similar between species. Thus, we would expect to see induction of B-cell aplasia or extremely low peripheral B-cell counts and hypogammaglobulinemia in dogs receiving CD19-targeted therapies. Human patients treated with CART19 receive intravenous immunoglobulin (IVIG) to counter the hypogammaglobulinemia. However, since CD19 is not expressed on plasma cells, the ability to produce IgG in adult human and canine patients should not be as affected as in pediatric patients, and it is possible that it will not be necessary to supplement adult canine patients with IVIG. Cytokine storms are also expected with CD19 CAR-T cell technology in canine patients, although clinical trials will need to be performed to confirm this hypothesis. With the advent of more potent, next-generation CAR-T cell designs, including armored CARs, TRUCKs, and inducible CARs, we predict that outbred, immunologically intact pet dogs with spontaneous cancer will play an important role in evaluating their safety profiles and potential superior efficacy prior to their use in human clinics.
Conclusions
We have generated an anti-canine CD19 mAb and its scFv derivative and have validated antigen-specific binding in vitro against a malignant B-cell line, peripheral blood B cells, and lymph node cells taken from a canine patient with B-NHL. We confirmed the specificity of antigen binding using flow cytometry, IHC, and Western blot and further confirmed specificity using peptide-blocking experiments. The 4E9 mAb has subnanomolar affinity, making it suitable for diagnostic or therapeutic targeting in vitro and in vivo. Given that one of the main causes for relapse following CD19-targeted immunotherapies is loss of target antigen and emergence of splice variants that are invisible to the targeted therapeutic, it is valuable to employ the same antibody recognizing the same target antigen epitope for diagnostic and therapeutic use so that adaptive resistance can be readily identified. Thus, 4E9 may be valuable for the development and evaluation of next-generation immunotherapies that target CD19 in canine patients with B-cell malignancies and possibly autoimmune diseases, such as systemic lupus erythematosus, and serve as an important diagnostic tool in the veterinary clinic. This work will serve the dual purpose of enabling pet dogs with spontaneous cancer to serve as a clinically relevant large animal model to accelerate application in the human clinics as well as provide much-needed targeted immunotherapeutics for use in pet dogs with B-cell malignancies for which there are no currently available, safe, and effective therapies that induce durable remissions.
Supplementary Material
Acknowledgements
We thank Dr. Matthew Atherton for his review of the manuscript and helpful comments and Mr. Edwin Colon-Acosta for technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a generous gift from Dave and Sandy Sabey.
Footnotes
Declaration of Conflicting Interests
NJM and AP report a financial interest in antibody Clone 4E9 with respect to licensing agreements. The other authors declare no conflict of interest.
Supplemental material for this article is available online.
References
- 1.Awan FT, Lapalombella R, Trotta R, et al. CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain-engineered monoclonal antibody. Blood. 2010;115(6):1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach PB, Giralt SA, Saltz LB. FDA approval of tisagenlecleucel: promise and complexities of a $475000 cancer drug. JAMA. 2017;318(19):1861–1862. [DOI] [PubMed] [Google Scholar]
- 3.Barbas CF. Phage Display: A Laboratory Manual. Cold Spring Harbor, NY:Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 4.Beirao BC, Raposo T, Jain S, et al. Challenges and opportunities for monoclonal antibody therapy in veterinary oncology. Vet J. 2016;218:40–50. [DOI] [PubMed] [Google Scholar]
- 5.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buie LW, Pecoraro JJ, Horvat TZ, et al. Blinatumomab: a first-in-class bispecific T-cell engager for precursor B-cell acute lymphoblastic leukemia. Ann Pharmacother. 2015;49(9):1057–1067. [DOI] [PubMed] [Google Scholar]
- 7.Carter RH, Barrington RA. Signaling by the CD19/CD21 complex on B cells. Curr Dir Autoimmun. 2004;7:4–32. [DOI] [PubMed] [Google Scholar]
- 8.Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther. 2015;15(8):1145–1154. [DOI] [PubMed] [Google Scholar]
- 9.Chong EA, Melenhorst JJ, Lacey SF, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)-modified T cells: refueling the CAR. Blood. 2017; 129(8):1039–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraietta JA, Beckwith KA, Patel PR, et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127(9): 1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goulart MR, Pluhar GE, Ohlfest JR. Identification of myeloid derived suppressor cells in dogs with naturally occurring cancer. PLoS One. 2012;7(3):e33274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol. 2018;183(3): 364–374. [DOI] [PubMed] [Google Scholar]
- 15.Kalos M, June CH. Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity. 2013;39(1):49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz BZ, Herishanu Y. Therapeutic targeting of CD19 in hematological malignancies: past, present, future and beyond. Leuk Lymphoma. 2014;55(5): 999–1006. [DOI] [PubMed] [Google Scholar]
- 17.LeBlanc AK, Breen M, Choyke P, et al. Perspectives from man’s best friend: national academy of medicine’s workshop on comparative oncology. Sci Transl Med. 2016;8(324):324ps5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makita S, Tobinai K. Antibody therapy targeting CD19 for B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2018;29(5):1086–1089. [DOI] [PubMed] [Google Scholar]
- 19.Maldini CR, Ellis GI, Riley JL. CAR T cells for infection, autoimmunity and allotransplantation. Nat Rev Immunol. 2018;18(10):605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mata M, Vera JF, Gerken C, et al. Toward immunotherapy with redirected Tcells in a large animal model: ex vivo activation, expansion, and genetic modification of canine T cells. J Immunother. 2014;37(8):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monjazeb AM, Kent MS, Grossenbacher SK, et al. Blocking indolamine-2,3dioxygenase rebound immune suppression boosts antitumor effects of radioimmunotherapy in murine models and spontaneous canine malignancies. Clin Cancer Res. 2016;22(17):4328–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mucha J, Rybicka A, Dolka I, et al. Immunosuppression in dogs during mammary cancer development. Vet Pathol. 2016;53(6):1147–1153. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson IC, Lenton KA, Little DJ, et al. Construction and characterisation of afunctional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol Immunol. 1997;34(16–17):1157–1165. [DOI] [PubMed] [Google Scholar]
- 25.Ninomiya S, Narala N, Huye L, et al. Tumor indoleamine 2,3-dioxygenase(IDO) inhibits CD19-CAR T cells and is downregulated by lymphodepleting drugs. Blood. 2015;125(25):3905–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overgaard NH, Fan TM, Schachtschneider KM, Principe DR, Schook LB,Jungersen G. Of mice, dogs, pigs, and men: choosing the appropriate model for immuno-oncology research. ILAR J 2018;59(3):247–262. [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Withers SS, Modiano JF, et al. Canine cancer immunotherapy studies: linking mouse and human. J Immunother Cancer. 2016;4:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parry RV, Rumbley CA, Vandenberghe LH, et al. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J Immunol. 2003;171(1):166–174. [DOI] [PubMed] [Google Scholar]
- 29.Pinheiro D, Chang YM, Bryant H, et al. Dissecting the regulatory microenvironment of a large animal model of non-Hodgkin lymphoma: evidence of a negative prognostic impact of FOXP3+ T cells in canine B cell lymphoma. PLoS One. 2014;9(8):e105027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regan D, Guth A, Coy J, et al. Cancer immunotherapy in veterinary medicine: current options and new developments. Vet J. 2016;207:20–28. [DOI] [PubMed] [Google Scholar]
- 32.Richards KL, Suter SE. Man’s best friend: what can pet dogs teach us aboutnon-Hodgkin’s lymphoma? Immunol Rev. 2015;263(1):173–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson HR, Qi J, Cook EM, et al. A CD19/CD3 bispecific antibody for effective immunotherapy of chronic lymphocytic leukemia in the ibrutinib era. Blood. 2018;132(5):521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salter AI, Pont MJ, Riddell SR. Chimeric antigen receptor-modified T cells CD19 and the road beyond. Blood. 2018;131(24):2621–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanford M Blinatumomab: first global approval. Drugs. 2015;75(3):321–327. [DOI] [PubMed] [Google Scholar]
- 36.Schiltz PM, Lieber J, Giorno RC, et al. Mast cell immunohistochemistry: nonimmunological immunostaining mediated by non-specific F(ab0)2-mast cell secretory granule interaction. Histochem J. 1993;25(9):642–647. [DOI] [PubMed] [Google Scholar]
- 37.Sherger M, Kisseberth W, London C, et al. Identification of myeloid derived suppressor cells in the peripheral blood of tumor bearing dogs. BMC Vet Res. 2012;8:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siegler EL, Wang P. Preclinical models in chimeric antigen receptor-engineered T-cell therapy. Hum Gene Ther. 2018;29(5):534–546. [DOI] [PubMed] [Google Scholar]
- 39.Suhoski MM, Golovina TN, Aqui NA, et al. Engineering artificial antigen presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15(5):981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tedder TF, Isaacs CM. Isolation of cDNAs encoding the CD19 antigen of human and mouse B lymphocytes: a new member of the immunoglobulin superfamily. J Immunol. 1989;143(2):712–717. [PubMed] [Google Scholar]
- 41.Torikai H, Reik A, Liu PQ, et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric antigen-receptor and eliminate expression of endogenous TCR. Blood. 2012; 119(24):5697–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai AK, Davila E. Producer T cells: using genetically engineered T cells as vehicles to generate and deliver therapeutics to tumors. Oncoimmunology. 2016; 5(5):e1122158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turtle CJ, Hanafi LA, Berger C, et al. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med. 2016;8(355):355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vail DM, Young KM. Hematopoietic tumors In: Withrow VP, ed. Small Animal Clinical Oncology. Philadelphia, PA: Saunders; 2013:608–678. [Google Scholar]
- 45.Valli VE, Kass PH, San Myint M, Scott F. Canine lymphomas: association of classification type, disease stage, tumor subtype, mitotic rate, and treatment with survival. Vet Pathol. 2013;50(5):738–748. [DOI] [PubMed] [Google Scholar]
- 46.Villarnovo D, McCleary-Wheeler AL, Richards KL. Barking up the right tree: advancing our understanding and treatment of lymphoma with a spontaneous canine model. Curr Opin Hematol 2017;24(4):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiskopf K, Anderson KL, Ito D, et al. Eradication of canine diffuse largeB-cell lymphoma in a murine xenograft model with CD47 blockade and antiCD20. Cancer Immunol Res. 2016;4(12):1072–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitlow M, Bell BA, Feng SL, et al. An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Eng. 1993; 6(8):989–995. [DOI] [PubMed] [Google Scholar]
- 50.Yeku OO, Purdon TJ, Koneru M, et al. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci Rep 2017;7(1):10541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J, Lin Q, Song Y, Liu D. Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol. 2018;11(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.