Abstract
Prior meta‐analyses have shown that new‐onset atrial fibrillation (NOAF) occurs in up to 40% of patients following cardiac surgery and is associated with substantial major adverse cardiovascular events. The stroke and mortality implications of NOAF in isolated CABG without concomitant valve surgery is not known. We thought that NOAF would be associated with increased risk of stroke and mortality, even in patients undergoing isolated CABG. A blinded review of studies from MEDLINE, CENTRAL, and Web of Science was done by two independent investigators. Stroke, 30‐day/hospital mortality, long‐term cardiovascular mortality, and long‐term (>1 year) all‐cause mortality were analyzed. We used Review Manager Version 5.3 to perform pooled analysis of outcomes. Of 4461 studies identified, 19 studies (n = 129 628) met inclusion criteria. NOAF incidence ranged from 15% to 36%. NOAF was associated with increased risk of stroke (unadjusted OR 2.15 [1.82, 2.53] [P < .00001]; adjusted OR 1.88 [1.02, 3.46] [P = .04]). NOAF was associated with increased 30‐day/hospital mortality (OR 2.35 [1.67, 3.32] [P < .00001]) and long‐term cardiovascular mortality (OR 2.04 [1.35, 3.09] [P = .0007]) NOAF was associated with increased long‐term all‐cause mortality (unadjusted OR 1.79 [1.63, 1.96] [P < .00001]; adjusted OR 1.58 [1.24, 2.00] [P = .0002]). We found that the incidence of NOAF following isolated CABG is high and is associated with increased stroke rate and mortality. Early recognition and management of NOAF could improve outcomes.
Keywords: atrial fibrillation, coronary artery bypass surgery, postoperative atrial fibrillation, stroke
1. INTRODUCTION
Following coronary artery bypass grafting (CABG), patients frequently develop new‐onset atrial fibrillation (NOAF). Of approximately 800 000 patients who undergo CABG each year, over 264 000 with develop NOAF. 1 The clinical implications of this phenomenon are significant, given that the development of NOAF has been associated with up to a 56% greater risk of long‐term mortality. 2 NOAF has also been associated with increased risk of stroke. Three prior meta‐analyses have examined outcomes of patients who develop NOAF after CABG in a mixed group of patients undergoing cardiac and thoracic procedures concomitantly. 3 , 4 , 5 However, each of these analyses included patients who had undergone concomitant valvular surgery, which could prognosticate for the development of atrial fibrillation (AF). The incidence of AF has been shown to be higher in those patients undergoing valvular surgery (46%) than in those patients undergoing isolated CABG (29%). 6 Our goal was to perform a systematic review and meta‐analysis to provide the most current information surrounding outcomes in patients with NOAF undergoing isolated CABG. We excluded patients who underwent concomitant procedures, including valve repair or replacement, aneurysmectomy, or arrythmia surgery. This is the first meta‐analysis to focus on development of NOAF in patients undergoing isolated CABG.
While many studies have shown increased mortality and stroke incidence in patients with NOAF, fewer studies have reported adjusted outcomes. Adjusting for confounding variables is essential given the multiple comorbidities that may affect outcomes in this patient population. We hypothesized that patients with NOAF following isolated CABG would have a higher incidence of mortality and of stroke, even when outcomes have been adjusted.
2. METHODS
2.1. Eligibility criteria
We included studies that reported our outcomes of interest in patients with NOAF following isolated CABG. Any study that did not specify whether the procedure was an isolated CABG, or that included patients undergoing isolated CABG but did not stratify outcomes by that subgroup, was excluded. We excluded studies in which patients underwent concomitant valve surgery, given that the additional atriotomy sites and annulus manipulation act as independent risk factors for development of atrial arrhythmias. Studies that contained populations of vascular surgery patients were excluded. We excluded studies in which the population did not explicitly exclude those patients with a prior history of AF. We also excluded studies in languages other than English. We excluded abstracts and unpublished studies. We reviewed citations of included articles, which did not yield any new relevant studies.
2.2. Data collection and analysis
This review follows the MOOSE guidelines for meta‐analysis reporting. 7 Approval by an ethics committee was not required. A single author (Matthew Kerwin) abstracted data. Data was verified by a second author (Jonathan Saado). We collected data on author, year, study design, outcomes measured, follow‐up rate, and several other fields (Table S3). We collected absolute event counts. Several studies reported both adjusted and unadjusted data. In these cases, we collected both sets of data. When necessary, we contacted authors to clarify results. We assessed study quality using the Newcastle‐Ottawa scale. The assessment of study quality was done by two blinded investigators (Matthew Kerwin and Jonathan Saado). Any disagreements were resolved by consensus.
Statistical analysis was performed using Review Manager (Rev Man, version 5.3, London, UK). We performed pooled analysis of adjusted and, when available, unadjusted outcomes. Hazard ratios, odds ratios, and relative risk were assumed to be roughly equivalent. We used a random‐effects model, assuming that the analyzed studies were examining different populations. We determined 95% confidence intervals for all estimates. Heterogeneity was assessed using the I 2 statistic.
3. RESULTS
3.1. Characteristics of included studies
Of the 4461 studies identified, 49 studies were adjudicated as relevant and underwent full‐text review (Figure 1). Of these, 19 studies met the criteria and were included in the meta‐analysis. Any conflicts about inclusion status were resolved by consensus. These studies comprised 129 628 patients. Eleven studies reported on stroke outcomes. Five reported hospital or 30‐day mortality and 16 reported long‐term all‐cause mortality. Thirteen studies reported adjusted variables. The most common variables for which authors adjusted were age, sex, history of myocardial infarction, diabetes, hypertension, and tobacco use. We abstracted additional details for each study. All but one study was retrospective. The majority had been conducted at a single center.
FIGURE 1.
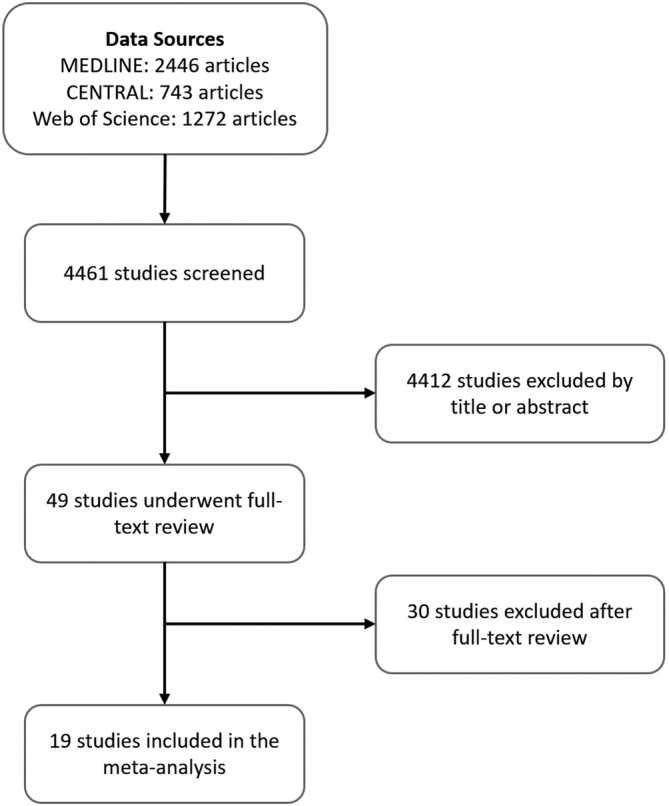
Study selection flowchart
3.2. Incidence of NOAF and use of anticoagulation
NOAF incidence ranged from 15% to 36%, with an average of 25.5%. There was variation in how AF was defined and detected (Table S3). The most common definition, used by six studies, was utilizing the contemporaneous STS definition of postoperative AF, namely AF/flutter requiring treatment. The rate of off‐pump CABG ranged from 0% to 94.3% in the 13 studies that reported this data. Only four studies measured recurrence of AF. 8 , 9 , 10 , 11 Among patients with NOAF, AF recurred in 22% to 39% of patients. Among patients without NOAF, AF occurred in 0% to 13% of patients. Three studies reported the rate of anticoagulation. 12 , 13 , 14 In those studies, patients who developed NOAF were anticoagulated 12% to 21% of the time. Patients who did not develop NOAF were anticoagulated 1% to 6% of the time.
3.3. Impact of NOAF on stroke and mortality
NOAF was associated with increased risk of stroke when examining unadjusted data (OR 2.15 [1.82, 2.53] [P < .00001]) as well as adjusted data (OR 1.88 [1.02, 3.46] [P = .04]; Figure 2). NOAF was associated with increased 30‐day/hospital mortality (OR 2.35 [1.67, 3.32] [P < .00001]; Figure 3) and long‐term cardiovascular mortality (OR 2.04 [1.35, 3.09] [P = .0007]; Figure 4). NOAF was associated with increased long‐term all‐cause mortality (unadjusted OR 1.79 [1.63, 1.96] [P < 0.00001]; adjusted OR 1.58 [1.24, 2.00] [P = .0002]; Figure 5). We conducted subgroup analyses stratified by study quality, which yielded similar results. We defined high‐quality studies as scoring 8 or 9 on the Newcastle‐Ottawa scale. NOAF remained associated with increased long‐term all‐cause mortality when high‐quality studies were analyzed alone (unadjusted OR 1.79 [1.65, 1.94] [P < .00001]; adjusted OR 1.74 [1.16, 2.62] [P = .008]).
FIGURE 2.
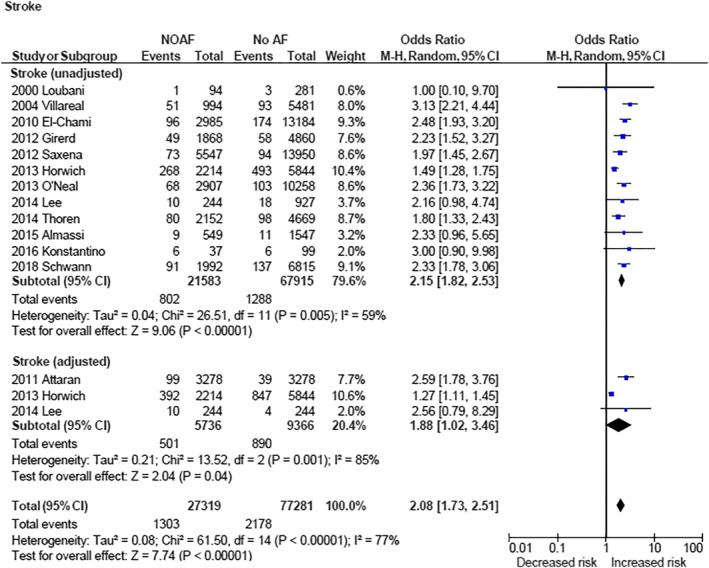
Forest plots (unadjusted and adjusted)—overall increased risk of stroke associated with new‐onset atrial fibrillation. CI, confidence interval; M‐H, Mantel‐Haenszel; NOAF, new‐onset atrial fibrillation
FIGURE 3.
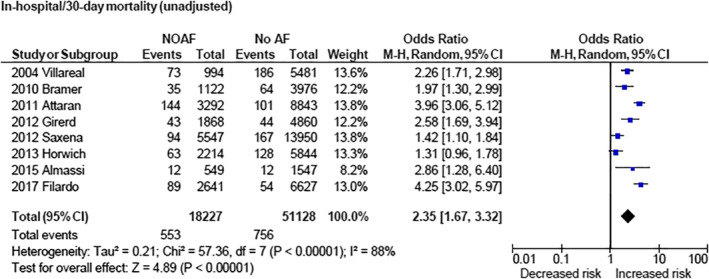
Forest plot (unadjusted)—overall increased risk of in‐hospital/30‐day mortality associated with new‐onset atrial fibrillation. CI, confidence interval; M‐H, Mantel‐Haenszel; NOAF, new‐onset atrial fibrillation
FIGURE 4.
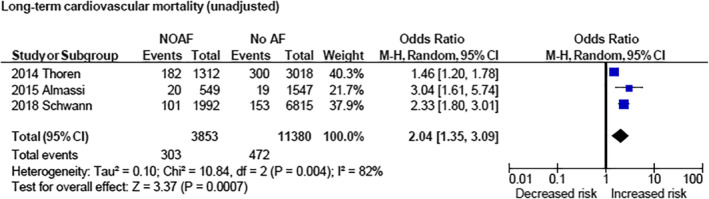
Forest plot (unadjusted)—overall increased risk of long‐term cardiovascular mortality associated with new‐onset atrial fibrillation. CI, confidence interval; M‐H, Mantel‐Haenszel; NOAF, new‐onset atrial fibrillation
FIGURE 5.
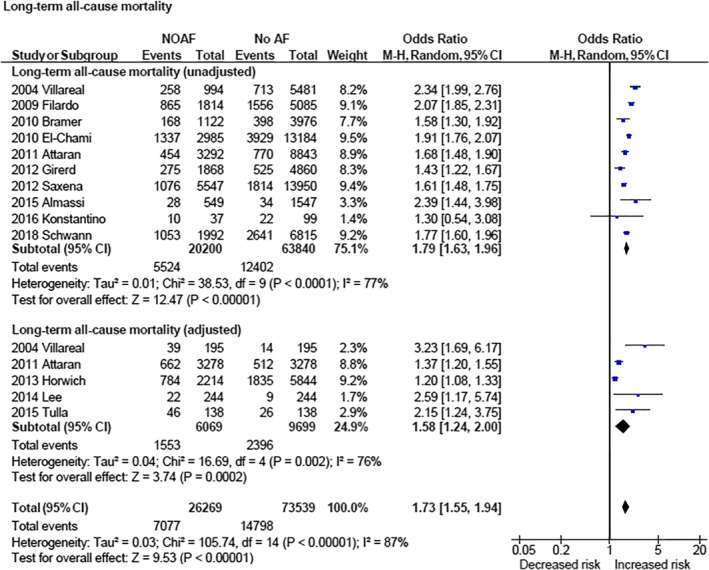
Forest plot (unadjusted and adjusted)—overall increased risk of long‐term all‐cause mortality associated with new‐onset atrial fibrillation. CI, confidence interval; M‐H, Mantel‐Haenszel; NOAF, new‐onset atrial fibrillation
3.4. Quality Assessment
There was substantial heterogeneity among studies. Among studies reporting stroke outcomes, there was substantial heterogeneity in the unadjusted data (I 2 = 59%) and adjusted data (I 2 = 85%). The data on 30‐day/hospital mortality had substantial heterogeneity (I 2 = 88%), as did the data on long‐term cardiovascular mortality (I 2 = 82%). Among the studies that reported long‐term all‐cause mortality outcomes, the degree of heterogeneity was substantial both for unadjusted data (I 2 = 77%) and adjusted data (I 2 = 76%). The degree of heterogeneity decreased when high‐quality studies were analyzed alone. For example, the degree of heterogeneity for long‐term mortality was lower for both unadjusted outcomes (I 2 = 69%) and adjusted outcomes (I 2 = 57%).
We assessed study quality using the Newcastle‐Ottawa Scale. Ninety percent of the studies scored 7 or higher (out of 9). There were two common issues we identified. Several of the studies used a multivariable analysis but did not explicitly state the variables included in that analysis. In addition, 75% of studies did not report, or did not have access to, data on follow‐up rates (Table S1).
4. DISCUSSION
Our main finding is that the development of NOAF after isolated CABG is associated with increased risk of stroke as well as short‐term, long‐term, and cardiovascular mortality. The unique aspect of this meta‐analysis is the focus on NOAF outcomes in patients undergoing CABG alone, unlike prior meta‐analyses that have reported on “all‐comers” cardiac surgery. 3 , 4 , 5 We excluded patients who underwent concomitant valvular procedures for two reasons. First, we considered that valvular procedures may predispose to a scarred atrial substrate, which could independently predispose to development of atrial arrhythmias. Second, patients undergoing valvular procedures may be anticoagulated a higher rate, reducing their risk of stroke. While the effect size appears to be larger in our meta‐analysis, it is primarily owing to the population of interest. Other meta‐analyses derive from patients who undergo valvular surgeries. We speculate a higher baseline rate of anticoagulation could skew to a lower effect size in this population. 5
We were able to use adjusted data for the outcomes of stroke and long‐term all‐cause mortality. The increased risk associated with NOAF remained significant in the adjusted data. There are several potential confounding factors in this population, including surgical technique, comorbidities, and demographic characteristics. The increased risk of poor outcomes seen even in the adjusted data lends further support to the argument that NOAF independently may portend poor outcomes.
There are several limitations to this analysis. Although there are several studies that have analyzed impact of AF in the postoperative setting, only 19 studies met our inclusion criteria for two reasons. The majority of these studies were retrospective. There was substantial heterogeneity between studies. This was not entirely unexpected, given the differences in populations included in these studies. However, there was variation among studies in how AF was defined and detected, which has been shown to have mortality implications. Among patients who develop NOAF after CABG, having classic NOAF (by STS definition) was associated with reduced mortality. Those patients who had NOAF that was missed by the STS definition had significantly higher risk‐adjusted 30‐day mortality. 15
We were unable to conduct systematic AF recurrence during follow‐up period owing to lack of studies reporting this consistently. Of the studies available, the recurrence rates were as high as 39%. 8 , 9 , 10 , 11 Unfortunately, none of the studies stratified outcomes based on recurrence of AF.
The timing of stroke varied between studies. Short‐term stroke rates were reported in three studies and showed increased stroke rate in the group with NOAF. 12 , 16 , 17 The majority of studies reported stroke at greater than 1 year of follow‐up. There were equivocal results for long‐term stroke rates, with rates in the group with NOAF ranging from similar to the group without AF 8 , 9 , 11 , 18 to higher than the group without AF. 13 , 14 , 19 , 20 , 21 , 22
None of the studies explicitly excluded patients with prior stroke. In five studies, there was a significantly greater history of stroke in the NOAF group compared to the group without AF. 12 , 13 , 16 , 19 , 23 In six of the other studies, either there was no significant difference in the history of stroke in the NOAF group compared to the group without AF, 10 , 15 or adjusted outcomes or matched groups were used. 14 , 17 , 21 , 22 It is likely that the unadjusted outcomes were affected by prior history of stroke. However, the association of NOAF with stroke and mortality was found even in the meta‐analysis of adjusted outcomes, indicating that NOAF is likely an independent risk factor.
The use of off‐pump CABG varied widely among these studies. The impact of off‐pump CABG on NOAF is unclear. Saxena et al showed that those patients who developed AF were significantly less likely to have undergone an off‐pump procedure. 16 Girerd et al showed no difference. 17 Schwann et al. showed that patients who developed NOAF were shown to have longer cardiopulmonary bypass and aortic cross‐clamp times than those without NOAF, even when receiving similar numbers of bypass grafts. 21 None of the studies reported on the prevalence of left atrial appendage occlusion. Left atrial appendage occlusion has been associated with decreased risk of thromboembolism among patients discharged without anticoagulation. 24 However, it has also been associated with increased risk of complications, including NOAF. 25 , 26 The unreported use of left atrial appendage occlusion could have impacted our results.
There are several mechanisms by which AF may lead to adverse outcomes. AF causes a number of physiologic abnormalities: left atrial stasis leading to thromboembolism, loss of atrioventricular synchrony, and decreased cerebral perfusion due to irregular ventricular contractions. 21 There is a theoretical mechanism by which irregular ventricular contractions, leading to decreased cerebral circulation, may predispose patients to noncardioembolic stroke. 22 , 27 NOAF may a surrogate for general poor health, or for a generalized inflammatory state that leads to increased risk of cardiovascular events. 17 A point supporting this theory is that stroke accounts for less than 10% of deaths in patients developing AF. 21
Whether anticoagulation would improve outcomes in this patient population is not clear. In one study, those patients discharged on warfarin had a 22% relative reduction in mortality over a mean follow‐up of 6 years, compared to those discharged without warfarin. 7 This study included over 600 patients with NOAF discharged on warfarin. One of the other studies included in this meta‐analysis did not find a mortality benefit, but was smaller, at 139 patients. 12 There was wide variability in the practice patterns, with anticoagulation rates ranging from 12% to 21% in NOAF patients to 1% to 6% in the non‐NOAF category. 12 , 13 , 14 The existing guidelines suggest that it is reasonable to manage NOAF with anticoagulation and cardioversion if AF does not spontaneously revert to AF during follow‐up, but no recommendation is made for those patients who develop NOAF and then revert to sinus rhythm. 28 The question of rate vs rhythm control, however, has been previously studied. In patients with NOAF after cardiac surgery, rate and rhythm control have been shown be associated with similar rates of persistent AF and similar rates of complications. 29
These results suggest two avenues for further research. The first is elucidating the true incidence of long‐term AF in this patient population. Implantable cardiac monitors provide a means with which to study this topic. Determining which patients have long‐term AF will further delineate which outcomes may due to AF and which outcomes are due to comorbidities. The second avenue of further research would guide the role of anticoagulation: whether it should be prescribed to all patients with NOAF, only those who have recurrent NOAF, or only those with concomitant indications. There is a current multicenter randomized trial comparing oral anticoagulation to no oral anticoagulation in patients who develop NOAF after CABG, which may provide further information on this topic. 30
5. CONCLUSIONS
NOAF is a common outcome following isolated CABG. It is associated with an increased risk of stroke and long‐term mortality, even when adjusted for confounding variables. It is also associated with an increased risk of short‐term mortality, an outcome for which we only had unadjusted data. Based on the existing data, NOAF is independently associated with worse outcomes. Early recognition of NOAF may help risk‐stratify patients for closer follow‐up and monitoring. The outcomes analyzed here raise the question of whether anticoagulation would improve outcomes in these patients. Further research, including randomized controlled trials, is needed to answer this question.
Supporting information
Table S1 Newcastle‐Ottawa Scale quality assessment results
Table S2. Search strategy
Table S3. Study characteristics. NOAF indicates new‐onset atrial fibrillation; SVA, supraventricular arrhythmia; AFl, atrial flutter; NR, not reported; LOS, length of stay; MI, myocardial infarction.
Kerwin M, Saado J, Pan J, et al. New‐onset atrial fibrillation and outcomes following isolated coronary artery bypass surgery: A systematic review and meta‐analysis . Clin Cardiol. 2020;43:928–934. 10.1002/clc.23414
REFERENCES
- 1. Filardo G, Damiano RJ, Ailawadi G, et al. Epidemiology of new‐onset atrial fibrillation following coronary artery bypass graft surgery. Heart. 2018;104(12):985‐992. 10.1136/heartjnl-2017-312150. [DOI] [PubMed] [Google Scholar]
- 2. Filardo G, Ailawadi G, Pollock BD, et al. Postoperative atrial fibrillation: sex‐specific characteristics and effect on survival. J Thorac Cardiovasc Surg. 2020;159(4):1419‐1425.e1. 10.1016/j.jtcvs.2019.04.097. [DOI] [PubMed] [Google Scholar]
- 3. Kaw R, Hernandez AV, Masood I, Gillinov AM, Saliba W, Blackstone EH. Short‐ and long‐term mortality associated with new‐onset atrial fibrillation after coronary artery bypass grafting: a systematic review and meta‐analysis. J Thorac Cardiovasc Surg. 2011;141(5):1305‐1312. 10.1016/j.jtcvs.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 4. Phan K, Ha HSK, Phan S, Medi C, Thomas SP, Yan TD. New‐onset atrial fibrillation following coronary bypass surgery predicts long‐term mortality: a systematic review and meta‐analysis. Eur J Cardiothorac Surg. 2015;48(6):817‐824. 10.1093/ejcts/ezu551. [DOI] [PubMed] [Google Scholar]
- 5. Megens MR, Churilov L, Thijs V. New‐onset atrial fibrillation after coronary artery bypass graft and long‐term risk of stroke: a meta‐analysis. J Am Heart Assoc. 2017;6(12):e007558 10.1161/JAHA.117.007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daoud EG, Strickberger SA, Man KC, et al. Preoperative amiodarone as prophylaxis against atrial fibrillation after heart surgery. N Engl J Med. 1997;337(25):1785‐1791. 10.1056/NEJM199712183372501. [DOI] [PubMed] [Google Scholar]
- 7. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008‐2012. 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8. Loubani M, Hickey MS, Spyt TJ, Galiñanes M. Residual atrial fibrillation and clinical consequences following postoperative supraventricular arrhythmias. Int J Cardiol. 2000;74(2–3):125‐132. 10.1016/s0167-5273(00)00229-1. [DOI] [PubMed] [Google Scholar]
- 9. Lee S‐H, Kang DR, Uhm J‐S, et al. New‐onset atrial fibrillation predicts long‐term newly developed atrial fibrillation after coronary artery bypass graft. Am Heart J. 2014;167(4):593‐600.e1. 10.1016/j.ahj.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 10. Tulla H, Hippelainen M, Turpeinen A, Pitkanen O, Hartikainen J. New‐onset atrial fibrillation at discharge in patients after coronary artery bypass surgery: short‐ and long‐term morbidity and mortality. Eur J Cardiothorac Surg. 2015;48(5):747‐752. 10.1093/ejcts/ezu526. [DOI] [PubMed] [Google Scholar]
- 11. Konstantino Y, Zelnik Yovel D, Friger MD, Sahar G, Knyazer B, Amit G. Postoperative atrial fibrillation following coronary artery bypass graft surgery predicts long‐term atrial fibrillation and stroke. Isr Med Assoc J. 2016;18(12):744‐748. [PubMed] [Google Scholar]
- 12. Villareal RP, Hariharan R, Liu BC, et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43(5):742‐748. 10.1016/j.jacc.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 13. El‐Chami MF, Kilgo P, Thourani V, et al. New‐onset atrial fibrillation predicts long‐term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55(13):1370‐1376. 10.1016/j.jacc.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 14. Horwich P, Buth KJ, Legare J‐F. New onset postoperative atrial fibrillation is associated with a long‐term risk for stroke and death following cardiac surgery. J Card Surg. 2013;28(1):8‐13. 10.1111/jocs.12033. [DOI] [PubMed] [Google Scholar]
- 15. Filardo G, Pollock BD, da Graca B, et al. Underestimation of the incidence of new‐onset post‐coronary artery bypass grafting atrial fibrillation and its impact on 30‐day mortality. J Thorac Cardiovasc Surg. 2017;154(4):1260‐1266. 10.1016/j.jtcvs.2017.05.104. [DOI] [PubMed] [Google Scholar]
- 16. Saxena A, Dinh DT, Smith JA, Shardey GC, Reid CM, Newcomb AE. Usefulness of postoperative atrial fibrillation as an independent predictor for worse early and late outcomes after isolated coronary artery bypass grafting (multicenter Australian study of 19,497 patients). Am J Cardiol. 2012;109(2):219‐225. 10.1016/j.amjcard.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 17. Girerd N, Pibarot P, Daleau P, et al. Statins reduce short‐ and long‐term mortality associated with postoperative atrial fibrillation after coronary artery bypass grafting: impact of postoperative atrial fibrillation and statin therapy on survival. Clin Cardiol. 2012;35(7):430‐436. 10.1002/clc.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Almassi GH, Wagner TH, Carr B, et al. Postoperative atrial fibrillation impacts on costs and one‐year clinical outcomes: the veterans affairs randomized on/off bypass trial. Ann Thorac Surg. 2015;99(1):109‐114. 10.1016/j.athoracsur.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 19. O'Neal WT, Efird JT, Davies SW, et al. Impact of race and postoperative atrial fibrillation on long‐term survival after coronary artery bypass grafting. J Card Surg. 2013;28(5):484‐491. 10.1111/jocs.12178. [DOI] [PubMed] [Google Scholar]
- 20. Thoren E, Hellgren L, Granath F, Horte L‐G, Stahle E. Postoperative atrial fibrillation predicts cause‐specific late mortality after coronary surgery. Scand Cardiovasc J. 2014;48(2):71‐78. 10.3109/14017431.2014.880793. [DOI] [PubMed] [Google Scholar]
- 21. Schwann TA, Al‐Shaar L, Engoren MC, et al. Effect of new‐onset atrial fibrillation on cause‐specific late mortality after coronary artery bypass grafting surgery. Eur J Cardiothorac Surg. 2018;54(2):294‐301. 10.1093/ejcts/ezy028. [DOI] [PubMed] [Google Scholar]
- 22. Attaran S, Shaw M, Bond L, Pullan MD, Fabri BM. Atrial fibrillation postcardiac surgery: a common but a morbid complication. Interact Cardiovasc Thorac Surg. 2011;12(5):772‐777. 10.1510/icvts.2010.243782. [DOI] [PubMed] [Google Scholar]
- 23. Bramer S, van Straten AHM, Soliman Hamad MA, Berreklouw E, Martens EJ, Maessen JG. The impact of new‐onset postoperative atrial fibrillation on mortality after coronary artery bypass grafting. Ann Thorac Surg. 2010;90(2):443‐449. 10.1016/j.athoracsur.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 24. Friedman DJ, Piccini JP, Wang T, et al. Association between left atrial appendage occlusion and readmission for thromboembolism among patients with atrial fibrillation undergoing concomitant cardiac surgery. JAMA. 2018;319(4):365‐374. 10.1001/jama.2017.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Melduni RM, Schaff HV, Lee H‐C, et al. Impact of left atrial appendage closure during cardiac surgery on the occurrence of early postoperative atrial fibrillation, stroke, and mortality: a propensity score‐matched analysis of 10 633 patients. Circulation. 2017;135(4):366‐378. 10.1161/CIRCULATIONAHA.116.021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elbadawi A, Ogunbayo GO, Elgendy IY, et al. Impact of left atrial appendage exclusion on cardiovascular outcomes in patients with atrial fibrillation undergoing coronary artery bypass grafting (from the National Inpatient Sample Database). Am J Cardiol. 2017;120(6):953‐958. 10.1016/j.amjcard.2017.06.025. [DOI] [PubMed] [Google Scholar]
- 27. Al‐Shaar L, Schwann TA, Kabour A, Habib RH. Increased late mortality after coronary artery bypass surgery complicated by isolated new‐onset atrial fibrillation: a comprehensive propensity‐matched analysis. J Thorac Cardiovasc Surg. 2014;148(5):1860‐1868.e2. 10.1016/j.jtcvs.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 28. January CT, Wann L, Samuel CH, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125‐e151. 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 29. Gillinov AM, Bagiella E, Moskowitz AJ, et al. Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. 2016;374(20):1911‐1921. 10.1056/NEJMoa1602002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anticoagulation for New‐Onset Post‐Operative Atrial Fibrillation After CABG (PACES) . ClinicalTrials.gov. https://www.clinicaltrials.gov/ct2/show/NCT04045665. Accessed March 28, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Newcastle‐Ottawa Scale quality assessment results
Table S2. Search strategy
Table S3. Study characteristics. NOAF indicates new‐onset atrial fibrillation; SVA, supraventricular arrhythmia; AFl, atrial flutter; NR, not reported; LOS, length of stay; MI, myocardial infarction.


