Coronavirus disease 2019 (COVID-19), caused by a severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which can lead to pneumonia and severe acute respiratory syndrome, continues to spread across the globe.[1] Due to strict precautionary measures, the epidemic situation of COVID-19 has been initially controlled in China. However, the numbers of confirmed positive cases of COVID-19 and deaths are increasing in other countries. In fact, the SARS-CoV-2 is more contagious than severe acute respiratory syndrome coronavirus (SARS-CoV)[2] and Middle East respiratory syndrome coronavirus (MERS-CoV).[3] Therefore, the rapid and accurate identification of pathogenic viruses plays a vital role in controlling epidemics. Computed tomography imaging and some hematologic parameters are the initial markers for COVID-19 infection,[4,5] while viral nucleic acid detection by reverse transcription-polymerase chain reaction is used as a gold standard for the clinical diagnosis of suspected cases.[1] However, nucleic acid detection is time consuming and requires certified laboratories and trained technicians, and the results are easily affected by the quality of the specimens. Detection of serum-specific antibodies using colloidal gold immunochromatography assay (GICA) is another key approach for the rapid and highly sensitive diagnosis of viral infection. More importantly, this method does not require special equipment and reagents and is ideal for point-of-care testing.
In this study, we aimed to develop efficient GICA kits to detect SARS-CoV-2-specific immunoglobulin (Ig) M and IgG antibodies in the blood of COVID-19 patients. Two kinds of detection kits were designed as follows: kit A uses a single test line to capture the specific IgM and IgG antibodies targeting the N protein of SARS-CoV-2. Kit B was developed based on kit A, in which the IgG and IgM test lines are printed on different locations to distinguish positive IgG or IgM antibodies. The colloidal gold-labeled N protein of SARS-CoV-2 was chosen as the antigen for both kits. For the control line, rabbit IgG and goat anti-rabbit IgG antibodies (Sigma Co., St. Louis, MO, USA) were selected as the antigen and antibody, respectively [Figure 1A].
Figure 1.
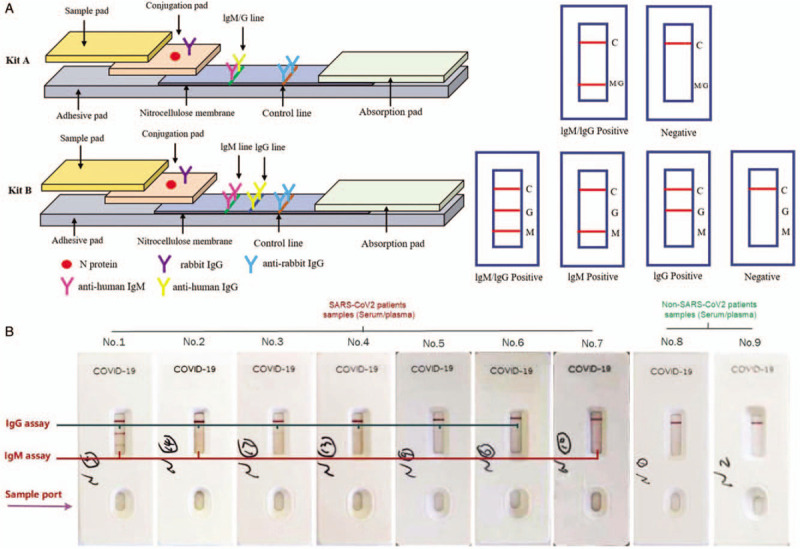
(A) Schematic diagram and interpretation standard of the SARS-COV-2 IgM/IgG antibody detection kit. (B) The representative test results using the developed kit B. COVID-19: Coronavirus disease 2019; Ig: Immunoglobulin; SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2.
To develop the GICA detection kits, the colloidal gold-labeled N protein and rabbit IgG were prepared according to the manufacturer's instructions. Briefly, 10 mL colloidal gold solution (40 nm; Bowendi Company, Changzhou, Jiangsu, China) was placed in a beaker and 65 μL of potassium carbonate (0.1 mol/L) was added to it. Recombinant SARS-CoV-2 N protein antigen (Feipeng Biological Co., Ltd, Shenzhen, Guangdong, China) was quickly added, and the solution was mixed well and incubated for 30 min at room temperature. Then, 100 μL of 20% bovine serum albumin solution was added to the mixture and incubated at room temperature for an additional 30 min. The mixture was centrifuged at 12,000 r/min for 20 min and washed with 5 mL phosphate-buffered saline (PBS; 50 mmol/L, pH 7.4). The precipitate was dissolved in 1.0 mL colloidal gold buffer. The antigen was loaded and printed on a conjugation pad (Schleicher & Schuell, Dassel, Germany) using the following printing conditions: the airbrush moving speed was 30 mm/s and the liquid advancing speed was 3 μL/cm. The mouse anti-human IgM/IgG antibody and goat anti-rabbit IgG antibody (Feipeng Biological Co., Ltd) were diluted with 50 mmol/L PBS (pH 7.4). The antibody was loaded and printed on a polyvinyl chloride sheet with nitrocellulose (Millipore, Bedford, MA, USA) using the following printing conditions: the airbrush moving speed was 30 mm/s and the liquid advancing speed was 0.5 μL/cm. The printed film was dried in a 37°C drying box for 6 h. All the prepared materials were assembled according to the design scheme shown in Figure 1A.
To test the sensitivity and specificity of these developed kits, we performed a clinical study using serum samples from 43 confirmed COVID-19 patients and 45 patients with unrelated disease (all the blood samples used for detecting specific antibodies were derived from the patients at the First Affiliated Hospital and Pulmonary Hospital of Shanxi Medical University, Taiyuan, China). All subjects were consecutively recruited during February 7 to 20, 2020. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki and approved by the Human Ethics Committee of the Shanxi Medical University (No. 2020001). To detect SARS-CoV-2-specific antibodies, 10 μL of the specimen was loaded into the sample pad, and 80 μL of dilution solution was added rapidly. The test results were determined qualitatively after 15 min by the coloration of the IgM and/or IgG line and control line. All procedures were performed in a biosafety level 3 laboratory. The test results from these kits should be interpreted as follows: the visual color in the control line and IgM and/or IgG test line indicate that the test specimen contains SARS-CoV-2-specific IgM and/or IgG antibodies. On the contrary, if only the control line is chromogenic, it represents that the IgM and IgG antibodies do not exist in the test specimen. In addition, no chromogenic reaction in the control line indicates an invalid test, possibly caused by the poor quality of kits or other unknown reasons [Figure 1A].
Representative test results of kit B are presented in Figure 1B. The visual band at both the detection line and control line can be observed in the COVID-19 patient samples, but not in the control samples. Furthermore, tests were performed using Human IgG Monoclonal Antibody to Nucleocapsid Protein Solution Reference Material of SARS-CoV-2, and confirmed by western blot. The results showed that the two methods were in agreement. Importantly, the results showed that the sensitivities of kits A and B were 88.3% and 93.0%, respectively, and the specificity was 100% for both kits. These results suggest that rapid and accurate detection of SARS-CoV-2 IgG and IgM antibodies can be achieved using our GICA kits. The higher sensitivity of kit B is due to the separately coated detection lines, which can effectively capture IgM or IgG antibodies against SARS-CoV-2. In this study, to avoid possible bias from operators, the blind method was used in the operation. All blood specimens were numbered and handled by a dedicated manager before testing. The information on the samples was kept confidential from the operators until the test was completed. It is worth noting, however, that the sensitivity and specificity of our pilot study may be overestimated because of the small sample size.
In summary, we developed two rapid, sensitive detection kits for SARS-CoV-2-specific IgG and IgM antibodies using colloidal gold immunochromatographic technology in this study. The rapid detection assay may be useful for COVID-19 clinical diagnosis and epidemiologic surveillance.
Funding
This study was supported by the Foundation of Shanxi Science and Technology Department (No. 202003D31004/GZ).
Conflicts of interest
None.
Footnotes
How to cite this article: Wang XL, Wang L, Hasi CL, Wang YP, Khan A, Ren BZ, Liu ZZ, Hou SL, Yang LH, Zhang LY, Dong YK, Xu J, Xie J. A rapid colloidal gold immunochromatographic assay for the diagnosis of coronavirus disease 2019. Chin Med J 2020;133:1986–1988. doi: 10.1097/CM9.0000000000000922
References
- 1.Jin YH, Cai L, Cheng ZS, Cheng H, Deng T, Fan YP, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020; 7:4.doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuiken T, Fouchier RA, Schutten M, Rimmelzwaan GF, van Amerongen G, van Riel D, et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 2003; 362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hui DS. Tracking the transmission and evolution of MERS-CoV. Lancet 2013; 382:1962–1964. doi: 10.1016/S0140-6736(13)61955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y, Liu X, Xiong L, Cai K. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Med Virol 2020; doi: 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]


