To the Editor: A first infection of varicella-zoster virus (VZV) during pregnancy can cause serious systemic disease to both mother and the fetus. Despite this maternal herpes zoster (HZ) does not increase fetal mortality, and the transmission of varicella-zoster virus to the fetus rarely occurs. Although HZ infection has a minimal effect on the fetus, maternal HZ can cause significant complications for the mother.[1]
Here, we present the promising response consisting of a treatment with both oral medication and nerve blocks to treat the neuralgic zoster pain of the pregnant woman.[2]
A 34-year-old woman in week 22 of pregnancy, with no significant medical history came to the pain clinic with flank pain and a rash (numeric rating scale [NRS] 4).[3] She noticed the lesions for the first time about 5 days earlier. On examination, an erythematous papulovesicular eruption was observed within the right thoracic six-seven dermatome [Figure 1].
Figure 1.
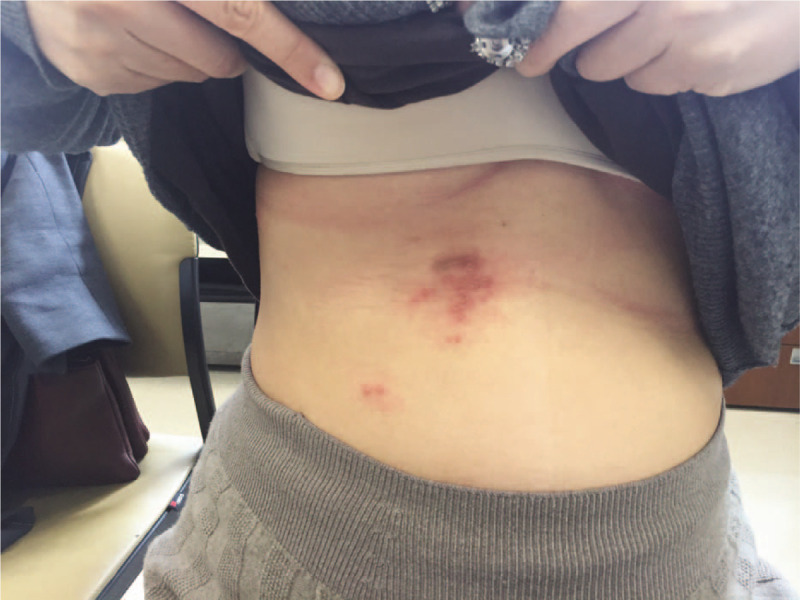
At the time of first outpatient visit, the patient was admitted with a rash that started 5 days ago, and all lesions at that time were observed in the thoracic six dermatome.
Under the diagnosis of HZ, a right thoracic six paravertebral block and right five, six, seven inter-costal nerve blocks were carried out under an ultrasound guidance. Paravertebral block was performed with 0.4% lidocaine 10 mL and inter-costal nerve blocks was performed with 0.4% lidocaine 5 mL per level. She was prescribed valtrex one gram and acetaminophen 300 mg pro re nata (PRN) for breakthrough pain over the next 3 days.[4]
At second visit (7 days after initial vesicle), she described that the previous pain intensity had been reduced to NRS 1-2. She had experienced sudden breakthrough pain of NRS 4 once or twice, and only then took acetaminophen. Nerve blocks were carried out again in the same way, and then she was prescribed valtrex 1 g tid for 7 days.
At third visit (12 days after initial vesicle), she no longer complained of pain and stated that she had not take any medicine. Four months later, via phone, we confirmed that she had given birth to a healthy child and had no further problems with pain.[5]
General treatment of HZ during pregnancy until now consisted of giving anti-viral drugs or taking acetaminophen regularly for pain. Although studies on the safety of anti-viral drugs in pregnancy are scarce, several studies report there is little effect on the fetus. Anti-viral drugs are classified for pregnant women in category B, acyclovir, and valacyclovir are recommended for mothers. Acetaminophen is the only category B oral analgesic for pregnant women and can be safely used on the second trimester. However, even when actively treated in the acute phase, it is difficult to significantly reduce the incidence of post-herpetic neuralgia. In this case, by performing nerve blocks, the intensity of the acute-phase pain was reduced and the rate of post-herpetic neuralgia was lowered. Steroids that can be commonly used in the acute phase were not used in category C, instead only local anesthetics (category B) were used. Nerve blocks were performed under ultrasound guidance in a safe and effective way for both mother and the fetus. It is thought that future research on nerve blocks will require large-scale prospective clinical studies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Conflicts of interest
None.
Footnotes
How to cite this article: Kim JY, Ko YR, Sim SE, Oh S, Lee MH, Park HJ. Safe drug treatment and procedure for herpes zoster in pregnancy: a case report. Chin Med J 2020;133:1999–2000. doi: 10.1097/CM9.0000000000000927
References
- 1.Robyn S, Melissa D, Julia C. Herpes zoster in pregnancy. J Midwifery Womens Health 2019; 64:230–235. [DOI] [PubMed] [Google Scholar]
- 2.Maryam S, Iman A. Severe herpes zoster neuralgia in a pregnant woman treated with acetaminophen. Acta Med Iran 2014; 52:238–239. [PubMed] [Google Scholar]
- 3.Hayward K, Cline A, Stephens A, Street L. Management of herpes zoster (shingles) during pregnancy. J Obstet Gynaecol 2018; 38:887–894. doi: 10.1080/01443615.2018.1446419. [DOI] [PubMed] [Google Scholar]
- 4.Young HJ. Herpes zoster and posttherapetic neuralgia: practical consideration for prevention and treatment. Korean J Pain 2015; 28:177–184. doi: 10.3344/kjp.2015.28.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yong IK, Kyung LL, Dong AA, In HK. The effect of regional sympathetic block in the treatment of herpes zoster. Korean J Anesthesiol 1983; 16:441–444. doi: 10.4097/kjae.1983.16.4.441. [Google Scholar]


