Abstract
Patient-derived tumor organoids (PDOs) currently represent important modeling tools in pre-clinical investigation of malignancies. Organoid cultures conserve the genetic and phenotypic characteristics of the original tumor and maintain its heterogeneity, allowing their application in many research fields. PDOs derived from colorectal cancer (CRC) have been used for genetic modeling to investigate the function of driver genes. Some researchers have been exploring the value of CRC PDOs in chemotherapy, targeted therapy, and radiotherapy response prediction. The successful generation of PDOs derived from CRC could deepen our understanding of CRC biology and provide novel tools for cancer modeling, for realizing precision medicine by assessing specimens from individual patients ex vivo. The present review discusses recently reported advances in CRC PDOs and the challenges they face as pre-clinical models in CRC research.
Keywords: Colorectal cancer, Patient-derived tumor organoids, Pre-clinical model
Introduction
Colorectal cancer (CRC) represents a major malignancy with an incidence increasing year by year in China, and ranks third in terms of cancer mortality.[1,2] The clinical outcomes and treatment responses of patients with CRC vary greatly,[3] yet remain unsatisfactory. The so-called standard treatment is limited in current clinical practice.[4,5] Meanwhile, a multitude of drugs demonstrating effectiveness in cancer models eventually failed in clinical trials, indicating a barrier between in vivo and in vitro model systems and clinical practice. Therefore, models should be developed, which can accurately reflect the genetic diversity and specificity of tumors, facilitating the understanding of specific changes in tumorigenesis, tumor maintenance, and therapeutic sensitivity.
Fluoropyrimidines remain the backbone of chemotherapeutic agents for metastatic/recurrent CRC.[6–9] Their treatment outcome has been improved greatly from 12 months to over 30 months in terms of overall survival.[10] With the inclusion of biologically targeted agents, such as monoclonal antibodies targeting epidermal growth factor receptor (EGFR) and anti-angiogenic agents regulating vascular endothelial growth factor signaling,[11] patient survival has been increased, especially after stratification by Ras/Raf mutation[12] and sidedness.[13] Studies have shown that instability-high CRC can benefit from programmed cell death 1 blockade.[14–16] Despite a step forward toward personalized treatment, it still is impossible to identify which patient might be responsive or not. The treatment is essentially experimental, and results are uncertain. Some patients may respond well and others not, or even be refractory after the initial treatment.[17]
Cancer, including CRC, involves multiple and complex events, with a series of genetic changes.[18] Tumor heterogeneity is particularly evident in CRC, caused by chromosome (CIN) or microsatellite instability,[19] which has an important impact on targeted therapy[3] and response prediction.[20]
Common cancer models, derived from primary tumors and mimicking tumor development, include malignant cell lines and xenografts in experimental animals. Human malignant cell lines are commonly cultured under two-dimensional culture conditions in vitro. In addition, only rare clones derived from primary patient tumors can be extended and maintained for many generations. Thus, such cell lines might have undergone tremendous genetic changes, resulting in the absence of genetic heterogeneity of the corresponding original tumors. Although animal cancer models are important in cancer research, their establishment takes time. In addition, animal models often fail to faithfully reflect the pathogenesis of patients since human cancer is histologically complex and genetically heterogeneous. Primary patient-derived tumor xenografts (PDTXs) are obtained via transplantation of freshly collected human tumors into immunodeficient mice. PDTXs enable in vivo assessment but are time-consuming and require large amounts of resources.
Recently, three-dimensional (3D) culture technology has led to the development of novel cancer models. When gut stem cells expressing leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) are supplemented with R-spondin-based culture conditions and maintained in a 3D extracellular matrix, they are capable of generating a steadily growing, self-organizing epithelial structure physiologically comparable to the non-diseased intestinal tissue. The newly developed culture system was termed organoid culture, and is eventually applicable for generating CRC organoids.[21] Subsequently, R-spondin-based culture conditions have been developed for noncancerous and cancer tissues of the pancreas,[22,23] stomach,[24] prostate,[25] and liver.[26] Compared with PDTXs, organoids from patients can be easily expanded for a long time. In this review, we discuss important advances in patients with CRC-derived tumor organoids [Table 1] and the challenges they face as pre-clinical models in CRC research.
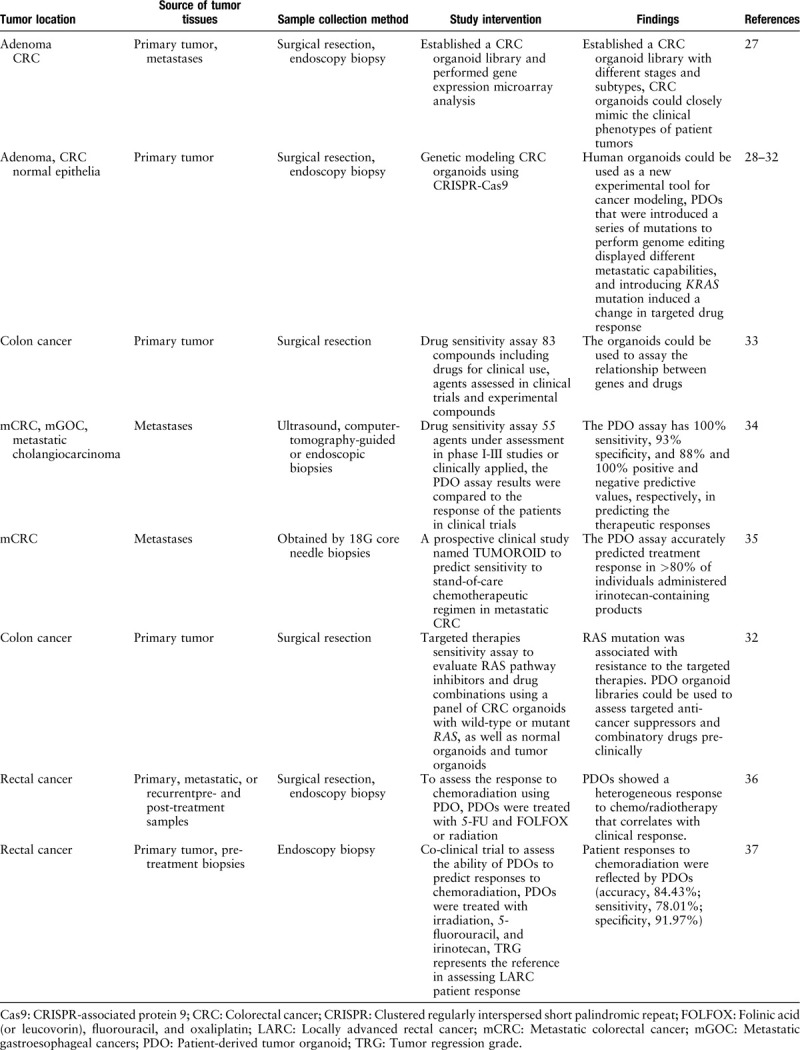
Table 1.
Examples of studies of CRC using human organoid models.
Organoid Culture: An in Vitro Model Mimicking the Primary Tumor
Human tumor organoids have been generated from colon,[21,33] pancreas,[23] prostate,[38] breast,[39] gastric,[40] lung,[41] esophageal,[42] bladder,[43] ovarian,[44] kidney,[45] and liver[46] tumor tissues. Many studies have demonstrated that cultured organoids closely reproduce the genetic and morphologic heterogeneity of malignant cells in the initial cancer tissue.[33,47,48] It was shown that primary tumors produce a large number of different organoids in the culture process, which suggests that the heterogeneity of primary tumors is largely conservative.[33] Transcriptome analysis also confirmed the heterogeneity of these cultures, which produce different expression profiles. A high degree of consistency in the morphologic and mutational characteristics of tumor organoids and matched patients’ tumors was demonstrated by histologic analysis and DNA sequencing.
Maintaining cancer cell heterogeneity in culture is critical in targeted drug development.[49,50] Gene expression assessment in solid tumor samples and respective cell lines has revealed substantial differences.[51] In addition, cell lines usually do not include all gene profiles of tumors. It is known that tumor tissues and cell lines retain common mutations rather than scarce mutations.[52] This could partially account for the low value of data generated in cell lines in translational research.[51,53] Of high importance, early and late-passage organoids display mostly identical mutations.[47,54]
Characteristics of Organoids for Clinical Application
Tumor organoids closely mimic the clinical phenotypes of human malignancy as well as cancer progression
CRC represents a heterogeneous malignancy with varying clinical features and prognoses. Organoids could routinely be obtained from cancer tissues at different clinical stages. For early stage malignancies, patient-derived tumor organoids (PDOs) could be employed for identifying molecular alterations potentially serving as biomarkers and targets for prevention. Fujii et al demonstrated that it is feasible to grow different types of precancerous colon neoplasia in culture. Organoids in all disease phases were independent of Wnt/R-spondin due to the activation of Wnt signaling while dependence on the remaining niche growth factors subsides during the adenoma to carcinoma transformation.[27]
Genetic cancer modeling in organoid cultures
Organoids can routinely be derived from normal human epithelia, enabling in vitro modeling of mutations in all cancer phases. Organoids can undergo subcloning following clustered regularly interspersed short palindromic repeat (CRISPR) modifications, allowing a comprehensive analysis of gene functions in cancer. Matano et al[28] used CRISPR-CRISPR-associated protein 9 (Cas9) for introducing many such mutations in organoids obtained from patients’ noncancerous gut epithelial specimens. Through culture condition changes to mimic the gut environment, the authors identified isogenic organoids mutated for tumor suppressor genes such as APC, SMAD4, and TP53, as well as for oncogenes including KRAS and PIK3CA. Organoids designed to harbor these five mutations showed normal growth without niche factors in culture. The mutated organoids generated tumors upon implantation, but could not form colonies in the liver. Meanwhile, organoids from chromosome-instable human adenoma specimens generated macrometastatic colonies. These findings indicate that mutation of driver pathways helps stem cells to survive a hostile tumor microenvironment, with further molecular alterations needed for invasiveness.
Another report by Drost et al[29] had a similar strategy. After KRAS, APC, P53, and SMAD4 mutation, human intestinal organoids can grow without stem-cell-niche molecules and with the P53 stabilizer nutlin-3 in culture, and as tumor tissues showing invasive cancer properties in vivo. In addition, they found that the engineered CRC organoids showed substantial CIN as well as aneuploidy. In a follow-on study, Fumagalli et al[30] orthotopically transplanted human colon organoids harboring various CRC mutation combinations, and demonstrated that sequential accumulation of oncogenic mutations in Wnt, EGFR, P53, and transforming growth factor-β(TGF-β) pathway genes promotes cancer cell growth, migration, and metastasis. Reconstituting specific niche signals could restore metastasis in cancer cells devoid of one of these oncogenic mutations, which implies that the capability of metastasizing, e.g., invading distant sites, directly results from loss of dependency upon particular niche factors.
Drost et al[31] employed CRISPR-Cas9 for deleting major DNA repair genes in colon organoids from humans, revealing that mutation accumulation in organoids not expressing the mismatch repair gene MLH1 was due to replication errors, which closely reflects the mutation patterns reported for mismatch repair-deficient CRC.
Verissimo et al[32] introduced an oncogenic KRAS mutation via CRISPR/Cas9-mediated homologous recombination in a patient-derived CRC organoid which was a wild type for the whole downstream EGFR pathway, and observed a marked change on targeted drug response.
Organoid culture can detect the genotype-to-drug association
Studies confirmed that organoid culture can detect the genotype-to-drug association. Van de Wetering et al[33] established tumor organoid cultures from 20 CRC cases enrolled consecutively. The organoids were obtained from surgically resected tissues from previously untreated patients with CRC. The most frequently altered CRC genes were also largely found in organoid cultures. Inhibiting changes to tumor-suppressive factors (APC, TP53, FBXW7, and SMAD4) and inducing alterations in KRAS (codons 12 and 146) and PIK3CA (codons 545 and 1047) were noted. Inducing BRAF and TGFBR1/2 gene alterations were found in organoids with hypermutation. Gene expression assessment revealed that the main CRC molecular subtypes were represented. These authors used a customized library of 83 compounds to screen drug sensitivity in 3D organoid cultures, including drugs for clinical use, chemotherapy, and agents previously assessed in or currently under clinical trials, as well as experimental molecules for different cancer targets. The relationship between genes and drugs could be detected by high-throughput drug screening. For instance, a particular organoid culture showing high sensitivity to Wnt secretion suppressors was mutated for ring finger protein 43 (RNF43), a negative feedback modulator of Wnt signaling, with no APC mutation.
Application in Colorectal Cancer
Organoid culture predicts sensitivity to chemotherapeutic agents in metastatic CRC
Chemotherapy markedly improves the overall survival in tumor patients. However, cancer cell chemosensitivity is highly heterogeneous, and individuals might undergo unnecessary therapy and be needlessly exposed to toxic products. It was reported that organoid cultures could be successfully initiated from metastatic biopsy samples in CRC, encompassing genetic features of the original metastatic lesion. Indeed, 90% of somatic mutations are present in both organoid and biopsy specimens from a given individual.[48]
Vlachogiannis et al[34] contemplated the possibility of employing PDOs from metastatic gastrointestinal cancer (CRC and gastroesophageal cancer) as drug screening tools. They ran 3D screening assays with 55 agents under assessment in phase I–III studies or clinically applied. The authors reported 100% sensitivity, 93% specificity, and 88% and 100% positive and negative predictive values, respectively, in predicting the therapeutic responses in patients.
Our team also established a CRC organoid model with robust growth over 25 days, which could be employed to screen drugs and perform individualized treatments.[55]
Subclonal populations of tumors were demonstrated to be resolved within the spheroid cultures,[56] which indicates that organotypic cultures could be a new tool for investigating the evolution of clonal architecture over time in response to therapies. In the above study, optical metabolic imaging was used to interrogate the metabolic activity at the single-cell level. Such single-cell analysis can dynamically quantify heterogeneous drug response, potentially identifying refractory sub-populations.
Recently, Ooft et al[35] performed a prospective clinical study based on PDOs from CRC metastatic tumors for identifying non-responders to the standard-of-care chemotherapeutic regimen. The study, named TUMOROID, involved multiple centers and assessed common treatment options applied in CRC, for example, a chemotherapeutic regimen based on 5-fluorouracil (5-FU) alone or with oxaliplatin, or irinotecan or irinotecan alone. Their PDO assay accurately predicted treatment response in >80% of individuals administered irinotecan-containing products without misclassification of cases who could have treatment benefits. Such correlation was specific to irinotecan-containing regimens, but could not reflect treatment outcome in patients administered the 5-FU-oxaliplatin combination. These findings indicate that PDOs could recapitulate patient response, with potential application in individualized treatment.
Organoid culture could help evaluate and optimize targeted anti-cancer therapies
Recently, treatments targeting the mitogen-activated protein kinase pathway have been applied in metastatic CRC. However, certain mutations confer resistance to such treatments, for example, in the KRAS and BRAF genes.[57–60] Recently, multiple reports have demonstrated that it is possible to overcome such resistance by simultaneously blocking the mutated molecules.[61] It is, therefore, necessary to identify suitable targeted anti-cancer inhibitors and drug combinations for patients. Verissimo et al[32] have assessed clinically employed KRAS-signaling suppressors and drug combinations against noncancerous colon and CRC organoids. RAS mutation was tightly associated with chemoresistance, in both malignant and noncancerous organoids. In addition, simultaneously inhibiting the EGFR and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways in organoids mutated for RAS transiently caused cell-cycle arrest but not cell death. Finally, EGFR pathway inhibition strongly sensitized for cell death induction in RAS-mutant CRCs by minimal addition of navitoclax, a known B-cell lymphoma-2/B-cell lymphoma XL suppressor. These results indicate a strong potential for patient-derived CRC organoid in assessing targeted anti-cancer suppressors and combinatory drugs pre-clinically.
Organoid culture predicts sensitivity to chemoradiotherapy in rectal cancer patients
Neoadjuvant chemoradiation (nCRT) and subsequent total mesorectal excision is routinely employed for treating locally advanced rectal cancer (LARC). The sensitivity of patients to radiotherapy presents a great heterogeneity. Some individuals show complete response to nCRT alone, with no need for operation,[62] unlike others who undergo radical surgery after nCRT.[63] Many efforts have been made to develop a method for predicting sensitivity to chemoradiotherapy to identify individuals sensitive to chemoradiation for decision making after nCRT.
The Memorial Sloan Kettering Cancer Center group[36] produced rectal cancer organoids (RCOs) from primary, metastatic, and recurrent cancer cases. They demonstrated that RCOs could help assess the responses of individuals to chemoradiation. RC tumoroids are heterogeneous in their responses to physiologic 5-FU and folinic acid (or leucovorin), FU, and oxaliplatin (FOLFOX) levels. These authors then employed areas under the dose-response curves (AUCs) to reflect ex vivo effects and found that AUCs for both 5-FU and FOLFOX in ex vivo assays were associated with progression-free survival of respective patients (Spearman r = 0.86, P = 0.024). They also exposed tumoroids to irradiation ex vivo, and a heterogeneous response was obtained. The association of ex vivo and patient-radiation sensitivities was further examined by comparing endoscopic tumors right pre- and post-radiation and assessing radiotherapy response. Interestingly, RC tumoroids displayed different sensitivities to radiation, corroborating patient response.
More recently, co-clinical trial data from Fudan University Shanghai Cancer Center indicated that PDOs could predict rectal cancer response clinically, and might constitute a tool for diagnosing rectal cancer. In this phase III study, 80 primary rectal cancer biopsies were collected from LARC cases. Pathologic tumor regression grade following total mesorectal excision represents the reference in assessing LARC patient response following nCRT. Cases had good treatment response with tumor organoids showing sensitivity to one or more of the three therapeutic components, including irradiation, 5-FU, and irinotecan (CPT-11). Patient responses to chemoradiation significantly reflected those of RCOs (accuracy, 84.43%; sensitivity, 78.01%; specificity, 91.97%).[37]
The data given earlier indicate that ex vivo chemoradiation systems could help assess clinical patient response prior to radiation and chemotherapy administration, which could avoid toxic effects of irradiation in resistant cases. On the contrary, in patients failing standard treatments or experiencing disease progression, these platforms might provide a possibility to investigate novel treatment options ex vivo using their own tumor tissues.
Current Challenges and Perspectives
Multiple anti-cancer compounds assessed clinically eventually fail to obtain approval because of inadequate safety and/or efficacy, suggesting that current pre-clinical predictive models are limited. The accuracy of in vitro high-throughput drug screening, therefore, depends on further optimization of drug screening platforms mirroring patient disease. Even though drug screening based on two-dimensional tumor cell lines provides important insights for predicting therapeutic response, poor reproducibility of the original cancer tissue may be the reason behind the frequent failure of novel drug in clinical studies. Tumor organoids derived from patients could better reproduce the primary tumor and may be an ideal model for identifying and testing new anti-cancer drugs. The development of high-throughput drug screening methods is just a start in PDOs
As demonstrated above, organoids have a great potential in CRC research, and their assessment could help clinical decision making and improve patient survival with CRC treatment. However, several challenges for the organoid system need to be addressed. One limitation is that organoids do not have multiple cell constituents of the tumor microenvironment, for example, stromal, vascular endothelial, and immune cells, which limits its ability to completely simulate the primary tumor. Although this limitation could be overcome by combining the PDO model with fibroblasts, and immune and endothelial cells. Many researchers are trying to solve this problem, but it remains a challenge for researchers to enhance mimicry of the tumor microenvironment.[64]
Although the success rate of PDO establishment is high, its efficiency needs to be improved. The time and costs of organoid generation also need to be reduced. The main issue hampering organoid cultures is insufficient fresh tissue with live cancer cells. CRC organoids are derived from biopsies of metastatic cancer or treatment-naive rectal cancer cases, but sometimes low amounts of live cancer cells are found in biopsies. The success rate of cultures could be further improved by obtaining many core biopsies and direct evaluation by pathologists.
To ease model establishment, multiple current protocols employed for CRC PDO culture should be optimized and standardized. The culture components are complex, which affects PDO growth and consequently the test results. In addition, standardized characterization by whole-exome sequencing, copy number assessment, RNA sequencing, and drug sensitivity tests, is required for achieving reproducibility.
Conclusions
CRC organoid cultures conserve the genetic and phenotypic heterogeneities of primary cancers. They could help detect gene-drug associations and perform high-throughput drug screening. CRC organoids thus constitute a model system comparable to genetically engineered mouse models, cell lines, and PDTXs. Additionally, they are expanded relatively faster, and can be cryopreserved and genetically altered. CRC PDOs can be used to generate living tumor organoid biobanks, and provide a platform for high-throughput drug screening, not only chemotherapeutic agents and targeted treatments, but also radiotherapy. The utility of organoids in CRC still warrants further evidence, especially those in clinical trials.
Funding
This work was supported by a grant from the National Natural Science Foundation (No. 81773214).
Conflicts of interest
None.
Footnotes
How to cite this article: Ji DB, Wu AW. Organoid in colorectal cancer: progress and challenges. Chin Med J 2020;133:1971–1977. doi: 10.1097/CM9.0000000000000882
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Linnekamp JF, Wang X, Medema JP, Vermeulen L. Colorectal cancer heterogeneity and targeted therapy: a case for molecular disease subtypes. Cancer Res 2015; 75:245–249. doi: 10.1158/0008-5472.CAN-14-2240. [DOI] [PubMed] [Google Scholar]
- 4.Dienstmann R, Salazar R, Tabernero J. Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. J Clin Oncol 2015; 33:1787–1796. doi: 10.1200/JCO.2014.60.0213. [DOI] [PubMed] [Google Scholar]
- 5.Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer 2017; 17:79–92. doi: 10.1038/nrc.2016.126. [DOI] [PubMed] [Google Scholar]
- 6.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol 2000; 18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004; 22:23–30. doi: 10.1200/JCO.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 8.Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008; 26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 9.Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol 2007; 25:1670–1676. doi: 10.1200/JCO.2006.09.0928. [DOI] [PubMed] [Google Scholar]
- 10.Cremolini C, Schirripa M, Antoniotti C, Moretto R, Salvatore L, Masi G, et al. First-line chemotherapy for mCRC-a review and evidence-based algorithm. Nat Rev Clin Oncol 2015; 12:607–619. doi: 10.1038/nrclinonc.2015.129. [DOI] [PubMed] [Google Scholar]
- 11.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. Lancet Oncol 2015; 16:499–508. doi: 10.1016/S1470-2045(15)70127-0. [DOI] [PubMed] [Google Scholar]
- 12.Van Cutsem E, Lenz HJ, Kohne CH, Heinemann V, Tejpar S, Melezinek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015; 33:692–700. doi: 10.1200/JCO.2014.59.4812. [DOI] [PubMed] [Google Scholar]
- 13.Zheng G, Tseng LH, Chen G, Haley L, Illei P, Gocke CD, et al. Clinical detection and categorization of uncommon and concomitant mutations involving BRAF. BMC Cancer 2015; 15:779.doi: 10.1186/s12885-015-1811-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015; 372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017; 18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammond WA, Swaika A, Mody K. Pharmacologic resistance in colorectal cancer: a review. Ther Adv Med Oncol 2016; 8:57–84. doi: 10.1177/1758834015614530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahouel K, Younes L, Danilova L, Giardiello FM, Hruban RH, Groopman J, et al. Revisiting the tumorigenesis timeline with a data-driven generative model. Proc Natl Acad Sci U S A 2020; 117:857–864. doi: 10.1073/pnas.1914589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang W, Kandimalla R, Huang H, Zhu L, Li Y, Gao F, et al. Molecular subtyping of colorectal cancer: recent progress, new challenges and emerging opportunities. Semin Cancer Biol 2019; 55:37–52. doi: 10.1016/j.semcancer.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baisse B, Bouzourene H, Saraga EP, Bosman FT, Benhattar J. Intratumor genetic heterogeneity in advanced human colorectal adenocarcinoma. Int J Cancer 2001; 93:346–352. doi: 10.1002/ijc.1343. [DOI] [PubMed] [Google Scholar]
- 21.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 2011; 141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 22.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015; 160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med 2015; 21:1364–1371. doi: 10.1038/nm.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, et al. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 2015; 148:126–136.e6. doi: 10.1053/j.gastro.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014; 159:176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huch M, Boj SF, Clevers H. Lgr5(+) liver stem cells, hepatic organoids and regenerative medicine. Regen Med 2013; 8:385–387. doi: 10.2217/rme.13.39. [DOI] [PubMed] [Google Scholar]
- 27.Fujii M, Shimokawa M, Date S, Takano A, Matano M, Nanki K, et al. A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 2016; 18:827–838. doi: 10.1016/j.stem.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med 2015; 21:256–262. doi: 10.1038/nm.3802. [DOI] [PubMed] [Google Scholar]
- 29.Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 2015; 521:43–47. doi: 10.1038/nature14415. [DOI] [PubMed] [Google Scholar]
- 30.Fumagalli A, Drost J, Suijkerbuijk SJ, van Boxtel R, de Ligt J, Offerhaus GJ, et al. Genetic dissection of colorectal cancer progression by orthotopic transplantation of engineered cancer organoids. Proc Natl Acad Sci U S A 2017; 114:E2357–E2364. doi: 10.1073/pnas.1701219114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drost J, van Boxtel R, Blokzijl F, Mizutani T, Sasaki N, Sasselli V, et al. Use of CRISPR-modified human stem cell organoids to study the origin of mutational signatures in cancer. Science 2017; 358:234–238. doi: 10.1126/science.aao3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verissimo CS, Overmeer RM, Ponsioen B, Drost J, Mertens S, Verlaan-Klink I, et al. Targeting mutant RAS in patient-derived colorectal cancer organoids by combinatorial drug screening. Elife 2016; 5: doi: 10.7554/eLife. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015; 161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernandez-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018; 359:920–926. doi: 10.1126/science.aao2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ooft SN, Weeber F, Dijkstra KK, McLean CM, Kaing S, van Werkhoven E, et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med 2019; 11: doi: 10.1126/scitranslmed.aay2574. [DOI] [PubMed] [Google Scholar]
- 36.Ganesh K, Wu C, O’Rourke KP, Szeglin BC, Zheng Y, Sauve CG, et al. A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med 2019; 25:1607–1614. doi: 10.1038/s41591-019-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L, et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell 2020; 26:17–26.e6. doi: 10.1016/j.stem.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Drost J, Karthaus WR, Gao D, Driehuis E, Sawyers CL, Chen Y, et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc 2016; 11:347–358. doi: 10.1038/nprot.2016.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 2018; 172:373–386.e10. doi: 10.1016/j.cell.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 40.Yan HHN, Siu HC, Law S, Ho SL, Yue SSK, Tsui WY, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell 2018; 23:882–897.e11. doi: 10.1016/j.stem.2018.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Sachs N, Papaspyropoulos A, Zomer-van Ommen DD, Heo I, Bottinger L, Klay D, et al. Long-term expanding human airway organoids for disease modeling. EMBO J 2019; 38: doi: 10.15252/embj.2018100300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X, Francies HE, Secrier M, Perner J, Miremadi A, Galeano-Dalmau N, et al. Organoid cultures recapitulate esophageal adenocarcinoma heterogeneity providing a model for clonality studies and precision therapeutics. Nat Commun 2018; 9:2983.doi: 10.1038/s41467-018-05190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullenders J, de Jongh E, Brousali A, Roosen M, Blom JPA, Begthel H, et al. Mouse and human urothelial cancer organoids: a tool for bladder cancer research. Proc Natl Acad Sci U S A 2019; 116:4567–4574. doi: 10.1073/pnas.1803595116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopper O, de Witte CJ, Lohmussaar K, Valle-Inclan JE, Hami N, Kester L, et al. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med 2019; 25:838–849. doi: 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 45.Schutgens F, Rookmaaker MB, Margaritis T, Rios A, Ammerlaan C, Jansen J, et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat Biotechnol 2019; 37:303–313. doi: 10.1038/s41587-019-0048-8. [DOI] [PubMed] [Google Scholar]
- 46.Li L, Knutsdottir H, Hui K, Weiss MJ, He J, Philosophe B, et al. Human primary liver cancer organoids reveal intratumor and interpatient drug response heterogeneity. JCI Insight 2019; 4: doi: 10.1172/jci.insight.121490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pauli C, Hopkins BD, Prandi D, Shaw R, Fedrizzi T, Sboner A, et al. Personalized in vitro and in vivo cancer models to guide precision medicine. Cancer Discov 2017; 7:462–477. doi: 10.1158/2159-8290.CD-16-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weeber F, van de Wetering M, Hoogstraat M, Dijkstra KK, Krijgsman O, Kuilman T, et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci U S A 2015; 112:13308–13311. doi: 10.1073/pnas.1516689112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discov 2016; 6:147–153. doi: 10.1158/2159-8290.CD-15-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol 2012; 13:e178–e185. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 51.Stein WD, Litman T, Fojo T, Bates SE. A Serial Analysis of Gene Expression (SAGE) database analysis of chemosensitivity: comparing solid tumors with cell lines and comparing solid tumors from different tissue origins. Cancer Res 2004; 64:2805–2816. doi: 10.1158/0008-5472.CAN-03-3383. [DOI] [PubMed] [Google Scholar]
- 52.Iorio F, Knijnenburg TA, Vis DJ, Bignell GR, Menden MP, Schubert M, et al. A landscape of pharmacogenomic interactions in cancer. Cell 2016; 166:740–754. doi: 10.1016/j.cell.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Begley CG, Ellis LM. Drug development: raise standards for preclinical cancer research. Nature 2012; 483:531–533. doi: 10.1038/483531a. [DOI] [PubMed] [Google Scholar]
- 54.Schütte M, Risch T, Abdavi-Azar N, Boehnke K, Schumacher D, Keil M, et al. Molecular dissection of colorectal cancer in pre-clinical models identifies biomarkers predicting sensitivity to EGFR inhibitors. Nat Commun 2017; 8:14262.doi: 10.1038/ncomms14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie BY, Wu AW. Organoid culture of isolated cells from patient-derived tissues with colorectal cancer. Chin Med J 2016; 129:2469–2475. doi: 10.4103/0366-6999.191782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pasch CA, Favreau PF, Yueh AE, Babiarz CP, Gillette AA, Sharick JT, et al. Patient-derived cancer organoid cultures to predict sensitivity to chemotherapy and radiation. Clin Cancer Res 2019; 25:5376–5387. doi: 10.1158/1078-0432.CCR-18-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008; 359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 58.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008; 26:1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 59.Peeters M, Oliner KS, Parker A, Siena S, Van Cutsem E, Huang J, et al. Massively parallel tumor multigene sequencing to evaluate response to panitumumab in a randomized phase III study of metastatic colorectal cancer. Clin Cancer Res 2013; 19:1902–1912. doi: 10.1158/1078-0432.CCR-12-1913. [DOI] [PubMed] [Google Scholar]
- 60.Loupakis F, Ruzzo A, Cremolini C, Vincenzi B, Salvatore L, Santini D, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer 2009; 101:715–721. doi: 10.1038/sj.bjc.6605177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhai Z, Yu X, Yang B, Zhang Y, Zhang L, Li X, et al. Colorectal cancer heterogeneity and targeted therapy: clinical implications, challenges and solutions for treatment resistance. Semin Cell Dev Biol 2017; 64:107–115. doi: 10.1016/j.semcdb.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 62.Smith JJ, Strombom P, Chow OS, Roxburgh CS, Lynn P, Eaton A, et al. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol 2019; 5:e185896.doi: 10.1001/jamaoncol.2018.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 2012; 30:1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harry Cheuk Hay Lau, Onno Kranenburg, Haipeng Xiao, Jun Yu. Organoid models of gastrointestinal cancers in basic and translational research. Nat Rev Gastroenterol Hepatol 2020; 17:203–222. doi: 10.1038/s41575-019-0255-2. [DOI] [PubMed] [Google Scholar]


