Abstract
Supplemental Digital Content is available in the text
To the Editor: Hyperuricemia, the second most common metabolic disease after diabetes mellitus in China, is characterized by the elevated serum uric concentration which is due to the overactive uric acid production or underexcretion.[1] Recent study supported the notion that hyperuricemia is implicated in renal tubular injury, subsequent tubulointerstitial fibrosis, and finally gouty nephropathy.[2] However, the lack of a suitable and stable mouse model to study hyperuricemia and gout nephropathy delays and limits the development of mechanism research and new drugs. Further understanding of the pathogenesis of hyperuricemia and gouty nephropathy has been largely hampered by a lack of preclinical animal models that reliably capture the characteristic phenotypes of hyperuricemia and gouty nephropathy. In this study, we aimed to develop a novel mouse model for hyperuricemia and gouty nephropathy.
Male C57BL/6J mice (30 g) were divided randomly into three groups the control group (treated with carboxymethyl cellulose sodium [CMC-Na] alone); the 100 mg/kg adenine and 500 mg/kg potassium oxonate group (Ade-100 + OXO-500); and the 150 mg/kg adenine and 300 mg/kg OXO group (Ade-150 + OXO-300). Mice were administered intragastrically with a mixture of adenine and OXO (diluted in 0.5% CMC-Na) daily consistently for 3 weeks [Figure 1A]. The food intake, water consumption and weight gain of mice were measured before administration. Mice were anesthetized with chloral hydrate and the blood samples were collected through the outer canthus for the measurement of serum uric acid and creatinine weekly. On the last day of the experiment, mice were kept in individual metabolic cages to collect 24-h urine for the determination of the urinary uric acid and protein. And then, mice were sacrificed 3 h after the last treatment. Blood was taken for the analysis of biochemistry indices. Kidneys were harvested for histologic examination and protein analysis.
Figure 1.
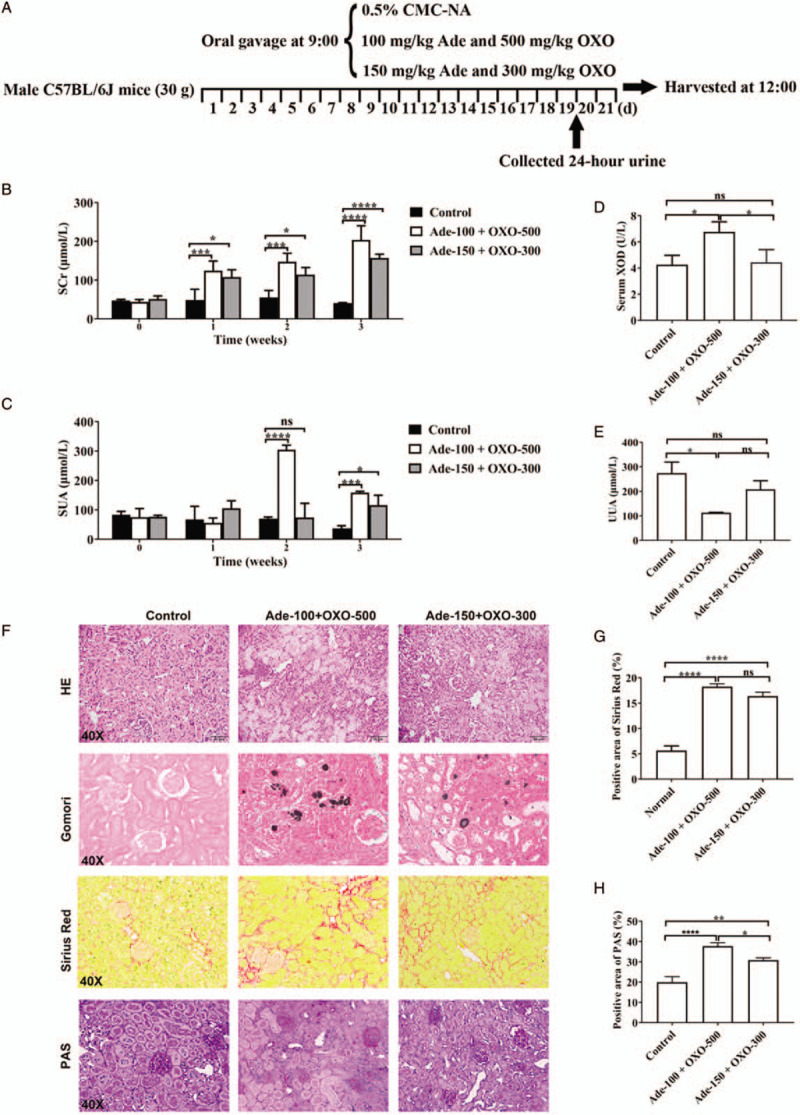
Effects of adenine (Ade) and OXO on renal function and uric acid distribution in mice. (A) Overview of the hyperuricemia model procedure. (B) Serum créatinine (SCr) level. (C) Serum uric acid (SUA) level. (D) Activity of serum XOD. (E) Urinary uric acid (UUA). (F) Representative histology of H&E, Gomori methenamine silver, Sirius red, and PAS staining (original magnification ×40). (G, H) Quantification of positive staining areas was measured by Image J software. Data are presented as mean ± standard deviation. n = 6 per group. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, ∗∗∗∗P < 0.0001. Similar results from three independent experiments are shown. CMC-Na: Carboxymethyl cellulose sodium; XOD: Xanthine oxidase; H&E: Hemotoxylin and eosin; OXO: Oxonate; PAS: Periodic acid-Schiff; ns: Not significant.
Mice treated with adenine and OXO exhibited severe kidney injury, as revealed by the elevated level of serum creatinine at the seventh day after the initiation of the study [Figure 1B]. And the elevated serum uric acid at the second and third weeks of the experiment suggested that the hyperuricemia mouse models were successfully established [Figure 1C].
In line with previous studies, adenine and OXO administration significantly decreased the body gain and food intake of mice but increased the water intake of mice. The 24-h urine volumes were also remarkably increased [Supplementary Figure 1, http://links.lww.com/CM9/A256]. Strikingly, the activity of serum xanthine oxidase, the key enzyme catalyzing the production of uric acid, was significantly up-regulated by the treatment with Ade-100 + OXO-500 resulting in an obvious decrease in urinary uric acid [Figure 1D and 1E]. In addition, histologic examination of kidney revealed that a significant damage by adenine and OXO treatment [Figure 1F]. Furthermore, not only the dilated kidney tubules and ectatic Bowman spaces, but also the dark needle-like urate crystals, focal tubulointerstitial fibrosis and glycometabolic disorder were more evident in the kidneys of mice treated with Ade-100 + OXO-500, further illustrating the more severe kidney injury [Figure 1G and 1H] in this group of mice. Immunohistochemistry of kidneys from mice administrated with adenine and OXO showed massive accumulation of URAT1 and GLUT9 in injured tubules, meaning the increase of uric acid re-absorption in the proximal renal tubules.[3] Western blotting assay also showed a remarkably increased renal expression of URAT1 and GLUT9 in mice treated with adenine and OXO treatment. Importantly, the increase of protein levels of URAT1 and GLUT9 in the kidney was further elevated in the Ade-100 + OXO-500 group [Supplementary Figure 2, http://links.lww.com/CM9/A256]. In addition, increased expression of inflammatory cytokine tumor necrosis factor (TNF)-α has been involved in the immune response during gout. Interleukin (IL)-1β, which is cleaved to the mature form by active Caspase 1, is also identified as the primary motivating factor of gouty inflammation. We next assessed the effects of adenine and OXO co-stimulation on systemic inflammation in mice by measuring the levels of serum TNF-α and IL-1β in mice. As expected, adenine and OXO treatment remarkably increased the serum concentration of TNF-α and IL-1β in mice [Supplementary Figure 3, http://links.lww.com/CM9/A256]. Consistently, mice administrated with Ade-100 + OXO-500 had exhibited severe inflammation. Taken together, our results suggest that mice treated with Ade-100 + OXO-500 is more favorable than Ade-150 + OXO-300 to generate disease phenotypes of mouse model of hyperuricemia and gouty nephropathy.
Accumulating evidences indicated that hyperuricemia was an independent risk factor for the new-onset and progression of chronic kidney disease. Methods such as adding yeast cream, gavaging adenine or potassium oxazinate, or intraperitoneally injecting inosine 5-monophosphate and guanosine monophosphate have been used to create mouse model of hyperuricemia with variable degree of reproductivity.[4] However, due to the inaccurate dose, high mortality rate and inconvenient operation, the hyperuricemia models in previous research were difficult to reproduced.[5] Initially, we started out the experiment by treating mice with 100 mg/kg adenine or 250 mg/kg OXO for 7 days. However, the serum concentration of uric acid in mice was increased provisionally and was less than 200 μmol/L. Hence, we attempted to create mouse model of hyperuricemia by injecting mouse with adenine and OXO. Notably, our results showed that administration of adenine and OXO (100 and 500 mg/kg, respectively) increases the intake of purine, inhibits the uricase activity, leading to the elevation of serum uric acid which eventually hyperuricemia and subsequent gouty nephropathy.
In summary, we have successfully established a mouse model of hyperuricemia and gouty nephropathy by administrating adenine and OXO. The validity of our mouse was confirmed by strict parameters of disease phenotypes such as serologic pathology, renal tissue histology examination, the expressions of uric acid transporters, and the inflammatory factors. Our animal model of hyperuricemia and gouty nephropathy can serve as an animal model to evaluate the efficacy of therapeutic agents.
Funding
This work was supported by grants from the Science and Technology Planning Project of Guangdong Province (No. 2017A020211007), China; the Key Project of Natural Science Foundation of Guangdong Province (No. 2016A030311014), China; the National Natural Science Foundation of China (No. 81870420); Special Project on the Integration of Industry, Education and Research of Guangdong Province (No. 2014B090902002); Science and Technological Program for Dongguan's Social Development (No. 2016108101043).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Guan J, Huang XQ, Dong JL, Lu HM, Lin YW, Liu M, Yi ZB, Wu LM, Huang YM, Lan T. A novel mouse model of hyperuricemia and gouty nephropathy. Chin Med J 2020;133:2012–2014. doi: 10.1097/CM9.0000000000000964
Jin Guan and Xiao-Qi Huang contributed equally to the work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.cmj.org).
References
- 1.Yong T, Chen S, Xie Y, Chen D, Su J, Shuai O, et al. Hypouricemic effects of ganoderma applanatum in hyperuricemia mice through OAT1 and GLUT9. Front Pharmacol 2017; 8:996.doi: 10.3389/fphar.2017.00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan J, Shi M, Li L, Liu J, Guo F, Feng Y, et al. Pterostilbene, a bioactive component of blueberries, alleviates renal fibrosis in a severe mouse model of hyperuricemic nephropathy. Biomed Pharmacother 2019; 109:1802–1808. doi: 10.1016/j.biopha.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 3.Zhou Y, Zhang X, Li C, Yuan X, Han L, Li Z, et al. Research on the pharmacodynamics and mechanism of Fraxini Cortex on hyperuricemia based on the regulation of URAT1 and GLUT9. Biomed Pharmacother 2018; 106:434–442. doi: 10.1016/j.biopha.2018.06.163. [DOI] [PubMed] [Google Scholar]
- 4.Han J, Wang X, Tang S, Lu C, Wan H, Zhou J, et al. Protective effects of tuna meat oligopeptides (TMOP) supplementation on hyperuricemia and associated renal inflammation mediated by gut microbiota. FASEB J 2020; 34:5061–5076. doi: 10.1096/fj.201902597RR. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Dalbeth N, Yin H, Li C, Merriman TR, Wei WH. Mouse models for human hyperuricaemia: a critical review. Nat Rev Rheumatol 2019; 15:413–426. doi: 10.1038/s41584-019-0222-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


