Abstract
Type 2 diabetes mellitus and metabolic disorders have become an epidemic globally. However, the pathogenesis remains largely unclear and the prevention and treatment are still limited. In addition to environmental factors during adulthood, early life is the critical developmental window with high tissue plasticity, which might be modified by external environmental cues. Substantial evidence has demonstrated the vital role of early-life nutrition in programming the metabolic disorders in later life. In this review, we aim to overview the concepts of fetal programming and investigate the effects of early-life nutrition on energy metabolism in later life and the potential epigenetic mechanism. The related studies published on PubMed database up to March 2020 were included. The results showed that both maternal overnutrition and undernutrition increased the riskes of metabolic disorders in offspring and epigenetic modifications, including DNA methylation, miRNAs, and histone modification, might be the vital mediators. The beneficial effects of early-life lifestyle modifications as well as dietary and nutritional interventions on these deleterious metabolic remolding were initially observed. Overall, characterizing the early-life malnutrition that reshapes metabolic disease trajectories may yield novel targets for early prevention and intervention and provide a new point of view to the energy metabolism.
Keywords: Early-life nutrition, Energy metabolism, Epigenetics, In later life
Introduction
Diabetes mellitus and other metabolic disorders have been characterized as pandemics. The World Health Organization reported that high blood glucose would be the third leading global risk factor for the mortality in the world, secondary to high blood pressure and tobacco use. In 2019, the Tnternational Diabetes Federation estimated that there were approximately 463 million people with diabetes among 20 to 79 years, which accounted for 9.3% of the adult population. If these trends continue, there would be 700.2 million diabetic patients by 2050, which will bring a huge economic burden and become a “catastrophe of the 21st century.”[1] China has become the country with the largest numbers of diabetic patients globally, and the prevalence of diabetes is still rising rapidly. Among them, type 2 diabetes mellitus (T2DM) accounts for the vast majority (approximately 90%) of global diabetes.[1] However, the etiology and pathogenesis of T2DM and other metabolic disorders are still largely unclear.
It is traditionally reported that genetic factors and adult lifestyles, such as obesity, high-calorie intake, physical inactivity, and smoke, have played an important role in the incidence and development of metabolic abnormalities. However, in recent years, a growing number of clinical studies[2–4] and experimental models[5–9] have shown that adverse early-life nutrition, including under-nutrition and over-nutrition, can reshape the disease trajectory and significantly increase the risk of metabolic abnormalities in adulthood. The Developmental Origins of Health and Disease hypothesis has been proposed and emphasized the link between the early life stages, including the perinatal fetus and infant, and the development of metabolic disorders in later life.[10,11] The fetus or newborn adjusts their homeostasis system by making predictive adaptations to the early life environment to help instant survival and to improve later survival in an expected postnatal environment of adversity. But inappropriate predictions of these changes to the early-life nutritional environment may lead to a mismatch between nutrition in early life and postnatal reality. As a result, these predictive responses lead to an increased risk of chronic disease in adulthood, which can be transmitted to future generations.[11]
Therefore, the incidence and prevention of metabolic disorders should be advanced to the early-life stage, and the pathogenesis of metabolic diseases should be explored from the perspective of different life-time windows, which would provide some new evidence, targets, and thoughts for the energy metabolism. This review summarized literature exploring the effects of early-life (perinatal fetuses or infants) malnutrition, including under-nutrition and over-nutrition, on energy metabolism later in life, and further investigated the role of epigenetics in mediating these effects, which could provide some evidence for the early and effective prevention and treatment of metabolic disorders from the perspective of early life [Figure 1].
Figure 1.
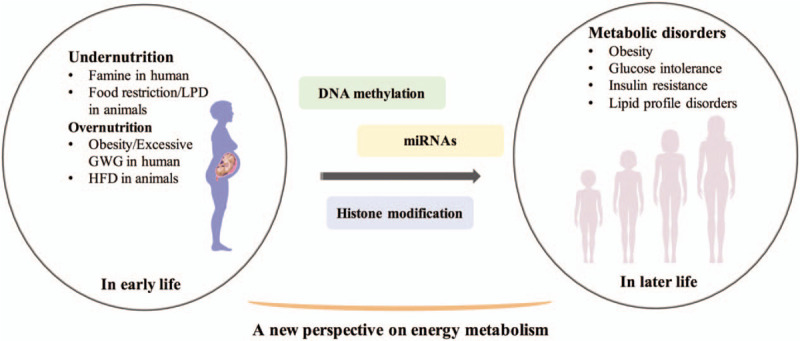
Overview of the relationship between early-life nutrition and energy metabolism in later life. GWG: Gestational weight gain; HFD: High-fat diet; LPD: Low-protein diet.
Early-life Under-nutrition and Metabolic Disorders in Later Life
Large numbers of epidemiological studies and experimental animal models have shown that the metabolic programming of energy balance can be attributed to the very early stages of development.[12–14] Thus, pregnancy and lactation have been recognized as the critical periods when nutritional modulation may lead to lasting effects on energy balance in offspring and shape health and disease trajectory in adulthood. Barker first reported that the death rates from ischemic heart diseases were higher in men with lower birth weight compared with those with normal birth weight and thus put forward the “the thrifty phenotype hypothesis,” which proposed that maternal under-nutrition during pregnancy and early post-partum programmed offspring to adapt to this thrifty environment and develop various dysfunctions in later life.[15,16] Consequently, the effects of early-life nutrition on metabolic diseases in later life gained increasing attention.
Clinical evidence
Famine is a natural model for investigating the effects of early-life poor nutritional environment on energy metabolism in later life in humans. Dutch winter famine (1944–1945) was one of the classic famine in the world with a 5-month period of severe undernutrition in the urban western part of the Netherlands at the end of World War II. The Dutch Famine cohort was also one of the most cited human cohorts to detect the role of early-life undernutrition on later metabolic health. Lumey et al[17] established a birth cohort of 3307 singletons born between 1945 and 1946. Their mothers experienced a food restriction of 900 Cal per day during pregnancy. This Dutch Hunger Winter Families Study showed that maternal undernutrition (famine exposure) during pregnancy was associated with a higher prevalence of T2DM and disorders of lipid profiles in adult offspring aged 59. Furthermore, another Dutch Famine Birth Cohort Study done by Ravelli et al[18] also demonstrated that prenatal famine exposure led to significant glucose intolerance and insulin resistance in offspring at ages of both 50 and 58.
In addition to Dutch, several other countries also experienced famine in the 20th century, including Austria, Leningrad, British Channel Island, and China. Data from the Great Chinese famine cohort detected the relationship between famine exposure in early life and metabolic diseases in later life. Several studies showed that maternal undernutrition during pregnancy and lactation significantly increased the risks of metabolic syndrome, non-alcohol fatty liver disease, and visceral fat dysfunction in adult offspring.[19–25] We also worked on the effects of adverse intrauterine nutritional environment on metabolic health in later life. A cohort of 2019 subjects born at the Peking Union Medical College Hospital between 1921 and 1954 was established. And the results showed that those with low birth weight had increased risks of developing diabetes mellitus, impaired glucose tolerance, and metabolic syndrome in their elderly life compared with those with normal birth weight.[26,27] Therefore, the relationship between maternal undernutrition exposure and energy metabolism in later life has been well demonstrated in humans.
Animal models
Human evidence is limited because of long generation times for prospective studies, large individual differences, and the quality of data for retrospective studies. Therefore, a growing number of animal model studies were designed to explore and validate the effects of early-life poor nutrition on metabolism in later life. Isganaitis et al[28] established a mouse model of low birth weight through restricting food by 50% during gestational days 12.5 to 18.5, which reduced offspring birth weight by 25%. Consistent with human studies, they also found that maternal food restriction resulted in significantly increased glucose intolerance, lipogenic gene expression, and adipocyte size in adipose tissue of adult offspring, accompanied by catch-up growth. Interestingly, when they further restricted food intake in offspring to inhibit catch-up growth, the adult offspring had better glucose and lipid metabolism than those experiencing postnatal catch-up growth.[29] This experiment verified the “metabolic memory” hypothesis that early-life malnutrition programmed their energy balance to adapt to the poor nutritional environment. If the postnatal nutrition mismatches the prenatal environment, the metabolic disorders would happen.
Furthermore, the maternal low-protein diet is another well-established early life undernutrition model. Our team established the early-life poor nutrition mouse model through feeding pregnant females a low-protein diet (9.6% protein, 80.2% carbohydrate, and 10.2% fat as kcal%, D02041002, Research Diets) during pregnancy and lactation. Compared with offspring of dams fed the isocaloric normal control diet (23.5% protein, 66.3% carbohydrate, and 10.2% fat as kcal%, D02041001, Research Diets), maternal low-protein diet led to lower birth weight, impaired glucose tolerance, and decreased fasting serum insulin levels in offspring at weaning.[8]
Therefore, maternal malnutrition during pregnancy and lactation programmed increased risks of developing glucose intolerance and lipid metabolic disorders in offspring. Recently, with the development of economy and society, as well as changes in diet and lifestyle, early-life overnutrition has become another severe issue and should also gain increasing attention.
Over-nutrition in Early Life and Energy Metabolism in Later Life
Clinical evidence
Maternal obesity during pregnancy and lactation is one of the most typical representatives of early-life overnutrition in humans. A significant volume of evidence have shown that the prevalence of obesity in women of childbearing ages is still growing rapidly,[30,31] which has been shown to increase the risks of metabolic disorders in offspring.[32] In the Helsinki Birth Cohort Study, Eriksson et al[33] reported that maternal body mass index (BMI) during pregnancy was positively associated with offspring health outcomes in later life, especially including cardiovascular disease and T2DM. Similarly, in another population-based prospective cohort study, Gaillard et al[34] showed that compared with children from normal-weight mothers, those from obese mothers during pregnancy had higher risks of childhood obesity and adverse cardiometabolic outcomes, such as higher systolic blood pressure, insulin levels, and lower high-density lipoprotein cholesterol levels. Furthermore, a recent multi-center Hyperglycemia and Adverse Pregnancy Outcome study by Tam et al[35] included a total of 905 mother-child pairs and analyzed the gestational weight gain, which is another parameter to evaluate nutrition during pregnancy, and metabolic health in offspring at 7 years of ages. They showed that offspring of mothers with higher body weight gain during pregnancy than the Institute of Medicine guideline recommendations had increased body weight, adiposity, and risks of hypertension and insulin resistance compared with those of women with normal gestational weight gain, which was independent of maternal pre-pregnancy BMI, gestational hyperglycemia, and other factors. Consistent with this study, other cohorts also demonstrated that maternal over-nutrition characterized by increased gestational weight gain was an independent predictor for increased BMI, adiposity, and cardiovascular risks in offspring.[36–38]
Animal experiments
Furthermore, emerging animal studies were designed to verify the clinical finding and explore the specific mechanisms. Maternal high-fat diet (HFD) intake during pregnancy is a common mouse model for overnutrition, which mimics the continuous high-fat food intake in humans. This overnutrition leads to an adverse intrauterine environment, which predisposes offspring to develop metabolic disorders. Some scholars have established this animal model and showed that the offspring of HFD fed dams through pregnancy and lactation gained more body weight and adiposity than the control diet-fed littermates.[39,40] Bringhenti et al[41] found that offspring of HFD fed dams developed metabolic disorders, including hypercholesterolemia, hypertriglyceridemia, and hyperinsulinemia, and had an elevated level of serum leptin but a decreased level of adiponectin. They also explored the mechanisms mediating the metabolic abnormalities in adult offspring by analyzing the structure and function of the islet. The results showed significant increases in the masses of the islet, numbers of beta cells, and alpha cells, along with the migration of the alpha cells from edge to the core of the islet, whereas down-regulation of insulin receptor substrate 1, phosphatidylinositide 3-kinase, pancreatic and duodenal homeobox 1, and glucose transporter 2 protein levels in offspring of HFD fed dams compared with the chow diet-fed mice. This study indicated that maternal HFD was responsible for the impairments in the insulin signaling pathway and altered islet structure in adult offspring. At the same time, another animal experiment showed the increased predisposition to steatohepatitis and the decreased α-diversity of gut microbiota in the offspring of HFD fed dams in comparison to those in the normal control group.[42] In addition to the effects of maternal overnutrition on offspring peripheral metabolic health, increasing studies focused on the central metabolic changes in offspring. A recent study showed that maternal HFD resulted in an increase in the brain insulin receptors (IRβs) in offspring at birth followed by a long-term decrease in brain glucose uptake and expression of glucose transporter 4 and IRβ in minipigs, which may increase the offspring predisposition to metabolic-neurodegenerative diseases.[43] Our team also established the mouse model of maternal HFD during pregnancy and lactation and investigated the intergenerational effects of maternal overnutrition on offspring. Similarly, our results also verified that perinatal overnutrition (HFD) changed health and disease trajectory in offspring and significantly increased the risks of glucose and lipid metabolic disorders.[44]
Role of Epigenetics in Mediating the Metabolic Programming
What is the critical mechanism mediating the “metabolic memory”? Although emerging studies began to pay attention to the potential role of central hypothalamic regulation,[45] gut microbiota,[46,47] circadian rhythm,[48,49] and brown adipose tissue[50,51] in linking the early-life nutrition and metabolic health in later life, we mainly overviewed the evidence regarding the epigenetic regulation in this review. Sib-pairs study, one exposure to diabetes and the other to a non-diabetes in utero, indicated that metabolic programming was independent of genetic inheritance to some content.[52] Animal model experiments also demonstrated that epigenetics might play a leading role in mediating the effects of the early-life nutritional environment on offspring metabolic health rather than genetic material.[53,54] Epigenetics was first proposed by Dr. Waddington and was defined as heritable changes in gene expression without alterations in the DNA sequence.[55,56] The major epigenetic mechanisms include DNA methylation, histone modifications, and non-coding RNA (ncRNAs). As an important molecular mechanism that regulates transcription, genetic stability, nuclear structure, and genomic imprinting, the altered epigenetic state was reported by a large number of studies to be associated with various types of diseases, including cardiovascular diseases,[57] cancers,[58] neurodegenerative diseases,[59] mental disorders,[60] immune system diseases,[61] diabetes, and obesity.[62]
DNA methylation
DNA methylation is one of the most widely studied epigenetic mechanisms at present, which usually occurs in regions rich in CpG clusters (CpG islands). Most of the gene promoters are located in the CpG islands, and CpG methylation in these promoter regions is usually associated with transcriptional silencing. However, DNA methylation is not limited to the CpG islands and is also found in the vicinity of the CpG islands. DNA methylation is a covalent modification that the methyl group from donor S-adenosylmethionine is transferred to C5 of cytosine under the catalysis of DNA methyltransferase (DNMT). In mammals, there are five kinds of DNA methylation transferases, including DNMT1, DNMT2, DNMT3A, DNMT3B, and DNMT3L. But only DNMT1, DNMT3A, and DNMT3B have the activity of methyltransferase, of which DNMT1 is responsible for the maintenance of extrauterine DNA methylation, whereas DNMT3A and DNMT3B are responsible for the establishment of intrauterine DNA methylation (de novo methylation). DNA methylation is a reversible modification. The 10-11 translocation enzyme family acts as a demethylase and has the opposite effect to DNMTs. DNA methylation inhibits gene expression through two mechanisms. The first is that methylated cytosine can directly prevent the binding of transcription factors to the target DNA sequences; The second is that methylated CpG-binding domain proteins recognize and bind to the methylated cytosine to further recruit chromatin-inactivating complexes, resulting in silencing of transcription.[63] Persistent changes in the modifications of DNA methylation of metabolic associated genes might partly decipher the metabolic programming.
We have previously reviewed the published literature detecting the role of DNA methylation in mediating the interaction between early-life nutrition and glucose metabolism in later life before 2014.[64] After that, substantial associated studies were emerging. Shen et al[65] recently investigated the relationship between early-life exposure to the Chinese Great Famine (1959–1961) and DNA methylation in insulin-like growth factor 2 (IGF2) and its effects on blood lipid levels in late adulthood in the Genomic Research of the Chinese Famine cohort. They found that elevated methylation level in IGF2 was associated with exposure to famine in early life, and this modification was also positively correlated with total cholesterol levels in late adulthood. Consistently, Tobi et al[66] also showed that DNA methylation changes in metabolic associated genes, including serine/threonine-protein kinase pim-3, thioredoxin-interacting protein, ATP-binding cassette sub-family G member 1, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3, and mRNA N(3)-methylcytidine methyltransferase 8, mediated the influence of prenatal famine exposure on long-term energy metabolic health. In addition to food restriction, early-life overnutrition also changed the DNA methylation in both placenta, umbilical cord, infants, and adult. The placenta is widely accepted to have multiple functions, including nutrients transportation for the fetus and exchanges of a wide range of cytokines, endocrine signals, and growth factors between the mothers and the fetuses. A recent clinical study showed that maternal obesity was associated with down-regulation in DNA methylation of the adiponectin and leptin in the third-trimester placenta of humans.[67] The changes in DNA methylation of critical metabolic genes were also reported to be associated with maternal obesity in the human umbilical cord, infant mesenchymal stem cells, offspring spermatozoa, and other tissues.[68–73] Furthermore, experimental animal models also provide strong supporting evidence. Modulation of maternal diet, including a HFD,[74,75] low-protein diet,[76,77] and calorie restriction[78–80] during pregnancy and lactation resulted in a persistent shift in DNA methylation levels of metabolism specific genes in offspring, which were associated with their metabolic disorders.
Overall, DNA methylation is an important mechanism mediating the effects of early-life nutrition on energy metabolism in later life. However, the specific regulating mechanisms are still largely unclear and require further exploration.
miRNAs
In addition to the programming effects of DNA methylation, there is also evidence for the changes of miRNAs after early-life malnutritional exposure.[81,82] Over the past decade, the importance of ncRNAs in most epigenetic regulatory events, such as gene transcription regulation, transposon activity and silencing, and X chromosome inactivation, has been gradually revealed. ncRNAs include a variety of RNA transcripts, such as miRNAs, long non-coding RNAs, small nucleolar RNAs, and circular RNAs, which can regulate the gene expression at a post-transcriptional level.[83–86] Currently, the most widely studied ncRNAs are miRNAs, which are small molecular RNAs of 21 to 25 nucleotides in length.[87] They are usually transcribed from the introns and exons of non-coding genes and the introns of protein-coding genes, partly from transposons and processed pseudogenes. miRNAs recruit RNA-induced silencing complex predominantly through complementary pairing with the 3′-untranslated-region of the target genes to further induce degradation or inhibition of translation.
miRNAs have been shown to play an important role in insulin sensitivity[88,89] and glucose homeostasis.[90,91] T2DM, obesity, and metabolic syndrome were all associated with dysregulation of miRNAs (eg, miR-103, miR-107, and the let-7 family).[92–94] Lie et al[95] showed that periconceptional undernutrition induced changes in the expression of 22 miRNAs, which might mediate the altered expression of the key insulin-signaling molecules in skeletal muscle and insulin resistance in adult life. This was also confirmed in the sheep model.[96,97] Our team detected the hepatic miRNAs levels in the intergenerational HFD and low-protein diet mouse models and found that both of maternal high-calorie intake and undernutrition significantly changed several important miRNAs in offspring liver, which were involved in mitogen-activated protein kinase signaling pathway, transforming growth factor-beta signaling pathway, Toll-like receptor signaling pathway, Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway, nucleotide-binding and oligomerization domain (NOD)-like receptor signaling pathway, cytokine-cytokine receptor interaction, chemokine signaling pathway, and adipocytokine signaling pathway.[8,82] However, how early-life nutrition changes levels of miRNAs and how altered miRNAs regulate metabolic health remain unknown.
Histone modifications
Histone modification is another important epigenetic mechanism. In eukaryotic cells, the nucleosome is the basic structural and functional unit of chromatin. It is composed of about 147 bp of DNA wrapped around a histone octamer. Histone modification occurs at the tail of histones, including methylation, acetylation, phosphorylation, ubiquitination, and hepatization. Histone modification usually regulates the structure and function of chromatin by changing the interaction between histones and DNA, further regulating gene expression. Among them, the methylation and acetylation of histones mediate the formation of transcriptionally active euchromatin and transcriptionally silent heterochromatin. In mammalian cells, heterochromatin has higher levels of DNA methylation, histone deacetylation, and trimethylation of H3K9, H3K27, and H4K20. Overall, different types of modification of histones and modification of different histone residues may produce different regulations of gene expression.[98] Studies linking histone modification changes with metabolic programming remain limited. One animal study explored the transgenerational effects of prenatal restricted diet on histone modifications in rats and found that maternal food restriction affected the level of global histone H3 acetylation in fetal liver.[99] Yang et al[100] reported that decreased Wnt1 gene expression induced by perinatal HFD in the neonatal liver was associated with changes in acetylation of H4 at its promoter as well as acetylation of H4 and methylation of H3K9 at the coding region.
Therefore, epigenetic modification might be an essential mediator for the influence of early-life nutrition on energy metabolism in later life. Nevertheless, there are still a lot of questions that need to be answered. Which epigenetic modification is the most important mediator? How do they mediate the effects? How long could they persistent? Which is the most important tissue for the epigenetic regulation of metabolic homeostasis? Can these epigenetic alterations be reversed?
Overview of Early-life Interventions and Metabolism in Later Life
Early life is the critical window for tissue plasticity, thus it can be modified by external environmental cues. Malnutrition in early life can program increased susceptibility to energy metabolic disorders in later life. Can this deleterious metabolic memory be blocked fundamentally? During the last decade, early-life interventions have gained increasing attention. For safety and effectiveness of fetal or infant stages, published studies mainly detected the effects of lifestyle management, supplementation of specific types of bioactive ingredients and nutritional interventions during the critical developmental period, especially pregnancy and lactation. Numerous prospective clinical trials have done lifestyle intervention in obese women during pregnancy and detected offspring metabolic health. Parts of these studies had positive outcomes, such as avoiding excessive gestational weight gain and decreasing adverse perinatal outcomes.[101,102] However, there was still a large proportion of these studies which showed limited success in improving both maternal and offspring parameters.[103–105] Although poor compliance during pregnancy and lactation in human and statistical power might be important reasons for the lack of success, the initiation of lifestyle intervention, duration, and specific means of intervention are also significant influencing factors.[106]
Besides, emerging evidence demonstrated the beneficial effects of dietary bioactive compounds on energy metabolism. Resveratrol (trans-3,5,4′-trihydroxystilben) is a natural polyphenol mainly from plants, fruits, peanuts, and red wine. It has been widely reported that resveratrol has multiple health benefits against cancer,[107] aging,[108] obesity,[109,110] diabetes mellitus,[111] and cardiovascular disease.[112] Several experimental animal models also validated the benefits of maternal resveratrol intake on offspring health. Maternal resveratrol supplementation during pregnancy and lactation could fight against the adverse effects of the HFD or low-protein diet on offspring, such as glucose intolerance, obesity, cholesterol metabolic disorders, non-alcoholic fatty liver disease, or even hypothalamic leptin signaling dysregulation.[113–118] As for the metabolic reprogramming of maternal bioactive ingredients intake, our team also established a mouse model to detect the intergenerational effects of maternal genistein intake by feeding dams a chow diet, HFD, or a HFD supplemented with genistein 3 weeks pre-pregnancy and during gestation and lactation. Genistein is one of the most important ingredients of soy isoflavone and also plays a vital role in metabolic metabolism. Our results showed that perinatal genistein intake significantly countered the adverse effects of maternal HFD on glucose and lipid metabolism in offspring, which can persist to later adult from weaning and is body weight independent.[119,120] Thus, both our research and previous studies confirmed that dietary bioactive compounds supplementation in early life can block the adverse metabolism trajectory in later life induced by early-life poor nutritional exposure. However, almost all of the present evidence is from animal models and is preliminary. In the future, more animal and clinical evidence should be provided to transfer this basic finding to human physiology. And the specific mechanism still needs to be explored.
Furthermore, nutritional interventions were another potential way to prevent the intergenerational transmission of metabolic disorders, especially those associated with epigenetic regulation. Cordero et al[121] supplemented methyl donors (choline, betaine, folic acid, vitamin B12) to high-fat-sucrose fed dams during lactation and showed that this can protect offspring against hepatic fat accumulation when challenged by an obesogenic diet, which also indicated that DNA methylation might be an important mediator. Andraos and colleagues summarized the randomized controlled trials assessing the impact of nutritional interventions in pregnant women on DNA methylation patterns of the offspring. The results showed that maternal micronutrient supplementation did not substantially change DNA methylation in neonatal tissues. However, the effects of nutritional interventions on DNA methylation depended on maternal baseline health and nutritional status, including BMI and smoking status. Folic acid has been recognized as an important nutritional supplementation during pregnancy and was also a regulator of DNA methylation. Clinical studies in human and animal experiments both detected the effects of folic acid supplementation during pregnancy on offspring energy metabolism.[122–125] Most of the studies indicated the metabolic protects of folic acid supplementation during pregnancy on offspring. However, Lewis et al gave folic acid to the mothers at 18 and 32 weeks of pregnancy and evaluated body composition in offspring at age 9 years among 5783 children from a population-based birth cohort study in the UK. But they did not find the relationship between intrauterine exposure to folate and childhood body composition.[126] These inconsistent results might be attributed to the differences in population and the dosage and duration of folic intake. Thus, larger cohort studies are urgently needed to verify the effect.
Conclusion and Future Prospect
This review summarized recently representative articles about the important role of early-life nutrition in metabolic health. Perinatal nutrition intake plays a vital role in shaping the health and disease trajectory in later life. Maternal malnutrition programs significantly increased risks of developing energy metabolic disorders in offspring. These studies indicated that metabolic disorders should not only be attributed to the genetic factors and the inappropriate lifestyle in adulthood, but also to the nutritional environment in early life. Epigenetics are reported to be the potential mechanisms mediating the “metabolic memory.” Early-life interventions, such as lifestyle modification (food management and physical activity), dietary bioactive ingredients supplementation, and nutritional interventions, are considered as promising strategies to block the deleterious effects of maternal malnutrition on offspring metabolic health and further correct the disease trajectory.
Overall, this review proposes a new perspective on energy metabolism that early-life nutrition is another important influencing factor determining the development of metabolic diseases other than environmental exposures. Therefore, it is suggested that prevention and management of metabolic disorders are extended to early life, even during the fetal and infant stages, which is the critical window for physiological programming. We emphasize and call for more widespread attention to early-life environments. The treatment, management, and especially prevention actions in early life might dramatically restrain the rapid increase of patients with T2DM and other metabolic disorders and alleviate the huge economic burden globally.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81870579, 81870545, 81170736, 81570715).
Conflicts of interest
None.
Footnotes
How to cite this article: Zhou LY, Deng MQ, Zhang Q, Xiao XH. Early-life nutrition and metabolic disorders in later life: a new perspective on energy metabolism. Chin Med J 2020;133:1961–1970. doi: 10.1097/CM9.0000000000000976
References
- 1. IDF Diabetes Atlas, 9th edn. Available from: http://www.idf.org:diabetesatlas. Accessed November 14, 2019. [Google Scholar]
- 2.Finer S, Iqbal MS, Lowe R, Ogunkolade BW, Pervin S, Mathews C, et al. Is famine exposure during developmental life in rural Bangladesh associated with a metabolic and epigenetic signature in young adulthood? A historical cohort study. BMJ Open 2016; 6:e011768.doi: 10.1136/bmjopen-2016-011768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wankhade UD, Thakali KM, Shankar K. Persistent influence of maternal obesity on offspring health: mechanisms from animal models and clinical studies. Mol Cell Endocrinol 2016; 435:7–19. doi: 10.1016/j.mce.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Zambrano E, Ibanez C, Martinez-Samayoa PM, Lomas-Soria C, Durand-Carbajal M, Rodriguez-Gonzalez GL. Maternal obesity: lifelong metabolic outcomes for offspring from poor developmental trajectories during the perinatal period. Arch Med Res 2016; 47:1–12. doi: 10.1016/j.arcmed.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Kruse M, Keyhani-Nejad F, Isken F, Nitz B, Kretschmer A, Reischl E, et al. High-fat diet during mouse pregnancy and lactation targets GIP-regulated metabolic pathways in adult male offspring. Diabetes 2016; 65:574–584. doi: 10.2337/db15-0478. [DOI] [PubMed] [Google Scholar]
- 6.Tain YL, Lee WC, Wu KLH, Leu S, Chan JYH. Maternal high fructose intake increases the vulnerability to post-weaning high-fat diet-induced programmed hypertension in male offspring. Nutrients 2018; 10:56.doi: 10.3390/nu10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Piccolo BD, et al. Maternal high-fat diet programs offspring liver steatosis in a sexually dimorphic manner in association with changes in gut microbial ecology in mice. Sci Rep 2018; 8:16502.doi: 10.1038/s41598-018-34453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng J, Xiao X, Zhang Q, Wang T, Yu M, Xu J. Maternal low-protein diet modulates glucose metabolism and hepatic microRNAs expression in the early life of offspring dagger. Nutrients 2017; 9:205.doi: 10.3390/nu9030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Sousa SM, Braz GRF, Freitas CM, de Santana DF, Sellitti DF, Fernandes MP, et al. Oxidative injuries induced by maternal low-protein diet in female brainstem. Nutr Neurosci 2018; 21:580–588. doi: 10.1080/1028415x.2017.1325974. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson JG. Developmental origins of health and disease - from a small body size at birth to epigenetics. Ann Med 2016; 48:456–467. doi: 10.1080/07853890.2016.1193786. [DOI] [PubMed] [Google Scholar]
- 11.Norris SA, Daar A, Balasubramanian D, Byass P, Kimani-Murage E, Macnab A, et al. Understanding and acting on the developmental origins of health and disease in Africa would improve health across generations. Glob Health Action 2017; 10:1334985.doi: 10.1080/16549716.2017.1334985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dearden L, Ozanne SE. Early life origins of metabolic disease: developmental programming of hypothalamic pathways controlling energy homeostasis. Front Neuroendocrinol 2015; 39:3–16. doi: 10.1016/j.yfrne.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Menchetti L, Brecchia G, Canali C, Cardinali R, Polisca A, Zerani M, et al. Food restriction during pregnancy in rabbits: effects on hormones and metabolites involved in energy homeostasis and metabolic programming. Res Vet Sci 2015; 98:7–12. doi: 10.1016/j.rvsc.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Yang H, Yan Q, Ren A, Kong Z, Tang S, et al. Evidence for liver energy metabolism programming in offspring subjected to intrauterine undernutrition during midgestation. Nutr Metab (Lond) 2019; 16:20.doi: 10.1186/s12986-019-0346-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35:595–601. doi: 10.1007/bf00400248. [DOI] [PubMed] [Google Scholar]
- 16.Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull 2001; 60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 17.Lumey LH, Stein AD, Kahn HS, Romijn JA. Lipid profiles in middle-aged men and women after famine exposure during gestation: the Dutch Hunger Winter Families Study. Am J Clin Nutr 2009; 89:1737–1743. doi: 10.3945/ajcn.2008.27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998; 351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, He Y, Qi L, Jaddoe VW, Feskens EJ, Yang X, et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes 2010; 59:2400–2406. doi: 10.2337/db10-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang N, Wang X, Li Q, Han B, Chen Y, Zhu C, et al. The famine exposure in early life and metabolic syndrome in adulthood. Clin Nutr 2017; 36:253–259. doi: 10.1016/j.clnu.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Li C, Yang Z, Ma J, Zou Z. Fetal and infant exposure to severe Chinese famine increases the risk of adult dyslipidemia: results from the China health and retirement longitudinal study. BMC Public Health 2017; 17:488.doi: 10.1186/s12889-017-4421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X, Ren W, Gong L, Long J, Luo R, Wang Y. The great Chinese famine exposure in early life and the risk of nonalcoholic fatty liver disease in adult women. Ann Hepatol 2017; 16:901–908. doi: 10.5604/01.3001.0010.5281. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Zhao L, Ning Z, Li Q, Han B, Cheng J, et al. Famine exposure in early life is associated with visceral adipose dysfunction in adult females. Eur J Nutr 2019; 58:1625–1633. doi: 10.1007/s00394-018-1707-0. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Song J, Li Y, Dong B, Zou Z, Ma J. Early-life exposure to the Chinese famine is associated with higher methylation level in the INSR gene in later adulthood. Sci Rep 2019; 9:3354.doi: 10.1038/s41598-019-38596-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Z, Ji L, Ma RCW, Zimmet P. Early life exposure to 1959-1961 Chinese famine exacerbates association between diabetes and cardiovascular disease. J Diabetes 2020; 12:134–141. doi: 10.1111/1753-0407.12975. [DOI] [PubMed] [Google Scholar]
- 26.Xiao X, Zhang ZX, Cohen HJ, Wang H, Li W, Wang T, et al. Evidence of a relationship between infant birth weight and later diabetes and impaired glucose regulation in a Chinese population. Diabetes Care 2008; 31:483–487. doi: 10.2337/dc07-1130. [DOI] [PubMed] [Google Scholar]
- 27.Xiao X, Zhang ZX, Li WH, Feng K, Sun Q, Cohen HJ, et al. Low birth weight is associated with components of the metabolic syndrome. Metabolism 2010; 59:1282–1286. doi: 10.1016/j.metabol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isganaitis E, Jimenez-Chillaron J, Woo M, Chow A, DeCoste J, Vokes M, et al. Accelerated postnatal growth increases lipogenic gene expression and adipocyte size in low-birth weight mice. Diabetes 2009; 58:1192–1200. doi: 10.2337/db08-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jimenez-Chillaron JC, Hernandez-Valencia M, Lightner A, Faucette RR, Reamer C, Przybyla R, et al. Reductions in caloric intake and early postnatal growth prevent glucose intolerance and obesity associated with low birthweight. Diabetologia 2006; 49:1974–1984. doi: 10.1007/s00125-006-0311-7. [DOI] [PubMed] [Google Scholar]
- 30.Araya BM, Padilla O, Garmendia ML, Atalah E, Uauy R. Prevalence of obesity among Chilean women in childbearing ages. Rev Med Chil 2014; 142:1440–1448. doi: 10.4067/s0034-98872014001100011. [DOI] [PubMed] [Google Scholar]
- 31.McCloud MB. Health behavior change in pregnant women with obesity. Nurs Womens Health 2018; 22:471–480. doi: 10.1016/j.nwh.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Leonard SA, Rasmussen KM, King JC, Abrams B. Trajectories of maternal weight from before pregnancy through postpartum and associations with childhood obesity. Am J Clin Nutr 2017; 106:1295–1301. doi: 10.3945/ajcn.117.158683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long-term consequences of maternal overweight in pregnancy on offspring later health: findings from the Helsinki Birth Cohort Study. Ann Med 2014; 46:434–438. doi: 10.3109/07853890.2014.919728. [DOI] [PubMed] [Google Scholar]
- 34.Gaillard R, Steegers EA, Duijts L, Felix JF, Hofman A, Franco OH, et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension 2014; 63:683–691. doi: 10.1161/hypertensionaha.113.02671. [DOI] [PubMed] [Google Scholar]
- 35.Tam CHT, Ma RCW, Yuen LY, Ozaki R, Li AM, Hou Y, et al. The impact of maternal gestational weight gain on cardiometabolic risk factors in children. Diabetologia 2018; 61:2539–2548. doi: 10.1007/s00125-018-4724-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dello Russo M, Ahrens W, De Vriendt T, Marild S, Molnar D, Moreno LA, et al. Gestational weight gain and adiposity, fat distribution, metabolic profile, and blood pressure in offspring: the IDEFICS project. Int J Obes (Lond) 2013; 37:914–919. doi: 10.1038/ijo.2013.35. [DOI] [PubMed] [Google Scholar]
- 37.Hrolfsdottir L, Rytter D, Olsen SF, Bech BH, Maslova E, Henriksen TB, et al. Gestational weight gain in normal weight women and offspring cardio-metabolic risk factors at 20 years of age. Int J Obes (Lond) 2015; 39:671–676. doi: 10.1038/ijo.2014.179. [DOI] [PubMed] [Google Scholar]
- 38.Karachaliou M, Georgiou V, Roumeliotaki T, Chalkiadaki G, Daraki V, Koinaki S, et al. Association of trimester-specific gestational weight gain with fetal growth, offspring obesity, and cardiometabolic traits in early childhood. Am J Obstet Gynecol 2015; 212:502.e1–502.e14. doi: 10.1016/j.ajog.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hallam MC, Reimer RA. A maternal high-protein diet predisposes female offspring to increased fat mass in adulthood whereas a prebiotic fibre diet decreases fat mass in rats. Br J Nutr 2013; 110:1732–1741. doi: 10.1017/s0007114513000998. [DOI] [PubMed] [Google Scholar]
- 40.Desai M, Jellyman JK, Han G, Beall M, Lane RH, Ross MG. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am J Obstet Gynecol 2014; 211:237.e1–237.e13. doi: 10.1016/j.ajog.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bringhenti I, Ornellas F, Mandarim-de-Lacerda CA, Aguila MB. The insulin-signaling pathway of the pancreatic islet is impaired in adult mice offspring of mothers fed a high-fat diet. Nutrition 2016; 32:1138–1143. doi: 10.1016/j.nut.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Thakali KM, et al. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS One 2017; 12:e0175675.doi: 10.1371/journal.pone.0175675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanguinetti E, Liistro T, Mainardi M, Pardini S, Salvadori PA, Vannucci A, et al. Maternal high-fat feeding leads to alterations of brain glucose metabolism in the offspring: positron emission tomography study in a porcine model. Diabetologia 2016; 59:813–821. doi: 10.1007/s00125-015-3848-5. [DOI] [PubMed] [Google Scholar]
- 44.Zheng J, Xiao X, Zhang Q, Yu M, Xu J, Wang Z. Maternal high-fat diet modulates hepatic glucose, lipid homeostasis and gene expression in the PPAR pathway in the early life of offspring. Int J Mol Sci 2014; 15:14967–14983. doi: 10.3390/ijms150914967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomes RM, Bueno FG, Schamber CR, de Mello JCP, de Oliveira JC, Francisco FA, et al. Maternal diet-induced obesity during suckling period programs offspring obese phenotype and hypothalamic leptin/insulin resistance. J Nutr Biochem 2018; 61:24–32. doi: 10.1016/j.jnutbio.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Guo Y, Wang Z, Chen L, Tang L, Wen S, Liu Y, et al. Diet induced maternal obesity affects offspring gut microbiota and persists into young adulthood. Food Funct 2018; 9:4317–4327. doi: 10.1039/c8fo00444g. [DOI] [PubMed] [Google Scholar]
- 47.Zhou L, Xiao X. The role of gut microbiota in the effects of maternal obesity during pregnancy on offspring metabolism. Biosci Rep 2018; 38:BSR20171234.doi: 10.1042/bsr20171234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D, Chen S, Liu M, Liu C. Maternal obesity disrupts circadian rhythms of clock and metabolic genes in the offspring heart and liver. Chronobiol Int 2015; 32:615–626. doi: 10.3109/07420528.2015.1025958. [DOI] [PubMed] [Google Scholar]
- 49.Zhou L, Kang L, Xiao X, Jia L, Zhang Q, Deng M. Gut microbiota-circadian clock axis” in deciphering the mechanism linking early-life nutritional environment and abnormal glucose metabolism. Int J Endocrinol 2019; 2019:5893028.doi: 10.1155/2019/5893028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Almeida MM, Dias-Rocha CP, Souza AS, Muros MF, Mendonca LS, Pazos-Moura CC, et al. Perinatal maternal high-fat diet induces early obesity and sex-specific alterations of the endocannabinoid system in white and brown adipose tissue of weanling rat offspring. Br J Nutr 2017; 118:788–803. doi: 10.1017/s0007114517002884. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Chen Y, Mao X, Du M. Maternal obesity impairs fetal mitochondriogenesis and brown adipose tissue development partially via upregulation of miR-204-5p. Biochim Biophys Acta Mol Basis Dis 2019; 1865:2706–2715. doi: 10.1016/j.bbadis.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000; 49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 53.Langie SA, Achterfeldt S, Gorniak JP, Halley-Hogg KJ, Oxley D, van Schooten FJ, et al. Maternal folate depletion and high-fat feeding from weaning affects DNA methylation and DNA repair in brain of adult offspring. FASEB J 2013; 27:3323–3334. doi: 10.1096/fj.12-224121. [DOI] [PubMed] [Google Scholar]
- 54.Marco A, Kisliouk T, Tabachnik T, Meiri N, Weller A. Overweight and CpG methylation of the Pomc promoter in offspring of high-fat-diet-fed dams are not “reprogrammed” by regular chow diet in rats. FASEB J 2014; 28:4148–4157. doi: 10.1096/fj.14-255620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tronick E, Hunter RG. Waddington, dynamic systems, and epigenetics. Front Behav Neurosci 2016; 10:107.doi: 10.3389/fnbeh.2016.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nicoglou A, Merlin F. Epigenetics: a way to bridge the gap between biological fields. Stud Hist Philos Biol Biomed Sci 2017; 66:73–82. doi: 10.1016/j.shpsc.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Rosa-Garrido M, Chapski DJ, Vondriska TM. Epigenomes in cardiovascular disease. Circ Res 2018; 122:1586–1607. doi: 10.1161/circresaha.118.311597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang YP, Lei QY. Metabolic recoding of epigenetics in cancer. Cancer Commun (Lond) 2018; 38:25.doi: 10.1186/s40880-018-0302-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roubroeks JAY, Smith RG, van den Hove DLA, Lunnon K. Epigenetics and DNA methylomic profiling in Alzheimer's disease and other neurodegenerative diseases. J Neurochem 2017; 143:158–170. doi: 10.1111/jnc.14148. [DOI] [PubMed] [Google Scholar]
- 60.Kular L, Kular S. Epigenetics applied to psychiatry: clinical opportunities and future challenges. Psychiatry Clin Neurosci 2018; 72:195–211. doi: 10.1111/pcn.12634. [DOI] [PubMed] [Google Scholar]
- 61.Tost J, Gay S, Firestein G. Epigenetics of the immune system and alterations in inflammation and autoimmunity. Epigenomics 2017; 9:371–373. doi: 10.2217/epi-2017-0026. [DOI] [PubMed] [Google Scholar]
- 62.Rosen ED, Kaestner KH, Natarajan R, Patti ME, Sallari R, Sander M, et al. Epigenetics and epigenomics: implications for diabetes and obesity. Diabetes 2018; 67:1923–1931. doi: 10.2337/db18-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeltsch A, Gowher H. Editorial-Role of DNA methyltransferases in the epigenome. Genes (Basel) 2019; 10:574.doi: 10.3390/genes10080574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng J, Xiao X, Zhang Q, Yu M. DNA methylation: the pivotal interaction between early-life nutrition and glucose metabolism in later life. Br J Nutr 2014; 112:1850–1857. doi: 10.1017/s0007114514002827. [DOI] [PubMed] [Google Scholar]
- 65.Shen L, Li C, Wang Z, Zhang R, Shen Y, Miles T, et al. Early-life exposure to severe famine is associated with higher methylation level in the IGF2 gene and higher total cholesterol in late adulthood: the genomic research of the Chinese famine (GRECF) study. Clin Epigenetics 2019; 11:88.doi: 10.1186/s13148-019-0676-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tobi EW, Slieker RC, Luijk R, Dekkers KF, Stein AD, Xu KM, et al. DNA methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in adulthood. Sci Adv 2018; 4:eaao4364.doi: 10.1126/sciadv.aao4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nogues P, Dos Santos E, Jammes H, Berveiller P, Arnould L, Vialard F, et al. Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta. Clin Epigenetics 2019; 11:20.doi: 10.1186/s13148-019-0612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ge ZJ, Liang QX, Hou Y, Han ZM, Schatten H, Sun QY, et al. Maternal obesity and diabetes may cause DNA methylation alteration in the spermatozoa of offspring in mice. Reprod Biol Endocrinol 2014; 12:29.doi: 10.1186/1477-7827-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharp GC, Lawlor DA, Richmond RC, Fraser A, Simpkin A, Suderman M, et al. Maternal pre-pregnancy BMI and gestational weight gain, offspring DNA methylation and later offspring adiposity: findings from the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2015; 44:1288–1304. doi: 10.1093/ije/dyv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boyle KE, Patinkin ZW, Shapiro ALB, Bader C, Vanderlinden L, Kechris K, et al. Maternal obesity alters fatty acid oxidation, AMPK activity, and associated DNA methylation in mesenchymal stem cells from human infants. Mol Metab 2017; 6:1503–1516. doi: 10.1016/j.molmet.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thakali KM, Faske JB, Ishwar A, Alfaro MP, Cleves MA, Badger TM, et al. Maternal obesity and gestational weight gain are modestly associated with umbilical cord DNA methylation. Placenta 2017; 57:194–203. doi: 10.1016/j.placenta.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Hjort L, Martino D, Grunnet LG, Naeem H, Maksimovic J, Olsson AH, et al. Gestational diabetes and maternal obesity are associated with epigenome-wide methylation changes in children. JCI Insight 2018; 3:e122572.doi: 10.1172/jci.insight.122572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin CL, Jima D, Sharp GC, McCullough LE, Park SS, Gowdy KM, et al. Maternal pre-pregnancy obesity, offspring cord blood DNA methylation, and offspring cardiometabolic health in early childhood: an epigenome-wide association study. Epigenetics 2019; 14:325–340. doi: 10.1080/15592294.2019.1581594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schellong K, Melchior K, Ziska T, Ott R, Henrich W, Rancourt RC, et al. Hypothalamic insulin receptor expression and DNA promoter methylation are sex-specifically altered in adult offspring of high-fat diet (HFD)-overfed mother rats. J Nutr Biochem 2019; 67:28–35. doi: 10.1016/j.jnutbio.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 75.Zhang Q, Xiao X, Zheng J, Li M, Yu M, Ping F, et al. A maternal high-fat diet induces DNA methylation changes that contribute to glucose intolerance in offspring. Front Endocrinol (Lausanne) 2019; 10:871.doi: 10.3389/fendo.2019.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gong L, Pan YX, Chen H. Gestational low protein diet in the rat mediates Igf2 gene expression in male offspring via altered hepatic DNA methylation. Epigenetics 2010; 5:619–626. doi: 10.4161/epi.5.7.12882. [DOI] [PubMed] [Google Scholar]
- 77.Carr SK, Chen JH, Cooper WN, Constancia M, Yeo GS, Ozanne SE. Maternal diet amplifies the hepatic aging trajectory of Cidea in male mice and leads to the development of fatty liver. FASEB J 2014; 28:2191–2201. doi: 10.1096/fj.13-242727. [DOI] [PubMed] [Google Scholar]
- 78.Paradis F, Wood KM, Swanson KC, Miller SP, McBride BW, Fitzsimmons C. Maternal nutrient restriction in mid-to-late gestation influences fetal mRNA expression in muscle tissues in beef cattle. BMC Genomics 2017; 18:632.doi: 10.1186/s12864-017-4051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X, Yan Q, Tang S, Tan Z, Fitzsimmons CJ, Yi K. Effects of maternal feed intake restriction during pregnancy on the expression of growth regulation, imprinting and epigenetic transcription-related genes in foetal goats. Anim Reprod Sci 2018; 198:90–98. doi: 10.1016/j.anireprosci.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 80.Miyoshi M, Sato M, Saito K, Otani L, Shirahige K, Miura F, et al. Maternal protein restriction alters the renal Ptger1 DNA methylation state in SHRSP offspring. Nutrients 2018; 10:1436.doi: 10.3390/nu10101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Benatti RO, Melo AM, Borges FO, Ignacio-Souza LM, Simino LA, Milanski M, et al. Maternal high-fat diet consumption modulates hepatic lipid metabolism and microRNA-122 (miR-122) and microRNA-370 (miR-370) expression in offspring. Br J Nutr 2014; 111:2112–2122. doi: 10.1017/s0007114514000579. [DOI] [PubMed] [Google Scholar]
- 82.Zheng J, Zhang Q, Mul JD, Yu M, Xu J, Qi C, et al. Maternal high-calorie diet is associated with altered hepatic microRNA expression and impaired metabolic health in offspring at weaning age. Endocrine 2016; 54:70–80. doi: 10.1007/s12020-016-0959-9. [DOI] [PubMed] [Google Scholar]
- 83.Koduru SV, Tiwari AK, Hazard SW, Mahajan M, Ravnic DJ. Exploration of small RNA-seq data for small non-coding RNAs in human colorectal cancer. J Genomics 2017; 5:16–31. doi: 10.7150/jgen.18856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Das A, Samidurai A, Salloum FN. Deciphering non-coding RNAs in cardiovascular health and disease. Front Cardiovasc Med 2018; 5:73.doi: 10.3389/fcvm.2018.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ignarski M, Islam R, Muller RU. Long non-coding RNAs in kidney disease. Int J Mol Sci 2019; 20:3276.doi: 10.3390/ijms20133276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Watson CN, Belli A, Di Pietro V. Small non-coding RNAs: new class of biomarkers and potential therapeutic targets in neurodegenerative disease. Front Genet 2019; 10:364.doi: 10.3389/fgene.2019.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yaribeygi H, Katsiki N, Behnam B, Iranpanah H, Sahebkar A. MicroRNAs and type 2 diabetes mellitus: molecular mechanisms and the effect of antidiabetic drug treatment. Metabolism 2018; 87:48–55. doi: 10.1016/j.metabol.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Koh EH, Chernis N, Saha PK, Xiao L, Bader DA, Zhu B, et al. miR-30a remodels subcutaneous adipose tissue inflammation to improve insulin sensitivity in obesity. Diabetes 2018; 67:2541–2553. doi: 10.2337/db17-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma E, Fu Y, Garvey WT. Relationship of circulating miRNAs with insulin sensitivity and associated metabolic risk factors in humans. Metab Syndr Relat Disord 2018; 16:82–89. doi: 10.1089/met.2017.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castano C, Kalko S, Novials A, Parrizas M. Obesity-associated exosomal miRNAs modulate glucose and lipid metabolism in mice. Proc Natl Acad Sci U S A 2018; 115:12158–12163. doi: 10.1073/pnas.1808855115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang M, Wang W, Wang J, Zhang J. MiR-182 promotes glucose metabolism by upregulating hypoxia-inducible factor 1alpha in NSCLC cells. Biochem Biophys Res Commun 2018; 504:400–405. doi: 10.1016/j.bbrc.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 92.Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature 2011; 474:649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- 93.Frost RJ, Olson EN. Control of glucose homeostasis and insulin sensitivity by the Let-7 family of microRNAs. Proc Natl Acad Sci U S A 2011; 108:21075–21080. doi: 10.1073/pnas.1118922109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosado JA, Diez-Bello R, Salido GM, Jardin I. Fine-tuning of microRNAs in type 2 diabetes mellitus. Curr Med Chem 2019; 26:4102–4118. doi: 10.2174/0929867325666171205163944. [DOI] [PubMed] [Google Scholar]
- 95.Lie S, Morrison JL, Williams-Wyss O, Suter CM, Humphreys DT, Ozanne SE, et al. Periconceptional undernutrition programs changes in insulin-signaling molecules and microRNAs in skeletal muscle in singleton and twin fetal sheep. Biol Reprod 2014; 90:5.doi: 10.1095/biolreprod.113.109751. [DOI] [PubMed] [Google Scholar]
- 96.Lie S, Morrison JL, Williams-Wyss O, Suter CM, Humphreys DT, Ozanne SE, et al. Impact of embryo number and maternal undernutrition around the time of conception on insulin signaling and gluconeogenic factors and microRNAs in the liver of fetal sheep. Am J Physiol Endocrinol Metab 2014; 306:E1013–E1024. doi: 10.1152/ajpendo.00553.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nicholas LM, Rattanatray L, MacLaughlin SM, Ozanne SE, Kleemann DO, Walker SK, et al. Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling pathways in the offspring. FASEB J 2013; 27:3786–3796. doi: 10.1096/fj.13-227918. [DOI] [PubMed] [Google Scholar]
- 98.Horikoshi M. Histone acetylation: from code to web and router via intrinsically disordered regions. Curr Pharm Des 2013; 19:5019–5042. doi: 10.2174/1381612811319280002. [DOI] [PubMed] [Google Scholar]
- 99.Nowacka-Woszuk J, Szczerbal I, Malinowska AM, Chmurzynska A. Transgenerational effects of prenatal restricted diet on gene expression and histone modifications in the rat. PLoS One 2018; 13:e0193464.doi: 10.1371/journal.pone.0193464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yang KF, Cai W, Xu JL, Shi W. Maternal high-fat diet programs Wnt genes through histone modification in the liver of neonatal rats. J Mol Endocrinol 2012; 49:107–114. doi: 10.1530/jme-12-0046. [DOI] [PubMed] [Google Scholar]
- 101.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG 2010; 117:1316–1326. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- 102.Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 2012; 344:e2088.doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tanvig M, Vinter CA, Jorgensen JS, Wehberg S, Ovesen PG, Beck-Nielsen H, et al. Effects of lifestyle intervention in pregnancy and anthropometrics at birth on offspring metabolic profile at 2.8 years: results from the lifestyle in pregnancy and offspring (LiPO) study. J Clin Endocrinol Metab 2015; 100:175–183. doi: 10.1210/jc.2014-2675. [DOI] [PubMed] [Google Scholar]
- 104.Ronnberg AK, Hanson U, Nilsson K. Effects of an antenatal lifestyle intervention on offspring obesity - a 5-year follow-up of a randomized controlled trial. Acta Obstet Gynecol Scand 2017; 96:1093–1099. doi: 10.1111/aogs.13168. [DOI] [PubMed] [Google Scholar]
- 105.Grotenfelt NE, Wasenius N, Eriksson JG, Huvinen E, Stach-Lempinen B, Koivusalo SB, et al. Effect of maternal lifestyle intervention on metabolic health and adiposity of offspring: findings from the Finnish Gestational Diabetes Prevention Study (RADIEL). Diabetes Metab 2019; 46:46–53. doi: 10.1016/j.diabet.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 106.Catalano P, deMouzon SH. Maternal obesity and metabolic risk to the offspring: why lifestyle interventions may have not achieved the desired outcomes. Int J Obes (Lond) 2015; 39:642–649. doi: 10.1038/ijo.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han Y, Jo H, Cho JH, Dhanasekaran DN, Song YS. Resveratrol as a tumor-suppressive nutraceutical modulating tumor microenvironment and malignant behaviors of cancer. Int J Mol Sci 2019; 20:925.doi: 10.3390/ijms20040925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li YR, Li S, Lin CC. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors 2018; 44:69–82. doi: 10.1002/biof.1400. [DOI] [PubMed] [Google Scholar]
- 109.Mousavi SM, Milajerdi A, Sheikhi A, Kord-Varkaneh H, Feinle-Bisset C, Larijani B, et al. Resveratrol supplementation significantly influences obesity measures: a systematic review and dose-response meta-analysis of randomized controlled trials. Obes Rev 2019; 20:487–498. doi: 10.1111/obr.12775. [DOI] [PubMed] [Google Scholar]
- 110.Springer M, Moco S. Resveratrol and its human metabolites-effects on metabolic health and obesity. Nutrients 2019; 11:143.doi: 10.3390/nu11010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hoseini A, Namazi G, Farrokhian A, Reiner Z, Aghadavod E, Bahmani F, et al. The effects of resveratrol on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Food Funct 2019; 10:6042–6051. doi: 10.1039/c9fo01075k. [DOI] [PubMed] [Google Scholar]
- 112.Dyck GJB, Raj P, Zieroth S, Dyck JRB, Ezekowitz JA. The effects of resveratrol in patients with cardiovascular disease and heart failure: a narrative review. Int J Mol Sci 2019; 20:904.doi: 10.3390/ijms20040904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Franco JG, Dias-Rocha CP, Fernandes TP, Albuquerque Maia L, Lisboa PC, Moura EG, et al. Resveratrol treatment rescues hyperleptinemia and improves hypothalamic leptin signaling programmed by maternal high-fat diet in rats. Eur J Nutr 2016; 55:601–610. doi: 10.1007/s00394-015-0880-7. [DOI] [PubMed] [Google Scholar]
- 114.Vega CC, Reyes-Castro LA, Rodriguez-Gonzalez GL, Bautista CJ, Vazquez-Martinez M, Larrea F, et al. Resveratrol partially prevents oxidative stress and metabolic dysfunction in pregnant rats fed a low protein diet and their offspring. J Physiol 2016; 594:1483–1499. doi: 10.1113/jp271543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zou T, Chen D, Yang Q, Wang B, Zhu MJ, Nathanielsz PW, et al. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J Physiol 2017; 595:1547–1562. doi: 10.1113/jp273478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tiao MM, Lin YJ, Yu HR, Sheen JM, Lin IC, Lai YJ, et al. Resveratrol ameliorates maternal and post-weaning high-fat diet-induced nonalcoholic fatty liver disease via renin-angiotensin system. Lipids Health Dis 2018; 17:178.doi: 10.1186/s12944-018-0824-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zheng S, Feng Q, Cheng J, Zheng J. Maternal resveratrol consumption and its programming effects on metabolic health in offspring mechanisms and potential implications. Biosci Rep 2018; 38:BSR20171741.doi: 10.1042/bsr20171741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamasaki S, Tomihara T, Kimura G, Ueno Y, Ketema RM, Sato S, et al. Long-term effects of maternal resveratrol intake during lactation on cholesterol metabolism in male rat offspring. Int J Food Sci Nutr 2020; 71:226–234. doi: 10.1080/09637486.2019.1639638. [DOI] [PubMed] [Google Scholar]
- 119.Zhou L, Xiao X, Zhang Q, Zheng J, Li M, Yu M, et al. Improved glucose and lipid metabolism in the early life of female offspring by maternal dietary genistein is associated with alterations in the gut microbiota. Front Endocrinol (Lausanne) 2018; 9:516.doi: 10.3389/fendo.2018.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhou L, Xiao X, Zhang Q, Zheng J, Deng M. Maternal genistein intake mitigates the deleterious effects of high-fat diet on glucose and lipid metabolism and modulates gut microbiota in adult life of male mice. Front Physiol 2019; 10:985.doi: 10.3389/fphys.2019.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cordero P, Milagro FI, Campion J, Martinez JA. Supplementation with methyl donors during lactation to high-fat-sucrose-fed dams protects offspring against liver fat accumulation when consuming an obesogenic diet. J Dev Orig Health Dis 2014; 5:385–395. doi: 10.1017/s204017441400035x. [DOI] [PubMed] [Google Scholar]
- 122.Aleliunas RE, Aljaadi AM, Laher I, Glier MB, Green TJ, Murphy M, et al. Folic acid supplementation of female mice, with or without vitamin B-12, before and during pregnancy and lactation programs adiposity and vascular health in adult male offspring. J Nutr 2015; 146:688–696. doi: 10.3945/jn.115.227629. [DOI] [PubMed] [Google Scholar]
- 123.Henderson AM, Tai DC, Aleliunas RE, Aljaadi AM, Glier MB, Xu EE, et al. Maternal folic acid supplementation with vitamin B(12) deficiency during pregnancy and lactation affects the metabolic health of adult female offspring but is dependent on offspring diet. FASEB J 2018; 32:5039–5050. doi: 10.1096/fj.201701503RR. [DOI] [PubMed] [Google Scholar]
- 124.Morakinyo AO, Samuel TA, Awobajo FO, Oludare GO, Mofolorunso A. High-dose perinatal folic-acid supplementation alters insulin sensitivity in sprague-dawley rats and diminishes the expression of adiponectin. J Diet Suppl 2019; 16:14–26. doi: 10.1080/19390211.2018.1426076. [DOI] [PubMed] [Google Scholar]
- 125.Burdge GC, Lillycrop KA, Phillips ES, Slater-Jefferies JL, Jackson AA, Hanson MA. Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J Nutr 2009; 139:1054–1060. doi: 10.3945/jn.109.104653. [DOI] [PubMed] [Google Scholar]
- 126.Lewis SJ, Leary S, Davey Smith G, Ness A. Body composition at age 9 years, maternal folate intake during pregnancy and methyltetrahydrofolate reductase (MTHFR) C677T genotype. Br J Nutr 2009; 102:493–496. doi: 10.1017/s0007114509231746. [DOI] [PubMed] [Google Scholar]


