Supplemental Digital Content is available in the text
Keywords: Atrial fibrillation, Heart valve prosthesis implantation, Propensity score
Abstract
Background
Surgical left atrial appendage occlusion (SLAAO) may be associated with a lower risk of thromboembolism in patients with atrial fibrillation undergoing cardiac surgery. However, evidence regarding the effectiveness of SLAAO in patients undergoing mechanical heart valve replacement (MHVR) is lacking. Therefore, we aimed to evaluate the association between SLAAO and the cardiovascular outcomes in patients with atrial fibrillation undergoing MHVR.
Methods
We retrospectively analyzed data for 497 patients with atrial fibrillation; 27.6% of the patients underwent SLAAO, and the remainder of the patients did not (No-SLAAO group). The primary outcome was a composite of ischemic stroke, systemic embolism, and all-cause mortality. Cumulative event-free survival rates were estimated using Kaplan-Meier curves, and we performed multivariate Cox analyses to evaluate the association between SLAAO and outcomes. We used one-to-one propensity score matching to balance patients’ baseline characteristics, and analyzed 120 matching pairs.
Results
Five patients died within 30 days postoperatively, and there were no significant differences between the two groups regarding in-hospital complications (all P > 0.05). After a median follow-up of 14 months, 14 primary events occurred. Kaplan-Meier curves showed no difference in the cumulative incidence of freedom from the primary outcome (log-rank P = 0.830), hemorrhagic events (log-rank P = 0.870), and the secondary outcome (log-rank P = 0.730), between the two groups. Multivariable Cox proportional hazards regression analysis showed no association between SLAAO and any outcome (all P > 0.05). After propensity score matching, cardiopulmonary bypass time and aortic cross-clamp time, and the postoperative length of stay were significantly longer in the SLAAO group (all P < 0.05); results were similar to the unadjusted analyses.
Conclusions
Concomitant SLAAO and MHVR was associated with longer length of stay, and cardiopulmonary bypass time and aortic cross-clamp time, but was not associated with additional protective effects against thromboembolic events and mortality during the 14-month follow-up.
Introduction
Atrial fibrillation is the most common arrhythmia and is expected to affect 33.5 million people worldwide.[1] Studies report that the left atrial appendage (LAA) plays a role in thrombus formation, and is considered the source of 90% of the embolisms in non-valvular atrial fibrillation and 57% of the embolisms in valvular atrial fibrillation.[2,3] Furthermore, atrial fibrillation is associated with an estimated three- to five-fold increase in the risk of stroke.[4] Therefore, in addition to conventional anti-thrombotic therapy, the impact of concomitant surgical LAA occlusion (SLAAO) through suture exclusion/excision, stapler exclusion/excision, snares/suture loops, or epicardial exclusion clips has also received widespread attention.[5]
Theoretically, SLAAO may be associated with a reduced risk of thromboembolism, but the procedure also comes with risks such as prolonged operation time, damage to the circumflex coronary arteries, and incomplete LAA occlusion. However, data are limited regarding the effectiveness of SLAAO in open cardiac surgery, which contributes to its IIa or IIb recommendation in recent European and American guidelines.[6–8] Several studies have examined the relationship between concomitant SLAAO during cardiac surgery and cardiovascular outcomes, but with divergent results. Recently, two large observational studies[9,10] and several meta-analyses[11–13] comparing patients who underwent SLAAO with those who did not, demonstrated that SLAAO during cardiac surgery was associated with a lower risk of thromboembolism, stroke, and mortality. Conversely, other studies found that concomitant SLAAO and cardiac surgery did not influence the risk of stroke or mortality.[14–16]
Because of the relatively small percentage of patients undergoing mechanical heart valve replacement (MHVR) in previous studies,[9,10] limited information is available regarding the effectiveness of SLAAO in patients undergoing MHVR. Therefore, we performed this retrospective observational study and enrolled patients with valve disease and atrial fibrillation who underwent MHVR with or without SLAAO in our center, to evaluate the association between SLAAO and the risk of ischemic stroke, systemic embolism, and mortality in patients with atrial fibrillation undergoing MHVR.
Methods
Ethical approval
The study was performed in accordance with the principles of the Declaration of Helsinki and was approved by our Hospital Ethical Review Board (No. 2017-880). The requirement for written informed consent was waived because of the retrospective design.
Study population
This was a single-center, retrospective, observational study. We consecutively enrolled adult patients with atrial fibrillation who underwent MHVR with or without coronary artery bypass grafting (CABG) from July 1, 2017 to June 30, 2018 at our center. Patients were excluded if they had undergone prior open-heart valve surgery, CABG, or atrial fibrillation ablation procedures.
Baseline data
Patients’ clinical data, namely age, height, weight, blood pressure, smoking status, and history of comorbidity were obtained by experienced physicians and nurses when patients were first hospitalized. We obtained all variables by database review or from the electronic medical record system in our center, which contained detailed baseline demographic information, comorbidities, surgical procedures, and medication records at discharge for all enrolled patients. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation,[17] as follows: eGFR = 141 × min(Scr/κ, 1)α × max(Scr/κ, 1)−1.209 × 0.993Age × 1.018 (if female), where Scr is serum creatinine, κ is 0.7 for women and 0.9 for men, α is −0.329 for women and −0.411 for men, min indicates the minimum Scr/κ or 1, and max indicates the maximum Scr/κ or 1. We also calculated the congestive heart failure, hypertension, age ≥75 years, diabetes mellitus, stroke, vascular disease, age 65 to 74 years, sex category (CHA2DS2-VASc) score (range: 0–9) as 1 point each for heart failure, hypertension, diabetes, vascular disease, age 65 to 74 years, and female sex; and 2 points for ≥75 years, and prior stroke, transient ischemic attack, or thromboembolism.
Surgical techniques
All surgeries were performed through a median sternotomy under cardiopulmonary bypass with SLAAO performed simultaneously in 137 (27.6%) patients using one of three surgical techniques, at the surgeon's discretion. When LAA occlusion was performed, the LAA ostium was oversewn in two layers of polypropylene suture from inside the left atrium or the LAA was ligated epicardially with suture. In a minority of patients, the LAA was amputated and its opening was oversewn with two layers of polypropylene suture, epicardially. The completeness of SLAAO was assessed by surgeons visually.
Follow-up and outcomes
Patients were encouraged to return for a routine outpatient visit 3 months and 1 year after surgery. Those who were not assessed in-person were interviewed by telephone or mail 1 year after surgery according to our standard institutional procedure. Follow-up began the day after surgery and continued for 1 year after surgery, or death, whichever occurred first. All related prognostic information was collected.
The primary outcome was a composite of thromboembolic events (ischemic stroke and systemic embolism) and all-cause mortality. Ischemic stroke and systemic embolism were defined as a primary or secondary diagnosis during an emergency department visit or an inpatient stay (diagnosis codes: International Classification of Diseases-9 codes 433, 434, and 444; and International Classification of Diseases-10 codes I63 and I74). We identified mortality according to telephone interviews or discharge status. Hemorrhagic events constituted major bleeding, which was diagnosed as requiring treatment during an emergency department visit or an inpatient stay, and minor bleeding identified according to self-reported patient data. The secondary outcome was defined as a composite of ischemic stroke, systemic embolism, major bleeding, and all-cause mortality.
We also compared in-hospital complication rates by treatment group, which included 30-day mortality, re-exploration, length of stay after surgery, red blood cell transfusion, acute kidney injury, and the composite in-hospital events of death, re-exploration, and cerebrovascular accidents. Acute kidney injury was defined as an increase in serum creatinine of ≥0.3 mg/dL (≥26.4 μmol/L) or an increase of ≥150% to 200% (1.5- to two-fold) from baseline (stage 1); an increase in serum creatinine of >200% to 300% (>two- to three-fold) from baseline (stage 2); or an increase in serum creatinine of >300% (>three-fold) from baseline (or serum creatinine ≥4.0 mg/dL [≥354 μmol/L] with an acute increase of at least 0.5 mg/dL [44 μmol/L]) (stage 3).[18]
Statistical analysis
Continuous data were expressed as mean ± standard deviation or median (interquartile range), and categorical variables were presented as counts with percentages. Differences between groups were tested using the t test or Wilcoxon rank-sum test for continuous variables and the Chi-squared test or Fisher exact test for categorical variables, depending on the nature of the distribution.
We compared observed event rates between the groups (SLAAO group and No-SLAAO group) and used a logistic regression model to calculate event rates. Comparisons of Kaplan-Meier curves were performed with the log-rank test. Additionally, univariate and multivariate Cox proportional hazards regression models were used to test for the association between SLAAO and the risk of primary and second outcomes and to estimate the corresponding relative hazard ratios (HRs) with 95% confidence intervals (CIs). The assumption of proportional hazard for the final models was checked using Schoenfeld residuals.
Propensity score matching (PSM) was used to balance differences in patients’ baseline characteristics using multiple logistic regression analysis. All pre-specified covariates were included in the final models for the SLAAO vs. No-SLAAO groups [Table 1 and Supplementary Table 1, http://links.lww.com/CM9/A257]. One-to-one nearest-neighbor matching was used to match patients according to the propensity score, without replacement, using a variable-width caliper matching algorithm (5:1 digit matching). The standardized differences were used to compare balance in baseline covariates between groups, and an absolute standardized difference of <10% was considered acceptable.
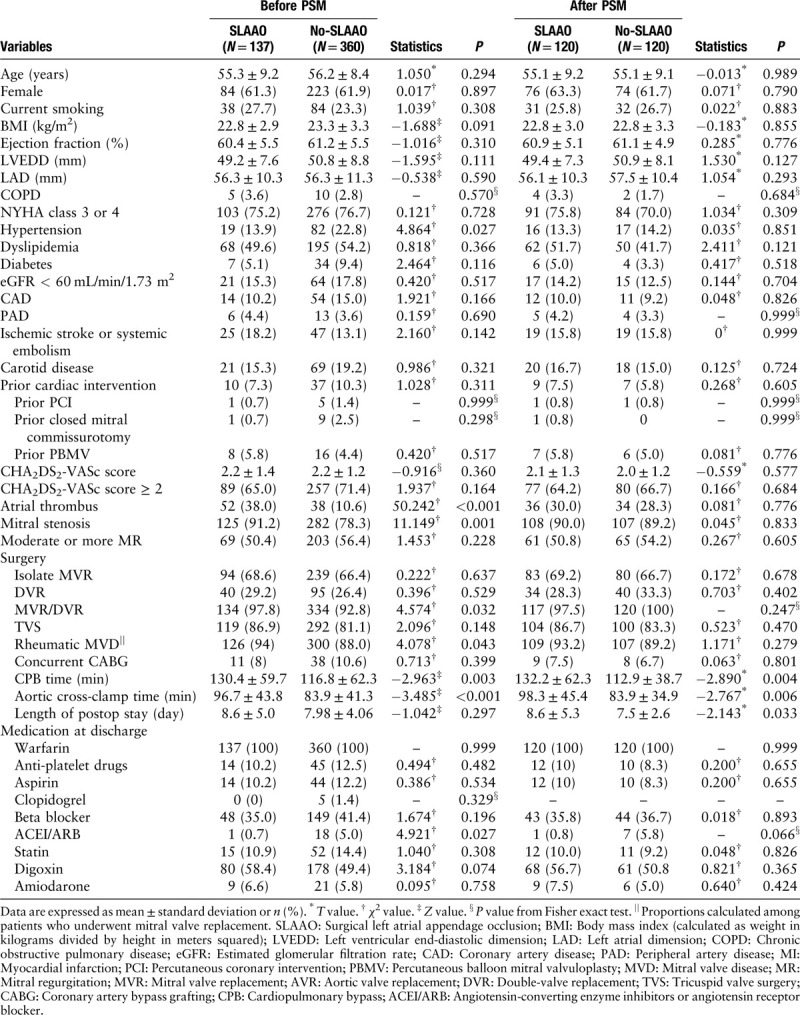
Table 1.
Baseline patients’ characteristics, detailed surgical information, and medication at discharge before and after propensity score matching.
Subgroup analyses for thromboembolism events and mortality were stratified by sex, BMI, CHA2DS2-VASc score, coronary artery disease, and left atrial dimension. Likelihood ratio tests were performed for interactions. Statistical significance was set at P < 0.05, and all statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) and SPSS software (version 24; IBM Corp., Armonk, NY, USA).
Results
Baseline characteristics
Between July 1, 2017 and June 30, 2018, 623 consecutive patients with atrial fibrillation underwent MHVR. After excluding patients who underwent prior open-heart valve or CABG procedures (n = 32), or concomitant atrial fibrillation ablation procedures (n = 94), 497 patients met the study criteria [Supplementary Figure 1, http://links.lww.com/CM9/A258]. Patients’ baseline characteristics and detailed surgical information for the overall cohort appear in Table 1. The mean (standard deviation) age of the study cohort was 55.9 (8.6) years; 307 (61.8%) patients were women; 379 (76.3%) patients had a New York Heart Association score of III or IV; and 47 (9.5%) patients had undergone prior cardiac intervention (24 underwent prior percutaneous balloon mitral valvuloplasty, ten underwent prior closed mitral commissurotomy, and 13 underwent other procedures). Most patients underwent mitral valve replacement or double-valve replacement because of rheumatic heart disease. Concomitant SLAAO was performed in 137 patients (27.6%), with ligation performed in 62 patients, intra-atrial oversewing in 61, and amputation in 14, while 49 (9.9%) underwent concomitant CABG, and 90 (18.1%) had an atrial thrombus. Compared with the No-SLAAO group, patients who underwent SLAAO had a lower incidence of hypertension (13.9% vs. 22.8%, P = 0.027) but were more likely to have an atrial thrombus (38.0% vs. 10.6%, P < 0.001), rheumatic mitral valve disease (94.0% vs. 88.0%, P = 0.043), and mitral stenosis (91.2% vs. 78.3%, P = 0.001), and to have undergone mitral valve surgery (97.8% vs. 92.8%, P = 0.032) (all comparison: SLAAO vs. No-SLAAO, respectively). As shown in Table 1, medication at discharge showed non-statistical differences between the SLAAO group and the No-SLAAO group other than for angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (0.7% vs. 5.0%, respectively; P = 0.027). All patients were prescribed warfarin after surgery, and the target international normalized ratio range was 1.8 to 2.5.
Surgical complications
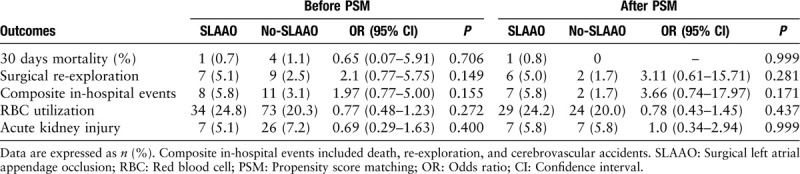
In the overall study cohort, five patients died within 30 days after surgery (one cardiac death, one neurogenic death, and three multiple organ system failures). Although cardiopulmonary bypass (CPB) time and aortic cross-clamp time were significantly longer in the SLAAO group, the length of stay after surgery was similar between the groups (8.6 ± 5.0 days in the SLAAO group vs. 8.0 ± 4.1 days in the No-SLAAO group; P = 0.297) [Table 1]. The rate of postoperative complications, including the incidence of postoperative surgical re-exploration, postoperative red blood cell transfusion, and acute kidney injury did not differ between patients in the SLAAO and No-SLAAO groups. In the unadjusted logistic regression analyses, SLAAO was not associated with 30-day mortality (odds ratio [OR], 0.65; 95% CI, 0.07–5.91; P = 0.706). Similar results were observed between the groups regarding the other in-hospital complications [Table 2].
Table 2.
Unadjusted associations between SLAAO vs. No-SLAAO and in-hospital outcomes before and after PSM.
Primary and secondary outcomes
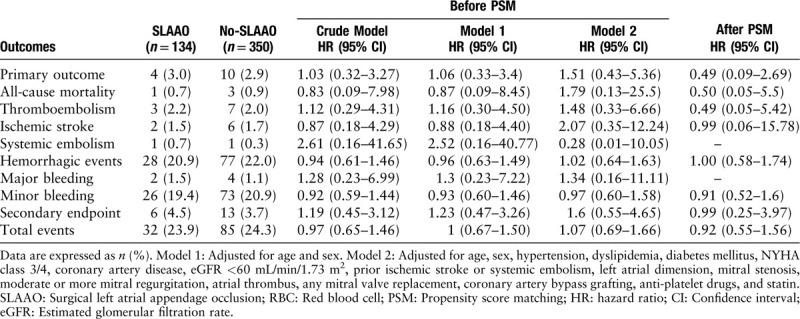
After a median follow-up of 14 months (13–16 months), eight patients were lost to follow-up, and 117 (24.2%) events occurred among the remaining 484 patients. Ischemic stroke occurred in 1.7% of the patients, systemic embolism in 0.4%, all-cause deaths constituted 0.8% of the patients, hemorrhagic events occurred in 21.9% (major bleeding in 1.3%, minor bleeding in 20.6%), and 3.9% of the patients experienced the secondary outcome. Kaplan-Meier curves showed no differences in the cumulative incidence of the primary outcome, thromboembolic events, all-cause mortality, hemorrhagic events, and the secondary outcome between groups (all log-rank, P > 0.05) [Figure 1]. The results of the univariate and multivariate COX analyses are presented in Table 3. Compared with No-SLAAO, SLAAO was not associated with lower risks of the primary outcome (HR, 1.03; 95% CI, 0.32–3.27; P = 0.965), hemorrhagic events (HR, 0.94; 95% CI, 0.61–1.46; P = 0.796), and the secondary outcome (HR, 1.19; 95% CI, 0.45–3.12; P = 0.730). Similar results were found when we adjusted for age, sex, comorbidities, type of surgery, and prescriptions at discharge.
Figure 1.
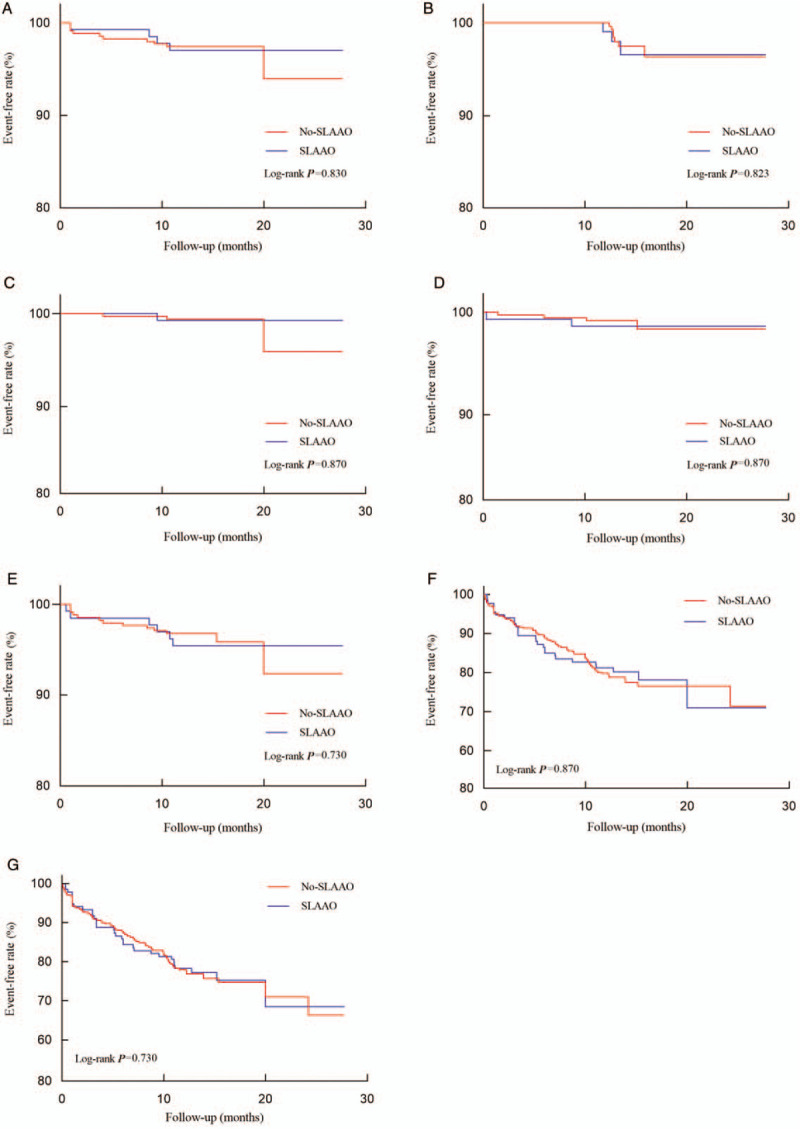
Kaplan-Meier curves for the cumulative event-free survival analyses. Primary outcome (A), thromboembolism event (B), all-cause mortality (C), major bleeding (D), secondary outcome (E), hemorrhagic events (F), and total event (G) among those with or without concomitant SLAAO. SLAAO: Surgical left atrial appendage occlusion.
Table 3.
Association between SLAAO vs. No-SLAAO and follow-up outcomes before and after PSM.
Propensity score matching
PSM matched 120 patients at a ratio of 1:1 between the SLAAO and No-SLAAO groups according to similar propensity scores. The performance of the PSM model was assessed using the C statistic (0.725) and the Hosmer-Lemeshow goodness-of-fit test (P = 0.520), which indicated good discrimination between the two groups. The baseline characteristics of the propensity-matched pairs between the groups were identical [Table 1].
After PSM, CPB time and aortic cross-clamp time were significantly longer in the SLAAO group (both comparisons, P < 0.05), and the postoperative length of stay was longer. The results were similar to the unadjusted analyses, and SLAAO was not associated with any in-hospital complications or follow-up outcomes [Tables 2 and 3].
Subgroup analyses
As presented in Figures 2 and 3, we also performed subgroup analyses stratified by sex, BMI, CHA2DS2-VASc score, coronary artery disease, peripheral artery disease, and anti-platelet drug use. The results of the subgroup analyses were consistent with the main findings before and after PSM. P values for the interactions were non-significant for sex, BMI, CHA2DS2-VASc score, coronary artery disease, left atrial dimension, and anti-platelet drug use.
Figure 2.
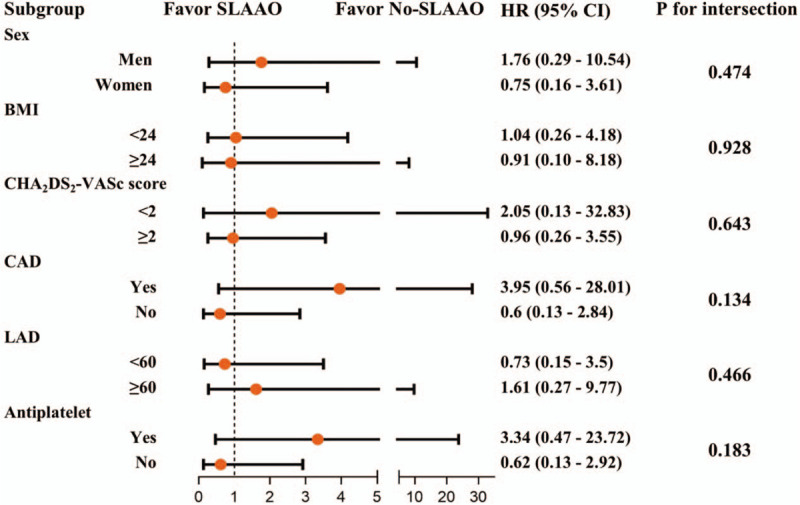
Subgroup analysis for the primary outcome in all patients. SLAAO: Surgical left atrial appendage occlusion; BMI: Body mass index (calculated as weight in kilograms divided by height in meters squared); CAD: Coronary artery disease; LAD: Left atrial dimension; HR: Hazard ratio; CI: Confidence interval.
Figure 3.
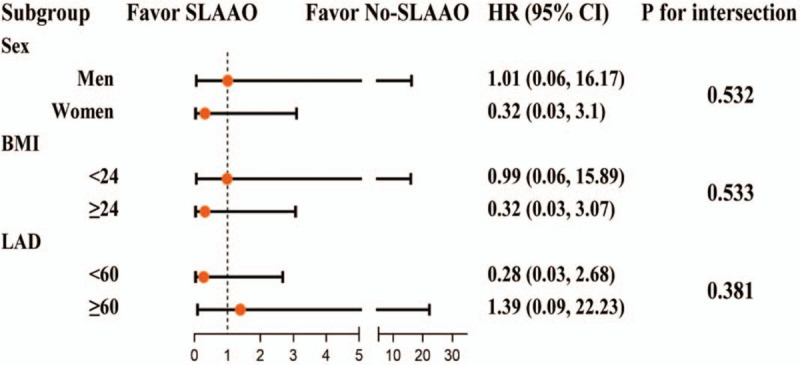
Subgroup analysis for the primary outcome in propensity-score matched patients. SLAAO: Surgical left atrial appendage occlusion; BMI: Body mass index (calculated as weight in kilograms divided by height in meters squared); LAD: Left atrial dimension; HR: Hazard ratio; CI: Confidence interval.
Discussion
In this retrospective study, we sought to evaluate the impact of concomitant SLAAO during MHVR on cardiovascular outcomes in patients with atrial fibrillation. We found that SLAAO was not associated with a lower incidence of in-hospital complications, or with primary or secondary outcomes observed during follow-up. However, the data showed that concomitant SLAAO significantly increased CPB time, aortic cross-clamp time, and length of stay compared with patients not undergoing SLAAO, in the propensity-matched cohort.
Emerging studies suggest that patients with atrial fibrillation have a higher risk of stroke or thromboembolism,[4] and LAA was reported to be the main source of thrombus formation leading to thromboembolic events. With these concerns, surgical LAA occlusion has interested cardiac surgeons for decades, to reduce thromboembolic events.[5,19] However, there is no conclusive evidence that SLAAO reduces stroke risk in patients with atrial fibrillation undergoing cardiac surgery. Recently, several retrospective studies[9,10,20] and meta-analyses[11–13] showed that SLAAO is associated with a lower incidence of ischemic stroke or systemic embolism and mortality in patients undergoing cardiac surgery. Friedman et al[10] published a retrospective study involving 10,524 patients aged ≥65 years with atrial fibrillation undergoing cardiac surgery and found that SLAAO was associated with a significantly lower risk of readmission for thromboembolism and all-cause mortality during a 3-year follow-up. Later, Yao et al[9] used a large administrative database from the United States containing data for 75,782 adults who underwent cardiac surgery, to evaluate the association between SLAAO during cardiac surgery and the risk of ischemic stroke, systemic embolism, and mortality. The authors demonstrated similar results to those from Friedman et al in patients with atrial fibrillation during a mean follow-up of 2.1 years. More recently, a meta-analysis of 22 studies involving 280,585 patients showed that stroke/embolic events and mortality in the mid- and long-term follow-up were all significantly lower in patients with preoperative atrial fibrillation who underwent SLAAO.[11] However, a recent study from the Mayo Clinic[14] using a propensity-matched analysis did not demonstrate that SLAAO significantly influenced the risk of stroke or long-term mortality. Furthermore, Johnsrud et al,[15] also from the Mayo Clinic, published a PSM analysis and stated that SLAAO did not appear to reduce the incidence of early or late stroke.
Despite this evidence, limited data are available to support the efficacy of SLAAO to reduce events in patients undergoing MHVR. Garcia-Fernandez et al[21] claimed that SLAAO was associated with a lower risk of late embolism in a retrospective study involving 205 patients (91.2% received a mechanical prosthesis). The different conclusions reached by this study and our study may relate to the quality of anti-coagulant therapy and the success rate of SLAAO. For example, studies[22,23] have reported that high warfarin treatment quality was associated with better outcomes (including thromboembolic events, major bleeding complications, and death) after MHVR. Furthermore, several studies[21,24,25] raised concerns that incomplete SLAAO may be associated with subsequent thromboembolic sequelae. However, the fact that transient ischemic attack was not included as a thromboembolic event in the current study may also have played a role in the different results between our study and previous studies. It is important to note that the Kaplan-Meier curves crossed each other regarding cardiovascular outcomes, indicating that the results might be influenced by other risk factors. Although we performed subgroup and PSM analyses, the results were similar in the different subgroups and after PSM. Nevertheless, further studies are needed to verify our results.
Notably, a common conclusion in previous studies was that SLAAO was not associated with thromboembolic events in patients discharged with oral anti-coagulant therapy.[9,10,14] However, one retrospective study of 136 patients who underwent SLAAO during mitral valve surgery reported a high incidence of thromboembolic events among patients not receiving anti-coagulant therapy on hospital discharge.[26] Studies have reported that SLAAO was associated with a significantly lower rate of thromboembolism in patients discharged without oral anti-coagulation therapy.[9,10] Consequently, we speculate that anti-coagulant therapy may have already reduced the incidence of thromboembolic events, and that the additional risk reduction from SLAAO was insignificant. Thus, our findings are consistent with the 2016 European Society of Cardiology guidelines stating that SLAAO might not be needed in patients with atrial fibrillation undergoing MHVR, if they are able to receive full anti-coagulation therapy with warfarin.[6]
We did not identify any differences between the groups regarding in-hospital complications, similar to previous studies.[11,13,27] However, our results showed that SLAAO was associated with longer length of stay, and longer CPB and aortic cross-clamp times, suggesting that this technique may still increase the burden of perioperative complications, to some extent.
Studies and guidelines have verified that anti-coagulation therapy could effectively reduce the risk of thromboembolism and mortality in patients with atrial fibrillation undergoing MHVR.[7,28,29] Therefore, the clinical efficacy of SLAAO in reducing thromboembolic events remains unclear, especially in patients requiring lifelong anti-coagulant therapy, although complete LAA occlusion should theoretically reduce the risk of thrombus formation. Additionally, studies have identified several roles that LAA plays in maintaining systemic homeostasis, such as its electrical, mechanical/reservoir, and neurohormonal properties.[30–32] Furthermore, studies have reported that SLAAO was associated with a higher burden of subsequent atrial fibrillation because of increased left atrial filling pressures, inflammation, and sympathovagal imbalance after the procedure.[9,14] With these considerations, concomitant SLAAO may be safe but may not effectively reduce the incidence of thromboembolic events while increasing the burden of subsequent atrial fibrillation, and SLAAO may also affect systemic homeostasis. Therefore, careful consideration is necessary before deciding to perform this additional procedure, and further studies are needed to evaluate whether SLAAO can be performed routinely in patients requiring lifelong anti-coagulant therapy.
Limitations
This study has several limitations. First, treatment (SLAAO vs. No-SLAAO) was not randomly assigned, and therefore, selection bias and uncontrolled confounding are possible. To address these issues, we performed a comprehensive PSM analysis to balance differences between groups, but the potential for residual and unmeasured confounding cannot be ruled out. Second, this study may be underpowered for the outcome because of the small number of events, and the fact that patients were enrolled from a single center. Therefore, we cannot exclude the possibility of type II error. Third, the study population included only adults with atrial fibrillation who underwent MHVR; we excluded patients who underwent atrial fibrillation ablation procedures. Therefore, the results may not be generalizable to individuals undergoing atrial fibrillation ablation or those with less risk of thromboembolic events. Fourth, since this is a retrospective study, the type of atrial fibrillation cannot be accurately distinguished by electronic medical records. So, we cannot rule out that different types of atrial fibrillation may have different outcomes. Fifth, patients in this study were all instructed by experienced anti-coagulant physicians or nurses to take life-long warfarin, but we did not capture information regarding adherence to the therapy or international normalized ratio values during the follow-up, which may have influenced outcomes. Finally, although we performed multivariable Cox regression analyses and included several confounding variables, the possibility of residual confounding cannot be ruled out. Larger sample-size studies with long-term follow-up are necessary to validate our findings.
Conclusions
This single-center retrospective analysis demonstrated that in patients with atrial fibrillation undergoing MHVR, concomitant SLAAO was associated with longer length of stay, and longer CPB and aortic cross-clamp times, but was not associated with additional protective effects against thromboembolic events and mortality 1 year after surgery. This study focusing on patients undergoing MHVR suggested that SLAAO might not be needed in patients with atrial fibrillation treated with lifelong anti-coagulant therapy. Further studies are needed to confirm our results.
Acknowledgements
The authors thank Jane Charbonneau, DVM, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript and all the staff and participants of this study for their important contributions.
Funding
This study was supported by the grants from the Ministry of Science and Technology of China and the Prevention and Control Project of Major Chronic Non-infection Disease during the 13th 5-year plan period (No: 2016YFC1302000) and the Beijing Municipal Science and Technology Commission (D171100002917001).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Zheng Y, Rao CF, Chen SP, He L, Hou JF, Zheng Z. Surgical left atrial appendage occlusion in patients with atrial fibrillation undergoing mechanical heart valve replacement. Chin Med J 2020;133:1891–1899. doi: 10.1097/CM9.0000000000000967
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.cmj.org).
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation. Circulation 2014; 129:837–847. doi: 10.1161/circulation/aha.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996; 61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 3.Karim N, Ho SY, Nicol E, Li W, Zemrak F, Markides V, et al. The left atrial appendage in humans: structure, physiology, and pathogenesis. Europace 2020; 22:5–18. doi: 10.1093/europace/euz212. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol 1998; 82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 5.Squiers JJ, Edgerton JR. Surgical closure of the left atrial appendage: the past, the present, the future. J Atr Fibrillation 2018; 10:1642.doi: 10.4022/jafib.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016; 18:1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 7.Badhwar V, Rankin JS, Damiano RJ, Gillinov AM, Bakaeen FG, Edgerton JR, et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the surgical treatment of atrial fibrillation. Ann Thorac Surg 2017; 103:329–341. doi: 10.1016/j.athoracsur.2016.10.076. [DOI] [PubMed] [Google Scholar]
- 8.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol 2019; 74:104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Yao X, Gersh BJ, Holmes DR, Melduni RM, Johnsrud DO, Sangaralingham LR, et al. Association of surgical left atrial appendage occlusion with subsequent stroke and mortality among patients undergoing cardiac surgery. JAMA 2018; 319:2116–2126. doi: 10.1001/jama.2018.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman DJ, Piccini JP, Wang T, Zheng J, Malaisrie SC, Holmes DR, et al. Association between left atrial appendage occlusion and readmission for thromboembolism among patients with atrial fibrillation undergoing concomitant cardiac surgery. JAMA 2018; 319:365–374. doi: 10.1001/jama.2017.20125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martín Gutiérrez E, Castaño M, Gualis J, Martínez-Comendador JM, Maiorano P, Castillo L, et al. Beneficial effect of left atrial appendage closure during cardiac surgery: a meta-analysis of 280 585 patients. Eur J Cardiothorac Surg 2019; 57:252–262. doi: 10.1093/ejcts/ezz289. [DOI] [PubMed] [Google Scholar]
- 12.Ando M, Funamoto M, Cameron DE, Sundt TM. Concomitant surgical closure of left atrial appendage: a systematic review and meta-analysis. J Thorac Cardiovasc Surg 2018; 156:1071–1080. doi: 10.1016/j.jtcvs.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Atti V, Anantha-Narayanan M, Turagam MK, Koerber S, Rao S, Viles-Gonzalez JF, et al. Surgical left atrial appendage occlusion during cardiac surgery: a systematic review and meta-analysis. World J Cardiol 2018; 10:242–249. doi: 10.4330/wjc.v10.i11.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melduni RM, Schaff HV, Lee H, Gersh BJ, Noseworthy PA, Bailey KR, et al. Impact of left atrial appendage closure during cardiac surgery on the occurrence of early postoperative atrial fibrillation, stroke, and mortality. Circulation 2017; 135:366–378. doi: 10.1161/CIRCULATIONAHA.116.021952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnsrud DO, Melduni RM, Lahr B, Yao X, Greason KL, Noseworthy PA. Evaluation of anticoagulation use and subsequent stroke in patients with atrial fibrillation after empiric surgical left atrial appendage closure: a retrospective case-control study. Clin Cardiol 2018; 41:1578–1582. doi: 10.1002/clc.23066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee C, Kim JB, Jung S, Choo SJ, Chung CH, Lee JW. Left atrial appendage resection versus preservation during the surgical ablation of atrial fibrillation. Ann Thorac Surg 2014; 97:124–132. doi: 10.1016/j.athoracsur.2013.07.073. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AR, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11:R31.doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piccini JP, Sievert H, Patel MR. Left atrial appendage occlusion: rationale, evidence, devices, and patient selection. Eur Heart J 2017; 38:869–876. doi: 10.1093/eurheartj/ehw330. [DOI] [PubMed] [Google Scholar]
- 20.Elbadawi A, Olorunfemi O, Ogunbayo GO, Saad M, Elgendy IY, Arif Z, et al. Cardiovascular outcomes with surgical left atrial appendage exclusion in patients with atrial fibrillation who underwent valvular heart surgery (from the National Inpatient Sample Database). Am J Cardiol 2017; 119:2056–2060. doi: 10.1016/j.amjcard.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Fernandez MA, Perez-David E, Quiles J, Peralta J, Garcia-Rojas I, Bermejo J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol 2003; 42:1253–1258. doi: 10.1016/s0735-1097(03)00954-9. [DOI] [PubMed] [Google Scholar]
- 22.Grzymala-Lubanski B, Svensson PJ, Renlund H, Jeppsson A, Själander A. Warfarin treatment quality and prognosis in patients with mechanical heart valve prosthesis. Heart 2017; 103:198–203. doi: 10.1136/heartjnl-2016-309585. [DOI] [PubMed] [Google Scholar]
- 23.Butchart EG, Payne N, Li H, Buchan K, Mandana K, Grunkemeier GL. Better anticoagulation control improves survival after valve replacement. J Thorac Cardiovasc Surg 2002; 123:715–723. doi: 10.1067/mtc.2002.121162. [DOI] [PubMed] [Google Scholar]
- 24.Healey JS, Crystal E, Lamy A, Teoh K, Semelhago L, Hohnloser SH, et al. Left Atrial Appendage Occlusion Study (LAAOS): results of a randomized controlled pilot study of left atrial appendage occlusion during coronary bypass surgery in patients at risk for stroke. Am Heart J 2005; 150:288–293. doi: 10.1016/j.ahj.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 25.Aryana A, Singh SK, Singh SM, Gearoid O, Neill P, Bowers MR, Allen SL, et al. Association between incomplete surgical ligation of left atrial appendage and stroke and systemic embolization. Heart Rhythm 2015; 12:1431–1437. doi: 10.1016/j.hrthm.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Almahameed ST, Khan M, Zuzek RW, Juratli N, Belden WA, Asher CR, et al. Left atrial appendage exclusion and the risk of thromboembolic events following mitral valve surgery. J Cardiovasc Electrophysiol 2007; 18:364–366. doi: 10.1111/j.1540-8167.2006.00755.x. [DOI] [PubMed] [Google Scholar]
- 27.Juo Y, Lee Bailey K, Seo Y, Aguayo E, Benharash P. Does left atrial appendage ligation during coronary bypass surgery decrease the incidence of postoperative stroke? J Thorac Cardiovasc Surg 2018; 156:578–585. doi: 10.1016/j.jtcvs.2018.02.089. [DOI] [PubMed] [Google Scholar]
- 28.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J 2017; 38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 29.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Fleisher LA, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017; 135:e1159–e1195. doi: 10.1161/CIR.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 30.Lakkireddy D, Sridhar MA, Kanmanthareddy A, Lee R, Badhwar N, Bartus K, et al. Left atrial appendage ligation and ablation for persistent atrial fibrillation: the LAALA-AF registry. JACC Clin Electrophysiol 2015; 1:153–160. doi: 10.1016/j.jacep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Maybrook R, Pillarisetti J, Yarlagadda V, Gunda S, Sridhar ARM, Deibert B, et al. Electrolyte and hemodynamic changes following percutaneous left atrial appendage ligation with the LARIAT device. J Interv Card Electrophysiol 2015; 43:245–251. doi: 10.1007/s10840-015-0012-6. [DOI] [PubMed] [Google Scholar]
- 32.Lakkireddy D, Turagam M, Afzal MR, Rajasingh J, Atkins D, Dawn B, et al. Left atrial appendage closure and systemic homeostasis: the LAA HOMEOSTASIS study. J Am Coll Cardiol 2018; 71:135–144. doi: 10.1016/j.jacc.2017.10.092. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


