Abstract
Background
Uveal melanoma (UM) is the most common primary intraocular malignancy in adults. It has been demonstrated that microRNA-145 (miR-145) is correlated with the progression of various cancers by regulating the expression of multiple target genes, especially a number of genes that regulate angiogenesis and proliferation. However, the underlying mechanisms of miR-145 in tumor angiogenesis of UM are still not well illustrated. Thus, we aimed to explore the potential target genes or pathways regulated by miR-145 in UM and the effect of miR-145 on invasion and angiogenesis.
Methods
Totally, 24 choroid samples were collected in our study, including 12 UM samples and 12 normal uveal tissues. The expression of neuroblastoma RAS viral oncogene homolog (N-RAS), phosphorylated protein kinase B (p-AKT), and vascular endothelial growth factor (VEGF) in UM tissues and normal uveal tissues was analyzed using Western blotting analysis. Lentivirus expression system was used to construct MUM-2B and OCM-1 cell lines with stable overexpression of miR-145. Transwell and endothelial cell tube formation assay were used to measure the effects of miR-145 on the invasion and angiogenesis of UM in vitro. The downstream target genes of miR-145 were predicted by bioinformatics and confirmed using a luciferase assay. BALB/c nude mice models were established to investigate the mechanisms of miR-145 on tumor growth and angiogenesis in vivo. Group data comparisons were performed using analysis of Student's t test. A two-tailed P < 0.05 was considered as statistically significant.
Results
The results of Western blotting analysis indicated that the expressions of N-RAS (1.10 ± 0.35 vs. 0.41 ± 0.36, t = 3.997, P = 0.012), p-AKT (1.16 ± 0.22 vs. 0.57 ± 0.03, t = 7.05, P = 0.001), and VEGF (0.97 ± 0.32 vs. 0.45 ± 0.21, t = 3.314, P = 0.008) in UM tumor tissues were significantly higher than those in normal uveal tissue. Luciferase assay demonstrated N-RAS and VEGF as downstream targets of miR-145. Moreover, tube formation assay revealed that miR-145-transfected human microvascular endothelial cell line formed shorter tube length (36.10 ± 1.51 mm vs. 42.91 ± 0.94 mm, t = 6.603, P = 0.003) and less branch points (350.00 ± 19.97 vs. 406.67 ± 17.62, t = 3.685, P = 0.021) as compared with controls. In addition, the numbers of invaded MUM-2B and OCM-1 cells with miR-145 overexpression were significantly lower than the controls (35.7 ± 3.3 vs. 279.1 ± 4.9, t = 273.75, P < 0.001 and 69.5 ± 4.4 vs. 95.6 ± 4.7, t = 21.27, P < 0.001, respectively). In vivo, xenografts expressing miR-145 had smaller sizes (miR-145 vs. miR-scr, 717.41 ± 502.62 mm3vs. 1694.80 ± 904.33 mm3, t = 2.314, P = 0.045) and lower weights (miR-145 vs. miR-scr, 0.74 ± 0.46 g vs. 1.65 ± 0.85 g, t = 2.295, P = 0.045).
Conclusion
Our results indicated that miR-145 is an important tumor suppressor and the inhibitory strategies against N-RAS/VEGF signaling pathway might be potential therapeutic applications for UM in the future.
Keywords: Uveal melanoma, Vascular endothelial growth factor A, Neuroblastoma RAS viral oncogene homolog, microRNA-145, Angiogenesis
Introduction
Uveal melanoma (UM), which arises from melanocytes, is the most common primary intraocular malignancy in adults and is associated with the development of systemic metastases in over half of the patients.[1] Previous studies have revealed that increased angiogenesis is associated with higher metastasis and mortality rate in UM.[2] However, the precise molecular underpinnings are still unknown. Therefore, strategies based on antiangiogenic and antimetastatic mechanisms may provide potential treatment for UM. MicroRNAs (miRNAs) are a group of endogenous non-coding RNAs that regulate gene expression by repressing the stability of conventional mRNAs and/or inhibiting their translation into proteins.[3] Recent studies have demonstrated that miRNAs are differentially expressed in lung, nasopharyngeal, gastric and breast cancers, and may function as either oncogenes or tumor suppressors by controlling the expression of their target genes.[4–6] The overexpression of several miRNAs, such as miR-145 and miR-224–5p, has been demonstrated an inhibitory role in migration, proliferation, and invasion of UM cells.[7]
MicroRNA-145 (miR-145), a widely studied miRNA, is downregulated in various tumors.[8,9] In our previous study, we reported that miR-145 was downregulated in UM tissues and cell lines [10] In addition, both bioinformatics prediction and experimental results indicated that miR-145 might inhibit tumor angiogenesis and growth in UM. Angiogenesis is of great importance in tumorigenesis and tumor development.[11] Vascular endothelial growth factor (VEGF), regulated by Ras, is crucial in angiogenesis among all the angiogenic factors.[12] Thus, we may speculate that miR-145 regulates the downstream genes of Ras signaling, including neuroblastoma RAS viral oncogene homolog (N-RAS) and VEGF. Nevertheless, whether these genes or pathways are regulated by miR-145 in UM and the effect of miR-145 on invasion and angiogenesis still needs further study.
In this study, we aimed to explore the potential target genes or pathways regulated by miR-145 in UM and the effect of miR-145 on invasion and angiogenesis in vitro and in vivo.
Methods
Ethical approval
Animal studies were approved by the animal care committee (No. GSZE0199323) and clinical studies were approved by the ethics committee of Beijing Tongren Hospital of Capital Medical University (No.TRECKY2015-017). All UM patients provided written informed consent to this study.
Human tissue samples
Fresh UM tumor samples were obtained from Beijing Tongren Hospital, China, from November 2016 to December 2017. None of the UM patients were given radiation or chemotherapy therapy prior to the surgery. UM samples were collected at surgery, immediately frozen in liquid nitrogen and stored overnight after removal, and then were transferred to −80°C for storage until use. The normal uveal tissues were obtained from Tongren Eye Bank (Beijing, China).
Cell culture
Human UM cell lines (OCM-1 and MUM- 2B) were purchased from Procell (PROCELL Life Science & Technology Co., Ltd., Wuhan, China). MUM-2B and OCM-1 cells were maintained in RPMI 1640 (Gibco, California, USA) and Dulbecco's modified Eagle's medium (Gibco, California, USA), respectively, supplemented with 10% fetal bovine serum (HyClone, Logan, Utah, USA) in a humidified atmosphere at 37°C under 5% CO2. For experimental purposes, the cells were cultured in 35 or 60-mm plastic Petri dishes. Cells were seeded at a density of 3000–5000 cells/cm2, and experiments were initiated when the cells had reached subconfluence.
Western blotting analysis
Cell lysates were collected using RIPA lysis buffer (Shanghai Genechem Co., Ltd., China) supplemented with protease inhibitor and phosphatase inhibitor. The concentration of total protein was determined by the Pierce Coomassie (Bradford) Protein Assay Kit (Thermo Fisher Scientific, USA). Then, a total of 20 μg protein samples were electrophoresed on polyacrylamide gels and transferred to polyvinylidene fluoride membranes. The membranes were blocked with 5% fat-free milk powder in TBS-T (pH 7.6; 20 mmol/L Tris-HCl, 100 mmol/L NaCl, and 0.01% Tween 20) at room temperature for 2 hours and incubated with the primary antibodies targeting human mammalian target of rapamycin (mTOR), p-AKT, AKT (Cell Signaling Technology, USA), N-RAS, and VEGFA (Thermo Fisher Scientific, USA) overnight at 4°C. After washing three times, the membrane was hybridized with horseradish peroxidase-conjugated secondary antibody (dilution factor, 1:10,000) at room temperature for 2 hours. The concentrations of all antibodies used in this study were based on the manufacturer's instructions. The signal was developed using the Enhanced Chemiluminescence Plus kit. The images of Western blots were analyzed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) for densitometry calculation.
Target gene prediction
The miRNA target prediction was performed by miRanda (Memorial Sloan-Kettering Cancer Center, New York, USA) and TargetScan (Department of Biology, MA Institute of Technology, Cambridge, MA, USA) based on target sequence, structure, and function.
Lentivirus expression system
Viral supernatant was obtained by the transfection of HEK293T cells with pLemiR-145 or pLemiR-scrambled control (pLemiR-scr). For stable transfection of miR-145, MUM-2B and OCM-1 cells were initially plated at a density of 2 × 105 cells in a six-well plate for 24 hours and then infected with lentiviral particles using polybrene (8 mg/mL) (Sigma-Aldrich, Germany). After 24 hours of incubation at 37°C, the medium was removed and fresh medium was added. Stably transfected cells (miR-145 overexpressing or control) were selected using ampromycin (100 μg/mL, Invitrogen, Madison, Wisconsin, USA) for 4 days. The miR-145 positive colonies were identified by qRT-PCR.
Luciferase activity assay
The wild-type (WT) 3’untranslated region (3’-UTR) of NRAS or VEGFA containing the miR-145–5p binding sites was amplified and cloned downstream of the firefly luciferase gene in the pGL3 control vector (Promega, WI, USA). MiR-145–5p mimics or miR-NC together with pGL3-control vector carrying the WT or Mut 3’-UTR of NRAS or VEGFA were co-transfected into HEK 293T cells using Lipofectamine 2000 (Invitrogen). Luciferase activity was assessed using the Dual-Luciferase H Reporter Assay System (Promega) 48 hours after transfection.
Transwell and endothelial cell tube formation assays
OCM-1 (1 × 105) and MUM-2B (1 × 105) cells were seeded in each well with an 8-μm pore membrane in the upper chambers of transwell culture plates (Corning, Shanghai, China). After 16 hours of incubation, the non-invading cells on the upper chamber were scraped using a cotton swab, and the membranes were then fixed with 100% methanol and stained with 0.5% crystal violet. The cells attached to the lower surface were counted and imaged under an inverted microscope (Shanghai Caikon Optical Instrument Co., Ltd., China) at × 200 magnification in nine randomly chosen fields.
For the endothelial cell tube formation assay, 55-mL growth factor-reduced Matrigel was polymerized on 96-well plates. Human microvascular endothelial cells (HMEC-1) were serum-starved in medium for 2 hours. The cells were suspended in the medium extracted from each group, then added to the Matrigel-coated wells at a density of 3 × 104 cells/well and incubated at 37°C for 24 hours. Tube formation of the endothelial cell was defined as the appearance of intraluminal space inside the tube and the quantification of anti-angiogenic activity was calculated by measuring the tube length and the number of branch points under a light microscope with an image analyzer (Confocal Quantitative Image Cytometer CQ1 software, Japan).
Animal care and tumor implantation
For the in vivo tumor growth assay, 12 four-week-old female BALB/c nude mice (six per group), maintained in pathogen-free conditions with standard diets, were injected subcutaneously in the right side of the axilla with 3 × 106 OCM-1 cells transfected with miR-145–5p mimic vector or its negative control vector. Fifteen days after the injections, the tumor volumes and body weights of the animals were measured every 3 days. Mice were sacrificed and the xenografts were removed at the indicated time. All mice were sacrificed according to a standard procedure after 30 days, and the tumors were harvested and weighed. The volume of each tumor was calculated according to the formula V = π/6 × L × W × W (L and W are the length and the width of the tumor, respectively), measured with a sliding caliper. Analysis of the targets and signaling molecules, including N-RAS, AKT, p-AKT, mTOR, and VEGF, in the xenografts were examined by Western blotting.
Statistical analysis
All statistical analyses were performed using SPSS statistical software (version 25.0 for Windows, SPSS IBM, New York, USA). All experimental data are represented as mean ± standard deviation. Group data comparisons were performed using Student's t-test analysis. A two-tailed P < 0.05 was considered as statistically significant.
Results
Expression of N-RAS and VEGF in primary UM and normal controls
In total, 24 choroid samples were collected in our study, of which 12 were UM samples and 12 were normal uveal tissues. The UM patients and normal controls did not differ significantly in age or gender. No one has ciliary body involved in UM group. The mean tumor basal diameter was 15.14 ± 3.85 mm and mean tumor thickness was 12.55 ± 4.35 mm at diagnosis. Clinical and pathological information of UM samples used in current work is listed in Table 1. We examined the expression of N-RAS-VEGF pathway components, including N-RAS, AKT, p-AKT, mTOR, and VEGF, in UM tissues compared with normal controls. The results indicated that the expression of N-RAS (1.10 ± 0.35 vs. 0.41 ± 0.36, t = 3.997, P = 0.012), p-AKT (1.16 ± 0.22 vs. 0.57 ± 0.03, t = 7.05, P = 0.001) and VEGF (0.97 ± 0.32 vs. 0.45 ± 0.21, t = 3.314, P = 0.008) in UM tumor tissues was significantly higher than that in normal uveal tissue, as examined by Western blotting [Figure 1A and 1B].
Table 1.
Summary of clinical and pathological information of UM samples used in current work.
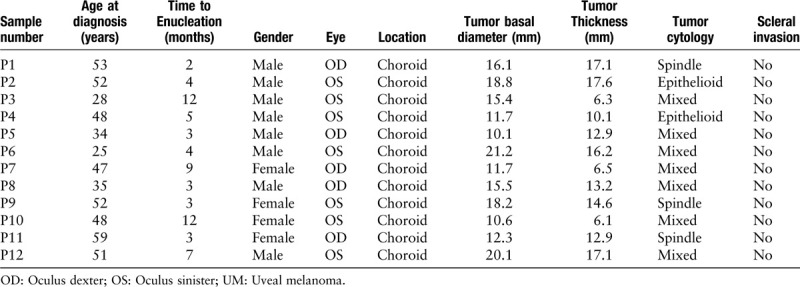
Figure 1.
Expression of N-RAS-VEGF pathway, including N-RAS, AKT, p-AKT, mTOR, and VEGF, in 12 UM tissues and 12 normal uveal tissue (normal control) examined by Western blotting. The β-actin level served as an internal control. ∗P < 0.05. AKT: Protein kinase B; mTOR: Mammalian target of rapamycin; N-RAS: neuroblastoma RAS viral oncogene homolog; p-AKT: phosphorylated protein kinase B; VEGF: Vascular endothelial growth factor.
MiR-145-5p directly targets N-RAS and VEGF
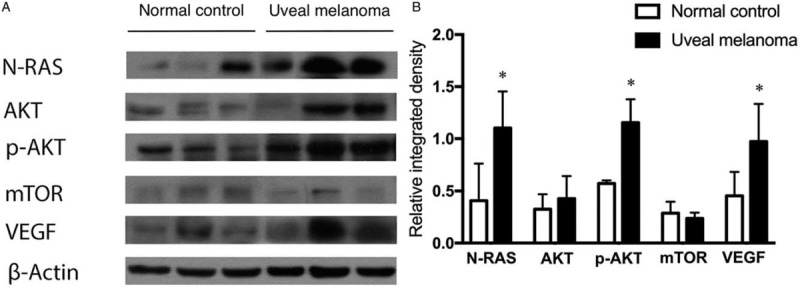
In our previous study,[10] we demonstrated that miR-145-5p was significantly downregulated in UM tissues compared with normal uveal tissues. Thus, it could be speculated that miR-145-5p might directly modulate N-RAS and VEGF expression in UM. To further understand the molecular mechanism of miR-145 in inhibiting angiogenesis and cell invasion, we searched for potential targets of miR-145 using miRanda (Memorial Sloan-Kettering Cancer Center, New York) and TargetScan (Department of Biology, MA Institute of Technology, Cambridge, MA). We found that NRAS and VEGFA could be the potential targets of miR-145 [Figure 2A]. To validate these miR-145 targets, luciferase activity assays were performed in HEK 293T cells. The luciferase activities of vectors carrying wild type VEGFA 3’-UTR (WT) and NRAS 3’-UTR (WT) were attenuated by co-transfection with miR-145-5p, while there was no significant difference in groups transfected with mutant 3’-UTR of VEGFA or NRAS and the negative control of miR-145-5p [Figure 2B]. These data demonstrated that VEGFA and NRAS might be the direct targets of miR-145-5p.
Figure 2.
NRAS and VEGFA were directly targeted by miR-145. (A) Predicted miR-145-5p binding site in NRAS 3’ UTR and VEGFA 3’ UTR and miR-145-5p sequence. (B) The effect of miR-145 on the luciferase activity assays of NRAS 3’ UTR and VEGFA 3’ UTR or NRAS 3’ UTR and VEGFA 3’ UTR with a mutated miR-145-5p binding site (mut). Overexpressed miR-145-5p resulted in a significant decrease in luciferase activity of vectors carrying NRAS 3’ UTR and VEGFA 3’ UTR, while there was no significant difference in groups transfected with mutant 3’-UTR of VEGFA or NRAS and negative control. ∗P < 0.001. miR: microRNA; NRAS: neuroblastoma RAS viral oncogene homolog; UTR: Untranslated regions; VEGFA: Vascular endothelial growth factor A.
MiR-145 inhibits angiogenesis and invasion in vitro
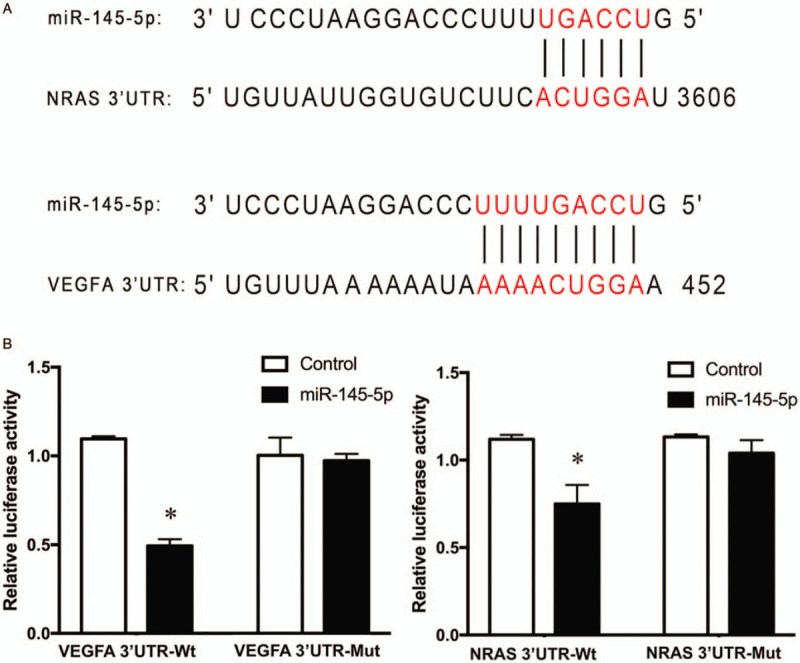
Since our data demonstrated that VEGFA and N-RAS were directly targeted genes regulated by miR-145-5p, we next investigated the effects of miR-145 on tumor angiogenesis and invasion in vitro. A tube formation assay using endothelial cells was performed and the quantification of angiogenic activity was calculated by measuring the tube length and number of branch points under a light microscope. The results showed that miR-145-transfected HMEC-1 cells formed shorter tube length (36.10 ± 1.51 mm vs. 42.91 ± 0.94 mm, t = 6.603, P = 0.003) and decreased branch points (350.00 ± 19.97 vs. 406.67 ± 17.62, t = 3.685, P = 0.021) as compared with controls, indicating a decreased angiogenic activity [Figure 3A]. Transwell assays were performed to investigate the effects of upregulation of miR-145-5p on the invasion of UM cells in vitro. MUM-2B and OCM-1 cells were transfected with miR-145-5p, which was confirmed by qPCR assay [Figure 3B]. The numbers of invaded MUM-2B and OCM-1 cells with miR-145 overexpression were significantly lower than the controls (35.7 ± 3.3 vs. 279.1 ± 4.9, t = 273.75, P < 0.001 and 69.5 ± 4.4 vs. 95.6 ± 4.7, t = 21.27, P < 0.001, respectively). These results suggested that cell invasion ability was significantly decreased by overexpression of miR-145 in MUM-2B and OCM-1 cells [Figure 3C and Figure 3D].
Figure 3.
Inhibiting effects of tumor angiogenesis and invasion caused by increasing expression of miR-145 in vitro. (A) Tube formation assay of HMEC-1 (original magnification, × 80). Significant decrease of tube length and branch points was observed in the cells transfected with miR-145 precursor when comparing with the scrambled control (miR-scr). ∗P < 0.05. †P < 0.01. (B) Relative miR-145 expression in MUM-2B and OCM-1 cells transfected with miR-scr and miR-145. P < 0.001. (C and D) Transwell assays of MUM-2B and OCM-1 cells transfected with miR-scr and miR-145 (original magnification, × 200). Cell invasion ability was significantly decreased by overexpression of miR-145-5p in MUM-2B and OCM-1 cells. ∗P < 0.001. HMEC-1: Human microvascular endothelial cell line; miR: microRNA; miR-scr: microRNA-scrambled control.
MiR-145 inhibits tumor growth and angiogenesis in vivo
To investigate the effect of miR-145 on tumor growth and angiogenesis in vivo, we injected lentivirus-miR-scr- and lentivirus-miR-145-transduced OCM-1 cells into nude mice. We found that miR-145 expression in UM cells significantly reduced xenograft tumor growth and angiogenesis compared with those transfected with empty vector (mock). At 30 days after subcutaneous injection, xenografts expressing miR-145 had smaller sizes (miR-145 vs. miR-scr, 717.41 ± 502.62 mm3vs. 1694.80 ± 904.33 mm3, t = 2.314, P = 0.045) and lower weights (miR-145 vs. miR-scr, 0.74 ± 0.46 g vs. 1.65 ± 0.85 g, t = 2.295, P = 0.045) [Figure 4A and 4B]. In addition, the expression of N-RAS (0.20 ± 0.09 vs. 0.70 ± 0.09, t = 9.715, P < 0.001), p-AKT (0.18 ± 0.09 vs. 0.72 ± 0.11, t = 9.304, P < 0.001), m-TOR (0.07 ± 0.02 vs. 0.40 ± 0.02, t = 27.116, P < 0.001) and VEGF (0.36 ± 0.07 vs. 0.76 ± 0.29, t = 3.327, P = 0.018) were also reduced in stable miR-145 precursor transfected xenografts compared with controls, as examined by Western blotting. [Figure 4C].
Figure 4.
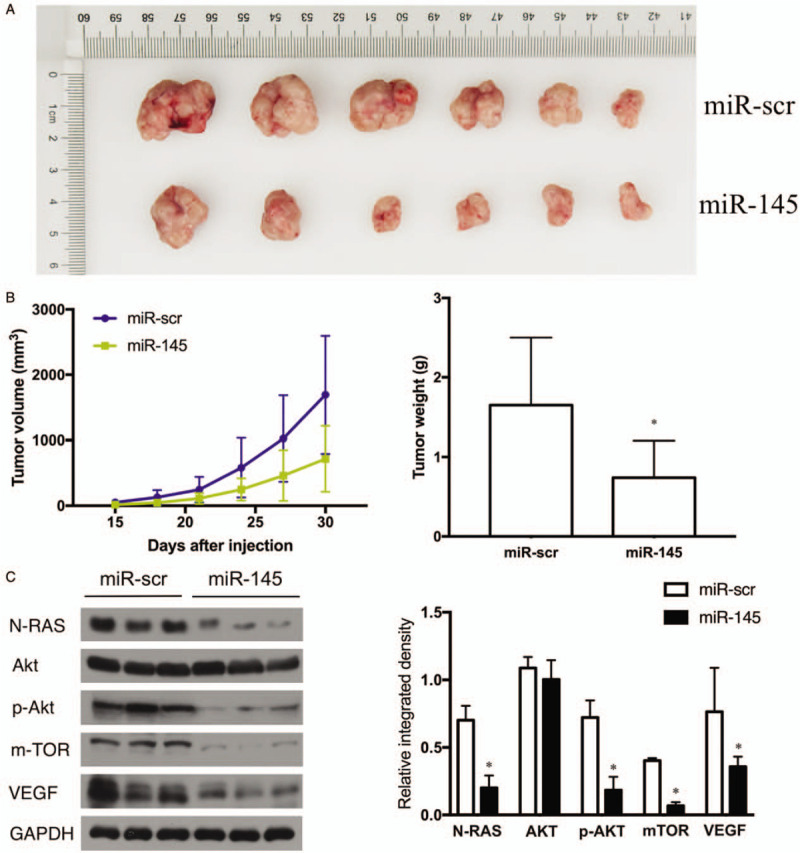
Inhibiting effects of tumor angiogenesis and invasion caused by increasing expression of miR-145 in vivo. (A) At 30 days after subcutaneous injection, all mice were sacrificed and the tumors were harvested, photographed, measured, and weighed. (B) The volume of each tumor was measured every 3 days, n = 6. ∗P < 0.05. (C) Analysis of the targets and signaling molecules, including N-RAS, AKT, p-AKT, mTOR, and VEGF were examined by Western blotting. GAPDH level served as an internal control. ∗P < 0.05. AKT: protein kinase B; GADPH: Glyceraldehyde-3-phosphate dehydrogenase; miR: microRNA; miR-scr: microRNA-scrambled control; mTOR: mammalian target of rapamycin; N-RAS: neuroblastoma RAS viral oncogene homolog; p-AKT: phosphorylated protein kinase B; VEGF: Vascular endothelial growth factor.
Discussion
It is well known that VEGF is produced by cells stimulating vasculogenesis and angiogenesis. Overexpression of VEGF could lead to disease when blood circulation is inadequate. Tumors cannot grow beyond a limited size without an adequate blood supply, while VEGF can promote endothelial cell proliferation and play a significant role in tumor angiogenesis and growth.[13,14] The VEGF gene has been demonstrated to be correlated with the invasion and metastasis of various cancers including UM.[15–17] In the current study, we investigated whether the VEGF and N-RAS-VEGF pathway components, including N-RAS, AKT, p-AKT, and mTOR, are involved in UM tissues. The results indicated that the expression of N-RAS, p-AKT, and VEGF in UM tumor tissues was significantly higher than that in normal uveal tissue, as examined by Western blotting. The results of our study were in agreement with former studies showing that VEGF-A is overexpressed in several UM cell lines, including MEL-270, OM-431, OMM-2.3, and OMM-2.5.[15,18,19]
It has been documented that the expression of miR-145 in vascular smooth muscle cells has specific connection with the proliferation of abnormal vascular intima.[20] MiR-145 is correlated with the progression of various cancers by regulating the expression of multiple target genes, especially a number of genes that regulate angiogenesis and proliferation.[21–23] Our previous work showed that the expression of miR-145 was significantly lower in UM tissues than normal uveal samples. Overexpression of miR-145 suppressed cell proliferation by blocking cells in G1 phase from entering S phase and promoted cell apoptosis in UM cells.[10] However, the underlying mechanisms by which miR-145–5p regulates UM angiogenesis remain unknown. To investigate the function of miR-145 in the invasion and metastasis of the cells, we performed transwell invasion assays and endothelial cell tube formation assays. The results revealed that the overexpression of miR-145 could inhibit the invasion and angiopoiesis of UM cells. The inhibitory effects of miR-145-5p on tumor cell lines have also been confirmed in several studies. Fan et al.[14] reported that VEGF expression in the miR-145 transfected group was significantly downregulated compared with that in the blank group in osteosarcoma cells. Boufraqech et al [24] suggested that miR-145 could regulate thyroid cancer growth by mediating the PI3K/Akt pathway and may serve as a useful diagnostic marker for thyroid cancer diagnosis. Therefore, miR-145 may be used as a potential diagnostic biomarker that plays an important role as a tumor suppressor in UM.
To further investigate the effect of miR-145 on tumor growth and angiogenesis in vivo, we injected lentivirus-miR-scr- and lentivirus-miR-145-transduced OCM-1 cells into nude mice. We found that xenografts expressing miR-145 had smaller sizes and lower weights. In addition, the expression of p-AKT, m-TOR, and VEGF was also reduced by stable transfection of the miR-145 precursor. Furthermore, we constructed luciferase-UTR vectors carrying VEGF 3’ UTR and N-RAS 3’ UTR as well as their corresponding mutants. The bioinformatic analysis results indicated that VEGF and N-RAS might be the direct targets of miR-145-5p in UM angiogenesis. These results were consistent with the previous findings. Shi et al [25] have revealed IGF-I/IRS1 as direct targets of miR-145 in colon cancer, indicating the complex regulatory network of this miRNA in tumors. It is also been demonstrated that miR-145 could suppress tumor angiogenesis and growth by targeting NRAS signaling in breast cancer and colorectal cancer.[26,27] The observed different effects of miR-145-5p in diverse tumors are very promising for application as diagnostic or prognostic biomarkers. To better understand the miR-145-mediated network on tumor angiogenesis, emphasis should be on searching for more target genes and exploring potential target therapies. When the results of our study are discussed, its limitations should be considered. First, although the ration of the tumor growth is higher in subcutaneous xenograft models and the lesions are easy to be removed, inoculated xenograft models (such as anterior chamber injection) could provide specific features of the ocular immune system leading to an intraocular tumor microenvironment. Further research on intraocular xenograft models is strongly needed, so that we can better understand the unique characteristics of UM. Second, since liver metastases occur in 95% of patients with metastatic UM, liver metastasis mouse models may offer a more detailed investigation of the biological behavior of metastatic UM cells. Such issues are expected to be addressed in our future work.
In conclusion, our results suggest that miR-145 suppresses tumor growth and invasion by directly targeting the N-RAS and VEGF signaling pathways in UM. The findings of our study also have identified miR-145 as an anti-angiogenic factor both in vitro and in vivo, which could have important implications for further understanding the signaling mechanisms involved in regulating UM angiogenesis. Accordingly, there is a good reason to speculate that miR-145 is an important tumor suppressor and the inhibitory strategies against the N-RAS/VEGF signaling pathway might be potential therapeutic applications for UM in the future.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81570891), Beijing Natural Science Foundation (No. 7151003), Beijing Municipal Administration of Hospitals’ Ascent Plan (No. DFL20150201), Advanced Health Care Professionals Development Project of Beijing Municipal Health Bureau (No. 2014-2-003), The Capital Health Research and Development of Special (No. 2016-1-2051), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. ZYLX201307), and Science & Technology Project of Beijing Municipal Science & Technology Commission (Nos. Z181100001818003 and Z151100001615052).
Conflicts of interest
None.
Footnotes
How to cite this article: Yang JY, Li Y, Wang Q, Zhou WJ, Yan YN, Wei WB. MicroRNA-145 suppresses uveal melanoma angiogenesis and growth by targeting neuroblastoma RAS viral oncogene homolog and vascular endothelial growth factor. Chin Med J 2020;133:1922–1929. doi: 10.1097/CM9.0000000000000875
References
- 1.Fry MV, Augsburger JJ, Corrêa ZM. Clinical Features, metastasis, and survival in patients younger than 21 years with posterior uveal melanoma. JAMA Ophthalmol 2019; 137:75–81. doi: 10.1001/jamaophthalmol.2018.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brouwer NJ, Gezgin G, Wierenga APA, Bronkhorst IHG, Marinkovic M, Luyten GPM, et al. Tumour angiogenesis in uveal melanoma is related to genetic evolution. Cancers (Basel) 2019; 11:pii: E979.doi: 10.3390/cancers11070979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Babaei K, Shams S, Keymoradzadeh A, Vahidi S, Hamami P, Khaksar R, et al. An insight of microRNAs performance in carcinogenesis and tumorigenesis; an overview of cancer therapy. Life Sci 2020; 240:117077.doi: 10.1016/j.lfs.2019.117077. [DOI] [PubMed] [Google Scholar]
- 4.Hata A, Lieberman J. Dysregulation of microRNA biogenesis and gene silencing in cancer. Sci Signal 2015; 8:re3.doi: 10.1126/scisignal.2005825. [DOI] [PubMed] [Google Scholar]
- 5.Sandoval-Bórquez A, Polakovicova I, Carrasco-Véliz N, Lobos-González L, Riquelme I, Carrasco-Avino G, et al. MicroRNA-335-5p is a potential suppressor of metastasis and invasion in gastric cancer. Clin Epigenetics 2017; 9:114.doi: 10.1186/s13148-017-0413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantini L, Bertoli G, Cava C, Dubois T, Zinovyev A, Caselle M, et al. Identification of microRNA clusters cooperatively acting on epithelial to mesenchymal transition in triple negative breast cancer. Nucleic Acids Res 2019; 47:2205–2215. doi: 10.1093/nar/gkz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu S, Chen H, Han N, Zhang C, Yan H. Long noncoding RNA PVT1 silencing prevents the development of uveal melanoma by impairing MicroRNA-17-3p-dependent MDM2 upregulation. Invest Ophthalmol Vis Sci 2019; 60:4904–4914. doi: 10.1167/iovs.19-27704. [DOI] [PubMed] [Google Scholar]
- 8.Zeinali T, Mansoori B, Mohammadi A, Baradaran B. Regulatory mechanisms of miR-145 expression and the importance of its function in cancer metastasis. Biomed Pharmacother 2019; 109:195–207. doi: 10.1016/j.biopha.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Xu WX, Liu Z, Deng F, Wang DD, Li XW, Tian T, et al. MiR-145: a potential biomarker of cancer migration and invasion. Am J Transl Res 2019; 11:6739–6753. [PMC free article] [PubMed] [Google Scholar]
- 10.Yang L, Qiming H, Xuehui S, Xiang J, Li S, Xiaolin X, et al. MicroRNA 145 may play an important role in uveal melanoma cell growth by potentially targeting insulin receptor substrate-1. Chin Med J 2014; 127:1410–1416. doi: 10.3760/cma.j.issn.0366-6999.20133206. [PubMed] [Google Scholar]
- 11.Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: is there a role for combinations with immunotherapy? Angiogenesis 2017; 20:185–204. doi: 10.1007/s10456-017-9552-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siveen KS, Prabhu K, Krishnankutty R, Kuttikrishnan S, Tsakou M, Alali FQ, et al. Vascular endothelial growth factor (VEGF) signaling in tumour vascularization: potential and challenges. Curr Vasc Pharmacol 2017; 15:339–351. doi: 10.2174/1570161115666170105124038. [DOI] [PubMed] [Google Scholar]
- 13.Jain L, Vargo CA, Danesi R, Sissung TM, Price DK, Venzon D, et al. The role of vascular endothelial growth factor SNPs as predictive and prognostic markers for major solid tumors. Mol Cancer Ther 2009; 8:2496–2508. doi: 10.1158/1535-7163.MCT-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan L, Wu Q, Xing X, Wei Y, Shao Z. MicroRNA-145 targets vascular endothelial growth factor and inhibits invasion and metastasis of osteosarcoma cells. Acta Biochim Biophys Sin (Shanghai) 2012; 44:407–414. doi: 10.1093/abbs/gms019. [DOI] [PubMed] [Google Scholar]
- 15.El Filali M, Missotten GSOA, Maat W, Ly LV, Luyten GPM, Van d VPA, et al. Regulation of VEGF-A in Uveal Melanoma. Investigative Opthalmology & Visual Science 2010; 51:2329.doi: 10.1167/iovs.09-4739. [DOI] [PubMed] [Google Scholar]
- 16.Barak V, Pe’er, Jacob, Kalickman I, Frenkel S. VEGF as a Biomarker for Metastatic Uveal Melanoma in Humans. Current Eye Research 2011; 36:386–390. doi: 10.3109/02713683.2010.534573. [DOI] [PubMed] [Google Scholar]
- 17.Filali ME, Velden PAVD, Luyten GPM, Jager MJ. Anti-angiogenic therapy in uveal melanoma. Dev Ophthalmol 2012; 49:117–136. doi: 10.1159/000329591. [DOI] [PubMed] [Google Scholar]
- 18.Notting IC, Missotten GSOA, Sijmons B, Boonman ZFHM, Keunen JEE, Gabriel VDP. Angiogenic profile of uveal melanoma. Curr Eye Res 2006; 31:775–785. doi: 10.1080/02713680600865052. [DOI] [PubMed] [Google Scholar]
- 19.Koch KR, Refaian N, Hos D, Schlereth SL, Bosch JJ, Cursiefen C, et al. Autocrine impact of VEGF-A on uveal melanoma cells. Invest Ophthalmol Vis Sci 2014; 55:2697–2704. doi: 10.1167/iovs.13-13254. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res 2009; 105:158–166. doi: 10.1161/circresaha.109.197517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Gong J, Zeng H, Chen N, Huang R, Huang Y, et al. MicroRNA145 targets BNIP3 and suppresses prostate cancer progression. Cancer Res 2010; 70:2728–2738. doi: 10.1158/0008-5472.can-09-3718. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, Yang F, Qi X, Li Q, Wang D, Yi T, et al. LncRNA DANCR promotes tumor growth and angiogenesis in ovarian cancer through direct targeting of miR-145.Mol. Mol Carcinog 2019; 58:2286–2296. doi: 10.1002/mc.23117. [DOI] [PubMed] [Google Scholar]
- 23.Docrat TF, Nagiah S, Krishnan A, Naidoo DB, Chuturgoon AA. Atorvastatin induces MicroRNA-145 expression in HEPG2 cells via regulation of the PI3K/AKT signalling pathway. Chem Biol Interact 2018; 287:32–40. doi: 10.1016/j.cbi.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Boufraqech M, Zhang L, Jain M, Patel D, Ellis R, Xiong Y, et al. miR-145 suppresses thyroid cancer growth and metastasis and targets AKT3. Endocr Relat Cancer 2014; 21:517–531. doi: 10.1530/ERC-14-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi B, Sepp-Lorenzino L, Prisco M, Linsley P, Deangelis T, Baserga R. Micro RNA 145 targets the insulin receptor substrate-1 and inhibits the growth of colon cancer cells. J Biol Chem 2007; 282:32582–32590. doi: 10.1074/jbc.m702806200. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Gao G, Yan D, Chen X, Yao X, Guo S, et al. Effects of miR-145-5p through NRAS on the cell proliferation, apoptosis, migration, and invasion in melanoma by inhibiting MAPK and PI3K/AKT pathways. Cancer Med 2017; 6:819–833. doi: 10.1002/cam4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Yin Y, Yan ZP, Lu NN, Xu Q, He J, Qian X, et al. Downregulation of miR-145 associated with cancer progression and VEGF transcriptional activation by targeting N-RAS and IRS1. Biochim Biophys Acta 2013; 1829:239–247. doi: 10.1016/j.bbagrm.2012.11.006. [DOI] [PubMed] [Google Scholar]


