Abstract
Background
The casein kinase 2-interacting protein-1 (CKIP-1) is important in the development of osteoblasts and cardiomyocytes. However, the effects of CKIP-1 on osteoblast precursor mesenchymal stem cells (MSCs) remain unclear. This study aimed to determine whether CKIP-1 affects osteogenic differentiation in MSCs and explore the relationship of CKIP-1 and inflammation.
Methods
Bone marrow MSCs of CKIP-1 wild type (WT) and knockout (KO) mice were cultivated in vitro. Cell phenotype was analyzed by flow cytometry, colony formation was detected to study the proliferative ability. Osteogenic and adipogenic induction were performed. The osteogenic ability was explored by alizarin red staining, alkaline phosphatase (ALP) staining and ALP activity detection. Quantitative real-time polymerase chain reaction (qRT-PCR) was carried out to determine the mRNA expression levels of osteoblast marker genes. The adipogenic ability was detected by oil red O staining. Content of the bone was analyzed to observe the differences of bone imaging parameters including trabecular bone volume/tissue volume (BV/TV), bone surface area fraction/trabecular BV, trabecular number (Tb.N), and trabecular spacing (Tb.sp). Interleukin (IL)-1β was injected on WT mice of 2 months old and 18 months old, respectively. Difference in CKIP-1 expression was detected by RT-PCR and western blot. The relationship between CKIP-1 and inflammation was explored by RT-PCR and western blot.
Results
ALP assays, alizarin red staining, and qRT-PCR showed that MSCs derived from CKIP-1 KO mice exhibited a stronger capability for osteogenesis. Micro-computed tomography detection showed that among 18-month-old mice, CKIP-1 KO mice presented significantly higher bone mass compared with WT mice (P = 0.02). No significant difference was observed in 2-month-old mice. In vivo data showed that expression of CKIP-1 was higher in the bone marrow of aging mice than in young mice (4.3-fold increase at the mRNA level, P = 0.04). Finally, the expression levels of CKIP-1 in bone marrow (3.2-fold increase at the mRNA level, P = 0.03) and cultured MSCs were up-regulated on chronic inflammatory stimulation by IL-1β.
Conclusions
CKIP-1 is responsible for negative regulation of MSC osteogenesis with age-dependent effects. Increasing levels of inflammation with aging may be the primary factor responsible for higher expression levels of CKIP-1 but may not necessarily affect MSC aging.
Keywords: Bone mesenchymal stem cell, Casein kinase 2-interacting protein-1, Interleukin-1β, Osteogenesis
Introduction
The mechanism behind the regeneration of mesodermal and neural crest-derived structural or connective tissues, such as bone, cartilage, muscles, and tendons, continues to be widely pursued due to their cellular properties. These structural tissues are generally homogeneous and comprise a predominant single-cell type or a limited number of cells contributing to the composition of the tissue.[1] Furthermore, precursors to their respective mature cell types can be found in adult tissues. These precursor cells, such as mesenchymal stem cells (MSCs), are generally multi-potent and can differentiate into a variety of lineages, including osteoblastic, chondrogenic, and adipogenic lineages. MSCs have no immunogenic effects and may be used to replace damaged tissues.[2]
MSCs found in bone marrow and adipose tissues are common precursor cells which are capable of differentiating into osteoblasts and adipocytes, respectively.[3] Several transcription factors and extracellular/intra-cellular signals that regulate osteoblastogenesis and adipogenesis have been identified. For instance, the wingless/integrated (Wnt)/β-catenin pathway has been shown to induce osteoblastogenesis and inhibit adipogenesis, whereas the peroxisome proliferator-activated receptor-γ is a potent inducer of adipogenesis and an inhibitor of osteoblastogenesis.[4] Osteoblast differentiation and bone development are regulated by a network of molecular signals and transcription factors induced by several proteins, including bone morphogenetic protein 2, osterix, and runx family transcription factor 2 (Runx2).[5]
Casein kinase 2-interacting protein-1 (CKIP-1), known as pleckstrin homology domain containing O1 (PLEKHO1), was originally identified as an interacting protein of the casein kinase 2 (CK2) kinase.[6]CKIP-1 is a negative regulator of osteogenesis. CKIP-1 interacts with SMAD-specific E3 ubiquitin protein ligase 1 (Smurf1), resulting in an increase in its E3 ligase activity. CKIP-1 specifically targets the linker region between the WW domains of Smurf1, thereby augmenting its affinity for and promoting the ubiquitination of Smurf1.[7]Smurf1, a C2-WW-HECT-domain E3, is crucial for bone homeostasis and suppresses osteoblast activity. Previous reports have shown that ribonucleic acid (RNA) interference-mediated CKIP-1 depletion in osteoblasts markedly promoted bone formation, enhanced bone micro-architecture, and increased whole bone mass in both healthy and osteoporotic rats, suggesting that CKIP-1 may be a potential therapeutic target in treating osteoporosis.[8]
MSCs derived from various sources, such as bone marrow or fat, have been expanded in cultures and differentiated in vitro into several lineages.[9] However, clinical experience and animal studies have shown that the regeneration potential of bone and other tissues declines in an age-dependent manner.[10] Age-related changes in MSCs may account for delayed regeneration and for the limited quality of the regenerated tissue in aging patients. Indeed, the aging of stem and progenitor cells has been suggested to affect the aging of tissues and whole organisms.[11] Regarding the differentiation potential of MSCs, they lose osteogenic potential in favor of adipogenic potential.[12] Further characteristics, such as the number of colony-forming units, telomere length, and telomerase activity, have been examined with a similar heterogeneity.[13]
In the stem-cell niche, the distortion of chronic inflammation can lead to the accumulation of fat deposition in bone and muscle tissue.[14] MSCs are multi-potent stem cells but can also specifically interact with a large variety of immune cells.[15] This study evaluated the effect of CKIP-1 on bone mass in aging mice and investigated whether MSCs had a regulating role in osteogenesis. Furthermore, it detected the association between CKIP-1 and aging and studied the factors of aging that regulate the CKIP-1 effects in mice.
Methods
Ethical approval
This study was approved by the Ethics Committee of Chinese People's Liberation Army (PLA) General Hospital (No. S2018-213-01).
Animals
CKIP-1 wild type (WT) and knockout (KO) C57BL/6J mice were provided by the Laboratory Animal Center of the Academy of Military Medical Sciences.[8] The MSCs used in this study originated from gender-matched 2-month-old (2M) CKIP-1 WT and KO mice.
Cell culture and identification
2M WT and CKIP-1 KO mice were executed by cervical dislocation. The tibias and femurs were immediately dissected from the attached muscles and tissues using aseptic techniques. The ends of the bones were removed, marrow plugs were flushed by repeated pipetting, and the cells were forcefully passed through a 19-gauge needle to obtain a single-cell suspension. The cells were cultured in a growth medium containing α-minimum eagle's medium (α-MEM; Invitrogen, Beijing, China) made of 10% fetal bovine serum and 1% penicillin. The cell suspensions were seeded in 10-cm tissue culture dishes and cultured in growth medium in a humidified atmosphere of 5% CO2 at 37°C. The culture media were changed every 2 to 3 days to remove non-adherent cells, and adherent cells were grown until they were confluent. At last, MSCs were sub-cultured after digestion with 0.25% trypsin and 1 mol/L ethylenediamine tetraacetic acid (EDTA) (Yaji Bio-technology Co., Ltd., Shanghai, China). A fluorescence-activated cell sorting analysis was used to analyze the expression of CD44, CD90, CD106, CD34, and CD45 (R&D Systems, Fushen Bio-technology Co., Ltd., Shanghai, China) according to the manufacturer's protocols.
Osteogenesis and adipogenesis
For the quantitative analysis of osteogenic induction, alkaline phosphatase (ALP) activity was assayed, and the suspensions of MSCs were seeded into 96-well plates at a density of 1 × 103 cells per well. After 7 days of osteogenic induction, the ALP activity of the MSCs was detected with a commercially available assay kit (Zhong Sheng Biochemical Sciences Co., Ltd., Guangzhou, China). After collecting the culture media, the cells were washed three times in phosphate-buffered saline (PBS) and incubated in 3% albumin bovine serum-0.1%Triton X-100 in PBS overnight at 4°C. Then, 100 μL of p-nitrophenol phosphate substrate solution was added to each well, and the plate was incubated for 40 min at 37°C. Then, the reaction was stopped with 0.1 mol/L NaOH. The optical density value was measured at 405 nm in a spectrophotometer using a micro-plate reader. After 21 days of osteogenic induction, mineral nodule formation was detected by staining the cultures with 2% alizarin red.
Similarly, WT and CKIP-1 KO MSCs of the same number were suspended in the adipogenic induction medium and re-seeded at the same density (1 × 104 cells/cm2). The adipogenic induction medium consisted of α-MEM with 10% fetal bovine serum (Hyclone, Logan, UT, USA), 10–6 mol/L dexamethasone (DEX) (Gibco, Gaithersburg, MD, USA), 500 mmol/L 3-isobutyl-1-methylxanthine (IBMX) (Gibco), and 10 mg/mL insulin (Fuzhou Maixin Biotech. Co., Fuzhou, China). The medium was changed every 2 days. Oil red O staining was performed as described before.[16] The adipogenesis assays of MSCs were performed in 48-well plates for adipocyte quantification. After adipogenic induction, the oil red O-positive cells of each group were counted in each well of the 48-well plate (in triplicate per group).
Micro-computed tomography
Micro-computed tomography (micro-CT) imaging analysis was performed as described in a previous study.[17] In brief, trabecular bone volume (BV)/tissue volume (TV) and the bone mineral density (BMD) of the femur were measured with a Scanco micro-CT 40 (Scanco Medical AG, Brüttisellen, Switzerland). The bones were placed vertically in 12-mm-diameter scanning holders and scanned at the following settings: 12-μm resolution, 55-kV energy, 145-μA intensity, and an integration time of 200 ms.
Interleukin-1β stimulation and injection treatment
MSCs were cultured with 10 ng/mL interleukin-1β (IL-1β) (BBI, Markham, Ontario, Canada) in vitro to detect the effect of inflammation on CKIP-1. When the cells were confluent, quantitative real-time polymerase chain reaction (qRT-PCR) and western blot were used to detect the expression of CKIP-1. In vivo, 2M mice were injected intra-peritoneally twice a week with 50 μg/kg body weight of IL-1β as the treatment group. Another group was also injected with vehicle control (PBS) for 12 weeks as the sham group. The mice were sacrificed by cervical dislocation, and all femurs were intactly isolated. The left femurs were used for micro-CT scanning.
Real-time reverse transcription-PCR of mRNA expression
The cells were harvested, and total RNA was isolated from renal tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer's protocols. For the reverse transcription of mRNA, cDNAs were synthesized from 2 mg of total RNA using a PrimeScript RT reagent kit (TaKaRa Biochemical Sciences Co., Ltd., Dalian, China). RT-PCR was performed using 2 μL of cDNA product in a 25-μL reaction volume with a 7500 RT-PCR System (Applied Biosystems). SYBR PremixExTaqII (TaKaRa Biochemical Sciences Co., Ltd.), specific primers, and 2 μL of cDNA were used in each PCR (95°C for 30 s, 40 cycles of denaturation at 94°C for 5 s, and annealing and extension at 60°C for 30 s). Sense and antisense primers were designed with Primer Express 5.0 based on published cDNA sequences. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control gene. All RT-PCR reactions were performed six times.
Western blot analysis
The cells were harvested in radioimmunoprecipitation assay (RIPA) lysis buffer (Beyotime Biochemical Sciences Co., Ltd., Shanghai, China). The whole cell protein extracts were quantified using bicinchoninic acid (BCA) assays. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. They were then blocked in 5% bovine serum albumin in Tris Buffered saline Tween (TBST) and hybridized with primary mouse β-actin antibody (1:2000; Beyotime Biochemical Sciences Co., Ltd.), primary rabbit β-tubulin antibody (1:2000; Abcam), or primary rabbit CKIP-1 antibody (1:100; Beyotime Biochemical Sciences Co., Ltd.). β-Actin was used as a loading control. Signals were displayed after incubation with anti-mouse immunoglobulin G secondary antibody (1:2000) connected with peroxidase using an electrochemiluminescence assay. The relative band intensities in the scanned images were analyzed with ImageJ software. Western blot experiments were performed in at least triplicate.
Statistical analyses
All statistical analyses were performed using SPSS24.0 (SPSS Inc., Chicago, IL, USA). Results conformed to normal distribution were expressed as mean ± standard deviation. Comparisons between groups (CKIP-1 KO group vs. WT group, young group vs. old group) were done using Student's t test and one-way analysis of variance was used for multiple comparisons. A P < 0.05 was considered statistically significant.
Results
CKIP-1 KO MSCs exhibited no differences in morphology or cell surface antigens but exhibited enhanced osteogenesis and adipogenesis
MSCs were isolated in vitro from CKIP-1 KO and WT mice. The CKIP-1 KO MSCs retained typical fibroblastic spindle shapes and maintained the ability to form colony-forming unit fibroblasts [Figure 1A]. Flow cytometry was used to characterize the surface molecules of CKIP-1 KO and WT MSCs. Both MSC subsets showed characteristic mesenchymal surface markers, including CD44, CD90, and CD106, and were negative for hematopoietic markers CD34 and CD45 [Figure 1B].
Figure 1.
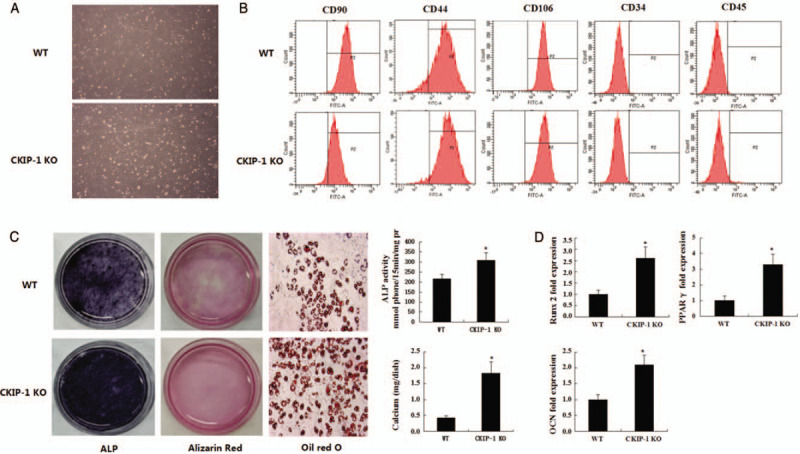
The morphology of MSCs acquired from WT and CKIP-1 KO mice (original magnificaiton ×100; A). Flow cytometry analysis of the surface antigens on the MSCs acquired from WT and CKIP-1 KO mice indicated positive straining for CD90, CD44, and CD106 and negative staining for CD34 and CD45 (B). The ALP activity was detected at day 7 culture. Mineralized nodules formed by MSCs after 21 days of culture were demonstrated by alizarin red staining, and volume of calcium sediment yield also detected at day 21. The representative oil red O staining of microscopic differentiated MSCs derived from WT and CKIP-1 KO mice after exposure to adipogenic induction medium for 6 days (original magnification ×200; C). RT-PCR analyses of osteogenic and adipogenic markers in the WT and CKIP-1 KO MSCs after treatment with osteogenic and adipogenic induction medium. The PCR data are from three independent experiments after normalization to β-actin (D). n = 6 per group. ∗P < 0.05, all quantitative data are shown as mean ± standard deviation. MSCs: Mesenchymal stem cells; ALP: Alkaline phosphatase; CKIP-1: Casein kinase 2-interacting proteiin-1; OCN: Osteocalcin; KO: Knockout; RT-PCR: Real-time polymerase chain reaction; WT: Wild type.
After cultured in osteogenic differentiation medium for 21 days, both MSC subsets formed distinct nodules in an induced time-dependent manner. The CKIP-1 KO MSCs produced a higher amount of extensive calcified deposits than the WT MSCs. The ALP activity was detected after osteogenic induction for 7 days. The ALP activity was higher in CKIP-1 KO MSCs than in WT MSCs [Figure 1C]. The quantification of the amount of alizarin red staining and ALP activity showed that the osteogenic capacity of CKIP-1 KO MSCs was significantly stronger than that of the WT MSCs [Figure 1C].
The quantitative PCR analyses showed that the expression of the osteogenic marker Runx2, and osteocalcin, increased 2.6- and 2.1-fold, respectively, in CKIP-1 KO MSCs compared with WT MSCs [Figure 1D]. Under adipogenic induction conditions for 6 days, both subsets of MSCs were able to form oil red O-positive lipid droplets. However, the amount of oil red O-positive lipid droplets was significantly higher in CKIP-1 KO MSCs than in WT MSCs [Figure 1C]. Adipogenic differentiation was further confirmed by the increased expression of specific adipogenic markers including peroxisome proliferator-activated receptor-γ, as determined by RT-PCR. No significant differences were observed between the MSCs. Adipogenesis was also enhanced in CKIP-1 KO MSCs compared with WT MSCs [Figure 1D].
Effect of CKIP-1 on in vivo bone mass was associated with aging
CKIP-1 is a negative control factor for osteogenesis. Therefore, bone formation was compared between CKIP-1 KO and WT mice. At 2 months of age, the mice body weights were not significantly different between the WT and CKIP-1 KO mice. As the mice aged, the difference in body weight between these two groups gradually increased. At 12 months, the average weight of the WT mice was 33 g, but the average weight of the KO mice was 39 g. At 18 months, the weight difference increased to 11 g [Figure 2A]. These results suggested that the CKIP-1 effects on bone formation or adipogenesis might be associated with aging. Further micro-CT analyses of trabecular bone from 2M CKIP-1 KO mice showed no significant changes in BV/TV or BMD compared with the WT mice. In correlation with the body weight, the micro-CT analysis of the 18-month-old (18M) CKIP-1 KO mice showed higher BV/TV and BMD compared with the WT mice [Figure 2B and 2C]. Taken together, these results indicated that CKIP-1 had negligible effects on bone formation in young mice but critical effects in aging mice, as observed by the increase in bone mass.
Figure 2.
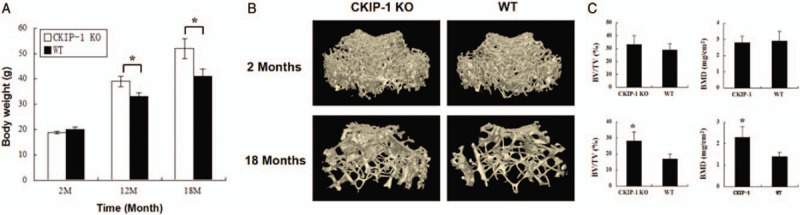
Body weight comparison between CKIP-1 KO and WT mice at 2, 12, and 18 months old (A). Femur bone mass was detected by micro- CT and was performed 3D reconstruction (B). Trabecular BV/TV and BMD of trabecular (C). n = 6 per group. ∗P < 0.05, all quantitative data are shown as mean ± standard deviation. CKIP-1: Casein kinase 2-interacting protein-1; KO: Knockout; WT: Wild type; CT: Computed tomography; BV: Bone volume; BMD: Bone mineral density; TV: Tissue volume.
Expression of CKIP-1 was up-regulated in vivo but not in cultured MSCs derived from aging mice
The expression of CKIP-1 was evaluated in WT mice. Quantitative PCR results showed that the expression of CKIP-1 gene from the MSCs derived from 2M mice was not significantly different from that of the MSCs derived from 18M mice [Figure 3A]. Under osteogenic induction conditions, the expression of CKIP-1 was also not significantly different between 2M and 18M mice [Figure 3B]. Western blot analyses showed that the expression of CKIP-1 could be detected but was weak in MSCs derived from young and old mice in normal or osteogenic induction culture conditions [Figure 3C]. Bone marrow was acquired in vivo from 2M and 18M mice to further detect the expression of CKIP-1. Quantitative PCR detected 4.3-fold higher gene expression levels in 18M mice compared with 2M mice [Figure 3D]. Western blot analysis results also revealed that the expression of CKIP-1 in old mice was significantly higher than that in young mice [Figure 3E]. The contradiction in the in vitro and in vivo data might be because the aging effect of the expression of CKIP-1 in MSCs was regulated via senescence rather than via MSC self-regulated aging.
Figure 3.
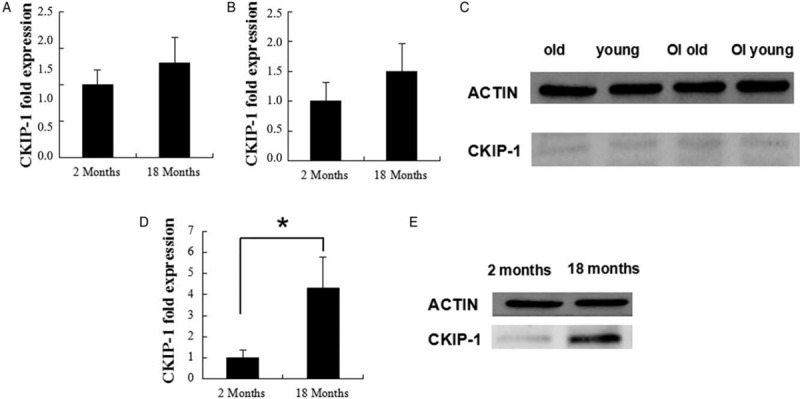
RT-PCR and western blot analyses of CKIP-1 expression in the MSCs derived from 2-month-old and 18-month-old mice after treatment with normal culture medium (A) and osteogenic induction medium (B; C). RT-PCR (D) and western blot analyses (E) of CKIP-1 expression in the bone marrow acquired from 2-month-old and 18-month-old mice. ∗P < 0.05, all quantitative data are shown as mean ± standard deviation. Western blot experiments were performed at least triplicates. RT-PCR experiments were performed at least sextuplicates. RT-PCR: Real-time polymerase chain reaction; CKIP-1: Casein kinase 2-interacting protein-1; MSCs: Mesenchymal stem cells; OI: osteogenic induction.
Inflammation had an important role in aging-associated CKIP-1 up-regulation
Concurrent with aging, the level of inflammation was gradually up-regulated. Previous studies have reported that inflammation can affect the differentiation of MSCs. Therefore, it was speculated that the up-regulation of inflammation promoted the high expression of CKIP-1 in aging MSCs. Quantitative PCR results showed that in vitro IL-1β significantly up-regulated the expression of CKIP-1 gene in MSCs derived from young and old mice [Figure 4A]. Western blot analyses showed that the expression of CKIP-1 protein was higher in inflammation-induced MSCs compared with normal MSCs [Figure 4B].
Figure 4.
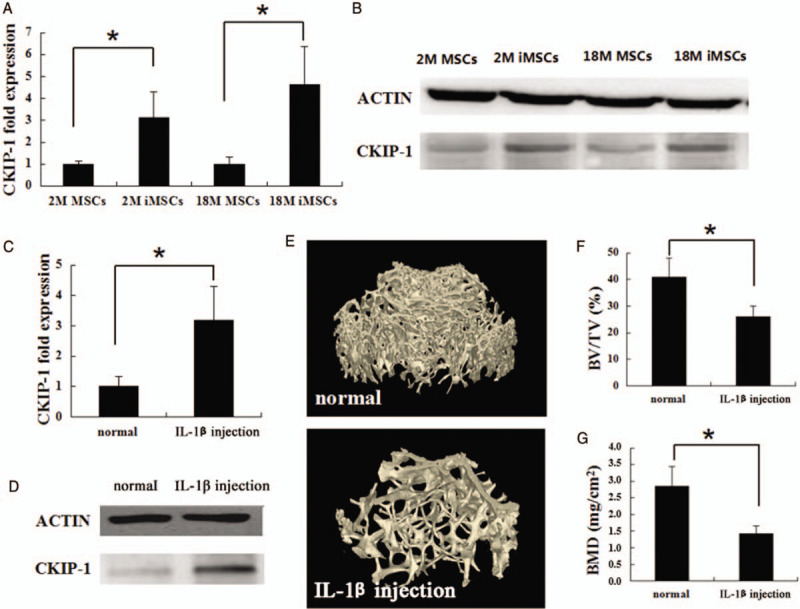
RT-PCR and western blot analyses of CKIP-1 expression in the MSCs derived from 2-month-old and 18-month-old mice after treatment with normal culture medium and inflammation stimulation medium (A and B). RT-PCR (C) and western blot analyses (D) of CKIP-1 expression in the bone marrow acquired from 2-month-old and 18-month-old mice after continue injection IL-1β 3 months. Comparison of the femur bone mass between normal mice and IL-1β injection mice using micro-CT (E). Trabecular BV/TV (F) and the BMD of trabecular (G). n = 6 per group. ∗P < 0.05, all quantitative data are shown as mean ± standard deviation. Western blot experiments were performed at least triplicates. RT-PCR experiments were performed at least sextuplicates. RT-PCR: Real-time polymerase chain reaction; CKIP-1: Casein kinase 2-interacting protein-1; MSCs: Mesenchymal stem cells; IL: Interleukin; CT: Computed tomography; BV: Bone volume; TV: Tissue volume; BMD: Bone mineral density.
IL-1β was regularly injected into 2M mice to simulate chronic inflammation so as to further detect the effect of inflammation on the expression of CKIP-1. At 3 months, the expression of CKIP-1 was assayed in the bone marrow of mice. The quantitative PCR results showed that the expression of CKIP-1 in the IL-1β injection group was 3.2-fold higher than that in the sham group [Figure 4C]. Western blot analyses also showed that the IL-1β up-regulation stimulated CKIP-1 up-regulation in vivo [Figure 4D]. In addition to the expression of CKIP-1, adult sham-injected mice were slightly larger in body size and heavier in body weight. As shown in Figure 4E, 4F, and 4G, the micro-CT analyses of the IL-1β injection group showed decreased femur bone masses compared with the sham-injected mice. From above all, these results indicated that inflammation in MSCs was critical in decreasing bone mass through the up-regulation of the expression of CKIP-1.
Discussion
CKIP-1 is a newly discovered intra-cellular negative regulator of bone formation. Previous studies have found that CKIP-1 siRNA can enhance osteogenic differentiation in vitro, activate bone formation, and invert bone loss in an osteoporotic mouse model.[18,19] MSCs are progenitors of osteoblasts having a critical role in bone differentiation and bone formation.[20] The role of CKIP-1 as a regulator of MSCs in osteogenesis remains unclear. In the present study, we employed a CKIP-1 KO mouse model to evaluate the effects of CKIP-1 on the potential of MSCs for osteogenesis and adipogenesis, as well as on the bone mass in mice of different ages. The results showed that MSCs derived from CKIP-1 KO mice exhibited a stronger capability for osteogenesis and adipogenesis in vitro. In line with that, CKIP-1 KO mice showed an increased bone mass as indicated by the higher BV/TV and BMD compared with the WT mice, however, at only old age (18M), when the bone marrow expression level of CKIP-1 in WT mice was much higher than that at young age (2M). As the inflammation level was gradually up-regulated with aging, its influence on the expression of CKIP-1 in MSCs was examined using IL-1β as an inflammatory stimulator. The result turned out that the expression levels of CKIP-1 in bone marrow and cultured MSCs were both up-regulated upon the stimulation. Our work presented for the first time the inflammation-mediated age-dependent effects of CKIP-1 on the osteogenic potential of MSCs.
MSCs isolated from CKIP-1 KO mice retained similar morphologies and surface molecule patterns as those from WT mice. These data suggested that CKIP-1 deficiency had no significant effects on the morphology or cell surface antigen expression of MSCs. These results agreed with previous report.[18] Further differentiation assays showed that CKIP-1 deficient MSCs acquired enhanced osteogenic and adipogenic ability. Generally, several molecules that promote osteogenesis are known to negatively regulate adipogenesis, some of which are implemented through the cell cycle regulation. However, CKIP-1 inhibits both osteogenesis and adipogenesis via distinct mechanisms.[21] Previous studies have shown that CKIP-1 inhibits osteogenesis and osteoprecursor cells through Smurf1 ligase-mediated degradation of Smad1/5 and mitogen-activated protein kinase kinase 2.[8,22] In adipogenic differentiation, CKIP-1 interacts with the histone deacetylase 1 in the nucleus and inhibits the transcription of CCAAT/enhancer-binding protein α, which is a crucial adipogenic transcription factor.[23]
The body weight and bone mass were not significantly different between young (2M) CKIP-1 KO mice and the age-matched WT mice. However, the CKIP-1 KO mice had higher body weights and bone masses at older age (18M). This finding indicated a role of CKIP-1 in negatively regulating osteogenesis and adipogenesis in aging mice other than young mice.
This observation contradicted the in vivo results, in which CKIP-1 enhanced osteogenesis primarily in aging mice but not in young mice. To further explore this discrepancy, the expression of CKIP-1 was detected in 2M and 18M mice in vivo and in vitro, respectively. In vitro, the difference in the expression of CKIP-1 was not significant between the MSCs derived from the 2M and 18M mice. However, CKIP-1 was more highly expressed in the bone marrow of aging mice compared with young mice. These results suggested that the MSC-intrinsic aging process was dispensable to regulate the expression of CKIP-1. Thus, it was hypothesized that the aging microenvironment around MSCs in vivo might up-regulate CKIP-1, leading to the inhibition of osteogenesis in MSCs.
As a function of ageing, inflammation has been well characterized in numerous epidemiologic studies. The levels of inflammatory mediators typically increase with age even in the absence of acute infection or other physiologic stresses.[24] For example, vascular cell adhesion molecule 1 increases in mesenchymal progenitor cells committed to lineage-specific differentiation. This marker is also up-regulated in primary MSCs derived from the bones of aged donors.[25] The delicate balance of regulatory networks necessary to govern tissue-specific regeneration and remodeling may be negatively impacted because inflammatory mediators interferon-γ and IL-1β activate the expression of vascular cell adhesion molecule 1 in MSCs as well as the levels of these pro-inflammatory cytokines increase in older individuals. Bone loss may occur once a sufficient level of inflammatory signals is achieved, whether acutely or chronically. Thus, dominant aberrations within the MSC microenvironment may arise from systemic chronic inflammation.[26] Therefore, in this study, it was speculated that the expression of CKIP-1 could be up-regulated in MSCs with increased inflammation levels, thus contributing to bone loss and osteoporosis in the aging body. The result that the inflammatory cytokine IL-1β up-regulated the expression of CKIP-1 in vitro verified the hypothesis. Subsequently, IL-1β was injected into 2M mice for 28 days to observe the changes in the expression of CKIP-1 and bone mass so as to further evaluate the inflammatory effects on CKIP-1-associated osteogenesis. The in vivo results showed that IL-1β increased the expression level of CKIP-1 in bone marrow and decreased the bone mass to similar levels as observed in aged mice. Taken together, it was concluded that CKIP-1-dependent decreases in bone mass in aged mice were due to the increase in chronic inflammation levels.
In conclusion, CKIP-1 is a negative regulator of osteogenic differentiation in MSCs. The effect of CKIP-1 on bone formation or bone mass is evidently age-dependent. Moreover, the CKIP-1-associated age dependence was not caused by MSC-intrinsic aging. Instead, the age dependence was attributed to the in vivo microenvironment around MSCs. Furthermore, this study found that the increased inflammation levels in aged individuals had a pivotal role in mediating this age-dependent feature by up-regulating the expression of CKIP-1. This study did not explore the mechanism by which inflammation regulates the expression of CKIP-1, which should be considered in future investigations.
Funding
This study was supported by the grants from the National Key Research and Development Project (No. 2017YFB1304300), Conversion Fund of PLA General Hospital (No. 2017tm-018), and the Clinical Research Support Fund of PLA General Hospital (No. 2017fc-tsys-2013).
Conflicts of interest
None.
Footnotes
How to cite this article: Tian XG, Gong FF, Li X, Meng FH, Zhou Z, Zhang HZ. Inflammation-mediated age-dependent effects of casein kinase 2-interacting protein-1 on osteogenesis in mesenchymal stem cells. Chin Med J 2020;133:1935–1942. doi: 10.1097/CM9.0000000000000951
References
- 1.Yamaguchi DT. Ins” and “outs” of mesenchymal stem cell osteogenesis in regenerative medicine. World J Stem Cells 2014; 6:94–110. doi: 10.4252/wjsc.v6.i2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol 2009; 217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Meng H, Wang X, Zhao C, Peng J, Wang Y. Differentiation of bone marrow mesenchymal stem cells in osteoblasts and adipocytes and its role in treatment of osteoporosis. Med Sci Monit 2016; 22:226–233. doi: 10.12659/msm.897044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallee A, Lecarpentier Y, Guillevin R, Vallee JN. Interactions between TGF-beta1, canonical WNT/beta-catenin pathway and PPAR gamma in radiation-induced fibrosis. Oncotarget 2017; 8:90579–90604. doi: 10.18632/oncotarget.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 6.Fakhry M. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J Stem Cells 2013; 5:136–148. doi: 10.4252/wjsc.v5.i4.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosc DG, Graham KC, Saulnier RB, Zhang C, Prober D, Gietz RD, et al. Identification and characterization of CKIP-1, a novel pleckstrin homology domain-containing protein that interacts with protein kinase CK2. J Biol Chem 2000; 275:14295–14306. doi: 10.1074/jbc.275.19.14295. [DOI] [PubMed] [Google Scholar]
- 8.Lu K, Yin X, Weng T, Xi S, Li L, Xing G, et al. Targeting WW domains linker of HECT-type ubiquitin ligase Smurf1 for activation by CKIP-1. Nat Cell Biol 2008; 10:994–1002. doi: 10.1038/ncb1760. [DOI] [PubMed] [Google Scholar]
- 9.Yang YK, Ogando CR, Wang See C, Chang TY, Barabino GA. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther 2018; 9:131.doi: 10.1186/s13287-018-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang G, Guo B, Wu H, Tang T, Zhang BT, Zheng L, et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat Med 2012; 18:307–314. doi: 10.1038/nm.2617. [DOI] [PubMed] [Google Scholar]
- 11.Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kühnisch J, et al. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem Cells 2009; 27:1288–1297. doi: 10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- 12.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol 2007; 8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 13.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 1971; 80:147–154. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev 2006; 5:91–116. doi: 10.1016/j.arr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Lepperdinger G. Inflammation and mesenchymal stem cell aging. Curr Opin Immunol 2011; 23:518–524. doi: 10.1016/j.coi.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371:1579–1586. doi: 10.1016/s0140-6736(08)60690-x. [DOI] [PubMed] [Google Scholar]
- 17.Sun S, Guo Z, Xiao X, Liu B, Liu X, Tang PH, et al. Isolation of mouse marrow mesenchymal progenitors by a novel and reliable method. Stem Cells 2003; 21:527–535. doi: 10.1634/stemcells.21-5-527. [DOI] [PubMed] [Google Scholar]
- 18.Guo B, Zhang B, Zheng L, Tang T, Liu J, Wu H, et al. Therapeutic RNA interference targeting CKIP-1 with a cross-species sequence to stimulate bone formation. Bone 2014; 59:76–88. doi: 10.1016/j.bone.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Ming L, Jin F, Huang P, Luo H, Liu W, Zhang L, et al. Licochalcone a up-regulates of FasL in mesenchymal stem cells to strengthen bone formation and increase bone mass. Sci Rep 2014; 4:7209.doi: 10.1038/srep07209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miura Y, Gao Z, Miura M, Seo BM, Sonoyama W, Chen W, et al. Mesenchymal stem cell-organized bone marrow elements: an alternative hematopoietic progenitor resource. Stem Cells 2006; 24:2428–2436. doi: 10.1634/stemcells.2006-0089. [DOI] [PubMed] [Google Scholar]
- 21.Peng X, Wu X, Zhang J, Zhang G, Li G, Pan X, et al. The role of CKIP-1 in osteoporosis development and treatment. Bone Joint Res 2018; 7:173–178. doi: 10.1302/2046-3758.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piacentino ML, Bronner ME. Intracellular attenuation of BMP signaling via CKIP-1/Smurf1 is essential during neural crest induction. PLoS Biol 2018; 16:e2004425.doi: 10.1371/journal.pbio.2004425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D, Zhu H, Liang C, Li W, Xing G, Ma L, et al. CKIP-1 suppresses the adipogenesis of mesenchymal stem cells by enhancing HDAC1-associated repression of C/EBPalpha. J Mol Cell Biol 2014; 6:368–379. doi: 10.1093/jmcb/mju034. [DOI] [PubMed] [Google Scholar]
- 24.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev 2011; 10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laschober GT, Brunauer R, Jamnig A, Singh S, Hafen U, Fehrer C, et al. Age-specific changes of mesenchymal stem cells are paralleled by upregulation of CD106 expression as a response to an inflammatory environment. Rejuvenation Res 2011; 14:119–131. doi: 10.1089/rej.2010.1077. [DOI] [PubMed] [Google Scholar]
- 26.Yang N, Wang G, Hu C, Shi Y, Liao L, Shi S, et al. Tumor necrosis factor alpha suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res 2013; 28:559–573. doi: 10.1002/jbmr.1798. [DOI] [PubMed] [Google Scholar]


