Abstract
Background
Due to airway remodeling and emphysematous destruction in the lung, the two classical clinical phenotypes of chronic obstructive pulmonary disease (COPD) are emphysema and bronchiolitis. The present study was designed to investigate the levels of small airway immunoglobulin A (IgA) in COPD with “emphysema phenotype.” The study also evaluated the associations between the small airway IgA levels and the severity of disease by the extent of emphysema versus airflow limitation.
Methods
Thirty patients (20 with COPD and ten healthy smokers) undergoing lung resection surgery for a solitary peripheral nodule were included. The study was conducted from January 2015 to December 2018 in the Shanxi Dayi Hospital. The presence of small airway IgA expression was determined in the lung by immunohistochemistry. In vivo, Wistar rats were exposed to silica by intratracheal instillation. Rats were sacrificed at 15 and 30 days after exposure of silica (n = 10 for each group). We also evaluated airway IgA from rats.
Results
Small airway secretory IgA (sIgA), dimeric IgA (dIgA), and dIgA/sIgA of Global Initiative for Chronic Obstructive Lung Disease grade 1–2 COPD patients showed no difference compared with smoking control subjects (5.15 ± 1.53 vs. 6.03 ± 0.85; 1.94 ± 0.66 vs. 1.67 ± 0.04; 41.69 ± 21.02 vs. 28.44 ± 9.45, all P > 0.05). dIgA/sIgA level in the lung of COPD patients with emphysema showed higher levels than that of COPD patients without emphysema (51.89 ± 24.81 vs. 31.49 ± 9.28, P = 0.03). The percentage of low-attenuation area below 950 Hounsfield units was positively correlated with dIgA/sIgA levels (r = 0.45, P = 0.047), but not associated with the severity of disease by spirometric measurements (forced expiratory volume in the first second %pred, P > 0.05). Likewise, in the rat study, significant differences in sIgA, dIgA, dIgA/sIgA, mean linear intercept, mean alveoli number, and mean airway thickness of bronchioles (VV airway, all P < 0.01) were only observed between control rats and those exposed for 30 days. However, in the group exposed for 15 days, although the VV airway was higher than that in normal rats (27.61 ± 2.26 vs. 20.39 ± 1.99, P < 0.01), there were no significant differences in IgA and emphysema parameters between the two groups (all P > 0.05).
Conclusion
Airway IgA concentrations in mild and moderate COPD patients are directly associated with the severity of COPD with “emphysema phenotype” preceding severe airway limitation. This finding suggests that small airway IgA might play an important role in the pathophysiology of COPD, especially emphysema phenotype.
Keywords: Immunoglobulin A, B cell, Emphysema, Chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD), as a global chronic respiratory disease, is characterized by progressive airflow obstruction involving emphysematous destruction of lung parenchyma and mucus hypersecretion with bronchiolitis.[1] With the aging of population and the increasingly prominent problem of air pollution,[2] this disease seriously affects the quality of life of patients and has become a major and growing cause of morbidity and mortality worldwide. In China, the mortality of COPD is more serious, and the overall prevalence of spirometry-defined COPD has increased to 8.6%.[3] The challenge of treating and managing this incurable lung disease stems from a lack of disease-modifying therapies and the clear pathogenesis.
Due to airway remodeling and emphysematous destruction in the lung contributing to disease severity and progression, the two classical clinical phenotypes of COPD are emphysema and bronchiolitis.[4] COPD patients with either the bronchiolitis-dominant or the emphysema-dominant phenotype differ in their clinical characteristics,[5] outcomes[6,7] as well as treatment response.[8] This may suggest that different phenotypes of COPD have different mechanisms.
The aberrant pulmonary inflammation resulted from repeated exposure to inhaled toxins (such as cigarette smoking and particulate matter [PM] 2.5) is at the core of the complex pathogenesis. This inflammation persists long after smoking cessation,[9] suggesting aberrant self-perpetuating immune responses similar to those in auto-immune diseases. Studies show that this chronic lung disease, particularly the emphysema-dominant phenotype, may originally result from B cells related immune response.[10] B cell activation factor of tumor necrosis factor family (BAFF), which is a key regulator of B cell homeostasis in several autoimmune diseases, is overexpressed and involved in the growth of lymphoid follicles (LFs) in the lungs of COPD patients.[11] In the severe stages, the size and number of B cell rich LFs increase.[12] Differential co-expression networks analysis of lung transcriptomics showed that B cell-related signaling was up-regulated in patients with emphysema phenotype, but not in patients with bronchiolitis phenotype.[13,14] The levels of B cell autoantibodies in blood and lung are both higher in COPD patients.[15] In other studies on the functional status of B cells in COPD, accumulation of immunoglobulin A (IgA) production occurs in peribronchiolar LFs from severe COPD.[16] Accordingly, the bronchial epithelium could induce B cells to switch into IgA-producing plasma cells through the interleukin (IL)-6/IL-6 receptor and BAFF-a proliferation-inducing ligand/transmembrane activator and calcium modulator ligand interactor pathways in COPD.[17]
In this study, we investigated the correlation between small airway IgA in lung tissue from COPD patients, healthy smokers, rats exposed to silica dust, and the extent of emphysema vs. airflow limitation.
Methods
Ethical approval
In adherence to the guidelines of the Declaration of Helsinki, this study was approved by the Ethics Committees of Shanxi Medical University (No. 2019LL090). All patients provided written informed consent to participate in the study.
Patients and grouping
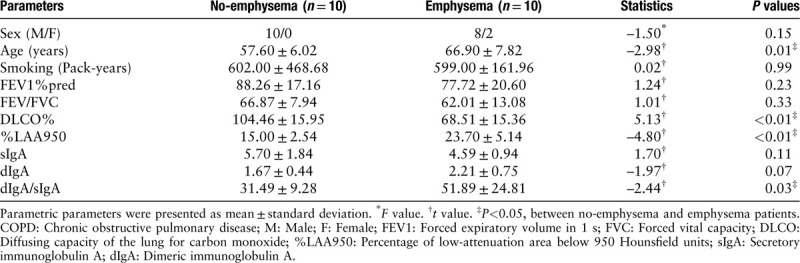
From January 2015 to December 2018, 30 patients (20 with COPD and ten smokers without COPD) undergoing lung resection surgery for a solitary peripheral nodule were included in the study. All patients were current smokers, and none of the subjects had evidence of acute exacerbation or respiratory tract infection at the time of the surgery. According to the spirometric criteria of COPD, subjects were divided into two groups: a COPD group (forced expiratory volume in the first second [FEV1]/forced vital capacity [FVC] <70% and FEV1%pred >50%) and a smoking control group (FEV1/FVC >70%). According to the results of a computed tomography (CT) scan and lung function test, the COPD patients were divided into two groups: an emphysema group (diffusing capacity of the lung for carbon monoxide [DLCO] <80% predicted and/or CT emphysema)[16] and a no-emphysema group (DLCO >80% predicted and absence of CT emphysema) [Tables 1 and 2].
Table 1.
Clinical characteristics of 20 patients with COPD and ten smokers without COPD undergoing lung resection surgery for a solitary peripheral nodule.
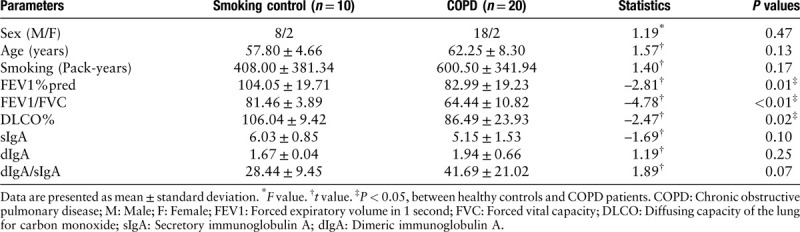
Table 2.
Clinical characteristics of 20 COPD patients.
Analysis of the CT scans
Two independent experts performed the analysis of the chest CT scans. As the percentage ratio of low-attenuation areas was below the −950 Hounsfield units threshold (%LAA950), the Chest Imaging Platform software was used to quantify emphysema.
Animal studies
A cross-sectional observational study demonstrated that self-reported exposure to workplace dust and fumes is not only associated with increased airflow limitation and respiratory symptoms, but also with more emphysema and gas trapping, assessed by CT scan, in both men and women.[18] Thus, we used rats exposed to silica dust to explore the relation of small airway IgA and emphysema. This study was approved by the Animal Welfare and Ethics Committee of Shanxi Medical University. Forty adult male Wistar rats were kindly provided by the Laboratory Animal Center of Capital Medical University in China. They were randomly assigned to four groups: a normal feeding 15 day group (15 normal, n = 10), a silica dust exposed 15 day group (15 exposed, n = 10), a normal feeding 30 day group (30 normal, n = 10), and a silica dust exposed 30 day group (30 exposed, n = 10).
The rats of normal feeding group received saline, while the rats of silica dust exposure group received silica by intratracheal instillation.[19] The lung tissues of rats were sampled on day 15 and day 30 after administration with silica dust, respectively. The lung tissues were then fixed, dehydrated, paraffin embedded, and stored at room temperature.
Lung tissue samples and lung histology
The formalin-fixed paraffin-embedded lung sections were collected. All lung tissues of patients at least 5 cm away from the nodule were studied. Lungs in the different groups of rats were equally inflated with the same volume of 4% paraformaldehyde solution to preserve the pulmonary architecture. Inflated right lungs of rats were fixed for 48 ho before the lungs were embedded in paraffin. The pathological change of lung sections from patients and rats stained with hematoxylin-eosin (HE) was observed under a microscope. The images of bronchioles and emphysema in five visual fields were collected. The mean airway wall thickness of terminal bronchioles (VV airway) was calculated. The extent of emphysema was assessed by calculating the mean linear intercept (MLI) and the mean alveoli number (MAN) in lung sections stained with HE at the same time. MLI and MAN were quantitated by using a light microscope (Leica DMLS, Solms, Germany) attached to an image analysis system (Image-Pro Plus, Chengdu Thai Union Technology Limited Company, China).
Immunohistochemistry
Primary antibodies were used as for anti-IgA monoclonal antibody (ab124716 and ab97181; Abcam, Cambridge, UK) followed by secondary anti-IgG H&L horseradish peroxidase (HRP) (ab6721 and ab6741; Abcam). The immunohistochemical staining was completed by automatic immunohistochemical instrument. The images were collected and imported into Image-Pro Plus software for quantitative analysis of IgA protein, then the average optical density of secretory IgA (sIgA) and dimeric IgA (dIgA) protein was calculated and recorded. The IgA expressed in intra-luminal is defined as sIgA, while dIgA is expressed in sub-epithelial.
Statistical analysis
Statistical analyses were performed by using SPSS 24.0 (SPSS Inc., Chicago, IL, USA). All study data are expressed as the mean ± standard deviation for normally distributed continuous variables; asymmetrically distributed variables were described as the median (interquartile range); categorical data are presented as numbers and percentages. Differences between two groups in the same category were compared by the independent samples t-test. Correlations were assessed by Spearman rank correlation coefficient (r). P values <0.05 were considered statistically significant.
Results
Subject characteristics
Twenty Global Initiative for Chronic Obstructive Lung Disease (GOLD) Grades 1–2 COPD patients and ten smoking controls were enrolled in this study. According to the results of CT scan and lung function test, COPD patients were assigned to two groups of ten patients each. Characteristics of the subjects are shown in Tables 1 and 2. There were only considerable differences in pulmonary function between the two groups. COPD patients had a significantly lower FEV1% predicted (P = 0.01), FEV1/FVC (P < 0.01), and DLCO% (P = 0.02) compared with smoking controls. There were no significant differences of FEV1% predicted (P = 0.23) or FEV1/FVC (P = 0.33) between each group. Emphysema patients had lower DLCO% (P < 0.01) and higher %LAA950 (P < 0.01) compared with patients with no emphysema. However, the ages of the emphysema patients were significantly higher than those of the no-emphysema patients (P = 0.01).
Expression levels of small airway sIgA and dIgA in subjects
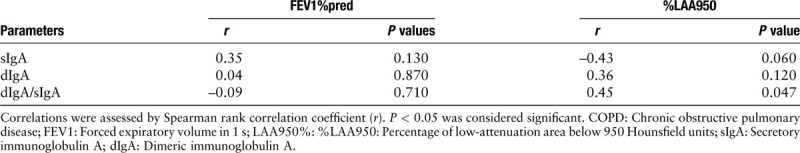
Small airway sIgA (P = 0.10), dIgA (P = 0.25), and dIgA/sIgA (P = 0.07) in GOLD Grades 1–2 COPD patients showed no significant difference compared with smoking controls [Table 1 and Figure 1]. Table 2 presents the distribution of dIgA/sIgA in COPD patients in relation to phenotype. Small airway dIgA/sIgA (P = 0.03) level was significantly greater in emphysema patients than that in no-emphysema patients. However, there was no significant difference in sIgA (P = 0.11) or dIgA (P = 0.07). As expected, %LAA950 and FEV1%pred correlated inversely with each other (r = –0.57, P < 0.01), but the levels of dIgA/sIgA (r = 0.45, P = 0.047) was only associated with %LAA950. There was no correlation between FEV1%pred and any parameters of IgA [Table 3].
Figure 1.
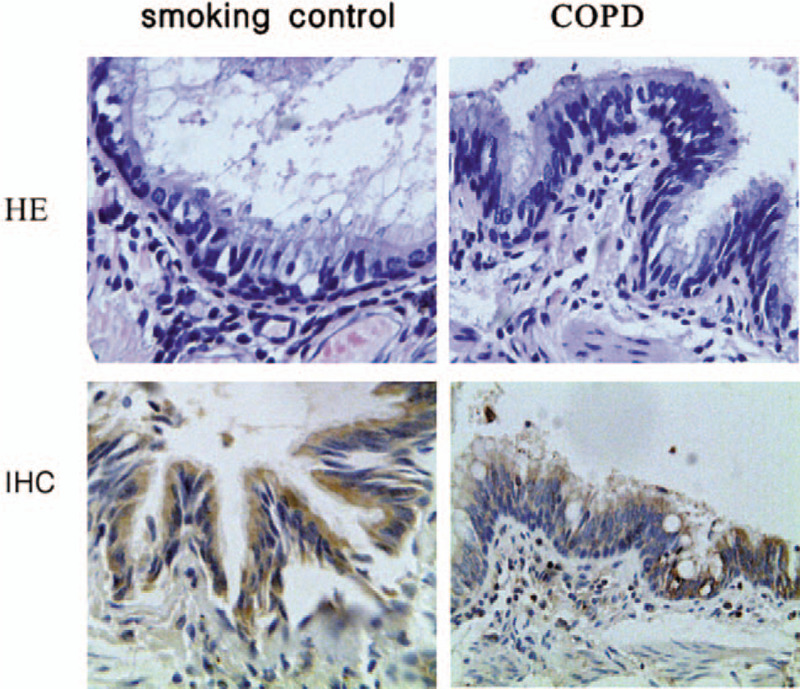
Pulmonary histology of smoking control patients and COPD patients (hematoxylin-eosin [HE] staining, original magnification ×400). Evaluation of sub-epithelial and intra-luminal IgA accumulation in small airway of smoking control patient and COPD patients (immunohistochemical [IHC] staining, original magnification ×400). COPD: Chronic obstructive pulmonary disease; IgA: Immunoglobulin A.
Table 3.
Correlation among small airway IgA and %LAA950 and FEV1%pred in COPD patients (n = 20).
Expression levels of small airway IgA in rats exposed silica dust
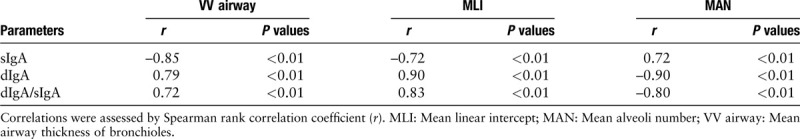
In the mild emphysema group (15 days), although the VV airway was higher in 15-day exposed rats than that in normal rats, there was no difference in sIgA (P = 0.17), dIgA (P = 0.21), dIgA/sIgA (P = 0.36), MLI (P = 0.20), and MAN (P = 0.17) between these two groups. However, in the severe emphysema group (30 days), there were significant differences in sIgA (P < 0.01), dIgA (P < 0.01), dIgA/sIgA (P < 0.01), MLI (P < 0.01), MAN (P < 0.01), and VV airway (P < 0.01) between exposed and control rats [Table 4 and Figure 2]. In addition, all IgA parameters were associated with MLI, MAN, and VV airway (all P < 0.01) [Table 5].
Table 4.
Levels of small airway IgA in all rats.
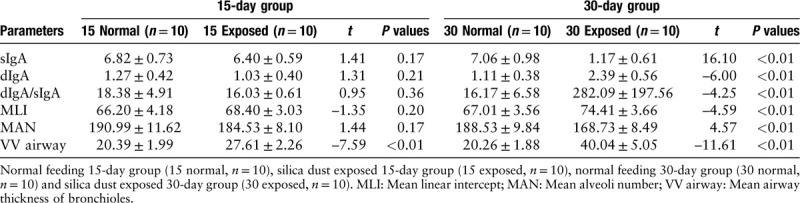
Figure 2.
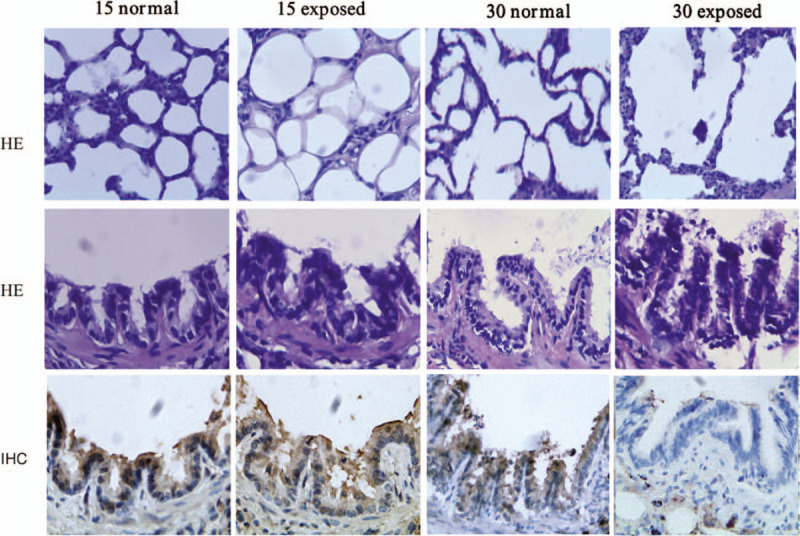
Pulmonary histology of different rat groups (hematoxylin-eosin [HE] staining, original magnification ×400). Evaluation of sub-epithelial and intra-luminal IgA accumulation in small airway of different rat groups. In lung tissue, with the aggravation of lung damage, less IgA accumulated in intra-luminal area, while more in sub-epithelial areas (immunohistochemical [IHC] staining, original magnification ×400). IgA: Immunoglobulin A.
Table 5.
Correlation among small airway IgA and VV airway, MAN as well as MLI in all exposed rats (n = 20).
Discussion
SIgA, together with mucociliary clearance, prevents adherence to airway or invasion of bronchial epithelium by bacterium and other foreign antigens, acting as “immune exclusion.” Therefore, impaired mucosal immunity could contribute to chronic and prolonged airway inflammation in COPD patients.[20] IgA is produced as dIgA by sub-epithelial follicular B cells in lung, which would bind to the epithelial polymeric immunoglobulin receptor (pIgR). The subsequent transcytosis of pIgR-IgA complex from the basolateral to the apical side of the mucosal epithelium is the basic mechanism of SIgA secretion.[21] The objective of this study was to evaluate the relationship between lung different isoforms of IgA levels and severity of COPD patients.
This study found the levels of sIgA in lungs from GOLD Grades 1–2 COPD patients is slightly lower, while the levels of dIgA is higher compared with smoking subjects, but both differences are not significant. In the study of rats, similar to the clinical test, the levels of lung sIgA and dIgA changed significantly in the 30-day exposed group, but not in the 15-day exposed group. Ladjemi MZ also report IgA was increased in COPD lung tissue, which led to its accumulation in sub-epithelial areas.[17] Other studies reported that the number of B cells and LF were only higher in severe and more severe COPD patients compared with mild or moderate COPD patients[12] and the IgA+ B cell numbers were increased in LF in distal lung parenchyma in severe COPD.[22] Because all COPD patients enrolled in this study would undergo lung resection surgery, the spirometrically-defined severity of these patients is mild or moderate. Thus, in this study, we could not identify the difference of the sIgA or dIgA levels in the small airways of COPD patients.
CT has been applied in identifying COPD sub-phenotypes, such as bronchiolitis and emphysematous destruction; the relative contribution of which varies from patient to patient. CT scans could detect emphysema in patients who do not fall under the spirometric criteria of COPD in 20% of the smokers.[23] Therefore, the use of lung function combined with chest CT may be able to detect early stage emphysema. In the present study, we found that the levels of dIgA/sIgA are higher in emphysema-dominant phenotype compared with bronchiolitis-dominant phenotype COPD patients. Previous studies reported that most B cellular parameters are significantly and strongly associated with only %LAA950, while FEV1%pred showed no association.[10] Similarly, this study showed that the levels of dIgA/sIgA are correlated with %LAA950, which reflects the severity of emphysema. Interestingly, in the study of rats, it was also found that the high expression of dIgA in lung tissue was related to emphysema, while in the 30-day exposed group, IgA (either dIgA or sIgA) was not only related to the severity of emphysema, but also related to airway wall thickness. Tanabe N also found that B cell infiltration in the walls of terminal bronchioles and alveolar tissue, which correlated with a reduced number of alveolar attachments to the airway walls,[24] and patients who have COPD with emphysema have a distinct B-cell transcriptomic signature.[13] These results suggested that B cells may participate in the pathology of emphysema before significant airflow limitation.
Our study found that in mild and moderate COPD patients, the level of dIgA/sIgA is correlated with LAA%, but not FEV1%. However, Vasiliy V. found that small airway surface sIgA was correlated with FEV1% in former smokers and COPD patients with Grade 1–2 or Grade 3–4.[25] The possible reason for this difference between the two study results is as followed. pIgR in patients with Grade 1–2 COPD, which is not significantly down-regulated compared with the smoking control group,[26] may slightly reduce the transport of IgA. However, derived from bronchial epithelium, the higher levels of IL-6 and BAFF imprint B cells with signals promoting maturation into IgA+ plasma cells.[17] Thus, sIgA is slightly reduced; and the correlation is not significant. The increase of B cells immune compartment associated with emphysema may lead to significant increase in dIgA/sIgA levels before significant airflow restriction. Therefore, IgA in lung would play the effect named “immune exclusion” in these stages of COPD (Grade 1–2). IgA could prevent the airway microbial colonization and maintain the stable airway microenvironment.
With the progression of COPD, areas of bronchial epithelial remodeling had reduced pIgR expression. Localized sIgA deficiency in small airway, leading to impaired mucosal immunity, is associated with pathogens translocation, small airway chronic inflammation, and airway remodeling.[27] At the same time, IgA-based immune response against Pseudomonas aeruginosa was low in severe COPD patients. This impaired local response against P. aeruginosa may favor chronic colonization, recurrent infections, and frequent acute exacerbation in severe COPD.[28] The continuous stimulation of lipopolysaccharide derived from colonized bacterium in the lung could promote the transformation of IgM + B cells to IgA + B cells,[22] which leads to the further increase of IgA secretion in severe COPD patients. Due to the reduced expression of pIgR in bronchial epithelial in severe COPD patients, dIgA could not accompany SC into the intra-lumen. Thus, the higher levels of dIgA and the lower levels of sIgA from lung correlated with airflow limitation in advanced stage patients. Using mice with genetic deficiencies of pIgR in lacking sIgA in the airways, Richmond BW found that airway bacteria provide the stimulus for deleterious neutrophilic inflammation, which in turns drives progressive emphysematous change in the lung parenchyma.[29] While treatment with broad-spectrum antibiotics inhibited development of both emphysema and small airway remodeling, it can be seen that in the late stage of COPD, chronic bacterial infection may become the core of this disease. High levels of dIgA may be coated with bacteria to form an IgA immune complex, which strengthens the affinity with CD89 expressed on inflammatory cells, and promotes the expression of inflammatory cytokines through Syk, PI3K, and tbk1 IKK ε.[30] Hence, in this stage of COPD, IgA in lung may have proinflammatory effects, but further basic animal research is required to confirm this.
These observations may open new therapeutic pathway for COPD patients, especially the emphysema phenotype. But there are also some limits in this paper. First, for surgery, there is a shortage of patients with Grade 3–4 COPD. Second, the number of clinical samples is small. Third, in the animal experiment, we evaluated airway IgA from rats exposed to silica dust. This needs to be further verified in rats exposed to tobacco smoke. Finally, it remains to be determined whether this IgA response is protective (immune exclusion) or harmful (proinflammatory effect). A better understanding of the role of dIgA and sIgA in COPD could help to define strategies to restore mucosal immune homeostasis in this disease and lead to more personalized therapeutic interventions which may further alleviate the burden of COPD.
Acknowledgements
The authors thank the Shanxi Dayi Hospital Affiliated to Shanxi Medical University for host the research.
Conflicts of interest
None.
Footnotes
How to cite this article: Liu H, Tang HY, Xu JY, Pang ZG. Small airway immunoglobulin A profile in emphysema-predominant chronic obstructive pulmonary disease. Chin Med J 2020;133:1915–1921. doi: 10.1097/CM9.0000000000000863
References
- 1.Agustí A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med 2019; 381:1248–1256. doi: 10.1056/NEJMra1900475. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Zhou Y, Liu S, Chen X, Zou W, Zhao D, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax 2017; 72:788–795. doi: 10.1136/thoraxjnl-2016-208910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet 2018; 391:1706–1717. doi: 10.1016/S0140-6736(18)30841-9. [DOI] [PubMed] [Google Scholar]
- 4.Page C, O'Shaughnessy B, Barnes P. Pathogenesis of COPD and asthma. Handb Exp Pharmacol 2017; 237:1–21. doi: 10.1007/164_2016_61. [DOI] [PubMed] [Google Scholar]
- 5.Izquierdo-Alonso JL, Rodriguez-Gonzálezmoro JM, de Lucas-Ramos P, Unzueta I, Ribera X, Antón E, et al. Prevalence and characteristics of three clinical phenotypes of chronic obstructive pulmonary disease (COPD). Respir Med 2013; 107:724–731. doi: 10.1016/j.rmed.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Golpe R, Suárez-Valor M, Martín-Robles I, Sanjuán-López P, Cano-Jiménez E, Castro-Añón O, et al. Mortality in COPD patients according to clinical phenotypes. Int J Chron Obstruct Pulmon Dis 2018; 13:1433–1439. doi: 10.2147/COPD.S159834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao W, Li M, Zhang Y, Zhang C, Wang P. Severity of chronic obstructive pulmonary disease with ’exacerbator with emphysema phenotype’ is associated with potential biomarkers. Postgrad Med J 2020; 96:28–32. doi: 10.1136/postgradmedj-2019-136599. [DOI] [PubMed] [Google Scholar]
- 8.Lee JH, Lee YK, Kim EK, Kim TH, Huh JW, Kim WJ, et al. Responses to inhaled long-acting beta-agonist and corticosteroid according to COPD subtype. Respir Med 2010; 104:542–549. doi: 10.1016/j.rmed.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 9.Lapperre TS, Postma DS, Gosman MM, Snoeck-Stroband JB, ten Hacken NH, Hiemstra PS, et al. Relation between duration of smoking cessation and bronchial inflammation in COPD. Thorax 2006; 61:115–121. doi: 10.1136/thx.2005.040519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan JL, Bagevalu B, Glass C, Sholl L, Kraft M, Martinez FD, et al. B cell adaptive immune profile in emphysema-predominant COPD. Am J Respir Crit Care Med 2019; 200:1434–1439. doi: 10.1164/rccm.201903-0632LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polverino F, Cosio BG, Pons J, Laucho-Contreras M, Tejera P, Iglesias A, et al. B cell-activating factor. An orchestrator of lymphoid follicles in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 192:695–705. doi: 10.1164/rccm.201501-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 13.Faner R, Cruz T, Casserras T, López-Giraldo A, Noell G, Coca I, et al. Network analysis of lung transcriptomics reveals a distinct B-cell signature in emphysema. Am J Respir Crit Care Med 2016; 193:1242–1253. doi: 10.1164/rccm.201507-1311OC. [DOI] [PubMed] [Google Scholar]
- 14.Qin J, Yang T, Zeng N, Wan C, Gao L, Li X, et al. Differential coexpression networks in bronchiolitis and emphysema phenotypes reveal heterogeneous mechanisms of chronic obstructive pulmonary disease. J Cell Mol Med 2019; 23:6989–6999. doi: 10.1111/jcmm.14585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiong Y, Gao S, Luo G, Cheng G, Huang W, Jiang R, et al. Increased circulating autoantibodies levels of IgG, IgA, IgM against cytokeratin 18 and cytokeratin 19 in chronic obstructive pulmonary disease. Arch Med Res 2017; 48:79–87. doi: 10.1016/j.arcmed.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Ladjemi MZ, Martin C, Lecocq M, Detry B, Nana FA, Moulin C, et al. Increased IgA expression in lung lymphoid follicles in severe COPD. Am J Respir Crit Care Med 2019; 199:592–602. doi: 10.1164/rccm.201802-0352OC. [DOI] [PubMed] [Google Scholar]
- 17.Ladjemi MZ, Lecocq M, Weynand B, Bowen H, Gould HJ, Van Snick J, et al. Increased IgA production by B-cells in COPD via lung epithelial interleukin-6 and TACI pathways. Eur Respir J 2015; 45:980–993. doi: 10.1183/09031936.00063914. [DOI] [PubMed] [Google Scholar]
- 18.Marchetti N, Garshick E, Kinney GL, McKenzie A, Stinson D, Lutz SM, et al. Association between occupational exposure and lung function, respiratory symptoms, and high-resolution computed tomography imaging in COPDGene. Am J Respir Crit Care Med 2014; 190:756–762. doi: 10.1164/rccm.201403-0493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright JL, Harrison N, Wiggs B, Churg A. Quartz but not iron oxide causes air-flow obstruction, emphysema, and small airways lesions in the rat. Am Rev Respir Dis 1988; 138:129–135. doi: 10.1164/ajrccm/138.1.129. [DOI] [PubMed] [Google Scholar]
- 20.Polosukhin VV, Richmond BW, Du RH, Cates JM, Wu P, Nian H, et al. Secretory IgA deficiency in individual small airways is associated with persistent inflammation and remodeling. Am J Respir Crit Care Med 2017; 195:1010–1021. doi: 10.1164/rccm.201604-0759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turula H, Wobus CE. The role of the polymeric immunoglobulin receptor and secretory immunoglobulins during mucosal infection and immunity. Viruses 2018; 10:E237.doi: 10.3390/v10050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis JL. B cells caught in the act: class switching to IgA in lung lymphoid follicles in COPD. Am J Respir Crit Care Med 2019; 199:548–550. doi: 10.1164/rccm.201810-1907ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med 2016; 374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanabe N, Vasilescu DM, Kirby M, Coxson HO, Verleden SE, Vanaudenaerde BM, et al. Analysis of airway pathology in COPD using a combination of computed tomography, micro-computed tomography and histology. Eur Respir J 2018; 51:1701245.doi: 10.1183/13993003.01245-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polosukhin VV, Cates JM, Lawson WE, Zaynagetdinov R, Milstone AP, Massion PP, et al. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011; 184:317–327. doi: 10.1164/rccm.201010-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gohy ST, Detry BR, Lecocq M, Bouzin C, Weynand BA, Amatngalim GD, et al. Polymeric immunoglobulin receptor down-regulation in chronic obstructive pulmonary disease. Persistence in the cultured epithelium and role of transforming growth factor-β. Am J Respir Crit Care Med 2014; 190:509–521. doi: 10.1164/rccm.201311-1971OC. [DOI] [PubMed] [Google Scholar]
- 27.Richmond BW, Du RH, Han W, Benjamin JT, van der Meer R, Gleaves L, et al. Bacterial-derived neutrophilic inflammation drives lung remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2018; 58:736–744. doi: 10.1165/rcmb.2017-0329OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millares L, Martí S, Ardanuy C, Liñares J, Santos S, Dorca J, et al. Specific IgA against Pseudomonas aeruginosa in severe COPD. Int J Chron Obstruct Pulmon Dis 2017; 12:2807–2811. doi: 10.2147/COPD.S141701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richmond BW, Brucker RM, Han W, Du RH, Zhang Y, Cheng DS, et al. Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat Commun 2016; 7:11240.doi: 10.1038/ncomms11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen IS, Baeten DLP, den Dunnen J. The inflammatory function of human IgA. Cell Mol Life Sci 2019; 76:1041–1055. doi: 10.1007/s00018-018-2976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


