Abstract
Background
There is a need for a rapid diagnostic point of care test to detect Neisseria gonorrhoeae (NG) infection to prevent incorrect, lack or excess of treatment resulting from current syndromic management in low-resource settings. An assay to identify NG antimicrobial resistance (AMR) is also highly desirable to facilitate antibiotic stewardship. Here we describe the development of two target product profiles (TPPs): one for a test for etiological diagnosis of NG and Chlamydia trachomatis (CT) (TPP1) and one for the detection of NG AMR/susceptibility (TPP2).
Methods
Draft TPPs were initially developed based on a landscape analysis of existing diagnostics and expert input. TPPs were refined via an online Delphi survey with two rounds of input from 68 respondents. TPP characteristics on which <75% of non-industry respondents agreed were further discussed and revised by an expert working group.
Results
The need for a test to identify NG in patients with urethral or vaginal discharge was identified as a minimal requirement of TPP1, with a test that can diagnose NG in asymptomatic patients as the optimal requirement. A sensitivity of 80% was considered acceptable, either in context of syndromic management or screening high-risk populations. For TPP2, the agreed minimal requirement was for a test to be used at level 2 healthcare facilities and above, with an optimal requirement of level 1 or above. A lateral flow format was preferred for TPP1, while it was considered likely that TPP2 would require a molecular format. A total of 31 test characteristics were included in TPP1 and 27 in TPP2.
Conclusions
Following the working group revisions, TPPs were posted online for public feedback for two months, and are now finalized. The final TPPs are currently guiding the development of new diagnostics that meet the defined characteristics to reach the market within two years.
Introduction
According to the World Health Organization (WHO) in 2016, there were an estimated 376 million new infections globally of the four curable sexually transmitted infections (STIs)–Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), Treponema pallidum subspecies pallidum and Trichomonas vaginalis. Gonorrhoea, caused by the bacterium Neisseria gonorrhoeae (NG), is the second most common bacterial STI with an estimated yearly burden of 87 million cases worldwide [1, 2]. Over time NG has progressively developed resistance to the majority of available antibiotics, raising concerns for the longevity of remaining and future therapies [3–5]. There are currently only two new antibiotics in development–zoliflodacin and gepotidacin—and, should either of them be successfully registered, their future conservation will be of primary importance [6, 7]. The WHO syndromic approach for the management of patients presenting with symptoms or signs of STIs has been in use since the 1990s in most resource-constrained settings due to the lack of affordable and appropriate diagnostic solutions [8]. However, its low sensitivity and specificity, and its lack of application for asymptomatic patients and women in general, drives both unnecessary use of antibiotics, and inadequate diagnosis and treatment [9–11]. In order to ensure that current and new antibiotics are used in a responsible manner and to prevent incorrect, lack of or excess of treatment, it is essential to improve and broaden diagnostic capabilities. A rapid point of care test (POCT) to detect NG would be a critical first step to avoid unnecessarily treating patients with STI syndrome, whilst a second assay to identify NG resistance/ susceptibility to existing antibiotics is highly desirable to optimize therapy and facilitate stewardship of both existing and future therapeutic options. To meet patient and market needs, the requirements of such assays should be specified in target product profiles (TPPs).
Gonorrhoea, a priority pathogen in the era of antimicrobial resistance (AMR)
Gonorrhoea affects high, middle and low-income countries with the African region having the highest incidence rates of infections worldwide, with approximately 50 and 100 estimated new infections per 1,000 women and men, respectively, every year [1, 12]. Urogenital gonorrhoea may be asymptomatic in 40% of men [13] and manifest most commonly as urethritis while it is also asymptomatic in more than half of the women, which increases the risk of misdiagnosis [14]. Women in resource-constrained settings are disproportionately affected by STIs, in particular by serious reproductive tract complications and sequelae [15, 16], such as infertility, pelvic inflammatory disease, ectopic pregnancy, spontaneous abortion, premature delivery and low birthweight. Furthermore, gonorrhoea enhances the transmission and acquisition of HIV infection [17]. Gay, bisexual and other men who have sex with men (MSM), racial or ethnic minorities, indigenous populations and sex workers appear to bear disproportionate burdens of NG. Asymptomatic, particularly extra-genital, gonorrhoea is significantly common in men who have sex with men (MSM) which remains undiagnosed and untreated and may lead to a reservoir which can result in widespread transmission among multiple partners [18, 19].
Appropriate STI diagnosis and treatment is crucial to prevent transmission and sequelae of NG infections [20]; however, access to diagnostic tools to be used at level 1; defined as a health post and centres where simple diagnostic technics, including collection of dried blood sports and rapid or dipstick test can be performed; is scarce in most low- and middle-income countries (LMICs), resulting in misdiagnosis and/or unnecessary antibiotic treatment [21–24]. NG identification can be done by Gram stained microscopy, although it cannot distinguish from other Neisseria species; culture, or the current gold standard, a nucleic acid amplification test (NAAT) [25–29]. A number of NAAT assays are available, however these platforms require significant infrastructure, resources and equipment, including continuous power, clean running water and climate control [27]. In order to reach patients in resource-limited settings, patient samples must be transported from urban, peri-urban and rural settings to the laboratory for processing. This is done using sample transport networks in-country; but, frequently, these services are not well developed, leading to long delays in returning sample results to patients and loss to follow-up [26, 27, 30].
A landscape analysis for STI POCTs performed by WHO showed that there were a limited number of immunoassays designed for use at the point of care available, four of which have been evaluated: the ACON Duo [31], NG ACON Plate (ACON Laboratories, USA) [31], BioStar Optical ImmunoAssay-Gonorrhea (BioStar, Inc., USA) [32], and the One Step Gonorrhea RapiCard InstaTest (Cortez Diagnostics, USA) [33]. The conclusion of this analysis was that although current lateral flow assays (LFA) rapid diagnostic test (RDT) for NG often have specificities of >90%; the sensitivities are often 50% or lower, and as such, do not perform adequately enough to be used for NG diagnosis. Accordingly, improved assays are required; this need is particularly acute with respect to women, where the syndromic approach to managing STIs is inadequate [34, 35]. There are several molecular platforms available and under development for the rapid detection of NG at level 1, some of them include the detection of resistance to ciprofloxacin but still there is no molecular platform able to detect resistance to broad spectrum cephalosporin, and some of them could potentially close the diagnostic gap in LMICS in the future [28, 35–37].
NG has a remarkable ability to develop or acquire resistance to antibiotics, and in the absence of a vaccine antibiotics are almost exclusively relied upon globally for gonococcal disease management and control. NG can very efficiently develop or acquire AMR through mutations, and via horizontal gene transfer (transformation), especially with the non-gonococcal Neisseria species of the pharynx. NG has developed resistance to the majority of available classes of therapeutic antibiotics including sulphonamides, penicillins, early-generation cephalosporins, macrolides, tetracyclines and fluoroquinolones, which cannot now be used in most areas of the world [5, 38–40]. Decreased susceptibility or resistance in NG to extended-spectrum cephalosporins, the last remaining option for first line empirical monotherapy, has been reported worldwide [41–43]. Determining whether antibiotic therapy will be effective ideally requires detection of AMR. This therefore poses the extraordinary challenge of developing a rapid diagnostic tool for NG that is also able to identify most AMR determinants [44].
The WHO, the Foundation for Innovative New Diagnostics (FIND) and the Global Antibiotic Research & Development Partnership (GARDP) have been collaborating to ensure antibiotic stewardship of existing and new antibiotics for NG since 2018. The need for more sensitive, easier-to-use and cheaper tests for both CT and NG that can deliver results in a single patient visit was the main rationale for the definition of the TPPs for diagnostics.
Two TPPs have been developed for NG POCT in resource-constrained settings; the first (TPP1) for a test for etiological diagnosis of NG and CT, and the second (TPP2) for the detection of NG AMR determinants that can predict resistance/susceptibility to existing and upcoming antibiotics. Novel technologies for NG detection and AMR prediction are being developed and it is important to ensure they meet the needs of end users particularly in resource-constrained settings [36, 45, 46].
Herein, we describe the TPP development process, which involved a meeting of sexual health, microbiology, diagnostic and global health experts, and the final consensus characteristics for tests to identify NG and CT and the susceptibility or resistance profile of NG to currently used antibiotics.
Methodology
The purpose of a TPP is to inform product developers of the technological performance standards and operational characteristics of a diagnostic test that are required to meet the end users’ needs for a defined use case [41]. A TPP must be sufficiently detailed to guide developers but not so restrictive as to hamper development.
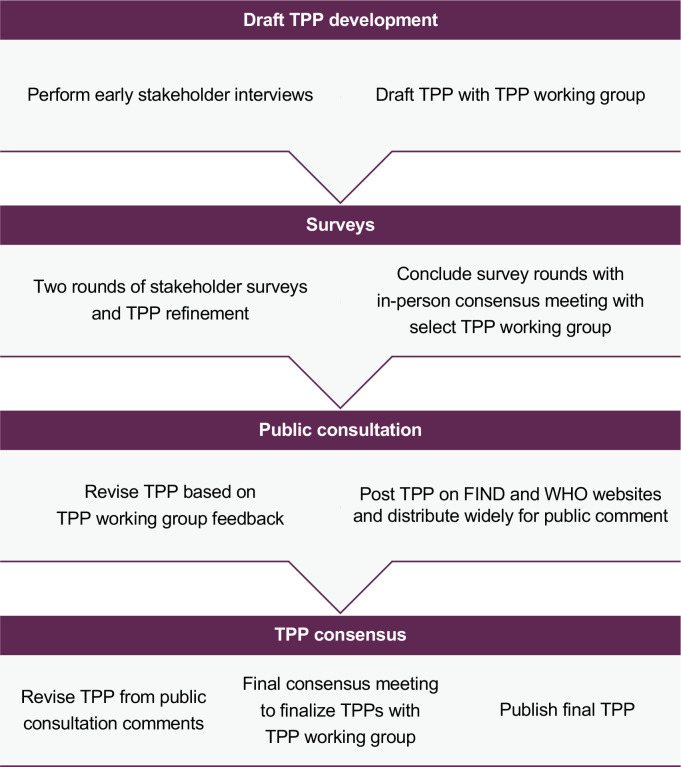
A landscape review of NG and/or NG/CT diagnostics available or in the pipeline was the initial step to define the TPP. An expert meeting was then jointly organized by GARDP, FIND and WHO in Geneva, Switzerland in May 2018 and involved key experts to identify the main test characteristics to inform draft TPPs. To further refine this draft, several interviews with key experts were conducted and the draft TPPs were further revised with a technical working group. These drafts were then included in an online Delphi survey process that involved two serial survey rounds of input from 68 survey respondents. Under the Delphi process, experts were asked to rate each TPP characteristic using a Likert scale of 1–5 to indicate level of agreement. The results were analysed with a pre-specified consensus threshold at >50% and >75% agreement (corresponding to a Likert score of either 4 or 5). Industry and non-industry responses were analysed separately; industry responses were only considered advisory and were not included in the main results. In total, 61 non-industry participants (primarily researchers, medical doctors, clinical officers and laboratory experts) and 7 industry participants responded to the Delphi survey rounds. Fig 1.
Fig 1. Overview of TPP development process.
The survey feedback was presented at a second expert meeting held in March 2019 in Montreux, Switzerland. The TPP requirements that had less than 75% agreement in the survey were discussed, proposed revisions were drafted in consultation with experts and then voted upon to confirm agreement. Any participants with conflicts of interest due to receipt of research funding, advisory fees or supplies from the diagnostic industry, or involvement in the development of diagnostic tests for NG, were excluded from voting. Final TPPs are published at websites of both FIND and WHO:
https://www.finddx.org/wp-content/uploads/2019/09/NG_CT-Test-TPP_20190731_clean-who.pdf
https://www.finddx.org/wp-content/uploads/2019/09/Comprehensive-NG-test-TPP_20190731_clean-who.pdf
Results
TPP1 (NG/CT diagnostic test): Scope of the test
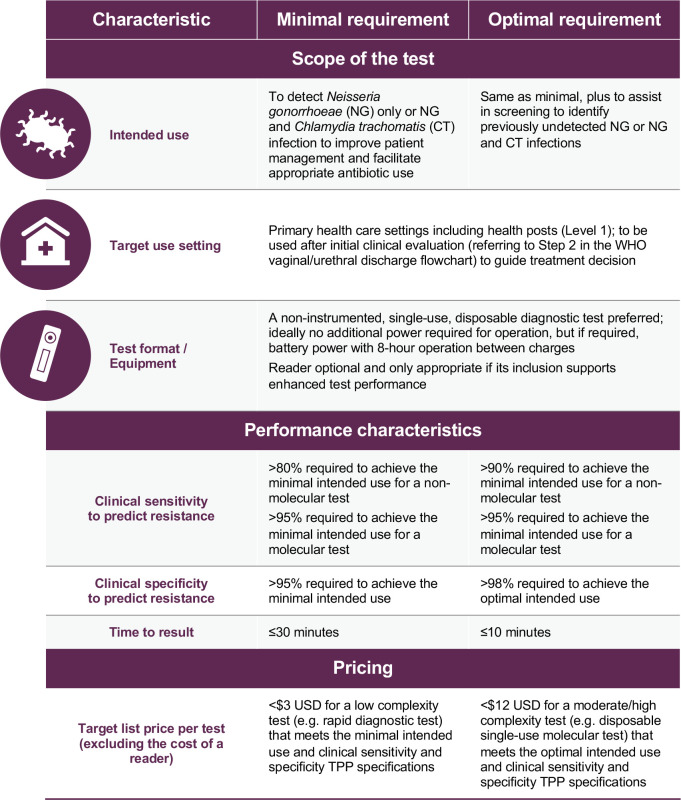
The working group identified the need for a test to identify NG in patients with urethral or vaginal discharge as a “minimal requirement” as most patients with symptoms are being managed with WHO syndromic approach and are frequently over-treated with several broad-spectrum antibiotics. The aim of such a test would be to ensure antibiotic stewardship providing NG treatment only to all who test NG positive, and the NG negative patients will likely be likely treated based on syndromic management. This test should be rapid, easy to use, and affordable, have acceptable sensitivity and specificity, be possible to implement at the primary health care (PHC) level in LMICs, and meet the ASSURED criteria [47].
The need for a test that could be used to diagnose NG in asymptomatic patients was highlighted and stated in the TPP as “optimal requirement”, acknowledging that such a test would be technically challenging because of the higher sensitivity required [34, 33]. Asymptomatic patients are the population driving NG transmission [46, 48, 49], hence screening was assigned as an optimal requirement particularly in the context of pre-exposure prophylaxis (PrEP) for HIV infection and antenatal care clinics (ANC).
As the WHO goal is to switch from syndromic management to etiologic diagnosis of STIs [50], it was proposed that the minimum requirement could be to assist in this shift and manage patient care and antibiotic usage more rationally, with an optimal requirement for additional use in etiologic screening to recognize undiagnosed cases and reduce the overall transmission of infection.
It was noted that the TPP should specify that the test can be for NG or NG and CT, to avoid implying that a CT-only test is acceptable. Furthermore, as it may take separate efforts to develop NG and CT tests, and performance characteristics will probably be different across the two, it would be advisable to allow for the option of an NG-only test.
The TPP working group agreed that 80% sensitivity would be acceptable, either in the context of syndromic management or in the context of screening of high-risk populations that cannot be screened using NAAT, as in the latter, there is no risk of overtreatment. Additionally, reaching 90% sensitivity using existing antibodies may be a challenge; while advancements in technology will allow some optimization, it is unlikely that they are sufficient to reach sensitivities of >90% while maintaining the rapidness, ease of use and low cost of the POCT. The appropriateness of implementing a test with lower sensitivity for use in LMICs has always been debated but has been shown to be more cost effective than a syndromic management approach lacking testing. Therefore, the experts agreed a sensitivity of 80% was the lowest acceptable sensitivity while still demonstrating benefit beyond syndromic management without diagnostic test [51–53]. The impact of sensitivity on uptake needs to be considered in conjunction with time, cost and ease of use. For example, a ‘pregnancy test’ style RDT would have greater uptake than a more complex and expensive test with the same sensitivity that takes 30 minutes to return a result [54–56]. The group agreed to break out the sensitivity and specificity in the TPP by test type to allow different values for molecular and non-molecular tests. It was agreed that a molecular test should not go below 90% sensitivity whereas a lateral flow assay could have a lower sensitivity and still be a significant improvement over syndromic management, particularly for symptomatic patients and women in general. The minimum and optimal sensitivity requirements for a non-molecular test could be the same, as it will be specificity that needs to differ.
WHO has developed simplified flowcharts to guide implementation of the syndromic management [57], which is based on the identification of symptoms and signs and the provision of treatment to deal with most of the organisms responsible for producing STIs. A recent systematic review and modelling study on vaginal discharge, performed to inform the TPP development, showed that the pooled sensitivity and specificity of the WHO flowchart 1, consisting of the basic clinical procedures such as history taking including risk assessment, physical examination and bimanual palpation which allows for immediate treatment of CT and NG, is 27.9% (24.7 to 31.1) and 57.0% (56.1 to 58.0) respectively [58]. The absolute effect of differences in prevalence using the pooled sensitivities and specificities of the different vaginal discharge flowcharts reveal that the low diagnostic accuracy of vaginal syndromic case management results in a high number of false positives (lower specificity), leading to overtreatment. The absolute effects on outcomes in settings with different CT/NG prevalence using hypothetical RDTs with sensitivities of 60%, 70%, 80% and a specificity of 90%, and using a POCT molecular assay (sensitivity of 95% and specificity of 98%), revealed fewer false positives and false negatives and more true positives compared with syndromic case management.
A total of 31 test characteristics were included in TPP1. Summary of TPP 1 is showed in Fig 2.
Fig 2. TPP1 for a rapid, low cost diagnostic to identify gonorrhoea with or without chlamydia infection.
TPP2 (AMR test): Scope of the test
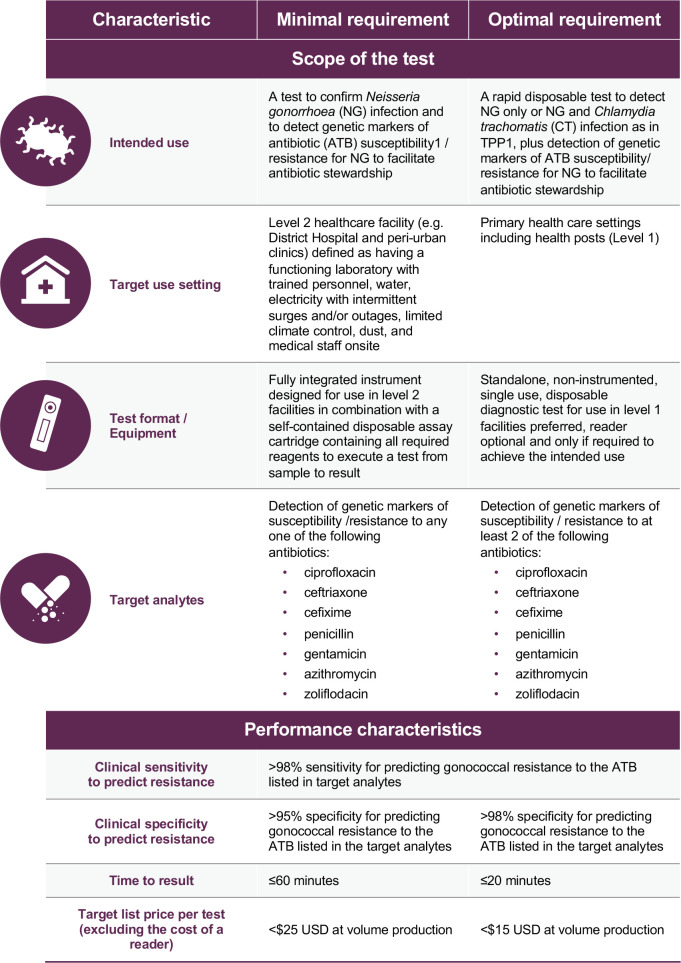
In the context of AMR, the expert group agreed on the need for a rapid test that allows resistance/susceptibility testing and individualized treatment for patients with confirmed NG, but also the need for increased AMR surveillance in LMICs, particularly in Africa, Eastern Europe, Central America, the Caribbean, and Asia, where substantially improved NG AMR data are urgently needed. However, the TPP2 used in the Delphi survey focused on patient management. The test was intended as a reflex test to be used after confirming NG infection in order to ensure antibiotic stewardship, as a minimum requirement. Individualized treatment is a more feasible use case but this is only likely to be possible at level 2 of the health care system–defined as a district hospital, which can act as a referral centre for specimens sent from level 1, include dedicated laboratory space, trained technicians and reagents) if an instrument is required for detection of antimicrobial resistance/susceptibility [22].
It was assumed that a test based on genotypic markers of infection would likely require a molecular testing format. For level 1 use [22], the most viable option would be a non-instrumented approach, such as a single use disposable LFA. However, if an instrument is required, it would be preferable for the instrument to have the capacity for testing of other diseases that commonly present at this level of care, which can be challenging from a cost perspective.
A test that could predict AMR/susceptibility to currently available antibiotics (particularly ceftriaxone and cefixime) and new antibiotics under development for NG would be ideal, however the technical challenges associated with developing such an assay mean that other key antibiotics might need to be chosen for inclusion in the assay. Several molecular tests under development include the detection of ciprofloxacin resistance/susceptibility [59, 60]; however, its utility in most African and South East Asian regions would be limited due to the high levels of resistance to this antibiotic. Whilst there is potential to develop a penicillin high level resistance test based on beta-lactamase production, the experts were concerned about how to deal with lower level or intermediate non-beta-lactamase (chromosomal) resistance to penicillin, as the predictive value of relevant markers is not fully understood, and treating people with intermediate resistance will likely drive more resistance.
Development of cephalosporin resistance tests would be extremely challenging but are also the most needed. Detection of mosaic penA alleles may have low specificity for predicting resistance (so as to rule out use of a cephalosporin) as susceptible strains can also carry most of the mosaic penA alleles. Additionally, in some countries such as China, Korea and Vietnam, much of the cephalosporin resistance reported has been due to mutations in non-mosaic alleles [5, 42, 61], therefore impacting upon the sensitivity of a mosaic penA based test. The possibility of including testing for novel antimicrobials, such as zoliflodacin, gepotidacin or other, was discussed, but it was noted that the knowledge relating to mechanisms of clinical resistance was limited for these drugs, and that whilst selecting for resistance in the laboratory is possible, the mechanism selected for will not necessarily be the dominant mechanism in a real-world situation once the antibiotic is in use.
Given the difficulties in developing AMR tests for many existing antibiotics, it was agreed to focus on tests for a single antibiotic for the minimum requirement, and a test including more than one antibiotic for the optimal requirement. This way, all of the antibiotics of interest could be listed, leaving options open for developers. A total of 27 test characteristics were included in TPP2. Summary of TPP 2 is showed in Fig 3. The final/revised TPP is shown in S2 File.
Fig 3. TPP2 for a test to identify susceptibility/resistance of gonorrhoea to antibiotics to facilitate antibiotic stewardship.
Discussion
Defining and disseminating TPPs for diagnostic tests identified as high priority helps to guide product developers. Consultation of a broad range of experts, and clarity of process, is required to make informed decisions about product features and specific performance specifications. The Delphi process and expert consultations used in the development of the NG TPPs provides confidence in the robustness of the final versions. This expert approach to define assay requirements should guide product development and enable targeted and timely efforts by industry partners and academic institutions.
It was agreed that the most suitable type of test for TPP1 for LMICs would be a LFA format due to the ease of use and low cost; although the few tests commercialized in the past showed poor performance and were abandoned years ago, several molecular assays are now in the pipeline [33, 35, 62]. The WHO syndromic approach has shown limitations in ensuring that patients with STIs are accurately diagnosed and treated, but most LMICs would face challenges in paying for a test that would be more expensive than the current combination of ceftriaxone and azithromycin (1 USD dollar) [63, 64, 65]. Patient health seeking behaviour and country guidelines for STI treatment are recognized as a major barrier for the implementation of such tests. Only half of patients with gonorrhoea have symptoms and only a third of them will present for health care [54, 66], meaning that many cases are missed from the health system, which would therefore benefit from a test with higher sensitivity that can be effectively used for screening as specified in the optimal requirements for TPP1.
Regarding TPP2, it was agreed that the minimum requirement should be for a test to be used at district hospital level and above, with an optimal requirement of use at PHC level or above; however, it will be important to specify that the minimum requirement test will not change patient management at PHC level. It was noted that if sample is sent to district hospital for testing, but the patient is lost to follow up, the test becomes a surveillance tool rather than a tool for patient management. If this is the intended use case, the TPP could be less prescriptive, as there is the potential to use a more complex sequencing-based system for surveillance purposes. Selecting the appropriate AMR determinants to be included in the test is a major challenge due to the genetic adaptability of NG allowing it to quickly develop or acquire AMR determinants; additionally, manufacturers might need to update the test as novel AMR mechanisms develop, which implies a need to resubmit the updated product for regulatory approval. However, this may be an opportunity for regulatory authorities to provide a more flexible framework, given the public health implications of being able to change the AMR determinants detected in a diagnostic assay.
The type of instrument and price were identified as some of the most important barriers for implementation of both TPPs. Most LMICs have a set of common barriers to implementation of diagnostic tests: a) deficient laboratory structures; b) lack of dependable sources of electricity and cold chain; and c) lack of trained staff [67]. Increased access to these diagnostic products will require the development and deployment of an instrument that can be used in remote LMICs by persons with little or no laboratory training. It was agreed that TPP1 should be ideally instrument free but given the technical challenges to develop such a tool, a small portable and battery-operated platform would be acceptable. TPP2 will require a higher level of infrastructure, which could be a district or regional hospital; however, this will not ensure individualized patient management if samples need to be referred. The experts acknowledged that the cost calculation would be different for both instruments, making TPP2 more expensive. A successful AMR test will be paradigm-shifting and likely to be included in a global AMR strategy rather than being restricted to LMICs, thus a strategy of income-based differential pricing might be possible. Additionally, fewer tests would be required as only those who have tested positive for NG would receive the AMR test, reducing overall funding needs.
Accurate diagnosis of STIs, and in particular for NG, is critical to ensure appropriate treatment, and for antibiotic stewardship to reduce the burden of AMR in the near future. However, structural challenges remain associated with the implementation of such tools once they are developed. Country adoption of new assays, and willingness to pay, are amongst the key barriers identified in a recent market assessment performed by FIND (manuscript under preparation). Acceptance of a new diagnostic approach will involve a cultural change from syndromic management in many LMICs. STI programmes are not sufficiently supported by donors in these settings, and even though some countries make efforts to integrate STIs into HIV programmes, integration challenges have been identified, including a lack of policy and guidelines, inadequately trained providers, vertical programming, provider work overload, and a weak health system [68].
As a next step these TPPs will serve to support the development of new diagnostics that meet the defined characteristics. It is anticipated that such tools may become available in the following two years.
Supporting information
(PDF)
(PDF)
Acknowledgments
The authors thank the experts and Delphi survey respondents for their contributions to the development of the TPPs. Editorial assistance for later drafts was provided by Rachel Wright, PhD, according to Good Publication Practice guidelines.
Data Availability
All relevant data are within the manuscript and its supporting information files.
Funding Statement
Our work on STIs and specifically on POC for NG and CT was enabled by the Global AMR Innovation Fund (GAMRIF).
References
- 1.Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019. August 1;97(8):548 10.2471/BLT.18.228486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2018 Report on global sexually transmitted infection surveillance [Internet]. 2018 [cited 2019 Jun 27]. Available from: http://apps.who.int/bookorders.
- 3.Wi T, Lahra MM, Ndowa F, Bala M, Dillon J-AR, Ramon-Pardo P, et al. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLOS Med [Internet]. 2017. July 7 [cited 2019 Oct 3];14(7):e1002344 Available from: 10.1371/journal.pmed.1002344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st Century: Past, evolution, and future. Clin Microbiol Rev. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unemo M, Lahra MM, Cole M, Galarza P, Ndowa F, Martin I, et al. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health [Internet]. 2019. August 23 [cited 2019 Sep 13];16(5):412 Available from: http://www.ncbi.nlm.nih.gov/pubmed/31437420 10.1071/SH19023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford PA, Miller AA, O’Donnell J, Mueller JP. Zoliflodacin: An oral spiropyrimidinetrione antibiotic for the treatment of Neisseria gonorrhoeae, including multi-drug resistant isolates. ACS Infect Dis. 2020. April 24; [DOI] [PubMed] [Google Scholar]
- 7.Taylor SN, Morris DH, Avery AK, Workowski KA, Batteiger BE, Tiffany CA, et al. Clinical Infectious Diseases Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose-Ranging, Single-Oral Dose Evaluation. [DOI] [PMC free article] [PubMed]
- 8.Implementation of the Global Strategy for Prevention and Control of Sexually Transmitted Infections: 2006–2015 PROGRESS REPORT [Internet]. 2015 [cited 2019 Jul 31]. Available from: www.who.int/about/licensing/copyright_form/en/index.html
- 9.Mlisana K, Naicker N, Werner L, Roberts L, van Loggerenberg F, Baxter C, et al. Symptomatic vaginal discharge is a poor predictor of sexually transmitted infections and genital tract inflammation in high-risk women in South Africa. J Infect Dis [Internet]. 2012. July 1 [cited 2019 Jul 31];206(1):6–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22517910 10.1093/infdis/jis298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickering JM, Whitworth JAG, Hughes P, Kasse M, Morgan D, Mayanja B, et al. Aetiology of sexually transmitted infections and response to syndromic treatment in southwest Uganda. Sex Transm Infect [Internet]. 2005. December [cited 2019 Jul 31];81(6):488–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16326853 10.1136/sti.2004.013276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wi TE, Ndowa FJ, Ferreyra C, Kelly-Cirino C, Taylor MM, Toskin I, et al. Diagnosing sexually transmitted infections in resource-constrained settings: challenges and ways forward. Vol. 22, Journal of the International AIDS Society. Wiley; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lowe S, Mudzviti T, Mandiriri A, Shamu T, Mudhokwani P, Chimbetete C, et al. Sexually transmitted infections, the silent partner in HIV-infected women in Zimbabwe. South Afr J HIV Med [Internet]. 2019. [cited 2019 Jun 27];20(1):849 Available from: http://www.ncbi.nlm.nih.gov/pubmed/30863622 10.4102/sajhivmed.v20i1.849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handsfield HH, Lipman TO, Harnisch JP, Tronca E, Holmes KK. Asymptomatic Gonorrhea in Men: Diagnosis, Natural Course, Prevalence and Significance. N Engl J Med. 1974. January 17;290(3):117–23. 10.1056/NEJM197401172900301 [DOI] [PubMed] [Google Scholar]
- 14.Van Der Pol B. Sexually transmitted infections in women. In: Scandinavian Journal of Clinical and Laboratory Investigation. Informa Healthcare; 2014. p. 68–74. [DOI] [PubMed] [Google Scholar]
- 15.Van Gemert C, Hellard M, Bradshaw C CS, Fowkes FJI, Agius PA, Bennett CM. Syndromic management of sexually transmissible infections in resource-poor settings: a systematic review with meta-analysis of the abnormal vaginal discharge flowchart for Neisseria gonorrhoea and Chlamydia trachomatis. [cited 2019 Apr 5]; Available from: 10.1071/SH17070 [DOI] [PubMed]
- 16.UNAIDS. Consultation on STD interventions for preventing HIV: what is the evidence? [Internet]. 2000 [cited 2019 Jul 31]. Available from: http://www.unaids.org
- 17.Cohen MS, Hoffman IF, Royce RA, Kazembe P, Dyer JR, Daly CC, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet (London, England) [Internet]. 1997. June 28 [cited 2019 Oct 7];349(9069):1868–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9217758 [DOI] [PubMed] [Google Scholar]
- 18.Budkaew J, Chumworathayi B, Pientong C, Ekalaksananan T. Prevalence and factors associated with gonorrhea infection with respect to anatomic distributions among men who have sex with men. PLoS One. 2019. April 1;14(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkcaldy RD, Weston E, Segurado AC, Hughes G. Epidemiology of gonorrhoea: A global perspective. Vol. 16, Sexual Health. CSIRO; 2019. p. 401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO | Global health sector strategy on Sexually Transmitted Infections, 2016–2021. WHO [Internet]. 2016 [cited 2019 Apr 4]; Available from: https://www.who.int/reproductivehealth/publications/rtis/ghss-stis/en/
- 21.Mayaud P, Mabey D. Approaches to the control of sexually transmitted infections in developing countries: old problems and modern challenges. Sex Transm Infect [Internet]. 2004. [cited 2019 Jul 31];80:174–82. Available from: www.stijournal.com 10.1136/sti.2002.004101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghani AC, Burgess DH, Reynolds A, Rousseau C. Expanding the role of diagnostic and prognostic tools for infectious diseases in resource-poor settings. Nature. 2015. December;528(7580):S50–2. 10.1038/nature16038 [DOI] [PubMed] [Google Scholar]
- 23.Verwijs MC, Agaba SK, Sumanyi J-C, Umulisa MM, Mwambarangwe L, Musengamana V, et al. Targeted point-of-care testing compared with syndromic management of urogenital infections in women (WISH): a cross-sectional screening and diagnostic accuracy study. Lancet Infect Dis [Internet]. 2019. April 25 [cited 2019 May 1]; Available from: https://www.sciencedirect.com/science/article/pii/S1473309918307242?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 24.Gaydos C, Hardick J. Point of care diagnostics for sexually transmitted infections: perspectives and advances. Expert Rev Anti Infect Ther [Internet]. 2014. June [cited 2019 Aug 8];12(6):657–72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24484215 10.1586/14787210.2014.880651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng L-K, Martin IE. The laboratory diagnosis of Neisseria gonorrhoeae. Can J Infect Dis Med Microbiol. 2005;16(1):15 10.1155/2005/323082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murtagh MM. The Point-of-Care Diagnostic Landscape for Sexually Transmitted Infections (STIs). [Google Scholar]
- 27.Vickerman P, Peeling RW, Watts C, Mabey D. Detection of gonococcal infection: pros and cons of a rapid test. Mol Diagn [Internet]. 2005. [cited 2020 May 6];9(4):175–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16392895 [PubMed] [Google Scholar]
- 28.Guy RJ, Causer LM, Klausner JD, Unemo M, Toskin I, Azzini AM, et al. Performance and operational characteristics of point-of-care tests for the diagnosis of urogenital gonococcal infections. Vol. 93, Sexually transmitted infections. BMJ Publishing Group Ltd; 2017. p. S16–21. [DOI] [PubMed] [Google Scholar]
- 29.Kelly H, Coltart CEM, Pant Pai N, Klausner JD, Unemo M, Toskin I, et al. Systematic reviews of point-of-care tests for the diagnosis of urogenital Chlamydia trachomatis infections. Vol. 93, Sexually transmitted infections. BMJ Publishing Group Ltd; 2017. p. S22–30. [DOI] [PubMed] [Google Scholar]
- 30.Moncada J, Clark CB, Holden J, Hook EW, Gaydos CA, Schachter J. Stability studies on dry swabs and wet mailed swabs for detection of chlamydia trachomatis and neisseria gonorrhoeae in aptima assays. J Clin Microbiol. 2017. March 1;55(3):971–7. 10.1128/JCM.02235-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nuñez-Forero L, Moyano-Ariza L, Gaitán-Duarte H, Ángel-Müller E, Ruiz-Parra A, González P, et al. Diagnostic accuracy of rapid tests for sexually transmitted infections in symptomatic women. Sex Transm Infect [Internet]. 2016. February [cited 2019 Oct 7];92(1):24–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26136508 10.1136/sextrans-2014-051891 [DOI] [PubMed] [Google Scholar]
- 32.Samarawickrama A, Alexander S, Ison C. A laboratory-based evaluation of the BioStar Optical ImmunoAssay point-of-care test for diagnosing Neisseria gonorrhoeae infection. J Med Microbiol [Internet]. 2011. December 1 [cited 2019 Mar 27];60(12):1779–81. Available from: http://jmm.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.034116-0 [DOI] [PubMed] [Google Scholar]
- 33.Abbai NS, Moodley P, Reddy T, Zondi TG, Rambaran S, Naidoo K, et al. Clinical Evaluation of the OneStep Gonorrhea RapiCard InstaTest for Detection of Neisseria gonorrhoeae in Symptomatic Patients from KwaZulu-Natal, South Africa. 2015. [cited 2019 Aug 8]; Available from: 10.1128/IAI.70.5.2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vickerman P, Watts C, Alary M, Mabey D, Peeling RW, Vickerman P. Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women [Internet]. [cited 2019 Mar 28]. Available from: www.stijournal.com [DOI] [PMC free article] [PubMed]
- 35.Murtagh MM. The Point-of-Care Diagnostic Landscape for Sexually Transmitted Infections (STIs) [Internet]. 2018 [cited 2019 Jan 28]. Available from: https://www.who.int/reproductivehealth/topics/rtis/Diagnostic_Landscape_2018.pdf
- 36.Toskin I, Murtagh M, Peeling RW, Blondeel K, Cordero J, Kiarie J. Advancing prevention of sexually transmitted infections through point-of-care testing: target product profiles and landscape analysis. Sex Transm Infect. 2017; [DOI] [PubMed] [Google Scholar]
- 37.Donà V, Low N, Golparian D, Unemo M. Recent advances in the development and use of molecular tests to predict antimicrobial resistance in Neisseria gonorrhoeae. Expert Rev Mol Diagn [Internet]. 2017. September 2 [cited 2019 Oct 17];17(9):845–59. Available from: https://www.tandfonline.com/doi/full/10.1080/14737159.2017.1360137 [DOI] [PubMed] [Google Scholar]
- 38.Wi T, Lahra MM, Ndowa F, Bala M, Dillon J-AR, Ramon-Pardo P, et al. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLOS Med [Internet]. 2017. July 7 [cited 2019 Feb 12];14(7):e1002344 Available from: https://dx.plos.org/10.1371/journal.pmed.1002344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unemo M, Nicholas RA. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiology. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alirol E, Wi TE, Bala M, Bazzo ML, Chen X-S, Deal C, et al. Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines. PLoS Med [Internet]. 2017. July [cited 2019 Feb 1];14(7):e1002366 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28746372 10.1371/journal.pmed.1002366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.FDA. Target Product Profile—A Strategic Development Process Tool | FDA [Internet]. 2007 [cited 2019 Aug 5]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/target-product-profile-strategic-development-process-tool
- 42.Unemo M, Golparian D, Nicholas R, Ohnishi M, Gallay A, Sednaoui P. High-level cefixime- and ceftriaxone-resistant Neisseria gonorrhoeae in France: novel penA mosaic allele in a successful international clone causes treatment failure. Antimicrob Agents Chemother [Internet]. 2012. March [cited 2019 Aug 8];56(3):1273–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22155830 10.1128/AAC.05760-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fifer H, Natarajan U, Jones L, Alexander S, Hughes G, Golparian D, et al. Failure of Dual Antimicrobial Therapy in Treatment of Gonorrhea. N Engl J Med [Internet]. 2016. June 23 [cited 2019 Aug 8];374(25):2504–6. Available from: http://www.nejm.org/doi/10.1056/NEJMc1512757 [DOI] [PubMed] [Google Scholar]
- 44.Ito M, Deguchi T, Mizutani K-S, Yasuda M, Yokoi S, Ito S-I, et al. Emergence and spread of Neisseria gonorrhoeae clinical isolates harboring mosaic-like structure of penicillin-binding protein 2 in Central Japan. Antimicrob Agents Chemother [Internet]. 2005. January [cited 2019 Aug 8];49(1):137–43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15616287 10.1128/AAC.49.1.137-143.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morikawa E, Mudau M, Olivier D, De Vos L, Joseph Davey D, Price C, et al. Acceptability and Feasibility of Integrating Point-of-Care Diagnostic Testing of Sexually Transmitted Infections into a South African Antenatal Care Program for HIV-Infected Pregnant Women. Infect Dis Obstet Gynecol. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner KM, Christensen H, Adams EJ, McAdams D, Fifer H, McDonnell A, et al. Analysis of the potential for point-of-care test to enable individualised treatment of infections caused by antimicrobial-resistant and susceptible strains of Neisseria gonorrhoeae: a modelling study. BMJ Open [Internet]. 2017. June 14 [cited 2018 Nov 30];7(6):e015447 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28615273 10.1136/bmjopen-2016-015447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toskin I, Peeling RW, Mabey D, Holmes K, Ballard R, Kiarie J, et al. Point-of-care tests for STIs: the way forward. Sex Transm Infect [Internet]. 2017. [cited 2018 Jul 12];93 Available from: 10.1136/sextrans-2016-053074 [DOI] [PubMed] [Google Scholar]
- 48.Zemouri C, Wi TE, Kiarie J, Seuc A, Mogasale V, Latif A, et al. The performance of the vaginal discharge syndromic management in treating vaginal and cervical infection: A systematic review and meta-analysis. PLoS ONE. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holley CE, Van Pham T, Mezzadra HM, Willis GC, Witting MD. Overtreatment of gonorrhea and chlamydial infections in 2 inner-city emergency departments. Am J Emerg Med [Internet]. 2015. September 1 [cited 2019 Mar 28];33(9):1265–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0735675715004817 10.1016/j.ajem.2015.06.009 [DOI] [PubMed] [Google Scholar]
- 50.WHO | Global strategy for the prevention and control of sexually transmitted infections: 2006–2015. WHO; 2018; [Google Scholar]
- 51.Pai NP, Vadnais C, Denkinger C, Engel N, Pai M. Point-of-Care Testing for Infectious Diseases: Diversity, Complexity, and Barriers in Low- And Middle-Income Countries. PLoS Med [Internet]. 2012. September 4 [cited 2019 May 24];9(9):e1001306 Available from: 10.1371/journal.pmed.1001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia PJ, You P, Fridley G, Mabey D, Peeling R. Point-of-care diagnostic tests for low-resource settings. Lancet Glob Heal [Internet]. 2015. May 1 [cited 2018 Aug 21];3(5):e257–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25889467 [DOI] [PubMed] [Google Scholar]
- 53.Vickerman P, Watts C, Alary M, Mabey D, Peeling RW. Sensitivity requirements for the point of care diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae in women. Sex Transm Infect. 2003. October;79(5):363–8. 10.1136/sti.79.5.363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosser E, Leslie Lugo M, Harvey SA, Cullen Balogun T, Barbu S, Beals J, et al. Willingness to Use and Pay for New Diagnostic Tests for Six Priority Diseases: Results from India [Internet]. [cited 2019 Jan 21]. Available from: www.chs-urc.org
- 55.Rivard KR, Dumkow LE, Draper HM, Brandt KL, Whalen DW, Egwuatu NE. Impact of rapid diagnostic testing for chlamydia and gonorrhea on appropriate antimicrobial utilization in the emergency department. Diagn Microbiol Infect Dis [Internet]. 2017. February 1 [cited 2019 Jan 21];87(2):175–9. Available from: https://www.sciencedirect.com/science/article/pii/S0732889316303467 10.1016/j.diagmicrobio.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 56.WHO STI POCT meeting report [Internet]. 2014 [cited 2019 Aug 5]. Available from: https://www.who.int/reproductivehealth/POTC-TPPs-2016.pdf?ua=1
- 57.Sexually Transmitted and Other Reproductive Tract Infections A guide to essential practice. 2005.
- 58.Wi TE, Ndowa FJ, Ferreyra C, Kelly‐Cirino C, Taylor MM, Toskin I, et al. Diagnosing sexually transmitted infections in resource‐constrained settings: challenges and ways forward. J Int AIDS Soc [Internet]. 2019. August 30 [cited 2019 Sep 2];22(S6). Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/jia2.25343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perera SR, Khan NH, Martin I, Taheri A, Parti RP, Levett PN, et al. Multiplex Real-Time PCR Assay for Simultaneous Identification of Neisseria gonorrhoeae and Its Ciprofloxacin Susceptibility Status. J Clin Microbiol [Internet]. 2017. November 1 [cited 2019 Feb 21];55(11):3201–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28814585 10.1128/JCM.00855-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allan-Blitz LT, Humphries RM, Hemarajata P, Bhatti A, Pandori MW, Siedner MJ, et al. Implementation of a rapid genotypic assay to promote targeted ciprofloxacin therapy of neisseria gonorrhoeae in a large health system. Clin Infect Dis. 2017;64(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gianecini RA, Golparian D, Zittermann S, Litvik A, Gonzalez S, Oviedo C, et al. Genome-based epidemiology and antimicrobial resistance determinants of Neisseria gonorrhoeae isolates with decreased susceptibility and resistance to extended-spectrum cephalosporins in Argentina in 2011–16. J Antimicrob Chemother [Internet]. 2019. June 1 [cited 2019 Jun 4];74(6):1551–9. Available from: https://academic.oup.com/jac/article/74/6/1551/5366949 10.1093/jac/dkz054 [DOI] [PubMed] [Google Scholar]
- 62.Miller E, Sikes HD. Addressing Barriers to the Development and Adoption of Rapid Diagnostic Tests in Global Health. Nanobiomedicine [Internet]. 2015. [cited 2019 Aug 8];2. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26594252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boadu NY, Amuasi J, Ansong D, Einsiedel E, Menon D, Yanow SK. Challenges with implementing malaria rapid diagnostic tests at primary care facilities in a Ghanaian district: a qualitative study. Malar J [Internet]. 2016. February 27 [cited 2019 Aug 8];15:126 Available from: http://www.ncbi.nlm.nih.gov/pubmed/26921263 10.1186/s12936-016-1174-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pai NP, Wilkinson S, Deli-Houssein R, Vijh R, Vadnais C, Behlim T, et al. Barriers to Implementation of Rapid and Point-of-Care Tests for Human Immunodeficiency Virus Infection: Findings From a Systematic Review (1996–2014). Point Care [Internet]. 2015. September [cited 2019 Aug 8];14(3):81–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26366129 10.1097/POC.0000000000000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Korenromp EL, Wi T, Resch S, Stover J, Broutet N. Costing of National STI Program Implementation for the Global STI Control Strategy for the Health Sector, 2016–2021. Barnabas R V., editor. PLoS One [Internet]. 2017. January 27 [cited 2019 Feb 21];12(1):e0170773 Available from: http://dx.plos.org/10.1371/journal.pone.0170773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urdea M, Penny LA, Olmsted SS, Giovanni MY, Kaspar P, Shepherd A, et al. Requirements for high impact diagnostics in the developing world. Nature [Internet]. 2006. November 22 [cited 2019 Jun 7];444 Suppl(S1):73–9. Available from: http://www.nature.com/articles/nature05448 [DOI] [PubMed] [Google Scholar]
- 67.Cooper D, Mantell JE, Moodley J, Mall S. The HIV epidemic and sexual and reproductive health policy integration: views of South African policymakers. BMC Public Health [Internet]. 2015. December 4 [cited 2019 Oct 9];15(1):217 Available from: http://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-015-1577-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whiticar P, Liberti T. Advancing Integration of HIV, STD, and Viral Hepatitis Services: State Perspectives. Public Health Rep [Internet]. 2007. [cited 2019 Oct 9];122(Suppl 2):91 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1831804/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.