Abstract
Dendrobium bibenzyls and phenanthrenes such as chrysotoxine, cypripedin, gigantol and moscatilin have been reported to show promising inhibitory effects on lung cancer growth and metastasis in ex vivo human cell line models, suggesting their potential for clinical application in patients with lung cancer. However, it remains to be determined whether these therapeutic effects can be also seen in primary human cells and/or in vivo. In this study, we comparatively investigated the immune modulatory effects of bibenzyls and phenanthrenes, including a novel Dendrobium bibenzyl derivative, in primary human monocytes. All compounds were isolated and purified from a Thai orchid Dendrobium lindleyi Steud, a new source of therapeutic compounds with promising potential of tissue culture production. We detected increased frequencies of TNF- and IL-6-expressing monocytes after treatment with gigantol and cypripedin, whereas chrysotoxine and moscatilin did not alter the expression of these cytokines in monocytes. Interestingly, the new 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl derivative showed dose-dependent immune modulatory effects in lipopolysaccharide (LPS)-treated CD14lo and CD14hi monocytes. Together, our findings show immune modulatory effects of the new bibenzyl derivative from Dendrobium lindleyi on different monocyte sub-populations. However, therapeutic consequences of these different monocyte populations on human diseases including cancer remain to be investigated.
Introduction
The Dendrobium plants are vastly distributed in a large area including tropical and subtropical regions of Asia and North Australia [1]. Because of the extensive geographical distribution, Dendrobium becomes one of the largest genera in Orchidaceae, composing of approximately 1500–2000 species [2]. Of these, about 41 species have been used in traditional Chinese medicine [3]. Various groups of secondary metabolites have been reported, for instance, alkaloids, coumarins, bibenzyls, fluorenones, phenanthrenes, and sesquiterpenoids. Some of these metabolites exhibit a wide spectrum of pharmacological activities, including antioxidant, anti-inflammatory, antidiabetic, antitumor, antimicrobial and neuroprotective activities [2, 4]. For example, purified bibenzyls and phenanthrenes, such as chrysotoxine from D. pulchellum, cypripedin from D. densiflorum, gigantol from D. draconis and moscatilin from D. brymerianum, have been demonstrated to show promising inhibitory effects on human in vitro cell line models of lung cancer growth and metastasis [5–9]. However, whether these therapeutic effects can be also seen in primary human cells and/or in vivo remains to be investigated. Nevertheless, it is also unclear whether systemic administration of these compounds would influence phenotypes and/or functions of other cell types such as circulating immune cells in the peripheral blood.
The lung is a highly specialized organ that is continuously exposed to numerous pathogens, pollutants, oxidants, gases, and toxicants from the outside ambient air, which makes it susceptible to varying degrees of oxidative and inflammatory injury. Under some circumstances, inflammatory responses can result in the release of proinflammatory cytokines and chemokines, which in turn stimulate the influx of polymorphonuclear leukocytes (PMNs) and monocytes into the lung to combat the invading pathogens. However, chronic inflammation-induced cytokine production of monocytes in the lung may predispose individuals to lung cancer [10]. Tumor-infiltrating myeloid cells (TIMs), comprising monocytes, macrophages, dendritic cells and neutrophils, have emerged as key regulators of cancer growth and recurrence [11, 12]. The infiltration of monocytes into human specimens of non-small-cell lung cancer was found to be increased compared with normal lung tissue [12]. Although, it remains elusive, which monocyte sub-populations recruit into tumors, these infiltrating cells showed increased expression of chemokines and chemotactic activity in tumor environment [11]. Increased numbers and inflammatory activity of tumor-associated, monocyte-derived macrophages were linked to poor prognosis in lung cancer patients [12, 13]. Amelioration of monocyte-induced inflammation and/or infiltration may synergize to inhibit tumor growth and metastasis, and lead to a better prognosis of patients with lung cancer.
Furthermore, monocytes are crucial for the efficiency of the immune system, since they act in numerous immunological mechanisms, such as replenishment of resident macrophages, production of dendritic cell subsets, and acute and chronic inflammatory responses [14,15]. The cellular activity of these cells involves the production of several molecules responsible for killing of pathogens (H2O2, NO), cellular recruitment (IL-8, CCL2, and CCL3), pro-inflammatory activation (TNF, IL-1β, and IL-6), polarization of adaptive immune response (IL-12), regulation of inflammation (IL-10 and TGF-β1), and tissue repair (TGF-β1 and bFGF) [16]. Monocyte dysregulation, which is generally indicated by intense production of inflammatory mediators, has been observed in several inflammatory diseases, including sepsis [17], atherosclerosis [18], rheumatoid arthritis (RA) [19], and hepatic fibrosis [20]. High levels of TNF and IL-6 are associated with worse prognoses in septicemia [21] and progression and worsening of RA [22].
In this study, we aimed to investigate the immune modulatory activities on circulating monocytes of four known Dendrobium substances, which have already been shown to exert therapeutic effects in in vitro models of lung cancer [5–9], as well as one newly identified bibenzyl Dendrobium compound. All five compounds were isolated, purified and identified from Dendrobium lindleyi Steud, known in Thai as “Ueang Pueng” [23]. This orchid is widely distributed in India (Sikkim), China (Guangdong, Hainan, Guangxi, and Guizhou), Hongkong, Bhutan and Southeast Asia (Thailand, Myanmar, Laos, and Vietnam) [3]. To our knowledge, chemical constituents and biological activities of compounds isolated from this orchid have never been investigated before. To assess their immune modulatory activities on circulating monocytes of the peripheral blood, we established an ex vivo model of primary human monocytes with low cell attachment, in which we attempted to minimize differentiation of monocyte into macrophage ex vivo. Our results revealed increased frequencies of TNF- and IL-6-expressing monocytes after treatment with gigantol and cypripedin, whereas chrysotoxine and moscatilin did not alter the expression of these cytokines in monocytes. Interestingly, the new 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl derivative alleviated lipopolysaccharide (LPS)-induced cytokine production of primary human monocytes, suggesting immune modulatory activity.
Materials and methods
Plant material
The whole plant of D. lindleyi was purchased in September 2009 from Chatuchak market, Bangkok, Thailand (13°47'57.1"N, 100°32'55.2"E). Plant identification was done by one of the authors (B. Sritularak). A voucher specimen (BS-DL-092552) has been deposited at the Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Chulalongkorn University.
Extraction and isolation
Dendrobium lindleyi (1.2 kg) was extracted as previously described [26]. Briefly, the dried whole plant was grounded extracted with methanol (MeOH) at room temperature (RT). The extract was then evaporated under reduced pressure. The MeOH residue (97 g) was suspended in water and partitioned with immiscible organic solvents comprising EtOAc and n-BuOH. The EtOAc extract (34 g) was fractionated on silica gel column (EtOAc-hexane, gradient) to yield in total of 10 fractions (A-J). Fraction F (3.2 g) was separated by column chromatography over silica gel (EtOAc -hexane, gradient) to obtain 13 fractions (FI-FXIII). Fraction FVIII (140 mg) was purified on Sephadex LH-20 column (MeOH) to furnish chrysotoxine (#2) (90 mg), gigantol (#3) (20 mg) and cypripedin (#4) (25 mg), respectively. Moscatilin (#5) (90 mg) was purified from fraction FX (340 mg) on Sephadex LH-20 (MeOH). Fraction G (2.6 g) was fractionated by CC ((silica gel, EtOAc-hexane, gradient) and then by Sephadex LH-20 (MeOH) to afford #1 (47 mg).
Analytical procedures
Bruker micro TOF mass spectrometer (ESI-MS) was used for mass spectrometric analysis and a Milton Roy Spectronic 300 Array spectrophotometer was utilized for UV spectroscopic measurement. NMR spectra were obtained on a Bruker Avance III HD 500 NMR spectrometer or a Bruker Avance DPX-300 FT-NMR spectrometer. The recording of IR spectra was done by a Perkin-Elmer FT-IR 1760X spectrophotometer. Column chromatography (CC) was conducted on different stationary phases. These were silica gel 60 (Merck, Kieselgel 60, 70–320 μm), silica gel 60 (Merck, Kieselgel 60, 230–400 μm), C-18 (Merck, Kieselgel 60 RP-18, 40–63 μm) and Sephadex LH-20 (25–100 μm, GE Healthcare).
Buffy coats and monocyte isolation
Buffy coats from healthy individuals were obtained from the German Red Cross (GRC). Blood donors gave informed consent to perform blood collection and to use the buffy coats samples for research. The research purpose was approved by the Ethics Committee of Charité –Universitätsmedizin Berlin.
Human peripheral blood mononuclear cells (PBMCs) were isolated and aliquoted at 20 x 106 PBMCs (per mL) were cryopreserved in liquid nitrogen tank until analysis. Frozen PBMCs of three biologically independent donors were thawed, washed and pooled in MACS buffer (0.5% BSA in PBS containing 2 mM EDTA). Monocytes were isolated using negative selection, pan-monocyte Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to manufacturer’s specifications. Briefly, PBMCs were resuspended in 120 μL of MACS buffer. Thirty micro liters of FcR blocking reagent and 30 μL of biotin-antibody cocktail were added, mixed thoroughly and incubated at 4°C. After 5 min incubation, 90 μL of MACS buffer and 60 μL of anti-biotin micro beads were added and incubated at 4°C for 10 min. Stained ells were then washed with MACS buffer and pelleted (4°C, 300 x g, 8 min). The pellet was then resuspended in 500 μL of MACS buffer and loaded onto the MACS column. The column was then washed twice with MACS buffer. The flow-through and washed fraction containing unlabeled monocytes was collected. Cell number and viability were determined by 0.2% trypan blue staining.
Cell culture and stimulation
Isolated monocytes were resuspended in 1 mL of RPMI1640 (Biochrom GmbH, Berlin, Germany) containing 10% heat-inactivated fetal calf serum (FCS) (Sigma-Aldrich, St. Louis, USA), penicillin (100 U/mL; Biochrom GmbH, Berlin, Germany) and streptomycin (100μg/mL; Biochrom GmbH, Berlin, Germany). Cell concentration was adjusted to ~ 2 x 106 cells/mL. About 2 x 105 cells (per well) were transferred into ultra-low-attachment 96-well plate (Corning, New York, USA). Different concentrations of compounds (Fig 3A) were added to the cell culture. To inhibit protein transport from Golgi apparatus to the endoplasmic reticulum, monensin (5 μg/mL; BioLegend, San Diego, USA) was also added right after the application of Dendrobium compounds. After two hours incubation, cells were stimulated by adding 100 ng/mL of lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, USA). Cells were cultured for 4 more hours or overnight (18 hours). Finally, cells were analysed by flow cytometry. Control conditions were naïve cells (without treatment of Dendrobium compounds and LPS) and unstimulated cells that were previously treated with the Dendrobium compounds.
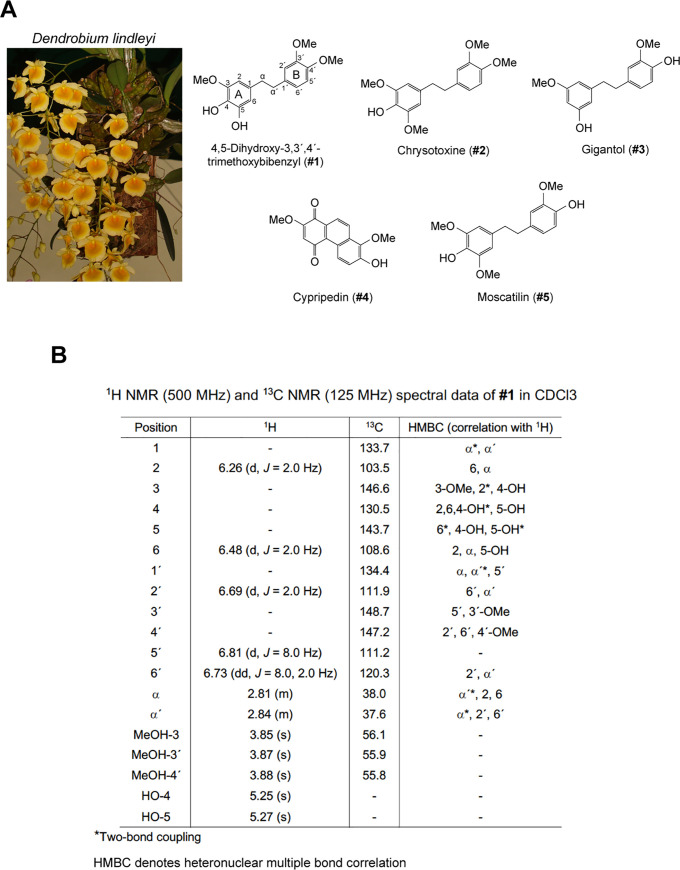
Fig 3. Isolated compounds from Dendrobium lindleyi.
(A) Chemical structures of five compounds isolated and purified from Dendrobium lindleyi. (B) Chemical structure characterization of 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl using 1H NMR and 13C NMR.
Flow cytometry
Monocytes were incubated for 10 min in FcR-blocking buffer (1:100; Miltenyi Biotec, Bergisch Gladbach, Germany) at 4°C, to block unspecific binding of antibodies to Fc-receptors. Next, cells were incubated with conjugated antibodies detecting extracellular epitopes (i.e. CD3-PE-Cy7 / SK7 / BioLegend; CD335-PE / 9E2 / BioLegend; CD16-APC-Cy7 / 3G8 / BioLegend; CD11c-APC / 3.9 / BioLegend; HLA-DR-pacific blue / L243 / BioLegend and CD14-FITC / RMO52 / Beckman) diluted in staining buffer (0.5% BSA in PBS containing 2 mM EDTA) for 20 min at 4°C. Cells were then washed with 1 mL of staining buffer, and further incubated in 200 μL of intracellular fixation/permeation buffer (eBioscience, California, USA) for 30 min at 4°C. After washing with 1 mL of permeation buffer (eBioscience, California, USA), 50 μL of antibody cocktail detecting intracellular proteins (i.e. TNF-brilliant violet / Mab11 / BioLegend; IL-6-PE-Cy7 / MQ2-13A5 / BioLegend; CCL2-PE / 5D3-F7 / BioLegend and CD68-PerCP-Cy5.5 / Y1/82A / BioLegend) was added and further incubated for 30 min at 4°C. Cells were then washed with staining buffer and pelleted at 300 x g, 4°C for 10 min. Stained cells were finally resuspended in staining buffer. Cell viability was determined using Propidium Iodide (25 μg/mL; Thermo Fisher Scientific, Massachusetts, USA). Protein expression was detected on a 3-laser BD FACSCanto II machine (BD Biosciences, New Jersey, USA) with software BD DIVA version 8.1. FlowJo software version 10.1 (Ashland, OR, USA) was used to determine the phenotype on the basis of fluorescence intensity.
Statistical analysis
Acquired FACS data were extracted in the Flow Cytometry Standard (*.fcs) format. Cell populations were gated in FlowJo (Becton, Dickinson and Company, New Jersey, USA). Frequency of cells positive for a given signal was extracted into GraphPad Prism 8 (GraphPad Software Inc., California, USA) and statistically analyzed by a one-way ANOVA or a multiple t-test (as indicated), with a confidence interval of 95% (α = 0.05).
Results
Establishment of low-attachment primary human monocyte cultures for functional analysis
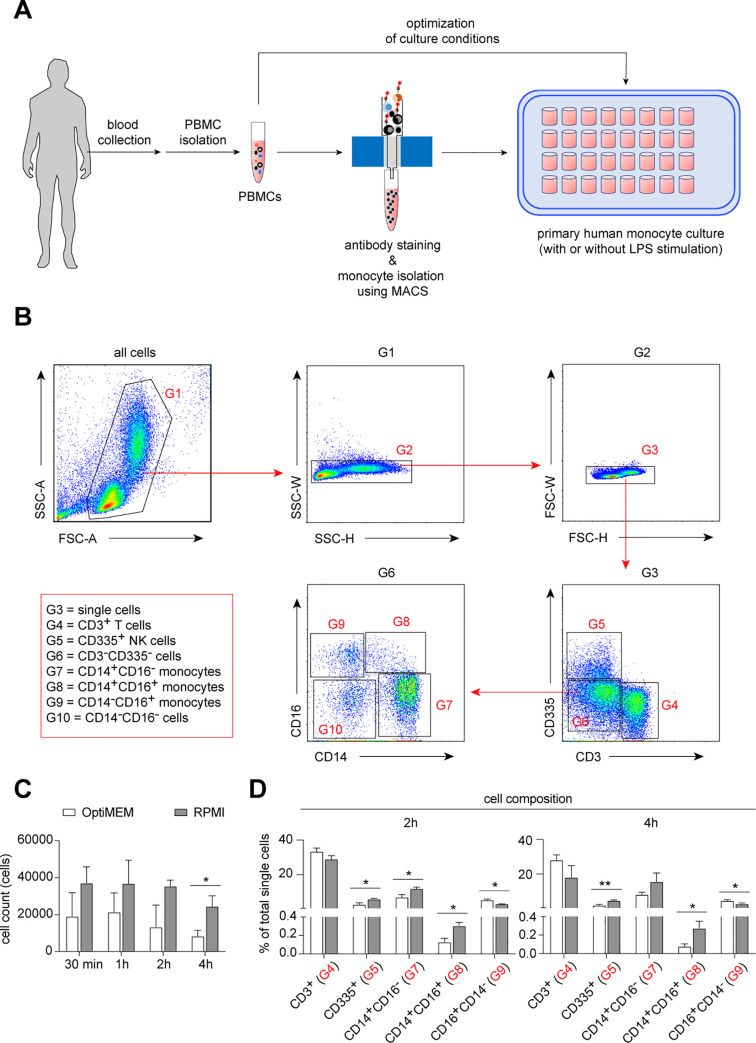
It has been demonstrated that ex vivo culture of human monocytes using classical polypropylene cell culture plates resulted in phenotypic and functional changes, such as an increased granularity and reduced transendothelial diapedesis function [24]. Therefore, we newly established an ex vivo culture system using an ultra-low attachment multiple well-plate (Fig 1A). First, we used peripheral blood mononuclear cells (PBMCs) to optimize the culture system (Fig 1A and 1B). We observed a better preservation of the cell composition in the culture system using RPMI-1640 medium compared with reduced serum medium OptiMEM (Fig 1C and 1D).
Fig 1. Low-attached in vitro cultured model of human monocytes.
(A) Schematic representation of experimental procedure. (B) Dot plots demonstrate flow cytometric gating strategy used to obtain monocytes (G7, G8 and G9) for all samples. (C) Bar graphs show cell numbers in different culture conditions at different time points in vitro. (D) Bar graphs show different cell composition of PBMCs that were cultured in OptiMEM or RPMI medium conditions at 2 and 4 hours in vitro. Multiple t-test, corrected for multiple comparisons using Holm-Sidak method, *P < 0.05, **P < 0.01.
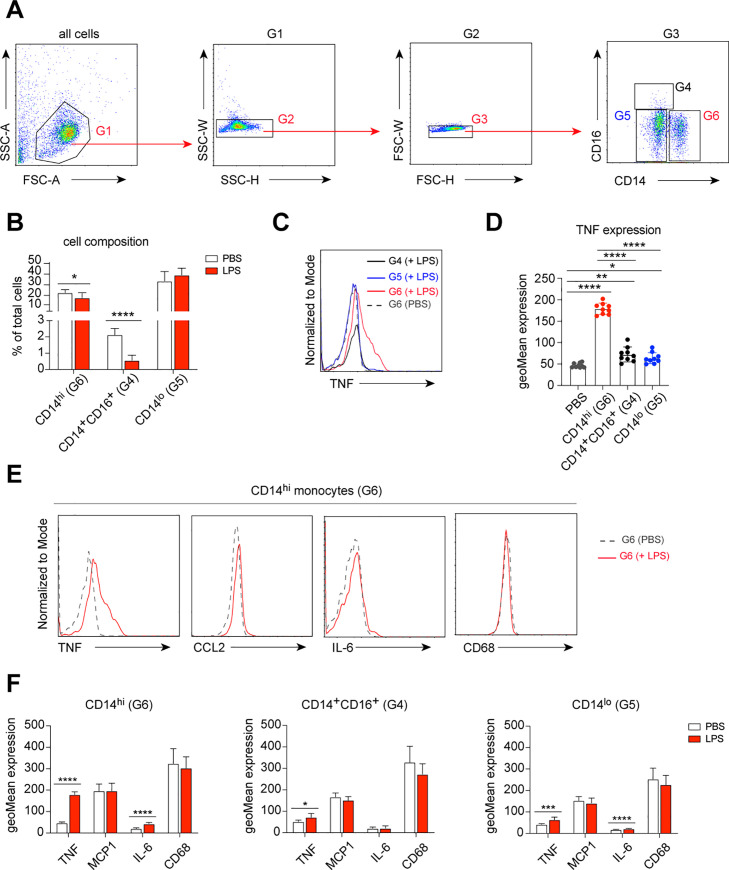
Next, we tested the LPS-induced inflammatory responses of monocytes under these culture conditions. We isolated monocytes (both CD14lo and CD14hi monocytes) from PBMCs using magnetic activated cell sorting (MACS) (Figs 1A and 2A). Isolated monocytes were then cultured in the established low-attachment culture system in the presence of 100 ng/mL LPS or PBS (control) for 6 hours. Compared to control, the cell composition of the monocyte population was changed after LPS stimulation. Namely, a strong reduction of CD14+CD16+ monocytes and a moderately decreased frequency of CD14hi monocytes were found (Fig 2B). As expected, increased expression of TNF was detected in all three monocyte populations after LPS stimulation, with the highest response in CD14hi inflammatory monocytes (Fig 2C and 2D). Furthermore, we found increased expression of IL-6 in CD14hi and CD14lo monocytes after LPS stimulation, whereas MCP-1 (or CCL2) and CD68 were unchanged under these conditions (Fig 2E and 2F).
Fig 2. LPS-induced cytokine production of cultured monocytes.
(A) Dot plots demonstrate flow cytometric gating strategy used to obtain monocytes (G4, G5 and G6) for all samples. (B) Bar graphs show changes in monocyte composition after LPS stimulation, compared with naïve monocytes. Multiple t-test, corrected for multiple comparisons using Holm-Sidak method, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (C) Histograms demonstrate fluorescent intensity representing LPS-induced TNF expression in different monocyte subsets, compared to naïve monocytes (unstimulated, PBS-treated). (D) Bar graphs show quantitative analysis of fluorescent intensity representing LPS-induced TNF expression in different monocyte subsets, compared to naïve monocytes (unstimulated, PBS-treated). Each dot represents an independent measurement. One-way ANOVA, corrected for multiple comparisons using Tukey Test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (E) Histograms demonstrate fluorescent intensity representing LPS-induced cytokine expression of CD14hi monocytes (red line), compared to naïve CD14hi monocytes (unstimulated, PBS-treated; dashed line). (F) Bar graphs show quantitative analysis of fluorescent intensity representing LPS-induced cytokine expression in different monocyte subsets, compared to naïve monocytes (unstimulated, PBS-treated). One-way ANOVA, corrected for multiple comparisons using Tukey Test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Dendrobium compound isolation, purification and identification
Phytochemical investigation from the whole plant of Dendrobium lindleyi (Fig 3A) revealed the isolation of a novel bibenzyl (Fig 3A, #1), along with four known compounds, which include chrysotoxine (Fig 3A, #2) [5, 6], gigantol (Fig 3A, #3) [8, 25–28], cypripedin (Fig 3A, #4) [7,29,30] and moscatilin (Fig 3A, #5) [9,27,31,32]. The structure of a new compound was elucidated through analysis of its spectroscopic data.
Compound #1 was obtained as a brown amorphous solid; UV (MeOH) λmax (log ε) 220 (4.09), 229 (4.08), 285 (3.76) nm; IR (film) νmax: 3428, 2924, 1724, 1629, 1605, 1515, 1453, 1263, 1093, 1027 cm-1; 1H NMR (500 MHz, CDCl3) and 13C NMR (125 MHz, CDCl3) spectral data, see Fig 3B; HR-ESI-MS: m/z 327.1212 [M+Na]+ (calcd. for C17H20O5Na, 327.1208). The positive HR-ESI-MS showed an [M+Na]+ ion at m/z 327.1212 (calcd. for C17H20O5Na, 327.1208), suggesting the molecular formula C17H20O5. The absorption bands of the IR spectrum were defined as hydroxyl (3428 cm-1), aromatic ring (2924, 1605 cm-1) and methylene (1453 cm-1) groups. The UV absorptions at 220, 229 and 285 nm were indicative of a bibenzyl nucleus [33]. This was confirmed by the presence of two pairs of methylene protons at δ 2.81 (H2-α) and 2.84 (H2-α′) ppm, which correlated to the carbon atoms at δ 38.0 (C-α) and 37.6 (C-α′) ppm, respectively. The 1H NMR spectrum (Fig 3B) also displayed five aromatic protons at δ 6.26–6.81 ppm and three methoxyl groups at δ 3.85 (3H, s, MeO-3), 3.87 (3H, s, MeO-3′) and 3.88 (3H, s, MeO-4′). On ring A, the 1H NMR spectrum showed signals for two doublets at δ 6.26 (J = 2.0 Hz, H-2) and 6.48 (J = 2.0 Hz, H-6), which correlated to C-α in the HMBC spectrum. The first methoxyl group (δ 3.85) was located at C-3 based on its NOESY correlation with H-2. The 1H NMR ABM coupling system of ring B appeared at δ 6.69 (1H, d, J = 2.0 Hz, H-2′), 6.73 (1H, dd, J = 8.0, 2.0 Hz, H-6′) and 6.81 (1H, d, J = 8.0 Hz, H-5′). The HMBC correlations of C-α′ with H-2′ and H-6′ and the NOESY cross-peak of two methoxy groups at δ 3.87 and δ 3.88 with H-2′ and H-5′, respectively, placed the second methoxyl group at C-3′ (δ 148.7) and the third methoxyl group at C-4′ (δ 147.2). Based on above spectral data, compound #1 was identified as 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl.
Evaluation of immune modulatory effects of Dendrobium compounds
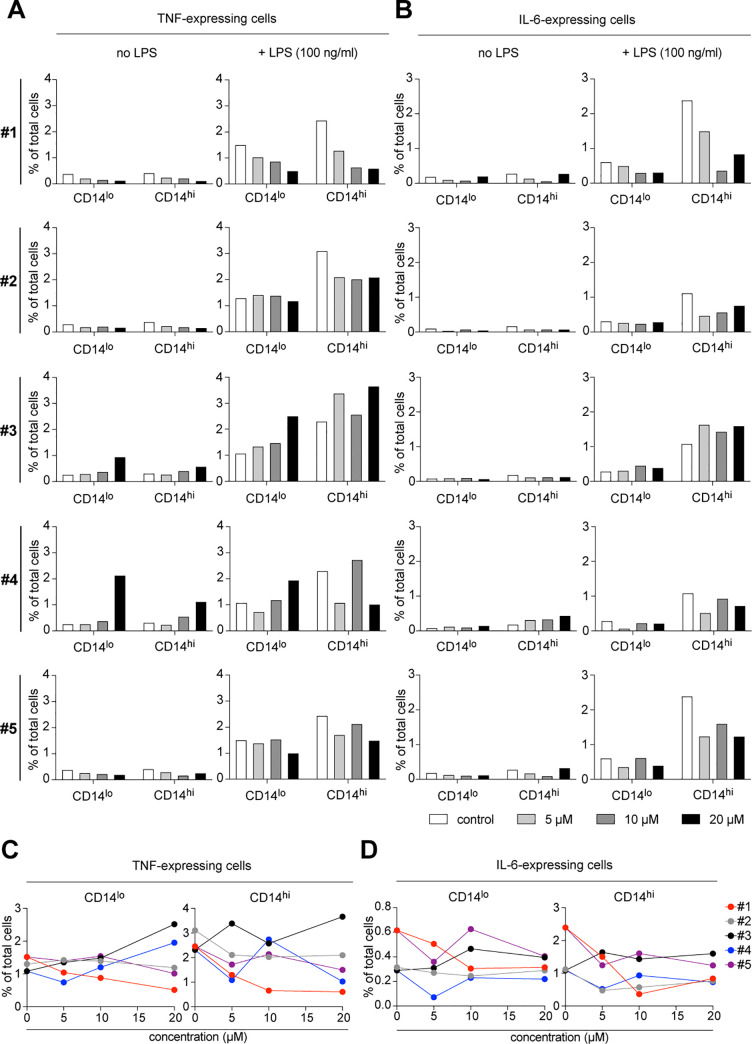
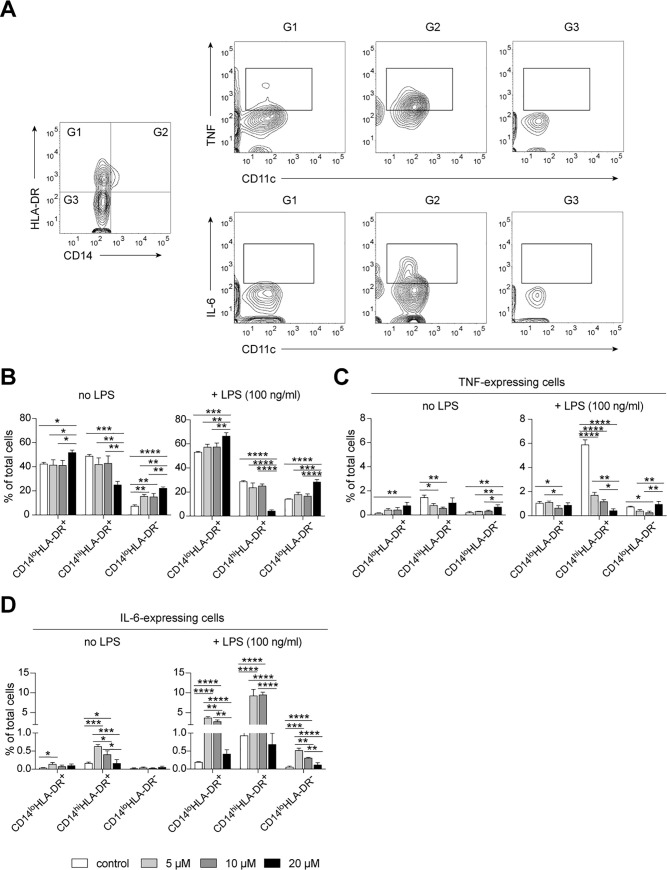
To assess immune modulatory effects of all five Dendrobium compounds, we cultured MACS-isolated primary human monocytes under our established culture conditions (Figs 1 and 2), including an inhibitor of intracellular protein transport–monensin, in the presence of Dendrobium compounds, which were 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl (#1), chrysotoxine (#2), gigantol (#3), cypripedin (#4) and moscatilin (#5). Since these five compounds were diluted in dimethyl sulfoxide (DMSO), the same concentration of DMSO was added to the control culture without any compounds. We tested three known therapeutic concentrations [5–9], which were 5, 10 and 20 μM. After 2 hours of incubation, we stimulated monocytes by adding 100 ng/mL LPS and incubated for four more hours. We observed an increased frequency of TNF-expressing cells in gigantol (#3)- and cypripedin (#4)-treated monocytes without LPS stimulation, suggesting immune modulatory effects of these compounds on monocyte responses ex vivo (Fig 4A and 4B). After LPS stimulation, we detected increased frequencies of TNF- and IL-6-expressing monocytes, which were most pronounced in CD14hi inflammatory monocytes (Fig 4A and 4B), which is in line with the results shown in Fig 2. Although we observed decreased frequencies of LPS-induced TNF- and IL-6-expressing cells in monocytes treated with 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl (#1), chrysotoxine (#2) and moscatilin (#5), only the compound #1 showed promising dose-dependent positive effects (Fig 4A–4D). Therefore, we selected the compound #1 for a further evaluation. We tested whether the immune modulatory effects of Dendrobium compounds on monocytes persisted also after long-term exposure to LPS. To do so, the isolated monocytes were first incubated in the presence of the compound #1 for two hours followed by overnight LPS-stimulation in a presence of monensin (5 μg/mL). We observed decreased expression of CD14 of the overnight cultured monocytes, and an increased abundance of TNF- and IL-6-expressing CD11clo monocytes (Fig 5A), especially in the CD14hiHLA-DR+ sub-population. Overnight exposure of monocytes to compound #1 resulted in changes in the monocyte composition, indicating as an increased abundance of CD14loHLA-DR- and CD14loHLA-DR+ cells and a decreased abundance of CD14hiHLA-DR+ cells (Fig 5B). These changes were found at a highest degree in monocytes treated with 20 μM of #1. Furthermore, these observed changes were more pronounced in LPS-stimulated monocytes (Fig 5B, +LPS). Similar to the short-term incubation (Fig 4), CD14hi monocytes were the main source of TNF- and IL-6-expressing cells (Fig 5A, 5C & 5D). Overnight incubation with 20 μM of the compound #1 alone induced TNF expression in CD14loHLA-DR+ and CD14loHLA-DR- cells (Fig 5C), while under LPS-stimulation it was downmodulating the LPS-induced TNF-expression (Fig 5C). In comparison to the control cells (without the compound #1), a treatment with 10 μM of #1 could decreased LPS-induced TNF expression in all monocyte subsets (Fig 5C). The treatments with either 5 or 20 μM of #1 could only decreased the LPS-induced TNF expression in CD14hiHLA-DR+ monocytes (Fig 5C). Without LPS-stimulation, the compound #1 induced IL-6 expression in both CD14lo and CD14hiHLA-DR+ monocytes, but not in CD14loHLA-DR- cells (Fig 5D). Interestingly, the 5 and 10 μM of the compound #1 strongly induced IL-6 expression in all monocyte subsets under LPS stimulation conditions, whereas the 20 μM concentration did not affect the expression of IL-6 in all three subsets (Fig 5D). The findings demonstrate a dose-dependent activating and/or immune modulatory effects of the compound #1, which are observed to be different in different monocyte sub-populations.
Fig 4. Immune modulatory effect of isolated compounds from Dendrobium lindleyi on LPS-stimulated human monocytes.
Immune modulatory effect of all five Dendrobium compounds on LPS-induced TNF (A,C) and IL-6 (B,D) expression of monocytes in vitro were evaluated. (A-B) Bar graphs show the mean frequency (%) of CD14lo and CD14hi monocytes, which express TNF and IL-6, compared to naïve monocytes (no LPS). This is an explorative screening experiment, in which each condition (or each concentration) was analyzed in duplicate, thus no variation was shown. (C-D) The X-Y graphs show the correlation between the frequency (%) of TNF- or IL-6-expressing CD14hi or CD14lo monocytes and the concentration of all five Dendrobium compounds (#1 - #5).
Fig 5. Immune modulatory effect of 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl on long-term LPS-stimulation of human monocytes.
(A) Contour plots demonstrate flow cytometric gating strategy used to analyze the long-term culture of low-attached monocytes for all samples. Expression of TNF and IL-6 of three monocyte subsets (G1, G2 and G3) were shown. (B) Bar graphs show changes in monocyte composition after overnight incubation with 5, 10 or 20 μM of 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl with (+ LPS) or without LPS (no LPS) stimulation. (C) Bar graphs show the mean frequency (%) of TNF-expressing monocytes after overnight incubation with 5, 10 or 20 μM of 4,5-dihydroxy-3,3’,4’-trimethoxybibenzyl with (+ LPS) or without LPS (no LPS) stimulation. (D) Bar graphs show the mean frequency (%) of IL-6-expressing monocytes after overnight incubation with 5, 10 or 20 μM of 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl with (+ LPS) or without LPS (no LPS) stimulation. One-way ANOVA, corrected for multiple comparisons using Tukey Test, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
In this study, we demonstrated the use of low-attached culture model of primary human monocytes as a tool to study immune modulatory effects of purified natural products (the Dendrobium compounds). We showed the immune modulatory potential of a novel Dendrobium compound: 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl, which appeared to be dose-dependent and different between monocyte subsets. In addition, we have tested those known compounds including chrysotoxine (#2), gigantol (#3), cypripedin (#4) and moscatilin (#5), which were proposed as potential candidates for the development of cancer therapy. However, these compounds appeared to have either less immune modulatory activity or even more inflammatory effect on primary monocytes, compared with the compound #1. Our findings suggest an importance and/or feasibility of using ex vivo studied models of different primary human (immune) cells to estimate the therapeutic potential and/or any potential adverse effects of purified natural products, which will further facilitate the adjustment of developmental potential of each isolated compound in diseases.
In the low-attached monocyte culture model used in this study, we found stronger responses to TLR4 agonist LPS of CD14hi monocytes, compared to CD14lo monocytes, on the basis of the expression of inflammatory cytokines especially TNF and IL-6. Our results are in line with the study by Cros et al. in which CD14dim was demonstrated to respond poorly to agonists of TLR1, TLR2 and TLR4 [34]. The inflammatory cytokine TNF has been shown to be involved in many biological processes including fever, apoptosis, and infection-induced cachexia [16], as well as in inflammation-associated diseases, for example rheumatoid arthritis [35], acquired generalized lipodystrophy and combined Crohn’s disease [36], Crohn's disease [37], and type 2 diabetes [38]. Moreover, chemokine such as TNF can also induce activate inflammatory responses, and are implicated in the regulation of tumor development and growth via regulation of tumor-associated angiogenesis, by activation of host immunological responses or by direct inhibition of tumor cell proliferation [39]. It has been also shown that suppressing the increased expression of inflammation-related factors such as TNF is considered to potentially suppress the migration of macrophages into tumor tissues and regulate inflammatory changes in the tumor environments, respectively [40]. In this study, we detected Dendrobium compound-induced TNF expression in monocytes treated with 20 μM of gigantol (#3) or cypripedin (#4), suggesting potentially inflammatory effects of these compounds at high concentration. Whereas 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl (#1), chrysotoxine (#2) and moscatilin (#5) did not show obvious inflammatory effects on monocytes in vitro, only the novel compound #1 has shown promising immune modulatory effects in a dose-dependent manner. However, in a long-term LPS-stimulation, only the 10 μM of the compound #1 could downmodulate the LPS-induced TNF expression in all monocyte subsets, whereas the concentration of 20 μM induced TNF expression of unstimulated cells, suggesting dose- and treatment-duration-dependent immune modulatory effects and/or inflammatory effects of this compound.
Interleukin-6 (IL-6) is a cytokine with pleiotropic function, which are involved in host defense. In acute infection and/or tissue injury, monocytes/macrophages promptly produce IL-6 and contribute to removal of infectious agents and regeneration of damaged tissue through activation of immune, hematological, and acute-phase responses [41]. However, a dysregulation of IL-6 production and/or persistently increased IL-6 expression plays a pathological role in the development of various inflammatory diseases and cancers, thus IL-6 is a double-edged sword for the host [41]. Thus, the proper IL-6 expression is very important for host defense. In this study, although we did observe the reduction of the LPS-induced IL-6 production in monocytes, that were short-term treated with the compound #1, we found strongly increased production of IL-6 in long-term treated monocytes (Fig 5). However, since we detected a decrease in LPS-induced TNF-expression under the same conditions, an increased IL-6 expression observed in this study may refer to increased monocyte activity against LPS-induced inflammation.
Conclusions
In summary, we demonstrate herein immune modulatory effects of the new compound 4,5-dihydroxy-3,3′,4′-trimethoxybibenzyl on different monocyte subsets ex vivo, which can potentially be developed as in vivo immune modulatory drug in a broad spectrum of inflammation-driven diseases besides lung carcinomas. However, since Dendrobium lindleyi is widely distributed, using this orchid for drug development may have a risk of habitat destruction, over-collection and commercially trade due to low natural regeneration rates. Moreover, Dendrobium plants are listed on Appendix II (D. cruentum is the only species listed on Appendix I) of Convention on International Trade in Endangered Species of Wild Fauna and Flora [42], which monitors, regulates or bans the trade to ensure species survival [43]. Therefore, in parallel to drug development, an establishment and improvement of methodologies/techniques for seedling propagation and artificial cultivation technology such as tissue culture and artificial-sheltered cultivation [44–47] are required to ensure sustainable development and future marketing.
Acknowledgments
The authors are grateful to Christian Böttcher for providing helpful technical support with the generation of PBMCs.
Data Availability
All relevant data are within the paper.
Funding Statement
P.K. acknowledged the financial support of the scholarship from the Graduate School, Chulalongkorn University to commemorate the 72nd anniversary of his Majesty King Bhumibol Adulyadej. C.B. and J.P. were supported by the German Research Foundation (SFB TRR167, B05 & B07). J.P. received additional funding from the Berlin Institute of Health (CRG2aSP6) and the UK DRI (Momentum Award). B.S. is grateful to Faculty of Pharmaceutical Sciences, Chulalongkorn University for a research fund (Phar2563-RG010). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Hou B, Luo J, Zhang Y, Niu Z, Xue Q, Ding X. Iteration expansion and regional evolution: phylogeography of Dendrobium officinale and four related taxa in southern China. Sci Rep. 2017; 7: 43525 10.1038/srep43525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam Y, Ng TB, Yao RM, Shi J, Xu K, Sze SC, et al. Evaluation of chemical constituents and important mechanism of pharmacological biology in Dendrobium plants. Evid Based Complement Alternat Med. 2015; 2015: 841752 10.1155/2015/841752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng J, Dang PP, Zhao Z, Yuan LC, Zhou ZH, Wolf D, et al. An assessment of the Chinese medicinal Dendrobium industry: Supply, demand and sustainability. J Ethnopharmacol. 2019; 229: 81–88. 10.1016/j.jep.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 4.Ng TB, Liu J, Wong JH, Ye X, Wing Sze SC, Tong Y, et al. Review of research on Dendrobium, a prized folk medicine. Appl Microbiol Biotechnol. 2012; 93: 1795–1803. 10.1007/s00253-011-3829-7 [DOI] [PubMed] [Google Scholar]
- 5.Chanvorachote P, Kowitdamrong A, Ruanghirun T, Sritularak B, Mungmee C, Likhitwitayawuid K. Anti-metastatic activities of bibenzyls from Dendrobium pulchellum. Nat Prod Commun. 2013; 8: 115–118. [PubMed] [Google Scholar]
- 6.Bhummaphan N, Pongrakhananon V, Sritularak B, Chanvorachote P. Cancer stem cell-suppressing activity of chrysotoxine, a bibenzyl from Dendrobium pulchellum. J Pharmacol Exp Ther. 2018; 364: 332–346. 10.1124/jpet.117.244467 [DOI] [PubMed] [Google Scholar]
- 7.Treesuwan S, Sritularak B, Chanvorachote P, Pongrakhananon V. Cypripedin diminishes an epithelial-to-mesenchymal transition in non-small cell lung cancer cells through suppression of Akt/GSK-3β signalling. Sci Rep. 2018; 8: 8009 10.1038/s41598-018-25657-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unahabhokha T, Chanvorachote P, Sritularak B, Kitsongsermthon J, Pongrakhananon V. Gigantol inhibits epithelial to mesenchymal process in human lung cancer cells. Evid Based Complement Alternat Med. 2016; 2016: 4561674 10.1155/2016/4561674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busaranon K, Plaimee P, Sritularak B, Chanvorachote P. Moscatilin inhibits epithelial-to-mesenchymal transition and sensitizes anoikis in human lung cancer H460 cells. J Nat Med. 2016; 70: 18–27. 10.1007/s11418-015-0931-7 [DOI] [PubMed] [Google Scholar]
- 10.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008; 11: 1–15. 10.1080/10937400701436460 [DOI] [PubMed] [Google Scholar]
- 11.Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD, et al. Single-cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity. 2019; 50: 1317–1334. 10.1016/j.immuni.2019.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Strom SR, Burdick MD, et al. Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer Immunol Immunother. 2000; 49: 63–70. 10.1007/s002620050603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balli D, Ren X, Chou FS, Cross E, Zhang Y, Kalinichenko VV, et al. Foxm1 transcription factor is required for macrophage migration during lung inflammation and tumor formation. Oncogene. 2012; 31: 3875–3888. 10.1038/onc.2011.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010; 116: e74–e80. 10.1182/blood-2010-02-258558 [DOI] [PubMed] [Google Scholar]
- 15.Gordon S, Taylor P. Monocyte and macrophage heterogeneity. Nature reviews Immunology. 2005; 5: 953 10.1038/nri1733 [DOI] [PubMed] [Google Scholar]
- 16.Abbas AK, Lichtman AHH, Pillai S. Cellular and Molecular Immunology. Elsevier Health Sciences; 2014. [Google Scholar]
- 17.Campos DP, Silva MV, Machado JR, Castellano LR, Rodrigues V, Barata CH. Early-onset neonatal sepsis: cord blood cytokine levels at diagnosis and during treatment. J Pediatr (Rio J). 2010; 86: 509–514. 10.2223/JPED.2043 [DOI] [PubMed] [Google Scholar]
- 18.Tabas I, GarcõÂa-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015; 209: 13–22. 10.1083/jcb.201412052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan FM, Maini RN, Feldmann M. Role of pro-inflammatory cytokines in rheumatoid arthritis. Springer Semin Immunopathol. 1998; 20: 133–147. 10.1007/BF00832003 [DOI] [PubMed] [Google Scholar]
- 20.Hemmann S, Graf J, Roderfeld M, Roeb E. Expression of MMPs and TIMPs in liver fibrosis±a systematic review with special emphasis on anti-fibrotic strategies. J Hepatol. 2007; 46: 955–975. 10.1016/j.jhep.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 21.Meadow W, Rudinsky B. Inflammatory mediators and neonatal sepsis. Rarely has so little been known by so many about so much. Clin Perinatol. 1995; 22: 519–536. [PubMed] [Google Scholar]
- 22.Srirangan S, Choy EH. The Role of Interleukin 6 in the Pathophysiology of Rheumatoid Arthritis. Ther Adv Musculoskelet Dis. 2010; 2: 247–256. 10.1177/1759720X10378372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaddhanaphuti N. A field guide to the wild orchids of Thailand. 4th ed Chiang Mai: Silkworm Books; 2005 [Google Scholar]
- 24.Tsubota Y, Frey JM, Raines EW. Novel ex vivo culture method for human monocytes uses shear flow to prevent total loss of transendothelial diapedesis function. J Leukoc Biol. 2014; 95: 191–195. 10.1189/jlb.0513272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Xu J, Yu H, Qing C, Zhang Y, Wang L, et al. Cytotoxic phenolics from Bulbophyllum odoratissimum. Food Chem. 2008; 107: 169–73. 10.1016/j.foodchem.2007.07.077 [DOI] [Google Scholar]
- 26.Sarakulwattana C, Mekboonsonglarp W, Likhitwitayawuid K, Rojsitthisak P, Sritularak B. New bisbibenzyl and phenanthrene derivatives from Dendrobium scabrilingue and their α-glucosidase inhibitory activity. Nat Prod Res. 2018; 22: 1–8. 10.1080/14786419.2018.1527839 [DOI] [PubMed] [Google Scholar]
- 27.Klongkumnuankarn P, Busaranon K, Chanvorachote P, Sritularak B, Jongbunprasert V, Likhitwitayawuid K. Cytotoxic and antimigratory activities of phenolic compounds from Dendrobium brymerianum. Evid Based Complement Alternat Med. 2015; 2015: 350410 10.1155/2015/350410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sukphan P, Sritularak B, Mekboonsonglarp W, Lipipun V, Likhitwitayawuid K. Chemical constituents of Dendrobium venustum and their antimalarial and anti-herpetic properties. Nat Prod Commun. 2014; 9: 825–827. [PubMed] [Google Scholar]
- 29.Fan C, Wang W, Wang Y, Qin G, Zhao W. Chemical constituents from Dendrobium densiflorum. Phytochemistry. 2001; 57: 1255–1258. 10.1016/s0031-9422(01)00168-6 [DOI] [PubMed] [Google Scholar]
- 30.Wattanathamsan O, Treesuwan S, Sritularak B, Pongrakhananon V. Cypripedin, a phenanthrenequinone from Dendrobium densiflorum, sensitizes non-small cell lung cancer H460 cells to cisplatin-mediated apoptosis. J Nat Med. 2018; 72: 503–513. 10.1007/s11418-018-1176-z [DOI] [PubMed] [Google Scholar]
- 31.Sritularak B, Duangrak N, Likhitwitayawuid K. A new bibenzyl from Dendrobium secundum. Z Naturforsch C J Biosci. 2011; 66: 205–208. 10.1515/znc-2011-5-602 [DOI] [PubMed] [Google Scholar]
- 32.Majumder PL, Sen RC. Moscatilin, a bibenzyl derivative from the orchid Dendrobium moscatum. Phytochemistry. 1987; 26: 2121–2124. [Google Scholar]
- 33.Zhang X, Xu JK, Wang J, Wang NL, Kurihara H, Kitanaka S, et al. Bioactive bibenzyl derivatives and fluorenones from Dendrobium nobile. J Nat Prod. 2007; 70: 24–8. 10.1021/np060449r [DOI] [PubMed] [Google Scholar]
- 34.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010; 33: 375–86. 10.1016/j.immuni.2010.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neovius M, Arkema EV, Olsson H, Eriksson JK, Kristensen LE, Simard JF, et al. Drug survival on TNF inhibitors in patients with rheumatoid arthritis comparison of adalimumab, etanercept and infliximab. Ann Rheum Dis. 2015; 74: 354–360. 10.1136/annrheumdis-2013-204128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziegler JF, Böttcher C, Letizia M, Yerinde C, Wu H, Freise I, et al. Leptin induces TNFα-dependent inflammation in acquired generalized lipodystrophy and combined Crohn’s disease. Nat Commun. 2019; 10: 5629 10.1038/s41467-019-13559-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stidham RW, Lee TCH, Higgins PDR, Deshpande AR, Sussman DA, Singal AG, et al. Systematic review with network meta-analysis: the efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment Pharmacol Amp Ther. 2014; 39: 1349–1362. 10.1111/apt.12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Qiao Y, Xu Y, Ling W, Pan Y, Huang Y, et al. Serum TNF-α concentrations in type 2 diabetes mellitus patients and diabetic nephropathy patients: A systematic review and meta-analysis. Immunol Lett. 2017; 186: 52–58. 10.1016/j.imlet.2017.04.003 . [DOI] [PubMed] [Google Scholar]
- 39.Chada S, Ramesh R, Mhashilkar AM. Cytokine- and chemokine-based gene therapy for cancer. Curr Opin Mol Ther. 2003; 5: 463–74. [PubMed] [Google Scholar]
- 40.Honda T, Inagawa H. Usefulness of Monocytes/macrophages Activated with low-dose lipopolysaccharide in tumor tissue and adipose tissue of obesity. Anticancer Res. 2019; 39: 4475–4478. 10.21873/anticanres.13621 [DOI] [PubMed] [Google Scholar]
- 41.Tanaka T, Narazaki M, Masuda K, Kishimoto T. Regulation of IL-6 in Immunity and Diseases. Adv Exp Med Biol. 2016; 941: 79–88. 10.1007/978-94-024-0921-5_4 [DOI] [PubMed] [Google Scholar]
- 42.CITES, 2017. Criteria for the inclusion of species in Appendices I, II and III, valid from 2017-10-04. https://www.cites.org/eng/app/appendices.php.
- 43.Bai Y, Bao YH, Jin JX, Yan YN, Wang WQ. Investigation and study on medicinal Dendrobium resources in China. Chin Tradit Herbal. Drugs. 2006; 37: 1440–1442. [Google Scholar]
- 44.Tang GX, Huang FD, Zhou WJ. Studies on the seed embryo germination and propagation of Dendrobium candidum in vitro. China J Chin Mater Med. 2005; 30: 1583–1586. [PubMed] [Google Scholar]
- 45.Zheng ZR, Zhu JH, Li XG, Lou YX, Li WK. Culture in vitro and rapid propagation of Dendrobium officinale. Acta Agric Shanghai. 2008; 24: 19–23. [Google Scholar]
- 46.Zeng WY, Li JH, Wang Z, Yang YW, Hu Q. Research on the condition of aseptic germination and plantlet rapid propagation of Dendrobium officinale kimura et migo. J Wuhan Polytech Univ. 2012; 31: 10–13. [Google Scholar]
- 47.Zeng SH, Xiao FH, Zha YH, Wen GS, Yang SC, Duan CL. Preliminary evaluation of the population of Dendrobium officinale in Wenshan, Yunnan. Lishizhen Med Mater Med Res. 2012; 23: 468–470. [Google Scholar]