Abstract
The ORCHID (Outcomes Related to COVID-19 treated with Hydroxychloroquine among In-patients with symptomatic Disease) trial is a multicenter, blinded, randomized trial of hydroxychloroquine versus placebo for the treatment of adults hospitalized with coronavirus disease (COVID-19). This document provides the rationale and background for the trial and highlights key design features. We discuss five novel challenges to the design and conduct of a large, multicenter, randomized trial during a pandemic, including 1) widespread, off-label use of the study drug before the availability of safety and efficacy data; 2) the need to adapt traditional procedures for documentation of informed consent during an infectious pandemic; 3) developing a flexible and robust Bayesian analysis incorporating significant uncertainty about the disease, outcomes, and treatment; 4) obtaining indistinguishable drug and placebo without delaying enrollment; and 5) rapidly obtaining administrative and regulatory approvals. Our goals in describing how the ORCHID trial progressed from study conception to enrollment of the first patient in 15 days are to inform the development of other high-quality, multicenter trials targeting COVID-19. We describe lessons learned to improve the efficiency of future clinical trials, particularly in the setting of pandemics. The ORCHID trial will provide high-quality, clinically relevant data on the safety and efficacy of hydroxychloroquine for the treatment of COVID-19 among hospitalized adults.
Clinical trial registered with www.clinicaltrials.gov (NCT04332991).
Keywords: COVID-19, SARS-CoV-2, ARDS, hydroxychloroquine, ORCHID
Coronavirus Disease 2019 (COVID-19) is an acute respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1). Though most adults with COVID-19 recover after a mild course (2, 3), a minority develop pneumonia and hypoxemic respiratory failure requiring hospitalization. Severe illness may progress to acute respiratory distress syndrome (ARDS) and death (1, 2, 4). Hydroxychloroquine has generated substantial interest as a potential treatment for COVID-19 because of its widespread availability, antiviral and immunomodulatory activity, and established safety profile from historical use for other indications (5, 6).
Hydroxychloroquine is approved by the United States Food and Drug Administration (FDA) as an antiparasitic agent for malaria and an immunomodulatory agent for rheumatologic diseases (7–9). In vitro, hydroxychloroquine limits entry of SARS-CoV-2 into cells by inhibiting glycosylation of cell receptors targeted by coronaviruses, interfering with proteolytic processing, and increasing endosomal pH to limit endosome-mediated viral entry and late-stage viral replication (5, 6, 10–14). Furthermore, hydroxychloroquine reduces the production of several proinflammatory cytokines potentially involved in the development of ARDS among those infected with SARS-CoV-2 (8, 9, 15).
Based on these mechanisms of action and clinical experience early in the pandemic, hydroxychloroquine is being widely used off-label as a treatment for COVID-19 in routine clinical care (16). Hydroxychloroquine has been adopted into treatment guidelines for COVID-19 in China (17) and some U.S. hospitals (18–20). Interim guidance from an International Task Force for the American Thoracic Society suggested administering hydroxychloroquine to hospitalized COVID-19 patients with pneumonia (21). On March 28, 2020, the FDA issued an emergency use authorization to allow use of hydroxychloroquine from the Strategic National Stockpile to treat COVID-19 patients hospitalized in the United States when enrollment in a clinical trial is not feasible (22).
Despite widespread use and rapid incorporation into treatment guidelines, data informing the efficacy and safety of hydroxychloroquine as a treatment for COVID-19 remain very limited. In a small case series, hydroxychloroquine may have been associated with more rapid viral clearance (23). In a 62-patient randomized trial, hydroxychloroquine may have shortened the duration of fever and cough (24). In other studies, however, hydroxychloroquine failed to improve viral clearance or clinical endpoints (25, 26). In an observational study of 1,446 patients hospitalized at New York–Presbyterian Hospital with COVID-19, use of hydroxychloroquine was not associated with improved outcomes (27). Recently, concerns have been raised regarding QT prolongation and arrhythmias associated with hydroxychloroquine use, particularly among patients receiving high doses of chloroquine or hydroxychloroquine in combination with other QT-prolonging medications (28, 29).
On April 21, 2020, the U.S. National Institutes of Health posted COVID-19 treatment guidelines stating there are insufficient clinical data to recommend either for or against the use of hydroxychloroquine (30). In COVID-19 treatment guidelines published April 11, 2020, the Infectious Disease Society of America recommended hydroxychloroquine be used within the context of a clinical trial and called for additional high-quality clinical trial data on the safety and efficacy of hydroxychloroquine as a treatment for COVID-19 among hospitalized patients (31).
Given the urgent need for effective therapies for COVID-19 and the public health imperative to evaluate an unproven treatment being broadly administered to patients, we designed the ORCHID (Outcomes Related to COVID-19 treated with Hydroxychloroquine among In-patients with symptomatic Disease) trial.
Methods
Trial methods are summarized in Tables 1, 2, and 3 with the following sections providing additional context. The complete protocol, the Standard Protocol Items: Recommendations for Interventional Trials checklist, and a schedule of enrollment, interventions, and assessments are provided in the online supplement (32).
Table 1.
Eligibility criteria
| Inclusion criteria | 1. Age ≥18 yr |
| 2. Currently hospitalized or in an emergency department with anticipated hospitalization | |
| 3. Symptoms of acute respiratory infection, defined as one or more of the following: | |
| a. Cough | |
| b. Fever (>37.5°C/99.5°F) | |
| c. Shortness of breath (operationalized as any of the following: subjective shortness of breath reported by patient/surrogate; hypoxemia, defined as SpO2 < 92% on room air or increased oxygen requirement for a patient on chronic oxygen to maintain SpO2 ≥ 92%; tachypnea with respiratory rate ≥22/min). | |
| d. Sore throat | |
| 4. Laboratory-confirmed SARS-CoV-2 infection within the past 10 d before randomization | |
| Exclusion criteria | 1. Prisoner |
| 2. Pregnancy | |
| 3. Breast feeding | |
| 4. Unable to randomize within 10 d after onset of acute respiratory infection symptoms | |
| 5. Unable to randomize within 48 h after hospital arrival | |
| 6. Seizure disorder | |
| 7. Porphyria cutanea tarda | |
| 8. QTc > 500 ms on electrocardiogram within 72 h before enrollment | |
| 9. Diagnosis of long QT syndrome | |
| 10. Known allergy to hydroxychloroquine, chloroquine, or amodiaquine | |
| 11. Receipt in the 12 h before enrollment or planned administration during the 5-d study period that treating clinicians feel cannot be substituted for another medication of any of the following: amiodarone, cimetidine, dofetilide, phenobarbital, phenytoin, sotalol | |
| 12. Receipt of >1 dose of hydroxychloroquine or chloroquine in the 10 d before enrollment | |
| 13. Inability to receive enteral medications | |
| 14. Refusal or inability to be contacted on Day 15 for clinical outcome assessment if discharged before Day 15 | |
| 15. Previous enrollment in this trial | |
| 16. The treating clinical team does not believe equipoise exists regarding the use of hydroxychloroquine for the treatment of this patient |
Definition of abbreviations: QTc = corrected QT; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SpO2 = oxygen saturation as measured by pulse oximetry.
Table 2.
Trial outcomes
| Primary outcome | COVID Ordinal Outcomes Scale assessed 14 d after randomization (on Study Day 15) |
| 1. Death | |
| 2. Hospitalized on invasive mechanical ventilation or ECMO | |
| 3. Hospitalized on non-invasive ventilation or HFNC | |
| 4. Hospitalized on supplemental oxygen | |
| 5. Hospitalized not on supplemental oxygen | |
| 6. Not hospitalized with limitation in activity | |
| 7. Not hospitalized without limitation in activity | |
| Secondary outcomes | Time to recovery, defined as time to reaching level 5, 6, or 7 on the COVID outcomes scale, which is the time to the earlier of final liberation from supplemental oxygen or hospital discharge |
| All-location, all-cause 14-d mortality (assessed on Study Day 15) | |
| All-location, all-cause 28-d mortality (assessed on Study Day 29) | |
| COVID ordinal outcomes scale measured 2 d after randomization (assessed on Study Day 3) | |
| COVID ordinal outcomes scale measured 7 d after randomization (assessed on Study Day 8) | |
| COVID ordinal outcomes scale measured 28 d after randomization (assessed on Study Day 29) | |
| Composite of death or receipt of ECMO through Day 28 | |
| Oxygen-free days through Day 28 | |
| Ventilator-free days through Day 28 | |
| Vasopressor-free days through Day 28 | |
| ICU-free days through Day 28 | |
| Hospital-free days through Day 28 | |
| Safety outcomes | Seizure |
| Atrial or ventricular arrhythmia | |
| Cardiac arrest | |
| Elevation in AST or ALT to twice upper limit of normal | |
| Acute pancreatitis | |
| Acute kidney injury | |
| Receipt of renal replacement therapy | |
| Symptomatic hypoglycemia | |
| Neutropenia, lymphopenia, anemia, or thrombocytopenia | |
| Severe dermatologic reaction |
Definition of abbreviations: ALT = alanine aminotransferase; AST = aspartate aminotransferase; COVID = coronavirus disease; ECMO = extracorporeal membrane oxygenation; HFNC = high-flow nasal cannula; ICU = intensive care unit.
Table 3.
Allocation, blinding, and statistical methods
| SPIRIT | ORCHID |
|---|---|
| Allocation | |
| Sequence generation | Patient-level randomization |
| 1:1 ratio of hydroxychloroquine to placebo | |
| Randomized in permuted blocks of varying size, stratified by treatment site | |
| Allocation concealment enrollment and randomization | The randomized sequence is stored on a secure server and not available to site study personnel; patients are enrolled via central web-based randomization, accessible 24 h/d. |
| Blinding | Blinded, placebo-controlled |
| Statistical methods | Intention-to-treat comparison between groups using a proportional odds model with the COVID ordinal outcome score 14 d after randomization (assessed on Study Day 15) as the dependent variable, randomized group assignment as the primary independent variable, and the following covariates: age, sex, baseline COVID ordinal outcome score, baseline SOFA score, and duration of acute respiratory infection symptoms before randomization. |
| Interim analyses | Bayesian sequential design with interim analyses at least every 102 patients and suggested stopping rules for efficacy and futility. Statistician will present unblinded outcomes with Bayesian posterior probabilities to data and safety monitoring board at each interim analysis. |
Definition of abbreviations: COVID = coronavirus disease; ORCHID = Outcomes Related to COVID-19 treated with Hydroxychloroquine among In-patients with symptomatic Disease; SOFA = Sequential Organ Failure Assessment; SPIRIT = Standardized Protocol Items: Recommendations for Interventional Trials.
Trial Design
The ORCHID trial is a patient-level, parallel-group, blinded, randomized clinical trial evaluating the superiority of hydroxychloroquine compared with placebo. The trial aims to enroll patients early after hospital presentation, screening in emergency departments, inpatient floors, and intensive care units of participating hospitals. The trial protocol was approved by the single institutional review board (IRB) at Vanderbilt University Medical Center and is being conducted with an exception from Investigational New Drug application requirements. An independent Data and Safety Monitoring Board (DSMB) is monitoring the trial.
Study Sites
The ORCHID trial is being conducted by the National Heart, Lung and Blood Institute Prevention and Early Treatment of Acute Lung Injury (PETAL) Clinical Trials Network. The PETAL Network consists of acute care and critical care researchers at more than 50 enrolling centers dedicated to conducting randomized trials to treat patients with or at risk for ARDS (additional details in online supplement) (33, 34). Massachusetts General Hospital serves as the coordinating center for the PETAL Clinical Trials Network.
Population
The trial includes hospitalized adults with laboratory-confirmed SARS-CoV-2 and symptoms of acute respiratory infection. Given delays in SARS-CoV-2 testing early in the pandemic, the trial initially included hospitalized patients with suspected or confirmed SARS-CoV-2 infection, but as testing capabilities improved, the inclusion criteria were narrowed on April 21, 2020, to include only laboratory-confirmed cases. Key exclusion criteria are corrected QT (QTc) interval >500 milliseconds, history of long QT syndrome, seizure disorder, respiratory symptoms for more than 10 days, hospitalization for greater than 48 hours, and receipt of medications that may adversely interact with hydroxychloroquine. A complete list of exclusion criteria is presented in Table 1.
Justification for ORCHID Trial Population
Eligibility criteria focusing on hospitalization and duration of symptoms are intended to target a population that is at high risk for poor clinical outcomes while still being in the acute phases of illness in which viral replication may still play a pathophysiologic role. Remaining exclusion criteria serve to protect vulnerable populations (e.g., prisoners) and exclude patients for whom receipt of hydroxychloroquine might increase the risk for serious adverse events (e.g., patients with a prolonged QTc or seizure disorder). Pregnant women are excluded from ORCHID because 1) hydroxychloroquine crosses the placental barrier; 2) although hydroxychloroquine is sometimes used in pregnancy for malaria and rheumatologic conditions, there is known clinical efficacy for those conditions but not for COVID-19; 3) the trial would not enroll a sufficiently large number of pregnant women to be able to draw meaningful conclusions; and 4) the drug is available outside of the clinical trial if there are particular pregnant patients for whom a clinician believes the potential benefits outweigh potential risks.
Process of Informed Consent during a Pandemic
Conducting clinical research in the setting of a pandemic infection presents unique challenges. Bringing a paper consent form and pen to the bedside of a patient with COVID-19 and then taking these out of the room would violate infection prevention principles and policies. Furthermore, face-to-face interaction between patients and research personnel would expend valuable personal protective equipment, which has limited availability in many areas of the United States. Finally, legally authorized representatives (LARs) are often prohibited from in-person visits. Following guidance from the FDA and Office for Human Research Protections, the ORCHID trial therefore documents the completion of written informed consent from the patient or LAR using “no-touch” procedures (35). These include 1) electronic consent using a study device approved to store protected health information or the patient’s or LAR’s own smart phone with signatures uploaded directly to an electronic database, 2) paper-based consent with photographic documentation of signature pages, and 3) when the prior two are not feasible, signed attestation by study staff and an impartial witness that the patient reviewed and signed the paper informed consent document (details in online supplement).
Randomization and Blinding
Patients are randomized 1:1 to hydroxychloroquine or placebo via central web-based randomization in permuted blocks of varying size, stratified by treatment site. The randomized sequence is stored on a secure electronic server not available to site study personnel.
The patients, treating clinicians, study personnel, and outcome assessors are blinded to group assignment.
During trial planning, it was noted that many participating institutions already included hydroxychloroquine as part of treatment algorithms for COVID-19. Concerns were raised regarding the feasibility of conducting a trial in which participants might be randomized to a group that would not receive hydroxychloroquine. There was broad agreement that hydroxychloroquine administration as part of a clinical study was preferable to off-label clinical use because it would increase the quality of informed consent, improve safety monitoring, and contribute to understanding of possible efficacy. There were, however, discussions regarding alternative allocation strategies that would decrease the number of patients randomized to placebo. Ultimately, however, a 1:1 ratio to hydroxychloroquine versus placebo was chosen because it is the approach to allocation that most efficiently produces robust data on efficacy and safety while exposing the fewest patients to the study drug should it prove to be ineffective or harmful.
Study Interventions
Hydroxychloroquine group
Patients assigned to the hydroxychloroquine arm receive hydroxychloroquine sulfate enterally for a total of 5 days: 400 mg twice daily for the first two doses and then 200 mg twice daily for the subsequent eight doses.
Placebo group
Patients randomized to the placebo group receive placebo twice daily in a dosing regimen matching that described above for hydroxychloroquine.
The process of manufacturing placebo tablets that are identical to study drug may be time consuming. In the face of a rapidly evolving pandemic, investigators faced the options of either delaying enrollment to await manufacture of placebo tablets or conducting an open-label trial without blinding. Instead, the ORCHID trial developed a process to create identical hydroxychloroquine and placebos through encapsulation of commercially available hydroxychloroquine (details in online supplement). Because the manual encapsulation process was laborious and not available at all sites, it was replaced by centrally distributed, identical hydroxychloroquine and placebo tablets as soon as these were available (shipped to sites on April 23, 2020), but it allowed the rapid launch of the ORCHID trial while maintaining a high-quality double-blinded design.
Justification of drug and dosing regimen
Hydroxychloroquine was favored over chloroquine by the ORCHID investigators given in vitro data demonstrating more potent antiviral activity against SARS-CoV-2 (6) as well as lower toxicity (36). The dosing regimen in ORCHID was chosen for several reasons. This dosing regimen has demonstrated safety when used for other conditions. In vitro studies suggest that this dosing regimen is sufficient to achieve SARS-CoV-2 inhibition. This dosing regimen results in therapeutic drug concentrations in lung tissue for up to 10 days (6). A higher dose (400 mg twice daily) for 5 days was considere, but was not selected because of the overall risk-to-benefit balance, with higher doses potentially leading to increased risk for ventricular dysrhythmias (30).
Approach to cointerventions
The ORCHID trial restricts the use of open-label hydroxychloroquine or chloroquine during the 5-day intervention period. All other clinical treatment decisions are made by treating clinicians. Administration of other open-label antiviral and immune modulating medications is allowed at the discretion of treating clinicians and is recorded. Coenrollment in other interventional trials is allowed on a case-by-case basis after consideration of potential interactions between agents under investigation, safety assessment and adverse event reporting, and the interpretability of trial results.
Study monitoring and adherence
In addition to routine clinical monitoring (including a preenrollment electrocardiogram [EKG]), research staff monitor daily for adherence to study drug dosing and potential drug interactions. To assess for QTc prolongation, study personnel review all clinically obtained EKGs, and the protocol requires measuring the QTc by EKG or a telemetry tracing 24–48 hours after administration of the first dose of study drug (29). If the QTc is >500 milliseconds on any assessment during the course of the study drug, study drug is held for a minimum of 24 hours and is not restarted until a subsequent EKG demonstrates a QTc ≤500 milliseconds (details in online supplement).
Outcomes
Primary outcome
The primary outcome is patients’ clinical status 14 days after randomization (measured on Study Day 15) as assessed with the seven-category COVID Ordinal Outcome Scale (Table 2) (37). To distinguish between categories 6 (not hospitalized but unable to perform normal activities) and 7 (not hospitalized and able to perform normal activities), study personnel blinded to group assignment call patients or caretakers and assess the patient’s performance of “usual activities” with questions consistent with validated health status measures (38, 39). An answer of “no problems doing my usual activities” results in assignment to category 7.
The COVID Ordinal Outcomes Scale serves as the primary outcome in multiple ongoing COVID-19 trials and is recommended by the World Health Organization Research and Development Blueprint for COVID-19 (37). Although this novel outcome has not yet been validated in prospective studies, use of this standardized outcome facilitates comparison and combination of results across trials (40). There is a mandate for trial efficiency during a pandemic, and by capturing the broad spectrum of clinical outcomes experienced by patients with COVID-19, the COVID Ordinal Outcome Scale has the advantage of increasing statistical efficiency compared with dichotomous outcomes.
We selected 14 days after randomization (on Study Day 15) as the time point at which we would assess the patient’s clinical status for the primary analysis of the primary outcome. This time point was selected by many ongoing clinical trials for COVID-19 because it captured the majority of early deaths and hospital discharges and was felt to be sufficient to capture the patient’s clinical trajectory while also being available rapidly enough for use with frequent interim analyses. Measurement of the primary outcome 14 days after randomization could be insensitive to treatment differences that occur after this time point. However, the median length of stay among patients hospitalized with COVID-19 is approximately 4 days (41), and results from a comparison of the COVID Ordinal Outcomes Scale at 14 days after randomization were concordant with results of other 28-day outcomes in the largest trial of COVID-19 to date (42).
Secondary and safety outcomes
Secondary and safety outcomes are shown in Table 2. Key secondary outcomes include all-cause, all-location mortality at 14 and 28 days after randomization (assessed on Study Days 15 and 29, respectively); COVID Ordinal Outcomes Scale at 2, 7, and 28 days after randomization (assessed on Study Days 3, 8, and 29 respectively); a composite of death or receipt of extracorporeal membrane oxygenation through Study Day 28 (assessed on Study Day 29); and days alive and free of each individual organ support (e.g., ventilator-free days) (additional details in online supplement). To allow comparison with the Adaptive COVID-19 Treatment Trial trial (NCT04280705), “time to recovery” was added as a secondary outcome before the first interim analysis.
Safety outcomes focus on potential adverse effects of hydroxychloroquine, including atrial and ventricular dysrhythmias, cardiac arrest, seizure, acute hepatitis, acute pancreatitis, symptomatic hypoglycemia, bone marrow suppression, and severe dermatologic reactions.
Data Collection
Figure E1 in the online supplement depicts the timeline of study procedures. The ORCHID trial was designed to minimize research activities that require person-to-person contact between study personnel and patients. This aimed to conserve personal protective equipment, reduce the risk of infection among study personnel, reduce the risk of spreading the virus, and enable conduct of the trial despite prohibitions against research staff entering clinical areas at many institutions. The trial, therefore, primarily uses data that can be collected from the electronic health record and assessments that can be completed by telephone. No biological specimens are required as part of the trial.
The effects of acute illness from COVID-19 on long-term patient-important outcomes such as cognitive and physical function are uncertain. Although follow-up in the ORCHID trial ends 28 days after enrollment (and initial results from the ORCHID trial will be limited to outcomes in the first 28 days), an ancillary study will follow selected patients at 12 months to assess long-term patient-important outcomes, including survival, cognitive, physical, and psychological function.
Data quality monitoring
Structured data collection training is provided to centers before study initiation. The PETAL Clinical Coordinating Center ensures ongoing data quality by front-end range and logic checks at the time of data entry into the secure online database and back-end monitoring with query reports and virtual site visits.
Statistical Methods
Approach to analysis of the primary outcome
The primary analysis will be an intention-to-treat comparison of the COVID Ordinal Outcome score at 14 days after randomization (assessed on Study Day 15) between all patients randomized to hydroxychloroquine versus placebo (Table 3). This analysis will be conducted with a proportional odds model using the COVID Ordinal Outcome score as the dependent variable, randomized group assignment as the primary independent variable, and the following covariates: age, sex, baseline COVID Ordinal Outcome score, baseline Sequential Organ Failure Assessment score, and duration of acute respiratory infection symptoms prior to randomization. An odds ratio (OR) >1.0 indicates more favorable outcomes with hydroxychloroquine on the COVID Ordinal Outcome scale, whereas an OR <1.0 indicates more favorable outcomes with placebo. The small number of patients enrolled with suspected rather than confirmed COVID-19 during the first 19 days of the trial (prior to limiting eligibility to laboratory-confirmed cases) will be included in the primary analysis. Sensitivity analyses will include an intention-to-treat comparison between groups, limited to patients with laboratory-confirmed SARS-CoV-2 infection.
The trial will be analyzed using a Bayesian framework. In addition to flexibility in the number and timing of interim analyses, a Bayesian framework allows consideration of new external data on the efficacy of hydroxychloroquine, which may become available during the trial. For the purpose of declaring success, we will use a skeptical prior, which assumes an equal chance of harm or benefit (normal distribution with mean log OR of 0.0) and assumes that the chance of a large benefit is small (standard deviation of log OR is 0.352).
Approach to sample size calculation
Accurate sample size calculations using a frequentist approach require knowledge about the frequency and distribution of the trial outcome and estimates of the effect of the trial intervention on the outcome (40). At the time of trial planning, none of these data were available for the use of hydroxychloroquine among hospitalized patients with COVID-19. Given these uncertainties, we selected a Bayesian statistical framework because it permits flexibility in the number and timing of interim analyses, provides the best opportunity for the trial to be stopped early for efficacy or futility, and allows the trial to be continued if the clinical effect of hydroxychloroquine remains unclear after accrual of the initially planned sample size. Given the relative complexity of estimating sample sizes using a Bayesian approach and the need to rapidly finalize a protocol and start enrollment, the initial trial protocol included a frequentist sample size calculation with a prespecified plan to transition to a Bayesian approach. This calculation used data from a prior trial of patients at risk for ARDS, the VIOLET (Vitamin D to Improve Outcomes by Leveraging Early Treatment) trial, to estimate the expected outcomes for the placebo group on the COVID Ordinal Outcome scale at 14 days after randomization (assessed on Study Day 15) (Table E1) (43). In brief, the initial sample size calculation estimated that enrollment of 510 patients would provide 90% power to detect an OR of 1.82 with a two-sided significance level of P < 0.05 (details in online supplement).
The full Bayesian analysis plan was developed during the first 3 weeks of enrollment and before review of any trial data. It includes an interim analysis every 102 patients with the opportunity to increase the frequency of interim analyses as the trial approaches a stopping criterion. The DSMB will review the totality of accrued data at each interim analysis to inform their recommendation that enrollment continue or stop. The DSMB may consider stopping the trial if either of the following criteria is met:
>95% probability of the OR being >1.0 (suggesting high likelihood of at least some efficacy) or
>90% probability that the OR is <1.1 (suggesting futility or harm).
For the purpose of stopping the trial for efficacy, we will use a skeptical prior, as described above. A threshold of 1.1 was chosen for the stopping criterion for futility, as this was felt to be the minimal clinically significant difference for the primary outcome. This criterion can also be used to stop the trial if accrued data suggest harm (OR <1.0). For the purpose of stopping the trial for futility or harm, we will use a noninformative prior, which assumes an equal probability of benefit or harm but allows for the possibility of arbitrarily large treatment effects. The final sample size will be determined by when the stopping criteria are met. An illustration of the probability that the trial will meet the proposed efficacy or futility criteria at each interim analysis is provided in the online supplement using hypothetical effect sizes (Table E2). In trials designed using frequentist approaches, stopping a trial early for efficacy has been shown to systematically overestimate treatment effects, as large, random fluctuations of the estimated treatment effect are common early in a trial’s progress (44, 45). The ORCHID trial protects against this type of effect overestimation by using a skeptical prior for efficacy. If the trial is stopped early for efficacy, the estimate of the treatment effect will be “pulled back” by the prior. The prior distribution’s influence fades as the sample size grows with later interim analyses.
Trial status
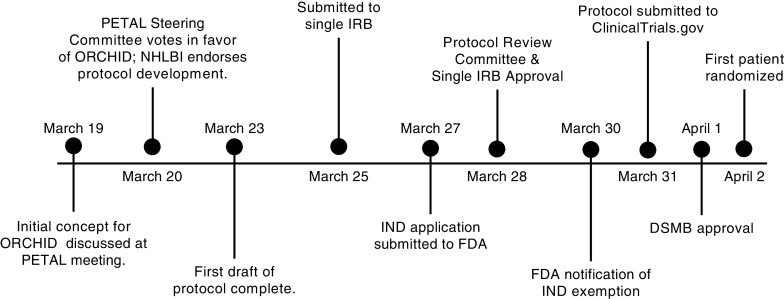
Figure 1 shows the 15-day timeline of study development from concept to enrollment of the first patient. The trial was registered (NCT04332991) prior to enrollment of the first patient on April 2, 2020. Though trial duration will depend on both the epidemiology of the COVID-19 pandemic and the efficacy of hydroxychloroquine, the anticipated timeline for completion of the trial is 3 months.
Figure 1.
Timeline from study conception to enrollment of the first patient. On February 28, 2020, the first death from coronavirus disease (COVID-19) in the United States was reported. On March 16, 2020, an initial Prevention and Early Treatment of Acute Lung Injury (PETAL) network conference call was held to discuss proposed interventions to treat COVID-19. On March 19, 2020, a brief trial concept and two-page summary was developed for a trial of hydroxychloroquine among hospitalized patients with COVID-19 and presented to the network along with other trial proposals. Following a PETAL Steering Committee vote on March 20, 2020, a trial of hydroxychloroquine was chosen as the first interventional trial for COVID-19 in the PETAL network. On the same day, the National Heart, Lung and Blood Institute reviewed the two-page summary and endorsed protocol development. A first draft of the trial protocol was completed in 72 hours and distributed to the PETAL Steering Committee. The trial protocol was finalized and submitted to the single institutional review board (IRB) on March 25, 2020. The trial was reviewed simultaneously by the single IRB and PETAL Protocol Review Committee, with both providing approval on March 28, 2020. Following an investigational new drug (IND) application submission to the Food and Drug Administration on March 27, 2020, a notification of IND exemption was received on March 30, 2020. The trial was submitted to clinicaltrials.gov on March 31, 2020. The trial was presented to the PETAL Data and Safety Monitoring Board on April 1, 2020, with approval granted on the same day. The first patient was randomized on April 2, 2020, with blinding maintained by encapsulation of hydroxychloroquine and placebo by local pharmacies. DSMB = Data and Safety Monitoring Board; FDA = Food and Drug Administration; NHLBI = National Heart, Lung, and Blood Institute; ORCHID = Outcomes Related to COVID-19 treated with Hydroxychloroquine among In-patients with symptomatic Disease.
Discussion
Since the first documented case in December 2019, COVID-19 has spread exponentially, with over 4 million confirmed cases and over 275,000 deaths as of May 11, 2020. The pandemic has brought unprecedented challenges to clinical research. Designing the ORCHID trial required solutions to several significant barriers, including widespread off-label use of hydroxychloroquine, the impracticability of traditional paper-based documentation of informed consent, the complexity of developing a flexible and robust Bayesian analysis plan under time constraints, avoiding delays typically required to obtain visually identical placebo pills, and the need to rapidly obtain administrative and regulatory approvals.
In the early stages of the COVID-19 pandemic, anecdotes and small case series about potential treatments for COVID-19 circulated on social medica, preprint servers, and the lay press. Some of these treatments were rapidly adopted into clinical care (46–50). Despite a lack of data from clinical trials informing efficacy and safety in the treatment of COVID-19, hydroxychloroquine was adopted as first-line treatment for adults hospitalized with COVID-19 in treatment guidelines at many U.S. medical centers (18–20). Administration of hydroxychloroquine to inpatients with COVID-19 became so common that questions were raised regarding the feasibility of conducting a randomized trial in which half the patients did not receive hydroxychloroquine (51). The investigators’ assessment that the benefits and risks to individual patients and to society favor preferentially administering hydroxychloroquine in a clinical trial rather than in clinical care has been confirmed by guidance from the Infectious Disease Society of America, the National Institutes of Health, and the Society of Critical Care Medicine (30, 31, 52).
An additional challenge has been documenting informed consent to participate in the trial. Traditional methods of written informed consent, in which a patient or LAR physically signs a paper document that is retained by study staff, are infeasible during an infectious pandemic. Fortunately, guidance released by FDA in 2016 provided information on obtaining written informed consent from patients or their LAR using electronic methods (53), which can be utilized in pandemic circumstances. However, developing consent procedures for an infectious pandemic, during which the patient, LAR, research staff, and witness may be in four physically distinct locations at the time of consent, has required the development of new operating procedures and adaptations of available technology.
Given the uncertainties regarding the epidemiology of COVID-19 and the efficacy of hydroxychloroquine, a Bayesian analytic framework was developed for ORCHID. High-quality data demonstrating efficacy, inefficacy, or harm associated with use of hydroxychloroquine for COVID-19 would immediately impact clinical care. Therefore, the design of ORCHID required frequent and flexible interim analyses to ensure that as soon as definitive results were known, the trial could be terminated and the results disseminated. Developing a robust Bayesian analysis requires time-consuming statistical simulations. Because the analysis plan would not affect any trial decisions prior to the first interim analyses, we chose to launch the trial with a preliminary frequentist analysis plan with the expectation of shifting to a Bayesian approach prior to the first interim analysis. This approach provided sufficient time to develop a robust analysis plan without delaying enrollment.
Because the manufacture and distribution of visually identical placebos is a potentially rate-limiting step in the launch of a randomized trial, many ongoing trials of COVID-19 interventions have chosen to forego blinding. By using encapsulation of a commercially available medication, the ORCHID trial demonstrates a method to maintain blinding without delaying enrollment.
The rapid launch of the ORCHID trial would not have been possible without a large, preexisting clinical trials network. The traditional process of designing a clinical trial within a trials network, however, can be time consuming. Trial networks function as large collaborations with existing agreements that govern trial selection, protocol development and review, and creation of study documents. These processes are accompanied by external reviews by IRBs, funding organizations, regulatory bodies such as the FDA, scientific review committees, and DSMBs. These tasks are designed to occur serially, with each step frequently occurring over weeks to months. Within the PETAL Network, the time from the selection of an idea for a new trial to the initiation of enrollment has been 12–18 months. Legitimate concerns have been raised regarding the feasibility of designing and conducting novel clinical trials within a discrete pandemic (49, 54). Some have suggested that preexisting platform or adaptive trials might be the only practicable options (55). However, the successful development, regulatory approval, and initiation of enrollment in the ORCHID trial in 15 days demonstrates that, within an established multicenter clinical trials network, large, novel trials can be conceived and launched within a timeframe relevant for pandemics. This rapid launch required flexibility and timely reviews, completed in parallel by multiple oversight bodies, including the funder (National Heart, Lung, and Blood Institute), the FDA, a single IRB, and the PETAL steering committee, coordinating center, Protocol Review Committee (the peer review group for PETAL trials), and DSMB.
Conclusions
We describe the rationale and design of the ORCHID trial, which is a multicenter, blinded, randomized trial comparing hydroxychloroquine versus placebo among hospitalized adults with COVID-19. This prespecified framework will enhance the rigor and reproducibility of the final report and will allow readers to better judge the impact of our findings. We also hope that publishing our full trial protocol and explaining how we overcame the unique challenges to conducting clinical research during the COVID-19 pandemic will assist other investigators working to address this public health crisis.
Footnotes
Supported by grants from the U.S. National Heart, Lung and Blood Institute (NHLBI): U01HL123009, U01HL122998, U01HL123018, U01HL123023, U01HL123008, U01HL123031, U01HL123004, U01HL123027, U01HL123010, U01HL123033, U01HL122989, U01HL123022, and U01HL123020. Massachusetts General Hospital was the sponsor. J.D.C. was supported in part by the NHLBI (K12HL133117). M.W.S. was supported in part by the NHLBI (K23HL143053). S.M.B. was supported in part by the NHLBI (1R01HL144624). F.H. and C.J.L.’s work on this paper was supported by Clinical and Translational Science Awards (UL1 TR002243) from the National Center for Advancing Translational Sciences. The content of this manuscript is the responsibility of the authors alone and does not necessarily reflect the views or policies of the National Institutes of Health, the NHLBI, the National Center for Advancing Translational Sciences, the Department of Health and Human Services, or the United States Government.
Author Contributions: All study authors approved the final version of this manuscript. Study concept and design: J.D.C., M.W.S., S.P.C., A.A.G., F.H., D.A.S., C.J.L., T.W.R., B.T.T., S.M.B., and W.H.S. Acquisition of data: J.D.C., N.J.J., M.W.S., S.P.C., S.Y.C., J.E., M.F., K.W.G., A.A.G., M.N.G., D.L.H., C.L.H., A.K., L.M.L., M.M., C.F.O., P.K.P., N.J.R., B.R.H.R., N.I.S., J.S.S., D.K.T., A.W., T.W.R., S.M.B., and W.H.S. Drafting of the manuscript: J.D.C., N.J.J., and W.H.S. Critical revision of the manuscript for important intellectual content: all authors. Study supervision: N.R.A., R.G.B., L.A.R., B.T.T., and W.H.S.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: for the ORCHID Protocol Committee and the National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury (PETAL) Network Investigators
References
- 1.Del Rio C, Malani PN. COVID-19-New insights on a rapidly changing epidemic. JAMA. doi: 10.1001/jama.2020.3072. [online ahead of print] 28 Feb 2020; DOI: 10.1001/jama.2020.3072. [DOI] [PubMed] [Google Scholar]
- 2.Fauci AS, Lane HC, Redfield RR. Covid-19 - navigating the uncharted. N Engl J Med. 2020;382:1268–1269. doi: 10.1056/NEJMe2002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. doi: 10.1093/cid/ciaa237. [online ahead of print] 9 Mar 2020; DOI: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ponticelli C, Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE) Expert Opin Drug Saf. 2017;16:411–419. doi: 10.1080/14740338.2017.1269168. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020;55:105982. doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 10.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. doi: 10.1001/jama.2020.6019. [online ahead of print] 13 Apr 2020; DOI: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 11.DrugBank. Hydroxychloroquine. 2020 [accessed 2020 Apr 22]. Available from: https://www.drugbank.ca/drugs/DB01611.
- 12.Al-Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5:e00293. doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colson P, Rolain J-M, Lagier J-C, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55:105932. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 18.Massachusetts General Hospital. Massachusetts General Hospital COVID-19 Treatment Guidance. 2020 [created 2020 Apr 5; accessed 2020 Apr 22] Available from: https://web.archive.org/web/20200410013441/https://www.massgeneral.org/assets/MGH/pdf/news/coronavirus/mass-general-COVID-19-treatment-guidance.pdf.
- 19.Brigham and Women’s Hospital. Brigham and Women’s Hospital COVID-19 critical care clinical guidelines. 2020 [created 2020 Mar 23; accessed 2020 Apr 22] Available from: https://web.archive.org/web/20200321221651/https://covidprotocols.org/
- 20.Yale School of Medicine. YNHHS initial treatment algorithm for hospitalized ADULTS with non-severe COVID-19. 2020 [created 2020 Apr 13; accessed 2020 Apr 22] Available from: https://files-profile.medicine.yale.edu/documents/f813a2c5-72bc-4a66-ae41-41249fa51443.
- 21.Wilson KC, Chotirmall SH, Bai C, Rello J on behalf of the International Task Force on COVID‐19. COVID-19: Interim Guidance on Management Pending Empirical Evidence. From an American Thoracic Society-led International Task Force. 2020 [created 2020 Apr 3; accessed 2020 Apr 22] Available from: https://www.thoracic.org/covid/covid-19-guidance.pdf.
- 22.Food and Drug Administration (FDA) Emergency use authorization for use of chloroquine phosphate or hydroxychloroquine sulfate supplied from the strategic national stockpile for treatment of 2019 coronavirus disease. 2020 [created 2020 Mar 28; accessed 2020 Apr 22]. Available from: https://www.fda.gov/media/136534/download.
- 23.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. doi: 10.1016/j.ijantimicag.2020.105949. [online ahead of print] 20 Mar 2020; DOI: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial [preprint] medRxiv 2020; Available from: https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v3.
- 25.Molina JM, Delaugerre C, Le Goff J, Mela-Lima B, Ponscarme D, Goldwirt L, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med Mal Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Liu D. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ (Med Sci) 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. doi: 10.1056/NEJMoa2012410. [online ahead of print] 7 May 2020; DOI: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roden Dan M, Harrington Robert A, Poppas A, Russo AM. Considerations for drug interactions on QTc in exploratory COVID-19 (coronavirus disease 2019) treatment. Circulation. doi: 10.1161/CIRCULATIONAHA.120.047521. [online ahead of print] 8 Apr 2020; DOI: 10.1161/CIRCULATIONAHA.120.047521. [DOI] [PubMed] [Google Scholar]
- 29.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institutes of Health. COVID-19 Treatment Guidelines. Therapeutic options for COVID-19 currently under investigation. 2020 [updated 2020 Apr 21; accessed on 2020 Apr 22]. Available from: https://web.archive.org/web/20200421163941/https://www.covid19treatmentguidelines.nih.gov/therapeutic-options-under-investigation/
- 31.Bhimraj A, Morgan R, Hirsch-Shumaker A, Lavergne V, Baden L, Chi-Chung Cheng V, et al. Infectious diseases society of America guidelines on the treatment and management of patients with COVID-19. 2020 [created 2020 Apr 11; accessed 2020 Apr 22] doi: 10.1093/cid/ciaa478. Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/ [DOI] [PMC free article] [PubMed]
- 32.Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang DT, Angus DC, Moss M, Thompson BT, Ferguson ND, Ginde A, et al. Reevaluation of Systemic Early Neuromuscular Blockade Protocol Committee and the National Institutes of Health National Heart, Lung, and Blood Institute Prevention and Early Treatment of Acute Lung Injury Network Investigators. Design and rationale of the reevaluation of systemic early neuromuscular blockade trial for acute respiratory distress syndrome. Ann Am Thorac Soc. 2017;14:124–133. doi: 10.1513/AnnalsATS.201608-629OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Self WH, Semler MW, Bellomo R, Brown SM, deBoisblanc BP, Exline MC, et al. CLOVERS Protocol Committee and NHLBI Prevention and Early Treatment of Acute Lung Injury (PETAL) Network Investigators. Liberal versus restrictive intravenous fluid therapy for early septic shock: rationale for a randomized trial. Ann Emerg Med. 2018;72:457–466. doi: 10.1016/j.annemergmed.2018.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Food and Drug Administration. FDA guidance on conduct of clinical trials of medical products during COVID-19 public health emergency. 2020 [created 2020 Mar 13; updated 2020 Apr 21; accessed 2020 Apr 22] Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency.
- 36.Lim H-S, Im JS, Cho JY, Bae KS, Klein TA, Yeom JS, et al. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob Agents Chemother. 2009;53:1468–1475. doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Coronavirus disease (COVID-2019) R&D: COVID-19 therapeutic trial synopsis. 2020 [created 2020 Feb 18; accessed: 2020 Apr 22]. Available from: https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf.
- 38.EuroQol Group. EuroQol: a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 39.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harhay MO, Casey JD, Clement M, Collins SP, Gayat É, Gong MN, et al. Contemporary strategies to improve clinical trial design for critical care research: insights from the First Critical Care Clinical Trialists Workshop. Intensive Care Med. 2020;46:930–942. doi: 10.1007/s00134-020-05934-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. ACTT-1 Study Group Members. Remdesivir for the treatment of covid-19 - preliminary report. N Engl J Med. [online ahead of print] 22 May 2020; DOI: 10.1056/NEJMoa2007764. [Google Scholar]
- 43.Ginde AA, Brower RG, Caterino JM, Finck L, Banner-Goodspeed VM, Grissom CK, et al. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Early high-dose Vitamin D 3 for critically ill, Vitamin D-deficient patients. N Engl J Med. 2019;381:2529–2540. doi: 10.1056/NEJMoa1911124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Briel M, Bassler D, Wang AT, Guyatt GH, Montori VM. The dangers of stopping a trial too early. J Bone Joint Surg Am. 2012;94:56–60. doi: 10.2106/JBJS.K.01412. [DOI] [PubMed] [Google Scholar]
- 45.Pocock SJ, Hughes MD. Practical problems in interim analyses, with particular regard to estimation. Control Clin Trials. 1989;10(Suppl):209S–221S. doi: 10.1016/0197-2456(89)90059-7. [DOI] [PubMed] [Google Scholar]
- 46.Rice TW, Janz DR. Ann Am Thorac Soc. In defense of evidence-based medicine for the treatment of COVID-19 ARDS. [online ahead of print] 22 Apr 2020; DOI: 10.1513/AnnalsATS.202004-325IP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodman JL, Borio L. Finding effective treatments for COVID-19: scientific integrity and public confidence in a time of crisis. JAMA. doi: 10.1001/jama.2020.6434. [online ahead of print] 16 Apr 2020; DOI: 10.1001/jama.2020.6434. [DOI] [PubMed] [Google Scholar]
- 48.Singer BD, Jain M, Budinger GRS, Wunderink RG. A call for rational intensive care in the era of COVID-19. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2020-0151LE. [online ahead of print] 21 Apr 2020; DOI: 10.1165/rcmb.2020-0151LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalil AC. Treating COVID-19-off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA. doi: 10.1001/jama.2020.4742. [online ahead of print] 24 Mar 2020; DOI: 10.1001/jama.2020.4742. [DOI] [PubMed] [Google Scholar]
- 50.Angus DC. Optimizing the trade-off between learning and doing in a pandemic. JAMA. [online ahead of print] 30 Mar 2020; DOI: 10.1001/jama.2020.4984. [Google Scholar]
- 51.Waterer GW, Rello J, Wunderink RG. COVID-19: first do no harm. Am J Respir Crit Care Med. 2020;201:1324–1325. doi: 10.1164/rccm.202004-1153ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alhazzani W, Møller MH, Arabi YM, Loeb M, Gong MN, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Crit Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.U.S. Food and Drug Administration. Research C for DE and use of electronic informed consent in clinical investigations: questions and answers. 2019 Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/use-electronic-informed-consent-clinical-investigations-questions-and-answers.
- 54.Rojek AM, Dunning J, Leliogdowicz A, Castle L, Van Lieshout M, Carson G, et al. Regulatory and operational complexities of conducting a clinical treatment trial during an ebola virus disease epidemic. Clin Infect Dis. 2018;66:1454–1457. doi: 10.1093/cid/cix1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rojek AM, Horby PW. Offering patients more: how the West Africa Ebola outbreak can shape innovation in therapeutic research for emerging and epidemic infections. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160294. doi: 10.1098/rstb.2016.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]