To the Editor:
In January 2020, the first cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection were reported in Europe. Multiple outbreaks have since then led to a global pandemic, as well as to massive medical, economic, and social repercussions (1, 2).
SARS-CoV-2 pneumonia can develop into acute respiratory distress syndrome (ARDS) when mechanical ventilation (MV) is needed (3, 4). ARDS produces abnormalities in gas exchange with a variable degree of shunt (5), high dead space ventilation (dead space volume [Vd]/tidal volume [Vt] ratio) (6), diminished pulmonary compliance (7), and alterations to the pulmonary circulation (8). The cornerstone of ARDS management is to provide adequate gas exchange without further lung injury as a result of MV. To date, information regarding the characteristics of SARS-CoV-2–induced ARDS is not completely known. However, this information is crucial to better apply MV and facilitate organ support strategies. We therefore present the characteristics of gas exchange, pulmonary mechanics, and ventilatory management of 50 patients with laboratory-confirmed SARS-CoV-2 infection, who developed ARDS and underwent invasive MV (IMV).
Methods
Descriptive analysis included 50 consecutive patients with laboratory-confirmed SARS-CoV-2 infection who developed ARDS (9) and underwent IMV. These patients were admitted to the SARS-CoV-2–dedicated intensive care units (ICUs) at Hospital Clinic of Barcelona, Spain, between March 7 and March 25, 2020.
Upon ICU admission, epidemiological characteristics, the severity of SARS-CoV-2 infection with the Acute Physiology and Chronic Health Evaluation II score, prognostic biomarkers of SARS-CoV-2 infection (described in Reference 4), time from hospital to ICU admission, time from ICU admission to intubation, oxygen therapy or noninvasive ventilation (NIV) use, and microbiology were investigated.
On the day that criteria for ARDS diagnosis were met (9) and IMV was needed, the following assessments were performed: impairment in oxygenation was analyzed with the partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio, and abnormalities of CO2 metabolism were studied with the ventilatory ratio (VR), a surrogate parameter of Vd/Vt (10).
In addition, adjunctive therapies and MV parameters related with ventilation-induced lung injury (VILI) described elsewhere (11–15) were investigated.
Correlations of SARS-CoV-2 prognostic biomarkers (4), pulmonary mechanics, and gas-exchange data were performed. Twenty-eight–day and hospital mortality, ventilator- and ICU-free days at Day 28, hospital and ICU lengths of stay, and need for tracheostomy were also evaluated (16). Finally, a subanalysis assessing differences before and after prone positioning was performed. For additional detail on the method, see the online supplement.
Results
By March 25th, 2020, 50 patients with laboratory-confirmed SARS-CoV-2 infection and ARDS had been admitted to our hospital. Table 1 shows the demographic and clinical characteristics of these patients. The median (interquartile range [IQR]) age was 66 (57–74) years. Thirty-six patients (72%) were men. Upon ARDS diagnosis, 44% of patients were initially classified as having moderate ARDS, whereas 24% were classified as having mild ARDS and 32% were classified as having severe ARDS. The outcomes of these patients are shown in Table 1. ICU and hospital lengths of stay were prolonged, and tracheostomy was performed in 30 (60%) patients. Hospital mortality was 34%.
Table 1.
Characteristics of 50 patients with SARS-CoV-2–induced ARDS
| Characteristic | Median (IQR) or n (%) |
|---|---|
| Baseline characteristics | |
| Age, yr | 66 (57–74) |
| Male | 36 (72) |
| BMI, kg/m2 | 27.68 (26.43–30.39) |
| Smoking status* | |
| Never | 23 (56) |
| Current | 4 (10) |
| Former | 14 (34) |
| Alcohol consumption habit | 6 (12) |
| APACHE II score at ICU admission | 13 (11–18) |
| SOFA score upon ARDS diagnosis | 7 (6–9) |
| Time from hospital admission to ICU admission, d | 2 (1–3.75) |
| Time from ICU admission to intubation, d | 1 (0–1) |
| Coinfection | 2 (4) |
| Oxygen therapy and NIV before intubation† | |
| Venturi and nonrebreathing oxygen mask | 43 (93) |
| High-flow oxygen therapy | 23 (50) |
| NIV | 5 (11) |
| ARDS severity | |
| Mild | 12 (24) |
| Moderate | 22 (44) |
| Severe | 16 (32) |
| Management factors | |
| Prone position | 11 (22) |
| Neuromuscular blockade use | 24 (48) |
| Recruitment maneuvers | 18 (36) |
| Vasopressor use | 16 (32) |
| Corticosteroid therapy | 18 (36) |
| Laboratory findings‡ | |
| Hemoglobin, g/dl | 12.80 (11.83–13.40) |
| White blood cell count, ×109/L | 8.11 (6.26–11.42) |
| Lymphocyte count, ×109/L | 0.60 (0.40–0.90) |
| Platelet count, ×109/L | 219 (174–274) |
| Creatinine, mg/ml | 0.98 (0.77–1.45) |
| Sodium, mEq/L | 138 (136–140) |
| Potassium, mEq/L | 3.90 (3.60–4.30) |
| Aspartate aminotransferase, U/L | 65 (48–82) |
| Alanine aminotransferase, U/L | 45 (28–76) |
| Total bilirubin, mg/dl | 0.50 (0.40–0.90) |
| γ-Glutamyltransferase, U/L | 54 (36–105) |
| Lactate, mmol/L | 1.33 (1–1.59) |
| Alkaline phosphatase, U/L | 67 (53–86) |
| Lactate dehydrogenase, U/L | 442 (386–541) |
| Albumin, g/L | 36 (33–38) |
| D-dimer, ng/ml | 1,100 (800–3,800) |
| Ferritin, ng/ml | 1,436.50 (951.25–2,149.25) |
| Procalcitonin, ng/ml | 0.27 (0.17–0.97) |
| C-reactive protein, mg/dl | 15.68 (9.64–27.03) |
| Outcomes§ | |
| Hospital mortality | 15 (34) |
| Mortality at Day 28 | 10 (20) |
| Ventilator-free at Day 28, d | 9 (0–16) |
| ICU-free at Day 28, d | 0 (0–9) |
| Hospital length of stay, d | 36 (24–44) |
| ICU length of stay, d | 26 (17–34) |
| Tracheostomy | 30 (60) |
Definition of abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II; ARDS = acute respiratory distress syndrome; BMI = body mass index; ICU = intensive care unit; IQR = interquartile range; NIV = noninvasive ventilation; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SOFA = sequential organ failure assessment.
Missing data from nine patients.
Missing data from four patients.
Laboratory findings upon ARDS diagnosis.
Six patients were still in the hospital after follow-up ending.
Table 2 shows the results for gas exchange, pulmonary mechanics, and variables associated with VILI upon ARDS diagnosis. Excluding baseline mechanical power, other MV parameters associated with VILI were within normal range.
Table 2.
Gas exchange, pulmonary mechanics, and VILI of 50 patients with SARS-CoV-2–induced ARDS upon ARDS diagnosis
| Measure | Median (IQR) |
|---|---|
| Arterial blood gas | |
| pH | 7.31 (7.28–7.37) |
| PaCO2, mm Hg | 47 (42.9–53.4) |
| PaO2, mm Hg | 107 (88.5–133.6) |
| PaO2/FiO2 | 174 (128–232) |
| Ventilatory ratio* | 1.93 (1.55–2.23) |
| Ventilator settings, pulmonary mechanics, and other variables associated with VILI | |
| Vt/PBW, ml/kg | 6.78 (6.30–7.32) |
| Respiratory rate, breaths/min | 22 (20–23) |
| PEEP, cm H2O | 13 (11–14) |
| FiO2, % | 62 (53–76) |
| Peak inspiratory pressure, cm H2O | 32 (29–34) |
| End-inspiratory plateau pressure, cm H2O | 23 (21–25) |
| Driving pressure, cm H2O† | 11 (9–13) |
| Mechanical power, J/min‡ | 22.32 (18.49–28.10) |
| Crs, ml × cm H2O−1§ | 40.13 (32.88–51.68) |
Definition of abbreviations: ARDS=acute respiratory distress syndrome; Crs = static compliance of the respiratory system; FiO2 = fraction of inspired oxygen; IQR = interquartile range; PaCO2 = partial pressure of carbon dioxide; PaO2 = partial pressure of oxygen; PBW = predicted body weight; PEEP = positive end-expiratory pressure; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; VILI = ventilation-induced lung injury; Vt = tidal volume.
Ventilatory ratio is defined as (minute ventilation [ml/min] × PaCO2 [mm Hg])/(PBW × 100 × 37.5).
Driving pressure is the difference between plateau pressure and PEEP.
Mechanical power was calculated following previously published formulas (11).
Crs is the ratio of tidal volume to driving pressure.
There was no correlation between PaO2/FiO2 and the static compliance of the respiratory system (Crs) (Figure 1A). Notwithstanding, a weak yet significant correlation was found between VR and both positive end-expiratory pressure and end-inspiratory plateau pressure (Figures 1C and 1D).However, D-dimer was not significantly correlated with VR (Figure 1B).
Figure 1.
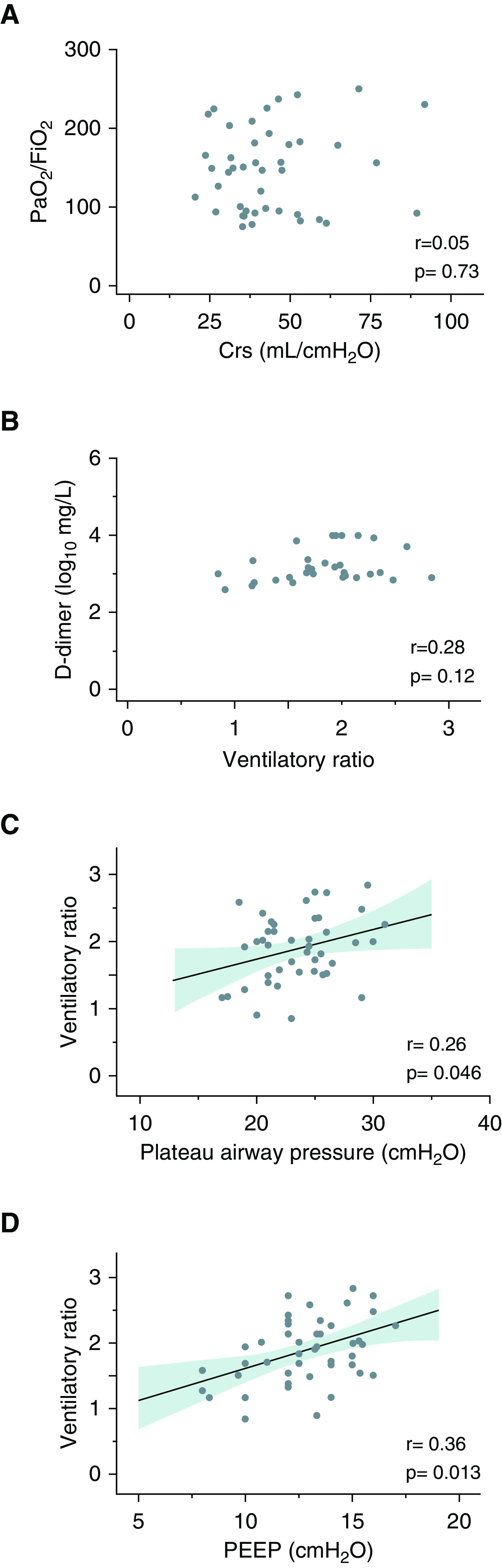
Correlation between pulmonary mechanics and gas-exchange abnormalities. (A) Correlation between Crs and PaO2/FiO2. (B) Correlation between ventilatory ratio and D-dimer concentration. (C) Correlation between plateau pressure and ventilatory ratio. (D) Correlation between PEEP and ventilatory ratio. Solid lines are the regression lines, whereas shaded bands display 95% confidence intervals. Crs = static compliance of the respiratory system; PaO2/FiO2 = ratio between partial pressure of oxygen and fraction of inspired oxygen, PEEP = positive end-expiratory pressure.
Eleven patients underwent prone positioning on the day of ARDS diagnosis (see Table E1 in the online supplement). On average, PaO2/FiO2 increased from the supine to the prone position by +59 (IQR, 32–143; P = 0.002). Significant differences were also found in Crs between supine to prone position with an increase of +5.45 (IQR, 4.32–18.25; P = 0.015).
Discussion
SARS-CoV-2–induced ARDS produced an impairment in gas exchange and pulmonary mechanics comparable with those of prior cohorts with non–SARS-CoV-2 ARDS (10, 13, 17, 18). As in other studies, VR was high, and the most frequent presentation was moderate ARDS (10, 17, 19). On average, Crs in the cohort with SARS-CoV-2–induced ARDS was also found to be comparable, but with remarkable heterogeneity (13, 18). Other studies have reported similar (20), higher (21), or lower Crs (19, 22, 23) in SARS-CoV-2–induced ARDS. As Crs decreases alongside the collapse of alveolar units due to lung edema, several factors may provide explanations for such reported differences, including treatments, intubation strategies, and the stage of the disease. In our cohort with early SARS-CoV-2–induced ARDS, the time from ICU admission to intubation was only 24 hours, despite the use of high-flow nasal cannula or NIV in some cases.
We found no correlation between Crs and PaO2/FiO2. Crs estimates the amount of aerated lung volume in ARDS (7). These results might therefore suggest that the proportion of nonaerated or poorly aerated to well-aerated lung volume is not the only determinant for such a degree of hypoxemia. This may not be specific to SARS-CoV-2–induced ARDS, as other factors apart from the amount of aerated lung tissue (i.e., lung perfusion) are largely known to influence pulmonary shunt (24). However, some authors have reported that lung perfusion in SARS-CoV-2–induced ARDS is more impaired than ARDS by other causes (21). We identified remarkable abnormal lung perfusion in computed tomographic scans performed in these patients (Figure E1).
We found no correlation between D-dimer and VR, suggesting that high Vd/Vt might not be related to a coagulation disorder (i.e., pulmonary microthrombosis). Nonetheless, as suggested by its association with VR, high end-inspiratory and end-expiratory pressures (i.e., mean airway pressures) could increase Vd/Vt if the lung is overdistended and perfusion is decreased.
Although driving pressure and end-inspiratory plateau pressure were within the protective range, mechanical power was found to be slightly high and might have promoted lung injury (25). In patients undergoing prone positioning, PaO2/FiO2 improvement was followed by an increase in Crs, suggesting recruitment and aeration of previously collapsed alveoli. In our study, mortality was similar to that reported in other studies of critically ill patients with SARS-CoV-2 pneumonia (3, 19).
This study presents some limitations. Four manual end-inspiratory and end-expiratory pauses could not be performed in all patients because of protective equipment shortages. However, all patients included had at least one end-inspiratory and end-expiratory pause done on the first day. These results cannot be extrapolated to late SARS-CoV-2–induced ARDS.
In summary, SARS-CoV-2–induced ARDS presents with an impairment in gas exchange and pulmonary mechanics comparable with those of prior ARDS cohorts. However, lung perfusion in SARS-CoV-2–induced ARDS warrants further investigation.
Acknowledgments
Acknowledgment
The authors thank Anthony Armenta for providing medical editing assistance of the publication at hand.
Covid Clinic Critical Care Group: A. Almuedo, J. R. Alonso, F. Aziz, X. Borrat, E. Bragulat, I. Carmona, O. De Diego, M. Farrero, S. Fernández, M. Forga, E. Guasch, M. Hernández-Tejero, A. Jacas, P. Leyes, T. López, J. A. Martínez, G. Martínez-Palli, J. Mercadal, G. Muñoz, J. Muñoz, R. Navarro, J. Ortiz, E. Poch, M. Pujol, E. Quintana, E. Reverter, J. Rosselló, I. Rovira, P. Ruiz, E. Sandoval, S. Schneider, O. Sibila, D. Soy, M. Suárez, A. Téllez, N. D. Toapanta, and X. Urra.
Footnotes
Supported by the Centro de Investigación Biomédica en Red de Enfermedades Respiratorias-Ciberes (CB 06/06/0028), which is an initiative of Instituto de Salud Carlos III (ISCIII), the Fondo de Investigación en Salud (FIS)–ISCIII (PI18/00974), the Spanish Society of Pneumology and Thoracic Surgery (SEPAR; 2017/537), and the August Pi i Sunyer Biomedical Research Institute (IDIBAPS), Suport Grups de Recerca (SGR) and BIOCAT (COVID-19), and CIBERESUCICOVD (ISCiii COV20/00110). E.B. is the recipient of a predoctoral grant from ISCIII (Rio Hortega; CM19/00133) and a predoctoral grant from the Catalan Society of Pneumology (2019). C.C. is the recipient of a postdoctoral grant from the Strategic Plan for Research and Innovation in Health (Strategic Plan for Research and Innovation in Health–Information and Communication Technologies [PERIS-ICT 2016–2020]). A.T. has been awarded with the Catalan Institution for Research and Advanced Studies (ICREA) Academy Award.
A complete list of Covid Clinic Critical Care Group members may be found before the beginning of the References.
Data Sharing Statement: Deidentified data including data dictionaries will be available when requested. Requests for data sharing to be made to the corresponding author atorres@clinic.cat.
Author Contributions: A.T. had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. E.B., A.M., and A.T. provided the concept and design. All authors contributed to acquisition. E.B., A.M., A.T., A.C., M.F., C.C., L.B., R.M., R.L.-A., H.Y., M.Y., L.F.-B., A.C.P., and I.V. provided analysis and interpretation of data. E.B., A.M., and A.T. drafted the manuscript. E.B. and A.M. provided statistical analysis. All authors contributed to the critical revision of the manuscript for important intellectual content.
This letter has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
Collaborators: The Covid Clinic Critical Care Group, A. Almuedo, J. R. Alonso, F. Aziz, X. Borrat, E. Bragulat, I. Carmona, O. De Diego, M. Farrero, S. Fernández, M. Forga, E. Guasch, M. Hernández-Tejero, A. Jacas, P. Leyes, T. López, J. A. Martínez, G. Martínez-Palli, J. Mercadal, G. Muñoz, J. Muñoz, R. Navarro, J. Ortiz, E. Poch, M. Pujol, E. Quintana, E. Reverter, J. Rosselló, I. Rovira, P. Ruiz, E. Sandoval, S. Schneider, O. Sibila, D. Soy, M. Suárez, A. Téllez, N. D. Toapanta, and X. Urra
References
- 1.Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2020. Coronavirus Disease 2019 (COVID-19): Cases in the U.S. [accessed 2020 Apr 6]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. [Google Scholar]
- 2.European Centre for Disease Prevention and Control. Solna, Sweden: European Centre for Disease Prevention and Control; 2020. COVID-19 situation update worldwide. [accessed 2020 Apr 6]. Available from: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases. [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radermacher P, Maggiore SM, Mercat A. Fifty years of research in ARDS: gas exchange in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;196:964–984. doi: 10.1164/rccm.201610-2156SO. [DOI] [PubMed] [Google Scholar]
- 6.Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in acute respiratory distress syndrome. N Engl J Med. 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 7.Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med. 2005;31:776–784. doi: 10.1007/s00134-005-2627-z. [DOI] [PubMed] [Google Scholar]
- 8.Boissier F, Katsahian S, Razazi K, Thille AW, Roche-Campo F, Leon R, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39:1725–1733. doi: 10.1007/s00134-013-2941-9. [DOI] [PubMed] [Google Scholar]
- 9.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 10.Sinha P, Calfee CS, Beitler JR, Soni N, Ho K, Matthay MA, et al. Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2019;199:333–341. doi: 10.1164/rccm.201804-0692OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cressoni M, Gotti M, Chiurazzi C, Massari D, Algieri I, Amini M, et al. Mechanical power and development of ventilator-induced lung injury. Anesthesiology. 2016;124:1100–1108. doi: 10.1097/ALN.0000000000001056. [DOI] [PubMed] [Google Scholar]
- 12.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 13.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 14.Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, et al. National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380:1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, et al. Dexamethasone in ARDS Network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 16.Schoenfeld DA, Bernard GR ARDS Network. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. LUNG SAFE Investigators; ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 18.Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, et al. Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology. 2002;96:795–802. doi: 10.1097/00000542-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically Ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201:1560–1564. doi: 10.1164/rccm.202004-1163LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan C, Chen L, Lu C, Zhang W, Xia JA, Sklar MC, et al. Lung Recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. 2020;201:1294–1297. doi: 10.1164/rccm.202003-0527LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenck EJ, Hoffman K, Goyal P, Choi J, Torres L, Rajwani K, et al. Respiratory mechanics and gas exchange in COVID-19 associated respiratory failure. Ann Am Thorac Soc. doi: 10.1513/AnnalsATS.202005-427RL. [online ahead of print] 20 May 2020; DOI: 10.1513/AnnalsATS.202005-427RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cressoni M, Caironi P, Polli F, Carlesso E, Chiumello D, Cadringher P, et al. Anatomical and functional intrapulmonary shunt in acute respiratory distress syndrome. Crit Care Med. 2008;36:669–675. doi: 10.1097/01.CCM.0000300276.12074.E1. [DOI] [PubMed] [Google Scholar]
- 25.Serpa Neto A, Deliberato RO, Johnson AEW, Bos LD, Amorim P, Pereira SM, et al. PROVE Network Investigators. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Med. 2018;44:1914–1922. doi: 10.1007/s00134-018-5375-6. [DOI] [PubMed] [Google Scholar]


