Abstract
Defective airway mucus clearance is a defining characteristic of cystic fibrosis lung disease, and improvements to current mucolytic strategies are needed. Novel approaches targeting a range of contributing mechanisms are in various stages of preclinical and clinical development. ARINA-1 is a new nebulized product comprised of ascorbic acid, glutathione, and bicarbonate. Using microoptical coherence tomography, we tested the effect of ARINA-1 on central features of mucociliary clearance in F508del/F508del primary human bronchial epithelial cells to assess its potential as a mucoactive therapy in cystic fibrosis. We found that ARINA-1 significantly augmented mucociliary transport rates, both alone and with CFTR (cystic fibrosis transmembrane conductance regulator) modulator therapy, whereas airway hydration and ciliary beating were largely unchanged compared with PBS vehicle control. Analysis of mucus reflectivity and particle-tracking microrheology indicated that ARINA-1 restores mucus clearance by principally reducing mucus layer viscosity. The combination of bicarbonate and glutathione elicited increases in mucociliary transport rate comparable to those seen with ARINA-1, indicating the importance of this interaction to the impact of ARINA-1 on mucus transport; this effect was not recapitulated with bicarbonate alone or bicarbonate combined with ascorbic acid. Assessment of CFTR chloride transport revealed an increase in CFTR-mediated chloride secretion in response to ARINA-1 in CFBE41o− cells expressing wild-type CFTR, driven by CFTR activity stimulation by ascorbate. This response was absent in CFBE41o− F508del cells treated with VX-809 and primary human bronchial epithelial cells, implicating CFTR-independent mechanisms for the effect of ARINA-1 on cystic fibrosis mucus. Together, these studies indicate that ARINA-1 is a novel potential therapy for the treatment of impaired mucus clearance in cystic fibrosis.
Keywords: mucus transport, mucus viscosity, cystic fibrosis, bicarbonate, glutathione
Cystic fibrosis (CF) is an autosomal recessive disease caused by absent or dysfunctional CFTR (cystic fibrosis transmembrane conductance regulator) protein, an ion channel that regulates the secretion of chloride, bicarbonate (1), and other molecules such as glutathione (2) across the epithelial cell surface. A hallmark of CF is the presence of copious amounts of hyperviscous mucus that is accompanied by delayed or absent mucociliary clearance and consequent obstruction of small and medium-sized airways, which results in a milieu that favors a feedback loop of bacterial colonization, chronic infection, and inflammation (3). Available therapy currently approved for clearance of CF mucus obstruction is limited to dornase alfa (Pulmozyme; Genentech Inc.), an inhaled recombinant human deoxyribonuclease that cleaves extracellular DNA accumulated in CF mucus (4), and hypertonic saline, which is believed to hydrate the mucus layer through osmotically driven fluid transfer (5). However, improvements in lung function, exacerbations, and quality of life observed with these agents are modest and variable (4, 6), accentuating the need for development of complementary or alternative strategies that address abnormal CF mucus and its clearance.
Although specific mechanisms driving the mucus clearance impairment in CF are still being elucidated, multiple contributing factors have been identified that can inform the development of novel therapeutic approaches. Dehydration of the airway surface liquid (ASL) layer, secondary to increased sodium and liquid absorption across the airway epithelia in the setting of deficient CFTR-mediated chloride transport (7), has been linked to abnormal mucus transport in vitro (8, 9) and in vivo (10–12) and through improvements in mucociliary clearance observed with clinical use of hydration therapies, such as hypertonic saline (13). Yet, impaired mucus transport has been observed even in a hydrated environment (9, 14), and airway dehydration has been observed even in the absence of delayed mucus transport (12). Together, these observations underscore the contribution of additional mechanisms that have been implicated in CF mucus abnormalities, such as bicarbonate deficiency (12, 15), abnormal rheological and biophysical properties of mucus (15, 16), and hyperacidic pH (12, 17). Recent studies have provided the first in vivo evidence that these features all play a complex and interactive role in generating the CF mucus defect (12) and emphasize the significance of therapeutic strategies capitalizing on multiple pathways to restoration of mucus transport.
ARINA-1 (Renovion) is a nebulized product currently in development for patients with CF and other chronic inflammatory lung diseases that is comprised of bicarbonate, glutathione, and ascorbic acid. Bicarbonate deficiency due to compromised CFTR-mediated efflux has long been hypothesized to promote abnormally viscous, static CF mucus (15), a link that has been supported by studies demonstrating the requirement of bicarbonate for normal secretion of mucins from airway submucosal glands and goblet cells as well as for mucin expansion from tightly packed macromolecules into a more transportable linear polymer form (18–20). Evidence derived from animal and cell models has directly shown the relationship between bicarbonate and effective mucus transport (9, 12, 18, 21). Glutathione is detected in high concentrations of respiratory epithelial lining fluid under normal conditions (22) but, like bicarbonate, is depleted in CF (23). Glutathione has properties that may also directly impact CF mucus (24). Notably, glutathione, which is a thiol derivative, consists of a sulfhydryl group that is capable of breaking down the disulfide bond structures that link mucin proteins (25); thus, increased mucus viscosity in the setting of glutathione depletion is plausible and potentially amendable to repletion. Low concentrations of ascorbic acid in CF are associated with high degrees of inflammation, consistent with its well-characterized antioxidant properties (26). Ascorbic acid interacts through a sequence of oxidation–reduction reactions with glutathione, and their concentrations have been shown to be strongly interrelated (27).
In this investigation, we tested the effect of ARINA-1 on key features of epithelial function that impact mucociliary clearance in vitro using 1-μm optical coherence tomography (μOCT), an imaging technology that enables simultaneous and colocalized assessment of airway functional microanatomy, including mucociliary transport (MCT) rate, ASL depth, and ciliary beat frequency (CBF), in addition to metrics of mucus viscosity (28, 29). Overall, findings implicated the potential of ARINA-1 as a novel mucolytic therapy for use in CF.
Methods
Detailed methods are provided in the data supplement.
Cell Culture
Primary human bronchial epithelial (HBE) cells harvested from lung explants of previously healthy (wild-type; WT) or F508del-CFTR homozygous CF donors were approved for use by the University of Alabama at Birmingham Institutional Review Board (IRB no. 160818002) and cultured according to a previously reported protocol (30). CFBE41o− cells expressing WT-CFTR or F508del-CFTR were also grown as previously described (31). F508del-CFTR CFBE41o− cells were treated with VX-809 (3 μM) for 48 hours before experimentation to correct for misfolding.
μOCT Imaging and Analysis
We used μOCT to obtain cross-sectional, video-rate images of functional microanatomic parameters of primary HBE cell cultures (29). ARINA-1 (1 M) was provided by Renovion and diluted to a range of concentrations (Table 1). For these studies, cells were treated apically with 1 μl of ARINA-1 (1:4 dilution) or PBS vehicle control. ARINA-1 was also assessed in combination with the CFTR modulator therapy lumacaftor (VX-809; 3 μM) and ivacaftor (VX-770; 10 μM), added basolaterally 18 hours before baseline addition of ARINA-1 to achieve its peak effect at 24–48 hours in this model system (32). μOCT images were collected at baseline before treatment (0 h) and at 6 and 24 hours after treatment, and MCT rate, ASL depth, and CBF were quantified (9, 26, 32).
Table 1.
Experimental Conditions for Testing ARINA-1 Components Using 1-μm Optical Coherence Tomography
| Solutions and Compounds | Concentration (mM) | Osmolality (mOsm/kg) | pH | ||
|---|---|---|---|---|---|
| 1 | ARINA-1 (high osmolality) | NaHCO3 | 1,000 | 1,812* | 5.56 |
| Glutathione | 488 | ||||
| Ascorbic acid | 400 | ||||
| 2 | 1:4 ARINA-1 (medium osmolality) | NaHCO3 | 250 | 479* | 6.25 |
| Glutathione | 122 | ||||
| Ascorbic acid | 100 | ||||
| 3 | 1:6 ARINA-1† (normosmolality) | NaHCO3 | 167 | 295* | 6.32 |
| Glutathione | 81 | ||||
| Ascorbic acid | 67 | ||||
| 4 | NaHCO3 |
250 | 437 | 8.09 | |
| 5 | NaHCO3 |
125 | 200 | 5.17 | |
| Glutathione |
122 | ||||
| 6 | NaHCO3 |
125 | 238 | 5.18 | |
| Ascorbic acid | 100 | ||||
Equal osmolality saline (NaCl) solutions were used as controls.
Has the same osmolality of 0.9% NaCl.
ARINA-1 and Component Studies
Studies exploring the effect of ARINA-1 components included bicarbonate only (250 mM; apical), bicarbonate with glutathione (bicarbonate, Letco 685940, 125 mM, and glutathione, G6013-5G/PCCA, 122 mM; apical; MilliporeSigma), and bicarbonate with ascorbic acid (bicarbonate 125 mM, and ascorbic acid, Letco 684471, 100 mM; apical) conditions.
ARINA-1 and Osmolarity Studies
To assess the effect of osmolarity, the osmolality of sodium chloride corresponding to normosmolar (297 mOsm/kg; diluted sixfold to achieve the same osmolality as PBS), medium osmolar (449 mOsm/kg; reflecting 25% dilution or the equivalent concentration of sodium chloride, 1.4%), and high osmolar (1,812 mOsm/kg; reflecting undiluted ARINA-1 as a comparator) ARINA-1 were prepared (Table 1) using the Fiske Model 210 Micro Osmometer (Fiske Associates). Changes in ASL depth and MCT rate on HBE cell cultures were quantified (9, 26, 32). Assuming 1) an average ASL height of 5 μm on the top of epithelial cells grown on 0.33 cm2 of a membrane insert and 2) an ASL osmolarity of 305 mOsm/kg, the osmolarities of the test compounds were mildly diluted (∼5%) upon their addition (1 μl) to the mucus layer of the cell culture based on resident ASL.
Mucus Reflectivity
Mucus reflectivity was evaluated on HBE cell cultures treated with ARINA-1 (1:4 dilution) or PBS for 6 hours at 37°C according to recently published methods, adapted for cell culture as described online (33).
Particle-tracking Microrheology
The viscosity of mucus lining HBE cell cultures was assessed upon treatment with 1 μl of ARINA-1 (1:4 dilution) or PBS using particle-tracking microrheological techniques (12, 32, 34).
Short-Circuit Current Measurements
CFTR function was measured in polarized WT-CFTR or F508del-CFTR CFBE41o− monolayers and in WT-CFTR or F508del-CFTR homozygous HBE cell cultures by Ussing chamber analyses (35).
Statistical Analyses
Prism software (GraphPad Software) was used for statistics, which are described in the data supplement.
Results
ARINA-1 Augments Mucus Transport in F508del/F508del HBE Cells
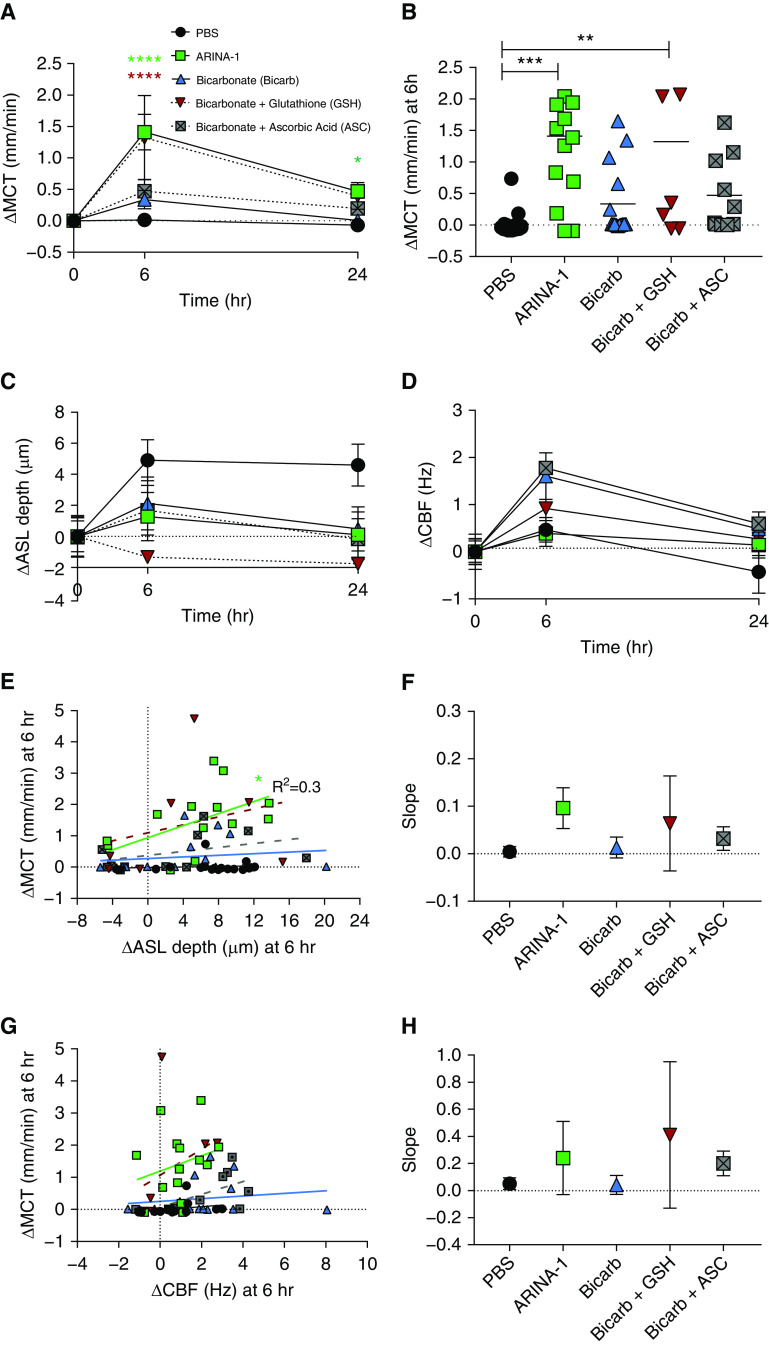
To evaluate the effect of ARINA-1 on functional parameters of the epithelial surface, the apical compartment of F508del/F508del primary HBE cells was treated with ARINA-1 for 6 or 24 hours at 1:4 dilution (Table 1). This concentration was established by preliminary dose– and time–response studies demonstrating its optimal effect on MCT (Figure E1 in the data supplement) and is believed to be achievable with inhalation in humans, accounting for dilution in the resident ASL. Subsequent replicates were conducted across various CF HBE donors. Before experimentation, HBE donors that were to receive ARINA-1 had an ASL depth of 9.8 ± 0.9 μm and an MCT of 0.1 ± 0.0 mm/min, and those assigned to the control group had an ASL depth of 8.0 ± 0.7 μm and an MCT of 0.1 ± 0.0 mm/min, reflecting similar status at baseline. Representative μOCT images are shown in Figures 1A–1H (also see Video E1), and quantification of these data as shown by relative change from baseline is presented in Figures 1I–1K. Absolute values are reported. Analyses demonstrated that monolayers treated with ARINA-1 exhibited higher absolute MCT rates peaking at 6 hours (2.8 ± 0.4 mm/min) than those treated with PBS vehicle control (0.2 ± 0.1 mm/min), representing a significant change from baseline pretreatment levels (P < 0.0001) (Figure 1I). As expected, ARINA-1 did not affect ASL depth (12.8 ± 1.2 μm) compared with vehicle control (12.8 ± 1.0 μm) after 6 hours of treatment (P = not significant [NS]). Similarly, although ARINA-1 increased CBF (9.3 ± 0.3 Hz), differences relative to PBS were not discernable (8.9 ± 0.2 Hz), even upon normalization to baseline values (P = NS) (Figures 1J and 1K).
Figure 1.
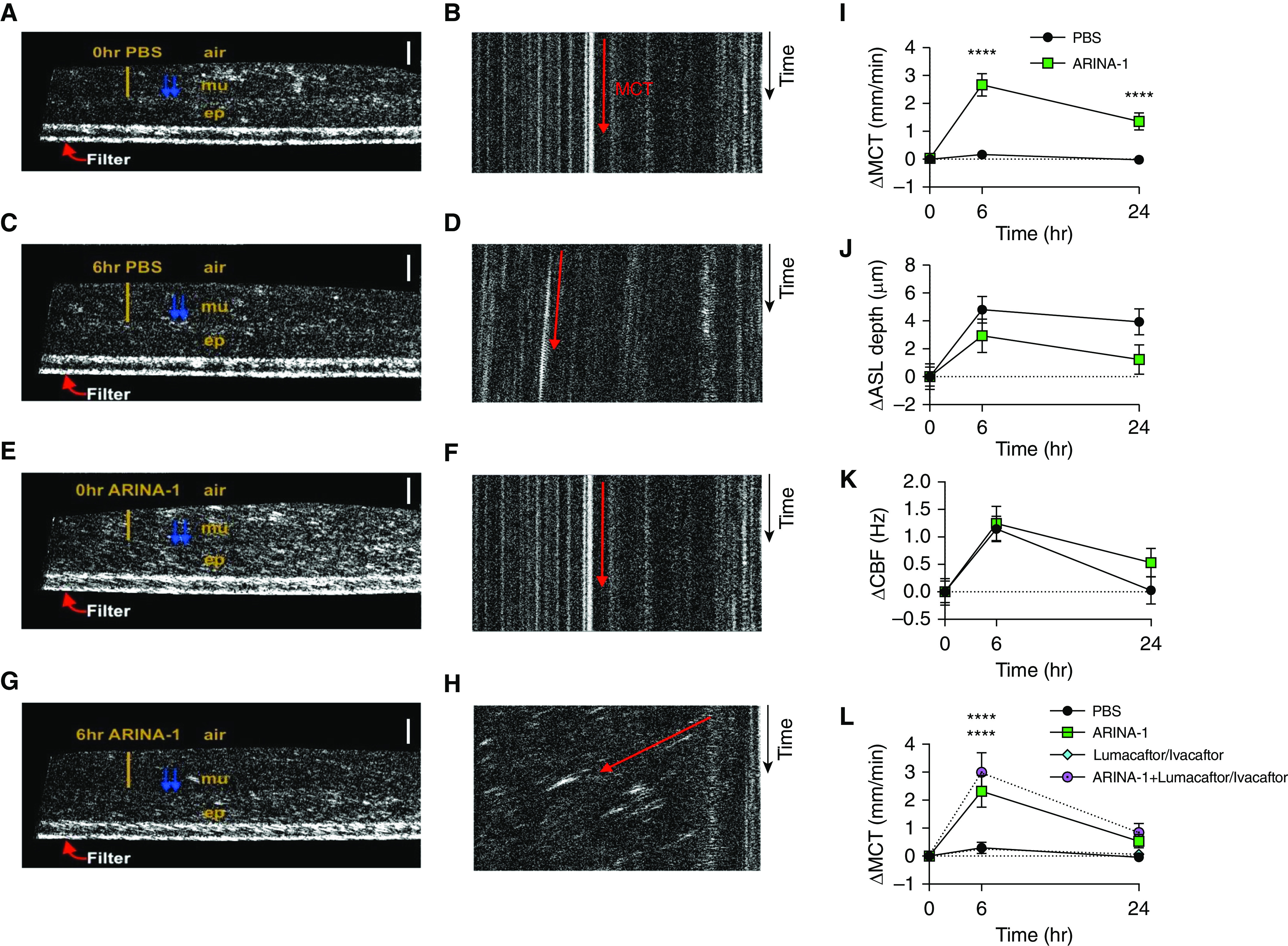
ARINA-1 improves mucus transport in F508del-homozygous primary human bronchial epithelial (HBE) cells. (A–H) Representative images of F508del/F508del HBE monolayers treated with PBS at 0 hours pretreatment (A and B), PBS at 6 hours (C and D), ARINA-1 at 0 hours pretreatment (E and F), and ARINA-1 at 6 hours (G and H). (A, C, E, and G) Text designates the epithelial cell monolayer (ep), mucus layer (mu), air interface (air), and cross-sectional view of the filter membrane (Filter). The yellow bar indicates airway surface liquid (ASL) layer depth, and the blue arrows denote cilia. Scale bars, 20 μm. (B, D, F, and H) Resliced views of images in which mucociliary transport (MCT) rate was measured as viewed from the apical surface. Orientation of the images is altered such that the slope of the diagonal streak (represented by the red arrow) represents the vectoral transport of mucus particles over time (time = y-axis). This enables visualization of differences in MCT rate (measured in video rate) using still images: steeper slope = slower transport; more horizontal slope = faster transport. (I–K) Change from baseline versus PBS in MCT rate (I), ASL depth (J), and ciliary beat frequency (CBF) (K) after 6 and 24 hours of treatment with PBS or ARINA-1; n = 44–45 monolayers per condition across four different donors with cystic fibrosis (CF). (L) Change from baseline versus PBS in MCT rate upon cotreatment with lumacaftor and ivacaftor. Two-way ANOVA and Tukey’s post hoc test. n = 20 monolayers per condition across three different donors with CF. Mean ± SEM. ****P < 0.0001 versus PBS control.
On the basis of these findings, noting that combination CFTR modulator therapy is currently approved for treatment of patients with F508del-CFTR and has been shown to improve MCT in F508del-homozygous primary HBE cells in correspondence to clinical benefit (32, 36), we assessed the impact of ARINA-1 in combination with the corrector lumacaftor and potentiator ivacaftor, applied basolaterally 18 hours before ARINA-1 addition to simulate chronic exposure (32). MCT rates at 6 hours seen with this multiagent treatment (3.0 ± 0.7 mm/min) exceeded those elicited by either approach alone (2.3 ± 0.6 mm/min for ARINA-1 and 0.3 ± 0.1 mm/min for lumacaftor and ivacaftor), conferring significant benefit over PBS (0.3 ± 0.2 mm/min; P < 0.0001) (Figure 1L). Together, these results suggest the potential of ARINA-1 as an efficacious approach to improve mucus clearance, both as a monotherapy and in addition to approved combination CFTR modulator therapy.
ARINA-1 Improves Airway Hydration and Mucus Transport above Equiosmolar Solutions of Saline
Because full-concentration ARINA-1 is hyperosmolar (1,812 mOsm/kg), its effect on airway hydration and improving MCT rate was assessed in direct comparison to three preparations of ARINA-1 with corresponding osmolarity solutions of saline to distinguish the response to ARINA-1 treatment from that secondary to solute concentration alone. We treated three groups of HBE monolayers from two separate donors homozygous for F508del with equiosmolar solutions of normosmolar, medium osmolar, and high osmolar saline or ARINA-1 (see Table 1); the medium osmolar solution was tested in Figure 1. This is estimated to be the final concentration in the airways when nebulized ARINA-1 is diluted with native airway fluid (37) and was slightly hyperosmolar, the equivalent of 1.4% saline when administered at typical doses (5 ml nebulized). Representative resliced images depicting the motion of mucus particles in time are shown in Figures 2A–2F (also see Video E2). The resulting effects of various solute concentrations of ARINA-1 relative to saline on airway hydration and mucus transport are shown in Figures 2G–2J. We observed abundantly increased airway hydration relative to baseline ASL in both high osmolar saline (18.6 ± 8.2 μm; P < 0.002) and high osmolar ARINA-1 (20.4 ± 7.7 μm; P < 0.0001) at 6 hours compared with lower osmolar solutions (Figure 2G). However, no statistically significant difference was found between ARINA-1 and saline within the three equiosmolar groups (Figure 2H). In contrast, a significant difference in MCT from baseline was found at 6 hours in high osmolar ARINA-1 (16.8 ± 4.4 μm; P ≤ 0.0001) (Figure 2I) compared with high osmolar saline (1.8 ± 1.2 μm; P = NS), representing a marked response that was maintained to the 24-hour time point (4.2 ± 1.6 μm; P < 0.001) (Figure 2I). Comparison of one-to-one equiosmolar concentrations with saline control showed a strong difference by treatment (P < 0.0001), with low (by 68%; 0.3 ± 0.6 PBS vs. 1.8 ± 1.6 ARINA-1; P < 0.05), medium (by 58%; 0.3 ± 0.5 saline vs. 1.6 ± 1.2 ARINA-1; P < 0.05), and high osmolar ARINA-1 (by 92%; 1.8 ± 2.9 saline vs. 16.8 ± 10.8 ARINA-1; P < 0.005) exceeding MCT rates observed with corresponding control (Figure 2J). These results suggest that the effect of ARINA-1 on mucus hydration is partially dependent on osmolarity, whereas the more prominent effects on mucus transport are largely independent of osmolality, particularly at concentrations expected to be achieved upon dilution into resident ASL.
Figure 2.

ARINA-1 mucus clearance effect exceeds equiosmolar treatments with hypertonic saline. Equiosmolar treatments of F508del/F508del HBE monolayers with normosmolar saline/normosmolar ARINA-1, medium osmolar saline/medium osmolar ARINA-1, and high osmolar saline/high osmolar ARINA-1 were performed as in Table 1. (A–F) Representative reslices of airway mucus transport (as in Figure 1) for each treatment condition at 6 hours after treatment. Slope of the diagonal streak (represented by the red arrow) represents the vectoral transport of mucus particles over time (time = y-axis [see Figures 1B, 1D, 1F, and 1H]), enabling visualization of differences in MCT rate. (G) Change in ASL depth from baseline at 6 and 24 hours after treatment. Top asterisks: high osmolar ARINA 1 versus normosmolar saline at same time point; bottom asterisks: high osmolar saline versus normosmolar saline at same time point, by two-way ANOVA and Tukey’s post hoc test. *P < 0.05, **P < 0.01, and ****P < 0.0001. (H) Paired comparisons of change in ASL depth at 6 hours of equiosmolar conditions (each point represents mean change per monolayer). ns = not significant. (I) Change in MCT rate relative to normosmolar saline and compared with baseline. (J) Change in MCT rate between dual groups of equiosmolar conditions at 6 hours, each point representing mean change from baseline per monolayer. One-way ANOVA and Tukey’s post hoc test. n = 7–8 monolayers per condition across two different donors with CF. Mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
ARINA-1 Improves Mucus Viscosity in F508del/F508del HBE Cells
We next evaluated the relationship between airway hydration (measured by ASL depth) and MCT. Disruptions of the normal linear relationship between these parameters when assessed on a colocalized basis has been characteristic of the CF defect in prior studies in cell and tissue cultures (8, 10) as well as in vivo (33). As assessed by linear regression analyses, changes in MCT after 6 hours of treatment with ARINA-1 were associated with changes in both ASL (R2 = 0.40; b = 0.22; P < 0.001) (Figure 3A) and CBF (R2 = 0.22; b = 0.52; P = 0.01) (Figure 3C), relationships characteristic of normal HBE cells (8, 38). In contrast, associations seen in PBS-treated monolayers were weaker (R2 = 0.14; b = 0.03; P < 0.05, ∆ASL vs. ∆MCT; R2 = 0.10; b = 0.10; P = 0.08, ∆CBF vs. ∆MCT), as also shown by direct comparison of regression slopes demonstrating significantly lower values for PBS relative to ARINA-1 (P < 0.001, ∆ASL vs. ∆MCT; P < 0.05, ∆CBF vs. ∆MCT) (Figures 3B and 3D). To probe whether this distinction was due to differences in viscosity between ARINA-1– and PBS-treated cells, we analyzed μOCT images to evaluate the reflectance intensity of the mucus layer for each condition. Our group has recently demonstrated that this technique provides an estimate of the viscosity of CF mucus, with greater reflectivity corresponding to greater viscosity (33), noting that other factors can certainly contribute to the backscatter properties of mucus. As shown in the representative images depicted in Figures 4A and 4B, the reflectance intensity of the mucus layer was diminished in F508del/F508del HBE cells treated with ARINA-1 for 6 hours as compared with those treated with PBS, in which the mucus layer exhibited larger particles and a greater backscattering of light. This was confirmed through quantification of pixel intensity demonstrating a significant forward (right) shift in the histogram curve for the PBS control group (P < 0.01) (Figure 4C), representative of a greater proportion of more highly reflective pixels and greater mucus viscosity for the PBS-treated cells. Correspondingly, mean reflectance intensity was significantly higher in PBS-treated cells (18.1 ± 0.9) than in ARINA-1–treated cells (12.7 ± 0.8; P < 0.001) (Figure 4D). To corroborate these findings indicating less viscous mucus in ARINA-1–treated monolayers, we performed additional evaluation of mucus viscosity using particle-tracking microrheology. In line with reflectivity analyses, representative tracings of particles added to F508del/F508del HBE monolayers after 6 hours of treatment with ARINA-1 or PBS revealed lower effective viscosity with ARINA-1 (Figure 5A), with graphical representation of the mean squared displacement of the tracked particles demonstrating less restricted particle movement in ARINA-1–treated monolayers (P < 0.0001) (Figure 5B). Viscoelastic curves confirmed that ARINA-1 significantly decreased the effective viscosity of the mucus (388.2 cP) compared with PBS (2,390 cP; P < 0.0001) (Figure 5C). Because some monolayers had both viscoelasticity and mucus reflectance intensity determined at the same time, we examined the relationship between those parameters, noting the associations were not statistically significant (Figure 5D). Together, these data indicate that ARINA-1 reduces the viscosity of CF mucus, suggesting a mechanistic pathway for its impact on mucus transport disproportionate to the effects on ASL depth alone.
Figure 3.
Changes in ASL depth and ciliary beating with ARINA-1 predict changes in mucus transport. (A) Linear regression analysis of the change in ASL depth versus the change in MCT at 6 hours for ARINA-1 and PBS-treated F508del/F508del HBE monolayers with (B) comparison of regression slopes. (C) Linear regression analysis of change in CBF versus change in MCT at 6 hours for ARINA-1– and PBS-treated monolayers with (D) comparison of regression slopes by unpaired two-tailed t test. n = 44–45 monolayers per condition across four different donors with CF. The ARINA-1 linear slopes are compatible with a normal relationship between ASL depth and CBF with MCT, often absent in CF. Mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 4.
ARINA-1 decreases reflectance intensity. Representative attenuation-corrected images of F508del/F508del HBE monolayers treated with (A) PBS or (B) ARINA-1 for 6 hours. Text designates the epithelial cell monolayer (ep), air interface (air), and cross-sectional view of the filter membrane (Filter). The yellow box circumscribes the ASL layer selected for analysis, and the blue arrows denote representative cilia. Scale bars, 20 μm. (C) Reflectivity histogram of the attenuation-corrected image plotted by treatment condition. Leftward shift of the histogram indicates a lower proportion of highly intense pixels, which has been shown to be proportionate to mucus viscosity. (D) Mean intensity of airway mucus plotted by treatment condition. **P < 0.01 and ***P < 0.001.
Figure 5.
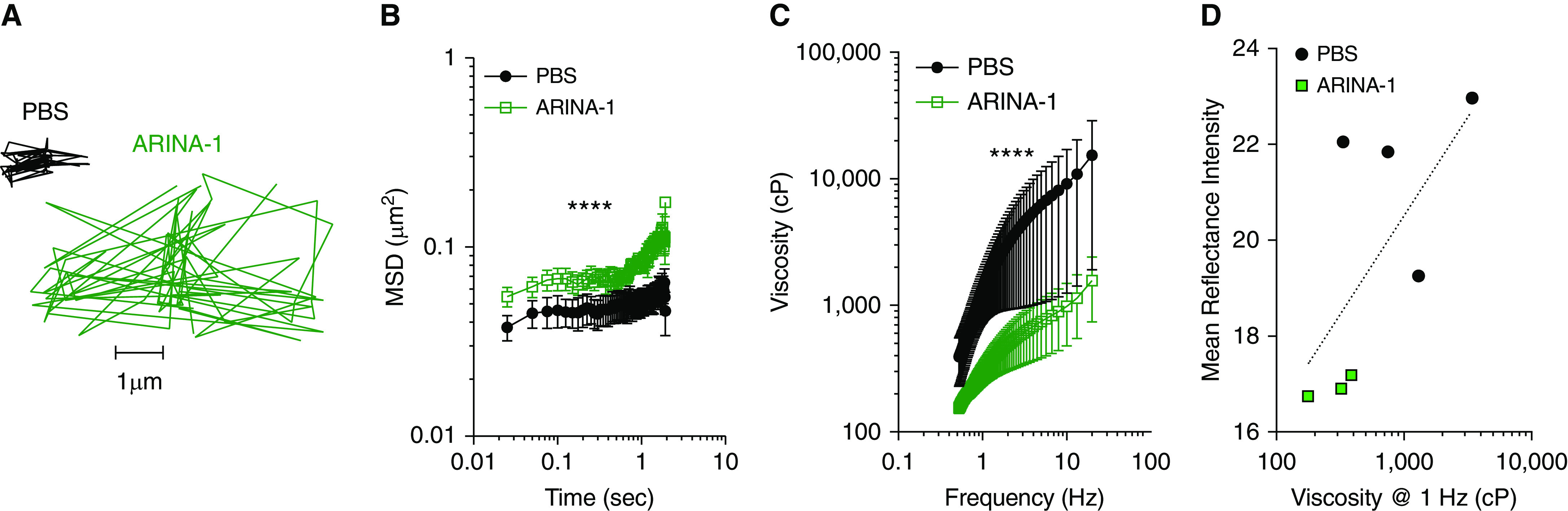
ARINA-1 decreases effective viscosity. (A) Representative tracings of beads in the mucus layer of F508del/F508del HBE monolayers treated with PBS or ARINA-1 for 6 hours as assessed by particle-tracking microrheology. (B) Plot of mean squared displacement (MSD) of each bead over time with (C) corresponding viscoelastic curves at frequency sweep ranging from 0.46 to 17.36 Hz. Statistics reported for comparison of ARINA-1 versus PBS. Two-way ANOVA and Sidak’s multiple comparisons test. n = 3–5 monolayers per condition from one donor with CF. Note logarithmic scale x- and y-axes. Mean ± SEM. ****P < 0.001. (D) Relationship between effective viscosity (at 1 Hz) and reflectance intensity. Data for same cells as in Figure 4 with four or five reflectance intensity replicates per well. Semilogarithmic regression relationship (dotted line) with R2 = 0.44; P = 0.10; n = 7. Note logarithmic scale x-axis.
Bicarbonate and Glutathione Combined Recapitulate ARINA-1 Effect on Mucus Transport
ARINA-1 is a combination of bicarbonate, glutathione, and ascorbic acid. Each component has previously been evaluated for its individual properties, but they have never been tested in combination. We assessed the contribution of these components to improvements in MCT rates seen with ARINA-1 (Table 1). To accomplish this, F508del/F508del HBE monolayers were treated apically with bicarbonate alone, bicarbonate combined with glutathione, bicarbonate combined with ascorbic acid, or ARINA-1 for 6 or 24 hours and evaluated by μOCT functional imaging. Glutathione-only and ascorbic acid–only conditions were not included, on the basis of low cell viability due to low pH (data not shown). Results demonstrated that the combination of bicarbonate with glutathione elicited absolute MCT rates at 6 hours (1.4 ± 0.7 μm) that were 92% of those seen with ARINA-1 (1.5 ± 0.3 μm), with both conditions driving a significant increase (P < 0.0001, bicarbonate + glutathione; P < 0.0001, ARINA-1) (Figure 6A) over PBS (0.1 ± 0.05 μm). Modest improvements in MCT were observed in monolayers treated with the combination of bicarbonate with ascorbic acid (0.5 ± 0.2 μm; 32% of ARINA-1) or bicarbonate alone (0.4 ± 0.1 μm; 23% of ARINA-1), reflecting prior reports of the beneficial effects of bicarbonate on MCT (12, 32) and mucus adhesion (39). Similar to ARINA-1, the effect of bicarbonate with glutathione on MCT rates was independent of changes in ASL depth or CBF relative to PBS (P = NS) (Figures 6C and 6D). Linear relationships between ASL depth or CBF and MCT rates were similar between bicarbonate combined with glutathione (R2 = 0.08; b = 0.07; P for slope = 0.6, ∆ASL vs. ∆MCT; R2 = 0.1; b = 0.41; P for slope = 0.5, ∆CBF vs. ∆MCT) and ARINA-1 (R2 = 0.30; b = 0.10; P for slope < 0.05, ∆ASL vs. ∆MCT; R2 = 0.06; b = 0.24; P for slope = 0.4, ∆CBF vs. ∆MCT) compared with the other conditions tested (Figures 6E–6H), suggesting a similar impact on mucus viscosity. These data indicate that the combination of bicarbonate with glutathione contributes significantly to the effect of ARINA-1 on MCT rates and, together with Figures 1 and 2, underscore the involvement of factors other than airway hydration or ciliary beating.
Figure 6.
The combination of bicarbonate with glutathione (GSH) recapitulates the effect of ARINA-1 on mucus transport. F508del/F508del HBE monolayers were treated with PBS vehicle control, ARINA-1, bicarbonate, bicarbonate + GSH, or bicarbonate + ascorbate (ASC) for 6 or 24 hours. (A) Change from baseline versus PBS in MCT rate by two-way ANOVA and Tukey’s post hoc test. At 6 hours, top asterisks = ARINA-1; bottom asterisks = bicarbonate + GSH (color-coded). (B) Change in MCT rate from baseline at 6 hours by treatment condition, with each point representing mean change per monolayer. One-way ANOVA and Tukey’s post hoc test. Change from baseline versus PBS in (C) ASL depth and (D) CBF. (E) Linear regression analysis of change in ASL depth versus change in MCT at 6 hours across treatment conditions with (F) comparison of regression slopes (one-way ANOVA = not significant; Bartlett’s test of homoscedasticity, P < 0.0001). (G) Linear regression analysis of change in CBF versus change in MCT at 6 hours with (H) comparison of regression slopes by unpaired two-tailed t test. n = 20–21 monolayers per condition across four different donors with CF. Mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
ARINA-1 and Ascorbic Acid Stimulate CFTR-dependent Chloride Transport in WT CFBE41o− Cells but Not in HBE Cells
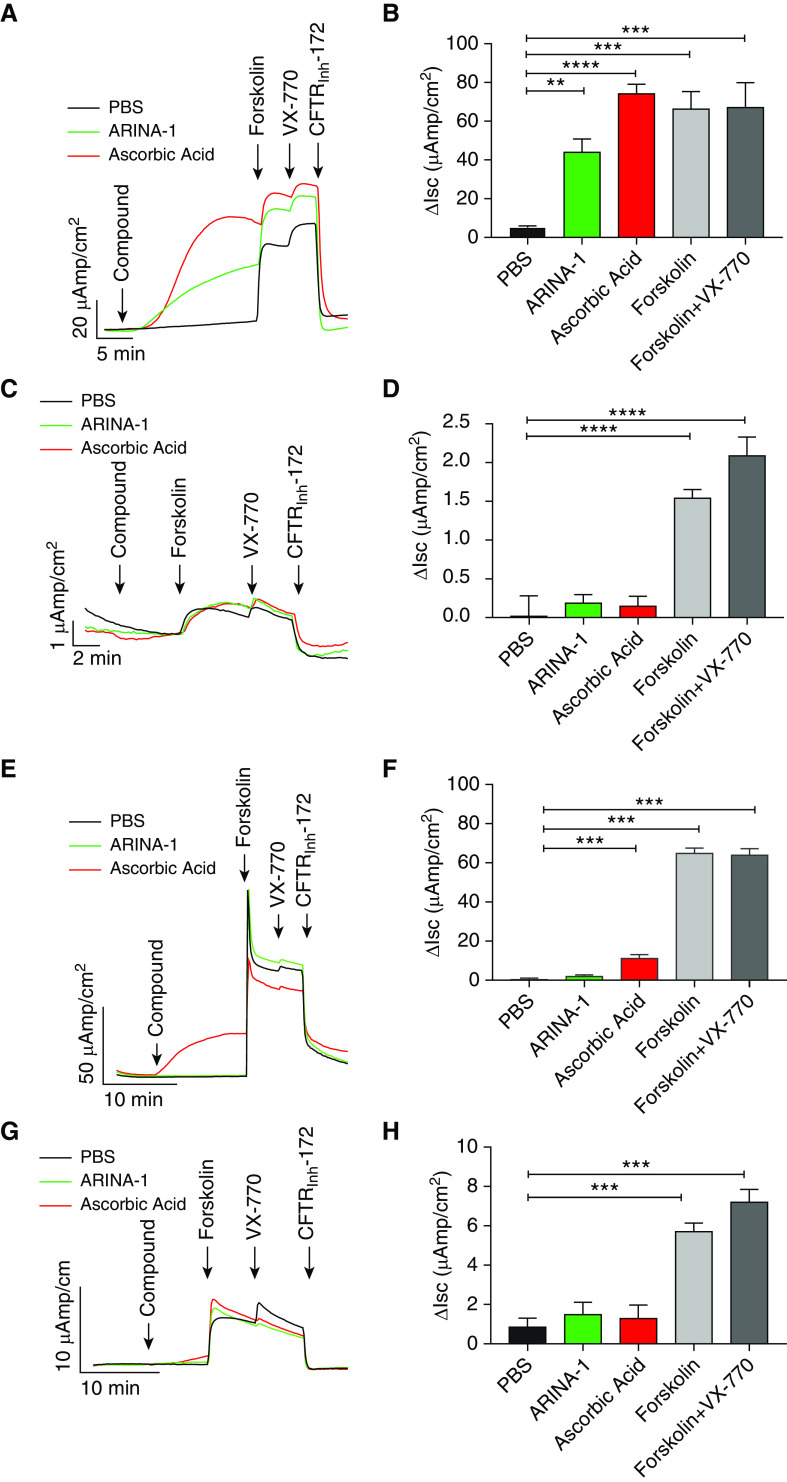
On the basis of prior reports demonstrating a stimulatory effect of ascorbic acid on CFTR-mediated chloride transport (40, 41), we hypothesized that ARINA-1 may impact CFTR channel function to impart beneficial effects on MCT. We therefore conducted Ussing chamber analyses in CFBE41o− cells expressing either WT or F508del-CFTR and in F508del/F508del or WT HBE monolayers. It should be noted that the CO2 buffer in the Ussing chamber reservoir neutralized pH, enabling assessment of ascorbic acid as an individual agent in this assay. As shown in Figures 7A and 7B, ARINA-1 significantly increased CFTR-dependent short-circuit current in WT CFBE41o− cells (44.0 ± 6.8 mV for ARINA-1 vs. 5.2 ± 0.8 mV for PBS; P < 0.01), with an equal concentration of ascorbic acid also eliciting a significant effect (74.2 ± 4.9 mV; P < 0.0001), consistent with previous work (40). Significant activation of CFTR-dependent currents was also observed with ascorbic acid (11.2 ± 1.9 mV for ascorbic acid vs. 0.4 ± 0.6 mV for PBS; P < 0.001) (Figures 7E and 7F), although not with ARINA-1 (1.9 ± 0.7 mV; P = NS), in WT HBE monolayers. The diminished effect of ARINA-1 was likely due to lower expression levels of CFTR present in primary cells compared with CFBE41o− cells (42), as also supported by the attenuated effect of ascorbic acid. In contrast to data with WT CFTR, neither ARINA-1 nor ascorbic acid notably altered CFTR ion transport in F508del-CFTR CFBE41o− (0.2 ± 0.1 mV for ARINA-1 and 0.1 ± 0.1 mV for ascorbic acid vs. 0.02 ± 0.3 mV for PBS; P = NS) (Figures 7C and 7D) or F508del-F508del HBE (1.5 ± 0.6 mV for ARINA-1 and 1.3 ± 0.7 mV for ascorbic acid vs. 0.9 ± 0.4 mV for PBS; P = NS) (Figures 7G and 7H), even when F508del-CFTR cell surface expression was corrected with VX-809. This suggests the requirement of fully functional CFTR beyond that provided by F508del-CFTR correction with lumacaftor for ARINA-1 to have a meaningful impact on CFTR function and further implicates ascorbic acid as a driver of this effect. Overall, these results indicate that the improvements in MCT induced by ARINA-1 in F508del/F508del HBE cells are largely independent of CFTR channel activity.
Figure 7.
ARINA-1 increases CFTR-mediated chloride secretion in wild-type (WT) CFBE41o− cells, but not in F508del-CFTR CFBE41o−, WT HBE, or F508del-F508del HBE cells. Transepithelial chloride currents across the epithelia of CFBE41o− cells and HBE monolayers were assessed by Ussing chamber analysis upon treatment with PBS, ARINA-1, or ASC. (A) Representative tracings with (B) summary bar graphs of chloride currents in CFBE41o− cells expressing WT-CFTR. (C) Representative tracings with (D) summary bar graphs of chloride currents in VX-809–corrected F508del-CFTR CFBE41o− cells. (E) Representative tracings with (F) summary bar graphs of chloride currents in WT HBE monolayers. (G) Representative tracings with (H) summary bar graphs of chloride currents in VX-809–corrected F508del-F508del HBE monolayers. One-way ANOVA and Tukey’s post hoc test. CFBE41o− cells: n ≥ 4 monolayers per condition. HBE monolayers: n = 8–12 monolayers per condition across three different donors with CF or WT donors. Mean ± SEM. **P < 0.01, ***P < 0.001, and ****P < 0.0001. Isc = short circuit current. ΔIsc = change in short-circuit current.
Discussion
Using μOCT analysis of primary HBE cell cultures, we demonstrated that the novel inhalational product ARINA-1 significantly improved MCT rate in the epithelial layer of F508del/F508del HBE cells, both alone and in combination with the commercially available CFTR modulator therapy lumacaftor-ivacaftor. Although the effect of ARINA-1 on mucus transport was largely independent of ASL hydration, ciliary beating, or solution osmolarity, studies using mucus reflectivity and particle-tracking microrheological techniques established that ARINA-1 decreased the viscosity of the mucus layer of these cells, bringing clarity to a plausible mechanism of action in improving MCT. To our knowledge, these significant findings are the first to implicate a therapeutic strategy consisting of bicarbonate, glutathione, and ascorbic acid combined as an approach to targeting defective mucus clearance in patients with CF. The magnitude of MCT rate changes elicited by ARINA-1 were particularly compelling, substantially exceeding effects seen with hypertonic saline in this study as well as those previously observed in the same model system using small-molecule modulators of CFTR activity (32). This may be a particular advantage of direct administration to abnormal mucus. It is notable that the effect was consistently observed at 6 hours and largely lost by 24 hours, indicating that two to three times daily dosing may be required for this mechanism. Furthermore, dose range finding may be useful because effective concentrations achieved upon nebulization and dilution with resident lung fluid are not known but were estimated conservatively in the present study with many studies conducted at 25% strength.
The hyperosmolarity of ARINA-1 suggests that osmolarity may drive hydration of the mucus layer, which could be responsible for accelerated MCT (43). However, comparisons between ARINA-1 dilutions and equiosmolar saline solution indicated the contribution of additional factors. Indeed, although the increases in ASL depths seen with ARINA-1 closely resembled those observed with equiosmolar saline at all osmolarities tested, changes in MCT rate in each corresponding osmolar solution were significantly greater with ARINA-1 than with equiosmolar saline, particularly at the high osmolar concentrations, revealing that neither the osmolarity of ARINA-1 nor its effect on airway hydration alone could fully account for its ability to improve mucus stasis. These findings are consistent with prior studies in CF tissues demonstrating that airway hydration is not the sole determinant of mucus transport and that mucus viscosity also plays a role (9, 12, 14). The data obtained using two complementary techniques to assess mucus viscoelasticity showed that ARINA-1 improved the viscosity of CF mucus and also its backscattering properties in concordance with a previous report establishing that viscosity can play a prevailing role over airway hydration in impacting MCT (14) and represents a likely mechanism in the significant impact of ARINA-1 on improving MCT. It is possible that spinnability, or the tendency of CF mucus to form adherent threads, could be a factor influencing the efficacy of ARINA-1 independent of mucus hydration, as also shown for hypertonic saline (44). Changes in hydration achieved by its osmolar properties could also have influences on reflectance intensity because this could also affect mucus backscatter properties. Evaluation of the correlations between changes in mucus transport and hydration, as well as ciliary beating, at earlier time points may be of clinical importance in future studies noting that dynamic regulation of ASL may occur in an earlier time frame than the 6-hour time point tested.
Studies evaluating the contribution of ARINA-1 components to its overall effect revealed that the combination of bicarbonate with glutathione was the key driver behind improvements in mucus transport. The influence of bicarbonate is consistent with a recent report demonstrating that bicarbonate replacement on the airway surface restored mucus transport in a rat model of CF, together with the finding that bicarbonate deficiency independently contributed to the mucus defect in the model (12). This is also consistent with other studies in the gastrointestinal tract of mice which have shown that CF mucus is highly adherent and can be released only when exposed to a high concentration of bicarbonate (18, 20). The adhesion of mucus strands to the outlet of CF gland ducts could be recapitulated in non-CF porcine trachea, but only when both bicarbonate and chloride secretion were pharmacologically blocked, noting that these studies were performed under submerged conditions in which chloride ions are already replete (14). Altogether, this points toward the potential benefit of a hyperosmolar agent that also provides sufficient bicarbonate ions to normalize mucin structure.
That glutathione enhanced the effect of bicarbonate as a dual agent is notable in light of the questionable clinical efficacy of glutathione as a monotherapy when administered by the inhaled route (45, 46). Glutathione deficiency has broadly recognized implications in inflammatory processes and lung damage because of its function as an electron donor in defense against oxidant-mediated CF lung tissue damage (24). Excess oxidation is also a plausible explanation for how CF mucus may be excessively cross-linked when released into an inflamed environment (47). Glutathione deficiency in CF led to clinical assessment of inhaled glutathione supplementation that demonstrated promise in improving lung function in preliminary studies but no long-term benefit in a 6-month, placebo-controlled, randomized trial (45). This may be due to the acidic nature of glutathione (including that used in the clinical studies) that may have mitigated its beneficial properties on oxidation, noting that we observed epithelial injury when glutathione solution was administered directly. Administering glutathione in a buffered solution with bicarbonate may explain its improved tolerance to epithelia and provide synergistic efficacy via two complementary mechanisms: antioxidation of disulfide bonds combined with improved mucin processing by bicarbonate.
Although ascorbic acid has been reported to have mucolytic properties (48), this was not substantiated by our data indicating that ascorbic acid did not appreciably contribute to increases in MCT rate. Ascorbic acid did promote CFTR ion transport in the setting of fully functional WT CFTR, consistent with reports in respiratory epithelia in vitro (40), ex vivo (41), and in vivo (40). This suggests that the contribution of ascorbic acid to the effect of ARINA-1 on mucus transport may be enhanced with the ongoing development of highly effective CFTR modulator therapies that restore CFTR function to levels beyond those achieved with lumacaftor correction used in our studies, which did not facilitate notable increases in ascorbic acid–stimulated current. Ascorbate may also provide immunomodulatory benefits (49) and may address ascorbic acid deficiency of the airways prevalent in CF (50); further studies will be needed to measure these effects more definitively. The well-studied interdependence between glutathione and ascorbic acid in the glutathione–ascorbate cycle may also impact its potential benefit.
Noting the safety and clinical experience with its individual constituents, ARINA-1 has been studied in a phase I clinical trial in patients with bilateral lung transplant, with a phase II clinical trial currently being planned for patients with bilateral lung transplant who have early bronchiolitis obliterans syndrome. A phase IIa trial in patients with CF assessing mucus clearance as an outcome is planned to validate the proof-of-concept studies presented in this article. Although animal studies would be useful in parsing additional questions regarding mechanisms and delivery to obstructed airways and could address efficacy in the context of chronic infection as opposed to the sterile cultures evaluated in the present study, they may not be adequately informative, because rodents, unlike humans, synthesize ascorbic acid endogenously and do not recapitulate deficient conditions seen in CF; CF ferrets or pigs could provide an appropriate model to test questions related to mucus, noting that they also synthesize ascorbic acid endogenously and thus cannot be used to evaluate all of the constituents of ARINA-1. Assessing the effect of delivered concentrations upon nebulization and inhalation might also influence expectations of efficacy during clinical development.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Heather Hathorne for her assistance with regulatory support for this work and the patients who donated cells for research. The authors also thank Hui Wen for her expert help with the Ussing chamber experiments. The authors acknowledge helpful contributions provided by investigators in the Therapeutics Development Network advisory meeting that included Scott Donaldson and Felix Ratjen, as well as members of the Cystic Fibrosis Foundation. The authors also acknowledge Roland Arnold, Ph.D., and David C. Henke, M.D., for their early research and development of ARINA-1.
Footnotes
Supported through direct funding from the Cystic Fibrosis Foundation (ROWE17XX2 [S.M.R.]), the Mucociliary Clearance Core Award of the Cystic Fibrosis Foundation (ROWE17XX1 [S.M.R.]), the Research Development Program (ROWE15R0 [S.M.R.]) of the Cystic Fibrosis Foundation, and the National Institute of Diabetes and Digestive and Kidney Diseases (grant P30 DK072482-12 [S.M.R.]). This work was also supported in part by an Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship (J.D.J.).
Author Contributions: A.T.A., E.F.L., L.F., D.C., C.D., and S.M.R. conception and design of the research. A.T.A., L.F., A.L., E.R.B., J.D.J., M.M., and S.M.R. performed experiments. A.T.A., E.F.L., L.F., E.R.B., and S.M.R. analyzed data. A.T.A., E.F.L., L.F., E.R.B., S.E.B., C.F.P., D.C., C.D., and S.M.R. interpreted the results of experiments. A.T.A., E.F.L., L.F., and S.M.R. prepared figures. A.T.A., E.F.L., L.F., and S.M.R. drafted and edited the manuscript. A.T.A., E.F.L., L.F., A.L., E.R.B., S.E.B., C.F.P., J.D.J., M.M., G.J.T., D.C., C.D., and S.M.R. approved the final version of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0287OC on May 6, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Tang L, Fatehi M, Linsdell P. Mechanism of direct bicarbonate transport by the CFTR anion channel. J Cyst Fibros. 2009;8:115–121. doi: 10.1016/j.jcf.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Kogan I, Ramjeesingh M, Li C, Kidd JF, Wang Y, Leslie EM, et al. CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J. 2003;22:1981–1989. doi: 10.1093/emboj/cdg194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe SM, Hoover W, Solomon GM, Sorscher EJ.Cystic fibrosis Broaddus VC, Mason RJ, Ernst JD, King TE, Lazarus SC, Murray JF.et al. editors. Murray and Nadel’s textbook of respiratory medicine Vol. 16th ed. Philadelphia, PA: Saunders; 2016822–852, e17. [Google Scholar]
- 4.Yang C, Chilvers M, Montgomery M, Nolan SJ. Dornase alfa for cystic fibrosis. Cochrane Database Syst Rev. 2016;4:CD001127. doi: 10.1002/14651858.CD001127.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–250. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 6.Henke MO, Ratjen F. Mucolytics in cystic fibrosis. Paediatr Respir Rev. 2007;8:24–29. doi: 10.1016/j.prrv.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Tarran R. Regulation of airway surface liquid volume and mucus transport by active ion transport. Proc Am Thorac Soc. 2004;1:42–46. doi: 10.1513/pats.2306014. [DOI] [PubMed] [Google Scholar]
- 8.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 9.Birket SE, Chu KK, Liu L, Houser GH, Diephuis BJ, Wilsterman EJ, et al. A functional anatomic defect of the cystic fibrosis airway. Am J Respir Crit Care Med. 2014;190:421–432. doi: 10.1164/rccm.201404-0670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 11.Donnelley M, Morgan KS, Awadalla M, Farrow NR, Hall C, Parsons DW. High-resolution mucociliary transport measurement in live excised large animal trachea using synchrotron X-ray imaging. Respir Res. 2017;18:95. doi: 10.1186/s12931-017-0573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birket SE, Davis JM, Fernandez CM, Tuggle KL, Oden AM, Chu KK, et al. Development of an airway mucus defect in the cystic fibrosis rat. JCI Insight. 2018;3:e97199. doi: 10.1172/jci.insight.97199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wark P, McDonald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev. 2018;9:CD001506. doi: 10.1002/14651858.CD001506.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–417. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- 16.Henderson AG, Ehre C, Button B, Abdullah LH, Cai LH, Leigh MW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124:3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang XX, Ostedgaard LS, Hoegger MJ, Moninger TO, Karp PH, McMenimen JD, et al. Acidic pH increases airway surface liquid viscosity in cystic fibrosis. J Clin Invest. 2016;126:879–891. doi: 10.1172/JCI83922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209:1263–1272. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang N, Garcia MA, Quinton PM. Normal mucus formation requires cAMP-dependent HCO3− secretion and Ca2+-mediated mucin exocytosis. J Physiol. 2013;591:4581–4593. doi: 10.1113/jphysiol.2013.257436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper JL, Quinton PM, Ballard ST. Mucociliary transport in porcine trachea: differential effects of inhibiting chloride and bicarbonate secretion. Am J Physiol Lung Cell Mol Physiol. 2013;304:L184–L190. doi: 10.1152/ajplung.00143.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 23.Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol (1985) 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- 24.Hudson VM. Rethinking cystic fibrosis pathology: the critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation. Free Radic Biol Med. 2001;30:1440–1461. doi: 10.1016/s0891-5849(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 25.Meldrum OW, Yakubov GE, Bonilla MR, Deshmukh O, McGuckin MA, Gidley MJ. Mucin gel assembly is controlled by a collective action of non-mucin proteins, disulfide bridges, Ca2+-mediated links, and hydrogen bonding. Sci Rep. 2018;8:5802. doi: 10.1038/s41598-018-24223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winklhofer-Roob BM, Ellemunter H, Frühwirth M, Schlegel-Haueter SE, Khoschsorur G, van’t Hof MA, et al. Plasma vitamin C concentrations in patients with cystic fibrosis: evidence of associations with lung inflammation. Am J Clin Nutr. 1997;65:1858–1866. doi: 10.1093/ajcn/65.6.1858. [DOI] [PubMed] [Google Scholar]
- 27.Winkler BS, Orselli SM, Rex TS. The redox couple between glutathione and ascorbic acid: a chemical and physiological perspective. Free Radic Biol Med. 1994;17:333–349. doi: 10.1016/0891-5849(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 28.Liu L, Shastry S, Byan-Parker S, Houser G, K Chu K, Birket SE, et al. An autoregulatory mechanism governing mucociliary transport is sensitive to mucus load. Am J Respir Cell Mol Biol. 2014;51:485–493. doi: 10.1165/rcmb.2013-0499MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Chu KK, Houser GH, Diephuis BJ, Li Y, Wilsterman EJ, et al. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PLoS One. 2013;8:e54473. doi: 10.1371/journal.pone.0054473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandez-Petty CM, Hughes GW, Bowers HL, Watson JD, Rosen BH, Townsend SM, et al. A glycopolymer improves vascoelasticity and mucociliary transport of abnormal cystic fibrosis mucus. JCI Insight. 2019;4:e125954. doi: 10.1172/jci.insight.125954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bebok Z, Collawn JF, Wakefield J, Parker W, Li Y, Varga K, et al. Failure of cAMP agonists to activate rescued ΔF508 CFTR in CFBE41o− airway epithelial monolayers. J Physiol. 2005;569:601–615. doi: 10.1113/jphysiol.2005.096669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birket SE, Chu KK, Houser GH, Liu L, Fernandez CM, Solomon GM, et al. Combination therapy with cystic fibrosis transmembrane conductance regulator modulators augment the airway functional microanatomy. Am J Physiol Lung Cell Mol Physiol. 2016;310:L928–L939. doi: 10.1152/ajplung.00395.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung HM, Birket SE, Hyun C, Ford TN, Cui D, Solomon GM, et al. Intranasal micro-optical coherence tomography imaging for cystic fibrosis studies. Sci Transl Med. 2019;11:eaav3505. doi: 10.1126/scitranslmed.aav3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu KK, Mojahed D, Fernandez CM, Li Y, Liu L, Wilsterman EJ, et al. Particle-tracking microrheology using micro-optical coherence tomography. Biophys J. 2016;111:1053–1063. doi: 10.1016/j.bpj.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu L, Rab A, Tang LP, Rowe SM, Bebok Z, Collawn JF. Dab2 is a key regulator of endocytosis and post-endocytic trafficking of the cystic fibrosis transmembrane conductance regulator. Biochem J. 2012;441:633–643. doi: 10.1042/BJ20111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. TRAFFIC Study Group; TRANSPORT Study Group. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buhl R, Vogelmeier C, Critenden M, Hubbard RC, Hoyt RF, Jr, Wilson EM, et al. Augmentation of glutathione in the fluid lining the epithelium of the lower respiratory tract by directly administering glutathione aerosol. Proc Natl Acad Sci USA. 1990;87:4063–4067. doi: 10.1073/pnas.87.11.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill DB, Long RF, Kissner WJ, Atieh E, Garbarine IC, Markovetz MR, et al. Pathological mucus and impaired mucus clearance in cystic fibrosis patients result from increased concentration, not altered pH. Eur Respir J. 2018;52:1801297. doi: 10.1183/13993003.01297-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ermund A, Trillo-Muyo S, Hansson GC. Assembly, release, and transport of airway mucins in pigs and humans. Ann Am Thorac Soc. 2018;15:S159–S163. doi: 10.1513/AnnalsATS.201804-238AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer H, Schwarzer C, Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci USA. 2004;101:3691–3696. doi: 10.1073/pnas.0308393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho DY, Hwang PH, Illek B. Effect of L-ascorbate on chloride transport in freshly excised sinonasal epithelia. Am J Rhinol Allergy. 2009;23:294–299. doi: 10.2500/ajra.2009.23.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowe SM, Pyle LC, Jurkevante A, Varga K, Collawn J, Sloane PA, et al. ΔF508 CFTR processing correction and activity in polarized airway and non-airway cell monolayers Pulm Pharmacol Ther 201023268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarran R, Grubb BR, Gatzy JT, Davis CW, Boucher RC. The relative roles of passive surface forces and active ion transport in the modulation of airway surface liquid volume and composition. J Gen Physiol. 2001;118:223–236. doi: 10.1085/jgp.118.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.King M, Dasgupta B, Tomkiewicz RP, Brown NE. Rheology of cystic fibrosis sputum after in vitro treatment with hypertonic saline alone and in combination with recombinant human deoxyribonuclease I. Am J Respir Crit Care Med. 1997;156:173–177. doi: 10.1164/ajrccm.156.1.9512074. [DOI] [PubMed] [Google Scholar]
- 45.Griese M, Kappler M, Eismann C, Ballmann M, Junge S, Rietschel E, et al. Glutathione Study Group. Inhalation treatment with glutathione in patients with cystic fibrosis: a randomized clinical trial. Am J Respir Crit Care Med. 2013;188:83–89. doi: 10.1164/rccm.201303-0427OC. [DOI] [PubMed] [Google Scholar]
- 46.Calabrese C, Tosco A, Abete P, Carnovale V, Basile C, Magliocca A, et al. Randomized, single blind, controlled trial of inhaled glutathione vs placebo in patients with cystic fibrosis. J Cyst Fibros. 2015;14:203–210. doi: 10.1016/j.jcf.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Yuan S, Hollinger M, Lachowicz-Scroggins ME, Kerr SC, Dunican EM, Daniel BM, et al. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci Transl Med. 2015;7:276ra27. doi: 10.1126/scitranslmed.3010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pillai K, Akhter J, Chua TC, Morris DL. Mucolysis by ascorbic acid and hydrogen peroxide on compact mucin secreted in pseudomyxoma peritonei. J Surg Res. 2012;174:e69–e73. doi: 10.1016/j.jss.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 49.Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galli F, Battistoni A, Gambari R, Pompella A, Bragonzi A, Pilolli F, et al. Working Group on Inflammation in Cystic Fibrosis. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim Biophys Acta. 2012;1822:690–713. doi: 10.1016/j.bbadis.2011.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.