Abstract
Electronic nicotine delivery system (ENDS) use is outpacing our understanding of its potential harmful effects. Homeostasis of the lung is maintained through proper balance of cell death, efferocytic clearance, and phagocytosis of pathogens. To investigate whether ENDS use has the potential to alter this balance, we developed physiologically relevant ENDS exposure paradigms for lung epithelial cells and primary macrophages. In our studies, cells were exposed directly to aerosol made from carefully controlled components with and without nicotine. We found that ENDS aerosol exposure led to apoptosis, secondary necrosis, and necrosis in lung epithelial cell models. In contrast, macrophages died mostly by apoptosis and inflammatory caspase–mediated cell death when exposed to ENDS aerosol. The clearance of dead cells and pathogens by efferocytosis and phagocytosis, respectively, is an important process in maintaining a healthy lung. To investigate the impact of ENDS aerosol on macrophage function independent of general toxicity, we used an exposure time that did not induce cell death in primary macrophages. Exposure to ENDS aerosol containing nicotine inhibited nearly all phagocytic and greatly reduced the efferocytic abilities of primary macrophages. When challenged with a bacterial pathogen, there was decreased bacterial clearance. The presence of nicotine in the ENDS aerosol increased its toxicity and functional impact; however, nicotine exposure alone did not have any deleterious effects. These data demonstrate that ENDS aerosol exposure could lead to increased epithelial cell and macrophage death in the lung and impair important macrophage functions that are essential for maintenance of lung function.
Keywords: ENDS aerosol, e-cigarette, programmed cell death, lung epithelial cells, macrophages
Clinical Relevance
The rate of use of electronic nicotine delivery systems (ENDS) is far outpacing our understanding of the impact that they may have on health. Overall, this study definitively shows that ENDS aerosols have toxic effects on cells that are important in lung function. These findings may indicate the potential danger of ENDS use and should be considered by relevant regulatory bodies.
Electronic cigarette (e-cig) sales in the United States began in 2007, and since then, they have grown into a complex class of products collectively known as electronic nicotine delivery systems (ENDS) (1). According to the Centers for Disease Control and Prevention’s latest report, 15.6% of adult Americans have tried ENDS (2). In addition, the number of youths that are using ENDS has been steadily increasing over the last decade. Currently, 42.2% of high school students have used ENDS products (3). These statistics are troubling considering the dearth of information regarding long-term health effects of ENDS aerosol exposure. In the past year, it has become apparent that there is the potential for short-term health impacts of ENDS use. There has been an outbreak of severe lung illnesses caused by vaping products. These illnesses have been classified as e-cigarette, or vaping, product use-associated lung injury (EVALI). EVALI cases have been predominantly associated with the use of tetrahydrocannabinol-containing products; however, there are EVALI cases with ENDS product use alone (4). As of February 18, 2020, there have been a total of 2,807 EVALI cases and 68 confirmed deaths (5). It is necessary to understand the mechanisms behind the potential toxicity of these products to have science-based policy regarding the regulation of ENDS products on the market.
All ENDS function on the same basic principle. A battery supplies an electric current to a heating element that contacts the e-liquid, generating heat sufficient to aerosolize the e-liquid. The important characteristics of this system for understanding toxicity are the composition of the e-liquid, the heating element, and the resultant aerosol. E-liquid is composed primarily of propylene glycol (PG) and glycerol, also known as vegetable glycerin (VG) (6). Contrary to popular belief, there is generally no water in e-liquids (6). E-liquid also contains a varying amount of nicotine and various flavors (7). Nicotine concentrations vary considerably across product ranges and from the labeled levels. A recent study found that many “nicotine-free” e-liquids contained nicotine (8). Heating elements are typically metal coils made of nickel–chromium alloys, but many different alloys are used (6, 9, 10). The heat-based aerosolization process causes degradation of the base e-liquid components into many toxic compounds (6, 9, 10). Chemical analyses of ENDS aerosol have shown the presence of PG, VG, nicotine, ethanol, acrolein, formaldehyde, and metal particulates (6). Although some of these compounds have known impacts on health individually (11), the effects of this combination of aerosolized compounds is unknown. In vitro studies on the effects of ENDS aerosol exposure have shown increased oxidative stress, DNA damage, and cell death. Such findings point generally to ENDS aerosol exposure being cytotoxic (12). Additional research has demonstrated an increase in proinflammatory cytokines in the respiratory tract of mice exposed to ENDS aerosol (13, 14).
The ENDS research field must eliminate the lack of consistency in experimental paradigms to understand the biological effects of ENDS use (15). The diversity of construction, output, and function of ENDS combined with the lack of regulation of e-liquid manufacturing processes makes standardization difficult. In addition, a significant portion of the literature regarding the toxicity of ENDS use is focused on the toxicity of flavoring components (16). We have taken a reductionist approach and developed a laboratory-produced e-liquid that eliminates the risk for variability in a commercial product. Importantly, we can separate out the impact of the carrier components, PG and VG, and the effects of nicotine. Our laboratory-produced e-liquid reflects the most common base composition of e-liquid on the market, a 50/50 mix of PG and VG (15). Nicotine is added at a concentration of 18 mg/ml, which is on the low end of the commercial e-liquids. We also use a direct aerosol exposure method, as it most closely recapitulates the exposure of an ENDS user. We have determined exposure based on usage reported by Hiler and colleagues, (17). Using this method, cells are exposed to particulate matter, reactive oxygen species, volatiles, and any other transiently present compounds generated in the device’s atomizer. This carefully controlled system allowed us to investigate the impacts of ENDS aerosol exposure on lung epithelial cells and macrophages.
An important aspect of maintaining the health of a tissue is programmed cell death (PCD). Although several studies have shown that ENDS aerosol can cause cell death, the mechanisms of cell death have not been thoroughly explored (11, 18, 19). Apoptotic cell death is considered noninflammatory and can be identified through the detection of activated executioner caspases, such as caspase-3 or 7 (20). In contrast, necrotic cell death is characterized by the loss of cell membrane integrity in a dysregulated fashion that results in the release of intracellular components. The release of intracellular components is inflammatory, as they are recognized by immune cells as damage-associated molecular patterns (DAMPs) (21). Inflammatory cell death does not always occur in a dysregulated fashion. Pyroptosis is a cell-intrinsic regulated form of inflammatory cell death (22). It has been described most often in macrophages and dendritic cells and is characterized by cell lysis and the release of the cytokines IL-1β and IL-18 (23). These are hallmarks of inflammasome activation. It is usually triggered by the detection of pathogen-associated molecular patterns, often during infection by intracellular pathogens (24). Often, it occurs in a caspase-1–dependent manner, but it can be executed by other inflammatory caspases such as 4 or 5 in humans and 11 in mice (25). It typically occurs independently of caspase-3 or 7 activation. The overall toxicity of ENDS aerosol exposure in the lung will be significantly influenced by the inflammation generated by cell death, which will be dependent on the mechanisms of cell death that occur.
At steady state, the primary immune cells found in the alveolar space are macrophages (26). They have important roles in mediating homeostasis of this tissue. Macrophages are important in phagocytosing and clearing pathogens (27). If pathogens are not cleared quickly from the lung, they can cause pneumonia (28). Macrophages also clear dead cells in a process called efferocytosis (29). Delayed clearance of apoptotic cells can cause the transition to inflammatory cell death in the form of secondary necrosis, which is the eventual dysregulation of the apoptotic process. Accumulation of these cells also causes inflammation and tissue damage (30). Determining how ENDS aerosol affects macrophage clearance of dead cells and pathogens is essential in understanding the potential for toxic effects on the lung.
Here we present data that epithelial cells undergo both apoptotic and necrotic cell death after exposure to ENDS aerosol. The addition of nicotine to the aerosol alters toxicity and increases the amount of both types of cell death. Primary human bronchial epithelial cells (HBECs) cultured on transwell inserts and differentiated into pseudostratified respiratory epithelial layers at the air–liquid interface (ALI) showed a disruption of their barrier function after ENDS aerosol exposure.
In primary macrophages, ENDS aerosol exposure induced apoptotic, secondary necrotic, and inflammatory caspase–mediated cell death. Macrophage cell death is not dependent on the presence of nicotine. Additionally, nicotine-alone treatment of cells did not replicate the phenotypes of ENDS aerosol exposure. Using a dose of ENDS aerosol that did not have cytotoxic effects, we examined the ability of macrophages to phagocytose bacteria, kill bacteria, and efferocytose dead cells. These abilities were decreased only when nicotine was present in the ENDS aerosol exposure. However, nicotine alone did not have any measurable impact on macrophage function.
Taken together, these data indicate that ENDS aerosol exposure in the lung could generate an inflammatory environment as a result of the accumulation of dead cells. The decreased phagocytic capacity of macrophages could result in suppression of bacterial clearance, leading to increased susceptibility to infection. Such a combination has the potential to generate acute inflammation, impair immune response to infection, and produce a chronic inflammatory environment that could ultimately decrease lung function.
Methods
Epithelial Cells
MLE12 (American Type Culture Collection [ATCC] CRL-2110) and BEAS-2B (ATCC CRL-9609) cells were grown in media with hydrocortisone, insulin, transferrin, estrogen, and selenium (HITES). Analyses were conducted 24 hours after exposure (AE). Cells were stained with CellEvent Caspase-3/7 Green and SYTOX Blue (Invitrogen) to evaluate cell death. All flow cytometry staining was conducted as per manufacturer protocols.
Bone Marrow–derived Macrophages
Bone marrow–derived macrophages (BMDMs) were generated from wild-type C57BL/6J mice. Bone marrow was cultured in RPMI 1640 media supplemented with 12% L929 supernatant, 1% penicillin/streptomycin, and 10% FBS. Differentiation occurred in 10 days. Analysis of cell death was conducted 4 hours AE. Cells were stained with CellEvent Caspase-3/7 Green, SYTOX Blue, and FLICA 660-YVAD-FMK Caspase-1 Assay Kit (ImmunoChemistry Technologies, LLC).
Primary Human Bronchial Epithelial Cells
Primary human bronchial epithelial cells (PCS-300–010; ATCC) were cultured on 24-well transwell inserts and grown in PneumaCult Medium system (Stemcell Technologies Inc.).
Transepithelial Electrical Resistance
Transepithelial electrical resistance (TEER) was measured in the ALI HBEC cultures using the EVOM2 Epithelial Volt/Ohm meter and STX3 electrode set (WPI). TEER readings were taken at the beginning and end of the 7 days ENDS aerosol exposure (4 min/d) and the change in resistance was calculated. Dissociated and dead cells were counted using trypan blue exclusion.
ENDS Aerosol Generation and Exposure
ENDS aerosol was generated using a Kangertech KBOW 160 with a Kangertech Protank 4. The atomizer had a rebuildable deck using Kangertech premade nickel–chromium alloy RBA coils and Kangertech cotton wicks. Separate tanks were used for e-liquid with and without nicotine. E-liquid was made using PG, VG, and nicotine. All liquids were made with a 50/50 PG/VG base. To that base was added 18 mg/ml nicotine (N3876; Sigma) or an equivalent volume of distilled water (PG/VG N−).
Using a peristaltic pump, high-efficiency particulate absolute (HEPA)-filtered air was pulled into an airtight chamber at a rate of 1 L/min. Another airtight box was used to house the vaporizer, allowing air to be HEPA filtered before entering the device. ENDS aerosol treatments were conducted with a 6-second puff once every 30 seconds.
Nicotine Treatment
Nicotine control samples were treated with media supplemented with 100 μM nicotine. This concentration of nicotine was determined through the measurement of aerosol deposition in exposure plates and the known concentration of the e-liquid. Nicotine treatment duration was equal to the time after ENDS aerosol exposure.
Phagocytosis Assay
Phagocytosis was evaluated using pHrodo Green Staphylococcus aureus Bioparticles. BMDMs were plated in Opti-MEM media. Treatments were 1.5 minutes and were assayed 24 hours AE. The assay was combined with a SYTOX Blue stain.
Efferocytosis Assay
Efferocytosis was evaluated in BMDMs using primary mouse thymocytes labeled with CellTracker Orange CMTMR. Apoptosis was induced in the thymocytes using 30 minutes of ultraviolet light exposure. Labeled apoptotic thymocytes were incubated with BMDMs 24 hours AE for 30 minutes. BMDMs were stained for F4/80. Flow cytometry was used to identify efferocytic macrophages (F4/80+ and CellTracker+).
Bacterial Clearance Assay
BMDMs were infected with Streptococcus pneumoniae at a multiplicity of infection (MOI) of 0.1 24 hours AE. Bacterial colony–forming units were measured at 3 hours after infection.
Full Methods can be found in the data supplement.
All animal studies were approved by the Brown University Institutional Animal Care and Use Committee and carried out in accordance with the Guide for the Care and Use of Animals of the National Institutes of Health. The University is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Brown University's PHS Assurance Number: D16-00183 (A3284-01), expiration date July 31, 2022. The U.S. Department of Agriculture Registration Number is 15-R-0003. Brown University Institutional Animal Care and Use Committee was approved on September 24, 2019, and the animal protocol number is 19-06-0002. All data generated or analyzed during this study are included in the published article.
Results
ENDS Aerosol Exposure Increases PCD in Lung Epithelial Cells and Disrupts Respiratory Epithelial Microtissues
To determine the impact of ENDS aerosol exposure on cell viability, we exposed human and mouse lung epithelial cell lines to ENDS aerosol for a total of 4 minutes (eight puffs). We exposed cells to ENDS aerosols derived from e-liquid containing only PG and VG (PG/VG N−), as these are the main ingredients in most commercial e-liquids (6). We also exposed cells to ENDS aerosols containing PG, VG, and nicotine (18 mg/ml) (PG/VG N+). As a control for air exposure of the plate, we treated a separate plate in the same manner but pumped in HEPA-filtered air. The untreated plate was exposed to room temperature for the experiment duration to account for changes in temperature during time outside of the incubator. Treated cells were analyzed 24 hours AE. To determine the potential cytotoxic effects of the ENDS aerosol, we used a flow cytometry–based method. Membrane permeability was measured using the SYTOX nucleic acid stain. Activation of caspase-3 and caspase-7 was quantified using CellEvent caspase-3/7 detection reagent, which becomes fluorescent when cleaved by active caspase-3 or 7. This combination allows for the detection of apoptotic cells (Caspase-3/7+ SYTOX−), necrotic cells (Caspase-3/7− SYTOX+), and secondary necrotic cells (Caspase-3/7+ SYTOX+).
Exposure of BEAS2B cells (BEAS2B; ATCC), a human bronchial epithelial cell line, to ENDS aerosol resulted in cell death 24 hours AE (Figure 1). The air control did not have statistically significant changes in cell death compared with the untreated cells (Figure 1), demonstrating that the cell death seen after ENDS aerosol exposure was due to the aerosol itself. ENDS aerosol with nicotine had higher levels of cell death compared with ENDS aerosol that did not contain nicotine (Figures 1A and 1B–1E). PG/VG N− exposure caused an increase in caspase-3/7+ cells, either apoptotic or secondary necrotic. Cells exposed to PG/VG N+ had higher levels of necrotic (Figure 1B), apoptotic (Figure 1D), and secondary necrotic cell death (Figure 1C) compared with all other treatments. Because mice are a common toxicology model system, we investigated the impact of ENDS aerosol exposure on a mouse lung epithelial cell line, MLE12 (MLE12 ATCC). The exposure paradigm and analysis were identical to the methods used for the BEAS2B cells. MLE12 cells were very sensitive to ENDS aerosol, with nearly 80% of the cells dead 24 hours AE (Figure 2). In contrast to BEAS2B cells, PG/VG N− aerosol also caused an increase in secondary necrotic cell death in MLE12 cells (Figure 2C). Additionally, there was a significant increase in necrotic cell death in MLE12 cells following PG/VG N+ aerosol exposure. Nicotine treatment alone did not cause cell death in this system. These data indicate that direct exposure of lung epithelial cells to ENDS aerosol causes an increase in cell death.
Figure 1.

Electronic nicotine delivery system (ENDS) aerosol exposure causes increased cell death in human lung epithelial cells. The BEAS-2B cell line was exposed to ENDS aerosol with (PG/VG N+) and without nicotine (PG/VG N−), 100 μM nicotine (Nicotine), high-efficiency particulate absolute (HEPA)-filtered air (Air), and room temperature (Untreated) for 4 minutes. Cell death was analyzed 24 hours after treatment by flow cytometry using Caspase-3/7 stain and SYTOX permeability dye. (A–E) Representative plots are shown in A and quantification in B–E. (B) Necrotic cells are defined as SYTOX+,Caspase-3/7−. (C) Secondary necrotic cells are dual positive. (D) Apoptotic cells are Caspase-3/7+,SYTOX−. (E) All cells with activated Caspase-3/7. Experiments were performed three times with an n ≥ 3 per group. *P < 0.05 compared with Untreated, #P < 0.05 compared with air, &P < 0.05 compared with PG/VG N−, and %P < 0.05 compared with nicotine. Data were analyzed using ANOVA followed by Tukey’s multiple comparisons test. Mean values are shown with SEM. BEAS-2B = human bronchial epithelial cells; PG = propylene glycol; VG = vegetable glycerin.
Figure 2.

ENDS aerosol exposure causes increased cell death in mouse lung epithelial cells. The MLE12 cell line was exposed to ENDS aerosol with (PG/VG N+) and without nicotine (PG/VG N−), 100 μM nicotine (Nicotine), HEPA-filtered air (Air), and room temperature (Untreated) for 4 minutes. Cell death was analyzed 24 hours after treatment by flow cytometry using Caspase-3/7 stain and SYTOX permeability dye. (A–E) Representative plots are shown in A and quantification in B–E. (B) Necrotic cells are defined as SYTOX+,Caspase-3/7−. (C) Secondary necrotic cells are dual positive. (D) Apoptotic cells are Caspase-3/7+,SYTOX−. (E) All cells with activated Caspase-3/7. Experiments were performed three times with an n ≥ 3 per group. *P < 0.05 compared with Untreated, #P < 0.05 compared with air, &P < 0.05 compared with PG/VG N−, and %P < 0.05 compared with nicotine. Data were analyzed using ANOVA followed by Tukey’s multiple comparisons test. Mean values are shown with SEM.
To better understand the impacts of ENDS aerosol exposure on human lung tissue, we used an ALI culture method for HBECs. This provides a more physiologically relevant system for ENDS aerosol exposure studies. As the primary role of the respiratory epithelium is barrier function, TEER was used to measure the health and function of the differentiated respiratory epithelial layers. Following 7 consecutive days of 4 min/d ENDS aerosol exposure, we found significant decreases in the resistance of HBEC cultures after exposure to ENDS aerosol with or without nicotine (Figure 3A). There was a small but significant decrease in resistance in the air-treated group compared with untreated. Treatment with nicotine alone did not have an impact on resistance. To confirm that our TEER findings were reflective of a dysregulation or disruption of the HBEC epithelial layers, we counted dissociated cells via trypan blue exclusion. Consistent with our TEER findings, we found increased numbers of dissociated cells, both alive and dead, only in the ENDS aerosol–treated groups (Figures 3B and 3C). This treatment paradigm resulted in an almost complete loss of barrier integrity in these differentiated respiratory epithelial microtissues.
Figure 3.
One week of ENDS aerosol exposure causes decreased transepithelial resistance, dissociation of cells, and cell death. Primary human bronchial epithelial cells were differentiated on transwell inserts into a pseudostratified epithelial layer. These cells were exposed to ENDS aerosol with (PG/VG N+) and without nicotine (PG/VG N−), HEPA-filtered air (Air), 100 μM nicotine (Nicotine), and room temperature (Untreated) for 4 minutes. Transepithelial resistance was measured using a WPI EVOM TEER meter and STX3 electrode. Measurements were taken in triplicate and averaged. Background readings of empty wells as well as baseline measurements of each sample were used to calculate the change in resistance over the course of the treatment, quantification shown in A. Dissociated and dead cells were detected using trypan blue exclusion and counting on a hemocytometer, quantification of live and dead cells shown in B and C, respectively. Treatments occurred once per day for 7 days. Experiments were performed two times with an n ≥ 3 per group. *P < 0.05 compared with untreated, #P < 0.05 compared with air, and %P < 0.05 compared with nicotine. Data were analyzed using ANOVA followed by Tukey’s multiple comparisons test. Mean values are shown with SEM.
Primary Macrophages Undergo PCD upon Exposure to ENDS Aerosol
The primary immune cell found in the lung at steady state is the macrophage. Macrophages in the lung have many functions including clearing pathogens, dead cells, and other debris (27). They also play important roles in mediating tissue repair processes (31). Alveolar macrophages are a self-renewing population; however, they can be replenished by infiltrating bone marrow–derived monocytes and macrophages (32, 33). To determine the impact of ENDS aerosol exposure on macrophages, we used primary mouse BMDMs. BMDMs are a useful cell type to model tissue-resident macrophages such as alveolar macrophages (34). They can be generated in large numbers and recapitulate the functions of many tissue-resident macrophages (35). In our system, BMDMs exposed to 4 minutes of ENDS aerosol had significantly increased levels of PCD (Figure 4). To examine the precise pathways, we used the FLICA reagent 660-YVAD-FMK to stain for active caspase-1, SYTOX, and CellEvent caspase-3/7 (Figures 4A and 4B). Apoptotic and secondary necrotic cells accounted for roughly one quarter of the cell death occurring in BMDMs exposed to ENDS aerosol (Figures 4A, 4C, and 4D). The data initially suggested a strong necrotic phenotype based on high levels of SYTOX+, caspase-3/7− cells; however, upon further investigation, we found a significant portion of active caspase-1+, SYTOX+ cells. This was assayed using FLICA 660-YVAD-FMK, which has specific binding affinity for active caspase-1; however, it can also bind other active inflammatory caspases such as caspase-4 or caspase-5 in humans and caspase-11 in mice. The large proportion of cells staining with SYTOX and active inflammatory caspases indicates that the pyroptotic machinery is activated. This could be in a canonical caspase-1 dependent manner or via a noncanonical pathway using caspase-11. In either scenario, the result is a cell death with membrane permeability and leakage of intracellular contents, thus causing inflammation. Pyroptosis usually occurs when macrophages are infected with intracellular pathogens (24), but here we demonstrate a putative mechanism by which ENDS exposure results in activation of this pathway. By combining SYTOX with FLICA 660-YVAD-FMK caspase-1 and CellEvent caspase-3/7, it shows that inflammatory caspase–mediated cell death was the most prevalent form of PCD (Figures 4B and 4F). In the ENDS aerosol–exposed BMDMs, there was no impact of nicotine presence on cell death, as the PG/VG N− groups and PG/VG N+ groups had similar levels of cell death. Nicotine-alone controls also showed no differences in cell death as compared with untreated or air controls. Additionally, there was not any change in the presence of SYTOX single positive cells (necrotic) in any treatment (Figure 4E).
Figure 4.

ENDS aerosol exposure induces apoptotic and inflammatory caspase–mediated cell death in primary macrophages. Mouse bone marrow–derived macrophages (BMDMs) were exposed to ENDS aerosol with (PG/VG N+) and without nicotine (PG/VG N−), 100 μM nicotine (Nicotine), HEPA-filtered air (Air), and room temperature (Untreated) for 4 minutes. Cell death was analyzed 4 hours after treatment by flow cytometry using Caspase-3/7 stain, SYTOX permeability dye, and Caspase-1 stain. Representative Caspase-3/7 versus side scatter (SSC) plots showing apoptotic cells shown in A. Representative plots of inflammatory cell death, gated on Caspase-3/7− cells, shown in B. Quantifications of cell populations shown in C–F. (C) Apoptotic cells are Caspase-3/7+,SYTOX+ and (D) Caspase-3/7+,SYTOX−. (E) Necrotic cells are Caspase-3/7−, Caspase-1−, and SYTOX+. (F) Cells dying from inflammatory caspase–mediated cell death are Caspase-3/7−, Caspase-1+, and SYTOX+. Caspase-1+,Caspase-3/7−,SYTOX− cells are seen in G. Experiments were performed three times with an n ≥ 3 per group. *P < 0.05 compared with untreated, #P < 0.05 compared with air, and %P < 0.05 compared with PG/VG N−. Data were analyzed using ANOVA followed by Tukey’s multiple comparisons test. Mean values are shown with SEM.
Macrophage Function Is Compromised after Exposure to Noncytotoxic Levels of ENDS Aerosol
One of the most important functions of macrophages is the phagocytosis and clearance of pathogens. BMDMs were incubated with fluorescent bioparticles following 1.5 minutes of ENDS aerosol exposure to evaluate their phagocytic capacity. The bioparticles were labeled with a pH-sensitive pHrodo dye that fluoresces once inside the phagolysosome. This allows for highly specific detection of successful phagocytosis. We found significant decreases in the number of phagocytic macrophages following ENDS aerosol exposure. Nicotine-alone treatment showed a slight decrease in phagocytic capacity that was not statistically significant. ENDS aerosol without nicotine caused a modest reduction in phagocytic capacity, whereas ENDS aerosol with nicotine caused an almost complete inhibition of phagocytosis (Figures 5A and 5B). To determine if this effect would also impact pathogen clearance, we infected BMDMs with S. pneumoniae at a multiplicity of infection of 0.1. Three hours after infection, supernatants and lysed cells were collected separately. There were no detectable intracellular bacteria in any group, indicating that the phagocytosed bacteria were rapidly cleared. However, there was a statistically significant increase in extracellular bacteria in ENDS-exposed macrophages (Figure 5C). The impact of these findings on the understanding of ENDS aerosol’s impact on macrophage function is bolstered by the lack of cytotoxicity of the 1.5-minute exposure. The phagocytosis assay was combined with a SYTOX stain, and it showed no significant differences across all treatment groups (Figure 5D). In a separate experiment, we examined both caspase-3/7 activation and SYTOX staining and found that even with 2 minutes of exposure, there is no increase in cell death (Figure E2).
Figure 5.
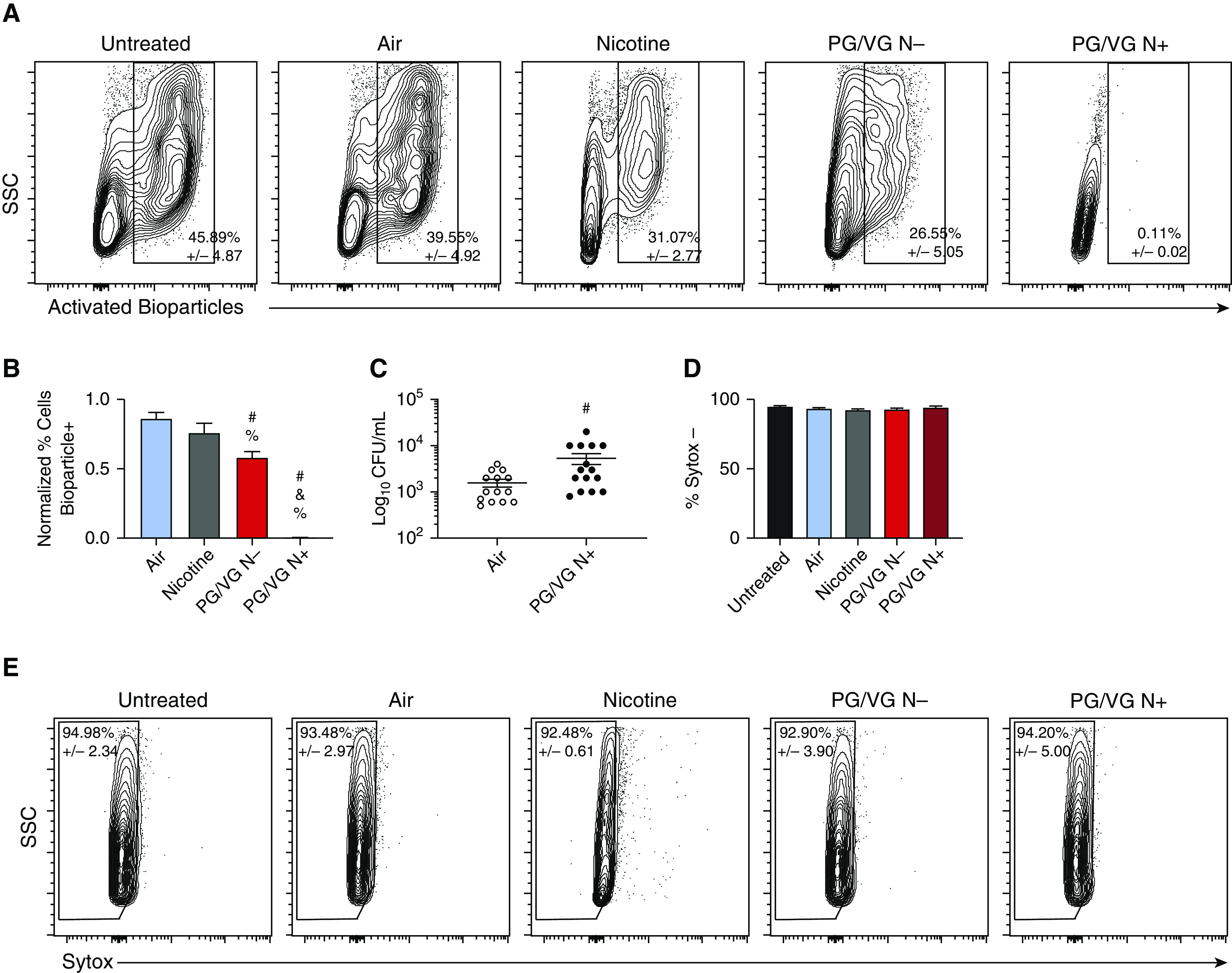
Low-level ENDS aerosol exposure causes decreased phagocytosis and bacterial clearance in primary macrophages. Mouse BMDMs were exposed to ENDS aerosol with (PG/VG N+) and without nicotine (PG/VG N−), 100 μM nicotine (Nicotine), HEPA-filtered air (Air), and room temperature (Untreated) for 1.5 minutes. Phagocytosis was evaluated 24 hours after treatment using pHrodo Green Staphylococcus aureus Bioparticles. Representative plots are shown in A. Quantification of bioparticle+ cells normalized to untreated is shown in B. Bacterial clearance was measured in macrophage cultures treated for 1.5 minutes with air or ENDS aerosol with nicotine by infecting with Streptococcus pneumoniae at a MOI of 0.1 24 hours after treatment. Bacteria from cultures was collected after 3 hours. CFU quantification is shown in C. Cell death was analyzed 24 hours after treatment by flow cytometry using SYTOX permeability dye. SYTOX+ cells are considered dead or dying. Quantification of cells with permeable membranes is shown in D, with representative plots shown in E. Experiments were performed three times with n ≥ 3 per group. #P < 0.05 compared with air, &P < 0.05 compared with PG/VG N−, and %P < 0.05 compared with nicotine. Data were analyzed using ANOVA followed by Tukey’s multiple comparisons test. Mean values are shown with SEM. CFU = colony-forming unit; MOI = multiplicity of infection.
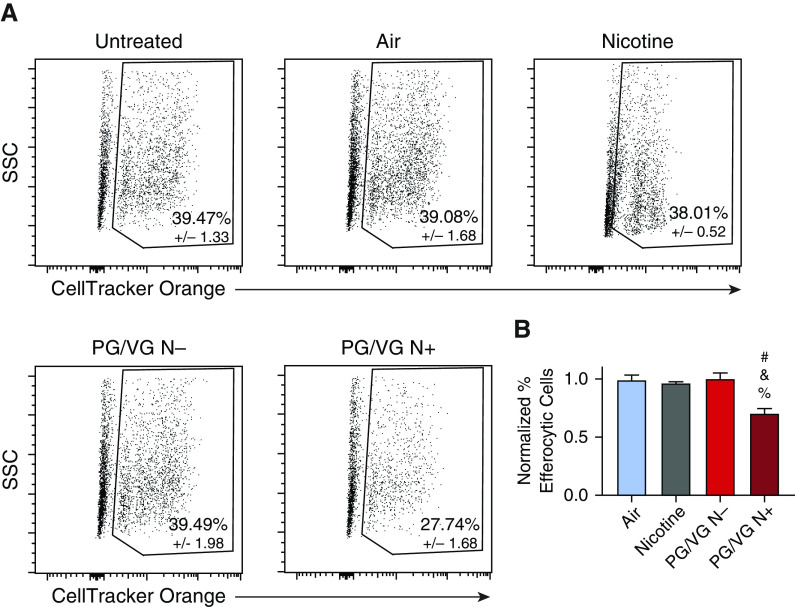
Given that ENDS aerosol exposure causes PCD in many cell types, there is potential for an accumulation of apoptotic cells or cell bodies in the lung following ENDS use. Therefore, it is important to evaluate macrophage efferocytic capacity following ENDS aerosol exposure. Efferocytic capacity was evaluated using CellTracker Orange labeled apoptotic primary mouse thymocytes. Following incubation with the thymocytes, macrophages that stained positively for F4/80 and CellTracker Orange were determined to be efferocytic. ENDS aerosol with nicotine exposure caused a decrease in the percentage of efferocytic macrophages (Figures 6A and 6B). Unlike in the phagocytosis assay, there was no detectable reduction in efferocytosis in the PG/VG N− treatment (Figures 6A and 6B). The presence of nicotine in the e-liquid is required for ENDS aerosol exposure to reduce efferocytic capacity of BMDMs; however, it is not sufficient on its own. Nicotine-alone treatment showed no decrease in efferocytic cells in this assay (Figures 6A and 6B). This demonstrates that nicotine only causes a decrease in efferocytosis when present with ENDS aerosol components.
Figure 6.
Low-level ENDS aerosol with nicotine exposure causes decreased efferocytosis in primary macrophages. Mouse BMDMs were exposed to ENDS aerosol with (PG/VG N+) and without nicotine (PG/VG N−), 100 μM nicotine (Nicotine), HEPA-filtered air (Air), and room temperature (Untreated) for 1.5 minutes. Efferocytosis was evaluated 24 hours after treatment using CellTracker Orange CMTMR Dye–labeled apoptotic primary mouse thymocytes uptake as the marker. Flow cytometry was used to identify macrophages with CellTracker Orange and F4/80 surface staining as efferocytic cells. Representative plots are shown in A. Quantification of efferocytic cells normalized to untreated is shown in B. Experiments were performed three times with n = 8 per group. #P < 0.05 compared with air, &P < 0.05 compared with PG/VG N−, and %P < 0.05 compared with nicotine. Data were analyzed using ANOVA followed by Tukey’s multiple comparisons test. Mean values are shown with SEM.
Discussion
This work demonstrates that ENDS aerosol exposure causes significant increases in PCD in lung epithelial cells and primary macrophages. Our study shows that ENDS aerosol induces apoptosis and secondary necrosis in human and mouse epithelial cells and inflammatory caspase–mediated cell death and apoptosis in primary macrophages. Noncytotoxic ENDS aerosol exposure causes decreased phagocytic and efferocytic capacity. A week of daily ENDS aerosol exposure was found to decrease barrier function of primary human bronchial epithelial cells, resulting in the near complete dissociation of ALI respiratory epithelial microtissues. These data have broad implications for the impacts of ENDS aerosol on the lung epithelium and the ability of macrophages to clear dead cells and pathogens. Additionally, the lack of recapitulation of the impacts of PG/VG N+ aerosol in the nicotine-alone–treated cells indicates an alternative role of nicotine in the aerosolization and decomposition processes that occur in the ENDS. Both ENDS aerosol and nicotine are required to produce the full suite of phenotypes described in our work. The combination of an increase in dead cells and a deficiency in their clearance would be highly inflammatory in the lung. This would likely result in acute damage and chronic inflammation in the lung. Decreased bacterial clearance can lead to increased susceptibility to severe lung infections. Therefore, ENDS aerosol exposure could cause both chronic and acute lung diseases.
Rigorous investigation of the biological impacts of ENDS aerosol exposure requires controllable reductionist approaches. Current literature on ENDS aerosol is inconsistent owing to variation in composition of e-liquids, duration of exposure, and the method of exposure. To investigate the impact of ENDS aerosol exposure on lung epithelial cells and macrophages, we developed an in-house e-liquid formulation and tractable method of exposure that eliminates any lag between aerosol generation and exposure to cells. This method also removes the possibility of variation or lack of quality control in the production of commercial e-liquid. Our method uses the base components of e-liquid, PG and VG, to determine the fundamental toxicity of vaping across all ENDS types. Also, we based our exposure paradigm on a study that found experienced ENDS users use relatively long average puff durations (17). Most studies are based on the average puff duration of inexperienced ENDS users, which is around 3 seconds. The most relevant metric of vaping behavior is that of experienced users, as that best replicates a chronic use scenario. Importantly, the puff duration in ENDS aerosol generation has the potential to drastically alter the composition of the aerosol (36). This is caused by increased temperatures in the atomizer during longer puffs. Considerable effort was put into making sure the vaporizer was operating within reasonable limits and avoiding combustion or “dry hits.” Our work points to a fundamental toxicity of ENDS based on the aerosol generated using only the shared components of all ENDS.
Several studies have shown that exposure to ENDS products causes cell death. However, there is not consensus on the specific types of cell death that occur (11). Additionally, multiple studies have shown limited amounts or no cytotoxicity from e-liquids lacking nicotine or flavorings (37, 10, 12, 37). In our study, using a reproducible and carefully controlled exposure paradigm, we investigate the type of cell death triggered. It is important to distinguish the pathways through which the cell death process occurs, as the results of different types of cell death are functionally distinct. There are distinct impacts of cell death depending on the precise type of cell death (38). Necrotic cell death causes damage and inflammation, as when the cell bursts it releases DAMPs that are recognized by innate immune cells, which mount an inflammatory response. These responses include upregulated expression of IL-1, IL-6, Il-8, TNFα, MIP-1 (macrophage inflammatory protein-1) (CCL3/4), and MCP1 (monocyte chemoattractant protein-1) (CCL2) (39). Apoptosis is a noninflammatory type of cell death. This regulated form of cell death can be antiinflammatory and prevents damage (40). Lung epithelial cells died by apoptosis, necrosis, and secondary necrosis after exposure to ENDS aerosols.
Pyroptosis is a distinct form of cell death that occurs primarily in immune cells (41). It is inflammatory by nature, but it does not occur in a dysregulated fashion. Pyroptosis is triggered by the inflammasome and results in the release of inflammatory cytokines such as IL-1β. It typically occurs in sentinel innate immune cells, including macrophages, as a signaling method of generating a robust inflammatory signal in response to the detection of a pathogen (22). Pyroptosis can occur in response to either DAMPs or pathogen-associated molecular patterns and serves to amplify those signals (42, 43). In the case of intracellular pathogens, pyroptosis can interrupt the pathogen’s replication cycle and release bacterial or viral products that lack immune evasion techniques of the fully formed pathogen. Although pyroptosis is an important part of the immune response, it can also cause inflammation and tissue damage. Nicotine has been shown to directly induce pyroptosis in endothelial cells and could contribute to the cardiovascular diseases seen in cigarette smokers (44). In our system, macrophages exposed to ENDS aerosol appear to be undergoing pyroptosis independent of the presence of nicotine. It is possible that this is induced through inflammasome activation as the result of “frustrated phagocytosis,” in which the cell attempts to phagocytose a large particle (45). ENDS aerosol has been shown to contain a wide variety of particulates, including metal particles and silica (46). Another method by which pyroptosis could be confirmed would be the production, enzymatic maturation, and release of IL-1β. We did not find IL-1β at significant levels in our experimental system (Figure E1 in the online supplement). A confounding factor here may be that the peak of BMDM cell death in response to ENDS aerosol is 4 hours AE. This timeframe may not allow for the production and processing of IL-1β. Additionally, the signaling initiated by the ENDS aerosol exposure may not induce IL-1β production regardless of the activation state of caspase-1, resulting in low levels of IL-1β. Although the specific mechanisms of pyroptosis activation remain elusive, it is clear that ENDS aerosol exposure causes significant cell death in a fashion that does not maintain membrane integrity. This finding suggests that ENDS aerosol exposure could be highly inflammatory and a potential detriment to the lung health of ENDS users.
Expanding the investigation of the impacts of ENDS aerosol on lung epithelial cells into the HBECs cultured at the ALI allows us to better recapitulate the human respiratory epithelium in vitro. The use of TEER as the readout of epithelial layer integrity and barrier function is the best way to understand the function of the microtissue. Near complete loss of barrier integrity in response to ENDS aerosol treatment represents a worrying potential for the use of ENDS products to have widespread negative impacts on respiratory epithelial layers after short-term use.
Given the ability of ENDS aerosol to cause cell death in lung epithelial cells lines, it is important for apoptotic cells to be cleared in a lung environment. Apoptotic bodies are cleared by resident phagocytes through efferocytosis (47). Our data show that ENDS aerosol–exposed primary macrophages have a decreased ability to clear apoptotic cells. This decrease in clearance was nicotine dependent; however, nicotine alone did not impact efferocytosis. Importantly, these assays were done at a low level of exposure that did not cause the macrophages cell death. This allowed us to probe macrophage function in a physiologically relevant state. These data demonstrate that a crucial homeostatic function of macrophages is significantly disrupted by exposure to ENDS aerosol.
Another necessary macrophage function is the phagocytic clearance of bacterial pathogens. Our data show a significant defect in bacterial clearance by macrophages exposed to ENDS aerosol. The suppression of phagocytosis occurs in treatments without nicotine; however, PG/VG aerosol with nicotine has a considerably stronger effect on phagocytic capacity of the macrophages. One similar study showed a reduction in the phagocytic capacity of the THP-1 cell line in response to nicotine alone, but not PG/VG vehicles (48). Another study investigated phagocytic capacity of primary murine alveolar macrophages after treatment with e-liquids in culture media without aerosolization (49). This is a common method of study for ENDS exposure; however, it fails to take into account the immense number of decomposition compounds that are produced in the heating and aerosolization processes in the vaporizer. They found a slight reduction in the number of phagocytic macrophages in response to the PG/VG vehicles as well as a strong inhibitory effect of cinnamon flavorings on phagocytosis. Findings in these different models are not imminently comparable, but there appears to be growing consensus that ENDS aerosol or e-liquid products can have a negative impact on phagocytic function. Our findings are particularly relevant as we used a direct ENDS aerosol exposure system in combination with primary macrophages. We also control for the impact that cell death would have on macrophage function. To our knowledge, this is the first report of ENDS aerosol exposure resulting in the inhibition of phagocytic capacity in primary macrophages.
Conclusions
This study represents novel findings regarding the biological effects of ENDS aerosol exposure. We report increased inflammatory and noninflammatory cell death in epithelial and innate immune cells, and decreased macrophage phagocytic capacity. Importantly, all findings were observed after exposure to e-liquid lacking flavorings. We found that the base components of e-liquid have toxicity on their own and this toxicity was enhanced by the addition of nicotine. Interestingly, nicotine must be present in the aerosol production phase to enhance impacts of ENDS aerosol. This points to a requirement of nicotine signaling for the toxicants generated by decomposition of PG or VG in the atomizer to have the full breadth of potential effects. Alternatively, the presence of nicotine could alter the decomposition reactions occurring in the atomizer and subsequently change the composition of the aerosol. The recent outbreak of EVALI demonstrates the need for renewed urgency in the regulation and control of ENDS devices. These findings are informative of the potential danger of ENDS use and should be considered as such by relevant regulatory bodies.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank John Murphy for assistance with apparatus construction and Kevin Carlson at the Brown University Flow Cytometry and Sorting Facility for assistance.
Footnotes
Supported by U.S. National Heart, Lung, and Blood Institute grant 1R01HL126887–01A1, Institutional Development Award Network for Biomedical Research Excellence from the U.S. National Institutes of Health/National Institute of General Medical Sciences under grant number P20GM103430, and National Institutes of Health/National Institute of Environmental Health Services Institutional National Research Service Award 5T32ES007272–24. This work was funded in part by the Rhode Island IDeA Network of Biomedical Research Excellence. The funding sources had no role in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
Author Contributions: G.L.S. collected most of the data, analyzed the data, made the final figures, and helped to write the manuscript. N.D.R. generated some of the data. N.L. and M.J.C. performed some of the experiments that were important in setting up the system. M.J.C. assisted in editing the manuscript. A.M.J. initiated the study, supervised the study design, supervised data analysis, obtained funding to do the study, and wrote the manuscript. All authors read and approved the final manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2019-0200OC on May 29, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Perikleous EP, Steiropoulos P, Paraskakis E, Constantinidis TC, Nena E. E-cigarette use among adolescents: an overview of the literature and future perspectives. Front Public Health. 2018;6:86. doi: 10.3389/fpubh.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics. CDC national health interview survey. 2018. [accessed 2019 May 22]. Available from https://www.cdc.gov/visionhealth/vehss/data/national-surveys/national-health-interview-survey.html.
- 3.Centers for Disease Control and Prevention. Youth risk behavior survey. 2017. [accessed 2019 May 22]. Available from https://www.cdc.gov/healthyyouth/data/yrbs/results.htm.
- 4.Moritz ED, Zapata LB, Lekiachvili A, Glidden E, Annor FB, Werner AK, et al. Lung Injury Response Epidemiology/Surveillance Group; Lung Injury Response Epidemiology/Surveillance Task Force. Update: characteristics of patients in a national outbreak of E-cigarette, or vaping, product use-associated lung injuries. United States, october 2019. MMWR Morb Mortal Wkly Rep. 2019;68:985–989. doi: 10.15585/mmwr.mm6843e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Outbreak of lung injury associated with the use of E-cigarette, or vaping, products, electronic cigarettes, smoking & tobacco use, CDC. [accessed 2020 Mar 26]. Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html.
- 6.Brown CJ, Cheng JM. Electronic cigarettes: product characterisation and design considerations. Tob Control. 2014;23:ii4–ii10. doi: 10.1136/tobaccocontrol-2013-051476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tierney PA, Karpinski CD, Brown JE, Luo W, Pankow JF. Flavour chemicals in electronic cigarette fluids. Tob Control. 2016;25:e10–e15. doi: 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond BH, Collette-Merrill K, Harrison RG, Jarvis S, Rasmussen RJ. The nicotine content of a sample of E-cigarette liquid manufactured in the United States. J Addict Med. 2018;12:127–131. doi: 10.1097/ADM.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 9.Goniewicz ML, Knysak J, Gawron M, Kosmider L, Sobczak A, Kurek J, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control. 2014;23:133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misra M, Leverette RD, Cooper BT, Bennett MB, Brown SE. Comparative in vitro toxicity profile of electronic and tobacco cigarettes, smokeless tobacco and nicotine replacement therapy products: e-liquids, extracts and collected aerosols. Int J Environ Res Public Health. 2014;11:11325–11347. doi: 10.3390/ijerph111111325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun LF, Moazed F, Calfee CS, Matthay MA, Gotts JE. Pulmonary toxicity of e-cigarettes. Am J Physiol Lung Cell Mol Physiol. 2017;313:L193–L206. doi: 10.1152/ajplung.00071.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor M, Carr T, Oke O, Jaunky T, Breheny D, Lowe F, et al. E-cigarette aerosols induce lower oxidative stress in vitro when compared to tobacco smoke. Toxicol Mech Methods. 2016;26:465–476. doi: 10.1080/15376516.2016.1222473. [DOI] [PubMed] [Google Scholar]
- 13.Sussan TE, Gajghate S, Thimmulappa RK, Ma J, Kim J-H, Sudini K, et al. Exposure to electronic cigarettes impairs pulmonary anti-bacterial and anti-viral defenses in a mouse model. PLoS One. 2015;10:e0116861. doi: 10.1371/journal.pone.0116861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberger CM, Podyminogin RL, Askovich PS, Navarro G, Kaiser SM, Sanders C, et al. Characterization of innate responses to influenza virus infection in a novel lung type I epithelial cell model. J Gen Virol. 2014;95:350–362. doi: 10.1099/vir.0.058438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margham J, McAdam K, Forster M, Liu C, Wright C, Mariner D, et al. Chemical composition of aerosol from an E-cigarette: a quantitative comparison with cigarette smoke. Chem Res Toxicol. 2016;29:1662–1678. doi: 10.1021/acs.chemrestox.6b00188. [DOI] [PubMed] [Google Scholar]
- 16.Sassano MF, Davis ES, Keating JE, Zorn BT, Kochar TK, Wolfgang MC, et al. Evaluation of e-liquid toxicity using an open-source high-throughput screening assay. PLoS Biol. 2018;16:e2003904. doi: 10.1371/journal.pbio.2003904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiler M, Breland A, Spindle T, Maloney S, Lipato T, Karaoghlanian N, et al. Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: influence of liquid nicotine concentration and user experience. Exp Clin Psychopharmacol. 2017;25:380–392. doi: 10.1037/pha0000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang JH, Lyes M, Sladewski K, Enany S, McEachern E, Mathew DP, et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J Mol Med (Berl) 2016;94:667–679. doi: 10.1007/s00109-016-1378-3. [DOI] [PubMed] [Google Scholar]
- 19.Leigh NJ, Lawton RI, Hershberger PA, Goniewicz ML. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS) Tob Control. 2016;25:ii81–ii87. doi: 10.1136/tobaccocontrol-2016-053205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wlodkowic D, Skommer J, Darzynkiewicz Z. Flow cytometry-based apoptosis detection. Methods Mol Biol. 2009;559:19–32. doi: 10.1007/978-1-60327-017-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18:e27. doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 24.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morales-Nebreda L, Misharin AV, Perlman H, Budinger GRS. The heterogeneity of lung macrophages in the susceptibility to disease. Eur Respir Rev. 2015;24:505–509. doi: 10.1183/16000617.0031-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aberdein JD, Cole J, Bewley MA, Marriott HM, Dockrell DH. Alveolar macrophages in pulmonary host defence the unrecognized role of apoptosis as a mechanism of intracellular bacterial killing. Clin Exp Immunol. 2013;174:193–202. doi: 10.1111/cei.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharif O, Matt U, Saluzzo S, Lakovits K, Haslinger I, Furtner T, et al. The scavenger receptor CD36 downmodulates the early inflammatory response while enhancing bacterial phagocytosis during pneumococcal pneumonia. J Immunol. 2013;90:5640–5648. doi: 10.4049/jimmunol.1202270. [DOI] [PubMed] [Google Scholar]
- 29.McCubbrey AL, Curtis JL. Efferocytosis and lung disease. Chest. 2013;143:1750–1757. doi: 10.1378/chest.12-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachet M, Liang YY, Oehler R. The immune response to secondary necrotic cells. Apoptosis. 2017;22:1189–1204. doi: 10.1007/s10495-017-1413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crane MJ, Lee KM, FitzGerald ES, Jamieson AM. Surviving deadly lung infections: innate host tolerance mechanisms in the pulmonary system. Front Immunol. 2018;9:1421. doi: 10.3389/fimmu.2018.01421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matute-Bello G, Lee JS, Frevert CW, Liles WC, Sutlief S, Ballman K, et al. Optimal timing to repopulation of resident alveolar macrophages with donor cells following total body irradiation and bone marrow transplantation in mice. J Immunol Methods. 2004;292:25–34. doi: 10.1016/j.jim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Misharin AV, Morales-Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie-Pimentel AC, et al. Monocyte-derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med. 2017;214:2387–2404. doi: 10.1084/jem.20162152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Yu X, Cao Q, Wang Y, Zheng G, Tan TK, et al. Characterization of murine macrophages from bone marrow, spleen and peritoneum. BMC Immunol. 2013;14:6. doi: 10.1186/1471-2172-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sala C, Medana C, Pellegrino R, Aigotti R, Bello FD, Bianchi G, et al. Dynamic measurement of newly formed carbonyl compounds in vapors from electronic cigarettes. Eur J Mass Spectrom (Chichester) 2017;23:64–69. doi: 10.1177/1469066717699078. [DOI] [PubMed] [Google Scholar]
- 37.Wu Q, Jiang D, Minor M, Chu HW. Electronic cigarette liquid increases inflammation and virus infection in primary human airway epithelial cells. PLoS One. 2014;9:e108342. doi: 10.1371/journal.pone.0108342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rock KL, Kono H. The inflammatory response to cell death. Annu Rev Pathol. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henson PM, Bratton DL. Antiinflammatory effects of apoptotic cells. J Clin Invest. 2013;123:2773–2774. doi: 10.1172/JCI69344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu T, Zhou YT, Wang LQ, Yue Li L, Bao Q, Tian S, et al. NOD-like receptor family, pyrin domain containing 3 (NLRP3) contributes to inflammation, pyroptosis, and mucin production in human airway epithelium on rhinovirus infection. J Allergy Clin Immunol. 2019;144:777–787. doi: 10.1016/j.jaci.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Ma Y, Jiang J, Gao Y, Shi T, Zhu X, Zhang K, et al. Research progress of the relationship between pyroptosis and disease. Am J Transl Res. 2018;10:2213–2219. [PMC free article] [PubMed] [Google Scholar]
- 43.Liao K-C, Mogridge J. Activation of the Nlrp1b inflammasome by reduction of cytosolic ATP. Infect Immun. 2013;81:570–579. doi: 10.1128/IAI.01003-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Zhang H, Qi W, Zhang Y, Li J, Li Z, et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018;9:171. doi: 10.1038/s41419-017-0257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakayama M. Macrophage recognition of crystals and nanoparticles. Front Immunol. 2018;9:103. doi: 10.3389/fimmu.2018.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mikheev VB, Brinkman MC, Granville CA, Gordon SM, Clark PI. Real-time measurement of electronic cigarette aerosol size distribution and metals content analysis. Nicotine Tob Res. 2016;18:1895–1902. doi: 10.1093/ntr/ntw128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolb JP, Oguin TH, III, Oberst A, Martinez J. Programmed cell death and inflammation: winter is coming. Trends Immunol. 2017;38:705–718. doi: 10.1016/j.it.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ween MP, Whittall JJ, Hamon R, Reynolds PN, Hodge SJ. Phagocytosis and inflammation: exploring the effects of the components of E-cigarette vapor on macrophages. Physiol Rep. 2017;5:e13370. doi: 10.14814/phy2.13370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clapp PW, Pawlak EA, Lackey JT, Keating JE, Reeber SL, Glish GL, et al. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am J Physiol Lung Cell Mol Physiol. 2017;313:L278–L292. doi: 10.1152/ajplung.00452.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.