Abstract
ROCHA, A. and Trujillo K. Title: Neurotoxicity of Low-Level Lead Exposure: History, Mechanisms of Action, and Behavioral Effects in Humans and Preclinical Models. NeuroToxicology.
Lead is a neurotoxin that produces long-term, perhaps irreversible, effects on health and well-being. This article summarizes clinical and preclinical studies that have employed a variety of research techniques to examine the neurotoxic effects of low levels of lead exposure. A historical perspective is presented, followed by an overview of studies that examined behavioral and cognitive outcomes. In addition, a short summary of potential mechanisms of action is provided with a focus on calcium-dependent processes. The current level of concern, or reference level, set by the CDC is 5 μg/dL of lead in blood and a revision to 3.5 μg/dL has been suggested. However, levels of lead below 3 μg/dL have been shown to produce diminished cognitive function and maladaptive behavior in humans and animal models. Because much of the research has focused on higher concentrations of lead, work on low concentrations is needed to better understand the neurobehavioral effects and mechanisms of action of this neurotoxic metal.
Keywords: low level lead exposure, cognitive function, educational achievement, mechanisms of action, neurological disorders, psychiatric disorders
1. Introduction
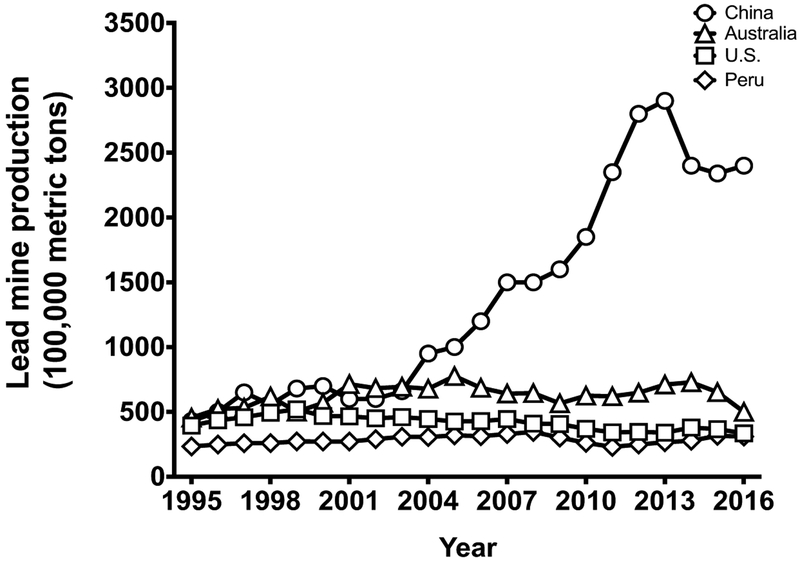
Lead is a naturally occurring heavy metal that has been used since ancient times for its malleability, low melting point, high resistance to corrosion, and versatility (Gilfillan, 1965; Lewis, 1985; Markowitz & Rosner, 2003; Needleman, 1991; Woolley, 1984). Despite its toxicity, lead remains ubiquitous in modern life [Table 1]. Even after the closing of the last lead smelter in 2013, the U.S. ranks as a major global producer of lead over the last 20 years, only third to China and Australia (See U.S. Geological Survey, 1997–2016) [Figure 1]. Modern uses of lead include leaded batteries, which have accounted for over 85% of lead production in the United States from 2005 to the present (See U.S. Geological Survey, 2006–2016). Several occupations remain at high risk for lead exposure, including workers who engage in the demolition of older buildings (ATSDR, 2007). In addition to the continued mining of lead and manufacturing of leaded products, the U.S. imports items with elevated lead levels, some of which are intended for use by children (CPSIA, 2012).
Table 1.
Milestones in the history of lead.
| Year | Event | Reference |
|---|---|---|
| 6500 B.C. | First evidence of lead mine dates back to this period in Turkey | Bochynska, 2013; Needleman, 1991 |
| 3000 B.C. | Chinese used lead to manufacture coins | Schafer, 1956 |
| 2000 B.C. | Spanish used lead to manufacture coins Egyptians used lead as pigment in cosmetics | Walter et al., 1999; Woolley, 1984 |
| 500 B.C.-300 A.D. | Romans used lead in cookware, food, and other applications | Needleman, 1991 |
| 200 B.C. | Nicander, a Greek physician-poet, offered an early description of lead poisoning | Needleman, 2009 |
| <100 A.D. | Dioscorides, the physician to the Roman Emperor Nero, asserted that “lead makes the mind give way,” providing one of the first direct medical diagnoses of lead poisoning | Needleman, 1991; Woolley, 1984 |
| 1621 | Lead first mined and smelted in the U.S. for production of bullets | Swiggett, 1917 |
| 1804 | First white lead factory in the U.S. | Kessler, 2014 |
| 1891 | National Lead Company is incorporated | Kessler, 2014 |
| 1897 | Jefferis Turner, Queensland Australia pediatrician, diagnosed lead poisoning in children | Rosner et al., 2005 |
| 1904 | Lockhart Gibson, American clinician, was attributed with the first scientific studies linking lead-based paint to neurotoxicity in children | Rosner et al., 2005; Gibson, 1904. |
| 1909 | France, Belgium, and Austria were among the first countries to ban or restrict white lead from interior paint | Markowitz & Rosner, 2000 |
| 1918 | Lead Industries Association promoted lead for use in children's products with a “Cater to the Children” campaign featuring the Dutch Boy | Markowitz & Rosner, 2000; 2003 |
| 1921 | Edward Cornish, president of the Lead Industries Association, acknowleded in a letter to Harvard that “lead is a poison” | Markowitz & Rosner, 2000 |
| 1922 | League of Nations banned white-lead interior paint and limited lead in exterior paint. Also they stated women and children under 16 years of age should not be employed where white lead is manufactured; the U.S. declined to adopt the ban. | Gilberts & Weiss, 2006; Markowitz & Rosner, 2003 |
| 1922 | Thomas Midgley Jr., first developed tetraethyl lead gasoline as an anti-knock compound | Kitman, 2000 |
| 1922 | William Mansfield Clark of the Public Health Service warns against use of tetraethyl, calling it a “serious menace to the public health” | Kitman, 2000; Nriagu, 1990 |
| 1923 | 13 to 15 deaths and over 300 men suffered mental health issues in three GM automobile plants due to working with tetraethyl lead | Needleman, 1991 |
| 1936 | U.S. increased production of leaded gasoline; 90% of gasoline sold in the U.S. contained tetraethyl lead | Nriagu, 1990 |
| 1955 | Voluntary standard adopted to limit lead in paint to 1% by weight in the U.S. | Markowitz & Rosner, 2000 |
| 1971 | Lead-Based Paint Poisoning Prevention Act passed to begin eliminating lead in paint in the U.S. |
MMWR, 2012 www.hud.gov/sites/documents/20258_legislativehistory.pdf |
| 1973 | Environmental Protection Agency (EPA) calls for a phasing out of lead in gasoline |
EPA (1996) https://archive.epa.gov/epa/aboutepa/epa-takes-final-step-phaseout-leaded-gasoline.html |
| 1976 | Toxic Substances Control Act enacted by EPA |
EPA(n.d.) https://www.epa.gov/laws-regulations/summary-toxic-substances-control-act. |
| 1978 | Consumer Product Safety Commission (CPSC) enacted in the U.S. to ban white lead paint from indoor, residential use over 50 years after the ban is recommended by the League of Nations |
MMWR, 2012 www.hud.gov/sites/documents/20258_legislativehistory.pdf |
| 1986 | Leaded material banned from the installation and repair of public water systems in both residential and non-residential facilities by the Safe Drinking Water Act (SDWA) [Proposition 65] |
EPA(n.d.) www.epa.gov/dwstandardsregulations/use-lead-free-pipes-fittings-fixtures-solder- and-flux-drinking-water |
| 1992 | Title X of the Housing and Community Development Act enacted—Residential Lead-Based Paint Hazard Reduction Act |
MMWR, 2012 www.hud.gov/sites/documents/20258_legislativehistory.pdf |
| 1994 | Third National Health and Nutrition Examination Survey (NHANES-III) study showed that U.S. blood lead levels declined by 78% from 1976–1991 |
MMWR, 1994 www.cdc.gov/mmwr/preview/mmwrhtml/00032080.htm |
| 1995 | U.S. banned use of lead in sealing canned foods | MMWR, 2012 |
| 1996 | Clean Air Act banned sale of leaded fuel for use in on-road vehicles in a final step to phaseout tetraethyl from gasoline | EPA, 1996 |
| 1996 | World Bank called for a worldwide phaseout of leaded gasoline and called lead one of the most serious health threats to large populations | Lovei, M., 1996 |
| 1999 | U.S. Department of Housing and Urban Development (HUD) enacted Lead-Safe Housing Rule | Federal Register, 2001 |
| 2001 | Lead dust and soil hazard standards are set | Federal Register, 2001 |
| 2000 | 42 countries phased out lead from petrol | Bulletin of the World Health Organization, 2002 |
| 2007 | CPSC recalled 2 million toy units due to excessive levels of lead in paint and Mattel and Fisher Price were fined $23 million for violations | CPSC, 2009 |
| 2010 | Healthy People 2020—Centers for Disease Control and Prevention (CDC) proposed health objectives to be achieved by the reduction of lead |
CDC(n.d.) www.cdc.gov/nceh/lead/publications/10_217029A_Walker_HealthyHomesBooklet_10131_Oupdated_WithCovers.pdf |
| 2011 | CPSC decreased the limit of lead by weight allowed in a product marketed toward children from 600 parts per million to 300 parts per million (ppm) in 2009 and to 100 ppm in 2011 | CPSIA, 2012 |
| 2011 | Reduction of Lead in Drinking Water Act lowered lead content in pipes and plumbing fixtures from 8% to .25% by weight | HUD, 2014 |
| 2014 | Paint companies in California ordered to pay $1.15 billion for selling leaded paint against regulations | Kessler, 2014 |
| 2014 | Flint Michigan's water system was contaminated by improperly treated water that caused leaching of lead from old plumbing. Measurements of lead in water were as high as 13,200 ppb. The level of concern set by the Environmental Protection Agency is 15 ppb |
DeWitt, 2017 EPA, 2016 Pell & Schneyer, 2016 Torrice, 2016 |
Figure 1.
World mine production of lead by year in 100,000 metric tons. The U.S. is third in the world production of lead behind China and Australia. Lead production in the U.S. has only minimally decreased over the decades in spite of the known harmful effects of lead.
Lead does not degrade once mobilized. Consequently, significant health risks persist due to residual lead particles released into the environment decades ago (Canfield et al., 2003; Mielke et al., 1999). Leaded paint continues to be a problem because of deteriorating older buildings and lead-based vehicle exhaust from decades ago. Approximately 24 million homes have hazardous levels of lead-based paint or dust, and low-income families are eight times more likely to be at risk (HUD, n.d.; Raymond & Brown, 2015) [See Table 1 for a short history of lead, including bans and regulations]. Leaded particles from these sources and others settle into the soil and resurface with rainfall and construction.
In addition to leaded paint, leaded plumbing remains a modem problem. Under suboptimal conditions, older plumbing leaches lead into the water system (Tiemann, 2017). The recent incidence in Flint, Michigan was primarily due to improperly treated water from a new source causing lead to leach from water pipes and contaminating the water supply (DeWitt, 2017; Young, 2017) [See Table 1 for a brief history of lead]. The concentration of lead in the water reached as high as 13,200 parts per billion (ppb), while the level of concern set by the Environmental Protection Agency (EPA) is 15 ppb (Torrice, 2016). Flint is not an anomaly (Pell & Schneyer, 2016). Lead is a modern problem that poses health risks and is easily transferred from the mother’s bloodstream to the fetus, thus transcending generations (Detroit Department of Health and Wellness Promotion, 2005; Gardella, 2001; Gulson et al., 1997; Tellez-Rojo et al., 2004; Weizsaecker, 2003).
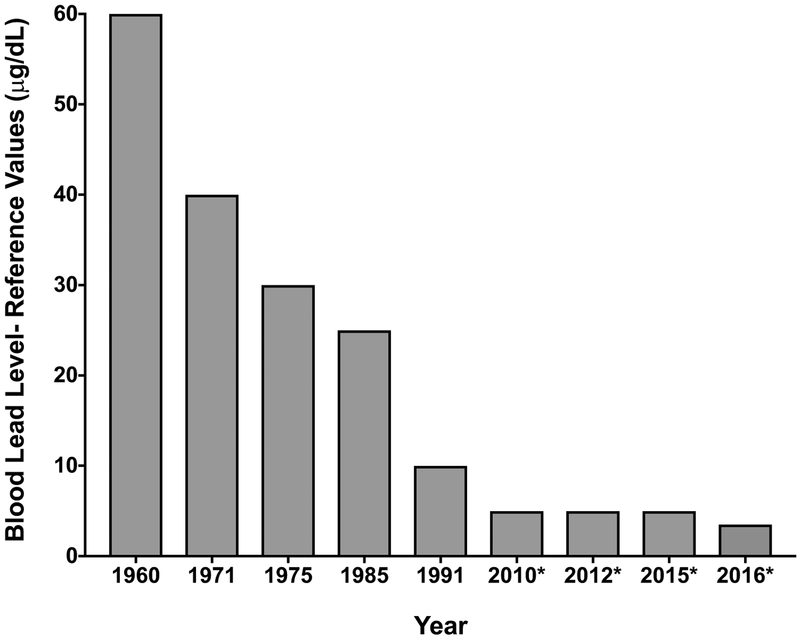
An individual’s lead burden is often measured by lead found in blood. The reference value, which is currently 5 μg/dL, is set according to the level of lead in blood in the highest 2.5% of children tested in the CDC National Health and Nutrition Examination Survey (NHANES) and is expected to be revised every four years using the two most recent NHANES surveys (CDC, 2017). Thus, the CDC level of concern is not based on toxicity as established by the scientific and medical literature. According to the latest NHANES data, the new 97.5th percentile of blood lead levels in this age group recommends a revision of the reference level down to 3.5 pg/dL (Tsoi et al., 2016) [See Figure 2]. This level of concern is down from 60 μg/dL in the 1960s to 10 μg/dL in 2015 (ATSDR, 2017; CDC, 2005; CDC, 2010a; 2010b; CDC, 2017; Gilbert & Weiss, 2006; National Research Council, 1993) [See Figure 2]). Blood lead levels are a good indicator of recent lead exposure due to a 30-day half-life. Bone lead levels are a better indicator of lifetime lead burden due to longer half-lives of 8–20 years and up to 50 years for trabecular/patella bone (e.g. knee cap) and cortical bone (e.g. tibia), respectively (Barry and Mossman, 1970; Farooqui, et al., Hu, 1998; Hu et al., 1989; 1991; Kim et al., 1997; Wilker et al., 2011).
Figure 2.
Blood lead levels determined to be levels of concern, or reference values, by the Advisory Committee on Childhood Lead Poisoning Prevention (ACCLPP), a Centers for Disease Control and Prevention (CDC) workgroup. In 2010, the reference value was decreased to 5 μg/dL only for pregnant women, followed by a similar decrease in 2012 to include children. By 2015, the decrease also applied to adults. In 2016, a suggested decrease to 3.5 μg/dL was based on the latest National Health and Nutrition Examination Survey (NHANES) data outlining the current 97.5th percentile of blood lead levels in children.
Though lead affects people of all races and socioeconomic backgrounds, there is a disproportionate lead burden placed on minority groups and lower income households (Glass et al., 2009; Lanphear et al., 2002: Mielke et al., 1999; Zierold, et al., 2007). The highest percentage of children with blood lead levels greater than 5 μg/dL are non-Hispanic African-American children (46.8%), followed by Mexican-American children (27.9%), and non-Hispanic Caucasian children (18.7%) (Bernard & McGeehin, 2003).
The purpose of the current paper is to provide an overview of lead’s neurotoxicity and mechanisms of action. This review is not intended to be comprehensive, but instead will highlight critical work in the field and offer recommendations for future research. Importantly, the evidence suggests that there are health consequences in humans and animal models at very low blood lead levels, below the concentration currently established by the CDC as a reference level.
2. Cognitive and Behavioral Effects of Lead in Humans
2.1. Cognitive Effects in Children
Effects of lead on cognition have long been known to occur at blood levels of 10 μg/dL and above. However, data suggest that significant effects are evident at levels below 5 μg/dL. Table 2 summarizes key studies on the cognitive and behavioral impacts of low blood lead levels in humans below 5 μg/dL and Table 3 summarizes those between 5–10 μg/dL. In children, blood lead levels below 5 μg/dL are associated with impulsivity and impairments in verbal processing, non-verbal reasoning, reading and arithmetic, as well as low scores in an array of achievement tests (Canfield et al., 2003; Chiodo et al., 2004; Lanphear et al., 2000). Attention is compromised at levels below 3 μg/dL in children as measured by simple reaction time, teacher report forms and neuropsychological tests (Chiodo et al., 2004; Després et al., 2005; Min et al., 2007). These results are not surprising. In a longitudinal study decrements in IQ scores were more pronounced at blood lead levels below 10 μg/dL than above this level (Canfield et al., 2003). For example, in a large longitudinal study 1,333 children were followed from birth or infancy until 5–10 years of age. Children with blood lead levels below 7.5 μg/dL showed a steeper decline in IQ for a given increase in lead concentration than children with lead levels higher than 7.5 μg/dL (Lanphear et al., 2005). These studies lend support for larger IQ decrements at lower blood lead levels.
Table 2.
Human <5 μg/dL. Summary of key studies in humans outlining the neurodevelopmental, cognitive, and achievement outcomes of blood lead levels below 5 μg/dL reported at p< .05. Excludes studies not explicitly reporting blood lead levels. Blood lead levels are listed as averages, unless otherwise noted, and appear as reported in respective studies. The most conservative adjusted statistical outcomes available are presented. Inclusion/exclusion criteria were established to determine study selection.1
| Test | Outcome (Using adjusted scores when available) | Mean Blood Lead Level (μg/dL) | Design | Age of Testing | Reference |
|---|---|---|---|---|---|
| <5 μg/dL, Human | |||||
| I. Verbal Processing/Memory | |||||
| Color Naming Task; Information Processing Speed | NS | <3.0 μg/dL | n=237 One test point Regression analysis using quartiles |
7.5 yrs | Chiodo et al., 2004 |
| No reference group | |||||
| Color Naming Task; Information Processing Speed | Deficits | <5.0 μg/dL | |||
| WRAML; Verbal Memory Index | NS | 3–4.0 μg/dL | n=389 One test point 1–2 μg/dL, reference group |
6–10 yrs | Surkan et al., 2007 |
| II. Intelligence Scale/Achievement Test | |||||
| WISC-III, Full-scale | NS | <5.0 μg/dL | n=237 One test point |
7.5 yrs | Chiodo et al., 2004 |
| Regression analysis using quartiles | |||||
| No reference group | |||||
| WISC-III, Full-scale | NS | 3–4.0 μg/dL | n=389 One test point |
6–10 yrs | Surkan et al., 2007 |
| 1–2 μg/dL, reference group | |||||
| WISC-IV, Short form & WIAT-II | Deficits | 0.4–3.47 μg/dL | n=150 Cross-sectional; Case control |
8–17 yrs | Nigg et al., 2008 |
| Regression-based path analysis | |||||
| No reference group | |||||
| WISC- III, Performance | NS | <3.0 μg/dL | n=237 One test point |
7.5 yrs | Chiodo et al., 2004 |
| Regression analysis using quartiles | |||||
| No reference group | |||||
| WISC-III, Performance | Deficits | <5.0 μg/dL | |||
| Stanford-Binet; Intelligence Scale |
Deficits | <5.0 μg/dL | n=172 Longitudinal Nonlinear mixed model |
24mos & 120 mos | Canfield et al., 2003 |
| No reference group | |||||
| WISC-R, Block Design | NS | <5.0 μg/dL | n=4,853 Cross-sectional, NHANES-III |
6–16 yrs | Lanphear et al., 2000 |
| Regression analysis using quartiles | |||||
| No reference group | |||||
| WISC-III, Block Design | NS | <3.0 μg/dL | n=237 One test point |
7.5 yrs | Chiodo et al., 2004 |
| Regression analysis using quartiles | |||||
| No reference group | |||||
| WISC-III, Block Design | Deficits | <5.0 μg/dL | |||
| WISC-III Block Design | NS | 3–4.0 μg/dL | n=389 One test point |
6–10 yrs | Surkan et al., 2007 |
| 1–2 μg/dL, reference group | |||||
| WRAT-R; Reading Subset | Deficits | <5.0 μg/dL | n=4,853 Cross-sectional, NHANES-III |
6–16 yrs | Lanphear et al., 2000 |
| Regression analysis using quartiles | |||||
| No reference group | |||||
| WIAT, Reading Composite | NS | 3–4.0 μg/dL | n=389 One test point |
6–10 yrs | Surkan et al., 2007 |
| 1–2 μg/dL, reference group | |||||
| WRAT, Arithmetic | Deficits | <5.0 μg/dL | n=4,853 Cross-sectional, NHANES-III |
6–16 yrs | Lanphear et al., 2000 |
| Regression analysis using quartiles | |||||
| No reference group | |||||
| WISC-III, Arithmetic | NS | 3–4.0 μg/dL | n=389 One test point 1–2 μg/dL, reference group |
6–10 yrs | Surkan et al., 2007 |
| III. Attention | |||||
| Child Behavior Checklist-Attention Teacher Report Form | Deficits | <3.0 μg/dL | n=237 One test point Regression analysis using quartiles |
7.5 yrs | Chiodo et al., 2004 |
| No reference group | |||||
| Child Behavior Checklist-Attention Teacher Report Form | NS | <5.0 μg/dL | |||
| Barkley Inattention | Deficits | <3.0 μg/dL | |||
| Barkley Off-Task | NS | <3.0 μg/dL | |||
| Barkley Off-Task | Deficits | <5.0 μg/dL | |||
| Continuous Performance Test-Sustained Attention | NS | <5.0 μg/dL | |||
| Mental Rotation; Reaction Time | NS | <5.0 μg/dL | |||
| Catsys System; Reaction Time | Deficits | <3.0 μg/dL | n=110 Longitudinal Regression analysis No reference group |
Birth & 4–6 yrs | Després et al., 2005 |
| IV. Impulsivity/Perseverative Errors/Cognitive Flexibility | |||||
| Stop Signal Reaction Time Task | Deficits | 0.4–3.4 μg/dL | n=150 Cross-sectional; Case-control Regression-based path analysis No reference group |
8–17 yrs | Nigg et al., 2008 |
| Wisconsin Card Sorting Task | NS | 3–4.0 μg/dL | n=389 One test point |
6–10 yrs | Surkan et al., 2007 |
| 1–2 μg/dL, reference group | |||||
| Matching Familiar Figures; Match to Sample | NS | <3.0 μg/dL | n=237 One test point Regression analysis using quartiles |
7.5 yrs | Chiodo et al., 2004 |
| No reference group | |||||
| Matching Familiar Figures; Match to Sample | Deficits | <5.0 μg/dL | |||
| V. Withdrawn | |||||
| Child Behavior Checklist-Withdrawn Teacher Report Form | Deficits | <3.0 μg/dL | n=237 One test point Regression analysis using quartiles No reference group |
7.5 yrs | Chiodo et al., 2004 |
| Child Behavior Checklist-Withdrawn Teacher Report Form | NS | <5.0 μg/dL | |||
| VI. Short-term Memory/Working Memory | |||||
| WISC-R; Digit Span | NS | <5.0 μg/dL | n=4,853 Cross-sectional, NHANES-III |
6–16 yrs | Lanphear et al., 2000 |
| Regression analysis using quartiles No reference group |
|||||
| WISC-III, Digit Span; Backwards | NS | <3.0 μg/dL | n=237 One test point Regression analysis using quartiles No reference group |
7.5 yrs | Chiodo et al., 2004 |
| Sternberg Short-Term Memory Task; Processing Speed | Deficits | <3.0 μg/dL | |||
| WISC-III, Digit Span; Backwards | Deficits | <5.0 μg/dL | |||
| Seashore Rhythm Test; Auditory Working Memory | Deficits | <5.0 μg/dL | |||
| WISC-III, Digit Span | Deficits | 3–4.0 μg/dL | n=389 One test point 1–2 μg/dL, reference group |
3–4 yrs | Surkan et al., 2007 |
| VII. Visual-Motor | |||||
| Beery Visual-Motor Integration | NS | <3.0 μg/dL | n=237 One test point Regression analysis using quartiles No reference group |
7.5 yrs | Chiodo et al., 2004 |
| Beery Visual-Motor Integration | Deficits | <5.0 μg/dL | |||
| Bruininks-Oseretsky; Visual-Motor Control | NS | Neonatal Day 10: 4.8 μg/dL | One test point 1–2 μg/dL, reference group |
6–10 yrs | Surkan et al., 2007 |
| VIII. Dexterity/Balance | |||||
| Catsys System; Balance | Deficits | 4.1 μg/dL | n=110 Longitudinal Regression analysis No reference group |
Birth & 4–6 yrs | Després et al., 2005 |
| Catsys System; Pointing Movements | Deficits | ||||
| IX. ADHD | |||||
| DSM-IV Criteria for ADHD | Association | lst tertile: 0.2–0.8 μg/dL 2nd tertile: 0.9–1.3 μg/dL 3rd tertile: >1.3 μg/dL |
n=2,588 Cross-sectional; NHANES (2001–2004) Regression analysis using tertiles No reference group |
8–15 yrs | Froehlich et al., 2009 |
| DSM-IV Criteria for ADHD-Combined | Association | 3.4 μg/dL | n=150 Cross-sectional; Case-control Regression-based path analysis No reference group |
8–17 yrs | Nigg et al., 2008 |
| DSM-IV Criteria for ADHD, Hyperactivity-Impulsivity Symptoms | Association | ||||
| DSM-IV Criteria for ADHD, Inattention-Disorganized Symptoms | NS | ||||
| DSM-IV Criteria for ADHD-Combined | Association | 0.3–2.2 μg/dL | n=150 Cross-sectional; Case-control Regression-based path analysis |
6–17 yrs | Nigg et al., 2010 |
| DSM-IV Criteria for ADHD-Predominantly Inattentive | NS | ||||
| DSM-IV Parent Report of Child's ADHD Symptoms (KSADS); Hyperactivity-Impulsivity | Association | ||||
| DSM-IV Parent Report of Child's ADHD Symptoms (KSADS); Inattention | NS | ||||
| DSM-IV Parent Report of Child's Cognition & Hyperactivity-Impulsivity (Conners) | Association | ||||
| DSM-IV Teacher Report of Child; ADHD Rating Scale & Inattention or Hyperactivity-Impulsivity | NS | ||||
| DSM-IV Teacher Report of Child's Cognition (Conners) | Association | ||||
| DSM-IV Teacher Report of Child's Hyperactivity & Impulsivity (Conners) | NS | ||||
| DSM-IV criteria for ADHD & ADHD-Combined | Increased incidence | lst tertile: 0.2–0.8 μg/dL 2nd tertile: 0.9–1.3 μg/dL 3rd tertile: >1.3 μg/dL |
n=2,588 Cross-sectional, NHANES (2001–2004) Regression analysis using tertiles No reference group |
8–15 yrs | Froehhch et al., 2009 |
| ADHD Assessed By Parent-Reported Medical Diagnosis of ADHD for Child & Child's Current Use of Stimulant Medication Use | Increased incidence (4.5-fold) | Lowest quintile (<0.7 μg/dL)vs Highest quintile (2–5.0 μg/dL) |
n=4,704 Cross-sectional, NHANES (1999–2002) Dose response measure 0.7 μg/dL or less, reference group |
4–15 yrs | Braun et al., 2006 |
| X. Conduct Disorder | |||||
| DSM-IV Criteria for Conduct Disorder | Increased incidence | 1st quartile: 0.2–0.7 μg/dL 2nd quartile: 0.8–1.0 μg/dL 3rd quartile: 1.1–1.4 μg/dL 4th quartile: 1.5–10 μg/dL |
n=3,081 Cross-sectional, NHANES (2001–2004) Regression analysis using quartiles No reference group |
8–15 yrs | Braun et al., 2008 |
Abbreviations: ADHD=Attention Deficit Hyperactivity Disorder; DSM-IV=Diagnostic and Statistical Manual of Mental Disorder; KSADS=Kiddie Schedule for Affective Disorders and Schizophrenia; MOS=Months; NHANES III=National Health and Nutrition Examination Survey III Version; NHANES (2001 −2004)=National Health and Nutrition Examination Survey 2001–2004 Version; NS=Not Significant; WIAT=Wechsler Individual Achievement Test; WIAT-II=Wechsler Individual Achievement Test II Version; WISC-III=Wechsler Intelligence Scale for Children III Version; WISC-IV=Wechsler Intelligence Scale for Children IV Version; WISC-R=Wechsler Intelligence Scale for Children-Revised; WRAML=Wide Range Assessment of Memory and Learning; WRAT=Wide Range Achievement Test; WRAT-R=Wide Range Achievement Test Revised; YRS=Years
Table 3.
Human 5–10 μg/dL. Summary of key studies in humans outlining the neurodevelopmental, cognitive, and achievement outcomes of blood lead levels between 5–10 μg/dL reported at p< .05. Excludes studies not explicitly reporting blood lead levels. Blood lead levels are listed as averages, unless otherwise noted, and appear as reported in respective studies. The most conservative adjusted statistical outcomes available are presented. Inclusion/exclusion criteria were established to determine study selection.1
| Test | Outcome (Using adjusted scores when available) | Mean Blood Lead Level (μg/dL) | Design | Age of Testing | Reference |
|---|---|---|---|---|---|
| 5–10 μg/dL, Human | |||||
| I. Reading | |||||
| WRAT-R; Reading Subset | Deficits | 7.5–10.0 μg/dL | n=4,853 Cross-sectional, NHANES-III Multiple linear regression No reference group |
6–16 yrs | Lanphear et al., 2000 |
| WIAT; Reading Composite | Deficits | 5–10.0 μg/dL | n=389 One test point 1–2 ug/dL, reference group |
6–10 yrs | Surkan et al., 2007 |
| II. Verbal Processing/Memory | |||||
| WRAML; Verbal Memory Index | Deficits | 5–10.0 μg/dL | n=389 One test point 1–2 μg/dL, reference group |
6–10 yrs | Surkan et al., 2007 |
| Color Naming Task; Information Processing Speed | NS | <7.5 μg/dL | n=237 One test point Multiple regression using quartiles No reference group |
7.5 yrs | Chiodo et al., 2004 |
| III. Intelligence Scale/Achievement Test | |||||
| WISC-III, Full-scale & Performance | Deficits | <7.5 μg/dL | n=237 One test point Multiple regression using quartiles No reference group |
7.5 yrs | Chiodo et al., 2004 |
| WISC-III, Full-scale & Performance | Deficits | 5–10.0 μg/dL | n=389 One test point 1–2 μg/dL, reference group |
6–10 yrs | Surkan et al., 2007 |
| WISC-R, Full-scale | Deficits | 6.3–6.5 μg/dL | n=148 Longitudinal Multiple regression No reference group |
Association between 24 mos & 57 mos or 10 yrs | Bellinger et al., 1992 |
| Full-scale | NS | 6.3–7.8 μg/dL | Longitudinal | Association between 6, 12, or 18 mos & either 57 mos or 10 yrs | |
| WIAT Listening Comprehension | Deficits | 5–10.0 μg/dL | n=389 One test point 1–2 μg/dL, reference group |
6–10 yrs | Surkan et al., 2007 |
| K-TEA, Battery Composite Score | Deficits | 6.3–6.5 μg/dL | n=148 Longitudinal Multiple regression No reference group |
Association between 24 mos & 57 mos; 24 mos & 10 yrs | Bellinger et al., 1992 |
| K-TEA, Battery Composite Score | NS | 6.3–7.8 μg/dL | Longitudinal | Association between 6, 12, or 18 mos & either 57 mos or 10 yrs | |
| Stanford-Binet Intelligence Scale, Composite Score | Deficits | 7.4 μg/dL (36 mos) 7.7 μg/dL (60 mos) |
n=172 Longitudinal Nonlinear mixed model No reference group |
36 mos 60 mos |
Canfield et al., 2003 |
| WISC-III, Verbal | Deficits | 5–10.0 μg/dL | n=389 One test point 1–2 μg/dL, reference group |
6–10 yrs | Surkan et al., 2007 |
| WISC-R, Verbal | Deficits | 6.3–6.5 μg/dL | n=148 Longitudinal Multiple regression No reference group |
Association between 24 mos & 57 mos or 10 yrs | Bellinger et al., 1992 |
| WISC-R, Verbal | NS | 6.3–7.8 μg/dL | n=169 Longitudinal Multiple regression No reference group |
Association between 6, 12, or 18 mos & either 57 mos or 10 yrs | |
| WISC-R, Block Design | Deficits | 7.5–10.0 μg/dL | n=4,853 Cross-sectional, NHANES-III Multiple linear regression using quartiles No reference group |
6–16 yrs | Lanphear et al., 2000 |
| WISC-III, Block Design | Deficits | <10.0 μg/dL | |||
| WRAT, Arithmetic | Deficits | 7.5–10.0 μg/dL | |||
| WIAT, Math Composite | Deficits | 5–10.0 μg/dL | n=389 One test point 1–2 μg/dL, reference group |
6–10 yrs | Surkan et al., 2007 |
| IV. Cognition | |||||
| McCarthy Scales of Children's Abilities General Cognitive Index; Verbal Subscale | NS | 6.4–6.8 μg/dL | n=170 Longitudinal Regression analysis No reference group |
24 mos Or 57 mos |
Bellinger et al., 1991 |
| McCarthy Scale of Children's Abilities; General Cognitive Index-Quantitative Subscale | NS | 6.4–6.8 μg/dL | n=170 Longitudinal Regression analysis No reference group |
24 mos Or 57 mos |
Bellinger et al., 1991 |
| McCarthy Scale of Children's Abilities; General Cognitive Index Perceptual-Performance Subscale | Deficits | 6.4–6.8 μg/dL | n=170 Longitudinal Regression analysis No reference group |
24 mos Or 57 mos |
Bellinger et al., 1991 |
| McCarthy Scale of Children's Abilities; General Cognitive Index Motor Subscale | NS | ||||
| McCarthy Scale of Children's Abilities; General Cognitive Index Memory Subscale | NS | ||||
| V. Attention | |||||
| Child Behavior Checklist-Attention Teacher Report Form | NS | <10.0 μg/dL | n=237 One test point Multiple regression using quartiles No reference group |
7.5 yrs | Chiodo et al., 2004 |
| Child Behavior Checklist-Attention Teacher Report Form | NS | <7.5 μg/dL | |||
| Barkley Off Task | NS | <7.5 μg/dL | |||
| Barkley Off Task | Deficits | <10.0 μg/dL | |||
| Continuous Performance Test; Sustained Attention | NS | <10.0 μg/dL | |||
| Mental Rotation; Reaction Time | Deficits | <7.5 μg/dL | |||
| VI. Withdrawn | |||||
| Child Behavior Checklist-Withdrawn Teacher Report Form | Deficits | <7.5 μg/dL | n=237 One test point Multiple regression using quartiles No reference group |
7.5 yrs | Chiodo et al., 2004 |
| Child Behavior Checklist-Withdrawn Teacher Report Form | NS | <10.0 μg/dL | |||
| VII. Short-term Memory/Working Memory | |||||
| Seashore Rhythm Test; Auditory Working Memory | NS | <7.5 μg/dL | n=237 One test point Regression analysis using quartiles No reference group |
7.5 yrs | Chiodo et al., 2004 |
| Seashore Rhythm Test; Auditory Working Memory | Deficits | <10.0 μg/dL | |||
| VIII. Visual-Motor | |||||
| Bruininks-Oseretsky; Visual-Motor Control | NS | Neonatal: 8.4 μg/dL 72 mos: 10.1 μg/dL |
n=245 Longitudinal Regression analysis |
Neonatal Day 10 association at 72 mos | Dietrich et al., 1993 |
| Bruininks-Oseretsky; Visual-Motor Control | Deficits | Neonatal: 8.4 μg/dL 72 mos: 10.1 μg/dL |
n=245 Longitudinal Regression analysis |
72 mos | Dietrich et al., 1993 |
| IX. Dexterity/Balance | |||||
| Bruininks-Oseretsky; Upper Limb Speed & Dexterity | Deficits | Neonatal: 8.4 μg/dL 72 mos: 10.1 μg/dL |
n=245 Longitudinal Regression Analysis |
Neonatal Day 10 association at 72 mos | Dietrich et al., 1993 |
| Bruimnks-Oseretsky; Fine Motor Composite | Deficits | ||||
| Bruininks-Oseretsky; Bilateral Coordination | NS | ||||
| Bruininks-Oseretsky; Upper Limb Speed & Dexterity | Deficits | 72 mos: 10.1 μg/dL | n=245 Longitudinal Regression analysis |
72 mos | Dietrich et al., 1993 |
| Bruininks-Oseretsky; Fine Motor Composite | Deficits | ||||
| Bruininks-Oseretsky; Bilateral Coordination | Deficits | ||||
| X. ADHD | |||||
| DSM-IV Criteria for ADHD | Association | lst tertile: 0.2–0.8 μg/dL 2nd tertile: 0.9–1.3 μg/dL 3rd tertile: >1.3 μg/dL | n=2,588 Cross-sectional, NHANES (2001–2004) Logistic regression No reference group |
8–15 yrs | Froehlich et al., 2009s |
| XI. Conduct Disorder | |||||
| DSM-IV Criteria for Conduct Disorder | Association | 1st quartile: 0.2–0.7 μg/dL 2nd quartile: 0.8–1.0 μg/dL 3rd quartile: 1.1–1.4 μg/dL 4th quartile: 1.5–10μg/dL |
n=3,081 Cross-sectional, NHANES (2001–2004) |
8–15 yrs | Braun et al., 2008 |
Abbreviations: ADHD=Attention Deficit Hyperactivity Disorder; DSM-IV=Diagnostic and Statistical Manual of Mental Disorder IV Version; K-TEA=Kaufman Test of Educational Achievement; MOS=Months; NS=Not Significant; NHANES (2001 −2004)=National Health and Nutrition Examination Survey 2001–2004 Version; NHANES III=National Health and Nutrition Examination Survey III Version; WAIS -IV=Wechsler Adult Intelligence Scale IV Version; WIAT=Wechsler Individual Achievement Test; WISC-III=Wechsler Intelligence Scale for Children III Version; WISC-R=Wechsler Intelligence Scale for Children Revised; WRAML=Wide Range Assessment of Memory and Learning; WRAT=Wide Range Achievement Test; YRS=Years
In other studies, blood concentrations at or below 10 μg/dL produced neurophysiological and neurobehavioral deficits that could affect academic outcomes, including distractibility, memory deficits, decreased verbal and quantitative scores, impaired visual-motor coordination, and longer reaction times (Bellinger et al., 1991; 1992; Canfield et al., 2003; Chiodo et al., 2004; Dietrich et al., 1993; Jusko et al., 2008; Lanphear et al., 2000; Min et al., 2007; Needleman et al., 1990; Reuben et al., 2017; Stiles and Bellinger, 1993; Surkan et al., 2007).
In an 11-year longitudinal study of children starting at 2 years of age, high dentin lead levels closely predicted future high school graduation drop-out rates, lower class standing, greater absenteeism, impaired reading skills, and deficits in vocabulary, fine motor skills, reaction time, and hand-eye coordination (Needleman et al., 1990). Lead exposure, as quantified through blood, dentin, or bone, is strongly associated with educational achievement outcomes, such as IQ tests, reading and arithmetic tests, tests of attention, short-term memory, verbal and non-verbal reasoning; these deficits are observed even after adjusting for socioeconomic status, mother’s age at the time of the child’s birth, the reference caregiver’s educational level and IQ (Bellinger et al., 1992; Braun et al., 2008; Canfield et al., 2003; Lanphear et al., 2000; 2005; Min et al., 2007; Needleman, 1979; Needleman et al., 1990; 1996; Stiles & Bellinger, 1993).
The impacts of lead on cognition were recently examined in another longitudinal study. A prospective cohort study of 1007 New Zealanders found that blood lead levels taken at 11 years of age (Mean: 10.99 μg/dL) accounted for lower cognitive function and a decline in socioeconomic mobility at 38 years of age (Reuben, et al., 2017). Declines in IQ as measured by WAIS-IV full-scale, perceptual reasoning, and working memory at 38 years of age were associated with blood lead levels at 11 years of age. IQ verbal comprehension showed less of an association and processing speed at 38 years of age was not associated with earlier blood lead levels (Reuben et al., 2017).
2.2. Behavioral Effects in Children and Young Adults
In addition to producing cognitive deficits, lead can affect educational achievement by increasing behavioral problems that run counter to academic success. Lead exposure during infancy has long been linked to violent, disruptive, and unpredictable behavior that contributes to academic failure and school dismissal (Byers and Lord, 1943). Dentin lead levels in children have been correlated with maladaptive classroom behavior, as reported by teachers (Needleman, 1979). In addition to teacher and parent reports, self-reports have correlated K-shell X-ray fluorescence (KXRF) measures of lead in tibia with aggression, attention, and delinquency in boys aged 7–11. KXRF is a reliable and non-invasive method used to measure long-term lead stores (Hu et al., 1991). Adolescents between the ages of 12–18 who were found guilty of committing a delinquent act were four times more likely to have elevated bone lead concentrations in tibia than a comparable non-delinquent group (Needleman et al., 1996). In a prospective, longitudinal study, prenatal exposure to lead was associated with increased parent reports of antisocial behavior in adolescent children and postnatal exposure was associated with the adolescents’ self-reported delinquent acts (Dietrich et al., 2001). In this study, 92% of the participants were African-American, 74% were of low socioeconomic status, and parental IQ was low, perhaps limiting generalizability; however, this study demonstrated that given a fairly homogenous population, lead levels are associated with delinquent behavior (Dietrich et al., 2001). In a cross-sectional study of 15–24 year olds, those with blood lead levels between 1.5–10 μg/dL were over 8 times more likely to meet the DSM-IV criteria for conduct disorder when compared to those in the lowest detectable range of less than .7 μg/dL (though the number of cases in each group was small) (Braun et al., 2008).
An examination of trends in lead contamination and delinquent behavior further supports a relationship between these two factors. After a delinquency rate that consistently increased over the years in the United States, a 13% decrease was observed in 2010 that is attributed to the phasing out of lead in gasoline years earlier (Department of Justice, 2012; FBI, 2011). The phasing out was not uniform across cities and, in fact, the lead burden remains variable throughout the nation. This variability can be used to calculate the effects of lead on delinquent behavior by comparing the lead burden in specific areas. The reduction in lead exposure in the 1970s may have contributed to a drop in violent crime in the 1990s when those people would have reached adolescence; these findings remain after controlling for several environmental, social, and economic factors (Jaeok et al., 2016; Nevin, 2000). The relationship between lead burden and crime trends remains on a global scale. One study estimated that 63–93% of the variability in international crime trends is explained by preschool blood lead levels from 19 years earlier (Nevin, 2007).
Behavioral parallels between children with attention deficit hyperactivity disorder (ADHD) and those exposed to lead are notable (Nigg et al., 2008; Rice, 2000). Children with ADHD and those exposed to lead exhibit pronounced impairments on discrimination reversal tasks such as the Wisconsin Card Sorting Test, spatial delayed alternation, go-no-go task, distractibility task and serial reaction tasks (e.g. review by Winneke, 2011). These effects are evident when blood lead levels are below 10 μg/dL, whereas comparably low levels of other toxic heavy metals, such as mercury and aluminum are not associated with ADHD-like effects (Ha et al., 2009; Nicolescu et al., 2010). Consistent with these findings, blood lead levels below 5 μg/dL are associated with combined hyperactive-inattentive ADHD symptoms, as described in the DSM-IV. This remains true even after co-varying for family income (Chiodo et al., 2007) and gender (Nigg et al., 2008). Lead levels below 5 pg/dL are associated with a more than 2-fold increased risk of diagnosis of ADHD when compared to children with undetectable levels of lead (Froehlich et al., 2009) [See Table 2]. Consistent with the increased rate of ADHD that is reported in males versus females, Denno observed that lead poisoning was the most significant predictor of delinquent and criminal behavior in males, but not in females (1984). These studies provide growing evidence that some diagnoses of ADHD may stem from the behavioral consequences of environmental lead exposure.
2.3. Cognitive and Behavioral Effects in the Elderly
In addition to lead effects in the young, lead that accumulates over a lifetime produces negative cognitive consequences in the elderly. In older individuals, lead stored in bone is released due to osteoporosis that occurs during decalcification (Rosin, 2009). Studies corroborate that lead exposure early in life produces latent cognitive effects that emerge later in life in the form of Alzheimer’s disease. Shih et al. (2006) reported that older adults with blood lead levels averaging 3.46 μg/dL had much higher cumulative levels of lead in tibia, averaging 18.7 μg/g. Importantly, tibia lead levels, but not blood lead levels, were significantly correlated with decreases in a wide range of cognitive tasks including language, processing speed, eye-hand coordination, executive functioning, verbal memory and learning, visual memory, and visuoconstruction. Though blood lead levels were low, the steady state and peak blood lead levels were not reported and presumably were high in order to produce the elevated levels of lead in bone. These studies suggest that lead impacts cognition later in life although results are mixed and ultimate susceptibility may be due to epigenetics and other factors (Basha & Reddy, 2010; Basha et al., 2005; Stewart et al., 2002; 2006; van Wijngaarden et al., 2009; Wu et al., 2008; Zawia & Basha, 2005).
Parkinson’s disease is another concern in the aging population that can be affected by lead exposure. Long-term occupational exposure to a combination of lead and copper (Gorell et al., 1999; 2004), or lead and iron (Gorell et al., 1999) increases the risk for Parkinson’s disease by twofold as assessed by retrospective, self-report measures. Objective measures such as K-shell X-ray fluorescence (KXRF) also correlate long-term lead exposure with Parkinson’s disease (Coon et al., 2006; Weisskopf et al., 2010). Furthermore, Parkinson’s disease is associated with KXRF measures of lead levels in cortical bone (e.g. tibia) where the half-life of lead is 20–30 years, but not in trabecular/patella bone (e.g. knee cap) where the half-life is 8 years (Weisskopf et al., 2010).
2.4. Gross Brain Structure in Humans
A prospective, longitudinal study identified brain correlates of youth lead exposure. Children’s blood lead levels were compared to morphometric brain images (obtained via MRI) when those same children reached 19 through 24 years of age (Cecil et al., 2008). A lead-induced, dose-dependent decline in the volume of gray matter in various areas of the prefrontal cortex was reported, including in the ventrolateral prefrontal cortex and anterior cingulate cortex in men; as well as in the inferior parietal lobe in women. These regions of the prefrontal cortex are associated with executive function, higher order thinking, decision-making, inhibitory control, mood regulation and fine motor control. Decreased functioning of these regions could lead to impulsive, aggressive, or violent behavior. In this study, males were more susceptible to lead-induced deficits given the same concentrations of lead and similar demographic factors to females. Perhaps not coincidentally, males are more prone to antisocial behavior. This study was conducted in economically impoverished areas of Cincinnati. While the socioeconomic and geographic homogeneity of the group may decrease generalizability, it provides a control for these two important factors and further supports the conclusion that dose-dependent lead levels are the reason for the observed morphological changes. Moreover, it is reasonable to suggest that neuroanatomical changes, such as these, contribute to cognitive and behavioral problems.
3. Cognitive, Behavioral and Environmental Effects in Animal Models
3.1. Cross-species Comparison
Animal models help clarify the consequences of lead exposure in a controlled laboratory setting. Neurodevelopmental, cognitive, and learning deficits following lead exposure are similar in humans, primates and rodents (Davis et al., 1990; Winder et al., 1983). Extrapolation from animal studies to humans is possible even though cross-species comparisons are complicated by differences in absorption, distribution, metabolism, and excretion. Physiologically-based pharmacokinetic (PBPK) models, for example, are successfully used to extrapolate information from animals to humans (Ginsberg et al., 2004).
Similarities in neurotoxic effects are evident across species despite pharmacokinetic differences. This is the case even though blood lead levels in small mammals are higher than in humans due to a faster metabolic rate in smaller organisms that affects gastrointestinal absorption and elimination of the metal. Summaries of neurodevelopmental, cognitive, and learning outcomes are outlined according to blood lead levels in Tables 4 and 5 for rodents (<5 μg/dL and 5–20 μg/dL, respectively), and Table 6 for non-human primates (< 20 μg/dL).
Table 4.
Animals <5 μg/dL. Summary of key studies in rodents outlining neurodevelopmental and cognitive outcomes of blood lead levels below 5 μg/dL reported at p< .05. Excludes studies where peak levels were above 5 μg/dL. Excludes studies not explicitly reporting blood lead levels. Blood lead levels are presented as averages, unless otherwise noted, and appear as reported in respective studies. Inclusion/exclusion criteria were established to determine study selection.1
| Test | Outcome (Using adjusted scores when available) | Mean Blood Lead Level Gig/dL), Strain, Species, & Sex | Exposure Protocol | Age of Testing | Reference |
|---|---|---|---|---|---|
| <5 μg/dL, Animals - Rodents | |||||
| I. Exploratory Activity | |||||
| Rearing During Spatial Memory Retrieval; Object-in-Place Task | Increased rearing in male & females | 3.3 μg/dL C57BL/6J Mice |
0 ppm n=18 (10 Males; 8 Females) | PND 28 | Sobin et al., 2017 |
| Males & Females | 30 ppm n=16 (6 Male; 10 Female) | ||||
| via lactation & drinking water PND 0-PND 28 | |||||
| Distance Traveled & Time Immobile During Spatial Memory Retrieval; Object-in-Place Task | NS | ||||
| Rearing During Object Memory Retrieval; Object-in-Place Task | Increased rearing in male & females | ||||
| Distance Traveled & Time Immobile during Object Memory Retrieval; Object-in-Place Task | NS | ||||
| III. Associative Learning | |||||
| Acquisition- Trace Fear Conditioning | NS | 5.65 μg/dL | 0 ppm & 150 ppm | PND 55 | Verma & Schneider, 2017 |
| Long Evans Rats Males | n=32 (16 Males; 16 Females, per group) | ||||
| via food Gestation to PND 21 | |||||
| Acquisition- Trace Fear Conditioning | NS | 4.38 μg/dL | |||
| Long Evans Rats Females | |||||
| Acquisition- Trace Fear Conditioning | NS | 5.42 μg/dL | 0 ppm & 150 ppm | ||
| Sprague Dawley Rats Males | n=36 (18 Males; 18 Females, per group) | ||||
| via food Gestation to PND 21 | |||||
| Acquisition- Trace Fear Conditioning | NS | 4.52 μg/dL | |||
| Sprague Dawley Rats Females | |||||
| Acquisition- Trace Fear Conditioning | NS | 5.95 μg/dL | 0 ppm & 150 ppm | ||
| Long Evans Rats Males | n=32 (16 Males; 16 Females, per group) | ||||
| via food PND 1-PND 21 | |||||
| Acquisition- Trace Fear Conditioning | NS | 5.38 μg/dL | |||
| Long Evans Rats Females | |||||
| Acquisition- Trace Fear Conditioning | NS | 5.45 μg/dL | 0 ppm & 150 ppm | ||
| Sprague Dawley Rats Males | n=36 (18 Males; 18 Females) | ||||
| via food PND 1-PND21 | |||||
| Acquisition- Trace Fear Conditioning | NS | 5.06 μg/dL | |||
| Sprague Dawley Rats Females | |||||
| II. Memory | |||||
| Memory Retention-Trace Fear Conditioning | Deficits | Long Evans Rats Males | 0 ppm & 150 ppm n=32 (16 Males; 16 Females, per group) |
PND 55 | Verma & Schneider, 2017 |
| via food Gestation to PND 21 | |||||
| Memory Retention-Trace Fear Conditioning | NS | Long Evans Rats Females | |||
| Memory Retention-Trace Fear Conditioning | NS | Sprague Dawley Rats Males | 0 ppm & 150 ppm n=36 (18 Males; 18 Females, per group) |
||
| via food Gestation to PND 21 | |||||
| Memory Retention-Trace Fear Conditioning | NS | Sprague Dawley Rats Females | |||
| Memory Retention-Trace Fear Conditioning | NS | Long Evans Rats Males | 0 ppm & 150 ppm | ||
| n=32 (16 Males; 16 Females, per group |
|||||
| via food PND1-PND21 | |||||
| Memory Retention-Trace Fear Conditioning | Deficits | Long Evans Rats Females | |||
| Memory Retention-Trace Fear Conditioning | NS | Sprague Dawley Rats Males | 0 ppm & 150 ppm n=36 (18 Males; 18 Females) |
||
| via food PND1-PND21 | |||||
| Memory Retention-Trace Fear Conditioning | NS | Sprague Dawley Rats | |||
| Spatial Memory Retrieval, Object-in-Place Task | NS | 0 ppm 0.2 μg/dL | 0 ppm n=18 (10 Males; 8 Females) |
PND 28 | Sobin et al., 2017 |
| 30 ppm 3.3 μg/dL | |||||
| 30 ppm | |||||
| C57BL/6J Mice | n=16 (6 Male; 10 Female) | ||||
| Males & Females | via lactation & drinking water PND 0- PND 28 | ||||
| (Note: BBLs were averaged from four previously published studies.) | |||||
| Object Memory Retrieval, Object-in-Place Task | NS | ||||
Abbreviations: BLL=Blood Lead Level; NS=Not Significant; PND=Postnatal Day; PPM=Parts Per Million
Table 5.
Animals 5–20 μg/dL. Summary of key studies in rodents outlining neurodevelopmental and cognitive outcomes of blood lead levels between 5–20 μg/dL reported at p< .05. Excludes studies where peak levels were above 20 μg/dL. Excludes studies not explicitly reporting blood lead levels. Blood lead levels are presented as averages, unless otherwise noted, and appear as reported in respective studies. Inclusion/exclusion criteria were established to determine study selection.1
| Test | Outcome (Using adjusted scores when available) | Mean Blood Lead Level (μg/dL), Strain, Species, & Sex | Exposure Protocol | Age of Testing | Reference |
|---|---|---|---|---|---|
| 5–20 μg/dL, Animals - Rodents | |||||
| I. Exploratory Activity | |||||
| Unbaited Nose Poke Task | Decreased explorator y activity | 0.2–15 μg/dL | 0 ppm n=19 | PND 28 | Flores-Montoya & Sobin, 2015 |
| C57BL/6J Mice | (8 Males; 11 Females) | ||||
| Males & Females | |||||
| 30 ppm n=26 (16 Males; 10 Females) | |||||
| 230 ppm n=16 (12 Males; 4 Females) | |||||
| via lactation & drinking water PND 0–28 (offspring tested) | |||||
| Regression analysis | |||||
| Open Field, Number of Quadrant Crosses | NS | ||||
| Rotarod | NS | ||||
| Total Exploration Time During Novel Odor Recognition Task for males, but not females | NS | 0.02–20.31 μg/dL C57BL/6J Mice Males & Females |
0 ppm n=10 (8 Males; 2 Females) | Flores-Montoya et al., 2015 | |
| 30 ppm n=10 (5 Males; 5 Females) | |||||
| 330 ppm n=13 (7 Males; 6 Females) | |||||
| via lactation & drinking water PND 0–28 (offspring tested) | |||||
| Regression analysis | |||||
| Open Field, Activity Counts | Decreased activity | <10 μg/dL, P | 0 ppm & 27 ppm | 1 yr | Leasure et al., 2008 |
| C57BL/6 Mice | (n= 6–8 Males; 6–8 Females, per group) | ||||
| Males | |||||
| via lactation & drinking water; Dam’s lead exposure: 14 days prenatal-PND 10 (offspring tested) | |||||
| Open Field, Activity Counts | NS | <10 μg/dL, P | |||
| C57BL/6J Mice | |||||
| Females | |||||
| II. Global Locomotor | |||||
| Amphetamine -Induced Motor Activity | Increased drug-induced locomotor activity | <10 μg/dL, P | 0 ppm & 27 ppm (n=6–9 Males; 6–9 | 1 yr | Leasure et al., 2008 |
| C57BL/6J Mice | Females, per group) | ||||
| Males | via lactation and drinking water; Dam’s lead exposure: 14 days prenatal- PND 10 (offspring tested) | ||||
| Amphetamine -Induced Motor | NS | <10 μg/dL, P | |||
| Activity | C57BL/6J Mice | ||||
| Female | |||||
| Running Wheel Activity | NS | <10 μg/dL, P | n=6 (Male) | ||
| C57BL/6J Mice | |||||
| Male | |||||
| III. Motor Coordination | |||||
| Rotarod | Deficits | <10 μg/dL, P C57BL/6J Mice Male |
|||
| Rotarod | NS | <10 μg/dL, P C57BL/6J Mice Female |
|||
| IV. Odor Recognition | |||||
| Novel Odor Recognition Task | Preference for familiar over novel odor | 0.02–20.31 μg/Dl C57BL/6J Mice |
0 ppm n=10 (8 Males; 2 Females) | PND 28 | Flores-Montoya et al., 2015 |
| Male | |||||
| 30 ppm n=10 (5 Males; 5 Females) | |||||
| & 330 ppm n=13 (7 Males; 6 Females) | |||||
| via lactation & drinking water | |||||
| PND 0–28 (offspring tested) | |||||
| Novel Odor Recognition Task | NS | 0.02–20.31 μg/dL | |||
| C57BL/6J Mice | |||||
| Female | |||||
| V. Cognitive Flexibility | |||||
| Delayed Spatial Alternation | Deficits | 19 μg/dl, SS Long-Evans Rats |
0 ppm & 15 ppm n=15 per group (Male) | 22 weeks | Alber & Strupp, 1996 |
| Male | via drinking water PND 25- through testing | ||||
| Cued Alternation | NS | ||||
| Spatial Alternation | NS | ||||
| VI. Delay of Reinforcement/Impulsivity | |||||
| Fixed-Ratio Waiting-for-Reward Schedule of Reinforcement (FR50) | Deficits | 10.8 μg/dL, SS Long-Evans Rats Male |
0 ppm & 50 ppm n=12 per group (Male) |
PND 60 | Brockel & Cory-Slechta, 1998 |
| via drinking water PND 21 through testing | |||||
| Fixed Interval Schedule-Controlled (FI 1 Minute) | NS | 11.4 μg/dL, SS Long-Evans Rats Male |
0 ppm & 50 ppm n=10 per group (Male) |
PND 21 through testing | Cory-Slechta & Brockel, 2002 |
| via drinking water PND 21 through testing | |||||
| Fixed Interval Schedule-Controlled (FI 1 Minute) | NS | 7.2 μg/dL, SS Long-Evans Rats Male |
0 ppm n=13 50 ppm n=14 |
PND 21 through testing | Cory-Slechta, O'Mara, Brockel, 1998 |
| (Male) | |||||
| via drinking water PND 21 through testing | |||||
| Fixed Interval Schedule-Controlled (FI 1 Minute) | NS | 15.9 μg/dL, Fischer-344 Rats Male |
0 ppm & 50 ppm n=16 per group (Male) via drinking water from arrival through testing |
16 mos old at arrival. Testing began after 6th mos of exposure through testing | Cory-Slechta & Pokora, 1991 |
| Fixed Interval Schedule-Controlled (FI 1 Minute) | NS | PND 21: 11.3 μg/dL | 2 mg/kg/day in 5 mis | PND 21, 8 mos. 16 mos | |
| Deficits | PND 8 months: 17.1 μg/dL | PND 21:n=30 PND 8mos:n=34 PND 16mos:n=42 |
at arrival. | ||
| NS | PND 16 months: 18.3μg/dL Fischer-344 Rats Male |
(Male) Via drinking tube from arrival through testing |
Testing began after 2.5 mos of lead exposure | ||
| Variable Interval Schedule-Controlled | NS | PND 21: 11.3 μg/dL | |||
| (Fl Minute) | NS | PND 8 months: 17.1 μg/dL | |||
| NS | PND 16 months: 18.3μg/dL Fischer- 344 Rats Male |
||||
| Fixed Interval Schedule-Controlled (FI 1 Minute) | Deficits | 15–20 μg/dL Long-Evans Rats |
0 ppm & 25 ppm n=12 per group (Male) |
PND 50 | Cory-Slechta et al., 1985 |
| Male | via drinking water PND 21 through testing |
||||
| Fixed Interval Schedule-Controlled (FI 1 Minute) | Deficits | <20 μg/dL Long-Evans Rats Male |
0 ppm & 50 ppm n=6 per group (Male) via drinking water PND 21-PND 178 or PND 20-PND 335 |
PND 55 | Cory-Slechta et al., 1983 |
| Fixed Interval Schedule-Controlled (FI 1 Minute) | Deficits | <10 μg/dL Sprague-Dawley Rats |
0 ppm n=8 50 ppm n=ll |
PND 55 | Cory-Slechta et al., 1979 |
| Male | |||||
| (Male) | |||||
| via drinking water from PND 21 through testing | |||||
Abbreviations: MOS=Months; NS=Not Significant; P=Peak; PND=Postnatal Day; PPM=Parts Per Million; SS=Steady State; YR=Year
Table 6.
Cynomolgus Monkeys. Summary of key studies in monkeys outlining neurodevelopmental and cognitive outcomes of blood lead levels below 20 μg/dL reported at p< .05. Excludes studies where peak levels were above 20 μg/dL. Excludes studies not explicitly reporting blood lead levels. Blood lead levels are presented as averages, unless otherwise noted, and appear as reported in respective studies. Inclusion/exclusion criteria were established to determine study selection.1
| Test | Outcome (Using adjusted scores when available) | Mean Blood Lead Level μg/dL) | Exposure Protocol | Age of Testing | Reference |
|---|---|---|---|---|---|
| <20 μg/dL, Cynomolgous Monkeys | |||||
| I. Attention/Impulsivity/Discrimination Task | |||||
| Discrimination Task Spatial Discrimination; No Irrelevant Cues | NS | 15.4 μg/dL, SS 10.9 μg/dL, P Controls: 3 μg/dL (Note: Controls had BLLs between 2.9 & 3.5 μg/dL perhaps masking an effect at the low level of lead tested) |
0 μg/kg/day (n=3 Males; n=3 Females) 50 μg/kg/day (4=Males; 4=Females) Experimenter administered PND 0 through testing |
9–10 yrs | Gilbert & Rice, 1987 |
| Irrelevant Form Cues | NS | 15 μg/dL, P 11 μg/dL, SS |
0 μg/kg/day (n=4 Males; n=3 Females) 50 μg/kg/day (n=4 Males; n=4 Females) Experimenter administered PND 0 through testing |
3–4 yrs | Rice, 1985 |
| Irrelevant Form & Color Cues | NS | ||||
| Acquisition of Nonspatial Form Discrimination | NS | ||||
| Acquisition of Nonspatial Color Discrimination with Irrelevant Form Cues | NS | ||||
| Acquisition of Nonspatial Form Discrimination with Irrelevant Color Cues | NS | ||||
| Spatial Form Discrimination | Deficits | ||||
| Nonspatial Color Discrimination with Irrelevant Form Cues | Deficits | ||||
| Nonspatial Form Discrimination with Irrelevant Color Cues | Deficits | ||||
| Delayed Alternation | Deficits | ||||
| II. Delay of Reinforcement | |||||
| Differential Reinforcement of Low Rate (DRL varied between 5–30 sec) | NS | 15.4 μg/dL, SS 10.9 μg/dL, P Controls: 3 μg/dL (Note: Controls had high levels of lead, perhaps masking an effect of the low level of lead tested) |
0 μg/kg/day (n=4 Males; n=3 Females) 50 μg/kg/day (n=4 Males; n=4 Females) Experimenter administered PND 0 through testing |
3 yrs | Rice & Gilbert, 1985 |
| III. Memory | |||||
| Acquisition of Alternation Tasks | NS | 15.4 μg/dL, P 10.9 μg/dL, SS |
0 μg/kg/day (n=4 Males; n=3 Females) 50 μg/kg/day (n=4 Males; n=4 Females) Experimenter administered PND 0 through testing |
7–8 yrs | Rice & Karpinski, 1988 |
| Acquisition of Delayed Alternation | Deficits | ||||
Abbreviations: =Blood Lead Level; NS=Not Significant; P=Peak; PND=Postnatal Day; SS=Steady State; YRS=Years
3.2. Cognitive and Behavioral Effects in Animals
In humans and animals lead exposure affects cognitive measures, such as learning and memory. Rodents exhibit memory impairments at blood lead levels as low as 3.58 μg/dL (Wang et al., 2012), discrimination tasks and increased distractibility at 15 μg/dL (Rice, 1985), and delayed spatial cognitive flexibility at 19 μg/dL (Alber & Strupp, 1996). Other characteristics of lead-exposed animals include perseveration of incorrect responses, impulsivity, increased rates of operant responding, and problems handling delays of reinforcement at, or below, 10 μg/dL (Brockel & Cory-Slechta, 1998; Cory-Slechta & Thompson, 1979), or below 20 μg/dL (Cory-Slechta et al., 1983; 1985) [See Tables 4 and 5, respectively]. Tasks that require higher-level learning reveal greater impairment than simpler, lower-level learning tasks (EPA, 2006; White et al., 2007).
In addition to cognitive deficits, locomotor effects are evident in rodents at blood lead levels below 10 μg/dL. Alterations include increased rearing during spatial memory retrieval at 3.3 μg/dL (Sobin et al., 2017) and decreased exploratory behavior at blood lead levels below 10 μg/dL in mice (Flores-Montoya & Sobin, 2015; Leasure et al., 2008). Likewise, a reduction of exploratory behavior on an open field task, and motor coordination on a rotarod are observed in rats administered lead at concentrations that yield blood lead levels in this range (Sabbar et al., 2012). These motor impairments accompany noradrenaline depletion and changes in the firing rate in the subthalamic nucleus while dopamine levels in the striatum remain unchanged (Sabbar et al., 2012). Interestingly, animals with lower blood lead levels (<10 μg/dL) than those with higher blood lead levels (24–27 μg/dL) show the greatest decreases in horizontal and vertical exploratory activity, amphetamine-induced increases in locomotion, and lack of motor coordination that is male-specific (Leasure et al., 2008).
3.2.1. Alteration of Drug Responses in Animals
Lead targets the frontal cortex, nucleus accumbens, dorsal striatum, and hippocampus with dopaminergic and glutamatergic changes in these regions that vary by dose, age, and duration of exposure as suggested by autoradiography receptor binding studies (Cory-Slechta, 1997; Cory-Slechta et al., 1993; 1997; 1998; Jett and Guilarte, 1995; Listos et al., 2013). Animals exposed to lead postweaning acquire drug discrimination more rapidly to SKF38393 (D1 agonist), quinpirole (D2 agonist), cocaine, apomorphine, and amphetamine suggesting a supersensitivity of the dopaminergic system. Similarly, increased sensitivity of the glutamatergic system in lead-exposed animals is evidenced by faster acquisition of a drug discrimination task when substituting NMDA for quinpirole in animals exposed to lead at postweaning (Cory-Slechta & Widzowski, 1991). Alternatively, hypoglutamatergic effects or no effects in animals exposed to lead at postweaning have also been reported along with glutamatergic sensitivity only in animals exposed to lead early in development (Cory-Slechta et al., 1997; Jett & Guilarte, 1995).
Age-dependent differences extend to drug self-administration in animals exposed to lead. While perinatal lead exposure increases sensitivity to dopaminergic systems producing hypersensitivity to cocaine, adult exposure to lead produces hyposensitivity to the same drug. Animals perinatally exposed to lead show sustained self-administration of cocaine at doses too low to sustain responding in control animals, exhibit a higher return to drug-seeking (relapse) at lower doses of a cocaine priming injection (Nation et al., 2004), and a higher rate of acquisition of cocaine self-administration (Rocha et al., 2005). Alternatively, animals exposed to lead in adulthood show an attenuated sensitization to the locomotor-stimulating properties of chronic cocaine (Nation et al., 1996) and the impact of cocaine on schedule-controlled operant responding is reduced when lead is presented in adulthood (Burkey et al., 1997).
These differences are consistent with other studies in which directionally opposite behavioral effects are observed depending on whether lead exposure occurs perinatally or postweaning (Areola & Jadhav, 2001; Pokora et al., 1996; Widzowski et al., 1994). Curiously, while perinatal lead exposure increases cocaine reward, it attenuates methamphetamine self-administration in a progressive ratio task, perhaps due to differences in the involvement of specific neurotransmitter systems (Rocha et al., 2008).
Age-dependent effects of lead exposure also apply to opiates suggesting interactions between the heavy metal and the opiate system. Specifically, exposure to lead during adulthood produces reduced locomotor activity in response to morphine while perinatal lead exposure produces an enhanced locomotor response (Miller et al., 2000), a downward vertical shift in a heroin dose-effect curve, and lower break points for heroin self-administration in a progressive ratio task (Rocha et al., 2004). A decreased sensitivity to morphine following lead exposure could contribute to use of the drug in larger quantities to get the same effect. Lead effects are age-dependent and the directional effects are drug-class dependent.
Studying lead effects on the responses to psychoactive drugs provides insights into the mechanisms of action of the heavy metal by pointing toward neurotransmitters and receptors that may be affected. Learning how lead modifies neurochemical systems that underlie the effects of psychoactive drugs has implications for substance abuse, addiction, and psychiatric and neurological disorders (Braun et al. 2008; Byers & Lord, 1943; Canfield et al., 2003; Cecil et al., 2008; Coon et al., 2006; Cory-Slechta et al., 1997; Jones & Miller, 2008; Jusko et al., 2008; Lanphear et al., 2000; Nation et al., 2004; Needleman et al., 1990; Reuben, et al., 2017; Rocha et al., 2005; van Wijngaarden et al., 2009; 2011; Weisskopf et al., 2010).
3.3. Environmental Effects in Animals
Research in animal models has revealed that environmental factors profoundly impact the severity of lead neurotoxicity. For example, Schneider et al. (2001) observed that an enriched environment consisting of playmates or novel objects could mitigate lead-induced deficits. Immediately after weaning, rat pups were put in either impoverished or enriched environments. Lead-exposed rats raised in an enriched environment performed similarly to control animals not exposed to lead, whereas lead-exposed animals reared in impoverished environments showed decreases in spatial ability in a Morris water maze relative to controls. Although the levels of lead in blood were relatively high (average: 26–34 μg/dL), the impact of environmental manipulation was striking. Similarly, Guilarte et al., (2003) found that lead-induced deficits were reversed by an enriched environment. Lead-exposed animals that were raised in isolation showed disruption of spatial learning and altered NMDA receptor gene expression, while those that were raised under social and environmental enrichment did not (average: 31.9 μg/dL blood lead). The translational implications of these studies are substantial, suggesting that even when lead burdens are elevated, environmental manipulations are enough to either mitigate or exacerbate lead-induced learning deficits (Schneider et al., 2001).
3.4. Summary
Low blood lead levels produce cognitive and behavioral effects in animal models; however, animal studies examining blood lead levels below 5 μg/dL are limited. To our knowledge, only three animal studies have been rigorously conducted and explicitly report blood lead levels below 5 μg/dL (Sobin et al., 2017; Verma & Schneider, 2017; Wang et al., 2012). Each of these studies found deficits. Other studies claim to use low levels of lead but include methods that initially introduce high peak levels of lead to dams and then study the offspring, expose animals to high peak levels of lead and then take blood samples after blood lead levels decline, or report deficits when comparing doses of lead below 5 μg/dL to high doses of lead, instead of comparing them to a control group with no detectable levels of lead. In other studies, blood lead levels are not measured at critical points during testing but are taken at the end of testing, thus missing the peak and steady state levels. In yet other studies, bone, dentin, or brain lead levels are reported but blood lead levels are absent, thus making comparisons difficult between the more traditional studies that report blood lead levels. This is further a problem because blood lead levels are a measure of current lead exposure and are not necessarily associated with bone lead levels that are a measure of lifetime lead exposure. In some instances, no lead levels are reported after a lead exposure regimen. Previously reported blood lead levels using similar dosing regimens are sometimes used to estimate blood lead levels for animals in the current study. Of note, thirty years ago it was common for control groups in animal studies to have blood lead levels as high as 3 μg/dL (Gilbert & Rice, 1987; Rice & Gilbert, 1985). These are levels that we now suspect produce cognitive and behavioral deficits. It is essential that animal studies examine lower blood lead levels in a highly controlled manner in order to better inform human findings and to better understand the threshold range for effects on the brain and behavior.
4. Mechanisms of Action: Calcium Dynamics
The mechanisms by which lead disrupts the brain and behavior are complex and poorly understood. Nonetheless, cellular and molecular work has resulted in a growing understanding of the effects of lead on brain function. Of particular importance are the effects of lead on calcium-dependent cellular processes. Calcium is a critical ion in neuronal function, including cell growth and differentiation, neurotransmitter release, and intracellular biochemical cascades.
Lead and calcium are divalent cations of similar size and ionic charge. The ability of lead to mimic or inhibit calcium-mediated effects is central to its biological and behavioral effects. Unlike calcium, which is a highly regulated ligand in the body, lead is an unregulated heavy metal. Lead binds to sites at which calcium acts and enters the cell through calcium channels, thus displacing, inhibiting, substituting, and/or activating calcium-dependent processes (Bridges & Zalups, 2005; Habermann et al., 1983; Kerper & Hinkle, 1997; Pounds, 1984). Given the ubiquity of calcium in cellular signaling, and the critical role of the spatial and temporal patterning of calcium signals in cell function, disruption of calcium-dependent processes can have profound cellular consequences (Berridge et al., 2000; 2003; Bootman, 2012; Bootman et al., 2001; 2002; Florea, et al., 2013). The effects of lead on neuronal calcium dynamics help to explain many far-reaching changes in brain function and behavior.
Below are short descriptions of some of the more well-known cellular actions of lead, including effects on NMDA receptors, presynaptic intracellular signaling proteins, mitochondria, and non-neuronal brain cells (for a more complete summary of targets involved in lead toxicity see CDC, 2005).
4.1. NMDA Receptors
Lead is a non-competitive, N-Methyl-D-aspartate receptor (NMDA-R) antagonist. NMDA-Rs are ionotropic receptors that are activated by the neurotransmitter glutamate, and are involved in many processes, including neural development, neuronal plasticity, learning and memory, and long-term potentiation (a physiological correlate of learning) (Cohn & Cory-Slechta, 1994; Cory-Slechta et al., 1997; Gilbert & Lasley, 2007; Hori et al., 1993; Hubbs-Tait et al., 2005; Nihei & Guilarte, 2001). Activation of NMDA-Rs by glutamate produces an influx of calcium through a ligand-gated ion channel, which can produce an excitatory post-synaptic potential, as well as strongly influence neuronal function by activating calcium-dependent second messenger cascades.
By blocking postsynaptic NMDA-Rs, lead inhibits activity-dependent calcium influx, which in turn can disrupt NMDA receptor-dependent developmental processes, neural plasticity, learning and memory, and long-term potentiation (LTP). Chronic, developmental lead exposure increases the threshold for induction of LTP at a wide range of lead concentrations and this is associated with impaired learning and memory (Cohn et al., 1993; Jett & Guilarte, 1995; Lasley & Gilbert, 2000; 2002; Lasley et al., 2001; Luo et al., 2011; Ma et al., 1997; Nihei & Guilarte, 2001; Ruan et al., 1998). Disruption of LTP and learning may be related to NMDA receptor blockade or other downstream effects of lead on calcium-dependent processes (Hori et al., 1993; Hussain et al., 2000).
Another consequence of NMDA receptor blockade is apoptosis, programmed cell death produced by a well-characterized biochemical cascade that leads to disruptions in normal brain development (Anastasio et al., 2009; Hansen et al., 2004; Ikonomidou et al., 1999; Léveillé et al., 2010; Lyall et al., 2009; Yuede et al., 2010). Apoptosis is normally involved in pruning unneeded connections and ‘sculpting’ the brain during development. However, under certain conditions pathological apoptosis can occur. Developmental exposure to lead has also been found to produce apoptosis and disrupt brain development at low concentrations in both mammalian and zebrafish models via blockade of NMDA receptors (Dou & Zhang, 2011; Dribben et al, 2011; Liu et al, 2010).
Given the critical role of NMDA receptors in a variety of neural and behavioral processes, and the ability of lead to block NMDA receptors, these receptors are essential to a complete understanding of the effects of lead on the brain and behavior.
4.2. Calmodulin
Another target of lead is calmodulin (CaM), or “calcium-modulated protein,” a major calcium-activated intracellular protein (Heizmann & Hunziker, 1991). Calmodulin is important in many neuronal processes, including transduction of calcium signaling, regulation of neurotransmitter receptors and ion channels, and neuronal plasticity (McCue et al., 2010; Sandhir & Gill, 1994). Calmodulin has four binding sites at which calcium is the natural ligand. When calcium is bound at all four sites, calmodulin is functionally active (Costa, 1998).
At physiologically-relevant levels, lead binds with greater affinity than calcium to calmodulin and activates the protein (Fullmer et al., 1985; Haberman et al., 1983; Sandhir & Gill, 1994; Shirran & Barran, 2009). When this occurs, calmodulin is activated in a non-physiological manner. Calmodulin signaling becomes tonically activated and stimulus-independent. Given the broad role of calmodulin in calcium signaling, unregulated calmodulin activation can have many consequences, ranging from disruption of calmodulin-dependent signal transduction to interference with calmodulin-dependent learning and memory (Goldstein, 1993; Goldstein & Ar, 1983; Habermann et al., 1983; Sandhir & Gill, 1994).
4.3. Protein Kinase C
Protein Kinase C (PKC) is a calcium- and phospholipid-dependent, intracellular signaling enzyme that is involved in a variety of cellular functions (Markovac & Goldstein, 1988). PKC phosphorylates proteins via the transfer of phosphate from ATP. PKC-mediated phosphorylation of transport proteins is important for the regulation of cellular growth and differentiation. PKC is also implicated in cytoskeletal function and signal transduction (Pears, 1995), and plays a role in learning and memory (Van der Zee et al., 1992; Xu et al., 2014).
At a clinically-relevant, picomolar concentration, lead substitutes for calcium in the activation of PKC, increasing intracellular calcium and interfering with neurotransmitter release (Goldstein, 1993). Specifically, at the synaptotagmin site, lead mimics and competes for calcium and does so with greater affinity than calcium (Bouton et al., 2001). Prolonged lead-induced increases of PKC activity produce a compensatory decrease in activity perhaps by downregulation or decreased efficacy of calcium activity (EPA, 2006). PKC is important for calcium-mediated LTP; in fact, PKC inhibitors, such as polymyxin B block the induction and maintenance of calcium-induced LTP (Cheng et al., 1994). Lead-induced impairment of learning and memory processes is thought to be due, at least in part, to disruption of normal PKC functioning. In addition, lead effects on PKC activity impact cell division, neuronal communication, neural plasticity, and structural organization of the cytoskeleton (Bressler et al., 1999; EPA, 2006), as well as cellular proliferation and differentiation (Markovac & Goldstein, 1988).
4.4. Neurotransmitter Release
Under normal circumstances, depolarization of neurons leads to the opening of voltage-gated calcium channels, which allows for influx of calcium into the presynaptic terminal. Calcium then activates a cascade of enzymes, which promotes fusion of the synaptic vesicle to the cell membrane and neurotransmitter release. Lead has converging effects on neurotransmitter release, binding to voltage-gated calcium channels and reducing the influx of calcium. In addition, lead competes with calcium for its binding sites on multiple proteins involved in neurotransmitter release, including calmodulin, CaM kinase II (CaMKII), and synaptotagmin (Bouton et al., 2001; Kern et al., 2000; Westerink et al., 2002). Together, these actions result in reduced neurotransmitter release at the presynaptic terminal. For example, nanomolar concentrations of lead have been found to inhibit neuronal release of glutamate and GABA (Braga et al, 1999). Disruption of normal neurotransmitter release can have a variety of consequences for the brain and behavior, depending on the specific neurotransmitter and its location in the brain.
4.5. Mitochondria
Mitochondria are organelles responsible for cellular respiration and energy production and, thus, are known as the powerhouses of eukaryotic cells. In addition to other functions, mitochondria store unbound calcium that regulates essential cellular activities such as cellular differentiation, neurogenesis, apoptosis, and signaling. Mitochondria can be found in dendrites and axon terminals where they are associated with the synthesis, storage, release, and reuptake of neurotransmitters.
Lead accumulates in mitochondria leading to oxidative stress and degradation of energy metabolism (Lidsky & Schneider, 2003; Silbergeld et al., 1980). Mitochondrial efflux of both calcium and lead occurs through a calcium uniporter (Chavez et al., 1987; Kamer & Mootha, 2015; Pounds, 1984). Lead competitively inhibits the energy-dependent intake of calcium into the mitochondrial matrix at the calcium uniporter, depleting necessary mitochondrial calcium stores (Galeotti et al., 1983; Goldstein, 1977; Holtzman et al, 1978; Parr & Harris 1976; Pounds, 1984). Depletion of mitochondrial calcium stores is implicated in lead-induced apoptosis (Kapoor & van Rossum, 1984) and excitotoxicity (Beal et al., 1993; Lidsky & Schneider, 2003) as well as oxidation of pyridine nucleotides, and a decay in membrane potential (Chavez et al., 1987, Kapoor & van Rossum, 1984). Lead depletion of mitochondria-generated ATP energy metabolism also contributes to disruption of neuronal function (Rafalowska et al., 1996).
4.6. Non-Neuronal CNS Targets
Although much of the research on lead neurotoxicity has focused on neuronal cells, attention has also been given to non-neuronal brain cells. Glial cells, for example, help protect the central nervous system against toxicants and provide support to neurons.
Astroglia are a type of glial cell that provide neurons with physical structure, necessary nutrients, chemicals, and clear debris. Astroglia sequester lead, exerting neuroprotectant effects in the short term (Holtzman et al., 1987; Tiffany-Castiglioni et al., 1986; 1989). However, after chronic or elevated blood lead burdens, astroglia gradually release sequestered lead, contributing to the neuronal effects of lead. The precise mechanism by which lead accumulates in astroglia is not completely understood; however, the transport site shares properties akin to an anion-dependent transporter and a divalent metal transporter 1 (DMT1) (Cheong et al., 2004). The mode of entry may include voltage-dependent calcium channels that are activated by depletion of intracellular calcium stores (Kerper & Hinkle, 1997). Via PKC activity, lead inhibits astroglia-induced microvessel formation and function that increases the permeability of the blood brain barrier to toxicants, particularly in the developing fetus and in young children. The increased absorption of toxicants into the brain increases the vulnerability to neurobehavioral deficits (Laterra et al., 1992).
Oligodendroglia are another type of glial cell. These are responsible for the production and maintenance of a protective myelin sheath that insulates axons (Tiffany-Castiglioni, 1993; Tiffany-Castiglioni et al., 1986). Oligodendroglia are believed to be the most lead-sensitive cell type in the brain (Tiffany-Castiglioni, 1993; Tiffany-Castiglioni et al., 1986). Lead can cause either hypomyelination or demyelination depending on concentration (Goyer, 1993; Krigman et al, 1974; Tennekoon et al., 1979; Toews et al., 1983). Lead produces a delay in the differentiation of oligodendroglia that may indirectly cause myelin damage, possibly as a result of impaired axonal growth and survival (Deng et al., 2001; Laterra et al., 1992; Tennekoon et al., 1979). Lead also interrupts the developmental maturation of oligodendrocytes by interfering with galactolipid metabolic enzymes (Deng et al., 2001). In addition, oligodendrocyte progenitors are vulnerable to low levels of lead exposure, while mature oligodendroglia are relatively resistant, suggesting a complex interaction (Deng et al., 2001).
4.7. Summary
Considerable progress has been made on the cellular mechanisms that mediate lead neurotoxicity and the resulting consequences for neuropathology, psychopathology, and behavior. However, many pioneering studies on the mechanisms of action of lead explored relatively high lead concentrations in vitro, but did not examine lower concentrations. It is possible that low levels of lead target a narrower range of cellular processes than higher levels. It would therefore be revealing to examine the targets of lead in vitro at concentrations that reflect blood levels below 5 μg/dL in vivo. It is possible that at these lower concentrations a clearer picture of the targets responsible for problems that arise from low-level lead exposure will be revealed.
5. Conclusions
Research on humans and in animal models has yet to determine a level of lead that is without neurobehavioral consequences. The CDC set 10 μg/dL as the reference level in 1991 and this was recently reduced to 5 μg/dL (ATSDR, 2017; CDC, 2005; 2017; 2018; Gilbert & Weiss, 2006; National Research Council, 1993). The reference level is now recommended to decrease to 3.5 μg/dL based on the most current NHANES data (Tsoi et al., 2016). However, levels as low as 2.5 μg/dL have been found to produce diminished cognitive function and maladaptive behavior in humans. Attention deficit hyperactivity disorder, Alzheimer’s disease, Parkinson’s disease and even criminal behavior have been associated with lead exposure. Of concern, there is an unequal lead burden placed on low-income and minority groups, contributing to income- and race-based health disparities. While the number of children with blood lead levels above the reference level has declined over the years following bans on leaded gasoline and leaded paint, considerable exposure continues to occur due to lead remaining in the environment from centuries of production and continued mining. Once lead is in the environment it does not dissipate.
The compilation of scientific research presented in this review argues that significant cognitive and behavioral consequences are evident at what previously were considered very low levels of lead. More controlled studies on low levels of lead would improve our understanding of the neurobehavioral consequences and mechanisms of action at these lower levels. Current human research has few prospective, longitudinal designs with lead measurements taken at critical points in development to provide us with an understanding of how early lead exposure could produce long-term effects on neuropsychological tests, health, and other outcomes. Studies on lead would also benefit from taking bone lead levels, in addition to blood lead levels. Bone lead levels are normally reserved for the elderly population, but may reveal important information about the lead burden across all ages because of its longer half-life (8 to 30 years, depending on bone type) compared to the half-life of lead in blood (30-days).
Likewise, more research using highly controlled animal studies is needed to better understand the neurobehavioral consequences and mechanisms of action of lower levels of lead. The current animal research has not adequately studied the impact of very low levels of lead. As with human studies, many animal studies rely on regression analyses across a range of blood lead concentrations that include high levels of lead. As an alternative, studies that compare low levels of lead directly with controls would help provide a threshold of effect for lead-induced deficits. Also, much of the animal work on lead is missing steady state or peak lead levels and often includes only one measurement at the end of the study. Some studies fail to report lead measurements, relying on previous lead levels reported by their group given similar dosing regimes. As with the clinical population, animal models will be improved by testing bone lead levels at critical points due to the longer half-life compared to blood lead levels.
With regard to policy implications, a clear understanding of the threshold for lead neurotoxicity would be a significant benefit to human health and well-being. Further research would allow for evidence-based guidance for health officials and federal agencies. This, in turn, would promote increased health and well-being by raising consumer awareness, improving environmental safety practices, and optimizing efforts to protect populations who are most vulnerable.
Highlights (to be submitted as a separate document):
Lead is a neurotoxin with a long history of use by humans.
Effects of low-level lead exposure on behavior and cognition are summarized.
Mechanisms of action for lead include neuronal and non-neuronal CNS targets.
The present level of concern set by the CDC for lead in blood is 5 μg/dL.
Lead levels below 3 μg/dL produce maladaptive behavior and cognitive deficits.
Acknowledgments
Preparation of this review was supported by the National Institutes of Health [NIGMS- 08807 and 64783] and the National Science Foundation [HRD-1302873]. We would like to thank Carlos Gonzalez for early discussions on this work and Veronika Espinoza for editorial assistance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
Footnotes
Inclusion and exclusion criteria for tables: Tables 2–6 summarize some key studies in the field. A Pubmed search was conducted using the phrase “low-level lead exposure.” Studies were included in the tables if they were performed in the United States and examined blood lead levels <10 μg/dL for humans, <15 μg/dL for rodents and <20 μg/dL for monkeys. Representative studies were included in cases where one author or group of authors conducted multiple studies using a similar paradigm over the years. Only outcomes for doses that yielded blood lead levels in the corresponding ranges were included (i.e. studies were excluded if regression analyses included blood lead levels outside of the set limits). Studies were excluded if blood lead levels were not explicitly reported. Studies were also excluded if peak levels or steady state levels were above the range indicated for each respective table. In human work, the most conservative statistical outcomes for each study were reported after adjusting for possible covariates such as sex, age, race, birth weight, iron status, maternal age at child’s birth, maternal education level, primary caregiver’s IQ, household income, medication used in the previous month, and other variables.
References
- Alber SA, & Strupp BJ (1996). An in-depth analysis of lead effects in a delayed spatial alternation task: Assessment of mnemonic effects, side bias, and proactive interference. Neurotoxicol Teratol, 18: 3–15. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Xia Y, O’Connor ZR, & Johnson KM (2009). Differential role of the N-methyl-D-aspartate receptor subunits 2A and 2B in mediating phencyclidine-induced perinatal neuronal apoptosis and behavioral deficits. Neuroscience, 163(4): 1181–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Areola OO, & Jadhav AL (2001). Low-level lead exposure modulates effects of quinpirole and eticlopride on response rates in a fixed-interval schedule. Pharmacol Biochem Behav, 69(1–2):151–6. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). (2007). Toxicological Profile for Lead. (p. 582). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service; Retrieved from http://www.atsdr.cdc.gov/toxprofiles/tp13.pdf. last accessed: January 2019. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). (2017). Lead toxicity: What are U.S. standards for lead levels? Retrieved from https://www.atsdr.cdc.gov/csem/csem.asp?csem=34&po=8. last accessed: January 2019.
- Barry PSI & Mossman DB (1970). Lead concentrations in human tissues. Br J Ind Med, 27(4): 339–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basha R, & Reddy GR (2010). Developmental exposure to lead and late life abnormalities of nervous system. Indian J Exp Biol, 48(7): 636–41. [PubMed] [Google Scholar]
- Basha MR, Wei W, Bakheet SA, Benitez N, Siddiqi HK, Ge YW, Lahiri DK, & Zawia NH (2005). The fetal basis of amyloidogenesis: Exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J Neurosci, 25(4): 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, Miller JM, Storey E, Srivastava R, Rosen BR, & Hyman BT (1993). Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci, 13(10): 4181–4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, & Waternaux C (1991). Low-level exposure and children’s cognitive function in the preschool years. Pediatrics, 87(2) 219–227. [PubMed] [Google Scholar]
- Bellinger DC, Stiles KM, & Needleman HL (1992). Low-level lead exposure, intelligence and academic achievement: A long-term follow-up study. Pediatrics, 90(6): 855–861. [PubMed] [Google Scholar]
- Bernard SM, & McGeehin MA (2003). Prevalence of blood lead levels > 5 μg/dL among U.S. children 1 to 5 years of age and socioeconomic and demographic factors associated with blood of lead levels 5 to 10 μg/dL, third National Health and Nutrition Examination Survey, 1988 – 1994. Pediatrics, 112(6): 1308–1313. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, & Roderick HL (2003). Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol, 4(7): 517–529. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, & Bootman MD (2000). The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol, 1(1): 11–21. [DOI] [PubMed] [Google Scholar]
- Bochynska K (2013). Facts and firsts of lead. The Lead Group Inc; Retrived from: http://www.lead.org.au/fs/fst29.html. last accessed: January 2019. [Google Scholar]
- Bootman MD (2012). Calcium signaling. Cold Spring Harb Perspect Biol, 4(7):a011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ, & Roderick HL (2002). Calcium signalling: More messengers, more channels, more complexity. Curr Biol, 12(16): R563–R565. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Lipp P, & Berridge MJ (2001). The organisation and functions of local CA(2+) signals. J Cell Sci. 114(Pt 12): 2213–2222. [DOI] [PubMed] [Google Scholar]
- Bouton CM, Frelin LP, Forde CE, Arnold Godwin H, & Pevsner J (2001). Synaptotagmin I is a molecular target for lead. J Neurochem, 76(6): 1724–1735. [DOI] [PubMed] [Google Scholar]
- Braga MF, Pereira EF, & Albuquerque EX (1999). Nanomolar concentrations of lead inhibit glutamatergic and GABAergic transmission in hippocampal neurons. Brain Res, 826(1): 22–34. [DOI] [PubMed] [Google Scholar]
- Braun JM, Froehlich TE, Daniels JL, Dietrich KN, Hornung R, Auinger P, & Lanphear BP (2008). Association of environmental toxicants and conduct disorder in U.S. children: NHANES 2001–2004. Environ Health Perspect, 116(7): 956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler J, Kim K, Chakraborti T, & Goldstein G (1999). Molecular mechanisms of lead neurotoxicity. Neurochem Res, 24(4): 595–600. [DOI] [PubMed] [Google Scholar]
- Bridges CC, & Zalups RK (2005). Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol, 204(3): 274–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockel BJ, & Cory-Slechta DA (1998). Lead, attention, and impulsive behavior: Changes in a fixed-ratio waiting-for-reward paradigm. Pharmacol Biochem Behav, 60(2), 545–552. [DOI] [PubMed] [Google Scholar]
- Bulletin of the World Health Organization (2002), 80(1): 82 Lead, unsafe at any level. Retrieved from:http://www.who.int/bulletin/archives/80(1)82.pdf. last accessed: January 2019. [PMC free article] [PubMed] [Google Scholar]
- Burkey RT, Nation JR, Grover CA, Bratton GR, & Al BET (1997). Effects of chronic lead exposure on cocaine-induced disturbance of fixed-interval behavior. Pharmacol Biochem Behav, 56(1), 117–121. [DOI] [PubMed] [Google Scholar]
- Byers RK, & Lord EE (1943). Late effects of lead poisoning on mental development. Am J Dis Child. 66(5): 471–494. [Google Scholar]
- Canfield RL, Henderson CR Jr, Cory-Slechta DA, Cox C, Jusko TA, & Lanphear BP (2003). Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. N Engl J Med, 348(16), 1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (n.d.) Developing a healthy homes program. Retrieved from: https://www.cdc.gov/nceh/lead/publications/10_217029A_Walker_HealthyHomesBooklet_101310_updated_WithCovers.pdf. last accessed: January 2019.
- CDC (Centers for Disease Control and Prevention). (2005). Preventing lead poisoning in young children: A statement by the Centers for Disease Control and Prevention, Report No. 99–2230. Atlanta, GA: Retrieved from https://www.cdc.gov/nceh/lead/publications/prevleadpoisoning.pdf. last accessed: January 2019. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). (2010a). Guidelines for the identification and management of lead exposure in pregnant and lactating women. Atlanta, GA: Retrieved from http://www.cdc.gov/nceh/lead/publications/leadandpregnancy2010.pdf. last accessed: January 2019. [Google Scholar]
- CDC (Centers for Disease Control and Prevention). (2010b). CDC response to advisory committee on childhood lead poisoning prevention recommendation in “Low level lead exposure harms children: A renewed call of primary prevention.” Retrieved from: https://www.cdc.gov/nceh/lead/acclpp/cdc_response_lead_exposure_recs.pdf. last accessed: January 2019.
- CDC (Centers for Disease Control and Prevention). (2017). What do parents need to know to protect their children? Retrieved from http://www.cdc.gov/nceh/lead/ACCLPP/blood_lead_levels.htm. last accessed: January 2019.
- CDC (Centers for Disease Control and Prevention). (2018). The National Institue for Occupational Safety and Health (NIOSH). Adult blood lead epidemiology and surveillance (ABLES) https://www.cdc.gov/niosh/topics/ables/description.html. last accessed: January 2019. [Google Scholar]
- Cecil KM, Brubaker CJ, Adler CM, Dietrich KN, Altaye M, Egelhoff JC, Wessel S, Elangovan I, Hornung R, Jarvis K, & Lanphear BP (2008). Decreased brain volume in adults with childhood lead exposure. PLoS Med, 5(5): e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez E, Jay D, & Bravo C (1987). The mechanism of lead-induced mitochondrial Ca2+ efflux. J Bioenerg Biomembr, 19(3): 285–295. [DOI] [PubMed] [Google Scholar]
- Cheng G, Rong XW, & Feng TP (1994). Block of induction and maintenance of calcium-induced LTP by inhibition of protein kinase C in postsynaptic neuron in hippocampal CA1 region. Brain Res, 646(2): 230–234. [DOI] [PubMed] [Google Scholar]
- Cheong JH, Bannon D, Olivi L, Kim Y, & Bressler J (2004). Different mechanisms mediate uptake of lead in a rat astroglial cell line. Toxicol Sci, 77(2), 334–40. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Jacobson SW, & Jacobson JL (2004). Neurodevelopmental effects of postnatal lead exposure at very low levels. 26(3): 359–371. [DOI] [PubMed] [Google Scholar]
- Chiodo LM, Covington C, Sokol RJ, Hannigan JH, Jannise J, Ager J, Greenwald M, & Delaney-Black V (2007). Blood lead levels and specific attention effects in young children. Neurotoxicol Teratol, 29(5): 538–546. [DOI] [PubMed] [Google Scholar]
- Cohn J, & Cory-Slechta DA (1994). Lead exposure potentiates the effects of NMDA on repeated learning. Neurotox Teratol, 16(5): 455–465. [DOI] [PubMed] [Google Scholar]
- Cohn J, Cox C, & Cory-Slechta DA (1993). The effects of lead exposure on learning in a multiple repeated acquisition and performance schedule. NeuroToxicology, 14(2–3), 329–346. [PubMed] [Google Scholar]
- Coon S, Stark A, Peterson E, Gloi A, Kortsha G, Pounds J, Chettle D, & Gorell J (2006). Whole-body lifetime occupational lead exposure and risk of Parkinson’s disease. Environ Health Perspect, 114(12): 1872–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA (1997). Relationships between Pb-induced changes in neurotransmitter system function and behavioral toxicity. NeuroToxicology, 18(3): 673–688. [PubMed] [Google Scholar]
- Cory-Slechta DA, McCoy L, & Richfield EK (1997). Time course and regional basis of Pb-induced changes in MK-801 binding: Reversal by chronic treatment with the dopamine agonist apomorphine but not the D1 agonist SKF-82958. J Neurochem, 68(5): 2012–23. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, O’Mara DJ, & Brockel BJ (1998). Nucleus accumbens dopaminergic medication of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J Pharmacol Exp Ther, 286: 794–805. [PubMed] [Google Scholar]
- Cory-Slechta DA, & Thompson T (1979). Behavioral toxicity of chronic postweaning lead exposure in the rat. Toxicol Appl Pharmacol, 47(1): 151–159. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Weiss B, & Cox C (1983). Delayed behavioral toxicity of lead with increasing exposure concentration. Toxicol Appl Pharmacol, 71: 342–352. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Weiss B, & Cox C (1985). Performance and exposure indices of rats exposed to low concentrations of lead. Toxicol Appl Pharmacol, 78 (2) 291–299. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, & Widzowski DV (1991). Low level lead exposure increases sensititivity to the stimulus properties of dopamine D1 and D2 agonists. Brain Res, 553(1): 65–74. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta DA, Widzowski DV, & Pokora MJ (1993). Functional alterations in dopamine systems assessed using drug discrimination procedures. NeuroToxicology, 14(2–3): 105–114. [PubMed] [Google Scholar]
- Costa LG (1998). Signal transduction in environmental neurotoxicity. Annu Rev Pharmacol Toxicol, 38: 21–43. [DOI] [PubMed] [Google Scholar]
- CPSC (U.S. Consumer Product Safety Commission) (June 5, 2009). Mattel, Fisher-Price to pay $2.3 million civil penalty for violating federal lead paint ban, penalty is highest ever for CPSC regulated product violations. https://www.cpsc.gov/content/mattel-fisher-price-to-pay-23-million-civil-penalty-for-violating-federal-lead-paint-ban. last accessed: January 2019.
- CPSIA (Consumer Product Safety Improvement Act) (2012). Section 101(b): Functional purpose exception from lead content limit for children’s products for a specific product, class of product, material, or component part. https://www.cpsc.gov/s3fs-public/lead101.pdf. last accessed: January 2019.
- Davis MJ, Otto DA, Weil DE, & Grant LD (1990). The comparative developmental neurotoxicity of lead in humans and animals. Neurotoxicol Teratology, 12(3): 215–229. [DOI] [PubMed] [Google Scholar]
- Deng W, McKinnon RD, & Poretz RD (2001) Lead exposure delays the differentiation of oligodendroglia progenitors in vitro. Toxicol Appl Pharmacol, 174(3): 235–244. [DOI] [PubMed] [Google Scholar]
- Denno DJ (1984). Neuropsychological and early environmental correlates of sex differences in crime. Int J Neurosci, 23(3): 199–213. [DOI] [PubMed] [Google Scholar]
- Department of Justice. (2012). Serious Violent Crime Against Youth Dropped 7 Percent from 1994 to 2010. https://www.bjs.gov/content/pub/press/vcay9410pr.cfm. last accessed: January 2019.
- Després C, Beuter A, François R, Poitras K, Veilleux A, Ayotte P, Dewailly É, Saint-Amour D, & Muckle G (2005). Neuromotor functions in Inuit preschool children exposed to Pb, PCBs, and Hg. Neurotoxicol Teratol, 27: 245–257. [DOI] [PubMed] [Google Scholar]
- Detroit Department of Health and Wellness Promotion (2005). Indicator: Lead poisoning in Detroit, Michigan. University of Windsor; Retrieved from: http://cronus.uwindsor.ca/units/stateofthestraight/softs.nsf/54ef3e94e5fe816e85256d6e0063d208/4506079a8efac1ca852573a3005000fa/$FILE/lead.pdf. last accessed: January 2019. [Google Scholar]
- DeWitt RD (2017). Pediatric lead exposure and the water crisis in Flint, Michigan. J Am Acad Phys, 30(2): 43–46. [DOI] [PubMed] [Google Scholar]
- Dietrich KN, Berger OG, & Succop PA (1993). Lead exposure and the motor developmental status of urban six-year-old children in the Cincinnati Prospective Study, 91(2): 301–307. [PubMed] [Google Scholar]
- Dietrich KN, Ris MD, Succop PA, Berger OG, & Bornschein RL (2001). Early exposure to lead and juvenile delinquency. Neurotoxicol Teratol, 23(6): 511–518. [DOI] [PubMed] [Google Scholar]
- Dou C, & Zhang J (2011). Effects of lead on neurogenesis during zebrafish embryonic brain development. J Hazard Mater, 194: 277–282. [DOI] [PubMed] [Google Scholar]
- Dribben WH, Creeley CE, & Farber N (2011). Low-level lead exposure triggers neuronal apoptosis in the developing mouse brain. Neurotoxicol Teratol, 33(4): 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA (n.d.). Laws and regulations. Retrieved from: https://www.epa.gov/laws-regulations/summary-toxic-substances-control-act. last accessed: January 2019.
- EPA (n.d.). Use of lead free pipes, fittings, fixtures, solder and flux for drinking water. Retrieved from: https://www.epa.gov/dwstandardsregulations/use-lead-free-pipes-fittings-fixtures-solder-and-flux-drinking-water. last accessed: January 2019.
- EPA (1996). EPA takes final step in phaseout of leaded gasoline. Retrieved from: https://archive.epa.gov/epa/aboutepa/epa-takes-final-step-phaseout-leaded-gasoline.html. last accessed: January 2019.
- EPA (Environmental Protection Agency) (2006). Air Quality Criteria for Lead Volume I of II. Research Triangle, Park, N.C. Retrieved from: https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=158823. last accessed: January 2019. [Google Scholar]
- Farooqui Z, Bakulski KM, Power MC, Weisskopf MG, Sparrow D, Spiro A III, Vokonas PS, Nie LH, Hu H FBI. (2011). FBI Releases 2010 Crime Statistics. Retrieved from: https://archives.fbi.gov/archives/news/pressrel/press-releases/fbi-releases-2010-crime-statistics. last accessed: January 2019.
- Federal Register: The daily journal of the United States government. (2001). Lead; Identification of dangerous levels of lead: A rule by the Environmental Protection Agency on 01/05/2001. Retrieved from: www.federalregister.gov/documents/2001/01/05/01-84/lead-identification-of-dangerous-levels-of-lead. last accessed: January 2019.
- Florea AM, Taban J, Varghese E, Alost BT, Moreno S, & Buselberg D (2013). Lead (Pb2+) neurotoxicity: Ion-mimicry with calcium (Ca 2+) impairs synaptic transmission. A review with animated illustrations of the pre- and post-synaptic effects of lead. J Loc Glob Health Sci, 4 Retrieved from: http://www.qscience.com/doi/abs/10.5339/jlghs.2013.4. last accessed: January 2019. [Google Scholar]
- Flores-Montoya MG, & Sobin C (2015). Early chronic lead exposure reduces exploratory activity in young C57BL/6J mice. J Appl Toxicol, 35(7): 759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich TE, Lanphear BP, Auinger P, Hornung R, Epstein JN, Braun J, & Kahn RS (2009). Association of tobacco and lead exposures with attention-deficit/hyperactivity disorder. Pediatrics, 124(6): e1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullmer CS, Edelstein S, & Wasserman RH (1985). Lead-binding properties of intestinal calcium-binding proteins. J Biol Chem, 260(11): 6816–6819. [PubMed] [Google Scholar]
- Galeotti T, Eboli ML, Palombini G, van Rossum GD, & Kapoor SC (1983). Inhibition of mitochondrial oxidative metabolism by SKF-525A in intact cells and isolated mitochondria. Biochem Pharmacol, 32(22): 3285–3295. [DOI] [PubMed] [Google Scholar]
- Gardella C (2001). Lead exposure in pregnancy: A review of the literature and argument for routine prenatal screening. Obstet Gynecol Surv, 56(4): 231–238. [DOI] [PubMed] [Google Scholar]
- Gibson JL (1904). A plea for painted railings and painted walls of rooms as the source of lead poisoning amongst Queensland children. 1904. Public Health Rep, 120(3): 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert ME, & Lasley SM (2007). Developmental lead (Pb) exposure reduces the ability of the NMDA antagonist MK-801 to suppress long-term potentiation (LTP) in the rat dentate gyrus, in vivo. Neurotoxicol Teratol, 29(3): 385–393. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, & Rice DC (1987). Low-level lifetime lead exposure produces behavioral toxicity (spatial discrimination reversal) in adult monkeys, Toxicol Appl Pharmacol, 91: 484–490. [DOI] [PubMed] [Google Scholar]
- Gilbert SG, & Weiss B (2006). A rationale for lowering the blood lead action level from 10 to 2 μg/dL. NeuroToxicology, 27(5): 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan SC (1965). Lead poisoning and the fall of rome. J Occup Med, 7(2): 53–60. [PubMed] [Google Scholar]
- Ginsberg G, Slikker W Jr., Bruckner J, & Sonawane B (2004). Incorporating children’s toxicokinetics into a risk framework. Environ Health Perspect, 112(2): 272–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass TA, Bandeen-Roche K, McAtee M, Bolla K, Todd AC, & Schwartz BS (2009). Neighborhood psychosocial hazards and the association of cumulative lead dose with cognitive function in older adults. Am J Epidemiol, 169(6): 683–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein GW (1977). Lead encephalopathy: The significance of lead inhibition of calcium uptake by brain mitochondria. Brain Res, 136(1): 185–188. [DOI] [PubMed] [Google Scholar]
- Goldstein GW (1993). Evidence that lead acts as a calcium substitute in second messenger metabolism. NeuroToxicology, 14(2–3): 97–101. [PubMed] [Google Scholar]
- Goldstein GW, & Ar D (1983). Lead activates calmodulin sensitive processes. Life Sci, 33(10): 1001–1006. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Kortsha GX, Brown GG, & Richardson RJ (1999). Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s Disease. NeuroToxicology, 20(2–3): 239–248. [PubMed] [Google Scholar]
- Gorell JM, Peterson EL, Rybicki BA, & Johnson CC (2004). Multiple risk factors for Parkinson’s disease. J Neurol Sci, 217(2): 169–174. [DOI] [PubMed] [Google Scholar]
- Goyer RA (1993). Lead toxicity: Current concerns. Environ Health Perspect, 100: 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte TR, Toscano CD, McGlothan JL, & Weaver SA (2003). Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Ann Neurol, 53(1): 50–56. [DOI] [PubMed] [Google Scholar]
- Gulson BL, Jameson CW, Mahaffey KR, Mizon KJ, Korsch MJ, & Vimpani G (1997). Pregnancy increases mobilization of lead from maternal skeleton. J Lab Clin Med, 130(1): 51–62. [DOI] [PubMed] [Google Scholar]
- Ha M, Kwon HJ, Lim MH, Jee YK, Hong YC, Leem JH, Sakong J, Bae JM, Hong SJ, Roh YM, & Jo SJ (2009). Low blood levels of lead and mercury and symptoms of attention deficit hyperactivity in children: a report of the children’s health and environment research (CHEER). NeuroToxicology, 30(1): 31–36. [DOI] [PubMed] [Google Scholar]
- Habermann E, Crowell K, & Janicki P (1983). Lead and other metals can substitute for Ca2+ in calmodulin. Arch Toxicol, 54(1): 61–70. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Briem T, Dzietko M, Sifringer M, Voss A, Rzeski W, Zdzisinska B, Thor F, Heumann R, Stepulak A, Bittigau P, & Ikonomidou C (2004). Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol Dis, 16(2): 440–453. [DOI] [PubMed] [Google Scholar]
- Heizmann CW & Hunziker W (1991). Intracellular calcium-binding protetins: more sites than insights. Trends Biochm Sci, 16(3): 98–103. [DOI] [PubMed] [Google Scholar]
- Holtzman D, Shen HJ, & Mortell P (1978). In vitro effects of inorganic lead on isolated rat brain mitochondrial respiration. Neurochem Res, 3(2): 195–206. [DOI] [PubMed] [Google Scholar]
- Holtzman D, DeVries C, Nguyen H, Olson J, & Bensch K (1987). Maturation of resistance to lead encephalopathy: Cellular and subellular mechanisms. NeuroToxicology, 5(3) 97–124. [PubMed] [Google Scholar]
- Hori N, Büsselberg D, Mathews MR, Parsons PJ, & Carpenter DO (1993). Lead blocks LTP by an action not at NMDA receptors. Exp Neurol, 119(2): 192–197. [DOI] [PubMed] [Google Scholar]
- Hu H (1998). Bone lead as a new biological marker of lead dose: recent findings and implications for public health. Environ Health Perspect, 106(4): 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Milder FL, & Burger DE (1989). X-Ray flourescence: Issues surrounding the application of a new tool for measuring burden of lead. Environ Res, 49: 295–317. [DOI] [PubMed] [Google Scholar]
- Hu H, Milder FL, & Burger DE (1991). The use of K X-Ray fluorescence for measuring lead burden in epidemiological studies: High and low lead burdens and measurement uncertainty. Environ Health Perspect, 94:107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbs-Tait L, Nation JR, Krebs NF, & Bellinger DC (2005). Neurotoxicants, micronutrients, and social environments: Individual and combined effects on children’s development. Psychol Sci Public Interest, 6(3): 57–121. [DOI] [PubMed] [Google Scholar]
- HUD U.S. Department of Housing and Urban Development (n.d.). Legislative history of lead-based paint. https://www.hud.gov/sites/documents/20258_legislativehistory.pdf. last accessed: January 2019.
- HUD U.S. Department of Housing and Urban Development (2014). The HUD regulation on controlling lead-based paint hazards in housing receiving federal assistance and federally owned housing being sold (24 CFR Part 35). Retrieved from: https://www.hud.gov/sites/documents/DOC_25476.PDF. last accessed: January 2019.
- Hussain RJ, Parsons PJ, & Carpenter DO (2000). Effects of lead on long-term potentiation in hippocampal CA3 vary with age. Brain Res Dev Brain Res, 121(2): 243–252. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, & Olney JW (1999). Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science, 283(5398): 70–74. [DOI] [PubMed] [Google Scholar]
- Jaeok K, Bushway S, & Tsao HS (2016). Identifying classes of explanations for crime drop: Period and cohort effects for New York State. J Quant Criminol, 32(3): 357–375. [Google Scholar]
- Jett DA, & Guilarte TR (1995). Developmental lead exposure alters N-methyl-D-Aspartate and nuscarinic cholinergic receptors in the rat hippocampus: an autoradiographic study. NeuroToxicology, 16(1): 7–18. [PubMed] [Google Scholar]
- Jones DC, & Miller GW (2008). The effects of environmental neurotoxicants on the dopaminergic system: A possible role in drug addiction. Biochem Pharmacol, 76(5): 569–81. [DOI] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, & Parsons PJ (2008). Blood lead concentrations <10 μg/dL and child intelligence at 6 years of age. Environ Health Perspect, 116(2): 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamer KJ, & Mootha VK (2015). The molecular era of the mitochondrial calcium uniporter. Nat Rev Mol Cell Biol, 16(9): 545–553. [DOI] [PubMed] [Google Scholar]
- Kapoor SC & van Rossum GD (1984). Effects of Pb2+ added in vitro on Ca2+ movements in isolated mitochondria and slices of rat kidney cortex. Biochem Pharmacol, 33(11): 1771–1778. [DOI] [PubMed] [Google Scholar]
- Kern M, Wisniewski M, Cabell L, & Audesirk G (2000). Inorganic lead and calcium interact positively in activation of calmodulin. NeuroToxicology, 21(3): 353–364. [PubMed] [Google Scholar]
- Kerper LE, & Hinkle PM (1997). Cellular uptake of lead is activated by depletion of intracellular calcium stores. J Biol Chem, 272(13): 8346–8352. [DOI] [PubMed] [Google Scholar]
- Kessler R (2014). Lead-based decorative paints: Where are they still sold-- and why? Environ Health Perspect, 122(4): A96–A103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Landrigan C, Mossman P, Sparrow D, & Hu H (1997). Age and secular trends in bone lead levels in middle-aged and elderly men: Three-year longitudinal follow-up in the normative aging study. Am J Epidemiol, 146(7): 586–591. [DOI] [PubMed] [Google Scholar]
- Kitman JL (March 20, 2000). The secret history of lead. The Nation; https://www.thenation.com/article/secret-history-lead. last accessed: January 2019. [Google Scholar]
- Krigman MR, Druse MJ, Traylor D, Wilson MH, Newell LR, & Hogan EL (1974). Lead encephalopathy in the developing rat: Effect upon myelination. J Neuropathol Exp Neurol, 33(1): 58–73. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Dietrich K, Auinger P, & Cox C (2000). Cognitive deficits associated with blood lead concentrations <10 μg/dL in US children and adolescents. Public Health Rep, 115(6): 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Ho M, Howard CR, Eberle S, & Knauf K (2002). Environmental lead exposure during early childhood. J Pediatr, 140(1): 40–7. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, Canfield RL, Dietrich KN, Bornschein R, Greene T, Rothenberg SJ, Needleman HL Schnaas L, Wasserman G, Graziano J, & Roberts R (2005). Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect, 113(7): 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasley SM, & Gilbert ME (2000). Glutamatergic components underlying lead-induced impairments in hippocampal synaptic plasticity. NeuroToxicology, 21(6): 1057–1068. [PubMed] [Google Scholar]
- Lasley SM, & Gilbert ME (2002). Rat hippocampal glutamate and GABA release exhibit biphasic effects as a function of chronic lead exposure level. Toxicol Sci, 66(1): 139–47. [DOI] [PubMed] [Google Scholar]
- Lasley SM, Green MC, & Gilbert ME (2001). Rat hippocampal NMDA receptor binding as a function of chronic lead exposure level. Neurotoxicol Teratol, 23(2): 185–189. [DOI] [PubMed] [Google Scholar]
- Laterra J, Bressler JP, Indurti RR, Belloni-Olivi L, & Goldstein GW (1992). Inhibition of astroglia-induced endothelial differentiation by inorganic lead: A role for protein kinase C. Proc Natl Acad Sci U S A, 89(22): 10748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Giddabasappa A, Chaney S, Johnson JE, Pothakos K, Lau YS, & Fox DA (2008). Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ Health Perspect, 116(3): 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léveillé F, Papadia S, Fricker M, Bell KF, Soriano FX, Martel MA, Puddifoot C, Habel M, Wyllie DJ, Ikonomidou C, Tolkovsky AM, & Hardingham GE (2010). Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci, 30(7): 2623–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J (1985). Lead poisoning: A historical perspective. Environmental Protection Agency (EPA). 11(4): 15–18. Retrieved from: https://archive.epa.gov/epa/aboutepa/lead-poisoning-historical-perspective.html. last accessed: January 2019. [Google Scholar]
- Lidsky TI, & Schneider JS (2003). Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain, 126: 5–19. [DOI] [PubMed] [Google Scholar]
- Listos J, Baranowska-Bosiacka I, Talarek S, Listos P, Orzelska J, Fidecka S, Gutowska I, Kolasa A, Rybicka M, & Chlubek D (2013). The effect of perinatal lead exposure on dopamine receptor D2 expression in morphine dependent rats. Toxicology 310, 73–83. [DOI] [PubMed] [Google Scholar]
- Liu J, Han D, Li Y, Zheng L, Gu C, Piao Z, Au WW, Xu X, & Huo X (2010). Lead affects apoptosis and related gene XIAP and Smac expression in the hippocampus of developing rats. Neurochem Res, 35(3): 473–479. [DOI] [PubMed] [Google Scholar]
- Lovei M The world’s bank approach to removing lead from gasoline (1996). Retrieved from: https://www.oecd.org/env/ehs/risk-management/1944711.pdf. last accessed: January 2019.
- Luo YY, Zhu DM, & Ruan DY (2011). Galantamine rescues lead-impaired synaptic plasticity in rat dentate gyrus. Toxicology, 289(1): 45–51. [DOI] [PubMed] [Google Scholar]
- Lyall A, Swanson J, Liu C, Blumenthal TD, & Turner CP (2009). Neonatal exposure to MK801 promotes prepulse-induced delay in startle response time in adult rats. Exp Brain Res, 197(3): 215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Chen HH, Lim DK, Hume AS, & Ho IK (1997). Effects of subacute lead exposure on [3H]MK-801 binding in hippocampus and cerebral cortex in the adult rat. Brain Res, 760(1–2): 187–192. [DOI] [PubMed] [Google Scholar]
- Markovac J, & Goldstein GW (1988). Picomolar concentrations of lead stimulate brain protein kinase C. Nature, 334(6177): 71–73. [DOI] [PubMed] [Google Scholar]
- Markowitz G, & Rosner D (2000). Cater to the children: The role of the lead industry in a public health tragedy, 1900–1955. Am J Pub Health, 90(1): 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz G, & Rosner D (2003). Deceit and Denial: The Deadly Politics of Industrial Pollution. The University of California Press. [Google Scholar]
- McCue HV, Haynes LP, & Burgoyne RD (2010). The diversity of calcium sensor proteins in the regulation of neuronal function. Cold Spring Harb Perspect Biol, 2(8): a004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke HW, Gonzales CR, Smith MK, & Mielke PW (1999). The urban environment and children’s health: Soils as an integrator of lead, zinc, and cadmium in New Orleans Louisiana, U. S. A. Environ Res, 81(2): 117–129. [DOI] [PubMed] [Google Scholar]
- Miller DK, Nation JR, Jost TE, Schell JB, & Bratton GR (2000). Differential effects of adult and perinatal lead exposure on morphine-induced locomotor activity in rats. Pharmacol Biochem Behav, 67(2): 281–290. [DOI] [PubMed] [Google Scholar]
- Min JY, Min KB, Cho SI, Kim R, Sakong J, & Paek D (2007). Neurobehavioral function in children with low blood lead concentrations. NeuroT oxicology, 28(2): 421–5. [DOI] [PubMed] [Google Scholar]
- MMWR (Morbidity and Mortality Weekly Report- CDC). August 5, 1994. Blood lead levels—United States, 1988–1991. 43(30): 545–548. Retrieved from: https://www.cdc.gov/mmwr/preview/mmwrhtml/00032080.htm. last accessed: January 2019. [PubMed] [Google Scholar]
- MMWR (Morbidity and Mortality Weekly Report- CDC). August 10, 2012. Lead in drinking water and human blood lead levels in the United States. Suppl (6). Retrieved from: https://www.cdc.gov/mmwr/pdf/other/su6104.pdf. last accessed: January 2019. [Google Scholar]
- Nation JR, Livermore CL, & Burkey RT (1996). Chronic lead exposure attenuates sensitization to the locomotor-stimulating effects of cocaine. Drug Alcohol Depend, 41(2): 143–149. [DOI] [PubMed] [Google Scholar]
- Nation JR, Smith KR, & Bratton GR (2004). Early developmental lead exposure increases sensitivity to cocaine in a self-administration paradigm. Pharmacol Biochem Behav, 77(1): 127–135. [DOI] [PubMed] [Google Scholar]
- National Research Council, Committee on measuring lead in critical populations (1993). Measuring lead exposure in infants, children, and other sensitive populations. Washington, DC: National Academy Press. [PubMed] [Google Scholar]
- Needleman HL (1979). Lead levels and children’s psychologic performance. N Engl J Med. 301(3): 163. [PubMed] [Google Scholar]
- Needleman HL (1991). History of lead poisoning in the world: Get the lead out. http://www.biologicaldiversity.org/campaigns/get_the_lead_out/pdfs/health/Needleman_1999.pdf. last accessed: January 2019.
- Needleman HL (2009). Low level lead exposure: History and Discovery. Ann Epidemiol, 19: 235–238. [DOI] [PubMed] [Google Scholar]
- Needleman HL, Riess JA, Tobin MJ, Biesecker GE, & Greenhouse JB (1996). Bone lead levels and delinquent behavior. J Am Med Assoc, 275(5): 363–369. [PubMed] [Google Scholar]
- Needleman HL, Schell A, Bellinger D, Leviton A, & Allred EN (1990). The long-term effects of exposure to low doses of lead in childhood: An 11-year follow-up report. N Engl J Med, 322(2): 83–88. [DOI] [PubMed] [Google Scholar]
- Nevin R (2000). How lead exposure relates to temporal changes in IQ, violent crime, and unwed pregnancy. Environ Res 83(1): 1–22. [DOI] [PubMed] [Google Scholar]
- Nevin R (2007). Understanding international crime trends: The legacy of preschool lead exposure. Environ Res, 104(3): 315–336. [DOI] [PubMed] [Google Scholar]
- Nicolescu R, Petcu C, Cordeanu A, Fabritius K, Schlumpf M, Krebs R, Kramer U, & Winneke G (2010). Environmental exposure to lead, but not other neurotoxic metals, relates to core elements of ADHD in Romanian children: Performance and questionnaire data. Environ Res, 110(5): 476–83. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Knottnerus GM, Martel MM, Nikolas M, Cavanagh K, Karmaus W, & Rappley MD (2008). Low blood lead levels associated with clinically diagnosed attention-deficit/hyperactivity disorder and mediated by weak cognitive control. Biol Psychiatry, 63(3): 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihei MK, & Guilarte TR (2001). Molecular changes in glutamatergic synapses induced by Pb2+: Association with deficits of LTP and spatial learning. NeuroToxicology, 22(5): 635–643. [DOI] [PubMed] [Google Scholar]
- Nriagu JO (1990). The rise and fall of leaded gasoline. Sci Total Environ, 92: 13–28. [Google Scholar]
- Parr DR, & Harris EJ (1976). The effect of lead on the calcium-handling capacity of rat heart mitochondria. Biochem J, 158(2): 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears C (1995). Structure and function of the protein kinase C gene family. J Biosci, 20(3): 311–332. [Google Scholar]
- Pell MB, & Schneyer J Special report: Thousands of U.S. areas afflicted with lead poisoning beyond Flint’s. Reuters; December 19, 2016. http://www.reuters.com/article/us-usa-lead-testing-specialreport-idUSKBN1481BT. last accessed: January 2019. [Google Scholar]
- Pokora MJ, Richfield EK, & Cory-Slechta DA (1996). Preferential vulnerability of nucleus accumbens dopamine binding sites to low-level lead exposure: Time course of effects and interactions with chronic dopamine agonist treatments. J Neurochem, 67(4):1540–50. [DOI] [PubMed] [Google Scholar]
- Pounds JG (1984). Effect of lead intoxication on calcium homeostasis and calcium-mediated cell function: A review. NeuroToxicology, 5(3): 295–332. [PubMed] [Google Scholar]
- Rafalowska U, Struzyńska L, Dabrowska-Bouta B, & Lenkiewicz Aa. (1996). Is lead toxicosis a reflection of altered energy metabolism in brain synaptosomes? Acta Neurobiol Exp, 56(2): 611–7. [DOI] [PubMed] [Google Scholar]
- Raymond J, & Brown MJ (2015). Childhood blood lead levels-- United States, 2007–2012. Centers for Disease Control and Prevention: Morbidity and Mortality Weekly Report (MMWR). 62(54): 76–80. Retrieved from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6254a5.htm?s_cid=mm6254a5_x. last accessed: January 2019. [DOI] [PubMed] [Google Scholar]
- Reuben A, Caspi A, Belsky DW, Broadbent J, Harrington H, Sugden K, Houts RM, Ramrakha S, Poulton R, & Moffitt TE (2017). Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. J Am Med Assoc, 317(12): 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DC (1985). Chronic low-lead exposure from birth produces deficits in discrimination reversal in monkeys. Toxicol Appl Pharmacol, 77(2): 201–210. [DOI] [PubMed] [Google Scholar]
- Rice DC & Gilbert SG (1985). Low lead exposure from birth produces behavioral toxicity (DRL) in monkeys. Toxicol Appl Pharmacol, 80(3): 421–426. [DOI] [PubMed] [Google Scholar]
- Rice DC (2000). Parallels between attention deficit hyperactivity disorder and behavioral deficits produced by neurotoxic exposure in monkeys. Environ Health Perspect, 108(Suppl 3), 405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha A, Valles R, Cardon AL, Bratton GR, & Nation JR (2004). Self-administration of heroin in rats: Effects of low-level lead exposure during gestation and lactation. Psychopharmacology, 174(2): 203–210. [DOI] [PubMed] [Google Scholar]
- Rocha A, Valles R, Cardon AL, Bratton GR, & Nation JR (2005). Enhanced acquisition of cocaine self-administration in rats developmentally exposed to lead. Neuropsychopharmacology, 30: 2058–2064. [DOI] [PubMed] [Google Scholar]
- Rocha A, Valles R, Hart N, Bratton GR, & Nation JR (2008). Developmental lead exposure attenuates methamphetamine dose-effect self-administration performance and progressive ratio responding in the male rat. Pharmacol Biochem Behavior, 89(4): 508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin A (2009). The long-term consequences of exposure to lead. Isr Med Assoc J, 11(11): 689–694. [PubMed] [Google Scholar]
- Rosner D, Markowitz G, & Lanphear B (2005). J. Lockhart Gibson and the discovery of the impact of lead pigments on children’s health: A review of a century of knowledge. Public Health Reports 120: 296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan DY, Chen JT, Zhao C, Xu YZ, Wang M, & Zhao WF (1998). Impairment of long-term potentiation and paired-pulse facilitation in rat hippocampal dentate gyrus following developmental lead exposure in vivo. Brain Res, 806(2): 196–201. [DOI] [PubMed] [Google Scholar]
- Sabbar M, Delaville C, De Deurwaerdère P, Benazzouz A, & Lakhdar-Ghazal N (2012). Lead intoxication induces noradrenaline depletion, motor nonmotor disabilities, and changes in the firing pattern of subthalamic nucleus neurons. Neuroscience, 210: 375–383. [DOI] [PubMed] [Google Scholar]
- Sandhir R, & Gill KD (1994). Calmodulin and cAMP dependent synaptic vesicle protein phosphorylation in rat cortex following lead exposure. Int J Biochem, 26(12): 1383–1389. [DOI] [PubMed] [Google Scholar]
- Schafer EH (1956). The early history of lead pigments and cosmetics in China. T’oung Pao, 44(2): 413–438. Retrieved from: https://www.jstor.org/stable/4527434. last accessed: January 2019. [Google Scholar]
- Schneider JS, Lee MH, Anderson DW, Zuck L, & Lidsky TI (2001). Enriched environment during development is protective against lead-induced neurotoxicity. Brain Res, 896(1–2): 48–55. [DOI] [PubMed] [Google Scholar]
- Shih R, Glass T, Bandeen-Roche K, Carlson M, Bolla KI, Todd AC, & Schwartz BS (2006). Environmental lead exposure and cognitive function in community-dwelling older adults. Neurology, 67(9): 1556–1562. [DOI] [PubMed] [Google Scholar]
- Shirran SL, & Barran PE (2009). The use of ESI-MS to probe the binding of divalent cations to calmodulin. J Am Soc Mass Spectrom, 20(6): 1159–1171. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK (1991). Lead in bone: Implications for toxicology during pregnancy and lactation. Environ Health Perspect, 91: 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbergeld EK, Hruska RE, Miller LP, & Eng N (1980). Effects of lead in vivo and in vitro on GABAergic neurochemistry. J Neurochem, 34(6): 1712–1718. [DOI] [PubMed] [Google Scholar]
- Sobin C, Flores-Montoya MG, & Alvarez JM (2017). Early chronic low-level Pb exposure alters global exploratory behaviors but does not impair spatial and object memory retrieval in an object-in-place task in pre-adolescent C57BL/6J mice. Neurotoxicol Teratol, 61: 204–114. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Simon D, Kelsey K, & Todd AC (2002). ApoE genotype, past adult lead exposure, and neurobehavioral function. Environ Health Perspect, 110(5): 501–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Davatzikos C, Shen D, Liu D, Wu X, Todd AC, Bassett S, & Youssem D (2006). Past adult exposure is linked to neurodegeneration measured by brain MRI. Neurology, 66(10): 1476–1484. [DOI] [PubMed] [Google Scholar]
- Stiles KM, & Bellinger DC (1993). Neuropsychological correlates of low-level exposure in school-age children: A prospective study. Neurotoxicol Teratol, 15 (1): 27–35. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Zhang A, Trachtenberg F, Daniel DB, McKinlay S, & Bellinger DC (2007). Neuropsychological function in children with blood lead levels <10 μg//dL. NeuroToxicology, 28:1170–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiggett GL (1917). Proceedings of the second Pan American Scientific Congress: Section VII, mining, metallurgy, economic geology and applied chemistry. Washington Government Printing Office, Washinton, D. C. last accessed: December 2018. [Google Scholar]
- Téllez-Rojo MM, Hernández-Avila M, Lamadrid-Figueroa H, Smith D, Hernández-Cadena L, Mercado A, Aro A, Schwartz J, & Hu H (2004). Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy. American J Epidemiol, 160(7): 668–78. [DOI] [PubMed] [Google Scholar]
- Tennekoon G, Aitchison CS, Frangia J, Price DL, & Goldberg AM (1979). Chronic lead intoxication: Effects on developing optic nerve. Ann Neurol, 5(6): 558–564. [DOI] [PubMed] [Google Scholar]
- Tiemann M (2017). Safe Drinking Water Act (SDWA): A summary of the act and its major requirements. Retrieved from: https://fas.org/sgp/crs/misc/RL31243.pdf. last accessed: January 2019.
- Tiffany-Castiglioni E (1993). Cell culture models for lead toxicity in neuronal and glial cells. NeuroToxicology, 14(4): 513–536. [PubMed] [Google Scholar]
- Tiffany-Castiglioni E, Neck KF, & Caceci T (1986). Glial culture on artificial capillaries: Electron microscopic comparisons of C6 rat glioma cells and rat astroglia. J Neurosci Res, 16(2): 387–396. [DOI] [PubMed] [Google Scholar]
- Tiffany-Castiglioni E, Sierra EM, Wu JN, & Rowles TK (1989). Lead toxicity in neuroglia. NeuroToxicology, 10(3): 417–443. [PubMed] [Google Scholar]
- Toews AD, Blaker WD, Thomas DJ, Gaynor JJ Krigman MR, Mushak P, & Morell P (1983). Myelin deficits produced by early postnatal exposure to inorganic lead or triethyltin are persistent. J Neurochem, 41(3): 816–882. [DOI] [PubMed] [Google Scholar]
- Torrice M (2016). How lead ended up in Flint’s tap water: Without effective treatment steps to control corrosion, Flint’s water leached high levels of lead from the city’s pipes. Chemical & Engineering News, 94(7), 26–29. Retrieved from: http://cen.acs.org/articles/94/i7/Lead-Ended-Flints-Tap-Water.html. last accessed: January 2019. [Google Scholar]
- Tsoi MF, Cheung CL, Cheung TT, & Cheung BM (2016). Continual decrease in blood lead level in Americans: United States National Health Nutrition and Examination Survey 1999–2014. Am J Med, 129(11): 1213–1218. [DOI] [PubMed] [Google Scholar]
- U.S. Geological Survey. Mineral commodity summaries, lead (1997). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/380397.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (1998). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/380398.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (1999). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/380399.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2000). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/380300.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2001). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/380301.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2002). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/380302.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2003). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/380303.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2004). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/leadmcs04.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2005). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/lead_mcs05.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2006). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/lead_mcs06.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2007). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/lead_mcs07.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2008). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2008-lead.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2009). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2009-lead.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2010). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2010-lead.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2011). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2011-lead.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2012). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2012-lead.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2013). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2013-lead.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2014). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2014-lead.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2015). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2015-lead.pdf. last accessed: January 2019.
- U.S. Geological Survey. Mineral commodity summaries, lead (2016). Available: http://minerals.usgs.gov/minerals/pubs/commodity/lead/mcs-2016-lead.pdf. last accessed: January 2019.
- Van der Zee EA, Compaan JC, de Boer M, & Luiten PG (1992). Changes in PKC gamma immunoreactivity in mouse hippocampus induced by spatial discrimination learning. J Neurosci, 12(12): 4808–4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijngaarden E, Campbell JR, & Cory-Slechta DA (2009). Bone lead levels are associated with measures of memory impairment in older adults. NeuroToxicology, 30(4): 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijngaarden E, Winters PC, & Cory-Slechta DA (2011). Blood lead levels in relation to cognitive function in older U.S. adults. NeuroToxicology, 32(1): 110–115. [DOI] [PubMed] [Google Scholar]
- Verma M, & Schneider JS (2017). Srain specific effects of low level lead exposure on associative learning and memory in rats. NeuroToxicology, 62: 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Martinetto P, Tscoucaris B, Bréniaux R, Lefebvre MA, Richard G, Talabot J, & Dooryhee E (1999). Making make-up in Ancient Egypt. Nature, 397: 483–484. [Google Scholar]
- Wang X-M, Liu W-J, Zhang R, & Zhou Y-K (2012). Effects of exposure to low-level lead on spatial learning and memory and the expression of mGluR1, NMDA receptor in different developmental stages of rats. Toxicol Ind Health, 29(8): 686–696. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Weuve J, Nie H, Saint-Hilaire MH, Sudarsky L, Simon DK, Hersh B, Schwartz J, Wright RO, & Hu H (2010). Association of cumulative lead exposure with Parkinson’s disease. Environ Health Perspect, 118(11): 1609–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weizsaecker K (2003). Lead toxicity during pregnancy. Prim Care Update Ob Gyns, 10(6): 304–309. [Google Scholar]
- Westerink RH, Klompmakers AA, Westenberg HG, & Vijverberg HP (2002). Signaling pathways involved in Ca2+- -and Pb2+ -induced vesicular catecholamine release from rat PC12 cells. Brain Res, 957(1), 25–38. [DOI] [PubMed] [Google Scholar]
- White LD, Cory-Slechta DA, Gilbert ME, Tiffany-Castiglioni E, Zawia NH, Virgolini M, Rossi-George A, Lasley SM Qian YC, & Basha MR (2007). New and evolving concepts in the neurotoxicology of lead. Toxicol Appl Pharmacol, 225(1): 1–27. [DOI] [PubMed] [Google Scholar]
- Widzowski DV, Finkelstein JN, Pokora MJ, & Cory-Slechta DA (1994). Time course of postnatal lead-induced changes in dopamine receptors and their relationship to changes in dopamine sensitivity. NeuroToxicology, 15(4):853–65. [PubMed] [Google Scholar]
- Winder C, Garten LL, & Lewis PD (1983). The morphological effects of lead on the developing central nervous system. Neuropathol Appl Neurobiol, 9: 87–108. [DOI] [PubMed] [Google Scholar]
- Winneke G (2011). Developmental aspects of environmental neurotoxicology: Lessons from lead and polychlorinated biphenyls. J Neurol Sci, 308(1–2): 9–15. [DOI] [PubMed] [Google Scholar]
- Wilker E, Korrick S, Nie LH, Sparrow D, Vokonas P, Coull B, Wright RO, Schwartz J, Hu H Longitudinal changes in bone lead levels: The VA normative aging study. J Occup Environ Med, 53(8): 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley DE (1984). A perspective of lead poisoning in antiquity and the present. NeuroToxicology, 5(3): 353–361. [PubMed] [Google Scholar]
- Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, & Zawia NH (2008). Alzheimer’s disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): Evidence for a developmental origin and environmental link for AD. J Neurosci, 28(1): 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Liu QY, & Alkon DL (2014). PKC activators enhance GABAergic neurotransmission and paired-pulse facilitation in hippocampal CA1 pyramidal neurons. Neuroscience, 268: 75–86. [DOI] [PubMed] [Google Scholar]
- Yuede CM, Wozniak DF, Creeley CE, Taylor GT, Olney JW, & Farber NB (2010). Behavioral consequences of NMDA antagonist-induced neuroapoptosis in the infant mouse brain. PLoS One, 5(6): e11374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J (2017). 1,000 days of toxic drinking water in Flint. Reuters; https://www.rt.com/usa/374545-flint-water-1000-days. last accessed: January 2019. [Google Scholar]
- Zawia NH, & Basha MR (2005). Environmental risk factors and the developmental basis for Alzheimer’s disease. Rev Neurosci, 16(4):325–37. [DOI] [PubMed] [Google Scholar]
- Zierold KM, Havlena J, & Anderson H (2007). Exposure to lead and length of time needed to make homes lead-safe for young children. Am J Public Health, 97(2), 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]