Abstract
Background
There are few descriptions of virologic failure (VF) and acquired drug resistance (HIVDR) in large cohorts initiating contemporary antiretroviral therapy (ART).
Methods
We studied all persons with HIV (PWH) in a California clinic population initiating ART between 2010 and 2017. VF was defined as not attaining virologic suppression, discontinuing ART, or virologic rebound prompting change in ART.
Results
During the study, 2315 PWH began ART. Six companion drugs were used in 93.3% of regimens: efavirenz, elvitegravir/c, dolutegravir, darunavir/r, rilpivirine, and raltegravir. During a median follow-up of 36 months, 214 (9.2%) PWH experienced VF (2.8 per 100 person-years) and 62 (2.7%) experienced HIVDR (0.8 per 100 person-years). In multivariable analyses, younger age, lower CD4 count, higher virus load, and atazanavir/r were associated with increased VF risk; lower CD4 count, higher virus load, and nevirapine were associated with increased HIVDR risk. Compared with efavirenz, dolutegravir, raltegravir, and darunavir were associated with reduced HIVDR risk. Risks of VF and HIVDR were not significantly associated with ART initiation year. Of the 62 PWH with HIVDR, 42 received an non-nucleoside RT inhibitor (NNRTI), 15 an integrase-strand transfer inhibitor (INSTI), and 5 a protease inhibitor (PI). Among those with HIVDR on an NNRTI or first-generation INSTI, 59% acquired dual class resistance and 29% developed tenofovir resistance; those receiving a PI or dolutegravir developed just M184V.
Conclusions
Despite the frequent use of contemporary ART regimens, VF and HIVDR continue to occur. Further efforts are required to improve long-term ART virological responses to prevent the consequences of ongoing HIV-1 replication including virus transmission and HIVDR.
Keywords: antiretroviral therapy, drug resistance, HIV-1, virological outcome
In a U.S. clinic population, the proportion of persons with HIV who experience virological failure or drug resistance on initial ART is low but may no longer be decreasing. Further efforts are required to improve long-term ART virological responses.
Many clinical trials and several longitudinal cohort studies in Europe and North America have reported that since 2000 antiretroviral therapy (ART) has become progressively more effective, with fewer persons with HIV-1 (PWH) developing virologic failure (VF) or acquiring drug resistance [1–11]. However, large numbers of PWH in these studies received ART regimens no longer recommended by current expert guidelines [12]. To evaluate the risk of VF and acquired HIV-1 drug resistance (HIVDR) in PWH receiving contemporary ART regimens, we examined the incidence of these end points in a large US clinic population initiating therapy during an 8-year period between 2010 and 2017.
We recently published a study of nucleoside RT inhibitor (NRTI)–, non-nucleoside RT inhibitor (NNRTI)–, and protease inhibitor (PI)–associated transmitted drug resistance (TDR) between 2003 and 2016 in the same clinic population described here [13]. The overall prevalence of TDR in that study was 13.9%, with a prevalence of 16% to 19% between 2012 and 2016. Many of the transmitted mutations were associated with drugs that had not been used since the early 2000s (eg, thymidine analogs) and likely resulted from ongoing transmission of drug-resistant strains that emerged years earlier. Therefore, in the current study, we sought to identify drug resistance mutations associated with contemporary ART regimens.
METHODS
Study Population
We identified all ART-naïve adult PWH in the Kaiser Permanente Northern California (KPNC) medical care program who had a baseline genotypic resistance test and initiated ART between January 1, 2010, and December 31, 2017. According to KPNC ART guidelines, genotypic resistance testing was recommended for all PWH initiating ART during this time period, and to our knowledge, adherence to this policy was close to 100%. PWH were followed on first-line ART until December 31, 2018, unless they experienced VF or were censored as defined below.
Demographic data, HIV-1 acquisition risk factors, virus load (VL), CD4 counts, and ART history data were obtained from an electronic KPNC research database. Genotypic resistance testing was performed at the Stanford University Healthcare Clinical Virology Laboratory. During the study, tenofovir alafenamide was substituted for tenofovir disoproxil fumarate after its approval in 2016. Here, we use tenofovir to refer to tenofovir disoproxil fumarate and tenofovir alafenamide. The Stanford University and KPNC institutional review boards approved this study.
VF and Censoring
Virologic suppression was defined as attaining a VL <200 copies/mL [14]. Between 2010 and 2014, plasma HIV-1 RNA levels (virus load [VL]) were monitored using the VERSANT assay (Siemans Molecular Diagnostics), which quantifies VL between 75 and 500 000 copies/mL. Between 2014 and 2018, VL was monitored using the Ampliprep/Cobas Taqman assay (Roche Laboratories), which quantifies VL between 48 and 10 million copies/mL. To adjust for this change, we used 500 000 copies/mL as the VL upper limit.
VF was defined as not attaining virological suppression or as experiencing virological rebound. The former was defined as displaying no evidence of virologic response, prompting a change in therapy within the first 6 months of starting ART, or not attaining virologic suppression after 6 months of starting ART. PWH who did not attain virologic suppression and died before completing 6 months of therapy were considered to have had VF. However, PWH who did not attain virologic suppression and discontinued KPNC care before completing 6 months of therapy were censored. Among PWH attaining initial virologic suppression, VF was defined as loss of virologic suppression prompting a change in therapy, as well as ART discontinuation for any reason. PWH were censored at the time of death, discontinuing KPNC medical care, switching to another regimen while virologically suppressed, or reaching December 31, 2018, without experiencing VF.
HIV Drug Resistance
TDR was defined as drug resistance before the start of ART. HIVDR was defined as acquiring 1 or more nonpolymorphic mutations associated with reduced susceptibility to NRTIs, PIs, or integrase-strand transfer inhibitors (INSTIs) that were not present before ART [15–17].
Statistical Methods
VF and HIVDR end points were reported as the proportion of PWH developing the end point, the incidence per 100 person-years, and the cumulative incidence for the population at risk following the start of therapy. Fisher exact tests were used for comparing proportions of categorical variables, and Wilcoxon rank sum tests were used for comparing continuous variables between PWH without VF and with VF, and between PWH without VF and with HIVDR.
Univariable and multivariable Cox regressions were used to estimate VF and HIVDR hazard ratio for age, gender, ethnicity (Caucasian, non-Caucasian), baseline CD4 cell count, baseline VL, companion ARVs, and presence of TDR. The effect of baseline CD4 cell count was estimated as a continuous variable and a categorical variable (<300, 300–500, ≥500 cells/μL). The effect of baseline VL was estimated as a continuous variable and a categorical variable (<4.0, 4.0–5.0, ≥5.0 copies/mL). Because of the strong association of abacavir/lamivudine with dolutegravir, we did not include the NRTI backbone in any of the regression models. Examining the Schoenfeld residuals of the model showed that the Cox regression proportional hazards assumption was not violated for any of the variables included in the final model [18]. Statistical significance was defined as P < .05.
RESULTS
Study Cohort
Between January 2010 and December 2017, 2315 PWH began ART following a baseline genotypic resistance test. The median age (interquartile range [IQR]) was 39 (29–49) years; 90.8% were male. Race/ethnicity was identified as Caucasian in 41.4%, Hispanic in 22.9%, black/African American in 21.5%, Asian in 10.1%, and unrecorded in 4.1%. HIV-1 acquisition risk factors included men who have sex with men (MSM) in 60.4%, heterosexual contact in 18.0%, bisexual contact in 11.9%, injection drug use in 5.5%, transfusion recipient in 0.4%, and unrecorded in 3.8%.
The median baseline CD4 count (IQR) was 373 (201–537) cells/μL, and the median baseline VL (IQR) was 4.5 (4.0–5.1) log copies/mL. TDR was present in 13.8% of PWH. The overall proportions with NNRTI, NRTI, PI, and multiclass TDR were 9.5%, 3.5%, 3.0%, and 2.0%, respectively. Of 140 PWH who underwent a baseline integrase genotypic resistance test, 4 (2.9%) had a nonpolymorphic INSTI-associated mutation. Three of these PWH also had NRTI- or NNRTI-associated TDR.
The median year of ART initiation (IQR) was 2014 (2011–2015). The median number of months of follow-up by year was 65 for the 302 starting ART in 2010, 56 for the 313 starting ART in 2011, 49 for the 307 starting ART in 2012, 55 for the 233 starting ART in 2013, 49 for the 329 starting ART in 2014, 39 for the 310 starting ART in 2015, 28 for the 300 starting ART in 2016, and 16 for the 221 starting ART in 2017. VL testing was performed a median (IQR) of every 4.6 (3.4–6.2) months. During the study period, there was a significant yearly decrease in the age of PWH starting ART (coefficient, –0.5 per year; P < .001), and there was a significant yearly increase in the proportions of non-MSM (OR, 1.08; P < .001) and non-Caucasians (OR, 1.06; P = .002). Baseline CD4 cell counts (coefficient, 20.3; P < .001) and baseline VL (coefficient, 0.03; P < .001) also significantly increased over the study period.
First-line ART Regimens
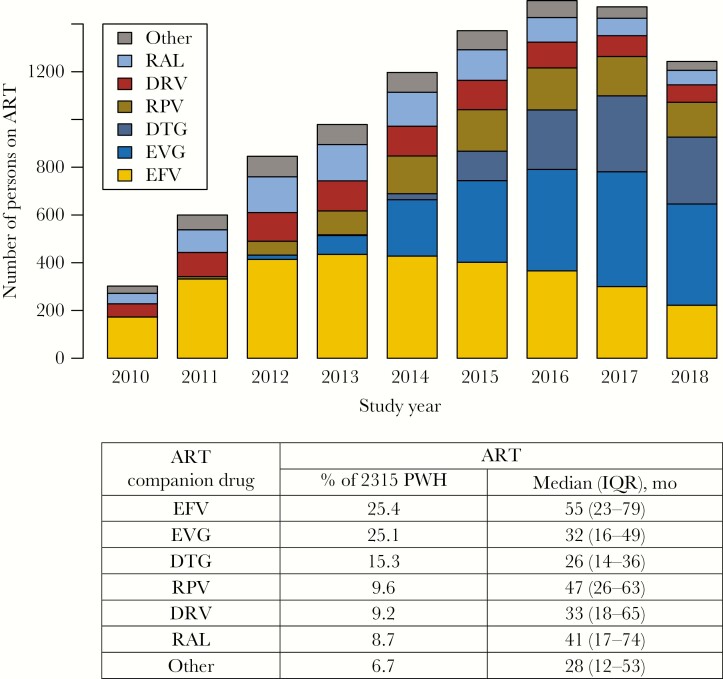
Figure 1 shows the proportion of the most common companion ARVs received as part of an ART regimen. Six ARVs—efavirenz, elvitegravir/cobicistat (elvitegravir/c), dolutegravir, rilpivirine, darunavir/ritonavir or darunavir/cobicistat (b-darunavir), and raltegravir—accounted for 93.3% of the 2315 ART regimens. The remaining companion ARVs included atazanavir/ritonavir or atazanavir/cobicistat (b-atazanavir; 2.9%), lopinavir/ritonavir (lopinavir/r; 0.6%), nevirapine (0.6%), and etravirine (0.3%). A regimen containing more than 1 companion ARV was received by 2.3% of the cohort—two-thirds of whom had baseline TDR.
Figure 1.
Yearly distribution of the 6 most common ART companion drugs used as part of the initial ART (n = 2315). Abbreviations: ART, antiretroviral therapy; DRV, darunavir; DTG, dolutegravir; EFV, efavirenz; EVG, elvitegravir; IQR, interquartile range; PWH, people with HIV; RAL, raltegravir; RPV, rilpivirine.
Single-tablet regimens accounted for 69.8% of the 2315 ART regimens. The most common NRTI backbones, tenofovir/emtricitabine and abacavir/lamivudine, were used in 82.3% and 12.2% of ART regimens, respectively. Among those receiving abacavir/lamivudine, 80.1% received it as part of a fixed-dose combination with dolutegravir.
Virological Failure and Acquired Drug Resistance
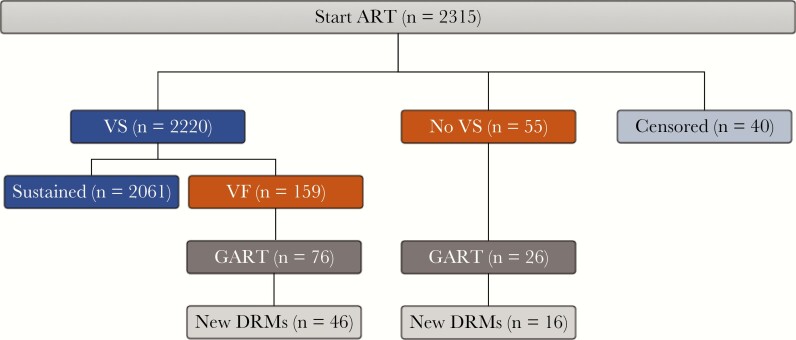
Of the 2315 PWH receiving ART, 214 (9.2%) experienced VF, including 55 (2.4%) who never attained virologic suppression and 159 (6.9%) who developed VF after attaining initial virologic suppression. Forty PWH (1.7%) left KPNC within 6 months of starting ART without attaining virologic suppression, and 2061 (89.0%) PWH never experienced VF (Figure 2).
Figure 2.
Disposition of virological outcomes, genotypic resistance testing, and acquired drug resistance in the overall cohort of PWH starting ART between January 2010 and December 2017. Forty PWH who left KPNC within 6 months of starting therapy without having attained virologic suppression were censored in the primary analysis. Abbreviations: ART, antiretroviral therapy; GART, genotypic antiretroviral resistance test; KPNC, Kaiser Permanente Northern California; New DRMs, drug-resistance mutations that emerged on therapy; PWH, people with HIV; VF, virological failure; VS, virologic suppression.
Of the 55 PWH not attaining virologic suppression, 7 died within 6 months of starting ART. Of the remaining 48 PWH, 26 underwent genotypic resistance testing, and 16 of these were found to have HIVDR. Five of the 10 PWH who underwent resistance testing but did not have HIVDR had discontinued therapy at least 3 months before testing. The 22 PWH with VF who did not undergo resistance testing included 11 who had discontinued ART before developing VF.
Of the 2220 PWH attaining virologic suppression, 159 (6.9% of the cohort) experienced VF after a median follow-up (IQR) of 21.4 (12.1–41.0) months, including 76 who underwent resistance testing, of whom 46 were found to have HIVDR. Fifteen of the 30 PWH who underwent resistance testing but did not have drug resistance had discontinued therapy at least 3 months before resistance testing. Of the 83 PWH with VF who did not undergo resistance testing, 59 discontinued therapy before developing VF and 9 discontinued KPNC care shortly after developing VF.
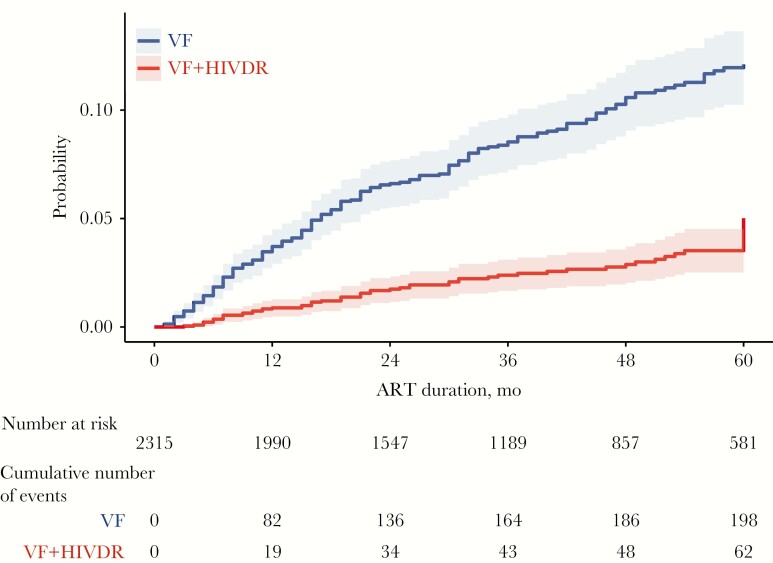
The rates of VF and HIVDR in the entire population were 2.8 and 0.8 cases per 100 person-years of follow-up, respectively. The rates of VF and of HIVDR in the subset of 2220 PWH attaining initial virologic suppression were 2.1 and 0.6 cases per 100 person-years of follow-up, respectively. Figure 3 shows that the yearly incidence of VF was highest in the first year of therapy, with 3.5 events per 100 person-years; it then decreased to 2.7 events per 100 person-years in the second year and 2.0 events per 100 person-years in the third year.
Figure 3.
Cumulative incidence of VF and VF plus acquired HIVDR for the 2315 PWH over a period of 60 months. PWH were censored if they died, discontinued KPNC care, switched to a third ART regimen, or reached December 31, 2018 without experiencing VF. Abbreviations: ART, antiretroviral therapy; HIVDR, HIV drug resistance; KPNC, Kaiser Permanente Northern California; HIVDR, drug-resistance mutations that emerged on therapy; PWH, people with HIV; VF, virological failure.
Among the 2061 PWH without VF (89.0% of the cohort), the median follow-up (IQR) was 38.1 (19.8–61.6) months. Of these PWH, 1035 (44.7% of the cohort) were followed until December 31, 2018; 331 (14.3% of the cohort) discontinued KPNC care; 575 (24.8% of the cohort) switched to a second regimen without experiencing VF; and 41 (1.8% of the cohort) died.
Factors Associated With VF and HIVDR
Table 1 shows the demographics, HIV acquisition risk factors, and baseline laboratory tests in the 2061 PWH without VF, the 214 with VF, and the 62 with VF and HIVDR. Compared with PWH without VF, those with VF were more likely to have lower baseline CD4 counts, higher baseline VLs, and to be non-Caucasian. Compared with PWH without VF, those with HIVDR were more likely to have lower baseline CD4 counts and higher baseline VL.
Table 1.
Demographics, HIV Risk Factors, and Baseline Laboratory Tests in People With HIV With Sustained Virologic Suppression, Virologic Failure, and Virologic Failure Plus Acquired HIVDR
| VS | Any VF | VF+HIVDR | |
|---|---|---|---|
| (n = 2061) | (n = 214) | (n = 62) | |
| Age, median (IQR), y | 39 (29–50) | 38 (29–49) | 43 (33–49) |
| Gender (male), % | 91.0 | 87.9 | 90.3 |
| Ethnicity, % | |||
| Caucasian | 42.6 | 33.2 a | 30.6 |
| Hispanic | 22.6 | 22.9 | 32.3 |
| Black/African American | 20.6 | 30.8 | 25.8 |
| Asian | 10.8 | 9.8 | 8.1 |
| Not recorded | 3.4 | 3.3 | 3.2 |
| HIV acquisition risk, % | |||
| MSM | 60.7 | 55.1 | 58.1 |
| Heterosexual contact | 18.0 | 20.6 | 21.0 |
| Bisexual | 12.2 | 9.8 | 8.1 |
| Intravenous drug use | 5.3 | 7.0 | 4.8 |
| Transfusion | 0.4 | 0.9 | 1.6 |
| Not recorded | 3.4 | 6.5 | 6.4 |
| Subtype B, % | 94.8 | 93.9 | 98.4 |
| CD4 count, median (IQR), cells/μL | 384 (217–551) | 275 (55–401) a | 109 (38–281)b |
| VL, median (IQR), log copies/mL | 4.5 (4–5) | 4.7 (4.3–5.2)a | 5.0 (4.6–5.5)b |
| TDR, % | 13.7 | 13.1 | 9.7 |
| NNRTI | 9.0 | 11.2 | 9.7 |
| NRTI | 3.8 | 1.9 | 1.6 |
| PI | 3.2 | 1.9 | 1.6 |
| Multiclass TDR | 2.0 | 1.9 | 3.2 |
Significant difference in people with HIV with virological failure and in people with HIV with virological failure plus acquired HIVDR compared with people with HIV with sustained virological suppression is indicated in boldface type.
Abbreviations: HIVDR, HIV-1 drug resistance; IQR, interquartile range; MSM, men who have sex with men; PWH, people with HIV; TDR, transmitted drug resistance; VF, virological failure; VL, virus load (plasma HIV-1 RNA log copies/mL); VS, virological suppression.
aCompared with PWH without VF, those with VF had lower baseline CD4 counts (P < .001), higher baseline VLs (P < .001), and were more likely to be non-Caucasian (P = .008).
bCompared with PWH without VF, those with VF plus HIVDR had lower baseline CD4 counts (P < .001) and higher baseline VLs (P < .001).
Table 2 shows the results of univariable and multivariable regression analyses of factors potentially associated with the incidence of VF. In multivariable regression analysis, there was a significantly increased incidence of VF per 100 person-years, with lower baseline CD4 counts, higher baseline VLs, and younger age but not non-Caucasian race/ethnicity. Compared with efavirenz, b-atazanavir was associated with an increased VF incidence. For each 100-cell CD4 count decrease, there was a 17.9% increased VF incidence. For each 1.0 log VL increase, there was a 47.0% increased VF incidence. For each 10-year decrease in age, there was a 26.1% increased VF incidence.
Table 2.
Association of Demographics, HIV Risk Factors, Baseline Laboratory Results, and Companion ARV With Virological Failure Incidence per 100 Person-Years
| Variable | No. of PWH | Person-Years | No. of VF | No. of VF/100 Person-Years | Unadjusted HRa (95% CI) | Adjusted HRb,c (95% CI) |
|---|---|---|---|---|---|---|
| Age | ||||||
| 10-y decrement | 1.13 (1.12–1.15) | 1.26 (1.24–1.28) | ||||
| Gender | ||||||
| M | 2103 | 7030 | 188 | 2.67 | Ref | |
| F | 212 | 691 | 26 | 3.76 | 1.40 (0.93–2.11) | 1.18 (0.697–2.01) |
| Race/ethnicity | ||||||
| Caucasian | 960 | 3358 | 71 | 2.11 | Ref | |
| Non-Caucasian | 1274 | 4150 | 136 | 3.28 | 1.53 (1.15–2.03) | 1.33 (0.97–1.84) |
| HIV acquisition risk | ||||||
| MSM | 1398 | 4774 | 118 | 2.47 | Ref | |
| Non-MSM | 830 | 2664 | 82 | 3.08 | 1.23 (0.93–1.63) | 1.12 (0.79–1.57) |
| CD4 count, cells/μL | ||||||
| ≥500 | 668 | 2095 | 31 | 1.48 | Ref | |
| 300–500 | 755 | 2686 | 63 | 2.35 | 1.64 (1.06–2.52) | 1.35 (0.85–2.14) |
| <300 | 838 | 2789 | 114 | 4.09 | 2.81 (1.89–4.19) | 2.08 (1.32–3.27) |
| VL, log copies/mL | ||||||
| <4.0 | 501 | 1834 | 24 | 1.31 | Ref | |
| 4.0–5.0 | 1086 | 3725 | 101 | 2.71 | 2.05 (1.31–3.20) | 2.10 (1.28–3.44) |
| ≥5.0 | 682 | 2041 | 83 | 4.07 | 2.98 (1.89–4.70) | 2.95 (1.74–5.0) |
| TDR | ||||||
| No | 1995 | 6734 | 186 | 2.76 | Ref | |
| Yes | 320 | 987 | 28 | 2.84 | 0.99 (0.67–1.49) | 0.97 (0.60–1.55) |
| Companion drug | ||||||
| Efavirenz | 588 | 2570 | 69 | 2.68 | Ref | |
| Elvitegravir/c | 580 | 1603 | 35 | 2.18 | 0.72 (0.47–1.07) | 0.65 (0.41–1.03) |
| Dolutegravir | 355 | 756 | 22 | 2.91 | 0.87 (0.53–1.41) | 0.90 (0.54–1.52) |
| b-darunavir | 213 | 739 | 28 | 3.79 | 1.33 (0.85–2.06) | 1.06 (0.65–1.71) |
| Rilpivirine | 222 | 836 | 17 | 2.03 | 0.72 (0.43–1.23) | 0.95 (0.53–1.69) |
| Raltegravir | 202 | 774 | 16 | 2.07 | 0.75 (0.44–1.29) | 0.87 (0.49–1.54) |
| b-atazanavir | 66 | 176 | 15 | 8.52 | 2.83 (1.61–4.96) | 2.37 (1.26–4.47) |
| Lopinavir/r | 15 | 31 | 3 | 9.68 | 3.23 (1.01–10.3) | 3.08 (0.72–13.3) |
| Nevirapine | 13 | 34 | 2 | 5.88 | 2.02 (0.50–8.26) | 3.02 (0.73–12.5) |
| Etravirine | 6 | 28 | 2 | 7.14 | 2.68 (0.66–10.9) | 1.43 (0.20–10.4) |
Variables associated with a significantly increased or decreased risk of virological failure per 100 person-years are indicated in boldface type.
Abbreviations: HR, hazard ratio; MSM, men who have sex with men; PWH, people with HIV; TDR, transmitted drug resistance; VL, virus load (plasma HIV-1 RNA log copies/mL).
aUnadjusted HRs derived from univariate Cox regression analysis.
bAdjusted HRs derived from multivariable Cox regression.
cFor the variables age, gender, TDR, and companion drug, there were no missing values. For the remaining 4 variables, baseline CD4 cell counts, baseline virus load, race/ethnicity, and HIV acquisition risk factor, the missing values varied between 1.5% and 4.1%. Thus, the multivariable Cox regression model was based on 89.5% (n = 2072) of the cohort.
Table 3 shows the results of univariable and multivariable regression analyses of potential factors associated with the incidence of HIVDR. In multivariable analysis, lower baseline CD4 counts and higher baseline VLs were associated with an increased risk of HIVDR. Compared with efavirenz, b-darunavir, dolutegravir, and raltegravir were associated with a decreased risk of HIVDR, while nevirapine was associated with an increased risk of HIVDR.
Table 3.
Association of Demographics, HIV Risk Factors, Baseline Laboratory Results, and Companion ARV With the Incidence of Virologic Failure Plus HIVDR Over 100 Person-Years
| Variable | No. of PWH | Person-Years | No. of VF | No. of VF/100 Person-Years | Unadjusted HRa (95% CI) | Adjusted HRb,c (95% CI) |
|---|---|---|---|---|---|---|
| Age | ||||||
| 10-y decrement | 1.03 (1–1.05) | 1.1 (1.08–1.13) | ||||
| Gender | ||||||
| M | 1931 | 6793 | 56 | 0.82 | Ref | |
| F | 192 | 648 | 6 | 0.93 | 1.12 (0.48–2.59) | 1.19 (0.39–3.6) |
| Race/ethnicity | ||||||
| Caucasian | 896 | 3258 | 19 | 0.58 | Ref | |
| Non-Caucasian | 1154 | 3981 | 41 | 1.03 | 1.77 (1.03–3.05) | 1.73 (0.94–3.16) |
| HIV acquisition risk | ||||||
| MSM | 1287 | 4609 | 36 | 0.78 | Ref | |
| Non-MSM | 761 | 2565 | 22 | 0.86 | 1.09 (0.64–1.86) | 0.87 (0.45–1.65) |
| CD4 count, cells/μL | ||||||
| ≥500 | 632 | 2048 | 4 | 0.20 | Ref | |
| 300–500 | 686 | 2572 | 10 | 0.39 | 2.00 (0.63–6.38) | 1.33 (0.407–4.37) |
| <300 | 757 | 2673 | 48 | 1.80 | 9.18 (3.31–25.5) | 5.4 (1.85–15.8) |
| VL, log copies/mL | ||||||
| <4.0 | 475 | 1793 | 3 | 0.17 | Ref | |
| 4.0–5.0 | 993 | 3569 | 25 | 0.70 | 4.24 (1.28–14.0) | 5.24 (1.22–22.5) |
| ≥5.0 | 612 | 1961 | 31 | 1.58 | 9.51 (2.90–31.1) | 8.68 (1.98–38) |
| TDR | ||||||
| No | 1834 | 6494 | 56 | 0.86 | Ref | |
| Yes | 289 | 948 | 6 | 0.63 | 0.73 (0.31–1.69) | 1.63 (0.616–4.29) |
| Companion drug | ||||||
| Efavirenz | 541 | 2480 | 32 | 1.29 | Ref | |
| Elvitegravir/c | 539 | 1565 | 12 | 0.77 | 0.56 (0.28–1.10) | 0.65 (0.31–1.37) |
| Dolutegravir | 328 | 728 | 1 | 0.14 | 0.09 (0.01–0.70) | 0.10 (0.01–0.79) |
| b-darunavir | 186 | 691 | 3 | 0.43 | 0.33 (0.09–1.06) | 0.08 (0.01–0.59) |
| Rilpivirine | 210 | 818 | 6 | 0.73 | 0.57 (0.24–1.38) | 0.96 (0.36–2.56) |
| Raltegravir | 185 | 754 | 2 | 0.27 | 0.20 (0.05–0.84) | 0.11 (0.01–0.80) |
| b-atazanavir | 53 | 155 | 2 | 1.29 | 0.96 (0.23–4.02) | 0.79 (0.18–3.41) |
| Lopinavir/r | 12 | 25 | 0 | 0.00 | 7.61e-08 (0–inf) | 1e-07 (0–inf) |
| Nevirapine | 13 | 34 | 2 | 5.88 | 4.40 (1.05–18.5) | 6.98 (1.58–30.9) |
| Etravirine | 6 | 28 | 2 | 7.14 | 5.64 (1.35–23.7) | 3.83 (0.49–30.1) |
Variables associated with a significantly increased or decreased risk of virological failure plus HIVDR per 100 person-years are indicated in boldface type.
Abbreviations: HR, hazard ratio; MSM, men who have sex with men; PWH, people with HIV; TDR, transmitted drug resistance; VL, virus load (plasma HIV-1 RNA log copies/mL).
aUnadjusted HRs derived from univariate Cox regression analysis.
bAdjusted HRs derived from multivariable Cox regression analysis.
cFor the variables age, gender, TDR, and companion drug, there were no missing values. For the remaining 4 variables, baseline CD4 cell counts, baseline virus load, race/ethnicity, and HIV acquisition risk factor, the missing values varied between 1.5% and 4.1%. Thus, the multivariable Cox regression model was based on 89.5% (n = 2072) of the cohort.
In a multivariable regression analysis including the year of ART initiation and all other covariates except for ART companion drug, the incidence of VF was not associated with calendar year (adjusted HR, 1.03; 95% CI, 0.95–1.12; P = .46). In this analysis, lower baseline CD4 cell counts, higher VL, and younger age remained associated with an increased incidence of VF. The ART companion drug variable was excluded from this analysis because it displayed high levels of collinearity with the year of ART initiation.
Acquired Drug Resistance Mutations
Table 4 summarizes the drug resistance mutations associated with the companion ARV received at the time of acquired HIVDR. Of the 62 PWH with HIVDR, 42 received an NNRTI-containing regimen, 15 an INSTI-containing regimen, and 5 a PI-containing regimen.
Table 4.
Drug Resistance Mutations in 62 People With HIV With Drug Resistance During First-line ART According to the Companion ARV at the Time of VF
| No. PWH (All) | Pretherapy CD4, Mediana | Pretherapy VL, Mediana | NRTI | NNRTI | INSTI | PI | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any | M184I/V | K65R | K70E/Q | Any | Any | Any | ||||
| NNRTI | ||||||||||
| Efavirenz | 32 | 93 | 4.9 | 18 | 16 | 3 | 4 | 31 | - | - |
| Rilpivirine | 6 | 155 | 5.0 | 6 | 5 | 1 | 2 | 5 | - | - |
| Nevirapine | 2 | 126 | 5.2 | 1 | 1 | 1 | - | 2 | - | - |
| Etravirine | 2 | 238 | 4.9 | 2 | 2 | - | 1 | 1 | - | - |
| INSTI | ||||||||||
| Elvitegravir/c | 12 | 114 | 5.0 | 11 | 11 | 4 | 0 | - | 10 | - |
| Raltegravir | 2 | 360 | 4.5 | 1 | 1 | - | - | - | 1 | - |
| Dolutegravir | 1 | 25 | 4.7 | 1 | 1 | - | - | - | - | - |
| PI | ||||||||||
| b-darunavir | 3 | 214 | 5.3 | 3 | 3 | - | - | - | - | 0 |
| b-atazanavir | 2 | 58 | 4.9 | 2 | 2 | - | - | - | - | 0 |
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; INSTI, integrase-strand transfer inhibitor; NNRTI, non-nucleoside RT inhibitor; NRTI, nucleoside RT inhibitor; PI, protease inhibitor; PWH, people with HIV; VL, virus load.
aThe median pretherapy CD4 and VL in those with VF and drug resistance.
Among the 42 PWH with HIVDR while receiving an NNRTI-containing regimen, NNRTI resistance mutations emerged in 39 (93%), including 31 receiving efavirenz, 5 receiving rilpivirine, 2 receiving nevirapine, and 1 receiving etravirine. Acquired NNRTI resistance mutations included K103N (n = 24), L100I (n = 5), G190A/S (n = 5), K101E (n = 5), Y181C (n = 4), and Y188L/H (n = 3) for those receiving efavirenz or nevirapine; E138K (n = 3), L100I (n = 2), and Y181C (n = 1) for those receiving rilpivirine; and K101E+Y181C for the 1 PWH receiving etravirine. Acquired NRTI resistance mutations included M184V/I in 24 (57%) and K65R or K70E in 10 (24%) PWH.
Among the 15 PWH with HIVDR while receiving an INSTI-containing regimen, INSTI resistance mutations emerged in 10 receiving elvitegravir/c and 1 receiving raltegravir. Acquired INSTI resistance mutations included E92Q (n = 6), T66I (n = 4), N155H (n = 2), E92G (n = 1), S147G (n = 1), Q148R (n = 1), F121Y (n = 1), and N155T (n = 1). M184V/I developed in 13 of 15 PWH, including 1 PWH receiving dolutegravir. K65R developed in 4 PWH receiving elvitegravir/c.
Among the 5 PWH with HIVDR while receiving a boosted PI-containing regimen, the NRTI resistance mutation M184V was the only acquired drug resistance mutation.
Supplementary Table 1 lists the drug resistance mutations emerging in the 62 PWH with HIVDR. It also shows that 6 of these PWH had TDR (ie, pretherapy drug resistance mutations) and that each of the pretherapy mutations was also present at VF.
Mortality
Overall, 48 PWH (2.1% of the cohort) died, including 7 not attaining virologic suppression and 41 not experiencing VF. The 7 PWH not experiencing virologic suppression died a median of 2 months following ART initiation. They were characterized by low CD4 counts (median [IQR], 36 [26–338]) and older age (median [IQR], 52 [44–62] years).
The 41 PWH without VF who died had lower CD4 counts (median [IQR], 273 [158–426]) and were older (median [IQR], 51 [41–58] years) than the remainder of the cohort. Although their causes of death were not known, only 7 had a CD4 count <200 at their last measurement. There were no apparent differences in the ART regimens of those who died compared with the complete cohort.
Discussion
This is one of the largest studies reporting the incidence of VF and HIVDR during first-line ART in a cohort of PWH receiving a contemporary regimen, defined as the regimen that was recommended or considered acceptable for first-line treatment by the US Public Health Service [12]. The study also describes factors associated with VF and HIVDR and the drug resistance mutations developing in PWH with HIVDR.
During a median follow-up of 36 months, the incidences of VF and HIVDR were 2.8 and 0.8 per 100 person-years, respectively. In multivariable analyses, younger age, lower baseline CD4 count, and higher baseline VL were associated with an increased risk of VF, while the same variables except for younger age were associated with an increased risk of HIVDR. Compared with efavirenz, dolutegravir, raltegravir, and b-darunavir were associated with a reduced risk of HIVDR. Of those developing HIVDR while receiving an NNRTI-containing or first-generation INSTI-containing regimen, 62% developed dual class resistance and 33% developed tenofovir resistance. Those developing resistance while receiving dolutegravir or a boosted PI developed just the cytidine analog resistance mutation M184V.
The finding that VF incidence was ~3.5 higher than that of HIVDR suggests that VF does not necessarily result in HIVDR and that HIVDR may not be the main cause of VF. Indeed, the relatively small proportion of PWH with VF who had HIVDR may reflect the increased use of single-tablet regimens, which are associated with a reduced risk of HIVDR in nonadherent PWH, as missed doses will be less likely to result in a partially suppressive regimen [19]. Additionally, a significant proportion of VF episodes resulted from treatment discontinuation, which is also less likely to result in HIVDR.
The low incidence of resistance should be qualified by the fact that just 102 (47.7%) of the 214 PWH with VF underwent resistance testing; thus, some proportion of cases of HIVDR may not have been detected. However, of the PWH with VF not undergoing resistance testing, most had discontinued ART or were unavailable for testing because they had low-level viremia or discontinued KPNC shortly after developing VF. Indeed, the absence of resistance testing appeared unexplained for just a small proportion of untested PWH with VF.
Several recently published cohort studies described reduced rates of VF and/or HIVDR over time between 1996–2000 and 2011–2015 [3–5, 9]. Our study, which began in 2010, did not demonstrate this trend. This suggests that despite the many improvements in ART efficacy and safety, treatment nonadherence and discontinuation remain a concern. The stable rate of VF and HIVDR throughout the study may reflect the fact that although treatments improved, PWH in the later years were more likely to be younger and non-Caucasian, demographic factors associated with an increased risk of nonadherence [4, 5, 20].
In the United States, between 2012 and 2016, newly diagnosed HIV among young adults age <30 increased by 6%, whereas newly diagnosed HIV among older adults declined or stabilized [21, 22]. In 2017, PWH under 30 accounted for 41% of new HIV-1 infections. We observed a similar trend, with 40% of those starting ART in 2017 being younger than 30. In 2017, 74% of newly diagnosed PWH in the United States were non-Caucasian. Although the overall proportion of non-Caucasian PWH in our cohort was 59%, it had increased to 70% by the final study year.
One important aspect of this study is that the population comprised PWHs receiving ART in an integrated health care system, defined as a network of hospitals, physicians, and other providers that coordinate care in return for fees covered by self- or employer-funded insurance. The population in our cohort is therefore more socioeconomically similar to the 34% of the US PWH population that has private insurance [23].
Our study has several limitations. First, because it was based solely on an electronic medical research database and not a chart review, we do not know the basis for VF or the decision of whether to do genotypic resistance testing in persons with VF. The PWH who discontinued therapy may have had intolerance, side effects, or been nonadherent for other reasons. Second, because it was based on clinical practice and not a prospectively followed cohort, the frequency of clinic visits and laboratory monitoring varied within the cohort. As in any multivariate analysis, residual confounding cannot be excluded, particularly with respect to ART, as PWH baseline characteristics often influence therapy and the perceived risk of VF [24]. Finally, 21% of the population was censored as a result of loss to follow-up; however, this occurs commonly in the United States as persons often leave a health care system as a result of moving, changing employment, or no longer being able to afford private insurance.
In conclusion, despite the frequent use of modern regimens, including an increasing number of single-tablet regimens, the proportion of PWH starting ART who experience VF or HIVDR is low but perhaps not decreasing. Public health efforts should focus on PWH of younger age, non-Caucasian race, or those who present to care late, as evidenced by lower CD4 counts and higher VLs. Further efforts to facilitate long-term treatment virological response rates will prevent the consequences of ongoing virus replication, including those associated with chronic inflammation, virus transmission, and the development of drug resistance.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This study was supported by a research grant from Janssen Scientific Affairs. S.Y.R. and R.W.S. were also supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institute of Health (NIH; award number AI136618). Janssen Scientific Affairs participated in the study design and reviewed drafts of the manuscript.
Potential conflicts of interest. This study was supported by a research grant from Janssen Scientific Affairs, which also participated in the study design and reviewed drafts of the manuscript. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Carr A, Richardson R, Liu Z. Success and failure of initial antiretroviral therapy in adults: an updated systematic review. AIDS 2019; 33:443–53. [DOI] [PubMed] [Google Scholar]
- 2. Weijsenfeld AM, Blokhuis C, Stuiver MM, et al. Longitudinal virological outcomes and factors associated with virological failure in behaviorally HIV-infected young adults on combination antiretroviral treatment in the Netherlands, 2000 to 2015. Medicine 2019; 98:e16357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 2017; 4:e349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tanner Z, Lachowsky N, Ding E, et al. ; Canadian Observation Cohort (CANOC) Collaboration . Predictors of viral suppression and rebound among HIV-positive men who have sex with men in a large multi-site Canadian cohort. BMC Infect Dis 2016; 16:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nance RM, Delaney JAC, Simoni JM, et al. HIV viral suppression trends over time among HIV-infected patients receiving care in the United States, 1997 to 2015: a cohort study. Ann Intern Med 2018; 169:376–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bontell I, Häggblom A, Bratt G, et al. Trends in antiretroviral therapy and prevalence of HIV drug resistance mutations in Sweden 1997–2011. PLoS One 2013; 8:e59337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Luca A, Dunn D, Zazzi M, et al. ; SEHERE collaboration in Chain . Declining prevalence of HIV-1 drug resistance in antiretroviral treatment-exposed individuals in Western Europe. J Infect Dis 2013; 207:1216–20. [DOI] [PubMed] [Google Scholar]
- 8. Davy-Mendez T, Eron JJ, Brunet L, et al. New antiretroviral agent use affects prevalence of HIV drug resistance in clinical care populations. AIDS 2018; 32:2593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scherrer AU, von Wyl V, Yang WL, et al. ; Swiss HIV Cohort Study . Emergence of acquired HIV-1 drug resistance almost stopped in Switzerland: a 15-year prospective cohort analysis. Clin Infect Dis 2016; 62:1310–7. [DOI] [PubMed] [Google Scholar]
- 10. Lodi S, Günthard HF, Dunn D, et al. ; HIV-CAUSAL Collaboration . Effect of immediate initiation of antiretroviral treatment on the risk of acquired HIV drug resistance. AIDS 2018; 32:327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li X, Brown TT, Ho KS, et al. Recent trends and effectiveness of antiretroviral regimens among men who have sex with men living with HIV in the United States: the Multicenter AIDS Cohort Study (MACS) 2008–2017. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. US Public Health Service. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2019. Available at: https://aidsinfo.nih.gov/guidelines. Accessed 6 December 2019.
- 13. Rhee SY, Clutter D, Fessel WJ, et al. Trends in the molecular epidemiology and genetic mechanisms of transmitted human immunodeficiency virus type 1 drug resistance in a large US clinic population. Clin Infect Dis 2019; 68:213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society-USA Panel. JAMA 2018; 320:379–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bennett DE, Camacho RJ, Otelea D, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 2009; 4:e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paredes R, Tzou PL, van Zyl G, et al. Collaborative update of a rule-based expert system for HIV-1 genotypic resistance test interpretation. PLoS One 2017; 12:e0181357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tzou PL, Rhee SY, Descamps D, et al. ; WHO HIVResNet Working Groups . Integrase strand transfer inhibitor (INSTI)-resistance mutations for the surveillance of transmitted HIV-1 drug resistance. J Antimicrob Chemother 2020; 75:170–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982; 69:239–41. [Google Scholar]
- 19. Gordon LL, Gharibian D, Chong K, Chun H. Comparison of HIV virologic failure rates between patients with variable adherence to three antiretroviral regimen types. AIDS Patient Care STDS 2015; 29:384–8. [DOI] [PubMed] [Google Scholar]
- 20. Youn B, Shireman TI, Lee Y, et al. Trends in medication adherence in HIV patients in the US, 2001 to 2012: an observational cohort study. J Int AIDS Soc 2019; 22:e25382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. Diagnoses of HIV infection in the United States and dependent areas. 2017. Available at: https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2017-vol-29.pdf. Accessed 1 May 2020.
- 22. Guilamo-Ramos V, Thimm-Kaiser M, Benzekri A, Futterman D. Youth at risk of HIV: the overlooked US HIV prevention crisis. Lancet HIV 2019; 6:e275–8. [DOI] [PubMed] [Google Scholar]
- 23. Dawson L, Kates J. An update on insurance coverage among people with HIV in the United States. 2019. Available at: https://www.kff.org/report-section/an-update-on-insurance-coverage-among-people-with-hiv-in-the-united-states-findings/. Accessed 1 May 2020.
- 24. Alejos B, Suárez-García I, Bisbal O, et al. ; CoRIS cohort . Choice of the initial antiretroviral treatment for HIV-positive individuals in the era of integrase inhibitors. PLoS One 2019; 14:e0221598. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.