From the Authors:
We received with great interest the letters by Tuffet and colleagues and by Michard and Shelley commenting on our work (1), which allows us to further explore the main findings of the study.
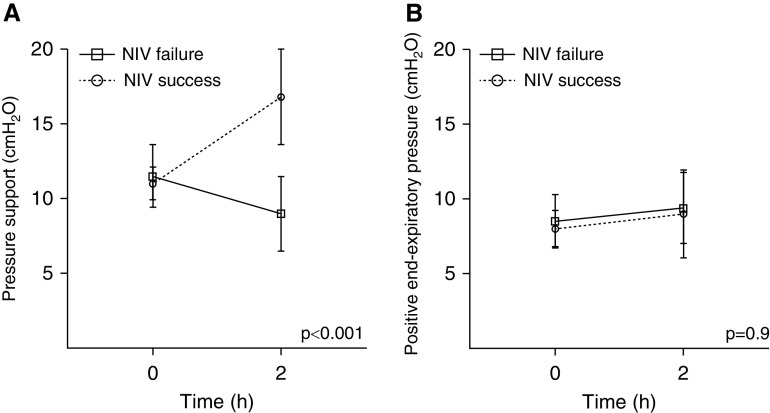
Tuffet and colleagues correctly noted that the different magnitude of dynamic transpulmonary pressure, as recorded after 2 hours of noninvasive ventilation (NIV) trial and compared with the baseline level of esophageal pressure swing, suggests different ventilator’s adjustments between patients who succeeded or failed NIV. In particular, the median value of pressure support (PS) set at 2 hours was different in the two groups (P < 0.001), whereas positive end-expiratory pressure level was not (Figure 1). With regard to this point, we have to stress that our study was observational and that NIV setting was adjusted by the attending physician who was blind to the study purpose and the physiological recording. PS was initially set at 10 cm H2O and then progressively modified to target an expiratory Vt below 9.5 ml/kg of predicted body weight to avoid a potentially harmful ventilation (2). Because the respiratory rates were reported similar for both groups after 2 hours, it is arguable that PS has been reduced with the aim to minimize Vt. The role of esophageal manometry has been strengthened as a reliable parameter with the potential to detect the actual magnitude of (inspiratory) effort, thus identifying those patients at major risk for NIV failure. Therefore, we agree with Tuffet and colleagues that the esophageal manometry–driven adjustment of respiratory support might avoid early intubation in a subset of patients with de novo respiratory failure. However, we also have to consider that in patients with acute respiratory distress syndrome, the inspiratory effort might be also affected by stimuli not subjected to ventilatory support (3), such as different degree of lung inflammation influencing the respiratory drive (4) through the activation of pulmonary C-fibers, vagal-nerve stimulation, and pulmonary stretch receptor inhibition (5). Hence, although NIV may decrease respiratory drive through the respiratory muscles’ unloading, there may be a subset of patients with acute respiratory distress syndrome in whom PS would not influence the magnitude of effort. Therefore, esophageal pressure monitoring will allow the optimization of ventilatory setting to target the best level of pressure on one hand and the identification of those patients whose effort would not be affected by NIV on the other.
Figure 1.
(A and B) Mean change of pressure support (A) and positive end-expiratory pressure (B) delivered after 2 hours in patients who failed or succeeded the noninvasive ventilation trial. NIV = noninvasive ventilation.
Michard and Shelley questioned the reproducibility of esophageal manometry because of the supposed scarce patient’s tolerance. We have used a nasogastric tube equipped with an integrated esophageal balloon (NutriVent) that did not modify the size of the probe and was thus less likely to reduce the patient’s tolerance. In addition, the oronasal face mask equipped with a dedicated output for probes (BluestarTM, KOO Medical Equipment) was able to minimize air leaks because of the tube placement. Furthermore, our patients needed prolonged NIV support, so gastric insufflation might occur as a common treatment complication (range, 10–50%) (6), even sonographically detectable at lower inspiratory pressures (e.g., 10–15 cm H2O) in up to 35% of patients (7). In line with these evidences, the insertion of a nasogastric tube during NIV has the theoretical advantage of reducing aerophagia and gastric distension, thus improving the patient to ventilator interaction. These colleagues also discussed the potential application of the pulse oximetry waveform in estimating the magnitude of inspiratory effort and suggested the use of Pleth Variability Index monitoring to assist clinicians in prompting intubation. This would allow a noninvasive, real-time, and easy-to-apply technique to quantify the patient’s respiratory swing. However, in the hypothesis that self-inflicted lung injury might constitute a major component of NIV failure, our study aimed at objectifying the influence of dynamic transpulmonary pressure and esophageal pressure swings on clinical outcome with this setting. Therefore, we relied on manometry as the optimal procedure to obtain reliable and objective measurements of inspiratory effort during spontaneous breathing (8). Although physiological investigations have addressed the rationale for pulse oximetry waveform analysis in spontaneously breathing healthy individuals (9) and in patients undergoing NIV (10), calibration of this measure is rather difficult to obtain, and cutoff values are not available from clinical studies so far. Furthermore, it is likely that inadequate peripheral perfusion, intrathoracic or total blood volume, and arrhythmias would significantly affect Pleth Variability Index monitoring under this condition, as described for all the noninvasive cardiovascular monitoring (11). Reproducible, well-standardized, but still to come, less invasive techniques to assess the patient’s respiratory effort are more than welcomed to enhance their usage in the critical care setting.
Supplementary Material
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202005-1730LE on June 3, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Tonelli R, Fantini R, Tabbì L, Castaniere I, Pisani L, Pellegrino MR, et al. Inspiratory effort assessment by esophageal manometry early predicts noninvasive ventilation outcome in de novo respiratory failure: a pilot study. Am J Respir Crit Care Med. doi: 10.1164/rccm.201912-2512OC. [online ahead of print] 23 Apr 2020; DOI: 10.1164/rccm.201912-2512OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carteaux G, Millán-Guilarte T, De Prost N, Razazi K, Abid S, Thille AW, et al. Failure of noninvasive ventilation for de novo acute hypoxemic respiratory failure: role of tidal volume. Crit Care Med. 2016;44:282–290. doi: 10.1097/CCM.0000000000001379. [DOI] [PubMed] [Google Scholar]
- 3.Spinelli E, Mauri T, Beitler JR, Pesenti A, Brodie D. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med. 2020;46:606–618. doi: 10.1007/s00134-020-05942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacono FJ, Peng YJ, Nethery D, Faress JA, Lee Z, Kern JA, et al. Acute lung injury augments hypoxic ventilatory response in the absence of systemic hypoxemia. J Appl Physiol (1985) 2006;101:1795–1802. doi: 10.1152/japplphysiol.00100.2006. [DOI] [PubMed] [Google Scholar]
- 5.Lin S, Walker J, Xu L, Gozal D, Yu J. Behaviours of pulmonary sensory receptors during development of acute lung injury in the rabbit. Exp Physiol. 2007;92:749–755. doi: 10.1113/expphysiol.2006.036673. [DOI] [PubMed] [Google Scholar]
- 6.Carron M, Freo U, BaHammam AS, Dellweg D, Guarracino F, Cosentini R, et al. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anaesth. 2013;110:896–914. doi: 10.1093/bja/aet070. [DOI] [PubMed] [Google Scholar]
- 7.Bouvet L, Albert ML, Augris C, Boselli E, Ecochard R, Rabilloud M, et al. Real-time detection of gastric insufflation related to facemask pressure-controlled ventilation using ultrasonography of the antrum and epigastric auscultation in nonparalyzed patients: a prospective, randomized, double-blind study. Anesthesiology. 2014;120:326–334. doi: 10.1097/ALN.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 8.Mauri T, Yoshida T, Bellani G, Goligher EC, Carteaux G, Rittayamai N, et al. PLeUral pressure working Group (PLUG—Acute Respiratory Failure section of the European Society of Intensive Care Medicine) Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med. 2016;42:1360–1373. doi: 10.1007/s00134-016-4400-x. [DOI] [PubMed] [Google Scholar]
- 9.Addison PS. Respiratory effort from the photoplethysmogram. Med Eng Phys. 2017;41:9–18. doi: 10.1016/j.medengphy.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Contal O, Carnevale C, Borel J-C, Sabil A, Tamisier R, Lévy P, et al. Pulse transit time as a measure of respiratory effort under noninvasive ventilation. Eur Respir J. 2013;41:346–353. doi: 10.1183/09031936.00193911. [DOI] [PubMed] [Google Scholar]
- 11.Monnet X, Guérin L, Jozwiak M, Bataille A, Julien F, Richard C, et al. Pleth variability index is a weak predictor of fluid responsiveness in patients receiving norepinephrine. Br J Anaesth. 2013;110:207–213. doi: 10.1093/bja/aes373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.