The slogan “Coughs and Sneezes Spread Diseases” was coined in the United States during the last great flu pandemic between 1918 and 1920 to highlight the role of coughs and sneezes in disseminating respiratory pathogens. A cough produces approximately 3,000 droplets, whereas a sneeze releases an estimated 40,000 (1–3). The recent pandemic of a novel infectious disease (coronavirus disease [COVID-19]) related to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (4) highlights the importance of understanding the generation and fate of the droplets created by coughing and sneezing and other aerosol-generating procedures (AGPs), so as to provide a scientific basis for various preventive measures used to limit the transmission of infection. This work has not previously been published or presented in abstract form.
Sneeze
Irritation of the mucous membranes of the nose or throat produces a deep inspiration followed by depression of the soft palate and palatine uvula with elevation of the back of the tongue that partially closes the passage to the mouth. Air bursts suddenly through the lungs with variable force, expelling mucus containing foreign particles or irritants from the nasal cavity.
Cough
Stimulation of sensory nerve fibers (branches of the vagus nerve) located in the ciliated epithelium of the upper airways and cardiac and esophageal branches from the diaphragm by infection, inflammation, or irritation provokes cough (5). Afferent impulses from these sensory fibers travel to the medulla where they are coordinated in the cough center. The efferent pathway of the reflex arc involves impulses that travel from the cough center via the vagus, phrenic, and spinal motor nerves to the diaphragm, abdominal wall, and muscles (5). Afferent inputs to the brain stem are also relayed to higher brain regions, where inputs are integrated in pontine, subcortical, and cortical nuclei (6). The motor and premotor cortical brain regions can voluntarily initiate a cough by descending pathways that may bypass brainstem integrative centers (7).
The cough maneuver includes an initial deep inhalation followed by a compression phase, in which contraction of the muscles of the chest wall, diaphragm, and abdominal wall along with closure of the glottis produces a rapid rise in intrathoracic pressure. In the subsequent expiratory phase, the glottis suddenly opens, and the high intrathoracic pressure generated during compression promotes an initial high expiratory airflow (up to 12 L/s) that breaks up mucus into smaller droplets and is accompanied by the sound of coughing (8).
The interaction between gas flow and mucus in the airways could be modeled as two-phase gas–liquid flow, that is, the simultaneous transport of gas and liquid in the same tube (9). Droplet formation in the respiratory tract probably occurs by three mechanisms (Figure 1). The first mechanism is the instability caused by the shear stress on the mucus–air interface by the high expiratory airflow generated during coughing, which dislodges mucus from the airways and breaks it up into smaller droplets. Compression of the airways creates waves of mucus, and the shear stresses from the high airflow causes small droplets to break off from the crest of these waves. The thickness of the mucus layer, its viscoelastic properties, and surface tension at the mucus–air interface influence the critical air speed required to initiate the instability. Such interfacial shearing has its peak within the trachea where airflow is the highest (10). Second, dynamic compression of the airways by high intrathoracic pressure makes them vibrate with their walls approximating each other. This compression squeezes and loosens mucus and promotes expulsion of foreign material from the airways. Vibration of the vocal cords and vocalization also contribute to droplet generation (2, 3). The third mechanism probably operates during normal tidal breathing when shear forces provided by respiratory airflow are insufficient to induce instabilities, and respiratory droplet formation probably occurs by reopening of collapsed terminal airways at the beginning of inspiration (Figure 1) (10, 11).
Figure 1.
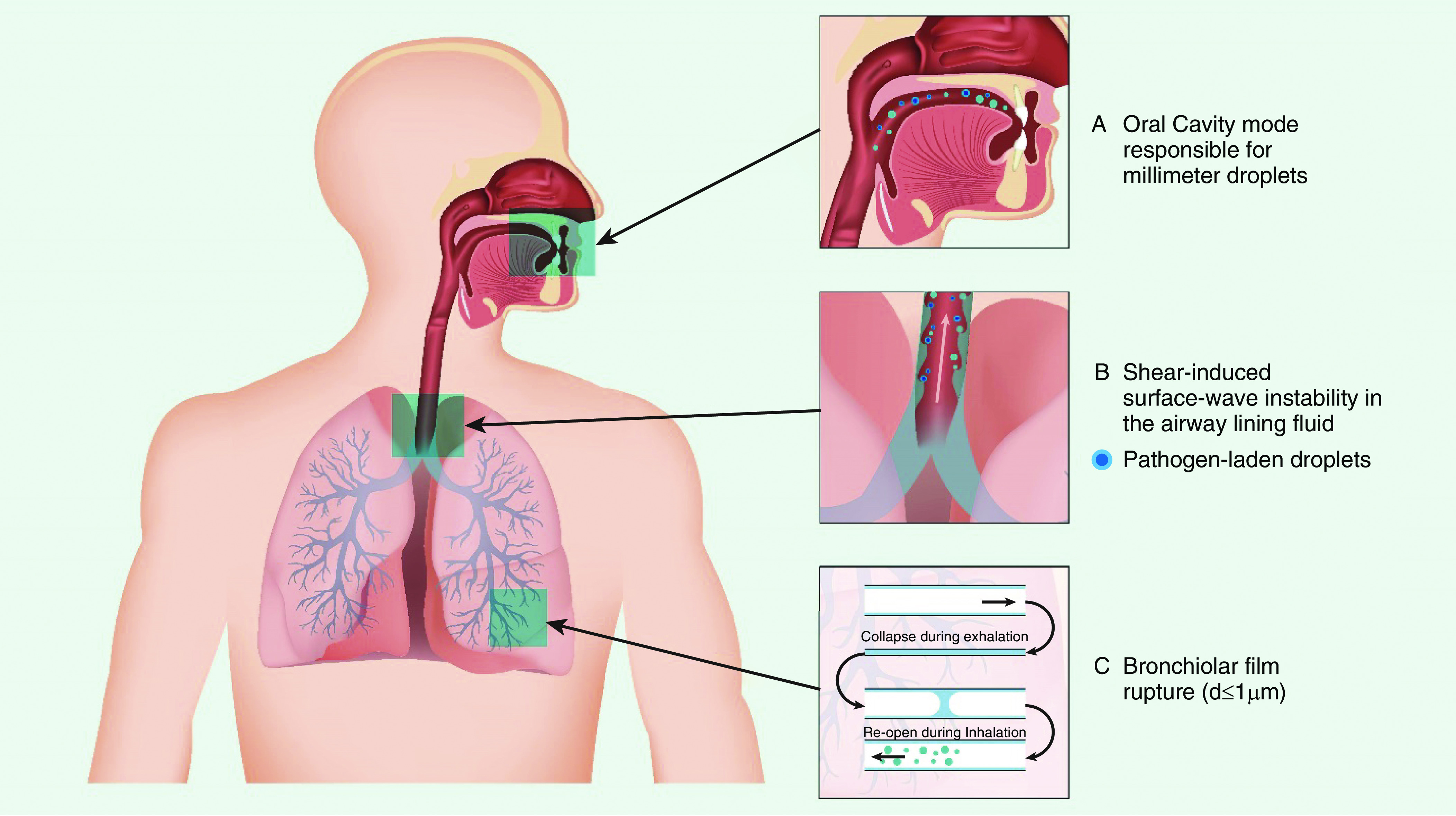
Schematic showing the site of origin and mechanisms of droplet generation from the respiratory tract. Adapted by permission from Reference 10.
The content and size of droplets expelled by an infected person depend largely on their site of origin. The oral cavity produces larger droplets (∼100 μm in size) during speech and coughing, whereas smaller droplets (1 μm) originate in the bronchioles during normal breathing and the larynx during talking and coughing (Figure 1) (12–14). The size of coughed droplets was reported to be between 0.62 and 15.9 μm (average mode size 8.35 μm) in one report (11), but the distribution of particle sizes could be altered by the presence of viral infections (15).
In several investigations, the number of droplets produced by various activities (coughing, sneezing, breathing, phonation, etc.) is very variable (16–18). By using a laser light scattering method, 1 minute of loud speaking was estimated to produce thousands of fluid droplets from the oral cavity per second; of these, at least 1,000 droplet nuclei contain virions, and under the conditions of the experiment, they could remain airborne for more than 8 minutes (19). Notably, patients infected with influenza virus exhaled aerosol particles containing infectious viral particles more frequently after coughing than after a forceful exhalation (20). Individuals who produce much higher quantities of infectious aerosols may be more likely to spread infection and be responsible for the “super spreader effect” in which an individual is responsible for infecting an unusually large number of susceptible individuals (17, 21). Other factors to consider in the spread of respiratory viral infections are the frequency of respiratory events, viral concentration in the exhaled fluid and its volume, and the duration of exposure to an infected individual (17). Because breathing and speaking occur more frequently than coughs and sneezes, they could have an important role in transmission of viral infections, especially from asymptomatic infected individuals.
Larger droplets settle quickly, whereas small airborne droplet nuclei are transported over longer distances by airflow (22). The distance droplets traverse depends on how forcefully a person coughs or sneezes. Large respiratory droplets containing pathogens like influenza can travel approximately 6 feet when a sick person coughs or sneezes (23–25). The aerosol expelled from the mouth during a cough emerges not as individual droplets but as a jet with a leading vortex (25) that has properties similar to those of a puff from a pressurized metered-dose inhaler (26, 27) and can penetrate an impressive distance into the surrounding ambient air before finally dissipating (28). Thus, emissions from coughs and sneezes contain droplets of various sizes suspended in a multiphase turbulent buoyant cloud (29). Turbulence sweeps around smaller particles, and eddies within the cloud resuspend the particles so that they settle more slowly, with some particles traveling more than 8 feet horizontally through the air (28, 29). Moreover, smaller droplets could spray 13–20 feet vertically in the air, which is theoretically high enough to enter and travel through ceiling ventilation systems in some buildings (29).
Most droplet transmission likely occurs at close range because of dilution and inactivation of viruses over longer periods and greater distances. In respiratory exhalation flows, the large droplets between 60 and 100 μm in size are expected to completely evaporate before traveling 2 m (30). These large droplets are carried farther away when they are expelled at high velocity, such as with coughs and sneezes. The time it takes particles to fall to the floor depends on their size; for example, particles 100 μm in diameter take about 10 seconds, whereas 10-μm–diameter particles are estimated to take 17 minutes to fall to the floor, and 1- to 3-μm–diameter particles could remain suspended almost indefinitely (31). Infectious droplets carried farther away by airflow from an air conditioner were suggested to have transmitted SARS-CoV-2 among diners at adjacent tables in a restaurant (32).
Airborne Transmission of Respiratory Viruses by Cough
Respiratory viruses transmit by multiple modes, including contact and by airborne transmission (Figure 2) (33). The SARS-CoV-2 virus primarily spreads by droplet transmission but has been reported in one experimental study to last for up to 4 hours on copper surfaces, 24 hours on cardboard, and 2–3 days on less porous surfaces such as plastic and stainless steel (34). These contaminated surfaces could be a potential source of transmission to other individuals who touch the same object or surface and then touch their mouth, nose, or eyes. Indirect transmission, through contaminated objects in a shopping mall in China, was probably responsible for a cluster of cases of COVID-19 (35).
Figure 2.
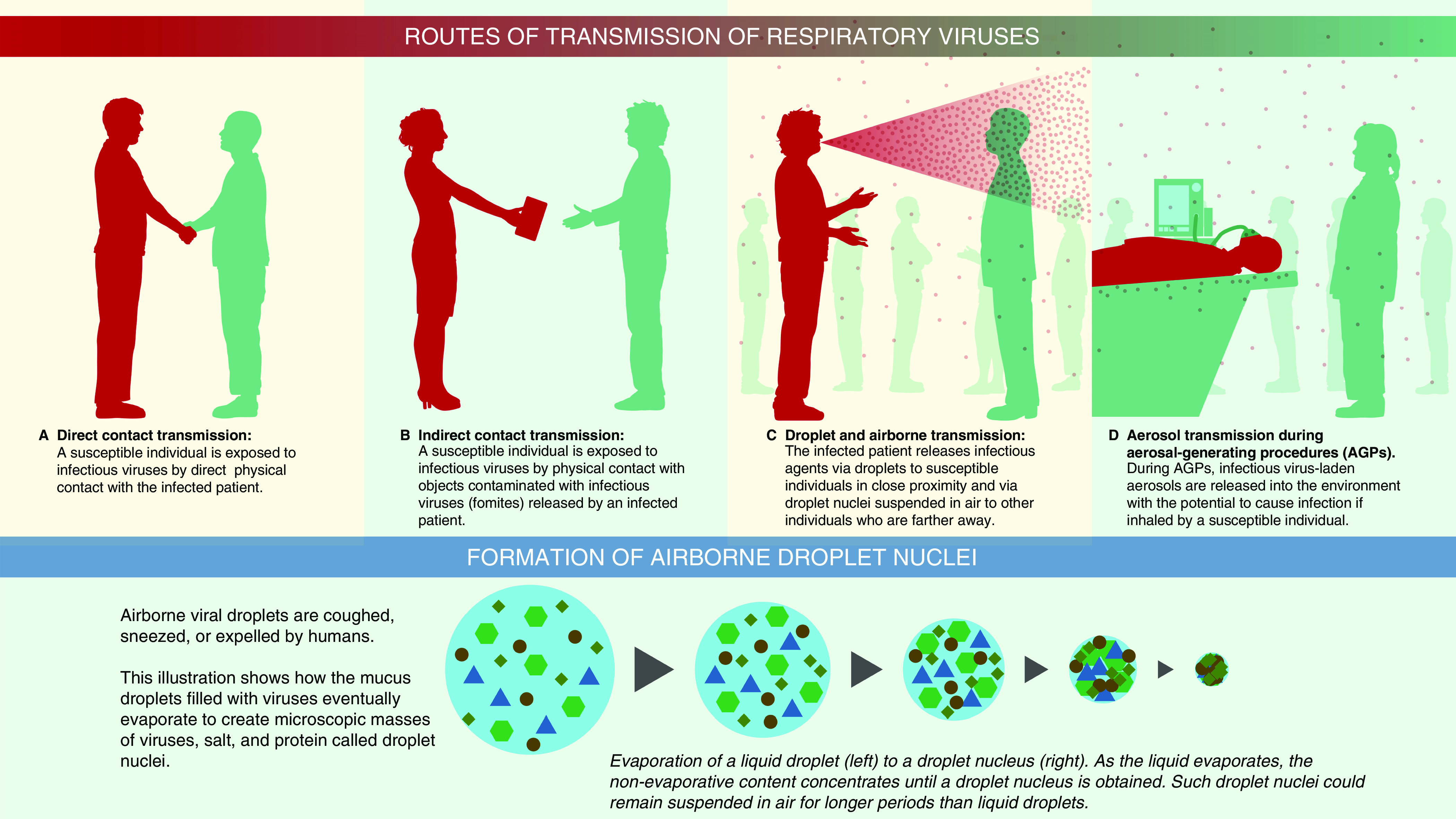
Illustration to show various routes of transmission. For contact transmission, an infected person can transfer virus-laden respiratory secretions by (A) direct physical contact or (B) indirectly. If an infected person sneezes or coughs and droplets deposit or if they have the virus on their hands from touching their face or blowing their nose and then touch an object or surface, then that object or surface serves as a repository for the contagion. When another individual touches the same object or surface that has the virus on it and then touches their mouth, nose, or eyes, the virus is transmitted to these mucosal surfaces. The most common mode of spread for respiratory viruses is via (C) respiratory droplet transmission. Virus-laden droplets (generated by coughing, sneezing, or talking) are propelled from an infected person directly onto the mucosal surfaces of a host. Respiratory droplets are larger and generally fall to the ground after traveling short distances. Transmission of infection can also occur indirectly after the infected droplets have deposited if a host touches the contaminated surface and then touches their face. (C) Airborne transmission occurs when virus-laden fine respiratory droplets remain viable in the environment and are inhaled by a susceptible individual. This transmission can occur either directly by inhalation of fine droplets expelled from (C) an infected person or (D) during aerosol-generating procedures on an infected individual. Larger droplets expelled by coughing or sneezing evaporate, and these smaller and drier droplet nuclei containing infective microorganisms (lower panel) remain suspended in air for extended periods. They have the potential to deposit in the lower respiratory tract after they are inhaled. Larger droplet nuclei that settle out from the air can potentially be resuspended after their size decreases from evaporation, in combination with an aerosol-generating activity such as making a bed or while doffing personal protective equipment.
Viral nucleic acids, and in some instances viable viruses, have been detected in environmental aerosols in healthcare settings (36–38). A rising plume of contaminated air, possibly owing to suction created by an exhaust fan, entered an air shaft and was thought to be responsible for an outbreak of SARS-CoV-1 in Hong Kong (39). Some preliminary evidence supports airborne transmission of SARS-CoV-2 virus (40–42). Santarpia and colleagues collected air and surface samples from rooms of patients with COVID-19 and found viral RNA in the air both inside and outside the rooms and on ventilation grates (40). Another study from Singapore (41) did not find SARS-CoV-2 in air samples in isolation rooms in an outbreak center. They reported the highest virus concentrations in toilet facilities and positive results from air outlet fans. Another investigation in Wuhan, the city at the epicenter of the original outbreak in China, also found low or undetectable airborne concentration of SARS-CoV-2 but recorded elevated airborne concentration of the virus inside mobile toilet facilities (42). Interestingly, they reported higher concentration of airborne SARS-CoV-2 in initial samples from medical staff areas and proposed that the virus was aerosolized while doffing personal protective equipment (PPE) (42). Most environmental sampling studies reported detection of viral RNA, but few studies demonstrated a recovery of viable virus, which limits the interpretation for the risk of airborne transmission. SARS-CoV-2 virus particles were detected in the air for a median of about 2.7 hours under the conditions of one experiment that may not have accurately reflected droplet production by coughs and sneezes (34). Current evidence does not establish effective spread of SARS-CoV-2 virus via airborne route between individuals. At the time of writing, the World Health Organization’s opinion is that SARS-CoV-2 is transmitted by respiratory droplets and by contact, and the virus could become airborne during procedures or treatments that generate aerosols (43).
Mechanisms of Particle Deposition in the Respiratory Tract
The air we breathe contains particles of various sizes. After airborne virus particles are inhaled, the nose effectively filters inhaled larger particles. However, the oropharynx is not as effective a filter as the nose, and smaller particles have a high probability of penetrating into the lower respiratory tract (44, 45). Therefore, mouth breathing increases the dose of respirable particles to the lung compared with nose breathing. Although the nose is an effective filter for most large particles, the optimal particle size that enables deposition in the respiratory tract is difficult to define precisely because of the changing diameter as droplets travel through air. Various cutoffs have been proposed, with some authors proposing a diameter ≤5 μm as a cutoff, but particles of ≤20 μm can desiccate to form droplet nuclei (46). In contrast, most particles >20 μm in diameter do not deposit in the lower respiratory tract (47).
The mass of the inhaled particles and their diameter and shape determines the rate at which they deposit onto airway surfaces (44, 45, 47). The most important characteristics of particles are the geometric size (d) and density (ρ) because these characteristics determine the particle’s inertia and transport velocity. Spheres that have the same transport velocity exhibit the same aerodynamic behavior and similar deposition patterns in the lung. Table 1 shows some commonly used terminology related to aerosol deposition in the respiratory tract, and Figure 3 shows the mechanisms involved in particle deposition in the respiratory tract (44).
Table 1.
Commonly Used Terminology to Describe Characteristics of Aerosols
| Parameter | Abbreviation | Comments |
|---|---|---|
| Aerodynamic diameter | AD | The AD is the diameter of a fictitious sphere of unit density (1 g cm−3) that has the same gravitational (settling) velocity in the same gas as the actual particle. |
| AD = d (sg)1/2; where sg = ρparticle/ρwater. | ||
| For particles of unit density, the AD is the same as the physical diameter. | ||
| Mass median aerodynamic diameter | MMAD | The MMAD divides the aerosol size distribution in half by mass. It is the diameter at which half the mass of the aerosol particles is contained in particles with larger diameter and the other half in particles with smaller diameter. |
| Geometric SD | GSD | GSD is a measure of dispersion of particle sizes within an aerosol. The GSD is the ratio of the median diameter to the diameter at ±1 SD from the median diameter. In a cumulative distribution plot of the AD and mass of particles, the GSD is calculated as the ratio of the median diameter to the diameter at 15.9% of the probability scale, or the ratio of the diameter at 84.1% on the probability scale to the median diameter. Aerosols with GSD ≥ 1.22 are considered polydisperse. |
Figure 3.
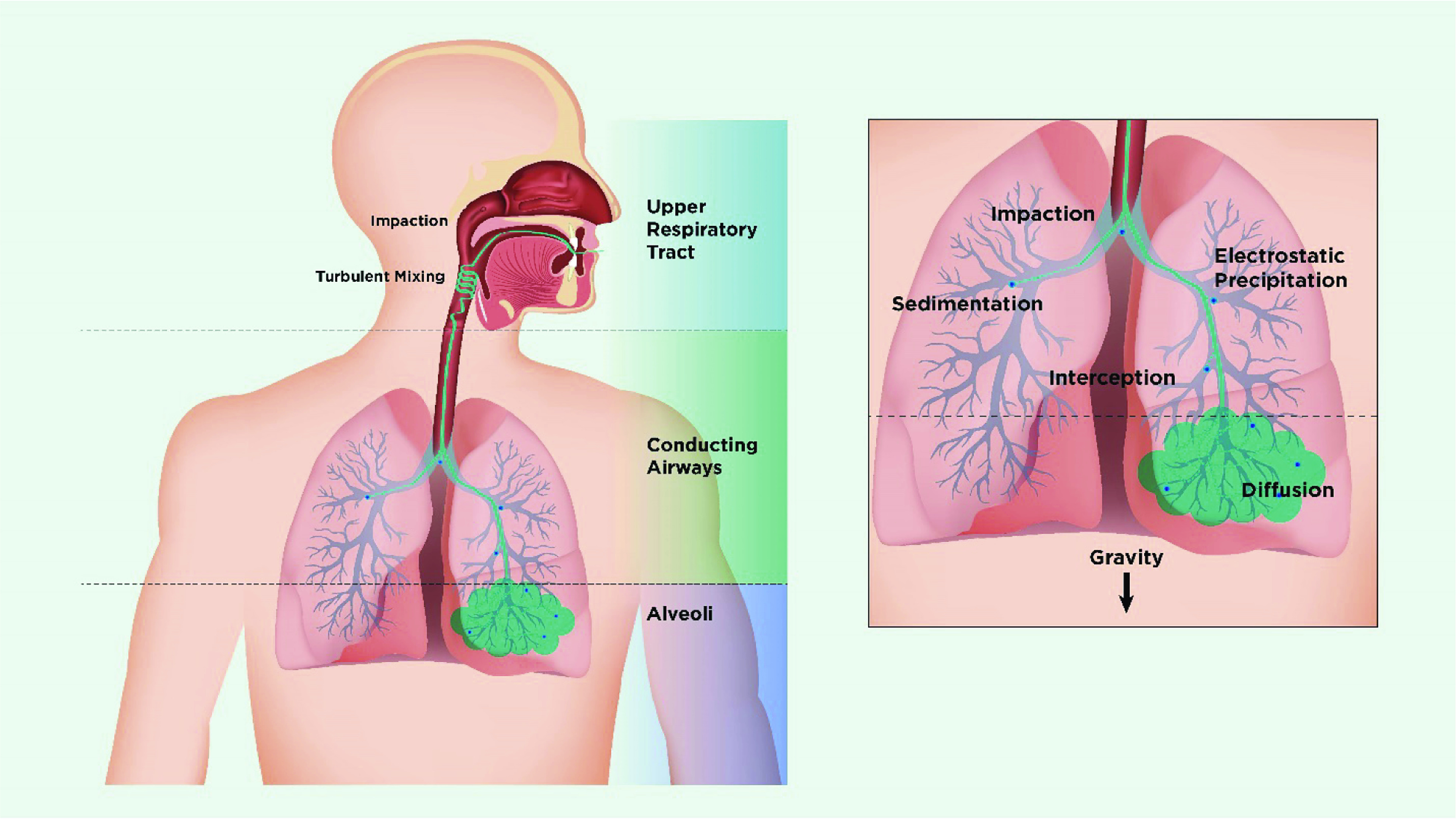
Schematic showing mechanisms of deposition of inhaled particles in the lung. On entering the nasal or oral cavity, particles deposit by impaction, turbulent mixing, sedimentation, and Brownian motion depending on their size. Particles >5 μm in aerodynamic diameter are most likely to deposit by impaction in the oropharynx and be swallowed, whereas particles <5 μm have the greatest potential for lung deposition. Particles between 4 and 5 μm deposit primarily in the bronchial/conducting airways, whereas smaller particles remain suspended in the airstream and penetrate to the peripheral airways and alveoli. In the lung periphery, a significant reduction in airflow rate allows particles to deposit predominantly by sedimentation, with gravity causing them to “rain out” and deposit. Most particles between 0.1 and 1 μm diffuse by Brownian motion and deposit when they collide with the airway wall. The longer the residence time in the smaller, peripheral airways, the greater the deposition from sedimentation and Brownian motion processes. Inhaled particles that do not deposit are exhaled. Adapted by permission from Reference 80.
The physical characteristics of the particle (e.g., mass and shape), the gas flow in which the particle is transported, the patient’s breathing pattern, velocity provided to the particle (e.g., by a propellant), and the airway anatomy (especially the presence of airway obstruction) determine the location of particle deposition within the airway (44, 45, 47). The inspiratory flow rate influences aerosol deposition, with slow, deep inspirations favoring deeper penetration in the lung and fast inspirations targeting the tracheobronchial region for deposition. Likewise, lung disease influences particle deposition, with higher deposition at the site of obstructed airways and reduced deposition in airways distal to the site of obstruction (44, 45).
Infection risk to the susceptible host caused by inhaled droplets depends on the quantity of the pathogen and on its site of deposition. The size of viruses varies from 0.02 to 0.3 μm, and that of bacteria from 0.5 to 10 μm in their naked form (2). During tidal breathing, virus particles may be contained in fine particles (48, 49). Analysis of aerosol particles from human coughs found that 35% of the influenza RNA detected was contained in particles >4 μm in aerodynamic diameter, 23% in particles 1–4 μm, and 42% in particles <1 μm, such that much of the viral RNA was contained within respirable particles with the potential to deposit in the lungs (50). The viral load within the droplets influences the probability of transmitting infection after inhalation. The probability of a droplet containing at least one virion depends on its initial hydrated volume. For COVID-19, the average virus RNA load in oral fluid has been estimated to be 7 × 106 copies/ml (51), but some patients may have a much higher titer (52). With this level of infection, there is a ∼37% probability that a droplet measuring 50 μm in diameter before dehydration contains at least one virion (19, 51), and this probability is reduced 100-fold in droplets with a diameter of 10 μm. Although very few particles actually carry pathogens (3), the number of small particles far exceeds the number of larger-sized droplets.
In exhaled breath, which has smaller particles than those in coughs and sneezes, quantitative PCR found greater influenza copy numbers in the fine (<5 μm) fraction compared with the coarse (>5 μm) fraction (49). This observation suggests that the infectious dose of influenza via aerosol may be lower than that with large droplets because of the greater likelihood of fine particles to deposit in the lower respiratory tract (49). Likewise, subjects infected with influenza expelled fine droplets (count median diameter, 0.57–0.71 μm; geometric SD, 1.54–1.83) with cough, and they produced a higher number of particles when they coughed compared with the same individuals after they recover from the infection and healthy subjects (53). Moreover, particles from infected persons contain viable virions (20, 49, 50).
Relative humidity of the indoor environment could alter the particle’s aerodynamic diameter, length of time airborne, and viability. Knight estimated that a 1.5-μm hygroscopic particle increases to 2.0 μm in diameter on passage through the nose and to 4.0 μm in the saturated air of the nasopharynx and the lung (54). Microorganisms are hygroscopic, and growth in particle size within airways could increase their retention in the tertiary bronchioles and alveolar ducts. This change is especially significant for viral aerosols because they are highly infectious for the peripheral airways in the lung (54). Other indoor environmental factors, besides relative humidity, that influence viral transmission include the temperature; ventilation; the size of the room; frequency of air exchanges; air turbulence; ultraviolet radiation (sunlight); inorganic and organic contents, such as mucus or saliva, to which the particles are attached; duration of exposure; the type of virus; and the use of disinfectants (17, 55–57).
Aerosol-Generating Procedures and Respiratory Tract Infections
As discussed above, dispersion effects of the virus in ambient air rely on the amount of virus production, particle size of patient-generated droplets, and the speed and distance of transportation (57). AGPs such as intubation, bronchoscopy, physiotherapy, and suctioning generate potential infectious bioaerosols by provoking cough (58) and are associated with increased infection rates among employees working in health care (59). In contrast, AGPs such as oxygen therapy, use of humidified high-flow nasal cannula (HFNC), noninvasive ventilation (NIV), and manual ventilation via mask are less about “generating” bioaerosols and more about “dispersing” bioaerosols farther away from the patient (Figure 4). Notably, evidence linking AGPs to spread of viral infections among healthcare providers is limited by the low quality of the studies (58).
Figure 4.
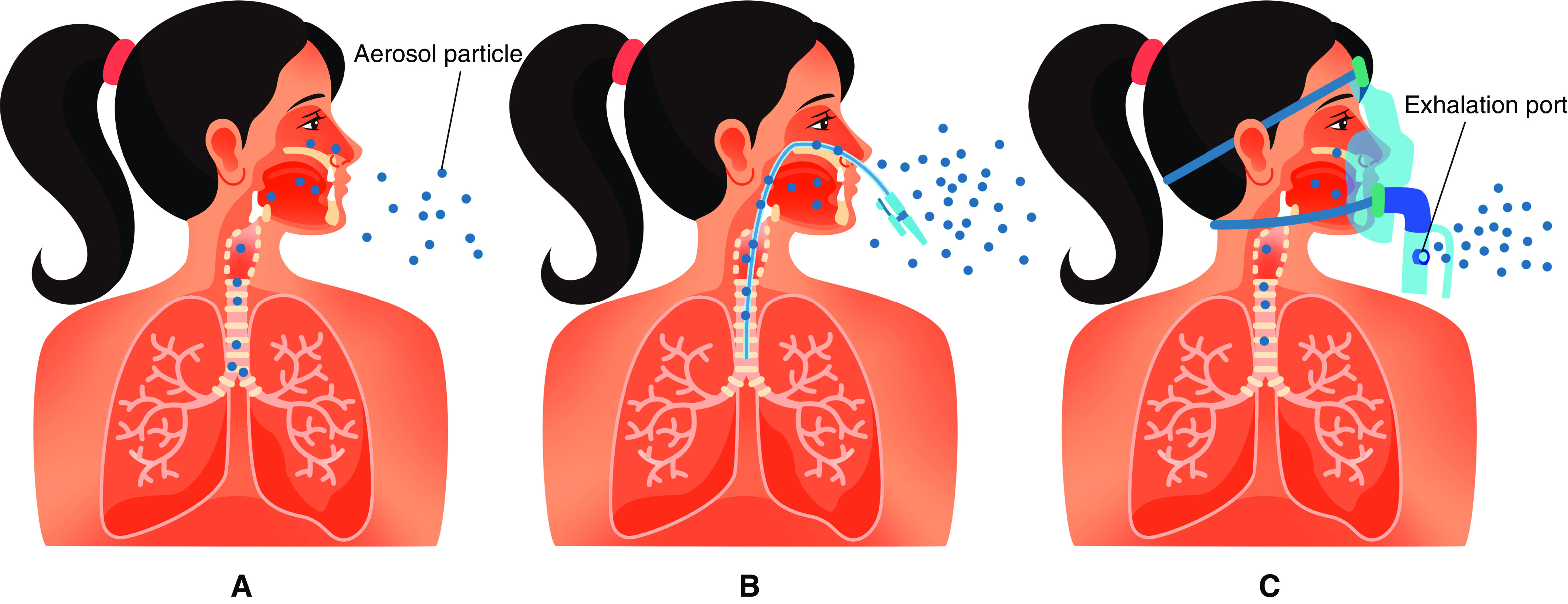
Illustration to show the difference between aerosol “generating” versus aerosol “dispersing” procedures. A shows that a small amount of aerosols generated during normal breathing travel short distances before evaporation. B shows a burst of aerosols generated during procedures that provoke coughing such as suctioning, intubation, or bronchoscopy. In C, administration of therapeutic aerosols by nebulizer, noninvasive ventilation, or use of high-flow nasal cannula could disperse aerosols from the patient as a jet to a greater distance.
Aerosol therapy significantly increases aerosol concentration in the patient’s vicinity (60, 61). Aerosols produced by medical aerosol generators do not contain pathogens unless the aerosol device is contaminated. Inhalers, including pressurized metered-dose inhalers, dry powder inhalers, or soft mist inhalers, have a low risk of contamination (62). However, the range of medications available in inhalers is limited, and drugs such as antivirals, antibiotics, mucokinetics, and prostacyclins are only available as solutions that require nebulization. The risk of medical aerosols as an AGP is, therefore, largely attributable to risk of contamination of nebulizers.
Primarily, care providers handling medication and the device may contaminate nebulizers, but contamination from the patient and nebulizer design also play important roles. Small-volume jet or ultrasonic nebulizers that are open to and positioned below the gas pathway can be contaminated by the patient’s secretions or exhaled bioaerosols when they are directly connected to the patient interface (mouthpiece or endotracheal tube) (62). In contrast, vibrating mesh nebulizers generate aerosols via mesh plates that separate the sealed medication reservoir from the patient interface. During nebulization, the aerosol derives from the fluid in the nebulizer chamber and does not carry patient-derived viral particles. In addition, residual drug remaining in jet or ultrasonic nebulizers at the end of treatment could act as a breeding environment for bacteria if the nebulizer remains in the circuit between treatments.
In simulation experiments that used smoke (an aerosol of solid particles <1 μm), in vitro studies found that HFNC and NIV dispersed exhaled air, as did other oxygen devices, including simple mask, venturi mask, and nonrebreather mask (63, 64). A randomized, controlled, crossover study in ICU patients with bacterial pneumonia who were treated with an oxygen mask at 8.6 ± 2.2 L/min versus HFNC at 60 L/min and had settle plates placed 0.4 and 1.5 m away found that bacterial counts were similar in the room air sample with each device (65). It is unclear, however, if the findings of this pragmatic study of bacterial transmission could be applied to transmission of viral infections. Personal observations (J.L.) of aerosol concentrations in the vicinity of patients with COVID-19 suggest that the masses of aerosols were not significantly different before and after HFNC use and were further reduced when a surgical mask was placed over the patient’s face. A computational fluid dynamic simulation also reached similar conclusions (66). However, if the connection of nasal cannula is loose during HFNC (63), a vented mask is used during NIV (67), or there is a large leak via the mask during NIV or manual ventilation (68), the leaking port functions as a jet that may spray the exhaled gas with virus in the ambient air, resulting in a longer dispersion distance. As such, using a tightly fitting nasal cannula (63) and placing a surgical mask over the patient’s face during HFNC (66) helps to reduce the dispersion distance of the exhaled aerosol. During NIV, vented masks should be avoided (67) and placement of a filter between a nonvented NIV mask and exhalation port or between the resuscitator bag and mask (68) is recommended for both NIV and manual resuscitators.
Prevention of Airborne Infection by Respiratory Viruses
Respiratory infection could be reduced or eliminated by interruptions of bioaerosol transmission in three phases: reducing the release of pathogen at the source, impeding pathogen transportation by air or by surface touch, and protecting susceptible persons. To reduce transmission of respiratory tract viruses:
-
1.
Avoid procedures that irritate airways and provoke violent coughing or reduce the exposure to infectious aerosol. Rapid sequence intubation is preferable because bioaerosol production is reduced by inhibiting patients’ breathing efforts and coughing with neuromuscular blockade and deep sedation (69, 70).
-
2.
If possible, caregivers should stay 6 feet away from infected patients, particularly when the patient is coughing or sneezing. Increasing air exchange frequency also helps reduce the bioaerosol concentration in the room air (65).
-
3.
Institute barriers to filter virus or reduce virus dispersion, for example, by placing a filter at the exhalation port of the mechanical ventilator or connecting a filter to the oxygen mask (e.g., HiOx Oxygen mask [Novus Medical Inc] or Respan’s Tavish mask). With the filter placed on the exhalation port of the modified nonrebreather mask, the HiOx Oxygen mask reduced the visible plume of exhaled droplets (71).
-
4.
In spontaneously breathing patients, placing a surgical mask on the patient’s face or using tissue to cover the mouth or nose, especially during coughing, sneezing, or talking, could reduce the dispersion distance (72) or virus load (73). Open systems with high-velocity gas flow, such as a vented NIV mask, should be avoided. Likewise, when invasive ventilation circuits need disconnection, such as changing an in-line suction catheter or switching ventilators, the endotracheal tube might be clamped and the ventilator turned off before disconnection.
-
5.
Use PPE for caregivers. For infection control and prevention in healthcare settings, standard precautions such as hand hygiene, respiratory hygiene, and the use of PPE are universally recommended to reduce contact transmission. The strictest precautions are needed for infections that may spread through the airborne route, such as requiring infected patients to stay in a single negative-pressure airborne isolation infection room (74). All healthcare providers and visitors who enter the patient’s room must wear a fit-tested N95 filtering respirator (38, 75, 76). Goggles/visors are also necessary for AGPs that require intimate contact (within 3 feet) with patients (76, 77). For AGPs that may generate a burst load of infectious droplets containing virus, such as intubation or bronchoscopy examination, powered air purification respirators, when available, are an alternative to N95 respirators (78). In such situations, the CDC recommend that healthcare providers in the room should wear an N95 or higher-level respirator such as disposable filtering face piece respirators, powered air purification respirators, and elastomeric respirators, with eye protection, gloves, and a gown (74). Ideally, such procedures should take place in an airborne isolation infection room.
-
6.
Droplet precautions, on the other hand, are less stringent. Infected patients should ideally be placed in single rooms, but it is acceptable to colocate patients infected by the same pathogen. Surgical masks are required when working within close distance with infected patients. However, special air-handling and ventilation in patient rooms are not recommended. PPE could be self-contaminated (42); virus positivity was found to be higher when PPE was worn for longer durations and after clinicians cared for many patients while wearing the same PPE (79). As such, special precautions are needed when removing PPE.
Conclusions
Coughs and sneezes create respiratory droplets of variable size that spread respiratory viral infections. Because these droplets are forcefully expelled, they are dispersed in the environment and can be inhaled by a susceptible host. Whereas most respiratory droplets are filtered by the nose or deposit in the oropharynx, the smaller droplet nuclei become suspended in room air and individuals farther away from the patient could inhale them. These finer particles are carried by the airstream into the lungs, where their site of deposition depends on their size and shape and is governed by various mechanisms, including impaction, sedimentation, Brownian diffusion, turbulent mixing, interception, and electrostatic precipitation. Various procedures and aerosol generators could also generate airborne particles. Methods for prevention of respiratory viral infections depend upon their propensity to be carried in respiratory droplets or as fine droplet nuclei (airborne transmission). The respiratory transmission of SARS-CoV-2 virus that causes COVID-19 is mainly by respiratory droplets. Respiratory transmission of this virus via aerosols has not been definitively established but is possible under certain circumstances. Appropriate protective measures are necessary to prevent SARS-CoV-2 virus transmission in various settings.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank Dr. J. Francis Turner, Dr. Mark Rasnake, and Dr. Paul Terry for critical review of the manuscript. They also thank Elana Smith, Cassandra Mosley, and J. J. Tracy for assistance in preparation of the manuscript.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202004-1263PP on June 16, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fitzgerald D, Sterling TR, Haas D.Mycobacterium tuberculosis Bennett JE, Dolin R, Blaser MJ.editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases; 9th ed.Philadelphia: Elsevier; 20202985–3021. [Google Scholar]
- 2.Cole EC, Cook CE. Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. Am J Infect Control. 1998;26:453–464. doi: 10.1016/S0196-6553(98)70046-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duguid JP. The numbers and the sites of origin of the droplets expelled during expiratory activities. Edinburgh Med J. 1945;52:385–401. [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet. 2008;371:1364–1374. doi: 10.1016/S0140-6736(08)60595-4. [DOI] [PubMed] [Google Scholar]
- 6.Mazzone SB, McGovern AE, Yang SK, Woo A, Phipps S, Ando A, et al. Sensorimotor circuitry involved in the higher brain control of coughing. Cough. 2013;9:7. doi: 10.1186/1745-9974-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee PC, Cotterill-Jones C, Eccles R. Voluntary control of cough. Pulm Pharmacol Ther. 2002;15:317–320. doi: 10.1006/pupt.2002.0365. [DOI] [PubMed] [Google Scholar]
- 8.McCool FD. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:48S–53S. doi: 10.1378/chest.129.1_suppl.48S. [DOI] [PubMed] [Google Scholar]
- 9.Leith DE, Butler JP, Sneddon SL, Brain JD.The respiratory system, section 3 Macklem PT, Mead J.editors. Handbook of physiology; Vol. 3.Bethesda, MD: American Physiological Society: 1986315–336. [Google Scholar]
- 10.Wei J, Li Y. Airborne spread of infectious agents in the indoor environment. Am J Infect Control. 2016;44:S102–S108. doi: 10.1016/j.ajic.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang S, Lee GWM, Chen CM, Wu CC, Yu KP. The size and concentration of droplets generated by coughing in human subjects. J Aerosol Med. 2007;20:484–494. doi: 10.1089/jam.2007.0610. [DOI] [PubMed] [Google Scholar]
- 12.Holmgren H, Ljungström E. Influence of film dimensions on film droplet formation. J Aerosol Med Pulm Drug Deliv. 2012;25:47–53. doi: 10.1089/jamp.2011.0892. [DOI] [PubMed] [Google Scholar]
- 13.Malashenko A, Tsuda A, Haber S. Propagation and breakup of liquid menisci and aerosol generation in small airways. J Aerosol Med Pulm Drug Deliv. 2009;22:341–353. doi: 10.1089/jamp.2008.0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fabian P, Brain J, Houseman EA, Gern J, Milton DK. Origin of exhaled breath particles from healthy and human rhinovirus-infected subjects. J Aerosol Med Pulm Drug Deliv. 2011;24:137–147. doi: 10.1089/jamp.2010.0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hersen G, Moularat S, Robine E, Géhin E, Corbet S, Vabret A, et al. Impact of health on particle size of exhaled respiratory aerosols: case-control study. Clean (Weinh) 2008;36:572–577. doi: 10.1002/clen.200700189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards DA, Man JC, Brand P, Katstra JP, Sommerer K, Stone HA, et al. Inhaling to mitigate exhaled bioaerosols. Proc Natl Acad Sci USA. 2004;101:17383–17388. doi: 10.1073/pnas.0408159101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2:143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindsley WG, Blachere FM, Thewlis RE, Vishnu A, Davis KA, Cao G, et al. Measurements of airborne influenza virus in aerosol particles from human coughs. PLoS One. 2010;5:e15100. doi: 10.1371/journal.pone.0015100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci USA. 2020;117:11875–11877. doi: 10.1073/pnas.2006874117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsley WG, Blachere FM, Beezhold DH, Thewlis RE, Noorbakhsh B, Othumpangat S, et al. Viable influenza A virus in airborne particles expelled during coughs versus exhalations. Influenza Other Respir Viruses. 2016;10:404–413. doi: 10.1111/irv.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Superspreading and the effect of individual variation on disease emergence. Nature. 2005;438:355–359. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones RM, Brosseau LM. Aerosol transmission of infectious disease. J Occup Environ Med. 2015;57:501–508. doi: 10.1097/JOM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 23.Bischoff WE, Swett K, Leng I, Peters TR. Exposure to influenza virus aerosols during routine patient care. J Infect Dis. 2013;207:1037–1046. doi: 10.1093/infdis/jis773. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. How infections spread. [accessed 2020 Apr 15]. Available from: https://www.cdc.gov/infectioncontrol/spread/index.html.
- 25.Tang JW, Liebner TJ, Craven BA, Settles GS. A schlieren optical study of the human cough with and without wearing masks for aerosol infection control. J R Soc Interface. 2009;6:S727–S736. doi: 10.1098/rsif.2009.0295.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vansciver M, Miller S, Hertzberg J. Particle image velocimetry of human cough. Aerosol Sci Technol. 2011;45:415–422. [Google Scholar]
- 27.Dhand R, Malik SK, Balakrishnan M, Verma SR. High speed photographic analysis of aerosols produced by metered dose inhalers. J Pharm Pharmacol. 1988;40:429–430. doi: 10.1111/j.2042-7158.1988.tb06308.x. [DOI] [PubMed] [Google Scholar]
- 28.Wei J, Li Y. Enhanced spread of expiratory droplets by turbulence in a cough jet. Build Environ. 2015;93:86–96. [Google Scholar]
- 29.Bourouiba L, Dehandschoewercker E, Bush JWM. Violent expiratory events: on coughing and sneezing. J Fluid Mech. 2014;745:537–563. [Google Scholar]
- 30.Xie X, Li Y, Chwang ATY, Ho PL, Seto WH. How far droplets can move in indoor environments: revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 31.Knight V. Viruses as agents of airborne contagion. Ann N Y Acad Sci. 1980;353:147–156. doi: 10.1111/j.1749-6632.1980.tb18917.x. [DOI] [PubMed] [Google Scholar]
- 32.Lu J, Gu J, Li K, Xu C, Su W, Lai Z, et al. COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. 2020;26:1628–1631. doi: 10.3201/eid2607.200764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Transmission-based precautions. [accessed 2020 Apr 15]. Available from: https://www.cdc.gov/infectioncontrol/basics/transmission-based-precautions.html.
- 34.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. Aerosol and surface stability of SARS-CoV-2 compared to SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai J, Sun W, Huang J, Gamber M, Wu J, He G. Indirect virus transmission in cluster of COVID-19 Cases, Wenzhou, China, 2020. Emerg Infect Dis. 2020;26:1343–1345. doi: 10.3201/eid2606.200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Interim guidance for infection control within healthcare settings when caring for confirmed cases, probable cases, and cases under investigation for infection with novel influenza A viruses associated with severe disease. 2014 [accessed 2020 Apr 15]. Available from: https://www.cdc.gov/flu/avianflu/novel-flu-infection-control.htm.
- 37.Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for hospitalized patients with Middle East respiratory syndrome coronavirus (MERS-CoV) 2017 [accessed 2020 Apr 15]. Available from: https://www.cdc.gov/coronavirus/mers/infection-prevention-control.html.
- 38.Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses: implication for infection prevention. Curr Opin Infect Dis. 2019;32:372–379. doi: 10.1097/QCO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 39.Yu IT, Li Y, Wong TW, Tam W, Chan AT, Lee JH, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–1739. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 40.Santarpia JL, Rivera DN, Herrera V, Morwitzer MJ, Creager H, Santarpia GW, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. medRxiv. 2020 [accessed 2020 Apr 19]. Available from: https://www.medrxiv.org/content/10.1101/2020.03.23.20039446v2.full.pdf. [Google Scholar]
- 41.Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;58:557–560. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 43.World Health Organization Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. Scientific Brief; 2020[accessed 2020 Apr 19]. Available from: who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations
- 44.Darquenne C. Aerosol deposition in health and disease. J Aerosol Med Pulm Drug Deliv. 2012;25:140–147. doi: 10.1089/jamp.2011.0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laube BL, Janssens HM, de Jongh FH, Devadason SG, Dhand R, Diot P, et al. European Respiratory Society; International Society for Aerosols in Medicine. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37:1308–1331. doi: 10.1183/09031936.00166410. [DOI] [PubMed] [Google Scholar]
- 46.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12:1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrow PE. Conference on the scientific basis of respiratory therapy: aerosol therapy. Aerosol characterization and deposition. Am Rev Respir Dis. 1974;110:88–99. doi: 10.1164/arrd.1974.110.6P2.88. [DOI] [PubMed] [Google Scholar]
- 48.Fabian P, McDevitt JJ, DeHaan WH, Fung ROP, Cowling BJ, Chan KH, et al. Influenza virus in human exhaled breath: an observational study. PLoS One. 2008;3:e2691. doi: 10.1371/journal.pone.0002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ. Influenza virus aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks. PLoS Pathog. 2013;9:e1003205. doi: 10.1371/journal.ppat.1003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindsley WG, Noti JD, Blachere FM, Thewlis RE, Martin SB, Othumpangat S, et al. Viable influenza A virus in airborne particles from human coughs. J Occup Environ Hyg. 2015;12:107–113. doi: 10.1080/15459624.2014.973113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 52.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindsley WG, Pearce TA, Hudnall JB, Davis KA, Davis SM, Fisher MA, et al. Quantity and size distribution of cough-generated aerosol particles produced by influenza patients during and after illness. J Occup Environ Hyg. 2012;9:443–449. doi: 10.1080/15459624.2012.684582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Knight V. Philadelphia: Lea and Febiger; 1993. Viral and mycoplasmal infections of the respiratory tract. pp. 1–9. [Google Scholar]
- 55.Tang JW. The effect of environmental parameters on the survival of airborne infectious agents. J R Soc Interface. 2009;6:S737–S746. doi: 10.1098/rsif.2009.0227.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gralton J, Tovey E, McLaws M-L, Rawlinson WD. The role of particle size in aerosolised pathogen transmission: a review. J Infect. 2011;62:1–13. doi: 10.1016/j.jinf.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bing-Yuan, Zhang YH, Leung NHL, Cowling BJ, Yang ZF. Role of viral bioaerosols in nosocomial infections and measures for prevention and control. J Aerosol Sci. 2018;117:200–211. doi: 10.1016/j.jaerosci.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Judson SD, Munster VJ. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11:940. doi: 10.3390/v11100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simonds AK, Hanak A, Chatwin M, Morrell M, Hall A, Parker KH, et al. Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess. 2010;14:131–172. doi: 10.3310/hta14460-02. [DOI] [PubMed] [Google Scholar]
- 61.O’Neil CA, Li J, Leavey A, Wang Y, Hink M, Wallace M, et al. Centers for Disease Control and Prevention Epicenters Program. Characterization of aerosols generated during patient care activities. Clin Infect Dis. 2017;65:1335–1341. doi: 10.1093/cid/cix535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Malley CA. Device cleaning and infection control in aerosol therapy. Respir Care. 2015;60:917–927. doi: 10.4187/respcare.03513. [Discussion, pp. 928–930] [DOI] [PubMed] [Google Scholar]
- 63.Hui DS, Chow BK, Lo T, Tsang OTY, Ko FW, Ng SS, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. Eur Respir J. 2019;53:1802339. doi: 10.1183/13993003.02339-2018. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Fink JB, Ehrmann S. High-flow nasal cannula for COVID-19 patients: low risk of bio-aerosol dispersion. Eur Respir J. 2020;55:2000892. doi: 10.1183/13993003.00892-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leung CCH, Joynt GM, Gomersall CD, Wong WT, Lee A, Ling L, et al. Comparison of high-flow nasal cannula versus oxygen face mask for environmental bacterial contamination in critically ill pneumonia patients: a randomized controlled crossover trial. J Hosp Infect. 2019;101:84–87. doi: 10.1016/j.jhin.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 66.Leonard S, Atwood CW, Jr, Walsh BK, DeBellis RJ, Dungan GC, Strasser W, et al. Preliminary findings on control of dispersion of aerosols and droplets during high-velocity nasal insufflation therapy using a simple surgical mask: implications for the high-flow nasal cannula. Chest. doi: 10.1016/j.chest.2020.03.043. [online ahead of print] 2 Apr 2020; DOI: 10.1016/j.chest.2020.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hui DS, Chow BK, Ng SS, Chu LCY, Hall SD, Gin T, et al. Exhaled air dispersion distances during noninvasive ventilation via different Respironics face masks. Chest. 2009;136:998–1005. doi: 10.1378/chest.09-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan MTV, Chow BK, Lo T, Ko FW, Ng SS, Gin T, et al. Exhaled air dispersion during bag-mask ventilation and sputum suctioning: implications for infection control. Sci Rep. 2018;8:198. doi: 10.1038/s41598-017-18614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao W, Wang T, Jiang B, Gao F, Wang L, Zheng H, et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020;125:e28–e37. doi: 10.1016/j.bja.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Somogyi R, Vesely AE, Azami T, Preiss D, Fisher J, Correia J, et al. Dispersal of respiratory droplets with open vs closed oxygen delivery masks: implications for the transmission of severe acute respiratory syndrome. Chest. 2004;125:1155–1157. doi: 10.1378/chest.125.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hui DS, Chow BK, Chu L, Ng SS, Lee N, Gin T, et al. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PLoS One. 2012;7:e50845. doi: 10.1371/journal.pone.0050845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson DF, Druce JD, Birch C, Grayson ML. A quantitative assessment of the efficacy of surgical and N95 masks to filter influenza virus in patients with acute influenza infection. Clin Infect Dis. 2009;49:275–277. doi: 10.1086/600041. [DOI] [PubMed] [Google Scholar]
- 74.Centers for Disease Control and Prevention. Interim infection prevention and control recommendations for patients with suspected or confirmed coronavirus disease 2019 (COVID-19) in healthcare settings. 2020 [accessed 2020 Apr 4]. Available from: https://www.cdc.gov/Coronavirus/2019-ncov/infection-control/control-recommendations.html.
- 75.Bałazy A, Toivola M, Adhikari A, Sivasubramani SK, Reponen T, Grinshpun SA. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control. 2006;34:51–57. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 76.Cohen HJ, Birkner JS. Respiratory protection. Clin Chest Med. 2012;33:783–793. doi: 10.1016/j.ccm.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Coia JE, Ritchie L, Adisesh A, Makison Booth C, Bradley C, Bunyan D, et al. Healthcare Infection Society Working Group on Respiratory and Facial Protection. Guidance on the use of respiratory and facial protection equipment. J Hosp Infect. 2013;85:170–182. doi: 10.1016/j.jhin.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yam LY, Chen RC, Zhong NS. SARS: ventilatory and intensive care. Respirology. 2003;8:S31–S35. doi: 10.1046/j.1440-1843.2003.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chughtai AA, Stelzer-Braid S, Rawlinson W, Pontivivo G, Wang Q, Pan Y, et al. Contamination by respiratory viruses on outer surface of medical masks used by hospital healthcare workers. BMC Infect Dis. 2019;19:491. doi: 10.1186/s12879-019-4109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Darquenne C.Deposition mechanisms. Chapter 2.2 Dhand R, editor. ISAM textbook of aerosol medicine. Knoxville: International Society for Aerosols in Medicine; 2016e73–e87. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


