Abstract
Ongoing thought patterns constitute important aspects of both healthy and abnormal human cognition. However, the neural mechanisms behind these daily experiences and their contribution to well-being remain a matter of debate. Here, using resting-state fMRI and retrospective thought sampling in a large neurotypical cohort (n = 211), we identified two distinct patterns of thought, broadly describing the participants’ current concerns and future plans, that significantly explained variability in the individual functional connectomes. Consistent with the view that ongoing thoughts are an emergent property of multiple neural systems, network-based analysis highlighted the central importance of both unimodal and transmodal cortices in the generation of these experiences. Importantly, while state-dependent current concerns predicted better psychological health, mediating the effect of functional connectomes, trait-level future plans were related to better social health, yet with no mediatory influence. Collectively, we show that ongoing thoughts can influence the link between brain physiology and well-being.
Keywords: Functional connectivity, Functional magnetic resonance imaging, Graph theory, Mental health, Resting state, Patterns of thought
Author Summary
Occupying a considerable portion of our waking lives, spontaneous thoughts constitute the foundations of our rich inner mental experiences and well-being. Nevertheless, the neural mechanisms behind this cognitive process and its relation to our mental health remain unresolved. In a large cohort of participants, we show that distinct dimensions of ongoing thoughts emerge from a broad set of whole-brain functional interactions, with certain patterns significantly mediating the link between brain connectivity and psychosocial health. Overall, these results highlight the heterogeneous nature of self-generated thoughts, the content and form of which have a significant influence on the association between our brain physiology and mental well-being.
INTRODUCTION
Recent advances in functional magnetic resonance imaging (fMRI) methods and data analysis techniques have facilitated a new era in the characterization of the neural representations that underlie human cognition and behavior (Barch, 2013). Big data-driven approaches are utilized to explore whole-brain functional interactions during unconstrained states of rest, explaining considerable levels of population-wise variation in complex traits, including intelligence (Greene, Gao, Scheinost, & Constable, 2018), personality (Toschi, Riccelli, Indovina, Terracciano, & Passamonti, 2018), daily habits (Cheng et al., 2019), and self-perceived quality of life (Kraft et al., 2018). In addition to helping researchers derive novel theories on healthy brain processing, one important goal of these functional connectomic mapping initiatives is to devise neural, cognitive, and behavioral links to better understand mental health disorders, and to identify “at-risk” groups for future preventative measures (Castellanos, Di Martino, Craddock, Mehta, & Milham, 2013; Van Essen & Barch, 2015). However, most studies often neglect the fact that periods of unconstrained states of rest can be characterized by patterns of spontaneous thought that are also associated with well-being (Andrews-Hanna et al., 2013), which may have unique neurocognitive correlates.
Research from psychology suggests that the ability to self-generate patterns of cognition is a core element of our mental lives, occupying a considerable portion of our daily mentation (Antrobus, Singer, & Greenberg, 1966; Klinger, 1971; Singer & Antrobus, 1963). Critically, these thoughts are particularly prevalent in situations with low external demands, such as periods of wakeful rest (Smallwood, Nind, & O’Connor, 2009), that is, the conditions when resting-state functional data are most commonly recorded. Importantly, measures of such experiences have a wide range of momentary correlates including indicators of stress (Engert, Smallwood, & Singer, 2014), ongoing physiology (Konishi, Brown, Battaglini, & Smallwood, 2017), and task performance (Smallwood, Beach, Schooler, & Handy, 2008), and also have documented links to both beneficial and deleterious aspects of psychological functioning. For example, patterns of ongoing thought have been previously linked to individuals’ ability to plan for future goals (Baird, Smallwood, & Schooler, 2011), wait for long-term rewards (Smallwood, Ruby, & Singer, 2013), and devise creative solutions to both personal (Baird et al., 2012) and social problems (Ruby, Smallwood, Sackur, et al., 2013). Other forms of self-generated mentation are associated with unhappiness (Killingsworth & Gilbert, 2010), as well as poor performance in sustained attention tasks (Allan Cheyne, Solman, Carriere, & Smilek, 2009) or measures of fluid intelligence (Mrazek et al., 2012). In fact, disruptive thought patterns are reported to underlie the absentminded mistakes in our everyday functioning (Carriere, Cheyne, & Smilek, 2008; McVay & Kane, 2009), including traffic accidents (Galera et al., 2012) or medical malpractice (Smallwood, Mrazek, & Schooler, 2011), and to form a potential basis for cognitive impairments reported in attention-deficit/hyperactivity disorder (Seli, Smallwood, Cheyne, & Smilek, 2015; Vatansever, Bozhilova, Asherson, & Smallwood, 2018) and clinical depression (Marchetti, Van de Putte, & Koster, 2014). Taken together, this body of evidence underlines the vital importance of both stable and transient thought patterns in our daily mentation and their variable influence on our psychological and social well-being.
Furthermore, there is now emerging evidence that links distinct profiles of neural activity to trait variance in specific patterns of thoughts. These investigations highlight that patterns of ongoing thought have complex and often heterogeneous neural correlates (Smallwood et al., 2016; Stawarczyk, Majerus, Maj, Van der Linden, & D’Argembeau, 2011), which depend on the functional interaction of multiple neural and cognitive components (Gorgolewski et al., 2014; Poerio et al., 2017; Wang, Poerio, et al., 2018). For example, Wang and colleagues identified distinct patterns of ongoing thoughts that were linked to reductions in within- and between-network connectivity for neural systems important for external attention (ventral, dorsal, and frontoparietal systems), which was in turn related to reduced performance in tasks of cognitive aptitude (Wang, Bzdok, et al., 2018). Other studies have illustrated that different types of spontaneous thought depend on differential patterns of functional connectivity between regions important for memory (e.g., temporal lobe) and those that form the core associative cortices (Golchert et al., 2017; Karapanagiotidis, Bernhardt, Jefferies, & Smallwood, 2017; Smallwood et al., 2016). Notably, tasks such as creative idea generation (Beaty, Benedek, Kaufman, & Silvia, 2015) and future planning (Spreng, Stevens, Chamberlain, Gilmore, & Schacter, 2010), which are collectively associated with unconstrained thought (Baird et al., 2012; Medea et al., 2016), depend on similar patterns of interaction between regions of the default mode and frontoparietal networks.
Converging bodies of evidence from contemporary cognitive neuroscience, therefore, highlight (a) that patterns of neural activity at rest have associated patterns of thought, and (b) that both neural and self-report descriptions of patterns of unconstrained activity are predictive of a wide range of psychological features of an individual, including mental well-being. Collectively, these observations highlight the need to understand the extent to which relationships between neural activity at rest and well-being are dependent upon the nature of patterns of ongoing experience that emerges while neural activity is recorded. Our current study reflects an attempt to address this issue by quantifying the relationship between patterns of thoughts during rest and the associated neural activity, and then exploring whether descriptions of neural organization gained in this fashion are associated with trait variance in a cross-culturally validated questionnaire that assessed both psychological and social well-being.
In the analysis of a large neurotypical sample (n = 211), we found differential profiles of functional interactions among multiple neural systems that explained individual variation among two distinct patterns of thought, broadly corresponding to the participants’ current concerns and future plans. Using a test-retest sample (n = 40), we found reasonable concordance between the tendency to generate future plans at rest and their brain basis, indicating that these measures are likely to reflect neurocognitive traits. On the other hand, current concerns were not stable across individuals, but showed evidence of common changes in terms of the pattern of thought and pattern of functional connectivity, suggesting that these neurocognitive links reflect a more transient state. Finally, we found that while current concerns indirectly mediated the effect of brain functional interactions on psychological well-being, future plans showed no such mediation effect.
RESULTS
Dimensions of Variation in Ongoing Thought Patterns
With the aim of investigating the differential influence of distinct thought patterns on the link between brain functional network topology and mental well-being, we collected 9 min of fMRI data from a large cohort of neurotypical participants (n = 211) during a period of wakeful rest. This neural measure was complemented with a session of retrospective thought sampling, administered immediately after the resting-state scanning, as well as self-assessed ratings of psychological and social well-being on a cross-culturally validated questionnaire from the World Health Organization Quality of Life (WHOQOL) group (WHOQOL Group, 1998), collected outside the scanner in a separate behavioral session. The workflow for the data collection and analysis techniques utilized in this study is presented in Figure 1, with further details provided in the Methods section and the Supporting Information. Parts of this dataset have been previously employed in our prior investigations (Vatansever, Bozhilova, et al., 2018; Vatansever, Bzdok, et al., 2017; Wang, Bzdok, et al., 2018; Wang, Poerio, et al., 2018).
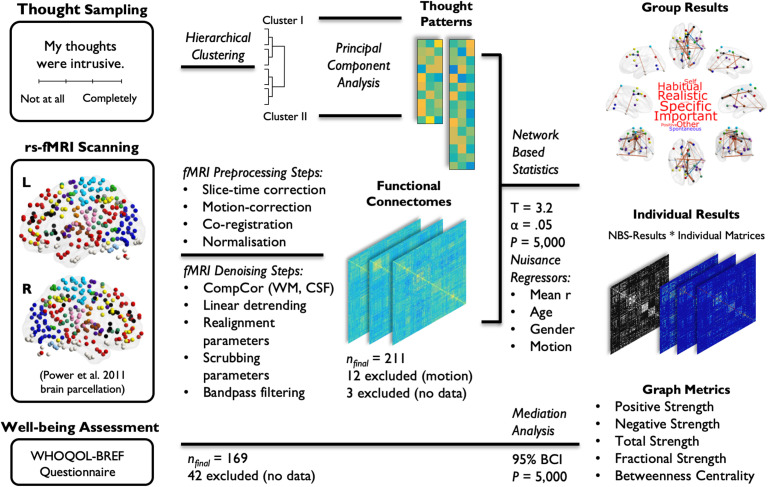
Figure 1. .
Experimental data collection and analysis pipeline. A large cohort of participants (n = 211) were fMRI scanned during a state of wakeful rest for a total of 9 min. A comprehensive pipeline of fMRI preprocessing and data denoising procedures were followed in order to ensure maximal removal of nuisance variables. The thought sampling scores collected at the end of the resting-state scanning session were first hierarchically clustered into two major groups, each of which was then reduced to three patterns of thought using principal component analysis. Fully connected, weighted correlation matrices based on the Power et al. (2011) parcellation (264 regions of interest) scheme and the individual component scores on the identified patterns of thought were then carried forward on to network-based statistics (NBS) with the aim of identifying components of brain connections that related to the participants’ thought patterns. For all six patterns of thought, t tests were carried out with an initial T score of 3.2 and a significance level of p < 0.05 over 5,000 permutations (mean connectivity, age, gender, and percentage of invalid scans based on the composite motion score from the scrubbing procedure were entered as group-level nuisance regressors). The identified brain components that significantly related to the participants’ thoughts were first characterized at the group level and then used as mask graphs to create thresholded connectivity matrices for each participant. Network metrics of positive, negative, total, and fractional strength (i.e., the ratio of positive to negative strength) as well as betweenness centrality were measured on individual thresholded matrices and were further used to characterize the identified neurocognitive profiles. Linear regressions and mediation analyses were then employed to investigate the mediatory effect of thought patterns on the link between brain connectivity and psychological and social well-being as measured using a cross-culturally validated World Health Organization Quality of Life group (WHOQOL-BREF) questionnaire.
Our first objective was to establish the dimensions of variation within the participants’ ongoing thought patterns. The employed retrospective thought sampling method required participants to subjectively characterize their thoughts using 4-choice (Likert scale) ratings on a set of questions derived from our previous investigations (Gorgolewski et al., 2014; Medea et al., 2016; Ruby, Smallwood, Engen, & Singer, 2013; Ruby, Smallwood, Sackur, et al., 2013; Smallwood et al., 2016; Supplementary Table S1). With the aim of reducing dimensionality and improving interpretability, these ratings were first hierarchically clustered and then decomposed into distinct dimensions using principal component analysis(PCA), which established the main patterns of thought reported in our sample (Figure 2). The total number of thought patterns was selected using scree plots, based on the eigenvalue (>1) and the explanatory power gained by each additional decomposition (Supplementary Figures S1 and S2). The robustness assessments and typical ratings from participants who scored highest on a given thought pattern are provided in Supplementary Figures S3–S5.
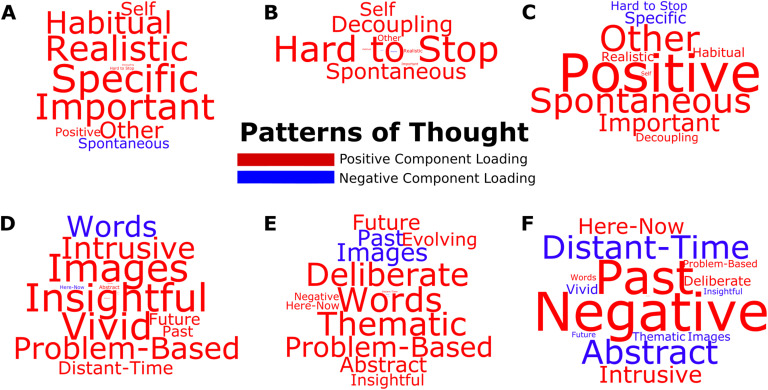
Figure 2. .
Decomposing distinct patterns of thought. Following the initial hierarchical clustering of the participants’ ratings on the experience sampling questionnaire, a total of six patterns of thought were identified using PCA. Three principal components in each of the two clusters explained 51% and 35% of the variability in the data, respectively. The Varimax rotated component loadings are visualized using word clouds. While the size of the text refers to the relative strength of the component loadings, positive and negative loadings are indicated via red and blue fonts, respectively. The components highlighted (A)important/specific thoughts, (B) perceptually decoupled/hard-to-stop thoughts, (C) positive/spontaneous thoughts, (D) insightful/image-based thoughts, (E) deliberate/verbal thoughts, and (F) negative/past-related thoughts. The individual variation on these patterns of thought were carried forward on to the NBS analysis as between-subject explanatory variables.
Principal component analysis, applied separately to each of the two initial hierarchical clusters on the self-reported ratings, revealed three patterns of thought each (a total of six), explaining 51% and 35% of the variance, respectively. The identified patterns highlighted aspects of ongoing thoughts that are commonly characterized in the existing literature (Smallwood & Schooler, 2015). These encompassed thoughts that were important and specific (Figure 2A); perceptually decoupled and hard-to-stop thoughts about the self (Figure 2B); positive, spontaneous thoughts about others (Figure 2C); insightful and image-based thoughts (Figure 2D); deliberate, verbal thoughts about the future (Figure 2E); and negative, past-related thoughts (Figure 2F).
Distinct Patterns of Thought Link to Differential Functional Connectomic Profiles
Next, we determined the extent to which the human functional connectomes were associated with individual variation on the identified patterns of thought. We used a whole-brain parcellation scheme (Power et al., 2011). Defining each of the 264 brain regions as nodes, and Pearson correlation coefficients among them as edges, we constructed fully connected, weighted functional connectomes for each participant. Utilizing network-based statistics (NBS; Zalesky, Fornito, & Bullmore, 2010), we entered the individual component scores for all six patterns of thought as variables of interest in a regression model, while removing the effects of nuisance variables including mean connectivity, age, gender, and the composite motion score (i.e., percentage of invalid volumes identified in the motion artifact detection procedure; Supplementary Figures S6–S7). This revealed two distinct patterns of thought that were significantly related to differential profiles of brain functional connectomic organization (Tthreshold = 3.2, 5,000 permutations, α = 0.05). Although we report on the initial Tthreshold = 3.2 threshold, comparable results for Tthreshold = 3.1 and Tthreshold = 3.3 are presented in the Supporting Information section (Supplementary Figure S9).
The first thought pattern that was significantly associated with neural patterns reflected high loadings on “habitual,” “realistic,” “specific,” and “important” elements of ongoing cognition, broadly corresponding to the construct of current concerns that has been argued to play an important motivational role in the content and form of ongoing thoughts (Klinger, 2013). Population variation in this experience was linked to a combination of positive and negative connections from a wide range of both unimodal and transmodal brain regions (Figure 3A) (Tthreshold = 3.2, 5,000 permutations, p = 0.015). These included areas associated with visual, auditory, somato-motor, dorsal/ventral attention, cingulo-opercular, salience, frontoparietal, default mode, subcortical networks, and a set of regions with memory-related functions, with the strongest link observed between the right middle frontal and middle cingulate gyri (salience network).
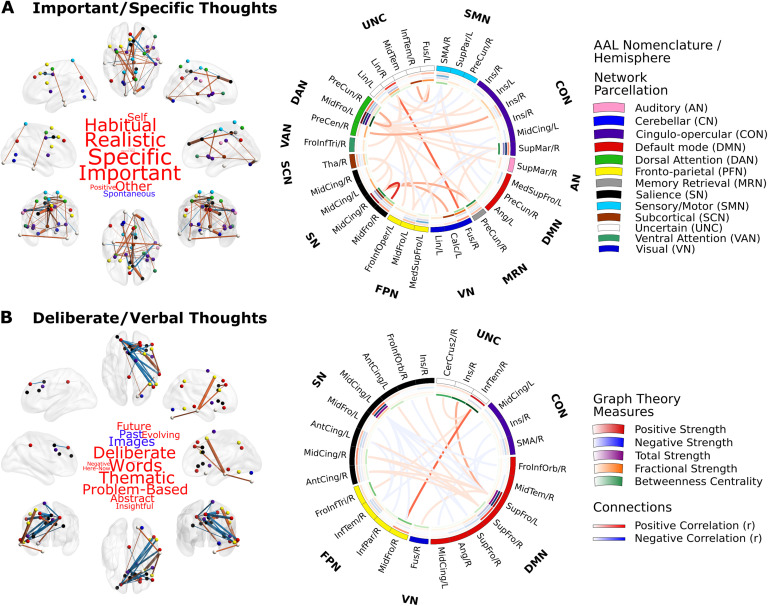
Figure 3. .
Distinct patterns of thought are linked to differential profiles of functional connectomic organization. NBS identified two connected components of brain functional interactions at rest that significantly related to the participants’ scores on distinct patterns of thought, which highlighted (A) important, specific, realistic, and habitual thoughts about the self and others that were not spontaneous; as well as (B) deliberate, verbal, thematic, abstract, problem-based, and future-oriented thoughts in words that were not about the past or in images. The average connectivity patterns of these brain graphs are visualized on an MNI152 smoothed glass brain, with the nodes color-coded according to the original Power et al. (2011) parcellation scheme, and the positive/negative connections colored in red and blue, respectively, in which the size of edges represents average strength. The average connectivity patterns of these brain graphs and the average graph theory metrics calculated across participants are visualized in circular representations. The Automated Anatomical Labeling (AAL) nomenclature, original network parcellation, average positive (red: [0–1] Pearson r scale), negative (blue: [0–1] Pearson r scale), total (purple: [0–2] Pearson r scale), and fractional strengths (orange: [0–10] arbitrary scale) as well as betweenness centrality (green: [0–6] arbitrary scale) of each region in the identified brain components are listed around the rings. The average positive and negative connections between these regions are represented by links color-coded red and blue, respectively ([0–1] Pearson r scale).
Overall, this neural component was composed of more positive than negative connections and involved a set of distributed brain regions that encompassed a diverse set of large-scale brain networks. Characterization of this brain component using graph theory measures revealed that an area in the left middle frontal gyrus, belonging to the dorsal attention network, showed the highest positive, negative, and total strength as well as betweenness centrality. This highlights the left middle frontal gyrus as a key node in the functional connectivity patterns linked to ongoing thoughts about the individuals’ current concerns. In addition, the highest fractional strength (i.e., the ratio of positive to negative strength) was observed in a region belonging to the salience network, namely the right middle cingulate gyrus.
The second pattern of thought that related to neural function reflected high loadings on the “future” rather than the “past,” as well as “deliberate” and “verbal” focus on “problems,” which is broadly consistent with studies of ongoing thought that emphasize a deliberate focus on future plans (Baird et al., 2011; Seli, Ralph, Konishi, Smilek, & Schacter, 2017). This thought pattern was related to the functional interaction of brain regions from a small set of large-scale brain networks mainly belonging to the higher order transmodal cortices (Tthreshold = 3.2, 5,000 permutations, p = 0.026; Figure 3B). Both positive and negative links between regions belonging to the default mode, frontoparietal, salience, cingulo-opercular, and visual regions correlated with higher scores reported on this pattern of thought, with the strongest link observed between regions in the right inferior temporal and middle frontal gyri. Relative to the “current concerns” component, this brain component was composed of more negative connections and was drawn from a more localized set of brain regions from a smaller number of large-scale brain networks. Network-level analysis indicated that a left superior frontal gyrus region belonging to the default mode network showed the highest positive, negative, and total strength, while a left middle cingulate region (salience network) displayed the greatest fractional strength. Betweenness centrality, however, was highest on a right inferior temporal gyrus region.
Test-Retest Reliability of Thought Patterns and Functional Connectomic Organization
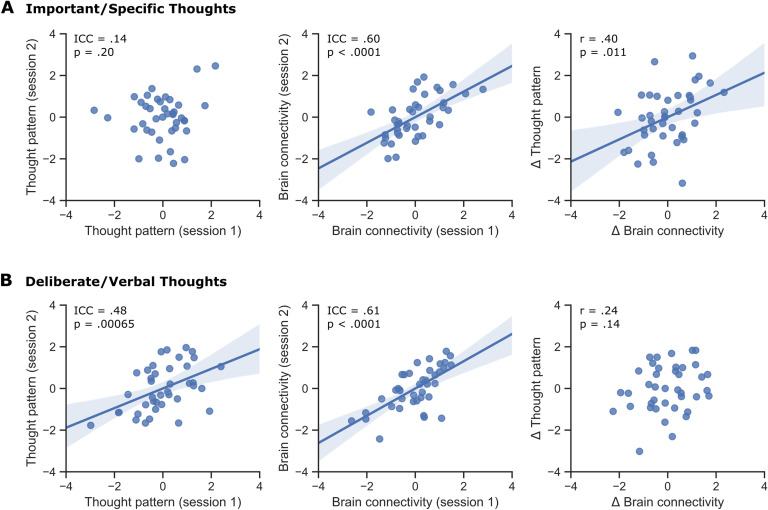
Next, we examined the stability of the two neurocognitive measures over time by exploring their intraclass correlation across two sessions in 40 participants for whom a second assessment was performed (i.e., resting-state scan and thought sampling). For “important and specific thoughts about the self and others” (i.e., current concerns), individual variability on this thought pattern displayed no significant intraclass correlation (ICC = 0.14, 95% BCI [−0.18, 0.43], p = 0.20); however, the associated brain connectivity (natural log of the fractional strength) was correlated between the first and second assessment sessions (ICC = 0.60, 95% BCI [0.36, 0.76], p < 0.0001). Importantly, there was a significant positive correlation between the change in component scores on this thought pattern and the change in brain connectivity between the two assessment sessions (Pearson r = 0.40, p = 0.011; Figure 4A). Together this pattern is broadly consistent with a more transient state.
Figure 4. .
Test-retest reliability of the identified thought patterns and the associated functional connectomic organization. A second session of resting-state fMRI scanning and thought sampling was carried out for a total of 40 participants. (A) For important/specific thoughts, while individual component scores on this thought pattern did not show a significant intraclass correlation (ICC = 0.14, CI [− 0.18,0.43], p = 0.20), the associated brain connectivity pattern (natural log of fractional strength) was largely consistent between the two repeated assessment sessions (ICC = 0.60, CI [0.36, 0.76], p < 0.0001). Moreover, the change in thought pattern was positively related to the change in brain connectivity (Pearson r = 0.40, p = 0.011). (B) For deliberate/verbal thoughts, both the component scores on this thought pattern (ICC = 0.48, CI [0.21, 0.69], p = 0.00065) and the associated brain connectivity (ICC = 0.61, CI [0.38, 0.55], p < 0.0001) showed significant intraclass correlations. However, there was no significant link between the change in this thought pattern and the change in brain connectivity between the two assessment sessions (Pearson r = 0.24, p = 0.14).
For “deliberate verbal thoughts about the future” (i.e., future plans) on the other hand, individual variability on both the thought pattern (ICC = 0.48, 95% BCI [0.21, 0.69], p = 0.00065) and the associated brain connectivity (ICC = 0.61, 95% BCI [0.38, 0.55], p < 0.0001) was consistent across the two assessment sessions. However, there was no significant correlation between the change in component scores on this thought pattern and the change in brain connectivity (Pearson r = 0.24, p = 0.14; Figure 4B). Collectively, these analyses show that the patterns of thought captured by “deliberate and verbal thoughts about the future” and their neural representations show greater trait-like stability over time than the participants’ state-like “important specific thoughts about the self and others.”
Distinct Patterns of Thought Mediate the Effect of Brain Connectivity on Well-Being
Finally, having identified two patterns of ongoing thought, each with an associated profile of complex brain functional interactions at rest, we tested whether these neurocognitive metrics derived from our study had mediatory influences on measures of mental health and well-being in daily life, as indicated by a cross-culturally validated World Health Organization questionnaire (i.e., WHOQOL-BREF). Incorporating the relative importance of both positive and negative connections (Fox, Zhang, Snyder, & Raichle, 2009; Keller et al., 2015), we first assessed the predictive power of fractional strength on psychological and social well-being via linear regressions, followed by mediation analyses to examine the indirect influence of brain connectivity on well-being, mediated by the participants’ reported patterns of thought.
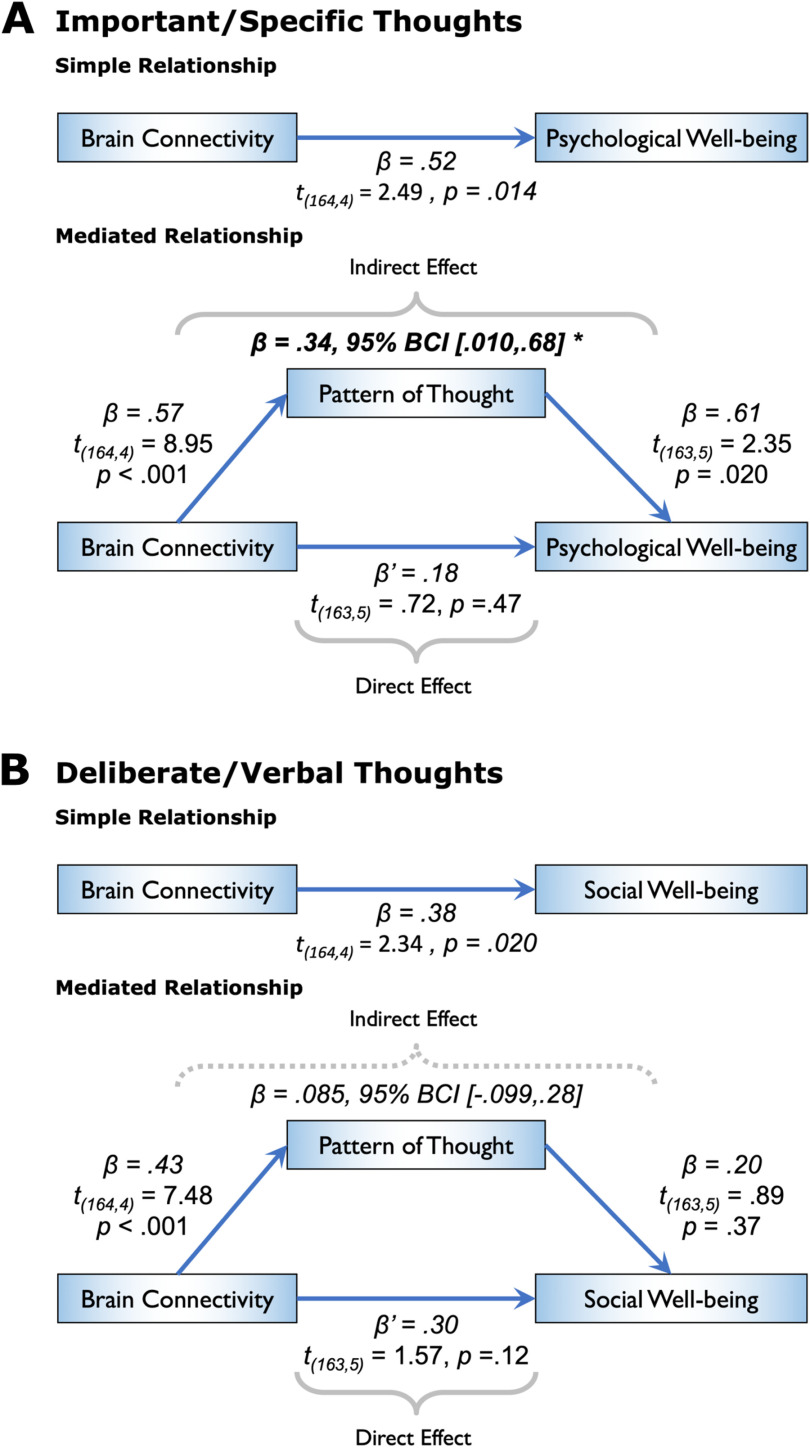
For “important and specific thoughts about the self and others” (i.e., current concerns), the fractional strength (natural log) of the associated brain connectivity component significantly predicted the participants’ psychological well-being score (β = 0.52, t(164,4) = 2.49, p = 0.014), while no significant link was observed with social well-being (β = 0.063, t(164,4) = 0.34, p = 0.74). A mediation analysis indicated that there was a significant indirect effect of brain connectivity on psychological well-being, mediated by the individuals’ scores on the identified pattern of thought (β = 0.34, SE = 0.17, 95% BCI [0.010, 0.68], corrected for age, gender, and in-scanner motion; Figure 5A). For “deliberate and verbal thoughts about the future” (i.e., future plans) on the other hand, the fractional strength (natural log) of the identified component of brain connectivity significantly predicted the participants’ social well-being score on the WHOQOL-BREF (β = 0.38, t(164,4) = 2.34, p = 0.020), while no significant link was observed with psychological well-being (β = −0.082, t(164,4) = −0.43, p = 0.67). Moreover, a mediation analysis revealed no evidence for an indirect effect of brain connectivity on social well-being through the individuals’ scores on the identified thought pattern (β = 0.085, SE = 0.096, 95% BCI [−0.099, 0.28], corrected for age, gender, and in-scanner motion; Figure 5B). Utilizing a more conventional total strength (sum of positive and negative strength) graph measure revealed comparable results, that is, β = 0.12 (SE = 0.045, 95% BCI [0.031, 0.22]) for current concerns, and β = −0.016 (SE = 0.019, 95% BCI [−0.062, 0.012]) for future plans, albeit with a smaller effect size.
Figure 5. .
Model of the functional connectome as a predictor of psychological and social well-being, mediated by patterns of thought. While brain connectivity refers to the natural log of the fractional strength, pattern of thought represents the individual component scores on the identified thought pattern, and the psychological/social well-being measures are self-reported ratings on the WHOQOL-BREF questionnaire. Only a subset of the participant cohort who fully completed the well-being questionnaire (n = 169) was utilized in this analysis. The calculation of confidence intervals (CI) for the mediation effect was based on the percentile bootstrap estimation approach with 5,000 samples (corrected for age, gender, and percentage of motion-related invalid scans identified by the scrubbing procedure). (A) A significant indirect effect of brain connectivity on psychological well-being was observed, mediated through the participants’ important/specific thoughts, broadly related to their current concerns. (B) There was no significant indirect effect of brain connectivity on social well-being, mediated through the participants’ deliberate/verbal thoughts on their future plans. Mediation results were comparable when using the total strength (sum of positive and negative strength) graph measure, albeit with a smaller effect size.
DISCUSSION
The aim of this study was to examine whether accounting for the patterns of ongoing thoughts that individuals experience during periods of wakeful rest (e.g., resting-state fMRI scanning) could allow for a more nuanced understanding of the relationships between neural organization and mental well-being. To achieve this goal, we first identified patterns of neural connectivity at rest that varied with aspects of self-reported experience during this period. One dimension of variation was along patterns of thinking that were indicative of a focus on current concerns. This variation was associated with functional connections from the middle frontal gyrus, a region within the salience network. While the neural pattern was a stable feature of the assessed individuals, the pattern of thinking did not depict such reliability. Nevertheless, both neural and self-report patterns changed concurrently across time, indicating that this neurocognitive profile described a transient mapping between brain and experience. A second pattern, associated with deliberate thoughts about the future, was dominated by functional connections from the superior frontal gyrus, situated within the default mode network. Both neural and experiential features of this mode were consistent across individuals and showed little evidence of common changes over time, suggesting a neural pattern that was relatively a stable trait. Importantly, these neural components had differential associations with measures of mental health and well-being. The transient neurocognitive component, linked to the participants’ focus on current concerns, was significantly associated with self-reported psychological well-being. Mediation analysis indicated that this brain and well-being relationship was fully mediated by the associated descriptions of ongoing experience. In contrast, while the dimension of brain connectivity variation was associated with social well-being, this relationship was independent of the associated patterns of ongoing experience.
Together these analyses indicate that important aspects of the commonly reported relationships between brain and well-being can partly be understood through their associations with patterns of ongoing thoughts that participants experience during fMRI scanning. First, our study shows that neural profiles, identified through their association with self-reported experience, have differential associations with well-being. Self-reports are often subject to factors that impact upon their credibility, however, the neural patterns of activity identified in this fashion have the advantage that they are embedded in a cognitive context without the need for reverse inference (Poldrack, 2011). Thus, experience sampling provides a complementary method for determining whether the source of observed neural patterns are cognitive in nature, or emerge for other reasons, such as cardiovascular function or motion-related confounds (Shen et al., 2017). Second, our study demonstrates that experience sampling is sensitive to patterns of neural activity that are stable across time and others that are transient. Our approach, therefore, may have direct relevance to studies that aim to explore the long-term stability of patterns of functional organization, or those that explore long-term changes in neural function. Based on the current data, for example, experience sampling may provide a reasonably direct way to test hypotheses into why certain neural patterns vary in their consistency across time. Despite the inherent weaknesses associated with retrospective experience sampling (Christoff, Irving, Fox, Spreng, & Andrews-Hanna, 2016), our study shows that the information it provides can address important shortcomings of the conceptual interpretations placed on functional connectivity patterns derived from resting-state analysis. Given the negligible cost associated with acquiring descriptions of experience after resting-state scans, and their prevalence as a tool of cognitive neuroscience, we see no reason why this method should not be employed as a community standard in similar studies moving forward.
As well as highlighting the value of experience sampling to studies investigating the relationship between functional organization and traits of well-being, our study provides valuable information into the neural processes that contribute to different types of spontaneous thought. Current concerns, in the form of either unfulfilled goals or personally relevant information, occupy a significant portion of the thoughts we experience in our daily lives, potentially constituting a determining factor in the functional outcomes of our ongoing cognition (Klinger, 2013; Marchetti et al., 2014). In line with these results, a key dimension of thought pattern that was reported by the participants in our cohort was related to “important and specific thoughts about the self and others” or more generally their current concerns. Our results revealed that important functional connections were observed in the salience network, commonly implicated in the detection of behaviorally important internal or external stimuli for the coordination of neural resources (Uddin, 2015). This neural system has been shown to causally influence the functional interaction between default mode and frontoparietal networks that are commonly anticorrelated at rest (Menon & Uddin, 2010). We recently combined momentary experience sampling with online neural activity and found that a prefrontal region of this network was associated with the ability to prioritize patterns of episodic social thoughts during periods when external demands were reduced (Turnbull et al., 2018). Together with such evidence our study suggests that the role of the salience network in patterns of ongoing thought emerges from its general capacity for prioritizing patterns of sensory, memorial, and affective content (Christoff et al., 2016) that is motivated by their contextual relevance (McMillan, Kaufman, & Singer, 2013; Smallwood, 2013). To this end, recent perspectives argue for the amplificatory influence of such transient thought patterns on functional outcomes under particular contexts (i.e., stress or anger; Marchetti, Koster, Klinger, & Alloy, 2016; Watkins, 2008). This may explain the state-like characteristic of the brain and well-being link that was observed in this study, and its significant mediation by the participants’ current concerns. Although our experiment was not formally set up to test an intervention-based mediatory relationship and thus could not assess the potential influence of other variables (Bullock, Green, & Ha, 2010), the findings of our study underline the central importance of at least one thought pattern in explaining the link between brain physiology and mental well-being.
A second component of “deliberate and verbal thoughts about the future” was linked to the connectivity of a limited number of nodes in transmodal cortices including regions of the default mode, cingulo-opercular, salience, and frontoparietal networks. This neural pattern was dominated by connections from a region of the superior frontal gyrus within the default mode network. A deliberate focus on future goals reflects our ability to simulate and envision the consequences of our actions based on our prior experiences (Schacter, Addis, & Buckner, 2008). Importantly, similar interactions between the default mode network and systems linked to executive control (e.g., frontoparietal and salience networks) are observed when participants engage in tasks that mimic this type of thought, such as creative problem-solving (Beaty et al., 2015), visuospatial (Vatansever, Manktelow, Sahakian, Menon, & Stamatakis, 2018) as well as autobiographical planning (Spreng et al., 2010), and imagining reward outcomes (Gerlach, Spreng, Madore, & Schacter, 2014). This pattern of thought was predictive of better social well-being, an observation consistent with previous studies linking patterns of ongoing thought to social problem-solving (Ruby, Smallwood, Sackur, et al., 2013) and our ability to infer the actions and mental states of others (Frith, 2007). Despite its significant link with the social health measure, however, the lack of a mediation effect of this thought pattern indicates the trait-like nature of future plans that the participants experienced inside the scanner. With recent hypotheses postulating the vital role of “goal attainment” in spontaneous thoughts (Klinger, 2013) and emerging reports indicating their links to personality traits (e.g., negative affectivity and neuroticism; Andrews-Hanna et al., 2013), we postulate that the observed brain and well-being link may reflect stable neurocognitive traits, particularly within thoughts that concern the participants’ social support network.
Furthermore, this component was also characterized by both positive as well as negative brain connections. Negative or anticorrelations have been historically assumed to arise from the analysis techniques employed, and head motion that is thought to lead to spurious connectivity measures (Murphy, Birn, Handwerker, Jones, & Bandettini, 2009). However, recent reports suggest a neurophysiological basis and a potential cognitive importance of such anticorrelations in healthy brain processing (Fox et al., 2009; Keller et al., 2015; Vatansever, Menon, & Stamatakis, 2017). Hence, our results raise the possibility that the tuning of interactions between neural systems may give rise to different types of ongoing experience—a hypothesis that requires further investigation. In addition, recent perspectives on the generation and maintenance of thought patterns highlight the vital importance of considering the dynamic nature of ongoing cognition and the within-individual variation in this process (Christoff et al., 2016). Although retrospective thought sampling methods provide the advantage of acquiring measures related to an undisrupted period of unconstrained cognition, online thought sampling and the assessment of the link between neural and experiential dynamics might constitute a fruitful route in deciphering the within- and between-individual variability in ongoing cognition and the underlying neural mechanisms (Kucyi, 2018). Taken together with the participants’ current concerns, further research will be required to deduce the differential influence of such stable and transitory thought patterns on functional outcomes and to assess their therapeutic potential as modulatory targets for intervention (Marchetti et al., 2016).
In summary, we have shown that distinct patterns of thought are reflected in the underlying brain functional connectomes at rest and that certain types of these experiences may mediate the influence of intrinsic brain connectivity organization on well-being. Our results highlight the importance of considering thought patterns when establishing predictive relationships between functional connectomes and complex traits, but also suggest that taking this aspect of human cognition into consideration may lead to better characterization of neural fingerprints of the connectome, with the potential for more useful clinical markers. In the future, it may be possible to tailor self-reported questions based on their neural associations, allowing development of measures targeting particular psychiatric populations with well-established neural hypotheses, such as mood and neurodegenerative disorders (Takamura & Hanakawa, 2017). Furthermore, the analysis method we employed could be useful in studies that test psychological or pharmacological interventions designed to improve well-being (Khalili-Mahani et al., 2017), allowing these investigations to disentangle whether their intervention targets the underlying neural architecture, changes in patterns of thought, or a combination of both.
METHODS
Participant Demographics
In accordance with the Declaration of Helsinki on the conduct of research involving human participants, ethical approval was obtained for this study from the Department of Psychology and York Neuroimaging Centre, University of York ethics committees. Following a standard informed consent procedure, a total of 226 healthy, right-handed (one left-handed), native English speaker undergraduate or postgraduate students with normal to corrected vision were recruited from the University of York. All volunteers received monetary compensation or course credit for their participation in line with the departmental policies. As per the exclusion criteria, none of the participants had a history of psychiatric or neurological illness, severe claustrophobia, anticipated pregnancy, or drug use that could alter cognitive functioning. Moreover, an extensive motion-correction procedure was followed (described in detail below) that resulted in the exclusion of 12 participants because of excessive head motion inside the scanner, and three participants were removed because of the impartial completion of the thought sampling method. In total, 211 participants’ imaging and thought sampling data were used in this analysis. The average age for this group was 20.85 years (range = 18–31, SD = 2.44) with a 129/82 female to male ratio.
Decomposition of Thought Patterns
To ascertain the principal dimensions of variation in the thought patterns of this participant cohort, a retrospective thought sampling questionnaire was administered immediately after the resting-state fMRI scanning. In this session, the participants were asked to subjectively rate their thoughts during the resting-state scan on a 4-choice Likert scale from “Not at all” to “Completely” based on a randomly presented set of questions that probed the content and form of thoughts. This set of questions and the accompanying analysis techniques have been extensively utilized in various thought sampling reports previously published in the literature (Supplementary Table S1; Gorgolewski et al., 2014; Medea et al., 2016; Ruby, Smallwood, Engen, & Singer, 2013; Ruby, Smallwood, Sackur, et al., 2013; Smallwood et al., 2016). First, the ratings from each participant were hierarchically clustered based on the similarity of responses using the Ward linkage method (squared Euclidean distance; Supplementary Figure S1). This technique was utilized in order to partition the thought ratings into two distinct groups, thus reducing the number of variables to be decomposed into interpretable patterns of thought (Andrews-Hanna et al., 2013). Subsequently, both groups of ratings were reduced to three factors each (six in total) using PCA in SPSS (Version 23; https://www.ibm.com/products/spss-statistics). The number of components was chosen based on scree plots indicating the eigenvalue of each subsequent decomposition, and its ability to explain variability in the data (Supplementary Figure S2). The component loadings for the total number of six decompositions were then rotated using the Varimax method and the resulting factors were visualized on word clouds (Figure 2) and heat maps (Supplementary Figure S3). The component scores of each participant on these patterns of thought were then used as between-subject covariates of interest in the subsequent analyses. Further results on the robustness of the employed hierarchical clustering and PCA procedures are provided in the Supporting Information and Supplementary Figures S1–S4.
Well-Being Assessment
For the assessment of the participants’ self-perceived well-being, we employed the brief version of a health questionnaire previously established by the World Health Organization Quality of Life (WHOQOL) group (WHOQOL Group, 1998). Termed WHOQOL-BREF, this quality of life assessment has been developed to provide a more comprehensive index of overall health of nations that extends beyond measures of mortality and morbidity; hence, it was designed to be readily administered across cultures and countries with different economic status. Extensively validated in a large number of centers, WHOQOL-BREF consists of 26 questions that broadly group into four domains of physical, psychological, social, and environmental health. All participants were asked to complete this questionnaire at a separate session administered outside the scanner. Out of 211 participants, 169 fully completed the questionnaire. The standardized responses for the psychological and social health domains were then used as covariates of interest in subsequent linear regression and mediation analyses.
MRI Data Acquisition
All MRI data acquisition was carried out at the York Neuroimaging Centre, York, with a 3T GE HDx Excite magnetic resonance imaging (MRI) scanner using an eight-channel phased array head coil. A single run of 9-min resting-state fMRI scan was carried out using single-shot 2D gradient-echo-planar imaging. The parameters for this sequence was as follows: TR = 3 s, TE = minimum full, flip angle = 90, matrix size = 64 × 64, 60 slices, voxel size = 3 × 3 × 3 mm3, 180 volumes. During resting-state scanning, the participants were asked to focus on a fixation cross in the middle of the screen. Subsequently, a T1-weighted structural scan with three-dimensional fast spoiled gradient echo was acquired (TR = 7.8 s, TE = minimum full, flip angle = 20, matrix size = 256 × 256, 176 slices, voxel size = 1.13 × 1.13 × 1 mm3).
MRI Data Preprocessing
All preprocessing and denoising steps for the MRI data were carried out using the SPM software package (Version 12.0; http://www.fil.ion.ucl.ac.uk/spm/) and CONN functional connectivity toolbox (Version 17.f; https://www.nitrc.org/projects/conn; Whitfield-Gabrieli & Nieto-Castanon, 2012), based on the MATLAB platform (Version 16.a; https://uk.mathworks.com/products/matlab.html). The first three functional volumes were removed in order to achieve steady-state magnetization. The remaining data were first corrected for motion using six degrees of freedom (x, y, z translations and rotations), and adjusted for differences in slice time. Subsequently, the high-resolution structural images were coregistered to the mean functional image via rigid-body transformation, segmented into gray/white matter and cerebrospinal fluid probability maps, and spatially normalized to Montreal Neurological Institute (MNI) space alongside all functional volumes using the segmented images and a priori templates. This indirect procedure utilizes the unified segmentation–normalization framework, which combines tissue segmentation, bias correction, and spatial normalization in a single unified model (Ashburner & Friston, 2005). No smoothing was employed, complying with recent reports on the negative influence of this procedure on the construction of functional connectomes and graph theoretic analyses (Alakorkko, Saarimaki, Glerean, Saramaki, & Korhonen, 2017).
Furthermore, a growing body of literature indicates the potential impact of volunteer head motion inside the scanner on the subsequent estimates of functional connectivity and graph theory metrics (Power, Barnes, Snyder, Schlaggar, & Petersen, 2012; Van Dijk, Sabuncu, & Buckner, 2012; Yan, Cheung, et al., 2013; Yan, Craddock, He, & Milham, 2013). In order to ensure that motion and other artifacts did not confound our data, we have employed an extensive motion-correction procedure and denoising steps, comparable to those reported in the literature (Ciric et al., 2017; Power et al., 2014). In addition to the removal of six realignment parameters and their second-order derivatives using the general linear model (GLM; Friston, Williams, Howard, Frackowiak, & Turner, 1996), a linear detrending term was applied as well as the CompCor method that removed five principal components of the signal from white matter (WM) and cerebrospinal fluid (CSF; Behzadi, Restom, Liau, & Liu, 2007). Moreover, the volumes affected by motion were identified and scrubbed based on the conservative settings of motion greater than 0.5 mm and global signal changes larger than z = 3. A total of 12 participants, who had more than 15% of their data affected by motion, were excluded from this study (Power et al., 2014). Though recent reports suggest the ability of global signal regression to account for head motion, it is also known to introduce spurious anticorrelations, and was thus not utilized in our analysis (Chai, Castanon, Ongur, & Whitfield-Gabrieli, 2012; Murphy et al., 2009; Saad et al., 2012). Nevertheless, the composite motion score (i.e., percentage of invalid scans) for each participant was also added as a covariate in group-level analyses to further account for the potential influence of head motion on functional connectome estimations. Finally, a band-pass filter between 0.009 Hz and 0.08 Hz was employed in order to focus on low-frequency fluctuations (Biswal, Yetkin, Haughton, & Hyde, 1995; Fox et al., 2005). The maximum, and mean motion parameters and global signal change, the percentage invalid volumes that were scrubbed, and the distribution of correlation coefficients before and after denoising steps are provided in Supplementary Figure S6. In addition, with the aim of ensuring that our results were not confounded by motion artifacts, we calculated mean framewise displacement using the Jenkinson formulation (Jenkinson, Bannister, Brady, & Smith, 2002), which showed no significant associations with the main variables of interest employed in this study (Supplementary Figures S7–S8).
Functional Connectome Analysis
Brain parcellation.
We adopted a set of 264 regions based on the Power et al. (2011) brain parcellation scheme that has been previously shown to produce reliable network topologies at rest and task conditions (Cole et al., 2013; Power et al., 2011; Vatansever, Menon, Manktelow, Sahakian, & Stamatakis, 2015). The network partitions outlined by Cole et al. (2013) were utilized to preassign each one of the 264 ROIs to one of the 13 large-scale networks documented in the original publication (Power et al., 2011). Namely, 10 well-established networks covering dorsal (DAN) and ventral attention (VAN), salience (SAN), cingulo-opercular (CON), frontoparietal control (FPN), default mode (DMN), visual (VN), auditory (AN), somato-motor (hand and mouth) (SMN), and subcortical networks (SCN), as well as 3 networks that fall into memory retrieval, cerebellum, and a network of uncertain function were used as the 13 network partitions (Power et al., 2011).
Connectome construction.
Fully connected, undirected, and weighted matrices (264 ROI × 264 ROI) of bivariate correlation coefficients (Pearson r) were constructed for each participant using the average BOLD signal time series obtained from the 6-mm (radius) spheres placed on the MNI coordinates of all the 264 ROIs described above. The matrices reflected both positive and negative weighted correlations. The arbitrary thresholding and binarization processes in graph theoretic analysis often lead to loss of information, especially in the case of negative correlations (Rubinov & Sporns, 2011). Given recent reports suggesting a neurophysiological basis and potential cognitive importance of such anticorrelations in healthy brain function (Fox et al., 2009; Keller et al., 2015; Spreng, Stevens, Viviano, & Schacter, 2016), we focused on fully connected, weighted connectomes.
Network-based statistic.
Next, we aimed to ascertain components of individual functional connectomes that significantly predicted the participants’ between-subject variation on the identified patterns of thought. For that purpose, we employed the network-based statistic (NBS) toolbox (Version 1.2; https://www.nitrc.org/projects/nbs/; Zalesky et al., 2010), which provides enhanced power to identify connected brain components formed by suprathreshold edge links that are associated with a covariate of interest, while controlling for family-wise error (FWE) at the component level. Utilizing this method, we entered the individual component scores for all six patterns of thought as variables of interest, while accounting for the effects of mean connectivity, age, gender, and percentage of motion-related invalid scans identified by the scrubbing procedure. Using these regressors, t tests were first carried out on fully connected whole-brain network edges for each pattern of thought to assess the relationship between the strength of an edge link and component scores on patterns of thought, storing the size of the connected components that survived the chosen T threshold. Next, over a total of 5,000 permutations in which the outcome measures were randomized, random null distributions of maximal component size above the chosen threshold were generated. The number of permutations in which the maximal component size was greater than the empirical component size, normalized by the total number of permutations, was used to estimate p values (0.05 level of significance). While the initial Tthreshold = 3.2 was used for the main analysis, comparable results for Tthreshold = 3.1 and Tthreshold = 3.3 are reported in the Supporting Information section (Supplementary Figure S9). The resulting connected brain components, the links of which showed a significant relationship with individual variability in thought patterns, were then defined as mask graphs to threshold individual functional connectomes, which were then carried forward onto graph theoretic analyses (Xia, Wang, & He, 2013).
Network neuroscience analysis.
Graph theoretic metrics in this study were calculated using MATLAB functions obtained from the publicly available Brain Connectivity Toolbox (https://sites.google.com/site/bctnet/). Commonly used in the identification of hub regions that greatly influence the efficiency of a network in distributing information (Rubinov & Sporns, 2010), network strength denotes one of the most fundamental measures in weighted functional connectomes and thus formed the basis of our network neuroscience analysis approach. Calculated as the sum of all neighboring link weights (Rubinov & Sporns, 2010), we measured the strength of connected components across all participants that were previously identified as illustrating significant relations to individual variability in thought patterns. Based on recent reports suggesting the importance of anticorrelations, we calculated positive, negative, as well as total strength for each individual. Furthermore, given recent evidence suggesting a contribution of the balance between both positive and negative correlations to healthy brain processing (Fox et al., 2009; Keller et al., 2015; Spreng et al., 2016), we aimed to utilize a metric that incorporated the importance of the interplay between these links when assessing its predictive power for explaining individual variability in self-reported mental well-being. Thus, we defined fractional strength as the ratio of the sum of positive to negative links, which was later used as the graph metric of interest in subsequent linear regression and mediation analyses. Finally, to identify central nodes in the functionally connected components that significantly related to the thought structures, betweenness centrality—the fraction of all shortest paths in the network that pass through a given node—was calculated. The average positive and negative links and the employed graph theoretic metrics were visualized on circular plots using Circos (Irimia, Chambers, Torgerson, & Van Horn, 2012).
Test-retest reliability analysis.
Our next aim in this study was to determine the reliability of the identified patterns of thought and brain connectivity measures. This would not only establish the generalizability of our results but would also allude to the potential differences in the state versus trait-level variability of our neurocognitive measures. For that purpose, a second session of resting-state scan and experience sampling was carried out for 44 participants using the same parameters outlined above. The thought ratings and brain imaging data for this second session were preprocessed using the same procedures, resulting in the exclusion of four participants because of excessive motion. For the thought decomposition scores, the hierarchical clustering and PCA decompositions obtained from the initial cohort were imposed on the ratings obtained from the second session (Wrigley, Albert, Deluzio, & Stevenson, 2006). Intraclass correlation coefficients (ICC) were employed to assess the reliability of thought component scores and the associated brain connectivity (as denoted by fractional strength) from the first and second sessions. Furthermore, we assessed a potential link between the change in thought patterns between the two sessions and the change in the associated brain functional connections using Pearson correlations.
Mediation analysis.
Finally, we used linear regressions to identify the relationship between component scores on the identified patterns of thought, the fractional strength (natural log) of connected components, and the participants’ self-reported scores on the psychological and social domains of the WHOQOL-BREF questionnaire. The relationships with health were Bonferroni corrected for multiple comparisons across the two health domains. After establishing linear relationships between all three measures, subsequent mediation analyses were carried out with the aim of determining the indirect effect of brain connectivity on psychological and social well-being through participants’ thought patterns. As it is suggested for small to medium sample sizes (Shrout & Bolger, 2002), the percentile bootstrap estimation approach with 5,000 samples was used to ascertain the presence of a mediation effect.
ACKNOWLEDGMENTS
The authors extend their gratitude to Mladen Sormaz, Charlotte Murphy, Hao-Ting Wang, and Giulia Poerio for their invaluable contribution to the scanning of participants. In addition, the authors thank Andre Gouws, Ross Devlin, Jane Hazell, and the rest of the York Neuroimaging Centre staff for their support in setting up the imaging protocol and scanning. Finally, we thank all the participants for their time and effort in taking part in this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
SUPPORTING INFORMATION
Supporting information for this article is available at https://doi.org/10.1162/netn_a_00137.
AUTHOR CONTRIBUTIONS
Deniz Vatansever: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing - Original Draft; Writing - Review & Editing. Theodoros Karapanagiotidis: Data curation; Methodology; Validation; Visualization. Daniel S Margulies: Conceptualization. Elizabeth Jefferies: Conceptualization; Investigation; Project administration; Resources; Supervision; Writing - Review & Editing. Jonathan Smallwood: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Visualization; Writing - Review & Editing.
FUNDING INFORMATION
Jonathan Smallwood, European Research Council (http://dx.doi.org/10.13039/501100000781), Award ID: 646927. Jonathan Smallwood, John Templeton Foundation (http://dx.doi.org/10.13039/100000925). Deniz Vatansever, Science and Technology Commission of Shanghai Municipality and ZJLab (http://dx.doi.org/10.13039/501100003399), Award ID: 2018SHZDZX01.
Supplementary Material
TECHNICAL TERMS
- Well-being:
The holistic experience of a state characterized by health and happiness.
- Functional connectivity:
Statistical dependencies among time series obtained from neurophysiological data.
- Mediation:
A statistical method to assess the mediatory influence of an external variable on the relationship between independent and dependent variables.
- Network-based statistic:
An open-source toolbox to statistically assess hypotheses related to the human connectome.
- Principal component analysis:
A method to reduce dimensionality of the variable space into a smaller number of orthogonal variables that maximize explained variance.
- Hierarchical clustering:
A clustering algorithm aiming to form a topological hierarchy among clusters of observations.
- Functional connectome:
Wiring diagram of a comprehensive set of functional connections in the brain.
- Graph theory:
A field of mathematics quantifying the topological properties of graphs formed by objects (nodes) and their pairwise relationships (edges).
Contributor Information
Deniz Vatansever, Institute of Science and Technology for Brain-inspired Intelligence, Fudan University, Shanghai, China; Department of Psychology, University of York, York, United Kingdom.
Theodoros Karapanagiotidis, Department of Psychology, University of York, York, United Kingdom.
Daniel S. Margulies, Brain and Spine Institute, French National Centre for Scientific Research, Paris, France
Elizabeth Jefferies, Department of Psychology, University of York, York, United Kingdom.
Jonathan Smallwood, Department of Psychology, University of York, York, United Kingdom.
REFERENCES
- Alakorkko T., Saarimaki H., Glerean E., Saramaki J., & Korhonen O. (2017). Effects of spatial smoothing on functional brain networks. European Journal of Neuroscience, 46(9), 2471–2480. 10.1111/ejn.13717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan Cheyne J., Solman G. J., Carriere J. S., & Smilek D. (2009). Anatomy of an error: A bidirectional state model of task engagement/disengagement and attention-related errors. Cognition, 111(1), 98–113. 10.1016/j.cognition.2008.12.009 [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Kaiser R. H., Turner A. E., Reineberg A. E., Godinez D., Dimidjian S., & Banich M. T. (2013). A penny for your thoughts: Dimensions of self-generated thought content and relationships with individual differences in emotional wellbeing. Frontiers in Psychology, 4, 900 10.3389/fpsyg.2013.00900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antrobus J. S., Singer J. L., & Greenberg S. (1966). Studies in the stream of consciousness: Experimental enhancement and suppression of spontaneous cognitive processes. Perceptual and Motor Skills, 23, 399–417 . [Google Scholar]
- Ashburner J., & Friston K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Baird B., Smallwood J., Mrazek M. D., Kam J. W., Franklin M. S., & Schooler J. W. (2012). Inspired by distraction: Mind wandering facilitates creative incubation. Psychological Science, 23(10), 1117–1122. 10.1177/0956797612446024 [DOI] [PubMed] [Google Scholar]
- Baird B., Smallwood J., & Schooler J. W. (2011). Back to the future: Autobiographical planning and the functionality of mind-wandering. Consciousness and Cognition, 20(4), 1604–1611. 10.1016/j.concog.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Barch D. M. (2013). Brain network interactions in health and disease. Trends in Cognitive Sciences, 17(12), 603–605. 10.1016/j.tics.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty R. E., Benedek M., Kaufman S. B., & Silvia P. J. (2015). Default and executive network coupling supports creative idea production. Scientific Reports, 5, 10964 10.1038/srep10964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., & Liu T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F. Z., Haughton V. M., & Hyde J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–541. [DOI] [PubMed] [Google Scholar]
- Bullock J. G., Green D. P., & Ha S. E. (2010). Yes, but what’s the mechanism? (Don’t expect an easy answer). Journal of Personality and Social Psychology, 98(4), 550–558. 10.1037/a0018933 [DOI] [PubMed] [Google Scholar]
- Carriere J. S., Cheyne J. A., & Smilek D. (2008). Everyday attention lapses and memory failures: The affective consequences of mindlessness. Consciousness and Cognition, 17(3), 835–847. 10.1016/j.concog.2007.04.008 [DOI] [PubMed] [Google Scholar]
- Castellanos F. X., Di Martino A., Craddock R. C., Mehta A. D., & Milham M. P. (2013). Clinical applications of the functional connectome. NeuroImage, 80, 527–540. 10.1016/j.neuroimage.2013.04.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai X. J., Castanon A. N., Ongur D., & Whitfield-Gabrieli S. (2012). Anticorrelations in resting state networks without global signal regression. NeuroImage, 59(2), 1420–1428. 10.1016/j.neuroimage.2011.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W., Rolls E. T., Robbins T. W., Gong W., Liu Z., Lv W., … Feng J. (2019). Decreased brain connectivity in smoking contrasts with increased connectivity in drinking. eLife, 8 10.7554/eLife.40765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K., Irving Z. C., Fox K. C., Spreng R. N., & Andrews-Hanna J. R. (2016). Mind-wandering as spontaneous thought: A dynamic framework. Nature Reviews Neuroscience, 17(11), 718–731. 10.1038/nrn.2016.113 [DOI] [PubMed] [Google Scholar]
- Ciric R., Wolf D. H., Power J. D., Roalf D. R., Baum G. L., Ruparel K., … Satterthwaite T. D. (2017). Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuro Image, 154, 174–187. 10.1016/j.neuroimage.2017.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. W., Reynolds J. R., Power J. D., Repovs G., Anticevic A., & Braver T. S. (2013). Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience, 16(9), 1348–1355. 10.1038/nn.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert V., Smallwood J., & Singer T. (2014). Mind your thoughts: Associations between self-generated thoughts and stress-induced and baseline levels of cortisol and alpha-amylase. Biological Psychology, 103, 283–291. 10.1016/j.biopsycho.2014.10.004 [DOI] [PubMed] [Google Scholar]
- Fox M. D., Snyder A. Z., Vincent J. L., Corbetta M., Van Essen D. C., & Raichle M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences, 102(27), 9673–9678. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. D., Zhang D., Snyder A. Z., & Raichle M. E. (2009). The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology, 101(6), 3270–3283. 10.1152/jn.90777.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. J., Williams S., Howard R., Frackowiak R. S., & Turner R. (1996). Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine, 35(3), 346–355. [DOI] [PubMed] [Google Scholar]
- Frith C. D. (2007). The social brain? Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences, 362(1480), 671–678. 10.1098/rstb.2006.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galera C., Orriols L., M’Bailara K., Laborey M., Contrand B., Ribereau-Gayon R., … Lagarde E. (2012). Mind wandering and driving: Responsibility case-control study. BMJ, 345, e8105 10.1136/bmj.e8105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach K. D., Spreng R. N., Madore K. P., & Schacter D. L. (2014). Future planning: Default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Social Cognitive and Affective Neuroscience, 9(12), 1942–1951. 10.1093/scan/nsu001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golchert J., Smallwood J., Jefferies E., Seli P., Huntenburg J. M., Liem F., … Margulies D. S. (2017). Individual variation in intentionality in the mind-wandering state is reflected in the integration of the default-mode, fronto-parietal, and limbic networks. NeuroImage, 146, 226–235. 10.1016/j.neuroimage.2016.11.025 [DOI] [PubMed] [Google Scholar]
- Gorgolewski K. J., Lurie D., Urchs S., Kipping J. A., Craddock R. C., Milham M. P., … Smallwood J. (2014). A correspondence between individual differences in the brain’s intrinsic functional architecture and the content and form of self-generated thoughts. PLoS ONE, 9(5), e97176 10.1371/journal.pone.0097176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene A. S., Gao S., Scheinost D., & Constable R. T. (2018). Task-induced brain state manipulation improves prediction of individual traits. Nature Communications, 9(1), 2807 10.1038/s41467-018-04920-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irimia A., Chambers M. C., Torgerson C. M., & Van Horn J. D. (2012). Circular representation of human cortical networks for subject and population-level connectomic visualization. NeuroImage, 60(2), 1340–1351. 10.1016/j.neuroimage.2012.01.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., & Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–841. 10.1016/s1053-8119(02)91132-8 [DOI] [PubMed] [Google Scholar]
- Karapanagiotidis T., Bernhardt B. C., Jefferies E., & Smallwood J. (2017). Tracking thoughts: Exploring the neural architecture of mental time travel during mind-wandering. NeuroImage, 147, 272–281. 10.1016/j.neuroimage.2016.12.031 [DOI] [PubMed] [Google Scholar]
- Keller J. B., Hedden T., Thompson T. W., Anteraper S. A., Gabrieli J. D., & Whitfield-Gabrieli S. (2015). Resting-state anticorrelations between medial and lateral prefrontal cortex: Association with working memory, aging, and individual differences. Cortex, 64, 271–280. 10.1016/j.cortex.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili-Mahani N., Rombouts S. A., van Osch M. J., Duff E. P., Carbonell F., Nickerson L. D., … van Gerven J. M. (2017). Biomarkers, designs, and interpretations of resting-state fMRI in translational pharmacological research: A review of state-of-the-art, challenges, and opportunities for studying brain chemistry. Human Brain Mapping, 38(4), 2276–2325. 10.1002/hbm.23516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth M. A., & Gilbert D. T. (2010). A wandering mind is an unhappy mind. Science, 330(6006), 932 10.1126/science.1192439 [DOI] [PubMed] [Google Scholar]
- Klinger E. (1971). Structure and functions of fantasy. New York, NY: Wiley-Interscience. [Google Scholar]
- Klinger E. (2013). Goal commitments and the content of thoughts and dreams: Basic principles. Frontiers in Psychology, 4, 415 10.3389/fpsyg.2013.00415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Brown K., Battaglini L., & Smallwood J. (2017). When attention wanders: Pupillometric signatures of fluctuations in external attention. Cognition, 168, 16–26. 10.1016/j.cognition.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Kraft I., Balardin J. B., Sato J. R., Sommer J., Tobo P., Barrichello C., … Kozasa E. H. (2018). Quality of life is related to the functional connectivity of the default mode network at rest. Brain Imaging and Behavior. 10.1007/s11682-018-9954-5 [DOI] [PubMed] [Google Scholar]
- Kucyi A. (2018). Just a thought: How mind-wandering is represented in dynamic brain connectivity. NeuroImage, 180(Pt. B), 505–514. 10.1016/j.neuroimage.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Marchetti I., Koster E. H. W., Klinger E., & Alloy L. B. (2016). Spontaneous thought and vulnerability to mood disorders: The dark side of the wandering mind. Clinical Psychological Science, 4(5), 835–857. 10.1177/2167702615622383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti I., Van de Putte E., & Koster E. H. (2014). Self-generated thoughts and depression: From daydreaming to depressive symptoms. Frontiers in Human Neuroscience, 8, 131 10.3389/fnhum.2014.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan R. L., Kaufman S. B., & Singer J. L. (2013). Ode to positive constructive daydreaming. Frontiers in Psychology, 4, 626 10.3389/fpsyg.2013.00626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay J. C., & Kane M. J. (2009). Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition, 35(1), 196–204. 10.1037/a0014104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medea B., Karapanagiotidis T., Konishi M., Ottaviani C., Margulies D., Bernasconi A., … Smallwood J. (2016). How do we decide what to do? Resting-state connectivity patterns and components of self-generated thought linked to the development of more concrete personal goals. Experimental Brain Research, 236, 2469–2481. 10.1007/s00221-016-4729-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., & Uddin L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214(5–6), 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek M. D., Smallwood J., Franklin M. S., Chin J. M., Baird B., & Schooler J. W. (2012). The role of mind-wandering in measurements of general aptitude. Journal of Experimental Psychology: General, 141(4), 788–798. 10.1037/a0027968 [DOI] [PubMed] [Google Scholar]
- Murphy K., Birn R. M., Handwerker D. A., Jones T. B., & Bandettini P. A. (2009). The impact of global signal regression on resting state correlations: Are anti-correlated networks introduced? NeuroImage, 44(3), 893–905. 10.1016/j.neuroimage.2008.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poerio G. L., Sormaz M., Wang H. T., Margulies D., Jefferies E., & Smallwood J. (2017). The role of the default mode network in component processes underlying the wandering mind. Social Cognitive and Affective Neuroscience, 12(7), 1047–1062. 10.1093/scan/nsx041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R. A. (2011). Inferring mental states from neuroimaging data: From reverse inference to large-scale decoding. Neuron, 72(5), 692–697. 10.1016/j.neuron.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J. D., Barnes K. A., Snyder A. Z., Schlaggar B. L., & Petersen S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage, 59(3), 2142–2154. 10.1016/j.neuroimage.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J. D., Cohen A. L., Nelson S. M., Wig G. S., Barnes K. A., Church J. A., … Petersen S. E. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. 10.1016/j.neuron.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J. D., Mitra A., Laumann T. O., Snyder A. Z., Schlaggar B. L., & Petersen S. E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage, 84, 320–341. 10.1016/j.neuroimage.2013.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., & Sporns O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52(3), 1059–1069. 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Rubinov M., & Sporns O. (2011). Weight-conserving characterization of complex functional brain networks. NeuroImage, 56(4), 2068–2079. 10.1016/j.neuroimage.2011.03.069 [DOI] [PubMed] [Google Scholar]
- Ruby F. J., Smallwood J., Engen H., & Singer T. (2013). How self-generated thought shapes mood—The relation between mind-wandering and mood depends on the socio-temporal content of thoughts. PLoS ONE, 8(10), e77554 10.1371/journal.pone.0077554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby F. J., Smallwood J., Sackur J., & Singer T. (2013). Is self-generated thought a means of social problem solving? Frontiers in Psychology, 4, 962 10.3389/fpsyg.2013.00962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad Z. S., Gotts S. J., Murphy K., Chen G., Jo H. J., Martin A., & Cox R. W. (2012). Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity, 2(1), 25–32. 10.1089/brain.2012.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter D. L., Addis D. R., & Buckner R. L. (2008). Episodic simulation of future events: Concepts, data, and applications. Annals of the New York Academy of Sciences, 1124, 39–60. 10.1196/annals.1440.001 [DOI] [PubMed] [Google Scholar]
- Seli P., Ralph B. C. W., Konishi M., Smilek D., & Schacter D. L. (2017). What did you have in mind? Examining the content of intentional and unintentional types of mind wandering. Consciousness and Cognition, 51, 149–156. 10.1016/j.concog.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli P., Smallwood J., Cheyne J. A., & Smilek D. (2015). On the relation of mind wandering and ADHD symptomatology. Psychonomic Bulletin and Review, 22(3), 629–636. 10.3758/s13423-014-0793-0 [DOI] [PubMed] [Google Scholar]
- Shen X., Finn E. S., Scheinost D., Rosenberg M. D., Chun M. M., Papademetris X., & Constable R. T. (2017). Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nature Protocols, 12(3), 506–518. 10.1038/nprot.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout P. E., & Bolger N. (2002). Mediation in experimental and nonexperimental studies: New procedures and recommendations. Psychological Methods, 7(4), 422–445. [PubMed] [Google Scholar]
- Singer J. L., & Antrobus J. S. (1963). A factor-analytic study of daydreaming and conceptually related cognitive and personality variables. Perceptual and Motor Skills, 17(1), 187–209. 10.2466/pms.1963.17.1.187 [DOI] [PubMed] [Google Scholar]
- Smallwood J. (2013). Distinguishing how from why the mind wanders: A process-occurrence framework for self-generated mental activity. Psychological Bulletin, 139(3), 519–535. 10.1037/a0030010 [DOI] [PubMed] [Google Scholar]
- Smallwood J., Beach E., Schooler J. W. & Handy T. C. (2008). Going AWOL in the brain: Mind wandering reduces cortical analysis of external events. Journal of Cognitive Neuroscience, 20(3), 458–469. 10.1162/jocn.2008.20037 [DOI] [PubMed] [Google Scholar]
- Smallwood J., Karapanagiotidis T., Ruby F., Medea B., de Caso I., Konishi M., … Jefferies E. (2016). Representing representation: Integration between the temporal lobe and the posterior cingulate influences the content and form of spontaneous thought. PLoS ONE, 11(4), e0152272 10.1371/journal.pone.0152272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J., Mrazek M. D., & Schooler J. W. (2011). Medicine for the wandering mind: Mind wandering in medical practice. Medical Education, 45(11), 1072–1080. 10.1111/j.1365-2923.2011.04074.x [DOI] [PubMed] [Google Scholar]
- Smallwood J., Nind L., & O’Connor R. C. (2009). When is your head at? An exploration of the factors associated with the temporal focus of the wandering mind. Consciousness and Cognition, 18(1), 118–125. 10.1016/j.concog.2008.11.004 [DOI] [PubMed] [Google Scholar]
- Smallwood J., Ruby F. J., & Singer T. (2013). Letting go of the present: Mind-wandering is associated with reduced delay discounting. Consciousness and Cognition, 22(1), 1–7. 10.1016/j.concog.2012.10.007 [DOI] [PubMed] [Google Scholar]
- Smallwood J., & Schooler J. W. (2015). The science of mind wandering: Empirically navigating the stream of consciousness. Annual Review of Psychology, 66, 487–518. 10.1146/annurev-psych-010814-015331 [DOI] [PubMed] [Google Scholar]
- Spreng R. N., Stevens W. D., Chamberlain J. P., Gilmore A. W., & Schacter D. L. (2010). Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage, 53(1), 303–317. 10.1016/j.neuroimage.2010.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R. N., Stevens W. D., Viviano J. D., & Schacter D. L. (2016). Attenuated anticorrelation between the default and dorsal attention networks with aging: Evidence from task and rest. Neurobiology of Aging, 45, 149–160. 10.1016/j.neurobiolaging.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D., Majerus S., Maj M., Van der Linden M., & D’Argembeau A. (2011). Mind-wandering: Phenomenology and function as assessed with a novel experience sampling method. Acta Psychologica, 136(3), 370–381. 10.1016/j.actpsy.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Takamura T., & Hanakawa T. (2017). Clinical utility of resting-state functional connectivity magnetic resonance imaging for mood and cognitive disorders. Journal of Neural Transmission (Vienna), 124(7), 821–839. 10.1007/s00702-017-1710-2 [DOI] [PubMed] [Google Scholar]
- Toschi N., Riccelli R., Indovina I., Terracciano A., & Passamonti L. (2018). Functional connectome of the five-factor model of personality. Personality Neuroscience, 1 10.1017/pen.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull A., Wang H. T., Schooler J. W., Jefferies E., Margulies D. S., & Smallwood J. (2018). The ebb and flow of attention: Between-subject variation in intrinsic connectivity and cognition associated with the dynamics of ongoing experience. NeuroImage. 10.1016/j.neuroimage.2018.09.069 [DOI] [PubMed] [Google Scholar]
- Uddin L. Q. (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16(1), 55–61. 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Van Dijk K. R., Sabuncu M. R., & Buckner R. L. (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage, 59(1), 431–438. 10.1016/j.neuroimage.2011.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D. C., & Barch D. M. (2015). The human connectome in health and psychopathology. World Psychiatry, 14(2), 154–157. 10.1002/wps.20228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatansever D., Bozhilova N. S., Asherson P., & Smallwood J. (2018). The devil is in the detail: Exploring the intrinsic neural mechanisms that link attention-deficit/hyperactivity disorder symptomatology to ongoing cognition. Psychological Medicine, 1–10. 10.1017/S0033291718003598 [DOI] [PubMed] [Google Scholar]
- Vatansever D., Bzdok D., Wang H. T., Mollo G., Sormaz M., Murphy C., … Jefferies E. (2017). Varieties of semantic cognition revealed through simultaneous decomposition of intrinsic brain connectivity and behaviour. NeuroImage, 158, 1–11. 10.1016/j.neuroimage.2017.06.067 [DOI] [PubMed] [Google Scholar]
- Vatansever D., Manktelow A., Sahakian B. J., Menon D. K., & Stamatakis E. A. (2018). Default mode network engagement beyond self-referential internal mentation. Brain Connectivity, 8(4), 245–253. 10.1089/brain.2017.0489 [DOI] [PubMed] [Google Scholar]
- Vatansever D., Menon D. K., Manktelow A. E., Sahakian B. J., & Stamatakis E. A. (2015). Default mode dynamics for global functional integration. Journal of Neuroscience, 35(46), 15254–15262. 10.1523/jneurosci.2135-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatansever D., Menon D. K., & Stamatakis E. A. (2017). Default mode contributions to automated information processing. Proceedings of the National Academy of Sciences, 114(48), 12821–12826. 10.1073/pnas.1710521114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. T., Bzdok D., Margulies D., Craddock C., Milham M., Jefferies E., … Smallwood J. (2018). Patterns of thought: Population variation in the associations between large-scale network organisation and self-reported experiences at rest. NeuroImage, 176, 518–527. 10.1016/j.neuroimage.2018.04.064 [DOI] [PubMed] [Google Scholar]
- Wang H. T., Poerio G., Murphy C., Bzdok D., Jefferies E., & Smallwood J. (2018). Dimensions of experience: Exploring the heterogeneity of the wandering mind. Psychological Science, 29(1), 56–71. 10.1177/0956797617728727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins E. R. (2008). Constructive and unconstructive repetitive thought. Psychological Bulletin, 134(2), 163–206. 10.1037/0033-2909.134.2.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., & Nieto-Castanon A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. 10.1089/brain.2012.0073 [DOI] [PubMed] [Google Scholar]
- WHOQOL Group. (1998). Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychological Medicine, 28(3), 551–558. [DOI] [PubMed] [Google Scholar]
- Wrigley A. T., Albert W. J., Deluzio K. J., & Stevenson J. M. (2006). Principal component analysis of lifting waveforms. Clinical Biomechanics (Bristol, Avon), 21(6), 567–578. 10.1016/j.clinbiomech.2006.01.004 [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., & He Y. (2013). BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS ONE, 8(7), e68910 10.1371/journal.pone.0068910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C. G., Cheung B., Kelly C., Colcombe S., Craddock R. C., Di Martino A., … Milham M. P. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage, 76, 183–201. 10.1016/j.neuroimage.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C. G., Craddock R. C., He Y., & Milham M. P. (2013). Addressing head motion dependencies for small-world topologies in functional connectomics. Frontiers in Human Neuroscience, 7, 910 10.3389/fnhum.2013.00910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., & Bullmore E. T. (2010). Network-based statistic: Identifying differences in brain networks. NeuroImage, 53(4), 1197–1207. 10.1016/j.neuroimage.2010.06.041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.