Abstract
BCG vaccination in children protects against heterologous infections and improves survival independently of tuberculosis prevention. The phase III ACTIVATE trial assessed whether BCG has similar effects in the elderly. In this double-blind, randomized trial, elderly patients (n = 198) received BCG or placebo vaccine at hospital discharge and were followed for 12 months for new infections. At interim analysis, BCG vaccination significantly increased the time to first infection (median 16 weeks compared to 11 weeks after placebo). The incidence of new infections was 42.3% (95% CIs 31.9%–53.4%) after placebo vaccination and 25.0% (95% CIs 16.4%–36.1%) after BCG vaccination; most of the protection was against respiratory tract infections of probable viral origin (hazard ratio 0.21, p = 0.013). No difference in the frequency of adverse effects was found. Data show that BCG vaccination is safe and can protect the elderly against infections. Larger studies are needed to assess protection against respiratory infections, including COVID-19 (ClinicalTrials.gov NCT03296423).
Keywords: vaccination, BCG, trained immunity, elderly, infection incidence, respiratory infections, cytokines, epigenetic modifications
Graphical Abstract
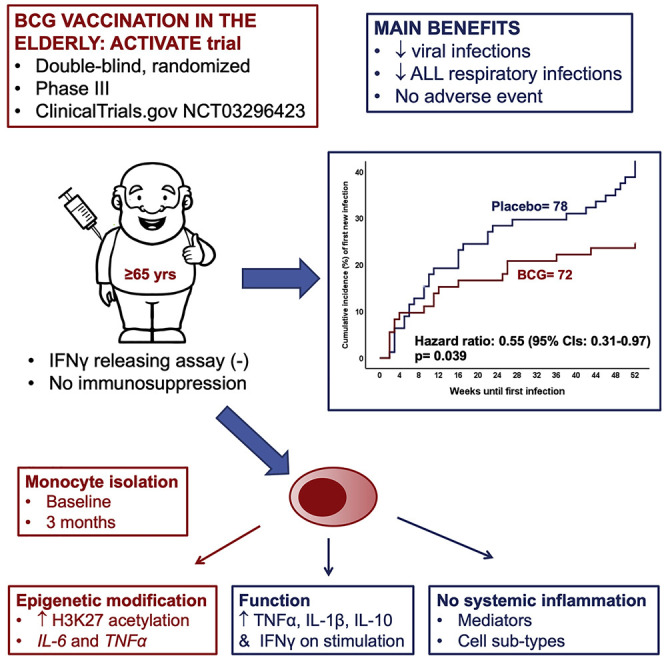
Interim analysis of the phase III ACTIVATE trial to evaluate protection against infection in elderly patients reveals that BCG vaccination is safe, increases the time to first infection, and shows protection against viral respiratory infections.
Introduction
Infection by the novel SARS-CoV-2 virus (also termed COVID-19) has a severe impact on both the health of the populations around the globe, and on the world economy. Many countries are in lockdown, with a third of the world population in some form of movement restrictions, which brings serious financial and societal consequences. The urgent need for the reversal of this situation can be met only through the generation of an immune defense shield to protect the populations from SARS-CoV-2 infection. Many efforts for the development of a vaccine are under way, but it is likely that at least 12–24 months will be needed until an effective vaccine could be available.
Interestingly, however, trained immunity induced by some already available vaccines such as Bacille Calmette-Guérin (BCG), oral polio vaccine (OPV), or the measles vaccine have been suggested to be used as a potential protective approach against COVID-19 to bridge the period until a specific vaccine is developed (Netea et al., 2020). Trained immunity is the process of epigenetic, transcriptional, and functional reprogramming of innate immune cells (such as myeloid cells or natural killer [NK] cells), leading to an increase in the cytokine production capacity and their antimicrobial function (Kleinnijenhuis et al., 2012; Netea et al., 2016). In models of experimental human infections such as yellow fever vaccine virus (Arts et al., 2018) or human experimental malaria (Walk et al., 2019), BCG vaccination was able to induce a non-specific protection.
These experimental data are accompanied by epidemiological studies in children and adults showing non-specific protection against infections and mortality by BCG vaccination. BCG vaccination reduced the incidence of respiratory syncytial virus infection in children in Africa (Stensballe et al., 2005) and protected the elderly against respiratory tract infections in Indonesia (Wardhana et al., 2011) and Japan (Ohrui et al., 2005). Finally, the concept was also successfully tested in healthy volunteers that were vaccinated with placebo or BCG vaccine and 14 days later received a tri-valent influenza A vaccine. Volunteers previous vaccinated by BCG developed significantly greater titers against hemagglutinin A of the influenza A virus, whereas their circulating monocytes were more potent for the production of interferon-gamma (Leentjens et al., 2015).
ACTIVATE (a randomized clinical trial for enhanced trained immune responses through BCG vaccination to prevent infections of the elderly) is a randomized trial in which hospitalized elderly patients were vaccinated on the day of hospital discharge with single doses of placebo or BCG. Patients were under follow-up for 12 months, with the last visit of the last patient scheduled for August 2020. However, the pressure rising from the need of protection of the elderly who are considered susceptible to infection by SARS-CoV-2 (Guan et al., 2020; Huang et al., 2020) led to an interim analysis of the results of the study. Results of this interim analysis clearly showed protection of the elderly from new infections with major effect on the prevention of respiratory infections.
Results
Baseline Characteristics Are Comparable between the Two Arms of Vaccination
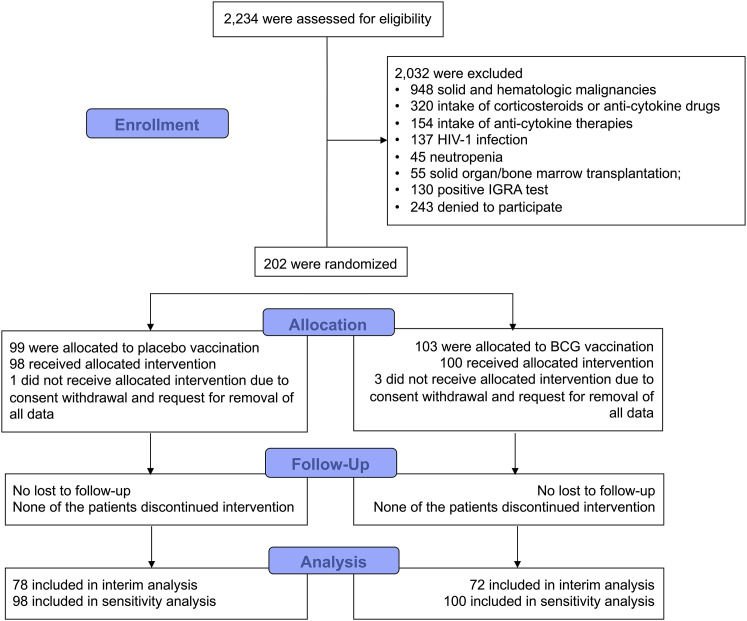
From September 2017 through August 2019, 202 patients were enrolled and randomized to double-blind vaccination with placebo or BCG; four patients withdrew consent and requested removal of all data, leaving a final intention-to-treat analysis cohort of 198 patients. No patient was reported as lost to follow-up (Figure 1 ). Interim analysis included 78 patients allocated to placebo vaccination and 72 patients allocated to BCG vaccination. Baseline characteristics were similar between the two arms (Table 1 ; Table S1).
Figure 1.
Study Flow Chart
BCG, Bacillus Calmette-Guérin; HIV, human immunodeficiency virus; IGRA, interferon-gamma releasing assay
Table 1.
Baseline Characteristics of Enrolled Patients
| Characteristic | Placebo (N = 78) | BCG (N = 72) | p value |
|---|---|---|---|
| Age, years, mean (SD) | 79.6 (7.8) | 79.9 (7.6) | 0.802 |
| Male gender, no. (%) | 35 (44.9) | 32 (44.4) | 1.000 |
| Charlson’s Comorbidity Index, mean (SD) | 5.5 (1.9) | 5.5 (2.2) | 0.909 |
| ΑPACHE II score on study enrolment, mean (SD) | 7.9 (3.0) | 8.1 (2.9) | 0.701 |
| SOFA score on study enrolment, mean (SD) | 1.2 (1.4) | 1.0 (1.1) | 0.586 |
| Comorbidities, no. (%) | |||
| Diabetes mellitus, no. (%) | 29 (37.2) | 23 (31.9) | 0.607 |
| Without organ damage, no. (%) | 23 (29.5) | 15 (20.9) | 0.262 |
| With organ damage, no. (%) | 6 (7.7) | 8 (11.1) | 0.578 |
| Chronic heart failure, no. (%) | 23 (29.5) | 20 (27.8) | 0.858 |
| Chronic renal disease, no. (%) | 14 (17.9) | 12 (16.7) | 1.000 |
| Chronic obstructive pulmonary disease, no. (%) | 12 (15.4) | 11 (15.3) | 1.000 |
| Cerebrovascular disease, no. (%) | 17 (21.8) | 21 (29.2) | 0.349 |
| Degenerative disease, no. (%) | 8 (10.3) | 6 (8.3) | 0.783 |
| Myocardial infarction, no. (%) | 13 (16.7) | 9 (12.5) | 0.498 |
| Biliary stones, no. (%) | 10 (12.8) | 11 (15.3) | 0.814 |
| Renal stones, no. (%) | 1 (1.3) | 1 (1.4) | 1.000 |
| Any surgery, no. (%) | 30 (38.5) | 30 (41.7) | 0.740 |
| Dementia, no. (%) | 15 (19.2) | 20 (27.8) | 0.249 |
| Hemiplegia, no. (%) | 1 (1.3) | 1 (1.4) | 1.000 |
| Peptic ulcer disease, no. (%) | 3 (3.8) | 3 (4.2) | 1.000 |
| Peripheral vascular disease, no. (%) | 1 (1.3) | 0 (0) | 1.000 |
| Liver disease, no. (%) | 1 (1.3) | 1 (1.4) | 1.000 |
| Hypertension, no. (%) | 56 (71.8) | 53 (73.6) | 0.856 |
| Atrial fibrillation, no. (%) | 30 (38.5) | 22 (30.6) | 0.391 |
APACHE, acute physiology and chronic health evaluation; SD, standard deviation; SOFA, sequential organ failure assessment.
BCG Vaccination Decreases the Incidence of New Infections with a Major Impact on the Incidence of Respiratory Tract Infections
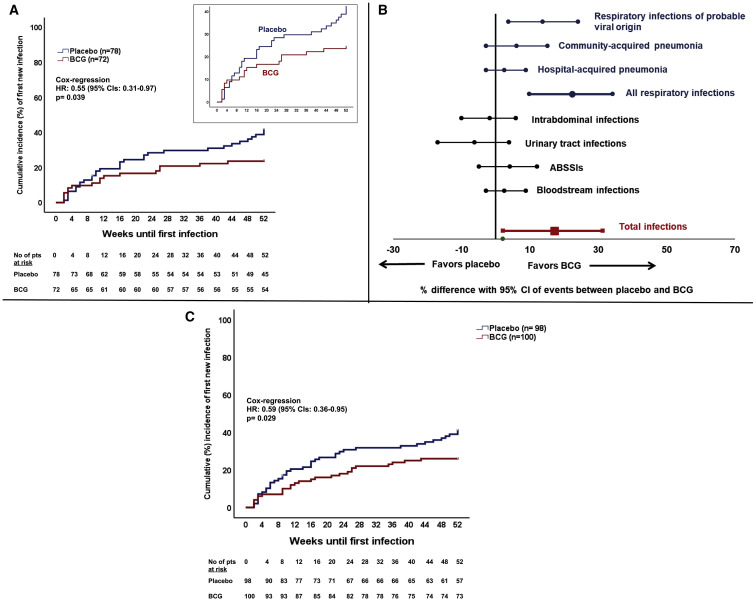
Regarding the primary endpoint of the study, BCG vaccination significantly increased the time to first infection: median 16 weeks after BCG vaccine compared to 11 weeks after placebo administration. The incidence of a new infection during the 12-month period of follow-up after vaccination was also significantly decreased; the statistically significant hazard ratio (HR) of 0.55 corresponds to 45% reduction in the risk of a new infection in the BCG group compared to the placebo group (Figure 2 A). The incidence of new infection was 42.3% (95% confidence intervals [CIs] 31.9%–53.4%) in the placebo group and 25.0% (95% CIs 16.4%–36.1%) in the BCG group. The difference in the incidence according to the type of infection showed most of the benefit on the prevention of respiratory infections of probable viral origin (Figure 2B); the HR in this case was 0.21 (95% CI 0.06–0.72) corresponding to 79% decrease in the risk for the BCG group in comparison to the placebo group. An analytical presentation of the efficacy of BCG vaccination for all primary and secondary study outcomes is shown in Table 2 .
Figure 2.
Primary Outcome of the ACTIVATE Trial
(A) Comparative time to first infection in the two groups of vaccination included in the interim analysis (placebo = 78 patients; BCG = 72 patients)
Infections counting against the primary endpoint were respiratory infection of probably viral origin necessitating medical attention, community-acquired pneumonia, hospital-acquired pneumonia, intraabdominal infections, urinary tract infections, bloodstream infections, and acute bacterial skin and skin structure infections (ABSSSIs). The inset shows the same data on an enlarged y axis. The hazard ratio (HR) and the 95% confidence intervals (CIs) of the Cox-regression analysis are shown along with the respective p value of comparison
(B) Percentage differences and 95% CIs of the incidence of each type of infection between the two groups of vaccination included in the interim analysis (placebo = 78 patients; BCG = 72 patients)
(C) Sensitivity analysis: time to first infection after placebo or BCG vaccination among all study participants (placebo = 98 patients; BCG = 100 patients). This analysis is done taking into consideration that the time of 12-month follow-up has not been completed for 48 patients. The HR and the 95% CIs of the Cox-regression analysis are shown along with the respective p value of comparison
Table 2.
Primary and Secondary Study Outcomes
| Study Outcome | Placebo (N = 78) | BCG (N = 72) | Hazard Ratio (95% CI) | p value |
|---|---|---|---|---|
| Incidence at least one new infection until month 12, no. (%)a | 33 (42.3) | 18 (25.0) | 0.55 (0.31–0.97) | 0.039 |
| Respiratory infections of probable viral origin necessitating medical treatment, no. (%) | 14 (17.9) | 3 (4.2) | 0.21 (0.06–0.72) | 0.013 |
| Community-acquired pneumonia, no. (%) | 8 (10.3) | 3 (4.2) | 0.38 (0.10–1.43) | 0.153 |
| Hospital-acquired pneumonia, no. (%) | 2 (2.6) | 0 (0) | – | 0.479 |
| All respiratory infections, no. (%) | 24 (30.1) | 6 (8.3) | 0.25 (0.10–0.60) | 0.002 |
| Intrabdominal infections, no. (%) | 3 (3.8) | 4 (5.6) | 1.39 (0.31–6.21) | 0.667 |
| Urinary tract infections, no. (%) | 6 (7.7) | 8 (11.1) | 1.38 (0.48–3.97) | 0.553 |
| Acute bacterial skin and skin structure infections, no. (%) | 6 (7.7) | 3 (4.2) | 0.51 (0.13–2.02) | 0.335 |
| Bloodstream infection, no. (%) | 2 (2.6) | 0 (0) | – | 0.497 |
| Incidence of second infection until month 12, no. (%) | 9 (11.5) | 5 (6.9) | 0.59 (0.20–1.77) | 0.349 |
| Incidence of third infection until month 12, no. (%) | 3 (3.8) | 1 (1.4) | 0.36 (0.04–3.45) | 0.375 |
| Rate of hospitalization/patient until month 12, mean (SD) | 0.49 (0.72) | 0.43 (0.72) | N/A | 0.383 |
| Patient-infections per year, per 100 patients, no. (%) | 45 (57.7) | 24 (33.3) | N/A | 0.003 |
| Courses of antibiotics until month 12, mean (SD) | 0.69 (1.66) | 0.60 (1.25) | N/A | 0.665 |
| One-year mortality, no. (%) | 14 (17.9) | 10 (13.9) | 0.68 (0.30–1.54) | 0.356 |
CI, confidence interval; N/A, not applicable; SD, standard deviation.
Some patients had more than one infection.
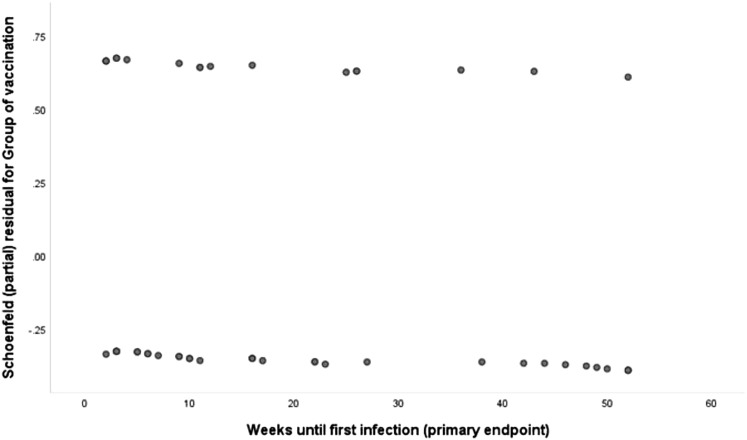
Sensitivity analysis was done for the total of 198 patients taking into consideration that the time of 12-month follow-up has not been completed for 48 patients (Figure 2C; Table S2). This sensitivity analysis confirmed the results of the primary outcome presented in Figure 2A. The confirmation of the primary endpoint of the interim analysis by the sensitivity analysis establishes the absence of any violations on the time-to-event analyses since individuals that are censored have the same probability of experiencing a subsequent event as individuals that remain in the study. The proportionality of the hazards over the total time period of follow-up was validated by plotting the Schoenfeld residuals (Figure S1 ).
Figure S1.
Plot of Schoenfeld Residuals against Time to Event, Related to Figure 2
If the assumption of proportionality of hazards across the two groups is not violated then residuals for the two groups should lie in two parallel straight lines. The above chart confirms this. Therefore the assumption of proportionality of the hazards across the whole time of follow-up is not violated since it remains practically the same.
Stepwise Cox regression analysis showed that BCG vaccination was an independent protective factor from the incidence of new infection until month 12 (HR 0.56; 95% CI, 0.32–0.99; p = 0.048) (Table 3 ).
Table 3.
Univariate and Multivariate Analysis of the Effects of Covariates on the Incidence of at Least One Infection until Month 12
| Covariates | No. Infection (n = 99) | At Least One New Infection (n = 51) | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |||
| BCG vaccination, no. (%)a | 54 (54.4) | 18 (35.3) | 0.55 | 0.31–0.97 | 0.035 | 0.56 | 0.32–0.99 | 0.048 |
| Male gender, no. (%)b | 40 (40.4) | 27 (52.9) | 0.66 | 0.38–1.15 | 0.145 | |||
| CCI > 4, no. (%)c | 61 (61.6) | 40 (78.4) | 2.11 | 1.08–4.12 | 0.028 | |||
| Type 2 diabetes mellitus, no. (%)b | 31 (31.6) | 21 (41.2) | 1.34 | 0.76–2.33 | 0.310 | |||
| Chronic heart failure, no. (%)b | 26 (26.5) | 17 (33.3) | 1.35 | 0.75–2.42 | 0.313 | |||
| Chronic renal disease, no. (%)a | 13 (13.3) | 13 (25.2) | 1.96 | 1.05–3.69 | 0.036 | 1.95 | 1.04–3.66 | 0.038 |
| COPD, no. (%)a | 10 (10.2) | 13 (25.5) | 2.14 | 1.14–4.02 | 0.018 | 2.12 | 1.13–3.99 | 0.019 |
| Cerebrovascular disease, no. (%)b | 27 (27.6) | 11 (21.6) | 0.77 | 0.40–0.51 | 0.450 | |||
| Degenerative disease, no. (%)b | 7 (7.1) | 7 (13.7) | 1.91 | 0.86–4.25 | 0.111 | |||
| Myocardial infarction, no. (%)c | 10 (10.2) | 12 (23.5) | 2.05 | 1.07–3.92 | 0.030 | |||
| Biliary stones, no. (%)b | 15 (15.3) | 6 (11.8) | 0.81 | 0.34–1.89 | 0.620 | |||
| Any surgery, no. (%)b | 40 (40.8) | 20 (39.2) | 0.92 | 0.53–1.62 | 0.783 | |||
| Dementia, no. (%)b | 22 (22.4) | 13 (25.5) | 1.26 | 0.67–2.37 | 0.472 | |||
| Peptic ulcer disease, no. (%)b | 4 (4.1) | 2 (3.9) | 0.88 | 0.22–3.63 | 0.863 | |||
| Hypertension, no. (%)b | 75 (76.5) | 34 (66.7) | 0.69 | 0.39–1.24 | 0.215 | |||
| Atrial fibrillation, no. (%)c | 29 (29.6) | 23 (45.1) | 1.72 | 0.99–2.99 | 0.053 | |||
CCI, Charlson’s comorbidity index; CI, confidence intervals; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
Covariates with a significant effect both in the univariate analysis and the multivariate model
Covariates without any significant effect in the univariate analysis and not entered in the multivariate stepwise Cox regression model
Covariates with a significant effect in the univariate analysis but failed to enter significantly in the multivariate stepwise Cox regression model
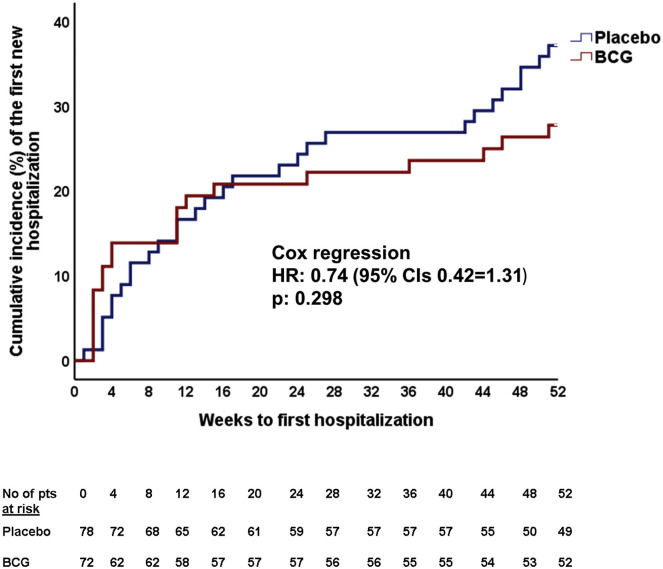
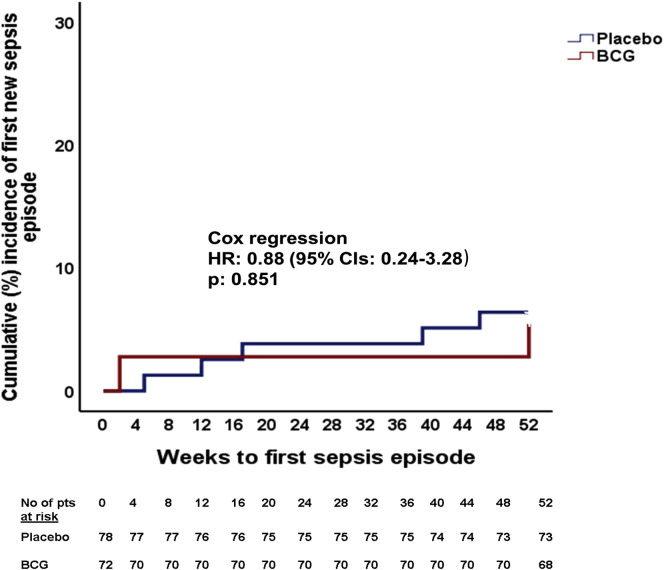
A major benefit from BCG vaccination was observed in the main secondary endpoint patient-infections per year. This was 57.7 per 100 patients in the placebo group and 33.3 per 100 patients in the BCG group (p = 0.003) (Table 2). No difference in the other secondary endpoints was found between the two groups (Table 2; Figures S2 and S3 ).
Figure S2.
Time to First Hospitalization after Placebo or BCG Vaccination, Related to Table 2
Figure S3.
Time to First Sepsis Episode after Placebo or BCG Vaccination, Related to Table 2
The Benefit from BCG Vaccination Is Associated with Modulation of the Pattern of Cytokine Production
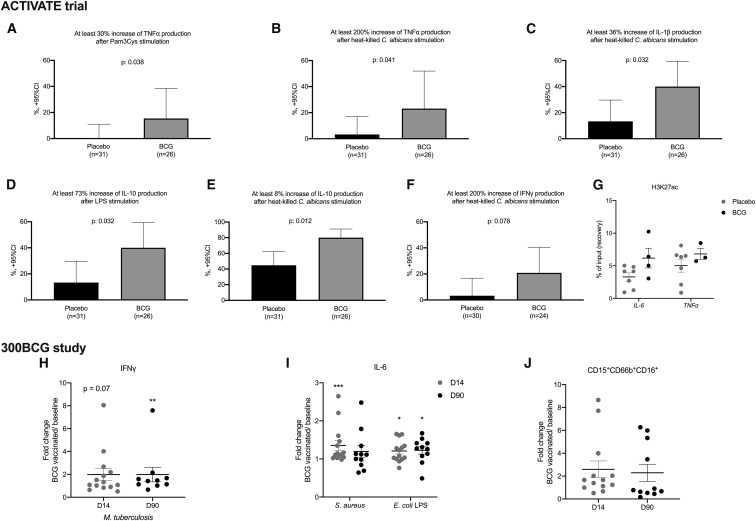
In a sub-group of 57 participants (31 placebo and 26 BCG vaccinated), we assessed production of innate immune responses at 2 time points (before and 3 months after vaccination) in peripheral blood mononuclear cells (PBMCs). Heterologous production of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and IL-10 (trained immunity induction) (Figures 3 A–3E) but not of IL-6 (data not shown) by PBMCs after stimulation with non-mycobacterial ligands was amplified among BCG-vaccinated individuals compared to placebo-vaccinated individuals. A trend toward amplified interferon-gamma (IFN-γ) (heterologous T cell responses) responses was also found (Figure 3F). Unfortunately, the number of BCG-vaccinated individuals in which cytokine data are available is too small to permit the prediction of trained immunity responses as correlates of protection.
Figure 3.
Immunological Effects of BCG Vaccination
Participants in the ACTIVATE trial were vaccinated with placebo or with BCG. (A)–(F) report PBMCs isolated at baseline (month 0) and 3 months after vaccination. PBMCs were stimulated for cytokine production. Blood sampling was not done for all participants after 3 months either because some individuals had died or because they were hospitalized at other study sites or because of denial for blood sampling.
(A) Percentage of patients vaccinated with placebo and BCG with more than 30% increase of the production of TNF-α after stimulation with Pam3Cys.
(B) Percentage of patients vaccinated by placebo and BCG with more than 200% increase of the production of tumor necrosis factor-alpha (TNF-α) after stimulation with heat-killed Candida albicans.
(C) Percentage of patients vaccinated by placebo and BCG with more than 36% increase of the production of interleukin (IL)-1β after stimulation with heat-killed C. albicans.
(D) Percentage of patients vaccinated by placebo and BCG with more than 73% increase of the production of IL-10 after stimulation with lipopolysaccharide (LPS) of Escherichia coli.
(E) Percentage of patients vaccinated by placebo and BCG with more than 8% increase of the production of IL-10 after stimulation with heat-killed C. albicans.
(F) Percentage of patients vaccinated by placebo and BCG with more than 200% increase of the production of interferon-gamma (IFN-γ) after stimulation with heat-killed C. albicans.
The p values of comparisons by the Fisher’s exact test are provided in (A)–(F).
(G) Monocytes were analyzed by chromatin immunoprecipitation (ChIP)-qPCR to determine H3K27ac levels at promoter sites of IL6 and TNFα (n = 7 placebo group, n = 4 BCG group).
Participants in the 300BCG study were vaccinated with BCG
(H and I) PBMCs were isolated and stimulated ex vivo with LPS, heat-killed Staphylococcus aureus, or Mycobacterium tuberculosis before vaccination and 14 days (D14) and 90 days (D90) after vaccination. Fold increases (compared to baseline) of IFN-γ and IL-6 are shown (n = 14, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 by the Wilcoxon’s signed-rank test.
(J) Cell-surface expression molecules in the granulocyte population were analyzed by flow cytometry before vaccination, and 14 days (D14) and 90 days (D90) after vaccination (fold change of median fluorescence intensity as compared to baseline).
Various studies have shown that the increased cytokine responses upon BCG vaccination are the result of epigenetic reprogramming of monocytes (Arts et al., 2018; Kleinnijenhuis et al., 2012). In order to examine potential differences in the epigenetic profile between BCG-vaccinated individuals and controls, we determined at pro-inflammatory genes the level of histone H3 acetylation at lysine 27 (H3K27ac), a mark of active promoters and enhancers. In line with previous findings, we observed increased levels of H3K27ac at the regions of IL-6 and TNF-α in BCG-vaccinated individuals as compared to individuals that received placebo, suggestive of epigenetic reprogramming upon BCG vaccination (Figure 3G).
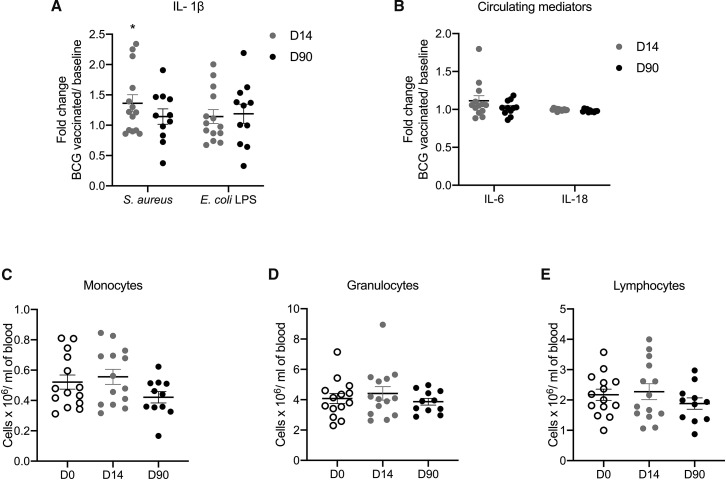
To further validate the solidity of the observation that BCG induces trained immunity responses in the elderly, we assessed immune responses before BCG vaccination, 2 weeks and 3 months after vaccination in 14 healthy volunteers aged 55 years or older that took part in an independent BCG-vaccination study (300BCG cohort, www.humanfunctionalgenomics.org). All individuals in this cohort were vaccinated with the same BCG strain used in the ACTIVATE trial and PBMCs were isolated and stimulated ex vivo with Staphylococcus aureus, lipopolysaccharide (LPS), or Mycobacterium tuberculosis, before and after vaccination to assess the magnitude of the immune memory responses. We observed a significant increase in IFN-γ upon stimulation with M. tuberculosis after BCG vaccination (Figure 3H), indicative of induction of adaptive immune memory response. In addition, cytokine production also significantly increased in the elderly when cells were exposed to non-mycobacterial stimuli such as S. aureus and LPS (Figure 3I; Figure S4 A), indicative of induction of trained immunity. Furthermore, we observed long-term changes in neutrophil phenotype 3 months upon BCG vaccination as compared to baseline (Figure 3J). Together, these findings indicate sustained trained immunity responses in the elderly and support our previous observation of non-specific beneficial effects against unrelated infections in the elderly upon BCG vaccination. In addition, we employed a targeted proteome platform to measure 92 inflammatory markers before and after BCG vaccination, which revealed no significant changes in the concentrations of circulating inflammatory proteins, including IL-6 and IL-18 after BCG (Figure S4B; Table S3). Similarly, no significant changes in monocyte, granulocyte, or lymphocyte count were observed upon vaccination (Figures S4C–S4E). This demonstrates that, while BCG vaccination induces trained immunity and cell responsiveness, it is not followed by excessive systemic inflammation.
Figure S4.
Immunological Effects of BCG Vaccination in the Elderly, Related to Figure 3
(A) Healthy volunteers (≥55 years) from the 300BCG cohort were vaccinated with BCG and peripheral blood mononuclear cells (PBMCs) were isolated and stimulated ex vivo with S. aureus or lipopolysaccharide (LPS) of Escherichia coli before vaccination, 14 days (D14) and 90 days (D90) after vaccination. Fold increases (compared to baseline) of interleukin (IL)-1β are shown (n = 14, ∗p < 0.05 by the Wilcoxon’s ranked sum test). (B) Fold changes of circulating IL-6 and IL-18 upon BCG vaccination as compared to baseline (n = 13). See also Table S3 for a complete list of circulating markers that were measured. (C) Absolute monocyte, granulocyte and lymphocyte counts in whole blood before vaccination, 14 days (D14) and 90 days (D90) after vaccination. Mean values and SE are provided in each panel.
Adverse Events
A trend for lower serious adverse events was recorded in the BCG vaccination group than in the placebo group (Table 4 ). Moreover, the incidence of non-serious adverse events did not differ between the two groups. None of the adverse events were related to the study intervention. None of the patients developed tuberculosis.
Table 4.
List of Severe Adverse Events and Non-severe Adverse Events Reported during the Study Period
| Serious Adverse Events (SAEs) | Placebo (N = 78) | BCG (N = 72) | p value |
|---|---|---|---|
| Presence of at least one SAE,a no. (%) | 30 (38.5) | 17 (23.6) | 0.055 |
| Death — no. (%) | 8 (10.3) | 5 (6.9) | 0.568 |
| SAEs with hospitalization,a no. (%) | 20 (25.6) | 10 (13.9) | 0.101 |
| Reason for hospitalization, no. (%) | |||
| Arrythmia | 1 (1.3) | 0 (0) | 1.000 |
| Stroke | 2 (2.6) | 1 (1.4) | 1.000 |
| Acute kidney injury | 0 (0) | 1 (1.4) | 0.480 |
| Deep vein thrombosis | 1 (1.3) | 0 (0) | 1.000 |
| Epilepsy | 1 (1.3) | 0 (0) | 1.000 |
| Electrolyte disturbance | 1 (1.3) | 0 (0) | 1.000 |
| Pulmonary edema | 1 (1.3) | 0 (0) | 1.000 |
| Anemia | 1 (1.3) | 0 (0) | 1.000 |
| ST-segment elevation at ECG | 1 (1.3) | 0 (0) | 1.000 |
| Elective surgery | 2 (2.6) | 2 (2.8) | 1.000 |
| SAEs without hospitalization, no. (%) | |||
| Stroke, no. (%) | 1 (1.3) | 0 (0) | 1.000 |
| Syncope | 0 (0) | 1 (1.4) | 0.480 |
| Anemia | 1 (1.3) | 0 (0) | 1.000 |
| Non-serious adverse events (AEs) | |||
| At least one non-serious AE,a no. (%) | 20 (25.6) | 26 (36.1) | 0.215 |
| Type of non-serious AE, no. (%) | |||
| Varicella-zoster eruption | 1 (1.3%) | 0 (0) | 1.000 |
| Helicobacter pylori infection | 3 (3.8) | 0 (0) | 0.246 |
| Dacryocystitis | 0 (0) | 1 (1.4) | 0.480 |
| Hip fracture | 2 (2.6) | 0 (0) | 0.490 |
| Non-infection associated cough | 4 (5.1) | 11 (15.3) | 0.055 |
| Asymptomatic bacteriuria | 2 (2.6) | 7 (9.7) | 0.088 |
| Breast cancer | 1 (1.3) | 1 (1.4) | 1.000 |
| Renal cancer | 0 (0) | 1 (1.4) | 0.480 |
| Squamous skin carcinoma | 0 (0) | 1 (1.4) | 0.480 |
| Rash at the injection site | 0 (0) | 2 (2.8) | 0.229 |
| Otitis | 0 (0) | 1 (1.4) | 0.480 |
| Dental infection | 2 (2.6) | 1 (1.4) | 1.000 |
SAEs and deaths due to infections counting against the primary endpoint are not encountered here since per protocol they should not be reported as adverse events.
Some patients had more than one SAE and/or more than one AE.
Discussion
The ACTIVATE study was conducted from 2017 with the aim to assess the potential of BCG vaccination to protect the elderly with an increased risk for infection against new infectious episodes. As a target population we have chosen to investigate elderly patients returning home from a hospital admission, as it is known that this population is at a high risk to develop infections (Bender, 2003). This approach using BCG vaccination is justified due to the increasing number of experimental and epidemiological studies suggesting that BCG can protect against respiratory infections in general, and viral infections, in particular (Moorlag et al., 2019). Indeed, the data shown here demonstrate that BCG vaccination led to a lower number of infections of all causes, and especially respiratory tract infections, arguing for a protective effect.
Epidemiological data suggest beneficial effects of BCG on all-cause mortality in children in countries with high infectious pressure. This protection has been attributed to lower incidence of neonatal sepsis and respiratory tract infections (Garly et al., 2003), which in children are often viral as etiological cause. This assumption is also supported by the data indicating protective effects of BCG vaccination against RSV infection (Stensballe et al., 2005). The protection in children was also complemented more recently by studies showing protective effects of BCG vaccination against respiratory tract infections in adolescents (Nemes et al., 2018) and in elderly individuals (Wardhana et al., 2011). In line with this, the incidence of infection in the ACTIVATE trial was significantly lower in the elderly individuals vaccinated with BCG, compared to the non-vaccinated participants. Moreover, this protection was mainly due to respiratory tract infections of probable viral origin, with HR 0.21 in the BCG vaccinated group, which is in line with the 70%–80% reduction in respiratory tract infections in studies done in Indonesia and Japan (Ohrui et al., 2005; Wardhana et al., 2011).
An important aspect that should be mentioned is that this interim analysis was performed earlier than the final visit of the planned study, resulting in 78 patients in the placebo group and 72 patients in the BCG group being able to complete the 12-month follow-up. The reason for this interim analysis that has been approved by National Ethics Committee and by the National Organization for Medicines of Greece was the emergence of the COVID-19 pandemic and the initiation of several major studies on the effect of BCG on the infection with SARS-CoV-2. In addition to the effectiveness aspect, another important question that needed to be urgently answered was that of the safety of BCG vaccination is the setting of COVID-19. An exaggerated inflammatory reaction has been described to contribute to severity and mortality in some patients with COVID-19 (Huang et al., 2020) raising concerns in the community that BCG vaccination may have deleterious effects due to the enhancement of innate immune responses. Indeed, circulating concentrations of proinflammatory cytokines are increased in severely ill COVID-19 patients (Huang et al., 2020). On the other hand, it can be also argued that vaccination with BCG leading to activation of antiviral mechanisms will lead to decreased viral loads and thus lower systemic inflammation, with milder disease and quicker recovery. This model is supported by our earlier studies showing that BCG vaccination decreased viral loads and systemic inflammation after yellow fever vaccine administration (Arts et al., 2018). It is reassuring to observe that this hypothesized protective effect of BCG vaccination is supported by the clinical data in ACTIVATE trial where most of benefit was observed for the incidence of respiratory infection of probable viral origin. Importantly, we observed no increase in the concentrations of pro-inflammatory proteins in the circulation of BCG-vaccinated individuals as compared to concentrations before vaccination, demonstrating that steady-state levels of inflammation are not increased by BCG.
The mechanism of protection induced by BCG vaccination could be either through heterologous T cell responses (Welsh et al., 2010) or though induction of trained immunity (Kleinnijenhuis et al., 2012). Our data showing enhanced cytokine responses to non-mycobacterial stimuli and epigenetic reprogramming of monocytes in BCG vaccinated individuals point toward the induction of trained immunity, although it is likely that a combination between innate and heterologous T cell immunity is responsible for the entire clinical effect.
The main limitations of the trial are (1) the relatively small sample size of our cohort that will need additional validation in a larger study, (B) the lack of repeat IGRA after vaccination, (C) the absence of serological information on the incidence of various respiratory infections, and (4) the lack of information on BCG vaccination at birth. It needs, however, to be mentioned that despite the small sample size significant differences were found.
The number of individuals participating in the trial is too low to permit us drawing any conclusions regarding the effect of BCG vaccination on coronaviruses in general, or COVID-19, in particular. For that, either much longer follow-up or much larger studies are necessary. Indeed, several clinical trials have been started or are under preparations to test this hypothesis. The majority of these trials are studying the protective effect of BCG in healthcare workers, and a synopsis of these trials is provided in Table S4. Serological assessment of the prevalence of antibodies against respiratory coronaviruses is also warranted in future studies.
In conclusion, in the present study we demonstrate that BCG vaccination is safe and decreases the number of infections in an elderly population at risk. While these data need to be interpreted with caution, they support the hypothesis that BCG-induced trained immunity protects against new infections, mostly against respiratory tract infections. Although results argue that BCG vaccination could be used to bridge the period until a specific vaccine for SARS-CoV-2 is developed and produced, larger randomized clinical trials to study the impact of BCG vaccination on morbidity and mortality due to SARS-CoV-2 infection are needed.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-H3K27ac | Diagendode | Cat#pab-196-050: RRID: AB_8580 |
| anti-CD16 FITC (clone 3G8) | Beckman Coulter | Cat#B49215: RRID: AB_2848116 |
| anti-CD10 PE (clone HI10A) | BioLegend | Cat#312203: RRID: AB_314914 |
| anti-CD11b PE-Dazzle (clone ICRF44) | BioLegend | Cat#301347: RRID: AB_2564080 |
| anti-CD14 PECy5.5 (clone M5E2) | BioLegend | Cat#301847: RRID: AB_2564058 |
| anti-CD62L PE-Cy7 (clone DREG-56) | BioLegend | Cat#301347: RRID: AB_830800 |
| anti-PD-L1 APC (clone MIH1) | ThermoFisher | Cat#17598342: RRID: AB_10597586 |
| anti-CD66b AF700 (clone G10F5) | BioLegend | Cat#305113: RRID: AB_2566037 |
| anti-CD15 Brilliant Violet 421 (clone W6D3) | BioLegend | Cat#323039: RRID: AB_2566519 |
| anti-CD45 Krome Orange (clone J33 | Beckman Coulter | Cat#A96416: RRID: AB_2833027 |
| Bacterial and Virus Strains | ||
| Heat-killed Mycobacterium tuberculosis | Gift | H37Rv |
| Bacille Calmette-Guérin Vaccine | Intervax | Bulgaria strain |
| Heat-killed Staphylococcus aureus | Gift | Clinical isolate |
| Chemicals, Peptides, and Recombinant Proteins | ||
| RPMI 1640 W/ Stable Glutamine W/ 25 MM HEPES | Biowest | L0496 |
| PBS Dulbecco’s Phosphate Buffered Saline w/o Magnesium, w/o Calcium | Biowest | L0615 |
| FBS Superior; standardized Fetal Bovine Serum, EU-approved | Biochrom | S0615 |
| Gentamycin Sulfate BioChemica | PanReac AppliChem | A1492 |
| Penicillin G Potassium Salt BioChemica | PanReac AppliChem | A1837 |
| Lipopolysaccharides from Escherichia coli O55:B5 | Sigma-Aldrich | L2880 |
| Ficoll-Paque | GE Healthcare | Cat#17-1440-03 |
| Roswell Park Memorial Institute medium (RPMI) | Invitrogen | Cat#22406031 |
| 16% Formaldehyde | Fisher Scientific | Cat#28908 |
| Protein A/G Magnetic beads | Diagenode | Cat#C03010021-150 |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | Cat#A7030 |
| Protease inhibitor cocktail | Sigma-Aldrich | Cat#P8465 |
| Ficoll-Paque | GE Healthcare | Cat#17-1440-03 |
| Critical Commercial Assays | ||
| Human TNFa uncoated ELISA | Invitrogen | 88-7346 |
| Human IL-6 ELISA | Invitrogen | 88-7066 |
| Human IL-1b uncoated ELISA | Invitrogen | 88-7261 |
| Human IL-10 uncoated ELISA | Invitrogen | 88-7106 |
| Human IFNγ ELISA | Diaclone | 950.000.192 |
| QuantiFERON®-TB Gold (QFT®) Tubes | QIAGEN | 590-0301 |
| QuantiFERON®-TB Gold (QFT®) ELISA | QIAGEN | 0594-0201 |
| MinElute PCR purification Kit | QIAGEN | 28006 |
| Olink Inflammation panel | Olink Proteomics | 95302 |
| Oligonucleotides | ||
| Primers for qPCR, see STAR Methods | This paper | N/A |
| Software and Algorithms | ||
| GraphPad Prism | Graphpad Software | https://www.graphpad.com |
| SPSS | IBM | https://www.ibm.com/analytics/spss-statistics-software |
| Cytobank platform | Beckman Coulter | https://www.beckman.com/flow-cytometry/software/cytobank-premium/premium-v-enterprise |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Evangelos J. Giamarellos-Bourboulis (egiamarel@med.uoa.gr).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Data of this study are available after communication with the Lead Contact. A material transfer agreement will be needed.
Experimental Model and Subject Details
ACTIVATE clinical trial
ACTIVATE was a prospective, double-blind, randomized and placebo-controlled phase III clinical trial conducted among patients of both genders hospitalized at the 4th Department of Internal Medicine of ATTIKON University General Hospital in Greece. The protocol and its subsequent amendments were approved by the National Ethics Committee and by the National Organization for Medicine of Greece, and by the Ethics Committees of ATTIKON hospital (EudraCT number, 2017-000596-87). The trial is conducted and funded by the Hellenic Institute for the Study of Sepsis. The funders have no role in the design, conduct, analysis and interpretation of data, and decision to publish. The laboratory of Immunology of Infectious Diseases of the 4th Department of Internal Medicine at ATTIKON University General Hospital served as a central laboratory for the study. The Sponsor initiative for an interim analysis was submitted to the National Ethics Committee and to the National Organization for Medicine of Greece on April 14th 2020 informing on the reasons for the analysis and on the procedure to be followed. The analysis was done by an independent committee of three experts who were appointed by the Sponsor through an invitation on April 16th 2020. The database lock was done on April 21st 2020 and the interim analysis was done by the Committee on April 29th 2020. The investigators remained blind to the intervention after the results of the analysis became known.
The study enrolled elderly patients (age ≥ 65 years) of both genders who were discharged from hospital after hospitalization for a medical cause. Exclusion criteria were: a) denial for written informed consent; b) solid organ malignancy or lymphoma diagnosed the last five years; c) treatment with oral or intravenous steroids defined as daily doses of 10mg prednisone or equivalent for longer than the last 3 months; d) severe immunodeficiency including infection by the human immunodeficiency virus (HIV-1), neutropenia (less than 500 neutrophils/mm3), history of solid organ and bone marrow transplantation, intake of chemotherapy, primary immunodeficiency, severe lymphopenia (less than 400 lymphocytes/mm3) and treatment with anti-cytokine therapies; and e) positive Interferon-gamma Release Assay (IGRA). All patients or their legal representatives provided written informed consent before enrolment STAR Methods.
On the day of hospital discharge and after careful recording of detailed medical history and laboratory examinations for the inclusion and exclusion criteria, patients remaining eligible underwent IGRA test. Those who had negative IGRA were allowed to be enrolled in the study. Participants were randomized to one intradermal vaccination with 0.1ml of sodium chloride 0.9% or with 0.1ml of BCG (BCG vaccine strain 1331; Intervax). Simple randomization was performed in a 1:1 ratio by a biostatistician and delivered to the investigators as an electronic file for treatment allocation at randomization. Blind administration was secured by two study pharmacists; one who was preparing the vaccine and another who was delivering the preparation to the investigators for vaccination.
The primary outcome was the time interval to the first infection post hospital discharge between the two groups of treatment. This was a composite endpoint involving any of the following infections: respiratory tract infections of probable viral origin necessitating medical attention; bloodstream infection; community-acquired pneumonia; hospital-acquired pneumonia; urinary tract infections; intraabdominal infection; and soft-tissue infection. Respiratory infections of probable viral origin necessitating medical attention were defined as body temperature above 38°C accompanied by at least two of the following: redness and purulent discharge of the throat; intense nasal discharge; cough; enlargement of cervical lymph nodes; enlargement of liver or of spleen found on deep abdominal palpation; and absolute lymphocyte count more than 4,000/mm3 (Antonopoulou et al., 2012). Bloodstream infection was defined as at least one positive blood culture for a pathogen not related to infection at other site (Calandra et al., 2005). Community-acquired pneumonia was defined as any new or evolving infiltrate on chest X-ray in a patient without any contact with the hospital environment the last 90 days and who was presenting with at least two of the following: new onset or worsening of cough; body temperature above 38°C; dyspnea; purulent expectoration; auscultatory findings compatible with pulmonary consolidation; and procalcitonin ≥ 0.25 ng/ml or absolute total white blood cell count ≥ 12,000/mm3 (Christ-Crain et al., 2006). Hospital-acquired pneumonia was defined as any new or evolving infiltrate on chest X-ray in a patient starting at least 48 hours after hospital admission and who was presenting with at least two of the following: new onset or worsening of cough; body temperature above 38°C; dyspnea; purulent expectoration or tracheobronchial secretions; auscultatory findings compatible with pulmonary consolidation; and procalcitonin ≥ 0.25 ng/ml or absolute total white blood cell count ≥ 12,000/mm3 (Kalil et al., 2016). The urinary tract infection was defined as the presence of ≥ 10 leukocytes/high power field in urine sediment or of positive urine culture with ≥ 105 colonies/ml in a patient who was presenting with at least two of the following: body temperature above 38°C; dysuria, increased urinary frequency or urgency; flank pain or lumbar pain at palpation; and ultrasound findings compatible with acute pyelonephritis (Pinson et al., 1997). One intraabdominal infection was defined as any radiological documentation of intraabdominal infection by abdominal X-ray or computed tomography of the abdomen accompanied by at least two of the following: body temperature above 38°C; nausea or vomiting; abdominal pain; and jaundice (Calandra et al., 2005). Acute bacterial skin and skin structure infection was defined as the acute onset of infection involving one upper extremity or one lower extremity or the abdominal wall accompanied by at least two of the following: redness, hotness and edema; well-circumscribed rim; ultrasound findings compatible with soft tissue infection; and absolute total white blood cell count ≥ 12,000/mm3 (Russo et al., 2016).
Secondary study outcomes included the rate of hospitalizations until month 12; the time to first sepsis episode; the total number of infections; the time to first hospitalization; the number of antibiotic courses; and one-year mortality. Sepsis was defined by the Sepsis-3 definitions, i.e., ≥ 2-point increase of the sequential organ failure assessment (SOFA) score measured on the day of study enrolment (Singer et al., 2016). For the analysis of both the primary and the secondary endpoints only events captured during the first 12 months after vaccination with placebo or BCG were encountered.
Patients’ visits were conducted every month for 12 months. During each visit the following data were captured: history of any new infection; any new hospitalization followed by thorough study of hospital discharge notes and contact with treating physicians if necessary; any need for antimicrobial prescription; and patient disposition. Ten ml of whole blood was sampled after venipuncture of one forearm vein under aseptic conditions before vaccination and repeated three months after vaccination with placebo/BCG. Blood was immediately transferred to the central lab for the isolation and culture of PBMCs.
Adverse events (AEs) and Serious Adverse Events (SAEs) were captured from baseline until the last patient’s evaluation. An adverse event was defined as any undesirable medical occurrence in a subject administered a pharmaceutical product and which does not necessarily have a causal relationship with this treatment. The adverse event could be a sign, a symptom, or an abnormal laboratory finding. AEs meeting any of the following criteria were considered SAEs: death; life-threatening situation; inpatient hospitalization or prolongation of existing hospitalization; persistent or significant disability/incapacity; congenital anomaly/birth defects; important medical events/experiences; and spontaneous and elective abortions experienced by study subject. All others AEs were reported as non-serious. All AEs were graded as: mild (transient and well-tolerated by the patient), moderate (causing discomfort and affecting the usual activities of the patient) and severe (affecting the usual activities to an important degree and causing disability or are life-threatening). The relationship of the AE to the study drug was reported as probably-related, possibly-related; probably not-related and unrelated
300BCG study
To study the immunological effects of BCG vaccination in elderly, immune responses were assessed in 14 healthy volunteers (4 females, 10 males) aged 55 years or older, that took part in the 300BCG study (ethical approval no. NL58553.091.16). Whole blood was drawn by venipuncture of one forearm vein under aseptic condition and collected into BD Vacutainer® spray-coated EDTA tubes. Blood sampling was done before BCG vaccination, two weeks and three months after vaccination. All volunteers received 0.1ml of BCG (BCG vaccine strain 1331; Intervax). Three volunteers did not show up at their three-month appointment, therefore the time point three months after vaccination contains data of 11 volunteers. The study was approved by the Ethical Committee (CMO) of Radboud University Nijmegen, the Netherlands. Inclusion of volunteers and experiments were conducted according to the principles expressed in the Declaration of Helsinki and all volunteers gave written informed consent before any material was taken.
Method Details
ACTIVATE clinical trial
For the IGRA test, venous blood was collected in four heparinized tubes, namely: one negative control; two tubes containing M. tuberculosis-specific antigens (ESAT-6, CFP-10); and one tube contained a positive control mitogen. Following incubation and centrifugation of the tubes, plasma was removed and the concentration of IFNγ produced in each tube was measured by an enzyme immunosorbent assay (QuantiFERON®-TB Gold, QFT® ELISA, QIAGEN). For the interpretation of the IGRA assay test, concentrations of IFNγ in the negative control were subtracted from the levels of antigen and mitogen stimulation respectively. The test was considered positive if IFNγ after antigen stimulation was higher than the negative control.
Peripheral blood mononuclear cells (PBMCs) were isolated from participants of the ACTIVATE study after gradient centrifugation over Ficoll (Biochrom, Berlin, Germany) for 20 minutes at 1400 g. After three washings in ice-cold PBS pH 7.2, PBMCs were counted in a Neubauer plate with trypan blue exclusion of dead cells. They were then diluted in RPMI 1640 enriched with 2mM of L-glutamine, 500 μg/ml of gentamicin, 100 U/ml of penicillin G, 10 mM of pyruvate, 10% fetal bovine serum (Biochrom) and suspended in wells of a 96-well plate. The final volume per well was 200 μL with a density of 2 x106 cells/ml. PBMCs were exposed in duplicate for 48 hours and 5 days at 37°C in 5% CO2 to different stimuli: 10 ng/ml of Escherichia coli O55:B5 lipopolysaccharide (LPS, Sigma, St. Louis, USA); 5 μg/ml of Pam3Cys-SKKK (EMC Microcollections, Tübingen, Germany); and 5x105 colony forming units of heat-killed Candida albicans. Following incubation, cells were removed and analyzed for flow cytometry. Concentrations of TNFα, IL-1β, IL-6, IL-10 and IFNγ were measured in cell supernatants in duplicate by an enzyme immunosorbent assay (Invitrogen, Carlsbad, California, USA). The lowest detections limits were: for TNFα 40 pg/ml; for IL-1β 20 pg/ml; for IL-6 10 pg/ml; for IL-10 157 pg/ml; and for IFNγ 12.5 pg/ml.
For chromatin immunoprecipitation, purified monocytes were fixed with 1% formaldehyde (Sigma) at concentration of 106 cells/ml. Fixed cell preparations were sonicated using a Diagenode Bioruptor UCD-300 for 3 × 10 min (30 s on; 30 s off) and incubated with protease inhibitor cocktail and H3K27ac antibody (Diagenode) and incubated overnight at 4°C with rotation. Protein A/G magnetic beads were washed in dilution buffer with 0.15% SDS and 0.1% BSA, added to the chromatin/antibody mix and rotated for 60 min at 4°C. Following five washes, chromatin was eluted using elution buffer for 20 min. Supernatant was treated with proteinase K for 4 h at 65°C. Finally, samples were purified using QIAGEN; Qiaquick MinElute PCR purification Kit. Primer pairs used for quantitative PCR were as follows: IL-6 forward: 5′-TGC ACA AAA TTT GGA GGT GA-3′; IL-6 reverse: 5′-ACC CAA CCT GGA CAA CAG AC-3′; TNFα forward: 5′- CTT GGG CCA GTG AGT GAA AG-3′; TNFα reverse: 5′-TAG CCA GGA GGG AGA ACA GA.
PBMCs were isolated from the whole blood of participants of the 300BCG study as described above. PBMCs were stimulated at counts of 5 × 106/ml in 96-well plates by 5 μg/ml of heat-killed M. tuberculosis H37Rv, 5x106 cfu/ml heat-killed S. aureus or 10 ng/ml of E. coli LPS. After 24 hours and 7 days of incubation at 37°C, supernatants were collected and stored at −20°C until analysis. Concentrations of IL-1β and IL-6 were measured in 24-hour supernatants and of IFNγ in 7-day supernatants by the enzyme immunosorbent assay described above.
300BCG study
Using whole blood of participants in the 300BCG study, blood cells were counted using a Coulter Ac-T Diff® cell counter (Beckman Coulter, Brea, USA). Following red blood cell lysis, white blood cells were washed twice with PBS and re-suspended in 300 μL of PBS + 0.2% BSA (Sigma-Aldrich, Zwijndrecht, Netherlands) and stained with the following monoclonal antibodies: anti-CD16 FITC (clone 3G8, Beckman Coulter; Suarlée, Belgium), anti-CD10 PE (clone HI10A, BioLegend, San Diego, CA, USA), anti-CD11b PE-Dazzle (clone ICRF44, BioLegend), anti-CD14 PE-Cy5.5 (clone M5E2, BioLegend), anti-CD62L PE-Cy7 (clone DREG-56, BioLegend), anti-PD-L1 APC (clone MIH1, ThermoFisher, Waltham, MA USA), anti-CD66b AF700 (clone G10F5, BioLegend), anti-CD15 Brilliant Violet 421 (clone W6D3, BioLegend) and anti-CD45 Krome Orange (clone J33, Beckman Coulter). Cells were analyzed on a 10-color Navios flow cytometer (Beckman Coulter). Acquired FACS data were analyzed using the cloud-based Cytobank platform.
Circulating plasma inflammatory markers were assessed in the plasma of participants of the 300 BCG study using the commercially available Olink Proteomics AB (Uppsala Sweden) Inflammation panel (92 inflammatory proteins), using a Proseek© Multiplex proximity extension assay (Assarsson et al., 2014). Of the 92 proteins in the panel, 73 proteins were detected in the plasma samples and included in the analysis. See Table S3 for an overview of all inflammatory markers that were analyzed.
Quantification and Statistical Analysis
The trial sample size was calculated assuming the median time to new infection would be 4 months in the placebo group and 7 months with BCG vaccination. To achieve so with 90% power at the 5% level of significance, 100 patients were allocated to each arm. Under these prerequisites, the study is sufficiently powered to prove that differences in the first time incidence of infection between the placebo and the BCG group of the order of 20% will be statistically significant.
Baseline qualitative data were presented as percentages and CIs and compared by the Fisher’s exact test. Baseline quantitative data were presented as mean and standard deviation and compared by the Student’ s “t-test” for variables that followed normal distribution; they were presented as mean and standard error and compared by the Mann-Whitney U test for variables that did not follow normal distribution.
The primary outcome was the time, in weeks, of the appearance of one first new infection, censored at twelve months after vaccination. Differences between the placebo and BCG vaccination groups were assessed with the HR of the Cox proportional hazards regression model with its 95% CIs. The corresponding p values were also reported. The effects of other confounders, both at the univariate and the multivariate Cox model, were also assessed with the corresponding HR. Only variables found to be significant in the univariate analysis entered in the stepwise multivariate analysis and they were retained in the model only if they had a significant effect after adjusting for the other effects. The proportionality of the hazard function at different levels throughout the follow-up period was assessed with the Schoenfeld residuals method (Xue et. al., 2013). Since this was an interim analysis, a sensitivity analysis was also performed for the total number of participants with the primary aim to show that individuals that are censored have the same probability of experiencing a subsequent event as individuals that remain in the study. The number of infections in each group was expressed as patient-infections per year. The same analysis was done for the secondary endpoints. The frequency of adverse events was compared by the Fisher’s exact test.
The interim analysis included only patients with completed 12-month of follow-up. In order to preserve the overall Type I error rate at 5%, an adjustment of the level of significance of the interim and final analyses was done by O’Brien-Fleming strict alpha adjustment. This adjustment provides significance a = 0.0054 at interim and a = 0.0492 at final (DeMets and Lan, 1994). The purpose of using this seemingly unattainable level of significance at interim analysis was to allow the study to conclude, at the same time providing evidence that the required level of significance will be attained at the final stage. Statistical analysis was performed with the IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, N.Y., USA) and corroborated with the R statistical package (R Core Team, 2013) (see supplementary Statistical Analysis plan, Data S1 and Methods S1).
Cytokine data were expressed for each group of vaccination as the ratio of the cytokine production at month 3 versus the production at month 0 (before vaccination). Receiver operator characteristic curve analysis was done to discriminate the ratio of each cytokine that can better differentiate the two groups of vaccination. The best cut-off of this ratio was selected by the co-ordinate points of the curve using the Youden index. Patients above and below this cut-off were compared between groups by the Fisher’s exact test.
Cytokine and protein data of participants in the 300BCG study were expressed as means ± SE. Data were analyzed using the Wilcoxon’s rank sum test.
Analysis was conducted using IBM SPSS Statistics v. 25.0. All p values were two-sided and any p value < 0.05 was considered as statistically significant.
Ongoing interventional clinical trials studying the protective effect of BCG vaccination on Covid-19 were reviewed using the Clinicaltrials.gov registry of trials (see Table S4).
Additional Resources
The study is registered at ClinicalTrials.gov NCT03296423
Acknowledgments
The study was funded in part by the Horizon 2020 grant ImmunoSep (#847422) and in part by the Hellenic Institute for the Study of Sepsis. The authors wish to acknowledge the contribution of Prof. Jos W.M. van der Meer, of Prof. Christine Stabell Benn, and of Miltiades Kyprianou for the interim analysis.
Author Contributions
E.J.G.-B. conceptualized and designed the study protocol, analyzed the data, wrote the manuscript, and gave approval of the final version to be submitted. M.T., N.A., E.K., M.-E.A., A.B., D.-I.D., G.R., and A.P. collected clinical information and blood samples, reviewed the manuscript, and gave approval of the final version to be submitted. A.K. performed study monitoring, reviewed the manuscript, and gave approval of the final version to be submitted. S.M., J.D.-A., T.G., G.D., P.K., A.K., and H.K. performed vaccine accountability and lab experiments, reviewed the manuscript, and gave approval of the final version to be submitted. R.v.C. and M.G.N. conceptualized the study, analyzed the data, drafted the manuscript, and gave approval of the final version to be submitted
Declaration of Interests
E.J.G.-B. has received honoraria from Abbott CH, Angelini Italy, bioMérieux Inc, InflaRx GmbH, MSD Greece, and XBiotech Inc.; independent educational grants from AbbVie, Abbott, Astellas Pharma Europe, AxisShield, bioMérieux Inc, InflaRx GmbH, ThermoFisher Brahms GmbH, and XBiotech Inc; and funding from the FrameWork 7 program HemoSpec (granted to the National and Kapodistrian University of Athens), the Horizon2020 Marie-Curie Project European Sepsis Academy (granted to the National and Kapodistrian University of Athens), and the Horizon 2020 European Grant ImmunoSep (granted to the Hellenic Institute for the Study of Sepsis). M.G.N. was supported by an ERC Advanced Grant (#833247) and a Spinoza grant of the Netherlands Organization for Scientific Research. M.G.N. is a scientific founder of TTxD. The other authors do not have any competing interests to declare.
Published: September 1, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.cell.2020.08.051.
Supplemental Information
References
- Antonopoulou A., Baziaka F., Tsaganos T., Raftogiannis M., Koutoukas P., Spyridaki A., Mouktaroudi M., Kotsaki A., Savva A., Georgitsi M., Giamarellos-Bourboulis E.J. Role of tumor necrosis factor gene single nucleotide polymorphisms in the natural course of 2009 influenza A H1N1 virus infection. Int. J. Infect. Dis. 2012;16:e204–e208. doi: 10.1016/j.ijid.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Arts R.J.W., Moorlag S.J.C.F.M., Novakovic B., Li Y., Wang S.Y., Oosting M., Kumar V., Xavier R.J., Wijmenga C., Joosten L.A.B., et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23:89–100. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Assarsson E., Lundberg M., Holmquist G., Björkesten J., Thorsen S.B., Ekman D., Eriksson A., Rennel Dickens E., Ohlsson S., Edfeldt G., et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS ONE. 2014;9:e95192. doi: 10.1371/journal.pone.0095192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender B.S. Infectious disease risk in the elderly. Immunol. Allergy Clin. North Am. 2003;23:57–64, vi. doi: 10.1016/s0889-8561(02)00078-4. [DOI] [PubMed] [Google Scholar]
- Calandra T., Cohen J., International Sepsis Forum Definition of Infection in the ICU Consensus Conference The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit. Care Med. 2005;33:1538–1548. doi: 10.1097/01.ccm.0000168253.91200.83. [DOI] [PubMed] [Google Scholar]
- Christ-Crain M., Stolz D., Bingisser R., Müller C., Miedinger D., Huber P.R., Zimmerli W., Harbarth S., Tamm M., Müller B. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am. J. Respir. Crit. Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- DeMets D.L., Lan K.K. Interim analysis: the alpha spending function approach. Stat. Med. 1994;13:1341–1352, discussion 1353–1356. doi: 10.1002/sim.4780131308. [DOI] [PubMed] [Google Scholar]
- Garly M.L., Martins C.L., Balé C., Baldé M.A., Hedegaard K.L., Gustafson P., Lisse I.M., Whittle H.C., Aaby P. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine. 2003;21:2782–2790. doi: 10.1016/s0264-410x(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalil A., Metersky M., Klompas M. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016;63:1–51. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinnijenhuis J., Quintin J., Preijers F., Joosten L.A., Ifrim D.C., Saeed S., Jacobs C., van Loenhout J., de Jong D., Stunnenberg H.G., et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA. 2012;109:17537–17542. doi: 10.1073/pnas.1202870109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leentjens J., Kox M., Stokman R., Gerretsen J., Diavatopoulos D.A., van Crevel R., Rimmelzwaan G.F., Pickkers P., Netea M.G. BCG vaccination enhances the immunogenicity of subsequent influenza vaccination in healthy volunteers: a randomized, placebo-controlled pilot study. J. Infect. Dis. 2015;212:1930–1938. doi: 10.1093/infdis/jiv332. [DOI] [PubMed] [Google Scholar]
- Moorlag S.J.C.F.M., Arts R.J.W., van Crevel R., Netea M.G. Non-specific effects of BCG vaccine on viral infections. Clin. Microbiol. Infect. 2019;25:1473–1478. doi: 10.1016/j.cmi.2019.04.020. [DOI] [PubMed] [Google Scholar]
- Nemes E., Geldenhuys H., Rozot V., Rutkowski K.T., Ratangee F., Bilek N., Mabwe S., Makhethe L., Erasmus M., Toefy A., et al. C-040-404 Study Team Prevention of M. tuberculosis infection with H4:IC31 vaccine or BCG revaccination. N. Engl. J. Med. 2018;379:138–149. doi: 10.1056/NEJMoa1714021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Joosten L.A., Latz E., Mills K.H., Natoli G., Stunnenberg H.G., O’Neill L.A., Xavier R.J. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea M.G., Giamarellos-Bourboulis E.J., Domínguez-Andrés J., Curtis N., van Crevel R., van de Veerdonk F.L., Bonten M. Trained immunity: a tool for reducing susceptibility and severity of SARS-CoV-2 infection. Cell. 2020;181:969–977. doi: 10.1016/j.cell.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrui T., Nakayama K., Fukushima T., Chiba H., Sasaki H. [Prevention of elderly pneumonia by pneumococcal, influenza and BCG vaccinations] Nippon Ronen Igakkai Zasshi. 2005;42:34–36. doi: 10.3143/geriatrics.42.34. [DOI] [PubMed] [Google Scholar]
- Pinson A.G., Philbrick J.T., Lindbeck G.H., Schorling J.B. Fever in the clinical diagnosis of acute pyelonephritis. Am. J. Emerg. Med. 1997;15:148–151. doi: 10.1016/s0735-6757(97)90087-5. [DOI] [PubMed] [Google Scholar]
- R Core Team . 2013. R: A language and environment for statistical computing.http://www.R-project.org/ [Google Scholar]
- Russo A., Concia E., Cristini F., De Rosa F.G., Esposito S., Menichetti F., Petrosillo N., Tumbarello M., Venditti M., Viale P., et al. Current and future trends in antibiotic therapy of acute bacterial skin and skin-structure infections. Clin. Microbiol. Infect. 2016;22(Suppl 2):S27–S36. doi: 10.1016/S1198-743X(16)30095-7. [DOI] [PubMed] [Google Scholar]
- Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensballe L.G., Nante E., Jensen I.P., Kofoed P.E., Poulsen A., Jensen H., Newport M., Marchant A., Aaby P. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine. 2005;23:1251–1257. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Walk J., de Bree L.C.J., Graumans W., Stoter R., van Gemert G.J., van de Vegte-Bolmer M., Teelen K., Hermsen C.C., Arts R.J.W., Behet M.C., et al. Outcomes of controlled human malaria infection after BCG vaccination. Nat. Commun. 2019;10:874. doi: 10.1038/s41467-019-08659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardhana D., Datau E.A., Sultana A., Mandang V.V., Jim E. The efficacy of Bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med. Indones. 2011;43:185–190. [PubMed] [Google Scholar]
- Welsh R.M., Che J.W., Brehm M.A., Selin L.K. Heterologous immunity between viruses. Immunol. Rev. 2010;235:244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X., Xie X., Gunter M., Rohan T.E., Wassertheil-Smoller S., Ho G.Y., Cirillo D., Yu H., Strickler H.D. Testing the proportional hazards assumption in case-cohort analysis. BMC Med. Res. Methodol. 2013;13:88. doi: 10.1186/1471-2288-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data of this study are available after communication with the Lead Contact. A material transfer agreement will be needed.