Abstract
Four GI-1/Massachusetts-type (GI-1/Mass-type) infectious bronchitis virus (IBV) strains were isolated and the complete genomes of these isolates, coupled with the Mass-type live-attenuated vaccine H120 and the Mass-type pathogenic M41 strains, were sequenced in the present study. Our results show that isolates LJL/140820 and I0306/17 may be derived from the Ma5 (another Mass-type live-attenuated vaccine strain) and H120 vaccine strains, respectively. The I1124/16 strain was found to be a M41 variant that likely resulted from nucleotide accumulated mutations in the genome. Consistently, the results of the virus neutralization test showed that isolate I1124/16 was antigenically related but slight different from the M41. Our results from the protection experiments pointed out that chickens immunized with H120 failed to eliminate viral shedding after infection with the isolate I1124/16, which was different from that of M41; this result was consistent to the field observation and further implicated that the variant IBV isolate I1124/16 was antigenic different from the M41 strain. Furthermore, the I1124/16 was found to have comparable but slightly lower pathogenicity with the M41 strain. More studies based on the reverse genetic techniques are needed to elucidate the amino acids in the S1 subunit of spike protein contributing to the altered antigenicity of the isolate I1124/16. In addition, an IBV isolate, LJL/130609, was found to be originated from recombination events between the I1124/16- and Connecticut-like strains. Our results from the virus neutralization test also showed that isolates LJL/130609 and I1124/16 were antigenic closely related. Hence, there are at least 3 different genetic evolution patterns for the circulation of the GI-1/Mass-type IBV field strains in China. The differences of vaccines used, the field conditions and genetic pressures between different flocks, likely account for the emergence, evolution patterns, and characteristics of the Mass-type IBV strains.
Key words: infectious bronchitis virus, GI-1/Massachusetts type, evolution patterns, antigenicity, pathogenicity
Introduction
Infectious bronchitis virus (IBV) is a Gammacoronavirus that belongs to the family Coronaviridae and has a positive sense single-stranded 27.6 kb RNA genome (Boursnell et al., 1987). The 1a and 1b open reading frames (ORFs) encode the 1ab polyprotein using a ribosomal frameshift event at the end of ORF1a. The large 1ab polyprotein is then post-translationally cleaved into 15 nonstructural polypeptides, which are required for RNA replication and translation. The 4 major IBV structural proteins, including the spike (S) glycoprotein, small envelope (E) protein, membrane (M) glycoprotein, and nucleocapsid (N) protein, were encoded by the ORFs 2, 3c, 4, and 6, respectively. The spike protein was further processed into the S1 and S2 subunits. The small accessory proteins, 3a, 3b, 5a, and 5b, which are not essential for viral replication (Cavanagh, 2007), are encoded by the ORF 3a, 3b, 5a, and 5b, respectively. The viral genome, especially the S1 gene which encodes the epitopes that induce virus neutralizing antibodies (Cavanagh et al., 1986), can evolve rapidly by mutation and recombination events, resulting in the occurrence of an array of different serotypes, genotypes, lineages, and variants. Thus, IBV strain classification and evolutionary analysis are typically accomplished by the phylogenetic analysis of the complete coding region of the S1 subunit.
The use of vaccination remains the most effective method for the prevention and control of infectious bronchitis (IB) to date. Live-attenuated vaccines were developed and used to protect chickens in an attempt to minimize the economic effects caused by IBVs (Winterfield, 1968). In addition, less attenuated strains, at a lower passage level in embryonated eggs (e.g., H52), were used to revaccinate future layers or breeders before the onset of lay (Bijlenga et al., 2004), albeit they are liable to cause disease (Alexander et al., 1978; Bijlenga et al., 2004). In general, a vaccine provides good clinical protection, and vaccination can be efficacious for protecting birds from IBV infection. However, outbreaks of IB-associated diseases occur frequently in vaccinated flocks throughout the world, which are caused by variable or poor cross-protection due to antigenic differences between the vaccine and field strain. With respect to the vaccine, it has been shown that the vaccine strains have a high relative risk, meaning that more cases of IB associated with the vaccine strain are observed than expected (Aleuy et al., 2018).
The recent comprehensive classification of phylogenetic analysis based on the complete S1 genes divided IBV strains in 36 lineages grouped in 7 genotypes (GI-1∼GI-29, GII-1, GII-2, GIII-1∼GVII-1), and a number of interlineage recombinants throughout the world (Valastro et al., 2016; Chen et al., 2017; Jiang et al., 2017; Ma et al., 2019; Molenaar et al., 2020). The Massachusetts (Mass)-type IBV, coupled with the Connecticut (Conn) serotype in the GI-1 lineage (Valastro et al., 2016), has been widely circulating worldwide for decades since the first description in 1931 (Shalk and Hawn, 1931). The Mass-type H120 vaccine virus has been successfully used worldwide as a primary vaccine in broilers for almost 50 yr (Bijlenga et al., 2004). China also includes Mass-type strains in its vaccination program; recently, the use of other strains, such as LDT3-A strain (Han et al., 2016), has been officially authorized. However, a number of Mass-type IBV strains were isolated from vaccinated chicken flocks (Liu et al., 2013; Chen et al., 2015). In addition, some of these strains were proposed to have been derived from recombination events between Mass-type IBVs, as well as between Mass type and other IBV types circulating in China (Liu et al., 2013, 2014; Chen et al., 2015). The isolated viruses had not exhibited extensive mutations with regard to both the S1 gene and complete genomic sequences of the H120 or M41 strains (Liu et al., 2013; Chen et al., 2015).
In the course of surveillance of IBV, we isolated 4 IBV strains from chicken flocks suspected to be infected with IBV in northeast China between 2013 and 2017. Classification based on the complete S1 sequence phylogenetic analysis has grouped the 4 isolates into GI-1/Mass lineage. To investigate the origin and evolution of the 4 GI-1/Mass lineage viruses, the complete genomic sequences of the 4 isolates were determined in this study. In addition, the other phenotypic characterization, including antigenicity, pathogenicity, and protective efficiency of the H120 vaccine against one of the isolates I1124/16 was evaluated in specific pathogen-free (SPF) chicks by vaccination-challenge test.
Materials and methods
Ethics
This study was conducted in accordance with the animal welfare guidelines of the World Organization for Animal Health. All animal protocols were reviewed and approved by the Agricultural Animal Care and Use Committee of Heilongjiang province, China.
Virus Isolation, Propagation, and Titration
Four field strains were used in this study. Strains ck/CH/LJL/130906 (LJL/130906) and ck/CH/LJL/140820 (LJL/140820) were isolated from the trachea of a 28-day-old layer in 2013 and the proventriculus of a 23-day-old layer in 2014 in Jilin province. Strains I1124/16 and I0306/17 were isolated in 2016 and 2017 from laying hens in Liaoning and Heilongjiang provinces in China, respectively (Xu et al., 2018). All the chicken flocks have been vaccinated with the Mass-type live-attenuated vaccine H120 strain (Bijlenga et al., 2004) at 1 D of age and boosted at 15 to 20 D. All the birds from which the viruses were isolated suffered respiratory signs.
The Mass-type live-attenuated vaccine H120 strain and the Mass-type pathogenic M41 strain (Chen et al., 2015) were also used in this study. Strains H120, M41, and 4 field isolates were used for complete genome sequencing and in the virus neutralization (VN) test. Isolate I1124/16 and strains H120 and M41 were used in the vaccination-challenge test. All of the viruses were passaged additional time in nine-day-old SPF chicken embryonated eggs to prepare the viral stocks. The viruses were titrated as previously described (Han et al., 2016). Virus titers were calculated using the Reed and Muench (1938) method and expressed as the 50% embryo infectious dose (EID50).
RNA Extraction, RT-PCR, Sequencing, and Genomic Sequence Determination
The complete genomic sequences of H120, M41, and the 4 field strains, LJL/130906, LJL/140820, I1124/16, and I0306/17 were determined. The RNA was extracted from the allantoic fluids using an RNAiso Plus kit (Takara Bio Inc., Shiga, Japan) in accordance with the manufacturer's protocol. RT-PCR was performed using a one-step RT-PCR kit (Takara Bio Inc.), in accordance with the manufacturer's instructions, with primers previously designed for IBV full genome sequencing (Liu et al., 2013). The 3′/5′ termini of each IBV strain were determined using 3′/5′ RACE protocols, as previously described (Liu et al., 2013). Each genome fragment was sequenced at least 3 times.
Chromatograms were analyzed using the program, Chromas (http://technelysium.com.au/wp/chromas/), the resulting complete genomic sequence of each virus was determined, aligned, and the ORFs were determined using BioEdit (http://www.mbio.ncsu.edu/bioedit/bioedit.html) based on the genomic sequence of the Massachusetts (GQ504724) strain.
S1 Subunit of the Spike Protein Sequence Analysis
To download all available IBV GI-1/Mass-type complete S1 gene sequences in the GenBank database (National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/), the S1 gene sequences of the H120 and M41 strains in this study were entered into the BLASTn program (http://www.ncbi.nlm.nih.gov/BLAST/). All sequences with a genetic distance higher than 95% were downloaded (equal or lower than 95% were Connecticut strains and other IBV types). In total, 275 GI-1/Mass-type complete S1 gene sequences were obtained and were merged with those of our 4 field isolates, one vaccine strain, and one pathogenic M41 strain. The S1 sequence of ck/CH/LDL/091022 (LDL/091022) was used as an outgroup. Of the 275 GI-1/Mass-type sequences, 140 were obtained from China, 49 from the USA, 27 from India, 12 from Brazil, 11 from Sweden, 6 each from Iran and Thailand, 5 from Korea, 3 each from Canada and Egypt, 2 each from Poland, South Africa, and Spain, and one sequence each from Japan, Jordan, Israel, Malaysia, the Netherlands, Pakistan, Saudi Arabia, and the UK. The nucleotide and the deduced amino acid sequences were generated using MegAlign program implemented in DNAStar (https://www.dnastar.com/software/lasergene/) (Burland et al., 2000), and phylogenetic trees were constructed via the maximum-likelihood and maximum parsimony methods, respectively, with 1,000 bootstrap replicates using MEGA 6.0 (http://www.megasoftware.net/). The detected strains were classified as vaccines when they were identical or closely related (p-distance <0.01) to one of the administered vaccine (Worthington et al., 2008; Legnardi et al., 2019). Hence, the S1 nucleotide and amino acid sequences of our 4 isolates were compared pairwise with those of the vaccine and M41 strains, and the p-distance was calculated via the Clustal W method in MegAlign program between our 4 field strains, vaccine strains, and M41 strain to clarify their genetic relationship with the reference IBV strains.
Complete Genomic Sequence Analysis
The BLASTn program was used to search GenBank for similar IBV sequences using the complete genomic sequences of the H120 and M41 strains in this study. All sequences with a genetic distance higher than 95% were downloaded (equal or lower than 95% were Connecticut and other IBV types). A total of 69 complete genomic sequences were obtained and merged with those of our 6 IBV strains and that of strain LDL/091022, which was used as a reference strain. Of the 69 sequences, 46 were obtained from China, 10 from the USA, 4 from Brazil, 3 from India, 2 from Canada, and one from Egypt, Jordan, Pakistan, and the UK. The MegAlign program implemented in DNAStar was used to align sequences and calculate the percentage identity between the selected sequences. Phylogenetic trees were constructed using the aforementioned methods.
To identify the genetic relationship among the GI-1/Mass-type viruses, the complete genomic sequences of our 6 IBV strains were compared with those of strains in the H120- and M41-clade based on the phylogenetic results of complete genomic sequences using Multiple Alignment with Fast Fourier Transformation (MAFFT) version v6 (http://mafft.cbrc.jp/alignment/software/) and Similarity plot (SimPlot) version 3.5.1 (http://sray.med.som.jhmi.edu/SCRopftware/simplot/) (Lole et al., 1999). The window width and step size in SimPlot were set to 500 bp and 50 bp, respectively. The complete genomic sequence of our H120 vaccine strain was used as a query.
The sequence fragments of the LJL/130906 and I1124/16 strains, which exhibited obvious diversity to those of our M41 strain by MAFFT and SimPlot, were then submitted to a BLAST search in the NCBI database to identify homologous sequences. The sequence of Conn-type virus (Connecticut vaccine) displayed higher close genetic relationships with the LJL/130906 in region III. Therefore, the complete genomic sequences of the Connecticut vaccine were used to further analyze the recombination events that possibly occurred in the genome of the LJL/130906 strain using SimPlot and bootscan analyses. The complete genomic sequences of our 4 isolates were also compared pairwise with those of the H120, Ma5, and M41 strains, and the percentage identities were calculated.
Accession Numbers
The complete genomic sequences of the H120, M41, LJL/130906, LJL/140820, I1124/16, and I0306/17 strains in this study have been deposited in the GenBank database under the following accession numbers: H120, MK937831; M41, MK937830; LJL/130906, MK937832; LJL/140820, MK937833; I1124/16, MK937828; and I0306/17, MK937829.
Virus Neutralization Test
To prepare hyperimmune sera for the VN assay, 24 one-month-old SPF layers were randomly divided into 6 groups consisting of 4 birds per group, and housed in separate isolators. The birds in each group were inoculated with strains H120, M41, LJL/130906, LJL/140820, I1124/16, and I0306/17 via the oculonasal route at a dose of 105 EID50 in 0.1 mL volume per chick. At 2 wk after inoculation, the birds in each group received an intravenous injection of the same dose of the corresponding virus. After another 2 wk, the blood was collected and the serum was harvested and pooled for each group, inactivated at 56°C for 30 min, and stored at −70°C until further use.
To determine the antigenic relationship between the 6 Mass-type IBV strains, cross-VN tests, with a fixed concentration of virus and serial dilutions of serum, were performed (Chen et al., 2015). The R values were calculated by the method described by Archetti and Horfall (1950). The tests were performed in the presence of appropriate controls. The antigenic difference between 2 given strains was defined as follows: an R value of 100% or close to 100% indicates viruses that are antigenically identical between 2 tested viruses, an R value > 70% indicates little or no difference, an R value between 33 and 70% indicates a minor subtype difference, an R value between 11 and 32% indicates a major subtype difference, and an R value between 0 and 10% indicates different serotypes (Choi et al., 2009).
Vaccination-Challenge Test
The protocols for the animal experiments were approved by the Animal Welfare Committee of Heilongjiang Province, China. Five groups of 10 one-day-old SPF White Leghorn chicks were used. Birds in groups 1 and 3 were experimentally vaccinated via the intraocular and intranasal routes with 0.1 ml of H120 vaccine (104.0 EID50/bird) (Zegpi et al., 2020). Birds in groups 2, 4, and 5 were mock-infected with SPF allantoic fluids and maintained under the same conditions. Blood samples were collected from each group at 4, 8, 12, 16, and 20 D of age. At 21 D, the chicks in groups 1 and 2 were experimentally infected with isolate I1124/16 and in groups 3 and 4 with the M41 strain via the intraocular and intranasal routes at a dose of 105 EID50 in 0.1 mL volume per chick. Birds in group 5 were mock-infected with SPF allantoic fluids and used as negative control. Each of the birds were marked and signs of virus effects (Ren et al., 2020), such as listlessness, huddling, and ruffled feathers, were investigated twice daily and recorded. Signs were scored as 0 (absent), 1 (mild), 2 (moderate), 3 (severe), and 4 (death). Clinical signs were investigated and recorded daily until 25 D post infection (dpi). The most severe clinical signs of each bird were used to calculate the mean score. Blood and tracheal samples were collected from the birds in each group at 4, 8, 12, 16, 20, and 24 dpi. Blood samples were used for antibody detection (ELISA; IDEXX Corporation, Westbrook, ME) in accordance with the manufacturer's instructions. To assess the protection efficiency, the tracheal samples were used for virus recovery using nine-day-old SPF chicken embryonated eggs (Liu et al., 2009).
Results
S1 Gene Characteristics of the Four Field IBV Strains
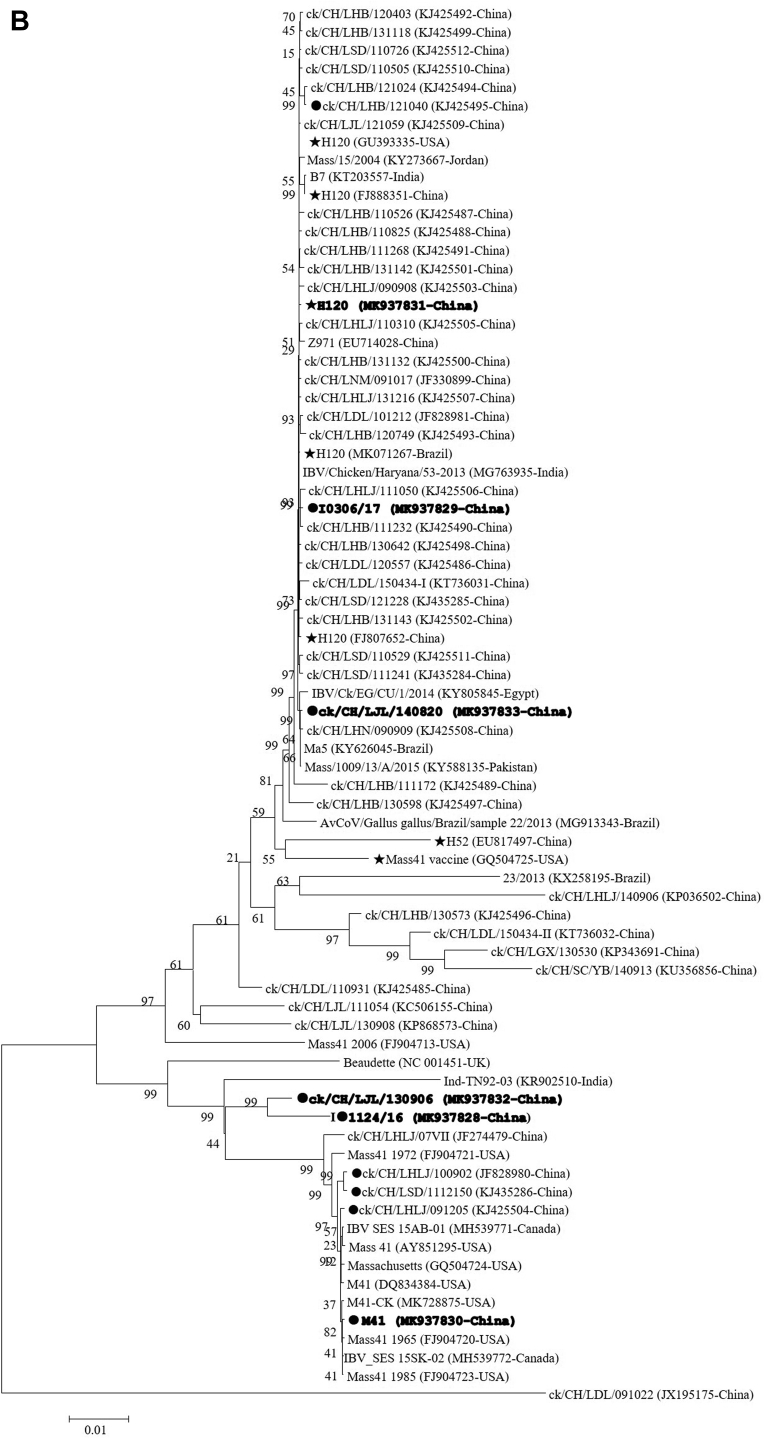
The S1 nucleotide sequences of the I2214/16 and I0306/17 strains were the same as previously reported (Xu et al., 2018). The phylogenetic trees constructed using the nucleotide and amino acid sequences of the S1 subunit of the spike glycoprotein with the maximum-likelihood and maximum-parsimony methods exhibited a very similar topology. Thus, the phylogenetic trees for amino acids via the maximum-likelihood method have been illustrated in this study (Figure 1A). Our 3 IBV isolates clustered together with the H120, Ma5, H52, and other Mass-type vaccine strains (H120-clade) (Liu et al., 2006), which were separate from M41 (M41-clade). Although all of the H120 vaccine strains were clustered in the same clade, they displayed amino acid substitutions compared with each other (Supplementary Figure 1).
Figure 1.
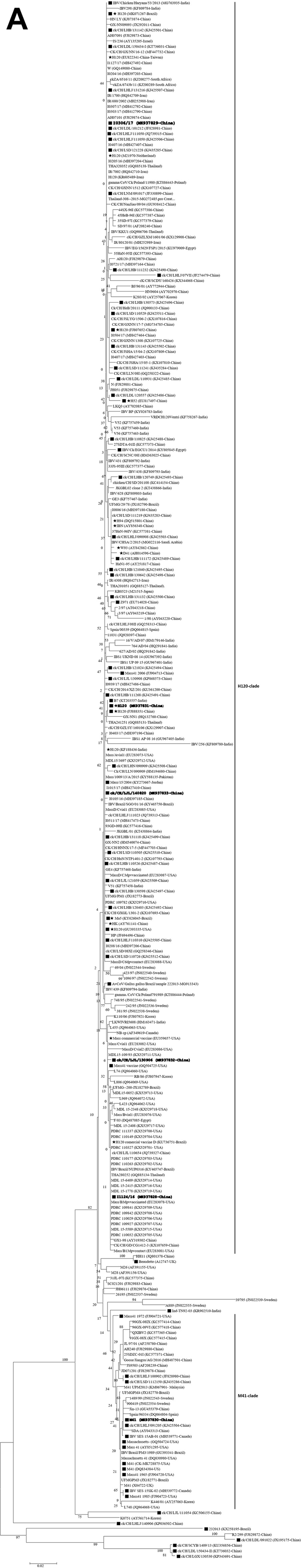
Phylogenetic trees based on the complete S1 amino acid sequences of 275 GI-1/Mass-type strains, including our 6 strains and 269 reference strains (A) and the complete genomic sequences of 69 IBV strains, including our 6 strains and 63 reference strains (B). The sequence of the IBV strain LDL/091022 was used as an outgroup. The phylogenetic trees were constructed using the maximum-likelihood with 1000 bootstrap replicates using MEGA 6.0 (http://www.megasoftware.net/). The stars indicate the vaccine strains. Our strains used in this study are shown in bold. The black squares indicate IBV strains with available complete genomes. Abbreviations: IBV, infectious bronchitis virus.
The p-distance of our isolates to the vaccine and M41 strains are listed in Table 1. The p-distances of strains LJL/140820 and I0306/17 to most of the Mass-type vaccine strains were lower than 0.01. The p-distances of strains LJL/140820 and I0306/17 to M41 strain were equal or higher than 0.025. These findings demonstrate that the LJL/140820 and I0306/17 strains were closely related to the vaccine. For the LJL/130906 and I1124/16 isolates, the p-distances to the H120 commercial vaccine D (a Mass-type live-attenuated vaccine strain derived from the H120 strain; GenBank accession no. KU736751) and Mass commercial vaccine were lower than 0.01. By contrast, the p-distances to other strains selected in this study were higher than 0.01.
Table 1.
The p-distances of S1 coding region1 between our IBV isolates and the vaccine strains.
| Strain | LJL/130906 |
LJL/140820 |
I1124/16 |
I0306/17 |
||||
|---|---|---|---|---|---|---|---|---|
| NT2 | AA2 | NT | AA | NT | AA | NT | AA | |
| H120 (M21970-Netherlands) | 0.020 | 0.024 | 0.002 | 0.004 | 0.020 | 0.024 | 0.001 | 0.000 |
| H120 (MK071267-Brazil) | 0.021 | 0.026 | 0.002 | 0.006 | 0.021 | 0.026 | 0.002 | 0.002 |
| H120 commercial vaccine D (KU736751-Brazil) | 0.003 | 0.007 | 0.018 | 0.020 | 0.002 | 0.004 | 0.020 | 0.020 |
| H120 (EU822341-China-Taiwan) | 0.019 | 0.022 | 0.002 | 0.006 | 0.019 | 0.022 | 0.002 | 0.002 |
| H120 (FJ807652-China) | 0.020 | 0.026 | 0.003 | 0.009 | 0.020 | 0.026 | 0.004 | 0.006 |
| H120 (MK937831-China) | 0.020 | 0.024 | 0.001 | 0.004 | 0.020 | 0.024 | 0.003 | 0.004 |
| H120 (FJ888351-China) | 0.020 | 0.024 | 0.001 | 0.004 | 0.020 | 0.024 | 0.003 | 0.004 |
| H120 (KF188436-India) | 0.019 | 0.022 | 0.001 | 0.002 | 0.019 | 0.022 | 0.002 | 0.002 |
| H120 (GU393335-USA) | 0.020 | 0.022 | 0.001 | 0.002 | 0.020 | 0.022 | 0.002 | 0.002 |
| H120 (KR605489-Iran) | 0.021 | 0.024 | 0.002 | 0.004 | 0.021 | 0.024 | 0.001 | 0.000 |
| W93 (AY842862-China | 0.022 | 0.028 | 0.003 | 0.007 | 0.022 | 0.028 | 0.005 | 0.007 |
| D41 (AH014596-China) | 0.022 | 0.030 | 0.004 | 0.009 | 0.022 | 0.030 | 0.006 | 0.009 |
| HK (AY761141-China) | 0.020 | 0.024 | 0.002 | 0.007 | 0.020 | 0.024 | 0.004 | 0.007 |
| H94 (DQ515801-China) | 0.022 | 0.028 | 0.003 | 0.007 | 0.022 | 0.028 | 0.005 | 0.007 |
| IBN (AY856348-China) | 0.021 | 0.026 | 0.002 | 0.006 | 0.021 | 0.026 | 0.004 | 0.006 |
| Ma5 (KY626045-Brazil) | 0.019 | 0.024 | 0.001 | 0.000 | 0.019 | 0.024 | 0.004 | 0.004 |
| Mass commercial vaccine (EU359657-USA) | 0.001 | 0.002 | 0.020 | 0.023 | 0.001 | 0.002 | 0.021 | 0.023 |
| H52 (EU817497-China) | 0.019 | 0.030 | 0.009 | 0.017 | 0.019 | 0.030 | 0.010 | 0.013 |
| M41 (MK937830-China) | 0.018 | 0.034 | 0.025 | 0.039 | 0.018 | 0.034 | 0.025 | 0.037 |
Abbreviations: IBV, infectious bronchitis virus; Mass, Massachusetts.
The first 1620 nucleotides, starting at the AUG translation start codon, of the S1 protein genes were compared.
NT, nucleotide; AA, amino acid.
Interestingly, the S1 amino acid sequence of strain I1124/16 was identical to that of the Thailand strain, THA280252, and 14 Mass-type strains (PDRC_110032, MDL_15-1778, MDL_15-2415, MDL_15-5589, MDL_15-6409, PDRC_109841, PDRC_109842, PDRC_109927, PDRC_110029, PDRC_110149, PDRC_110177, PDRC_110263, PDRC_110327, and PDRC_111337), which were isolated in America.
Complete Genomic Characteristics of the Four Field Strains
The complete genome lengths of strains LJL/130906, LJL/140820, I1124/16, and I0603/17 were 27,459, 27,630, 27,463, and 27,627 bp, respectively (Supplementary Table 1). The 4 IBV strains had a typical IBV genome organization consisting of 5′ UTR, ORF1a/ab, S glycoprotein, ORF3a, ORF3b, E protein, M protein, ORF 4b, ORF 5a, ORF 5b, N protein, 3′ UTR, and a poly (A) tail.
The phylogenetic trees constructed with complete genomic sequences revealed that the IBV strains, LJL/140820 and I0306/17, were clustered together with the different H120 and Ma5 vaccine strains (H120-clade), whereas the LJL/130906 and I1124/16 strains were separated from both H120-clade and M41-clade (Figure 1B). Comparatively, the LJL/130906 and I1124/16 strains were genetically closer to the M41-clade than that of the H120-clade. Both clades in which our 4 strains were included are clearly distinct from the H52, Mass41 vaccine, Mass41 2006, Beaudette, and viruses derived from the recombination between H120 and other IBV types (Liu et al., 2013; Chen et al., 2015; Han et al., 2016; 2018). Consistently, isolates LJL/140820 and I0306/17 displayed highly similar genomic patterns with those of viruses in the H120-clade using MAFFT and SimPlot analyses; however, the LJL/130906 and I1124/16 strains were clustered with viruses of the M41-clade (Supplementary Figures 3A and 3B).
Isolates LJL/140820 and I0306/17 May Be Derived From Vaccine Strains
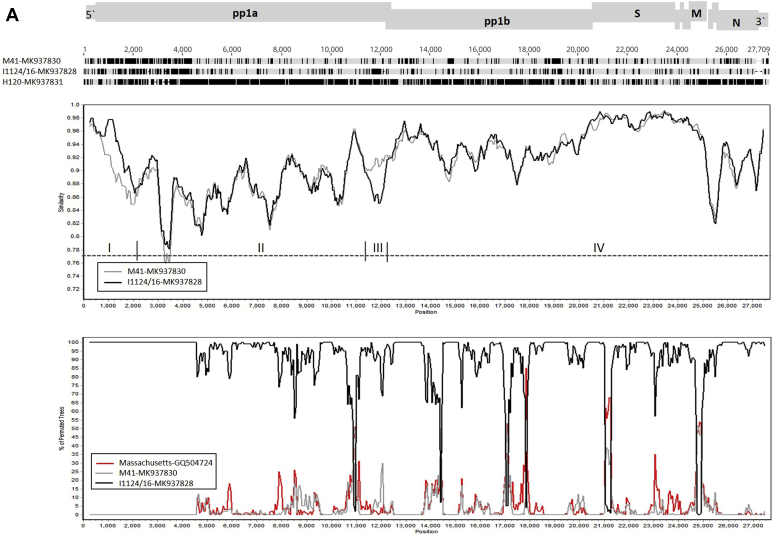
The results from MAFFT showed that the genomic sequence of isolate LJL/140820 showed high identity to that of Ma5; however, the genomic sequence of isolate I0603/17 showed high identity to that of the H120 vaccine strain (Figure 2). Only 10 nucleotide mutations were found across the genomes between LJL/140820 and Ma5 strains. However, 47, 885, and 2441 mutations were found between LJL/140820 and H120, H52, and M41, respectively. For isolate I0603/17, 34, 53, 876, and 2,447 nucleotide mutations were found when compared with H120, Ma5, H52, and M41 strains, respectively. Taken together, these results show that the LJL/140820 isolate was possibly a reisolated Ma5 strain and I0306/17 may be derived from the H120 vaccine strain.
Figure 2.
Analysis of the complete genomic sequences of our 4 IBV strains with those of the H120, Ma5, and M41 strains by using MAFFT. Nucleotide sequence disagreement at the indicated positions is shown in black, whereas the nucleotide sequence agreement is shown in gray. The GenBank accession numbers are shown at the end of the name of the viruses. The numbers indicate the genomic position at the top of the figure. Abbreviations: IBV, infectious bronchitis virus; MAFFT, Multiple Alignment with Fast Fourier Transformation.
Isolate I1124/16 Is Likely a Variant of the M41 Strain
Comparative genomic analyses revealed that the I1124/16 strain had the highest genome similarity with the LJL/130906 strain, sharing 98.4% overall nucleotide identity, compared with that of M41 (97.0%), H52 (93.1%), H120 (91.4%), and Ma5 (91.4%). These results were confirmed by an MAFFT analysis, which showed that the I1124/16 and LJL/130906 strains had comparatively similar patterns to each other and to that of the M41 strain, compared with the H120 and Ma5 vaccine strains (Figure 2).
To identify possible recombinant events, SimPlot analyses were performed on the genomes of the I1124/16 strain with that of the M41 strain, and the H120 genomic sequence used as a query (Figure 3A). Two regions (I and III) which showed relatively high diversity to the M41 strain were identified in the I1124/16 genome. However, these 2 regions still displayed a close relationship with other Mass-type viruses by a BLAST search in the NCBI database. In addition, bootscan did not identify an obvious potential recombination in the genome of the I1124/16 strain. These results suggest that the I1124/16 strain is a variant M41 strain that resulted from accumulated mutations after passaging in vaccinated chickens.
Figure 3.
MAFFT, SimPlot, and bootscan analyses were used to detect the genetic relationship of the complete genomes of isolates I1124/16 (A) and LJL/130906 (B). Nucleotide sequence disagreement at the indicated positions is shown in black, whereas the nucleotide sequence agreement is shown in gray in the MAFFT. The complete genomic sequence of the H120 strain (GenBank no. MK937831) was used as the query in the SimPlot and bootscan analysis. The window width and step size in SimPlot were set to 500 bp and 50 bp, respectively. Different regions in the genome of the isolates I1124/16 (I-IV) and LJL/130906 (I-III) showed different degrees of diversity. The hollow boxes indicate the different fragments in the bootscan analysis. Abbreviations: MAFFT, Multiple Alignment with Fast Fourier Transformation; SimPlot, Similarity plot.
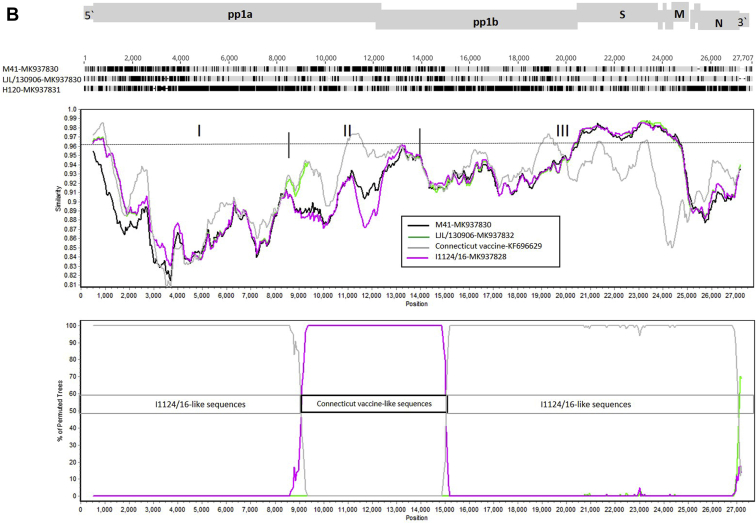
Strain LJL/130906 Isolate Is a Mosaic
One region (II) which showed a relatively high diversity with the I1124/16 strain was identified in the LJL/130906 genome by SimPlot analyses. A BLAST search in the NCBI database showed that sequences in region II of the LJL/130906 showed a higher close genetic relationship with the Conn-type viruses (Connecticut vaccine) (99% nucleotide identity). Further analysis using SimPlot and bootscan identified obvious recombination events in the genomic sequence of LJL/130906 when compared with those of M41, I1124/16, and Connecticut vaccine strains (Figure 3B). Thus, LJL/130906 might originate from recombination events between the I1124/16- and Connecticut-like strains. Two major recombination events were observed, one in the 5′ end of nsp 4 (approximately at nucleotides 8,848 to 8,853) and another in the 5′ end of nsp 12 (approximately at nucleotides 14,963 to 15,053) (Supplementary Figure 2).
We also compared the sequences of LJL/130906 from the 5′ end of nsp 4 to the 5′ end of nsp 12 with those of the Connecticut vaccine and 4 Conn-type field strains (Conn46 1966, Conn46 1972, Conn46 1983, and Conn46 1991), and did not identify whether the LJL/130906 strain was genetically closely related to the Connecticut vaccine or to 4 Conn-type field strains (Supplementary Table 2).
Antigenicity Between the Six Mass-type IBV Strains
The neutralization capacity of each antiserum was evaluated based on the viral replication by RT-PCR in embryonated chicken eggs inoculated with a virus-serum mixture, and the R value was calculated to evaluate the antigenic relatedness between viruses. The R value of homologous virus-serum was standardized to 100. The results showed that different Mass-type strains possess R values between 68.8 (I0306/17 and M41) and 85.6 (I1124/16 and LJL/130906) (Table 2). Comparatively, higher R values were shared between I0306/17, LJL/140820, and H120, comparing with those of other isolates, which were in line with the results of S1 and complete genomic identities between these IBV strains. However, isolates I1124/16 and LJL/130906 showed high R value (85.6) between each other but slight lower R values to the M41 and H120, suggesting difference in the antigenicity between the 2 isolates, and M41 and H120, although they belong to the same serotype. Hence, strain I1124/16 was used for further pathogenic and vaccination-challenge tests.
Table 2.
The R values between Mass-type IBV strains used in this study.
| Strain | R Value |
|||||
|---|---|---|---|---|---|---|
| I1124/16 | LJL/130906 | I0306/17 | LJL/140820 | H120 | M41 | |
| I1124/16 | 100 | 85.6 | 67.4 | 68.5 | 72.4 | 76.0 |
| LJL/130906 | 100 | 70.2 | 71.6 | 68.8 | 74.1 | |
| I0306/17 | 100 | 79.8 | 78.6 | 68.4 | ||
| LJL/140820 | 100 | 82.2 | 69.8 | |||
| H120 | 100 | 66.2 | ||||
| M41 | 100 | |||||
Abbreviations: IBV, infectious bronchitis virus; Mass, Massachusetts.
The Virulence of Isolate I1124/16 Was Comparable With That of Pathogenic M41
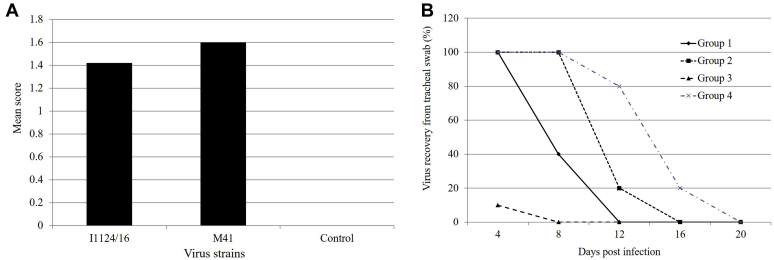
Clinical signs were observed in 7 of the I1124/16-infected chicks (70% morbidity) from postinoculation days 3 to 9, including listlessness, huddling, and ruffled feathers. The virulence of isolate I1124/16 was comparable but slightly lower than that of the pathogenic M41 strain (90% morbidity) to 21-day-old SPF chickens, as evaluated by the scores of clinical signs (Figure 4A). None of the chicks died during the experiment in both the I1124/16- and M41-infected groups. Chicks infected with I1124/16 and M41 were negative for IBV antibody at 4 dpi and approximately 50% of birds were positive at 8 dpi. All the birds were antibody positive at 8 dpi onward in both groups. No antibody was detected in the birds of the negative group.
Figure 4.
The vaccination-challenge test. The mean score was calculated from individual scores that were based on severity of respiratory signs detected in individual chickens (A). The virus recovery from the tracheal swabs collected from H120-vaccinated chickens at different time points after challenge with isolate I1124/16 and strain M41, comparing with those of the nonvaccinated and challenge chickens (B).
Protection of Vaccination With H120 Against Challenge of Isolate I1124/16
No clinical signs were observed in any of the birds in the two H120-vaccinated groups after challenge with the isolate I1124/16 and strain M41, comparing with those of nonvaccinated and challenged birds (Figure 4B). In terms of the viral shedding in the trachea, 100% of chicks vaccinated with H120 had not provided protection at 4 D against challenge of isolate I1124/16; however, only 10% of birds vaccinated with H120 shed viruses in the trachea after challenge with M41 strain. Furthermore, there are 40% of H120-vaccinated birds that shed viruses in the trachea at 8 D after challenge with I1124/16, which suggested only partial protection was provided with H120 vaccination.
Discussion
An IBV infection is one of the most important threats to the poultry industry in China despite extensive vaccination programs (Cook et al., 2012). Constant molecular surveillance indicated that at least 16 lineages of IBV strains, as well as a number of recombinants, are currently circulating in China (Ren et al., 2019). GI-1/Mass IBV is one of the most important serotypes/lineages, although Mass-type vaccines (e.g., H120, H52, and Ma5) are extensively used to control and prevent IBV outbreaks in China. The 4 Mass-type IBV strains in this study were isolated from the H120-vaccinated chickens between 2013 and 2017 in China. Although the 4 strains were genetically close to the H120 and Ma5 vaccines based on the results of S1 phylogeny analysis, they showed slightly different patterns in the phylogenetic trees, and deep investigation into the complete genomes of the LJL/140820 and I0306/17 strains using phylogenetic analysis, MAFFT, and a pairwise comparison suggested that LJL/140820 was most closely related to the Ma5 vaccine strain and I0306/17 exhibited a close relationship with the H120 vaccine strain. Further analysis showed that multiple nucleotide point mutations were observed between the genomes of I0306/17 and H120 strains, which suggests that the I0306/17 strain might have resulted from nucleotide accumulated point mutations after several passages in chickens, as previously reported (Liu et al., 2013; Chen et al., 2015). The fact that the Mass-type viruses were isolated from clinically diseased chickens indicates that vaccine-derived viruses have likely acquired the necessary sequence changes for pathogenicity (McKinley et al., 2011) and returned to a wild-type pathogenic state (Marandino et al., 2018).
The S1 sequences of the isolates LJL/130906 and I1124/16 showed obvious point mutations when compared with those of the M41 and H120 strains (Xu et al., 2018); however, other genes and regions were found to be affected by mutations in the genomes of the Mass-type strains which was consistent with previous observations (Amarasinghe et al., 2018). It is important to note that the sequence of the S1 subunit in the isolate I1124/16 was identical to that of the 14 Mass-type IBV strains in America and a Thailand strain. We cannot conclude that the I1124/16 strain originated from the 15 Mass-type IBV strains or vice versa because no additional information is available regarding these strains. However, the present results suggest that the I1124/16 strain and the 15 strains might share the same ancestor, and the evolutionary path of the strain I1124/16 was different from that of the LJL/140820 and I0306/17 strains. Comparatively, the I1124/16 strain was more closely related to the M41 strain than the H120, H52, and Ma5 vaccine strains; however, point mutations were found to be scattered across the genome of the I1124/16 strain when compared with that of the M41 strain. The IBV M41 is a virulent respiratory IBV strain (Wickramasinghe et al., 2011) and the M41-like virus is detected at a low frequency in chicken flocks (Chen et al., 2015). We hypothesized that the I1124/16 strain might have resulted from an accumulation of point mutations in the M41 strain when it spread in chickens in the presence of Mass-specific antibody; thus, it was an M41 variant. The results of the VN test revealed that the I1124/16 strain was antigenically related but different from the M41, which was consistent with the minor genetic differences in the S1 genes between them. Changes in the antigenicity are likely caused by S1 variability because it has been well established that even a small change in the amino acid sequence of the spike protein can result in the antigenic changes (Mockett et al., 1984; Cavanagh et al., 1992).
The Mass lineage virus is the current industry standard vaccine strain for IBV control. Our results from the protection experiments pointed out that chickens immunized with the H120 virus failed to eliminate viral shedding after infection with the isolate I1124/16. These results were consistent to the field observation because the I1124/16 was isolated from the H120-vaccinated diseased flock. Furthermore, the virus produced appreciable pathogenic effects in the immunized chickens, indicating that the H120 vaccine may not confer full cross-protection against the variant IBV isolate. It has been reported that there is an increased likelihood of a high level of cross-protection between strains with high nucleotide identity when compared with strains with a low homology. However, this correlation is not considered to be strong (de Wit et al., 2011) because strains which differed only by a few percentages in nucleotide similarity were associated with a significant drop in cross-protection (Meir et al., 2004; Abdel-Moneim et al., 2006). Thus, more studies based on the reverse genetic techniques are needed to elucidate the amino acids found in the S1 subunit possibly contributing to the altered antigenicity of the isolate I1124/16 comparing with M41 strain, as illustrated by the VN and vaccination-challenge tests. The IBV pathogenicity was shown to be polygenic, involving both the spike and replicase proteins (Hodgson et al., 2004; Ammayappan et al., 2009; Armesto et al., 2009; Oade et al., 2019). The I1124/16 was found to have comparable but slightly lower pathogenicity with the M41 strain. Hence, the amino acid substitutions in the S1 subunit of I1124/16 are likely to be of little or no consequence to the pathogenicity.
A unique mechanism of RNA synthesis involving polymerase jumping and discontinuous transcription might be responsible for recombination in coronaviruses (Lai and Cavanagh, 1997). Moreover, the occurrence of multiple recombination events has been reported to be related to Mass-type IBV strains, between field strains (Chen et al., 2015), between vaccine strains (Liu et al., 2014), and between field and vaccine strains (Liu et al., 2013, 2014; Chen et al., 2015), either within the same genotype (intragenotypic) or different genotypes (intergenotypic). The present findings indicate that LJL/130906 has undergone intragenotypic recombination between an M41 variant and Conn-type virus. Although the Conn-type field strain has not been detected in China to date, a recombinant IBV between H120 and the Connecticut vaccine has previously been detected (Liu et al., 2014). It is possible that the Connecticut vaccine was involved in the emergence of the LJL/130906 strain because the Mass-Conn bivalent vaccine is used extensively throughout China; however, we did not find any direct evidence. The 2 identified recombination sites are located at the 5′ ends of nsp 4 and nsp 12 of LJL/130906, respectively, which are not within the intergenic regions previously reported as recombination “hot spots” (Lee and Jackwood, 2000).
The results of the present study show multiple patterns of the spread of GI-1/Mass-type IBV field strains in China. These different Mass-type IBV strains may evolve independently from each other. The differences of the field conditions and genetic pressures between different flocks likely account for these evolution of the viruses although the successful spread of the GI-1/Mass-type IBVs is associated, at least in part, with the use of vaccines. These findings underscore the need for continuous surveillance to provide a better understanding of GI-1/Mass-type IBV molecular diversity. The GI-1/Mass-type is among the most prevalent and economical important IBV lineages worldwide; thus, the genetic and antigenic results obtained in this study are significant because these results extend our knowledge of the GI-1/Mass-type IBV strains and may potentially provide relevant elements to improve control programs.
Acknowledgments
This work was supported by grants from the China Agriculture Research System (No. CARS-40-K18), the Provincial Supported Science Foundation of Heilongjiang Province, China, for The National Key Technology R & D Program (GX16B003), and the National Key Research and Development Program of China (2017YFD0500105).
Conflict of Interest Statement: The authors declare that they have no conflict of interest and competing interests.
Footnotes
Supplementary data associated with this article can be found in the online version at http://doi.org/10.1016/j.psj.2020.08.037.
Supplementary Data
References
- Abdel-Moneim A.S., El-Kady M.F., Ladman B.S., Gelb J., Jr. S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virol. J. 2006;3:78. doi: 10.1186/1743-422X-3-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleuy O.A., Pitesky M., Gallardo R. Using multinomial and space-time permutation models to understand the epidemiology of infectious bronchitis in California between 2008 and 2012. Avian Dis. 2018;62:226–232. doi: 10.1637/11788-122217-Reg.1. [DOI] [PubMed] [Google Scholar]
- Alexander D.J., Gough R.E., Pattison M. A long-term study of the pathogenesis of infection of fowls with three strains of avian infectious bronchitis virus. Res. Vet. Sci. 1978;24:228–233. [PubMed] [Google Scholar]
- Amarasinghe A., De Silva Senapathi U., Abdul-Cader M.S., Popowich S., Marshall F., Cork S.C., van der Meer F., Gomis S., Abdul-Careem M.F. Comparative features of infections of two Massachusetts (Mass) infectious bronchitis virus (IBV) variants isolated from Western Canadian layer flocks. BMC Vet. Res. 2018;14:391. doi: 10.1186/s12917-018-1720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammayappan A., Upadhyay C., Gelb J., Jr., Vakharia V.N. Identification of sequence changes responsible for the attenuation of avian infectious bronchitis virus strain Arkansas DPI. Arch. Virol. 2009;154:495–499. doi: 10.1007/s00705-009-0325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archetti I., Horsfall F.L. Persistent antigenic variation of influenza A viruses after incomplete neutralization in ovo with heterologous immune serum. J. Exp. Med. 1950;92:441–462. doi: 10.1084/jem.92.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armesto M., Cavanagh D., Britton P. The replicase gene of avian coronavirus infectious bronchitis virus is a determinant of pathogenicity. PLoS One. 2009;4:e7384. doi: 10.1371/journal.pone.0007384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlenga G., Cook J.K.A., Gelb J., de Wit J.J. Development and use of the H strain of avian infectious bronchitis virus from The Netherlands as a vaccine: a review. Avian Pathol. 2004;33:550–557. doi: 10.1080/03079450400013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursnell M.E., Brown T.D., Foulds I.J., Green P.F., Tomley F.M., Binns M.M. Completion of the sequence of the genome of the coronavirus avian infectious bronchitis virus. J. Gen. Virol. 1987;68:57–77. doi: 10.1099/0022-1317-68-1-57. [DOI] [PubMed] [Google Scholar]
- Burland T.G. DNASTAR's Lasergene sequence analysis software. Methods Mol. Biol. 2000;132:71–91. doi: 10.1385/1-59259-192-2:71. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitisvirus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J. Coronavirus IBV: removal of spike glycopolypeptide S1 by urea abolishes infectivity and haemagglutination but not attachment to cells. J. Gen. Virol. 1986;67:1443–1448. doi: 10.1099/0022-1317-67-7-1443. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Cook J.K., Li D., Kant A., Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992;21:33–43. doi: 10.1080/03079459208418816. [DOI] [PubMed] [Google Scholar]
- Chen L., Zhang T., Han Z., Liang S., Xu Y., Xu Q., Chen Y., Zhao Y., Shao Y., Li H., Wang K., Kong X., Liu S. Molecular and antigenic characteristics of Massachusetts genotype infectious bronchitis coronavirus in China. Vet. Microbiol. 2015;181:241–251. doi: 10.1016/j.vetmic.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Jiang L., Zhao W., Liu L., Zhao Y., Shao Y., Li H., Han Z., Liu S. Identification and molecular characterization of a novel serotype infectious bronchitis virus (GI-28) in China. Vet. Microbiol. 2017;198:108–115. doi: 10.1016/j.vetmic.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K.S., Lee E.K., Jeon W.J., Park M.J., Kim J.W., Kwon J.H. Pathogenicity and antigenicity of a new variant of Korean nephropathogenic infectious bronchitis virus. J. Vet. Sci. 2009;10:357–359. doi: 10.4142/jvs.2009.10.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K., Jackwood M., Jones R.C. The long view: 40 years of infectious bronchitis research. Avian Pathol. 2012;41:239–250. doi: 10.1080/03079457.2012.680432. [DOI] [PubMed] [Google Scholar]
- de Wit J.J., Cook J.K., van der Heijden H.M. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40:223–235. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Gao M., Chen Y., Zhao W., Sun J., Zhao Y., Liu S. Genetics, antigenicity and virulence properties of three infectious bronchitis viruses isolated from a single tracheal sample in a chicken with respiratory problems. Virus Res. 2018;257:82–93. doi: 10.1016/j.virusres.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Zhang T., Xu Q., Gao M., Chen Y., Wang Q., Zhao Y., Shao Y., Li H., Kong X., Liu S. Altered pathogenicity of a tl/CH/LDT3/03 genotype infectious bronchitis coronavirus due to natural recombination in the 5'-17kb region of the genome. Virus Res. 2016;213:140–148. doi: 10.1016/j.virusres.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson T., Casais R., Dove B., Britton P., Cavanagh D. Recombinant infectious bronchitis coronavirus Beaudette with the spike protein gene of the pathogenic M41 strain remains attenuated but induces protective immunity. J. Virol. 2004;78:13804–13811. doi: 10.1128/JVI.78.24.13804-13811.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Zhao W., Han Z., Chen Y., Zhao Y., Sun J., Li H., Shao Y., Liu L., Liu S. Genome characterization, antigenicity and pathogenicity of a novel infectious bronchitis virus type isolated from south China. Infect. Genet. Evol. 2017;54:437–446. doi: 10.1016/j.meegid.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M., Cavanagh D. The molecular biology of coronaviruses. Adv. Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.W., Jackwood M.W. Evidence of genetic diversity generated by recombination among avian coronavirus IBV. Arch. Virol. 2000;145:2135–2148. doi: 10.1007/s007050070044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legnardi M., Franzo G., Koutoulis K.C., Wiśniewski M., Catelli E., Tucciarone C.M., Cecchinato M. Vaccine or field strains: the jigsaw pattern of infectious bronchitis virus molecular epidemiology in Poland. Poult. Sci. 2019;98:6388–6392. doi: 10.3382/ps/pez473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Chen J., Han Z., Zhang Q., Shao Y., Kong X., Tong G. Infectious bronchitis virus: S1 gene characteristics of vaccines used in China and efficacy of vaccination against heterologous strains from China. Avian Pathol. 2006;35:394–399. doi: 10.1080/03079450600920984. [DOI] [PubMed] [Google Scholar]
- Liu S., Xu Q., Han Z., Liu X., Li H., Guo H., Sun N., Shao Y., Kong X. Origin and characteristics of the recombinant novel avian infectious bronchitis coronavirus isolate ck/CH/LJL/111054. Infect. Genet. Evol. 2014;23:189–195. doi: 10.1016/j.meegid.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhang X., Gong L., Yan B., Li C., Han Z., Shao Y., Li H., Kong X. Altered pathogenicity, immunogenicity, tissue tropism and 3'-7kb region sequence of an avian infectious bronchitis coronavirus strain after serial passage in embryos. Vaccine. 2009;27:4630–4640. doi: 10.1016/j.vaccine.2009.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Shao Y., Ma H., Sun C., Zhang X., Li C., Han Z., Yan B., Kong X., Liu S. Comparative analysis of four Massachusetts type infectious bronchitis coronavirus genomes reveals a novel Massachusetts type strain and evidence of natural recombination in the genome. Infect. Genet. Evol. 2013;14:29–38. doi: 10.1016/j.meegid.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lole K.S., Bollinger R.C., Paranjape R.S., Gadkari D., Kulkarni S.S., Novak N.G., Ingersoll R., Sheppard H.W., Ray S.C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999;73:152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Xu L., Ren M., Shen J., Han Z., Sun J., Zhao Y., Liu S. Novel genotype of infectious bronchitis virus isolated in China. Vet. Microbiol. 2019;230:178–186. doi: 10.1016/j.vetmic.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marandino A., Vagnozzi A., Craig M.I., Tomás G., Techera C., Panzera Y., Vera F., Perez R. Genetic and antigenic heterogeneity of infectious bronchitis virus in South America: implications for control programmes. Avian Pathol. 2019;48:270–277. doi: 10.1080/03079457.2019.1583315. [DOI] [PubMed] [Google Scholar]
- McKinley E.T., Jackwood M.W., Hilt D.A., Kissinger J.C., Robertson J.S., Lemke C., Paterson A.H. Attenuated live vaccine usage affects accurate measures of virus diversity and mutation rates in avian coronavirus infectious bronchitis virus. Virus Res. 2011;158:225–234. doi: 10.1016/j.virusres.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir R., Rosenblut E., Perl S., Kass N., Ayali G., Perk S., Hemsani E. Identification of a novel nephropathogenic infectious bronchitis virus in Israel. Avian Dis. 2004;48:635–641. doi: 10.1637/7107. [DOI] [PubMed] [Google Scholar]
- Mockett A.P., Cavanagh D., Brown T.D. Monoclonal antibodies to the S1 spike and membrane proteins of avian infectious bronchitis coronavirus strain Massachusetts M41. J. Gen. Virol. 1984;65:2281–2286. doi: 10.1099/0022-1317-65-12-2281. [DOI] [PubMed] [Google Scholar]
- Molenaar R.J., Dijkman R., de Wit J.J. Characterization of infectious bronchitis virus D181, a new serotype (GII-2) Avian Pathol. 2020;7:1–8. doi: 10.1080/03079457.2020.1713987. [DOI] [PubMed] [Google Scholar]
- Oade M.S., Keep S., Freimanis G.L., Orton R.J., Britton P., Hammond J.A., Bickerton E. Attenuation of infectious bronchitis virus in eggs results in different patterns of genomic variation across multiple replicates. J. Virol. 2019;93:e00492-19. doi: 10.1128/JVI.00492-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty per cent endpoint. Am. J. Epidemiol. 1938;27:493–497. [Google Scholar]
- Ren M., Sheng J., Ma T., Xu L., Han Z., Li H., Zhao Y., Sun J., Liu S. Molecular and biological characteristics of the infectious bronchitis virus TC07-2/GVI-1 lineage isolated in China. Infect. Genet. Evol. 2019;75:103942. doi: 10.1016/j.meegid.2019.103942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M., Zhang L., Hou Y., Zhao Y., Han Z., Sun J., Liu S. Genetic, antigenic, and pathogenic characteristics of infectious bronchitis virus GI-7/TW-II in China. Avian Dis. 2020 doi: 10.1637/0005-2086-64.2.183. [DOI] [PubMed] [Google Scholar]
- Shalk A., Hawn M. An apparently new respiratory disease of baby chicks, 1931. J. Am. Vet. Med. Assoc. 1931;78:413. [Google Scholar]
- Valastro V., Holmes E.C., Britton P., Fusaro A., Jackwood M.W., Cattoli G., Monne I. S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infect. Genet. Evol. 2016;39:349–364. doi: 10.1016/j.meegid.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasinghe I.N., de Vries R.P., Grone A., de Haan C.A., Verheije M.H. Binding of avian coronavirus spike proteins to host factors reflects virus tropism and pathogenicity. J. Virol. 2011;85:8903–8912. doi: 10.1128/JVI.05112-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterfield R.W. Respiratory signs, immunity response, and interference from vaccination with monovalent and multivalent infectious bronchitis vaccines. Avian Dis. 1968;12:577–584. [PubMed] [Google Scholar]
- Worthington K.J., Currie R.J.W., Jones R.C. A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathol. 2008;37:247–257. doi: 10.1080/03079450801986529. [DOI] [PubMed] [Google Scholar]
- Xu L., Han Z., Jiang L., Sun J., Zhao Y., Liu S. Genetic diversity of avian infectious bronchitis virus in China in recent years. Infect. Genet. Evol. 2018;66:82–94. doi: 10.1016/j.meegid.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegpi R.A., Breedlove C., Gulley S., Toro H. Infectious bronchitis virus immune Responses in the Harderian Gland upon Initial vaccination. Avian Dis. 2020;64:92–95. doi: 10.1637/0005-2086-64.1.92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.