Abstract
In a few months, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has become the main health problem worldwide. Epidemiologic studies revealed that populations have different vulnerabilities to SARS-CoV-2. Severe outcomes of the coronavirus disease 2019 (COVID-19) with an increased risk of death are observed in patients with metabolic syndrome, as well as diabetic and heart conditions (frail population). Excessive proinflammatory cytokine storm could be the main cause of increased vulnerability in this frail population. In patients with diabetes and/or heart disease, a low inflammatory state is often associated with gut dysbiosis. The increase amount of microbial metabolites (i.e., trimethylamine N-oxide and lipopolysaccharide), which generate an inflammatory microenvironment, is probably associated with an improved risk of severe illness from COVID-19. Nutritional interventions aimed at restoring the gut microbial balance could represent preventive strategies to protect the frail population from COVID-19. This narrative review presents the possible molecular mechanisms by which intestinal dysbiosis that enhances the inflammatory state could promote the spread of SARS-CoV-2 infection. Some nutritional strategies to counteract inflammation in frail patients are also analyzed.
Keywords: SARS-CoV-2, COVID-19, metabolic syndrome, cardiovascular diseases, gut dysbiosis, nutraceuticals
Coronavirus disease 2019 pandemic
On March 11, 2020, the World Health Organization declared the coronavirus disease 2019 (COVID-19) a pandemic [1]. Just 3 mo ago (December 2019), the first cases of unexplained severe acute respiratory syndrome and pneumonia were reported in the province of Hubei, China [2], and subsequently this respiratory syndrome was associated with infections related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; previously named 2019-nCoV) infection [3], a novel human coronavirus that belongs to the β-coronavirus cluster that also includes SARS-CoV and Middle East respiratory syndrome CoV [3]. In the first week of May, approximately 4,000,000 individuals were infected, and 290,000 subjects had died in 208 countries [1]. These numbers highlight the global severity of this epidemic for health and economic development worldwide.
The cornerstone of the COVID-19 pandemic management is the early detection of positive patients and their isolation to prevent further infections, considering that SARS-CoV-2 spreads through respiratory droplets. The clinical strategy for COVID-19 treatment is represented by symptomatic treatments, because no specific protocols, antiviral drugs, or vaccines have been developed to date [4].
Epidemiologic studies indicate that the elderly, who are above all other subjects affected by metabolic syndrome, diabetes, and/or cardiovascular diseases (especially hypertension), are more susceptible to SARS-CoV-2 infection as highlighted by their increased symptom severity, disease rate progression, and mortality [2,5].
Given the high number of infected people who need intensive care and the ineffectiveness and aspecificity of currently adopted therapies, many countries are now committed to limiting SARS-CoV-2’s exponential growth by making extraordinary decisions, including the lockdown of certain regions or entire countries with severe economic impact [6].
While countries work to contain the COVID-19 pandemic and until a vaccine is developed, the problem of higher SARS-CoV-2-associated risk and mortality in frail subpopulations (eg, diabetic patients and patients with metabolic syndrome or cardiovascular diseases) remains. Some authors have strongly advised the implementation of blood glucose management in diabetic patients, using online services, and avoiding person-to-person contact [7]. Others have suggested a tighter nutritional control to avoid malnutrition conditions [8].
However, to protect frail subjects, investigating the mechanisms involved in the aberrant immune response triggered by SARS-Cov-2 and identifying possible therapeutic targets to counteract the infection are necessary.
Aberrant inflammatory response induction and SARS-CoV-2
SARS-CoV-2 activates intracellular stress pathways, and induces and takes advantage of aberrant responses by the host that are innate and adaptive to the immune system, which plays a crucial role in causing fatal pneumonia and death [1]. SARS-CoV-2 (as SARS-CoV) enters the host target cells by binding to the angiotensin-converting enzyme 2 (ACE2) protein [9]. The inflammatory process begins after the interaction between SARS-CoV-2 and the toll-like receptor (TLR), whose activation triggers a sequence of responses from leads to interleukin (IL) synthesis to leads the synthesis of interleukins (ILs) [10] and inflammasome activation [11].
Previous robust data show how SARS-CoV, whose sequence homology with SARS-CoV-2 is 96% [12], interacts with the NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome, indicating NLRP3 as a coronavirus inflammatory target [13,14]. NLRP3 inflammasome is a multiprotein complex that consists of NLRP3, adaptor protein apoptosis-associated speck-like protein-containing CARD adapter (ASC) molecule, and pro-caspase 1 effector molecule [15]. After an inflammatory insult, the assembly of these 3 complexes induces caspase-1 activation by cleavage of pro-caspase-1 and then pro-IL-1 and pro-IL-18 conversion in their active forms IL-1 and IL-18 [15], [16], [17], [18]. NLRP3 inflammasome is associated with various autoimmune, inflammatory, and metabolic diseases, and its formation is responsible for pyroptosis due to the loss of the ionic gradient after the formation of pores on the cell membrane [16].
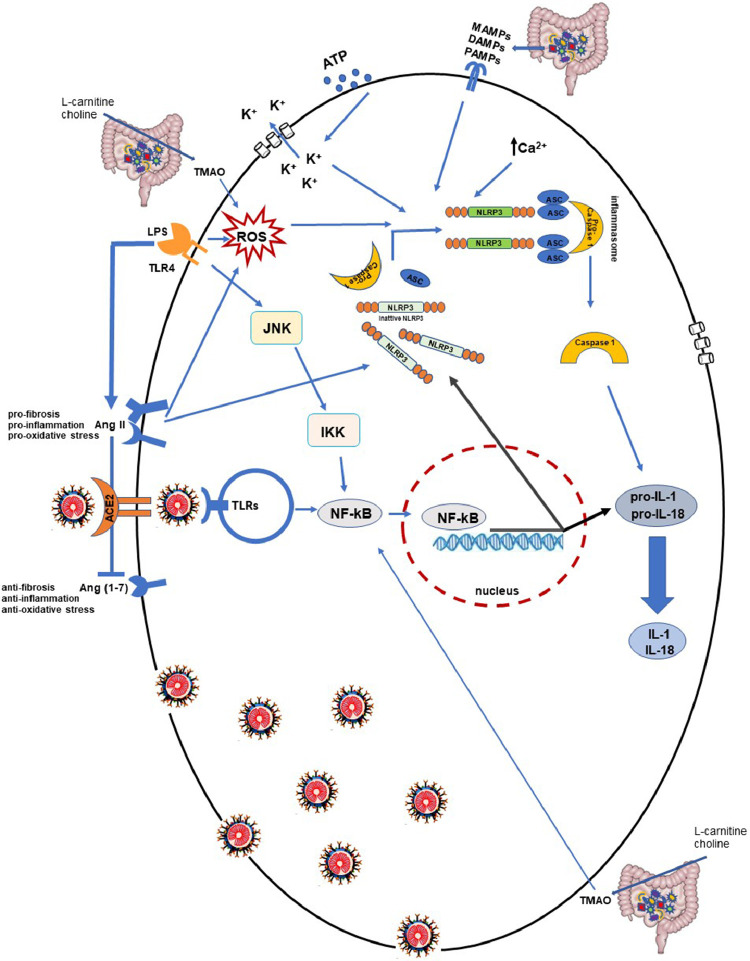
NLRP3 activation requires 2 steps, both generated by stressful conditions [15,17,18]: First, the transcriptional activation of inflammasome-related proteins (including NLRP3, pro-IL-1 beta, and pro-IL-18) and then their subsequent assembly and activation. The priming step occurs after nuclear factor kappa B (NF-kB) induction enhanced by the interaction between several microbial molecules or endogenous cytokines with different receptors, including TLRs [19]. The binding of lipopolysaccharide (LPS), the major structural component of Gram-negative bacteria membrane, to the TLR4 receptor represents a strong stimulus for NF-kB activation [20]. LPS–TLR4 binding, enhancing c-Jun N-terminal kinase and I-κB kinase intracellular signaling, leads to NF-kB activation through the dissociation of its inhibitor (Fig. 1 ). This process culminates in NF-kB translocation into the nucleus and the consequent mRNA expression of all inflammasome subunits [21].
Fig. 1.
Possible molecular mechanisms involved in aberrant inflammatory response to severe acute respiratory syndrome coronavirus 2 in frail patients. The binding of severe acute respiratory syndrome coronavirus 2 to angiotensin-converting enzyme 2 and its interaction with intracellular toll-like receptors (TLRs) triggers a sequence of responses, which leads to nuclear factor kappa B activation and interleukin synthesis. Host cell inflammation could be exacerbated by NOD-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome activation and proinflammatory cytokine production. NLRP3 inflammasome activation requires 2 steps generated by stressful conditions: Transcription of NLRP3 and adaptor protein apoptosis-associated speck-like protein-containing CARD adapter, pro-caspase-1, and their assembly and activation. The first step is consequent to nuclear factor kappa B activation and translocation into the nucleus, stimulated by lipopolysaccharide–TLR4 binding through c-Jun N-terminal kinase and IκB kinase intracellular signaling. The second step is induced by an abnormal mitochondrial reactive oxygen species production, or by pathogen-associated molecular patterns, damage-associated molecular patterns, and microbial-associated molecular pattern stimuli related to gut dysbiosis. Moreover, the K+ outflow, due to adenosine triphosphate extracellular stimulus, or intracellular and cytosolic Ca2+ fluxes imbalance induces NLRP3 inflammasome assembly. NLRP3 activation is also regulated by AngII overexpression stimulated by lipopolysaccharide–TRL4 interaction and gut dysbiosis-derived metabolite trimethylamine N-oxide.
Recent data suggest that TLR4 could also play an important role in the viral infection immune response [21]. TLR4 overstimulation is associated with an excessive inflammatory response and an unfavorable outcome of viral infections [22]. Olejnik et al. demonstrated that TLR4 signaling inhibition reduces excessive proinflammatory responses during Ebola virus infection [23]. In the same manner, TLR4 and LPS antagonists, which inhibit LPS–TLR4 binding, prevent receptor activation and significantly reduce the inflammatory state during respiratory syncytial virus infection [24]. On the other hand, other authors have suggested that a low degree of TLR4 activation could have positive effects on establishing a protective immune response during viral infection and that TLR4(-/-) mice are more sensitive to SARS-CoV compared with wild-type mice [25]. These data suggest that the TLR4 activation degree is crucial in SARS-CoV infection, and TLR4 overactivation is associated with a poor prognosis. In this context, concomitant pathologies that excessively activate TLR4 could conceivably aggravate the COVID-19 condition.
The second step of NLRP3 activation is represented by inflammasome subunits (NLRP3, ASC, and pro-caspase 1) assembling, which is essential for caspase-1 supply and IL-1 beta and IL-18 production and secretion [15,26]. This process is stimulated by different cellular stress conditions, including adenosine triphosphate extracellular stimulus, that determines an outflow of K+ and membrane pores formation or fluxes of intracellular and cytosolic Ca2+ (ie, mitochondrial dysfunction due to Ca2+ overload) [18,27]. In the same way, aberrant reactive oxygen species production, pathogen-associated molecular patterns (PAMPs), or damage-associated molecular patterns (DAMPs) induce NLRP3 assembly (Fig. 1) [28].
Of note, NLRP3 is also regulated by angiotensin II (AngII) [29], a key hormone of the renin-angiotensin system involved in blood pressure regulation [30]. AngII is highly responsible for oxidative stress induction, inflammation, and the fibrosis process characterizing numerous diseases, including hypertension [31] and insulin resistance [32]. These metabolic alterations predispose to metabolic syndrome and type 2 diabetes mellitus, which are associated with an increased risk of heart disease and lung dysfunction [33,34]. Numerous studies have shown that AngII stimulates NLRP3 inflammasome, promoting proinflammatory cytokines expression [31,35]. Interestingly, some authors have observed that the LPS–TLR4 interaction is involved in AngII overexpression and consequently in NLRP3/NF-kB activation (Fig. 1) [36,37]. This leads to the deduction that pathogenetic stimuli, capable of increasing NLRP3 activation, contribute to the worsening of proinflammatory conditions trigged by SARS-CoV-2.
Recent accumulating evidence indicates that Ang-(1-7), derived from AngII degradation by the ACE2 enzyme, is a counter-regulator for the AngII prooxidative and NLRP3 inflammasome activation [38]. Different authors have demonstrated that an increased Ang-(1-7) level, through exogenous administration or by molecules enhancing Ang-(1-7) signaling, improves lung pathology in mice models [39], [40], [41]. Of note, cardiovascular and pulmonary health is closely related to the AngII/Ang-(1-7) axis controlled by ACE2 and the SARS-2-CoV-2 functional receptor, and in patients infected with COVID-19, the ACE2/Ang-(1-7) pathway is downregulated [42]. In patients infected with COVID-19 who are affected by metabolic syndrome, diabetes, or cardiovascular diseases, SARS-CoV-2 exacerbates the ACE2/Ang-(1-7) axis, and the alteration worsens the inflammation state and causes multi-organ damage (Fig. 1).
During the first weeks of the pandemic, some authors and social media outlets speculated that ACE inhibitors and AngII type 1 receptor blockers (ARBs) used for the treatment of hypertension facilitate SARS-CoV-2 infection by increasing ACE2 expression. However, the data supporting this hypothesis are conflicting.
Although ACE and ACE2 enzymes are highly homologous, they catalyze different reactions of the renin-angiotensin system and ACE inhibitors are not able to inhibit ACE2. Moreover, different studies report that ARBs increase ACE2 expression, but ARB action is dose-related and tissue-specific. Inappropriate information about these antihypertensive agents causes stress and anxiety in patients and some have discontinued treatment, probably aggravating their condition.
Currently, ACE inhibitors or ARB correlation with SARS-CoV-2 severe illness is not clinically proven. Indeed, as mentioned, elevated Ang-(1-7) probably has a protective pulmonary action. For this reason, therapy with renin-angiotensin system blockers should not be withdrawn or modified to prevent SARS-CoV-2 infection [43,44].
Possible link between gut dysbiosis and COVID-19 severe illness
The gut microbiome has received growing attention over the last decades and accumulating data have shown how gut alterations are associated with physiological and pathologic conditions [45], [46], [47]. In particular, in diabetic patients and those with heart disease, gut microbiota is characterized by a decrease in microbiologic diversity associated with an altered ratio between Firmicutes and Bacteroidetes, the 2 principal phyla in intestinal microflora, in favor of Firmicutes [48], [49], [50].
An altered Firmicutes/Bacteroidetes ratio is known to decrease intestinal permeability by promoting PAMPs, DAMPs, and microbial-associated molecular pattern productions and contributing to the proinflammatory state [51]. Moreover, gut dysbiosis is associated with an increased level of trimethylamine N-oxide (TMAO), a gut microbiota-derived metabolite that is processed after choline and L-carnitine ingestion [52,53]. This molecule has been receiving interest as a mediator in systemic inflammation and atherosclerosis condition [54], [55], [56]. Both observational and experimental studies suggest that TMAO causes endothelial inflammatory injury [55,56]. Remarkably, recent studies have shown that TMAO recruits NLRP3 inflammasome by activating NF-kB and reactive oxygen species signaling, induces proinflammatory cytokines release, and promotes over-inflammatory conditions [57,58]. Therefore, high TMAO levels are correlated with an abnormal inflammation condition that could be exacerbated by a SARS-CoV-2 infection (Fig. 1). However, other elements seem to be involved.
LPS–TLR4 interaction [19,20] plays a role in the pathogenesis of insulin resistance [59,60]. Higher levels of plasma LPS concentration were found in obese and elderly patients, as well as those with type 2 diabetes mellitus, compared with healthy subjects [60]. LPS–TLR4 binding not only triggers inflammatory conditions, but also inhibits insulin-stimulated IRS1 phosphorylation and impairs glucose homeostasis control [61]. To confirm the LPS–TLR4 role in insulin resistance, recent evidence has revealed that NLRP3 activation represents an essential mechanism in metabolic inflammation and insulin-resistance induction [61], and its inhibition could prevent ischemic stroke in diabetic patients [62]. Furthermore, as previously reported, the LPS–TLR4 axis increases AngII overexpression that contributes to the destruction of the ACE2/Ang-(1-7) balance [36,37]. Of note, inflammation induced by gut microbiota-altered composition represents an important mediator in the cardiometabolic and diabetic pathogenesis and could contribute to aggravated SARS-CoV-2 infection in frail patients.
As further support for this hypothesis, high plasma LPS levels are correlated with lung diseases and IL-6 overproduction. Several studies have shown that LPS, which promotes IL-6 production, is strongly involved in lung pathologies that accelerate neutrophil recruitment and cell infiltration [63,64]. Chen et al. highlighted IL-6’s important pathophysiological role in COVID-19, and proposed IL-6 serum levels as a biomarker for SARS-CoV-2 prognosis. High IL-6 levels are associated with a poor prognosis [65]. Different groups have proposed to counteract IL-6 overproduction and tocilizumab (IL-6 inhibitor usually used to treat rheumatoid arthritis) has already been administered to Chinese patients infected with COVID-19. In Italy, a clinical trial is evaluating tocilizumab safety and efficacy, and the U.S. Food and Drug Administration has formally approved a tocilizumab phase 3 trial for patients who are severely ill with COVID-19 [66,67].
Recently, different authors have emphasized the important relationship between the gut microbiome and the lung assuming a gut–lung axis. The gut microbiome is crucial for immune homeostasis and above all pulmonary innate immunity against infections. The altered microbiota composition of mice, which was induced by treatment with antibiotic cocktails, is associated with an ineffective T- and B-cell immunity after an influenza virus infection. These animals displayed an impaired capacity to reduce viral replication and consequently increased morbidity and mortality [68,69].
Nutritional strategies to improve gut dysbiosis and reduce low chronic inflammation state
Intestinal dysbiosis could contribute to the aberrant inflammatory response triggered by SARS-CoV-2. Nutraceutical strategies aimed at restoring eubiosis conditions could represent a valid adjuvant intervention for COVID-19 management. To reduce TMAO production, limiting the ingestion of choline and carnitine, from which TMAO derives, is an option [70], but these molecules are essential to maintain a healthy state. Choline has a structural role and is a neurotransmitter precursor, while several groups, including ours, have demonstrated that L-carnitine improves musculoskeletal function [71,72] and has a crucial antioxidant action on the heart, in particular during a myocardial infarction [73].
Alternatively, the use of prebiotic and probiotic agents could be convenient to decrease TMAO production. Martin et al. demonstrated that Lactobacillus paracasei ingestion counteracts TMAO synthesis [74]. Moreover, Qui et al. studied the beneficial effect of Enterobacter aerogenes ZDY01 and Lactobacillus plantarum ZDY04 administration on TMAO levels [75,76]. Other authors obtained encouraging results promoting gut colonization with methanogenic archaea [77]. In addition, resveratrol [78] and berberine [79] nutraceutical supplementation appears able to modulate gut composition and decrease TMAO production. Moreover, a diet enriched with polyphenols ameliorates the metabolic syndrome that enhances gut eubiosis. In particular, Roopchand et al. studied the effect of concord grape polyphenols on a high-fat diet and observed that this nutraceutical intervention attenuated the production of inflammatory markers, including IL-6 and LPS. Moreover, grape polyphenols modify the Firmicutes/Bacteroidetes ratio, decreasing the growth of Firmicutes and increasing Akkermansia muciniphila bacteria [80].
The positive role of A. muciniphila on the metabolic syndrome and immune diseases has been well investigated in numerous animal models and human studies [81]. In obese mice, the administration of A. muciniphila decreases hepatic lipid accumulation and IL-6 expression, and ameliorates the altered Firmicutes/Bacteroidetes ratio [82]. Several studies have shown that inulin (an important soluble dietary fiber) or oligofructose consumption are involved in LPS reduction and the maintenance of the gut barrier integrity. Zhang et al. demonstrated that inulin decreases Desulfovibrio abundance and reduces LPS and IL-6 levels in mice models [83,84]. This inulin action was replicated in human clinical trials [85,86].
In addition, oligofructose consumption with/without inulin enrichment exerts a positive action on gut dysbiosis and inflammation. Parnell observed that 12 wk of oligofructose supplementation induces a significant plasma LPS reduction in overweight and obese adults [87]. In addition, oligofructose-enriched inulin treatment alleviates metabolic endotoxemia in diabetic women [88]. Oligofructose intake has been reported to increase the amount of Roseburia in the intestinal gut. Different studies demonstrated that Roseburia increased the differentiation of regulatory T cells and inhibited intestinal inflammation [59]. Moreover, probiotic and prebiotic food compounds can selectively modify the gut microbial community, enhancing the growth of health-promoting bacteria (ie, Lactobacillus spp., Bifidobacterium breve, and Bacteroides‐Prevotella spp.). Recently, Milajerdi et al. performed a meta-analysis and observed how probiotic supplementation significantly reduced several cytokines productions, including IL-6 [89]. In obese mice, Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 supplementation ameliorated the inflammatory state and gut dysbiosis, reducing Bacteroides and Desulfovibrio bacteria and increasing the Lactobacillus and Bifidobacterium population [90]. Supplementation with Bifidobacterium animalis subsp. lactis V9 inhibits the hepatic expression of TLR4 and NLRP3, reducing the secretion of inflammatory cytokines (IL-6, IL-1 beta, and TNF-α) [91].
A clinical trial performed by Ried et al. for 3 mo established that Kyolic-aged garlic extract has an important antihypertensive action, but above all decreases TNF-α and IL-6 and improved gut diversity, increasing the immune-stimulating bacteria Lactobacillus and Clostridia species [92]. Accumulated data confirm the beneficial effects of probiotic/prebiotic oral supplementation on inflammatory conditions. Many of these compounds are already marketed and personalized dietary regimens may be developed for patients who are most vulnerable to SARS-CoV-2 infection [93,94].
Conclusions
Overall, clinical data exist to show that patients with obesity, hypertension, diabetes, and general metabolic syndrome (i.e., frail patients) are at a higher risk for COVID-19 infection. As known, the interconnection between these pathologies and gut microbiota composition promotes an aberrant secretion of bacterial products, including TMAO and LPS. LPS binding to TRL4 induces a low-grade inflammation state. Data reported in the literature suggest that SARS-CoV-2 interacting with TRL activates NLRP3 inflammasome, which can also be activated by TMAO (Fig. 1), thereby worsening the host cellular proinflammatory microenvironment.
Pending the development of specific drugs or a vaccine, effective strategies to ensure good health for patients with metabolic syndrome must be identified. Modulation of the microbiome through diet or probiotic/prebiotic agents is well known to ameliorate an inflammation state. In this work, we reviewed some nutritional interventions, focusing on gut microbiota, aimed at improving low-grade inflammation conditions. Currently, the consumption of probiotic/prebiotic agents, polyphenols, or oligofructose/inulin counteracts gut dysbiosis and ameliorates the inflammation state. Future advances on the knowledge of COVID-19 molecular mechanisms would lead to an improved understanding of interactions between SARS-CoV-2 and gut microbiota. In perspective, after microbiota analyses, a novel personalized nutritional approach can be developed to restore a healthy gut microbiota and avoid SARS-CoV-2-induced exacerbated inflammation in frail patients.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Italian Ministry of Health-Ricerca Corrente-IRCCS Multimedica.
References
- 1.World Health Organization. Available at: https://www.who.int/.
- 2.Lake M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin Med (Lond) 2020;20:124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a, Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H., Zhou Y., Zhang M., Wang H., Zhao Q., Liu J. Updated approaches against SARS-CoV-2. Antimicrob Agents Chemother. 2020 doi: 10.1128/AAC.00483-20. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau H., Khosrawipour V., Kocbach P., Mikolajczyk A., Schubert J., Bania J., et al. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa037. taaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang A., Zhao W., Xu Z., Gu J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract. 2020;162 doi: 10.1016/j.diabres.2020.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: A systematic review. J Med Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., et al. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581:221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 11.Zhong Y., Kinio A., Saleh M. Functions of NOD-like receptors in human diseases. Front Immunol. 2013;4:333. doi: 10.3389/fimmu.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang P., Wang X. COVID-19: A new challenge for human beings. Cell Mol Immunol. 2020;17:555–557. doi: 10.1038/s41423-020-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi C.S., Nabar N.R., Huang N.N., Kehrl J.H. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siu K.L., Yuen K.S., Castaño-Rodriguez C., Ye Z.Y., Yeung M.L., Fung S.Y., et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019;33:8865–8877. doi: 10.1096/fj.201802418R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinon F., Burns K., Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen I., Miao E.A. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauernfeind F.G., Horvath G., Stutz A., Alnemri E.S., MacDonald K., Speert D., et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franchi L., Eigenbrod T., Muñoz-Planillo R., Ozkurede U., Kim Y.G., Arindam C., et al. Cytosolic double-stranded RNA activates the NLRP3 inflammasome via MAVS-induced membrane permeabilization and K+ efflux. J Immunol. 2014;193:4214–4222. doi: 10.4049/jimmunol.1400582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshino K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 20.Lai J.L., Liu Y.H., Liu C., Qi M.P., Liu R.N., Zhu X.F., et al. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation. 2017;40:1–12. doi: 10.1007/s10753-016-0447-7. [DOI] [PubMed] [Google Scholar]
- 21.Kuzmich N.N., Sivak K.V., Chubarev V.N., Porozov Y.B., Savateeva-Lyubimova T.N., Peri F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines (Basel) 2017;5:34. doi: 10.3390/vaccines5040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olejnik J., Hume A.J., Mühlberger E. Toll-like receptor 4 in acute viral infection: Too much of a good thing. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olejnik J., Forero A., Deflubé L.R., Hume A.J., Manhart W.A., Nishida A., et al. Ebolaviruses associated with differential pathogenicity induce distinct host responses in human macrophages. J Virol. 2017;91 doi: 10.1128/JVI.00179-17. e00179–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rallabhandi P., Phillips R.L., Boukhvalova M.S., Pletneva L.M., Shirey K.A., Gioannini T.L., et al. Respiratory syncytial virus fusion protein-induced toll-like receptor 4 (TLR4) signaling is inhibited by the TLR4 antagonists Rhodobacter sphaeroides lipopolysaccharide and eritoran (E5564) and requires direct interaction with MD-2. mBio. 2012;3 doi: 10.1128/mBio.00218-12. e00218–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Totura A.L., Whitmore A., Agnihothram S., Schäfer A., Katze M.G., Heise M.T., et al. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. mBio. 2015;6 doi: 10.1128/mBio.00638-15. e00638–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabeony H., Pohin M., Vasseur P., Petit-Paris I., Jégou J.F., Favot L., et al. IMQ-induced skin inflammation in mice is dependent on IL-1R1 and MyD88 signaling but independent of the NLRP3 inflammasome. Eur J Immunol. 2015;45:2847–2857. doi: 10.1002/eji.201445215. [DOI] [PubMed] [Google Scholar]
- 27.Shenderov K., Riteau N., Yip R., Mayer-Barber K.D., Oland S., Hieny S., et al. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1β in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J Immunol. 2014;192:2029–2033. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross O., Thomas C.J., Guarda G., Tschopp J. The inflammasome: An integrated view. Immunol Rev. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao M., Bai M., Ding G., Zhang Y., Huang S., Jia Z., et al. Angiotensin II stimulates the NLRP3 inflammasome to induce podocyte injury and mitochondrial dysfunction. Kidney Dis (Basel) 2018;4:83–94. doi: 10.1159/000488242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X.C., Zhang J., Zhuo J.L. The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol Res. 2017;125:21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero J.C., Reckelhoff J.F. State-of-the-art lecture. Role of angiotensin and oxidative stress in essential hypertension. Hypertension. 1999;34:943–949. doi: 10.1161/01.hyp.34.4.943. [DOI] [PubMed] [Google Scholar]
- 32.Olivares-Reyes J.A., Arellano-Plancarte A., Castillo-Hernandez J.R. Angiotensin II and the development of insulin resistance: Implications for diabetes. Mol Cell Endocrinol. 2009;302:128–139. doi: 10.1016/j.mce.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimura C., Oga T., Chin K., Takegami M., Takahashi K., Sumi K., et al. Relationships of decreased lung function with metabolic syndrome and obstructive sleep apnea in Japanese males. Intern Med. 2012;51:2291–2297. doi: 10.2169/internalmedicine.51.7427. [DOI] [PubMed] [Google Scholar]
- 34.Pinto Pereira L.M., Seemungal T.A., Teelucksingh S., Nayak B.S. Restrictive pulmonary deficit is associated with inflammation in sub-optimally controlled obese diabetics. J Thorac Dis. 2013;5:289–297. doi: 10.3978/j.issn.2072-1439.2012.07.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dikalov S.I., Nazarewicz R.R. Angiotensin II-induced production of mitochondrial reactive oxygen species: Potential mechanisms and relevance for cardiovascular disease. Antioxid Redox Signal. 2013;19:1085–1094. doi: 10.1089/ars.2012.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vieira L.D., Farias J.S., de Queiroz D.B., Cabral E.V., Lima-Filho M.M., Sant'Helena B.R.M., et al. Oxidative stress induced by prenatal LPS leads to endothelial dysfunction and renal haemodynamic changes through angiotensin II/NADPH oxidase pathway: Prevention by early treatment with α-tocopherol. Biochim Biophys Acta Mol Basis Dis. 2018;1864:3577–3587. doi: 10.1016/j.bbadis.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Hao X.Q., Zhang H.G., Yuan Z.B., Yang D.L., Hao L.Y., Li X.H. Prenatal exposure to lipopolysaccharide alters the intrarenal renin-angiotensin system and renal damage in offspring rats. Hypertens Res. 2010;33:76–82. doi: 10.1038/hr.2009.185. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L.L., Huang S., Ma X.X., Zhang W.Y., Wang D., Jin S.Y., et al. Angiotensin(1-7) attenuated angiotensin II-induced hepatocyte EMT by inhibiting NOX-derived H2O2-activated NLRP3 inflammasome/IL-1β/Smad circuit. Free Radic Biol Med. 2016;97:531–543. doi: 10.1016/j.freeradbiomed.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 39.Sun N.N., Yu C.H., Pan M.X., Zhang Y., Zheng B.J., Yang Q.J., et al. Mir-21 mediates the inhibitory effect of Ang (1-7) on AngII-induced NLRP3 inflammasome activation by targeting Spry1 in lung fibroblasts. Sci Rep. 2017;7:14369. doi: 10.1038/s41598-017-13305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao Y., Liu Y., Shang J., Yuan Z., Ping F., Yao S., et al. Ang-(1-7) treatment attenuates lipopolysaccharide-induced early pulmonary fibrosis. Lab Invest. 2019;99:1770–1783. doi: 10.1038/s41374-019-0289-7. [DOI] [PubMed] [Google Scholar]
- 41.Chen Q.F., Kuang X.D., Yuan Q.F., Hao H., Zhang T., Huang Y.H., et al. Lipoxin A4 attenuates LPS-induced acute lung injury via activation of the ACE2-Ang-(1-7)-Mas axis. Innate Immun. 2018;24:285–296. doi: 10.1177/1753425918785008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng H., Wang Y., Wang G.Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92:726–730. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: At present there is no evidence to abandon renin-angiotensin system blockers. Hypertension. 2020;75:1382–1385. doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Versmissen J., Verdonk K., Lafeber M., van den Akker J.P.C., Hunfeld N.G.M., Hoorn E.J., et al. Angiotensin-converting enzyme-2 in SARS-CoV-2 infection: Good or bad? J Hypertens. 2020;38:1196–1197. doi: 10.1097/HJH.0000000000002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallianou N.G., Stratigou T., Tsagarakis S. Microbiome and diabetes: Where are we now? Diabetes Res Clin Pract. 2018;146:111–118. doi: 10.1016/j.diabres.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Tang W.H., Kitai T., Hazen S.L. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim S., Jazwinski S.M. The gut microbiota and healthy aging: A mini-review. Gerontology. 2018;64:513–520. doi: 10.1159/000490615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spychala M.S., Venna V.R., Jandzinski M., Doran S.J., Durgan D.J., Ganesh P.B., et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. 2018;84:23–36. doi: 10.1002/ana.25250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad A., Yang W., Chen G., Shafiq M., Javed S., Zaidi S.S.A., et al. Analysis of gut microbiota of obese individuals with type 2 diabetes and healthy individuals. PLoS One. 2019;14 doi: 10.1371/journal.pone.0226372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang T., Santisteban M.M., Rodriguez V., Li E., Ahmari N., Carvajal J.M., et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feng Y., Huang Y., Wang Y., Wang P., Song H., Wang F. Antibiotics induced intestinal tight junction barrier dysfunction is associated with microbiota dysbiosis, activated NLRP3 inflammasome and autophagy. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anbazhagan A.N., Priyamvada S., Priyadarshini M. Gut microbiota in vascular disease: Therapeutic target? Curr Vasc Pharmacol. 2017;15:291–295. doi: 10.2174/1570161115666170105095834. [DOI] [PubMed] [Google Scholar]
- 53.Li Z., Wu Z., Yan J., Liu H., Liu Q., Denget Y. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest. 2019;99:346–357. doi: 10.1038/s41374-018-0091-y. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y., Dai M. Trimethylamine N-oxide generated by the gut microbiota is associated with vascular inflammation: New insights into atherosclerosis. Mediators Inflamm. 2020;2020 doi: 10.1155/2020/4634172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stubbs J.R., House J.A., Ocque A.J., Zhang S., Johnson C., Kimber C., et al. Serum trimethylamine-N-oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. 2016;27:305–313. doi: 10.1681/ASN.2014111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seldin M.M., Meng Y., Qi H., Zhu W.F., Wang Z., Hazen S.L., et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun X., Jiao X., Ma Y., Liu Y., Zhang L., He Y., et al. Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem Biophys Res Commun. 2016;481:63–70. doi: 10.1016/j.bbrc.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 58.Chen M.L., Zhu X.H., Ran L., Lang H.D., Yi L., Mi M.T. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fuke N., Nagata N., Suganuma H., Ota T. Regulation of gut microbiota and metabolic endotoxemia with dietary factors. Nutrients. 2019;11:2277. doi: 10.3390/nu11102277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang H., Hussey S.E., Sanchez-Avila A., Tantiwong P., Musi N. Effect of lipopolysaccharide on inflammation and insulin action in human muscle. PLoS One. 2013;8:e63983. doi: 10.1371/journal.pone.0063983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vandanmagsar B., Youm Y.H., Ravussin A., Galgani J.E., Stadler K., Mynatt R.L., et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong P., Gu R.N., Li F.X., Xiong X.X., Liang W.B., You Z.J., et al. NLRP3 inflammasome as a potential treatment in ischemic stroke concomitant with diabetes. J Neuroinflammation. 2019;16:121. doi: 10.1186/s12974-019-1498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nova Z., Skovierova H., Calkovska A. Alveolar-capillary membrane-related pulmonary cells as a target in endotoxin-induced acute lung injury. Int J Mol Sci. 2019;20:831. doi: 10.3390/ijms20040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voiriot G., Razazi K., Amsellem V., Tran Van Nhieu J., Abid S., Adnot S., et al. Interleukin-6 displays lung anti-inflammatory properties and exerts protective hemodynamic effects in a double-hit murine acute lung injury. Respir Res. 2017;18:64. doi: 10.1186/s12931-017-0553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen L., Liu H.G., Liu W., Liu J., Liu K., Shang J., et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E005. doi: 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 66.Agenzia Italiana del Farmaco. https://www.aifa.gov.it
- 67.U.S. Food and Drug Administration. Available at: https://www.fda.gov/.
- 68.Ichinohe T., Pang I.K., Kumamoto Y., Peaper D.R., Ho J.H., Murray T.S., et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci U S A. 2011;108:5354–5359. doi: 10.1073/pnas.1019378108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abt M.C., Osborne L.C., Monticelli L.A., Doering T.A., Alenghat T., Sonnenberg G.F., et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Janeiro M.H., Ramírez M.J., Milagro F.I., Martínez J.A., Solas M. Implication of trimethylamine N-oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients. 2018;10:1398. doi: 10.3390/nu10101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Montesano A., Senesi P., Luzi L., Benedini S., Terruzzi I. Potential therapeutic role of L-carnitine in skeletal muscle oxidative stress and atrophy conditions. Oxid Med Cell Longev. 2015;2015 doi: 10.1155/2015/646171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Terruzzi I., Montesano A., Senesi P., Villa I., Ferraretto A., Bottani M., et al. L-Carnitine reduces oxidative stress and promotes cells differentiation and bone matrix proteins expression in human osteoblast-like cells. Biomed Res Int. 2019;2019 doi: 10.1155/2019/5678548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vacante F., Senesi P., Montesano A., Frigerio A., Luzi L., Terruzzi I. L-carnitine: An antioxidant remedy for the survival of cardiomyocytes under hyperglycemic condition. J Diabetes Res. 2018;2018 doi: 10.1155/2018/4028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin F.P., Wang Y., Sprenger N., Yap I.K., Lundstedt T., Lek P., et al. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol. 2008;4:157. doi: 10.1038/msb4100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu L., Yang D., Tao X., Yu J., Xiong H., Wei H. Enterobacter aerogenes ZDY01 attenuates choline-induced trimethylamine N-oxide levels by remodeling gut microbiota in mice. J Microbiol Biotechnol. 2017;27:1491–1499. doi: 10.4014/jmb.1703.03039. [DOI] [PubMed] [Google Scholar]
- 76.Qiu L., Tao X., Xiong H., Yu J., Wei H. Lactobacillus plantarum ZDY04 exhibits a strain-specific property of lowering TMAO via the modulation of gut microbiota in mice. Food Funct. 2018;9:4299–4309. doi: 10.1039/c8fo00349a. [DOI] [PubMed] [Google Scholar]
- 77.Ramezani A., Nolin T.D., Barrows I.R., Serrano M.G., Buck G.A., Regunathan-Shenk R., et al. Gut colonization with methanogenic archaea lowers plasma trimethylamine N-oxide concentrations in apolipoprotein e-/- mice. Sci Rep. 2018;8:14752. doi: 10.1038/s41598-018-33018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen M.L., Yi L., Zhang Y., Zhou X., Ran L., Yang J., et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio. 2016;7 doi: 10.1128/mBio.02210-15. e02210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi Y., Hu J., Geng J., Hu T., Wang B., Yan W., et al. Berberine treatment reduces atherosclerosis by mediating gut microbiota in apoE-/- mice. Biomed Pharmacother. 2018;107:1556–1563. doi: 10.1016/j.biopha.2018.08.148. [DOI] [PubMed] [Google Scholar]
- 80.Roopchand D.E., Carmody R.N., Kuhn P., Moskal K., Rojas-Silva P., Turnbaugh P.J., et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes. 2015;64:2847–2858. doi: 10.2337/db14-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Y., Wang N., Tan H.Y., Li S., Zhang C., Feng Y. Function of Akkermansia muciniphila in Obesity: Interactions with lipid metabolism, immune response and gut systems. Front Microbiol. 2020;11:219. doi: 10.3389/fmicb.2020.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim S., Lee Y., Kim Y., Seo Y., Lee H., Ha J., et al. Akkermansia muciniphila prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Appl Environ Microbiol. 2020;86:e03004–e03019. doi: 10.1128/AEM.03004-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Q., Yu H., Xiao X., Hu L., Xin F., Yu X. Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. Peer J. 2018;6:e4446. doi: 10.7717/peerj.4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li K., Zhang L., Xue J., Yang X., Dong X., Sha L., et al. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019;10:1915–1927. doi: 10.1039/c8fo02265h. [DOI] [PubMed] [Google Scholar]
- 85.Costa G., Vasconcelos Q., Abreu G., Albuquerque A., Vilarejo J., Aragão G. Changes in nutrient absorption in children and adolescents caused by fructans, especially fructooligosaccharides and inulin. Arch Pediatr. 2020;27:166–169. doi: 10.1016/j.arcped.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 86.Nogacka A.M., Salazar N., Arboleya S., Ruas-Madiedo P., Mancabelli L., Suarez A., et al. In vitro evaluation of different prebiotics on the modulation of gut microbiota composition and function in morbid obese and normal-weight subjects. Int J Mol Sci. 2020;21:906. doi: 10.3390/ijms21030906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parnell J.A., Klancic T., Reimer R.A. Oligofructose decreases serum lipopolysaccharide and plasminogen activator inhibitor-1 in adults with overweight/obesity. Obesity (Silver Spring) 2017;25:510–513. doi: 10.1002/oby.21763. [DOI] [PubMed] [Google Scholar]
- 88.Dehghan P., Pourghassem Gargari B., Asghari Jafar-abadi M. Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized controlled clinical trial. Nutrition. 2014;30:418–423. doi: 10.1016/j.nut.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Milajerdi A., Mousavi S.M., Sadeghi A., Salari-Moghaddam A., Parohan M., Larijani B., et al. The effect of probiotics on inflammatory biomarkers: A meta-analysis of randomized clinical trials. Eur J Nutr. 2020;59:633–649. doi: 10.1007/s00394-019-01931-8. [DOI] [PubMed] [Google Scholar]
- 90.Wang T., Yan H., Lu Y., Li X., Wang X., Shan Y., et al. Anti-obesity effect of Lactobacillus rhamnosus LS-8 and Lactobacillus crustorum MN047 on high-fat and high-fructose diet mice base on inflammatory response alleviation and gut microbiota regulation. Eur J Nutr. 2020;59:2709–2728. doi: 10.1007/s00394-019-02117-y. [DOI] [PubMed] [Google Scholar]
- 91.Yan Y., Liu C., Zhao S., Wang X., Wang J., Zhang H., et al. Probiotic Bifidobacterium lactis V9 attenuates hepatic steatosis and inflammation in rats with non-alcoholic fatty liver disease. AMB Express. 2020;10:101. doi: 10.1186/s13568-020-01038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ried K., Travica N., Sali A. The effect of kyolic aged garlic extract on gut microbiota, inflammation, and cardiovascular markers in hypertensives: The GarGIC trial. Front Nutr. 2018;5:122. doi: 10.3389/fnut.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Di Renzo L., Merra G., Esposito E., De Lorenzo A. Are probiotics effective adjuvant therapeutic choice in patients with COVID-19? Eur Rev Med Pharmacol Sci. 2020;24:4062–4063. doi: 10.26355/eurrev_202004_20977. [DOI] [PubMed] [Google Scholar]
- 94.Dhar D., Mohanty A. Gut microbiota and COVID-19- Possible link and implications. Virus Res. 2020;285 doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]