Abstract
Background
Acute encephalopathy with COVID-19 has been reported in several studies but its impact on outcomes remains unclear. We hypothesized that hospitalized COVID-19 patients with encephalopathy have worse COVID-19 related outcomes.
Methods
We used TriNetX, with a large COVID-19 database, collecting real-time electronic medical records data. We included hospitalized COVID-19 patients since January 20, 2020 who had encephalopathy based on ICD-10 coding. We examined clinical outcomes comprising need for critical care services, intubation and mortality among these patients and compared it with patients without encephalopathy before and after propensity-score matching.
Results
Of 12,601 hospitalized COVID-19 patients, 1092 (8.7%) developed acute encephalopathy. Patients in the acute encephalopathy group were older (67 vs. 61 years) and had higher prevalence of medical co-morbidities including obesity, hypertension, diabetes, heart disease, COPD, chronic kidney and liver disease among others. Before and after propensity score-matching for co-morbidities, patients with acute encephalopathy were more likely to need critical care services (35.6% vs. 16.9%, p < 0.0001), intubation (19.5% vs. 6.0%, p < 0.0001) and had higher 30-day mortality (24.3% vs. 17.9%, p 0.0002).
Conclusion
Among hospitalized COVID-19 patients, acute encephalopathy is common and more likely to occur in patients with medical co-morbidities and are more likely to need critical care, intubation and have higher 30-day mortality even after adjusting for age and underlying medical co-morbidities.
Keywords: COVID-19, Encephalopathy, Outcomes
Highlights
-
•
Acute encephalopathy is common in COVID-19 patients.
-
•
Acute encephalopathy is more common in COVID-19 patients with co-morbidities.
-
•
Acute encephalopathy is associated with worse outcomes in COVID-19 patients.
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) causing the ongoing Coronavirus Disease 2019 (COVID-19) pandemic, has led to >11 million infections and >525,000 deaths worldwide. Studies have described neurological manifestations with COVID-19 ranging from mild symptoms such as headache, anosmia, ageusia, dizziness to more severe syndromes including acute encephalopathy, encephalitis, cerebrovascular disease, Guillian-Barré syndrome, myopathy among others (Varatharaj et al., 2020; Nalleballe et al., 2020; Helms et al., 2020; Jasti et al., 2020; Onteddu et al., 2020). Acute encephalopathy, a broad terminology used to describe altered sensorium and central nervous system (CNS) dysfunction, may be an indirect consequence of systemic/metabolic dysfunction or due to CNS involvement in the form of acute ischemic, hemorrhagic strokes, encephalitis, vasculitis among others (Varatharaj et al., 2020). Despite building evidence, there are no studies describing outcomes in acutely encephalopathic COVID-19 patients. We aimed to determine prevalence of acute encephalopathy in hospitalized COVID-19 patients and hypothesized that COVID-19 patients with acute encephalopathy are likely to have worse COVID-19 related outcomes.
2. Materials and methods
2.1. Data source
De-identified patient information for COVID-19 patients with and without acute encephalopathy were extracted using TriNetX, a global health collaborative clinical research platform collecting real-time electronic medical record data from a network of health care organizations across U.S.A. and some outside US territories. “COVID-19 Research Network” in TriNetX represents a large global COVID-19 database.
Queries were made through TriNetX using browser and real-time features. While, TriNetX does not allow data downloads, or individual patient data for review, it allows analysis in the form of queries. At University of Arkansas for Medical Sciences the data from TriNetX is managed by the Arkansas Clinical Data Repository and maintained by the Department of Biomedical Informatics.
2.2. Study protocol
Appropriate approval was obtained from the Institutional Review Board (IRB). The analysis was run on June 29th 2020 on TriNetX COVID-19 Research Network. Patients ≥18 years of age with COVID-19, hospitalized on or after January 20th 2020 (when the first case of COVID-19 was reported in the U.S.) were identified and among them patients with acute encephalopathy were identified using ICD-10 codes (Supplement for ICD codes). Then, baseline demographics, co-morbidities and clinical outcomes, including need for critical care services, intubation and mortality within 30 days from COVID-19 diagnosis were compared in patients with and without encephalopathy. 1:1 propensity-score matching was done for baseline characteristics and comorbidities between the two groups and outcomes were compared. Statistical analysis was performed through TriNetX analytics function. Descriptive statistics were reported as number of observations and percentage or mean ± standard deviation as applicable. These queries were performed independently by two physicians.
3. Results
In the TriNetX database, we identified 12,601 patients with COVID-19 that were hospitalized between January 20 and June 29, 2020. Among them, 5990 (47.5%) patients were female, 4232 (33.6%) patients were Caucasian, 2922 (23.2%) African-Americans, 775 (6.2%) Hispanic, 155 (1.23%) Asian and 4517 (35.8%) were other races/ethnicities/unknown. 1092 (8.7%) developed acute encephalopathy and 11,509 (91.3%) did not. Baseline characteristics, gender, race, comorbidities in the encephalopathy versus non-encephalopathy groups are detailed in Table 1.
Table 1.
Characteristics of patients hospitalized with COVID-19. Baseline demographics and comorbidities of Corona virus disease 2019 (COVID-19) hospitalized patients with and without encephalopathy. [NA: not applicable, ∗COPD: chronic obstructive pulmonary disease].
| Variable | With encephalopathy | Without encephalopathy | p-value |
|---|---|---|---|
| Number of patients | 1092 | 11,509 | NA |
| Age (years) | 67.2 ± 14.9 | 61 ± 18.1 | <0.0001 |
| Females | 488 (44.7%) | 5,502 (47.8%) | 0.048 |
| Race/Ethnicity | |||
| White | 519 (47.5%) | 3,713 (32.3%) | <0.0001 |
| African American | 384 (35.2%) | 2,538 (22.1%) | <0.0001 |
| Asian | 20 (1.8%) | 135 (1.2%) | 0.059 |
| Hispanic (ethnicity) | 116 (10.6%) | 659 (5.7%) | <0.0001 |
| Co-morbidities | |||
| Hypertension | 674 (61.7%) | 4,386 (38.1%) | <0.0001 |
| Diabetes mellitus | 437 (40.0%) | 2,446 (21.3%) | <0.0001 |
| Ischemic heart disease | 324 (29.7%) | 1,792 (15.6%) | <0.0001 |
| Chronic kidney disease | 312 (28.6%) | 1,537 (13.4%) | <0.0001 |
| Heart failure | 287 (26.3%) | 1,472 (12.8%) | <0.0001 |
| Atrial fibrillation & flutter | 218 (19.9%) | 1,155 (10.0%) | <0.0001 |
| COPD∗ | 164 (15.0%) | 1,040 (9.0%) | <0.0001 |
| Asthma | 101 (9.2%) | 915 (7.9%) | 0.13 |
| Ischemic stroke | 129 (11.8%) | 527 (4.6%) | <0.0001 |
| Liver Disease | 134 (12.3%) | 901 (7.8%) | <0.0001 |
| Overweight & obesity | 302 (27.7%) | 1,984 (17.2%) | <0.0001 |
| Nicotine dependence | 118 (10.8%) | 963 (8.4%) | 0.006 |
Among the two groups, patients with encephalopathy were older (67.2 vs 61.0 years; p < 0.0001), had significantly higher prevalence of co-morbidities including hypertension, diabetes mellitus, ischemic heart disease, chronic kidney disease, heart failure, atrial fibrillation, COPD, prior ischemic stroke, liver disease, obesity (p < 0.0001) (Table 1). In the encephalopathy group, overall 22.6% patients had a concomitant acute neurologic illness, with 11.7% with acute stroke (ischemic and hemorrhagic), 13.2% had seizure and 0.8% had encephalitis. When comparing outcomes, unmatched analysis showed statistically significant higher need for critical care services (35.5% vs 10.0%, p < 0.0001), intubation (19.5% vs 4.5%, p < 0.0001) and higher 30-day mortality (24.3% vs 13.9%, p < 0.0001) in the encephalopathy group (Table 2 & Fig. 1). After 1:1 propensity-score matching for demographics and all above listed comorbidities, each group had 1090 patients. The need for critical care services (35.6% vs. 16.9%, p < 0.0001), intubation (19.5% vs. 6%) and mortality (24.3% vs. 17.9%, p = 0.0002) remained significantly higher in the acute encephalopathy group (Table 2 & Fig. 1).
Table 2.
Comparison of outcomes in COVID-19 patients with and without encephalopathy. Baseline demographics and clinical outcome of Corona virus disease 2019 (COVID-19) hospitalized patients with and without encephalopathy. [NA: Not applicable].
| Unmatched analysis | |||
|---|---|---|---|
| Encephalopathy | Without encephalopathy | p-value | |
| Number of patients | 1092 | 11,509 | NA |
| Age (years) | 67.2 ± 14.9 | 61 ± 18.1 | <0.0001 |
| Female | 488 (44.7%) | 5,502 (47.8%) | 0.048 |
| Clinical outcome | |||
| Critical care services | 388 (35.5%) | 1,154 (10.0%) | <0.0001 |
| Intubation | 213 (19.5%) | 518 (4.5%) | <0.0001 |
| Death | 265 (24.3%) | 1,607 (13.9%) | <0.0001 |
| Propensity matched analysis | |||
| Encephalopathy | Without encephalopathy | p-value | |
| Number of patients | 1090 | 1090 | NA |
| Age (years) | 67.2 ± 14.9 | 67.7 ± 15.2 | 0.44 |
| Female | 488 (44.8%) | 476 (43.7%) | 0.60 |
| Clinical outcome | |||
| Critical care services | 388 (35.6%) | 184 (16.9%) | <0.0001 |
| Intubation | 213 (19.5%) | 65 (6.0%) | <0.0001 |
| Death | 265 (24.3%) | 195 (17.9%) | 0.0002 |
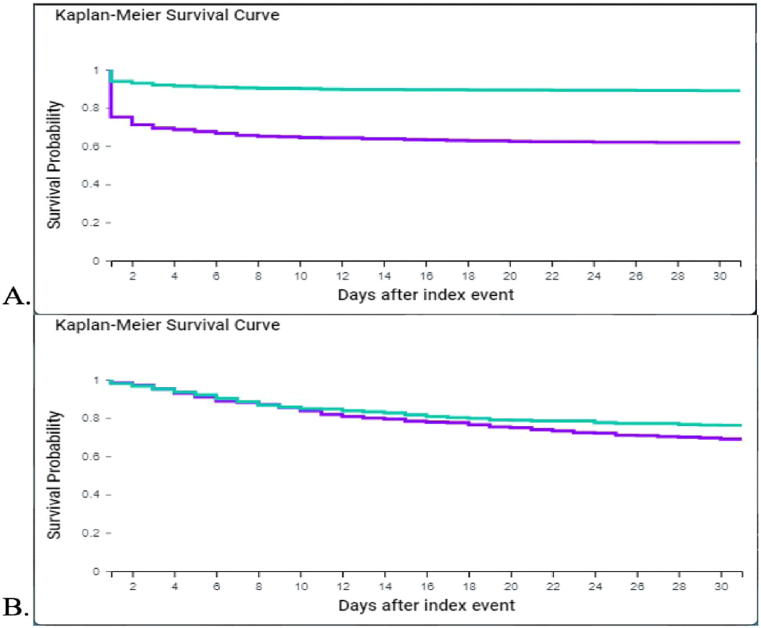
Fig. 1.
Kaplan-Meier Survival Curve for COVID-19 patients with and without encephalopathy 1.A.: unmatched analysis; 1.B.: 1:1 propensity score match analysis
 = with encephalopathy;
= with encephalopathy;  = no encephalopathy].
= no encephalopathy].
4. Discussion
Acute encephalopathy is a common neurologic syndrome seen in COVID-19 patients (Varatharaj et al., 2020; Nalleballe et al., 2020; Jasti et al., 2020; Berger, 2020). An Italian study (Helms et al., 2020) of COVID-19 patients with neurologic symptoms demonstrated that 69% had agitation, 65% had confusion on the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) scales and 36% had dysexecutive syndrome, all signs of acute encephalopathy. In another similar U.K. study (Varatharaj et al., 2020), 31% patients presented with altered mental status, 23% had unspecified encephalopathy and 18% had encephalitis. Above studies describe prevalence of acute encephalopathy amongst COVID-19 patients with neurologic symptoms. One study showed that 2.3% of hospitalized and non-hospitalized COVID-19 patients had acute encephalopathy (Nalleballe et al., 2020). However, no prior study has evaluated the overall prevalence of acute encephalopathy in COVID-19 hospitalized patients which are likely patients with severe COVID-19 infection and more likely to develop complications and encephalopathy. We found that 8.7% of 12,601 hospitalized COVID-19 patients developed acute encephalopathy. Of these 22.6% had an acute primary neurologic illness such as stroke, seizure or encephalitis.
Acute encephalopathy may be a direct consequence of underlying cytokine storm, severe systemic and metabolic dysfunction (Berger, 2020), which is more likely to occur in patients who have severe COVID-19 infection, which is in turn more common in patients with underlying medical co-morbidities as shown in our study.
Outcomes in COVID-19 patients with acute encephalopathy have not been described. We found that need for critical care services, intubation and 30-day mortality were significantly higher in patients who had acute encephalopathy and this difference persisted even after propensity-score matching for age, demographics and medical co-morbidities.
The mechanism of acute encephalopathy in COVID-19 patients and this difference in outcomes in encephalopathic patients remains to be determined. There are several plausible hypotheses. Higher mortality in acutely encephalopathic COVID-19 patients may likely be due to severe underlying infection and consequent systemic and metabolic dysfunction including hypoxia, however, given that severity of COVID-19 infection increases with age and underlying co-morbidities, in our study, even after propensity-score matching for these factors, patients with acute encephalopathy continued to demonstrate higher mortality. Acute encephalopathy is a known contributor to increased mortality in patients with underlying sepsis (Sprung et al., 1990) and this may apply to COVID-19 as well. In addition, higher prevalence of strokes in COVID-19 patients (Merkler et al., 2020) may contribute to acute encephalopathy and higher mortality. While other coronaviruses, such as SARS-CoV-1, have been shown to invade the CNS causing neuronal death in animal models (Netland et al., 2008), this has not been demonstrated with the SARS-CoV-2 virus yet. Moreover, neuropathological studies have shown that the predominant form of neurologic injury in COVID-19 is acute hypoxic injury to neurons in the cerebral cortex, hippocampus and Purkinje cell layer of the cerebellum with rare signs of focal perivascular lymphocytic infiltration (Solomon et al., 2020). In a retrospective study of 64 COVID-19 patients, the most common neuroimaging findings included ischemic stroke in 27%, leptomeningeal enhancement in 17% and encephalitis-like changes in 13% (Kremer et al., 2020).
There are several limitations in our study. First, our study is retrospective and the data used for this analysis is based on ICD-10 coding which is subject to reporting bias. Second, due to the same reason, we are unable to differentiate the etiology of encephalopathy, the differences in treatments between the two groups and are unable to determine predictors for acute encephalopathy in COVID-19. We are unable to determine if the worse outcomes are secondary to severe underlying infection versus a direct effect of encephalopathy leading to need for critical care admission, intubation and death.
5. Conclusion
Acute encephalopathy can occur in 8.7% patients hospitalized after COVID-19 infection and encephalopathic patients are more likely to have underlying medical co-morbidities. Hospitalized COVID-19 patients with acute encephalopathy are more likely to need critical care, intubation and have higher 30-day mortality even after matching for age and co-morbidities as surrogates for severity of infection. Further prospective studies are needed to confirm our findings and determine any specific etiologies, predictors of acute encephalopathy, which could help identify potential therapeutic targets.
Details page
-
1.
I confirm that the manuscript complies with instructions to authors
-
2.
I confirm that authorship requirements have been met and the final manuscript was approved by all authors.
-
3.
I confirm that this manuscript has not been published elsewhere and is not under consideration by another journal.
-
4.
I confirm adherence to ethical guidelines. Appropriate IRB approval was obtained prior to study and given data was de-identified study was provided an exempt status by local IRB.
-
5.
None of the authors have any relevant conflicts of interests and have nothing to disclose.
-
6.
The study required no funding.
Declaration of competing interest
-
1.
I confirm that the manuscript complies with instructions to authors
-
2.
I confirm that authorship requirements have been met and the final manuscript was approved by all authors.
-
3.
I confirm that this manuscript has not been published elsewhere and is not under consideration by another journal.
-
4.
I confirm adherence to ethical guidelines. Appropriate IRB approval was obtained prior to study and given data was de-identified study was provided an exempt status by local IRB.
-
5.
Vishank Arun Shah, MD has no relevant conflicts of interests and has nothing to disclose.
-
6.
Krishna Nalleballe, MD has no relevant conflicts of interest and has nothing to disclose.
-
7.
Mhd Ezzat Zaghlouleh, MD has no relevant conflicts of interest and has nothing to disclose.
-
8.
Sanjeeva Reddy Onteddu, MD has no relevant conflicts of interest and has nothing to disclose.
-
6.
The study required no funding.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2020.100136.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Berger J.R. COVID-19 and the nervous system. J. Neurovirol. 2020;26(2):143–148. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H. Neurologic features in severe SARS-CoV-2 infection. N. Engl. J. Med. 2020;382(23):2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti M., Nalleballe K., Dandu V., Onteddu S. A review of pathophysiology and neuropsychiatric manifestations of COVID-19. J. Neurol. 2020 doi: 10.1007/s00415-020-09950-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer S., Lersy F., Anheim M. Neurologic and neuroimaging findings in COVID-19 patients: A retrospective multicenter study. Neurology. 2020 doi: 10.1212/WNL.0000000000010112. [DOI] [PubMed] [Google Scholar]
- Merkler A.E., Parikh N.S., Mir S. Risk of ischemic stroke in patients with covid-19 versus patients with influenza. medRxiv. 2020 doi: 2020.05.18.20105494. [Google Scholar]
- Nalleballe K., Reddy Onteddu S., Sharma R. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.06.020. doi: S0889-1591(20)31008-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onteddu S.R., Nalleballe K.A., Department of Neurology, University of Arkansas for Medical Sciences. Sharma R., Brown A.T. Underutilization of health care for strokes during the COVID-19 outbreak. Int. J. Stroke. 2020 doi: 10.1177/1747493020934362. 1747493020934362. [DOI] [PubMed] [Google Scholar]
- Solomon I.H., Normandin E., Bhattacharyya S. Neuropathological features of covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprung C.L., Peduzzi P.N., Shatney C.H. Impact of encephalopathy on mortality in the sepsis syndrome. the veterans administration systemic sepsis cooperative study group. Crit. Care Med. 1990;18(8):801–806. doi: 10.1097/00003246-199008000-00001. [DOI] [PubMed] [Google Scholar]
- Varatharaj A., Thomas N., Ellul M.A. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30287-X. doi: S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.