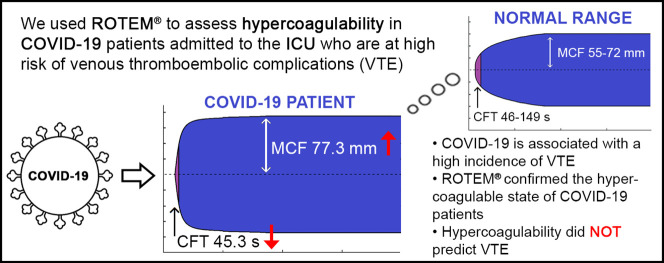
Graphical abstract
ROTEM® readout of EXTEM variables in healthy patients and COVID-19 patients.
Highlights
-
•
COVID-19 is associated with a high incidence of thromboembolic complications.
-
•
ROTEM® analysis confirmed the hypercoagulable state of COVID-19 patients admitted to the ICU.
-
•
ROTEM® had no additional value to identify COVID-19 patients at risk of developing thromboembolic complications.
Coronavirus disease 2019 (COVID-19) has spread rapidly around the world, affecting more than 23 million people so far [1]. Besides typical respiratory symptoms, COVID-19 is associated with coagulation abnormalities leading to thromboembolic complications [2]. In COVID-19 patients admitted to the Intensive Care Unit (ICU), the incidence of thromboembolic complications is approximately 31–49% [3,4].
Monitoring of standard coagulation variables in COVID-19 is recommended due to the associated coagulopathy. However, conventional coagulation tests reflect limited parts of the coagulation cascade. Currently, no test reliably identifies which COVID-19 patients are at the highest risk of developing thromboembolic complications.
Rotational thromboelastometry (ROTEM®) is a point-of-care device that evaluates viscoelastic changes during coagulation and enables identification of hypercoagulability [5]. Hence, ROTEM® profiles may be useful to identify COVID-19 patients at risk of developing thrombosis.
In this single-center retrospective observational study, we aimed to evaluate ROTEM® profiles of COVID-19 patients admitted to the ICU and to compare the ROTEM® profiles of COVID-19 patients with and without thromboembolic complications.
Patients diagnosed with COVID-19 admitted to the ICU in April 2020 were enrolled. COVID-19 was diagnosed according to World Health Organization (WHO) definition and was confirmed by RNA detection of the SARS-CoV-2 using the polymerase chain reaction (PCR)-based technique. Patients received thromboprophylaxis with low molecular weight heparin (LMWH) according to local protocol (at time of study, nadroparin 2850E od). Patient characteristics, clinical data, and outcomes were collected. Coagulation variables were collected as part of standard clinical care, including prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen, and platelet count.
Blood samples were collected and ROTEM® was carried out using a coagulation analyzer (ROTEM® Sigma; Werfen, The Netherlands). Three and a half milliliter of citrated blood was collected for each COVID-19 patient and processed within 6 h.
Coagulation patterns were analyzed using extrinsic and intrinsic activators (EXTEM and INTEM). The influence of fibrinogen on clot firmness was analyzed using the platelet inactivating FIBTEM test. All samples were run for at least 30 min. The following variables were recorded and analyzed: clotting time (CT), clotting formation time (CFT), clot firmness' amplitude after 5 min (A5) and after 10 min (A10), and maximum clot firmness (MCF). Hypercoagulability was defined as a reduction in CFT and an increase in MCF compared to reference values from healthy patients [5]. All normal values are displayed in the tables.
Patients who were clinically suspected of having a thromboembolic complication, underwent additional imaging during ICU admission. A thromboembolic complication was defined as a pulmonary embolism (PE) or deep venous thrombosis (DVT; either catheter-related or not). The decision for imaging was at the discretion of the attending intensivist.
Data are reported as mean with standard deviation (SD), median with interquartile range (IQR), or number with percentage. Student's t-test, Mann-Whitney U test, or the Chi-square test were used as appropriate. A p-value of <0.05 was considered statistically significant. SPSS Statistics version 23.0 for Windows was used for statistical analyses.
Forty-seven patients were included, of which 81% were male. The median age was 63 years (range 29–79), and the median BMI was 28.8 kg/m2 (range 24.4–48.4). Median length of stay at the ICU at the time of blood sampling for ROTEM® was 8 days (range 1–25). All patients required mechanical ventilation. None of the patients was diagnosed with a thromboembolic complication at the time of study. Development of thromboembolic complications was followed until death or discharge. Demographic and clinical data are shown in Table 1 .
Table 1.
Baseline characteristics of COVID-19 patients (n = 47).
| Age, years - median, IQR | 63 (29–79) |
| Sex - n (%) | |
| Male | 38 (81) |
| Female | 11 (19) |
| BMI, kg/m2 - median, IQR | 28.8 (24.4–48.4) |
| Mechanically ventilated - n (%) | 47 (100) |
| APACHE IV score - median, IQR | 63 (15–108) |
| SAPS II score - median, IQR | 42 (17–70) |
| CCI score - median, IQR | 3.6 (0−12) |
| Length of ICU admission at time of the study, days - median, IQR | 8 (1–25) |
| Total length of ICU stay, days - median, IQR | 18 (7–47) |
| Anticoagulant - n (%) | |
| Prophylactic LMWH | 41 (87%) |
| Therapeutic LMWH | 2 (4%) |
| Unfractionated heparin | 4 (9%) |
| CT - n (%) | |
| CT | 17 (36%) |
| No CT | 30 (64%) |
| Thromboembolic complication - n (%) | |
| Thromboembolic complication | 10 (21%) |
| No thromboembolic complication | 37 (79%) |
| ICU outcome - n (%) | |
| Alive to ward and home | 40 (85%) |
| Death | 7 (15%) |
Abbreviations: BMI = Body Mass Index; APACHE IV = Acute Physiology And Chronic Health Evaluation; SAPS = Simplified Acute Physiology Score; CCI = Charlson Comorbidity Index; LMWH = low molecular weight heparin; ICU = Intensive Care Unit; CT = Computed Tomography.
The ROTEM® variables are reported in Table 2 . CFT was decreased in EXTEM (45.3 s, SD 10.0 s; normal 46–149 s) and INTEM (45.6 s, SD 12.8 s; normal 62–130 s). MCF was increased in EXTEM (77.3 mm, SD 4.1 mm; normal 55–72 mm), INTEM (72.9 mm, SD 5.0 mm; normal 51–69 mm), and FIBTEM (32.9 mm, SD 6.4 mm; normal 6–21 mm). CT was slightly prolonged in EXTEM (85.5 s, SD 20.6 s; normal 53–83 s), but fell within normal ranges in INTEM (178 s, SD 25 s; normal 168–212 s).
Table 2.
ROTEM® variables and conventional coagulation variables in COVID-19 patients with and without thromboembolic complications.
| ROTEM® parameter | Normal range | All COVID-19 patients (n = 47), mean (SD) | Thromboembolic complications (n = 10), mean (SD) | No thromboembolic complications (n = 37), mean (SD) |
P-value |
|---|---|---|---|---|---|
| EXTEM | |||||
| CT, s | 53–83 | 85.5 (20.6) | 95.7 (17.4) | 82.8 (20.8) | 0.08 |
| CFT, s | 46–149 | 45.3 (10.0) | 54.1 (8.4) | 42.9 (9.2) | 0.001 |
| A5, mm | 32–52 | 63.1 (6.4) | 58.5 (7.8) | 64.3 (5.4) | 0.01 |
| A10, mm | 43–63 | 71.5 (5.3) | 68.0 (7.2) | 72.4 (4.4) | 0.02 |
| MCF, mm | 55–72 | 77.3 (4.1) | 75.0 (5.9) | 77.9 (3.3) | 0.05 |
| INTEM | |||||
| CT, s | 168–212 | 177.6 (25.0) | 184.5 (18.5) | 175.7 (26.3) | 0.30 |
| CFT, s | 62–130 | 45.6 (12.8) | 53.3 (15.9) | 43.5 (11.2) | 0.03 |
| A5, mm | 33–52 | 55.8 (7.4) | 51.4 (8.4) | 57.0 (6.7) | 0.03 |
| A10, mm | 43–62 | 65.3 (6.4) | 61.6 (7.5) | 66.2 (5.8) | 0.04 |
| MCF, mm | 51–69 | 72.9 (5.0) | 70.5 (6.1) | 73.5 (4.5) | 0.09 |
| FIBTEM | |||||
| MCF, mm | 6–21 | 32.9 (6.4) | 31.1 (8.3) | 33.3 (5.8) | 0.33 |
| Conventional coagulation variables | |||||
| Platelet count, 109/L | 150–400 | 404 (154) | 350 (208) | 419 (135) | 0.21 |
| PT, s | 10.2–13.3 | 15.3 (2.0) | 14.9 (2.1) | 15.4 (1.9) | 0.47 |
| APTT, s | 25–36 | 34.7 (8.7) | 32.6 (3.4) | 35.3 (9.6) | 0.40 |
| Fibrinogen, g/L | 2.0–3.9 | 7.2 (1.6) | 7.1 (1.9) | 7.3 (1,5) | 0.76 |
Abbreviations: ROTEM® = rotational thromboelastometry; INTEM = intrinsic rotational thromboelastometry; EXTEM = extrinsic rotational thromboelastometry; FIBTEM = fibrinogen rotational thromboelastometry; CT = clotting time; CFT = clot formation time; A5 = amplitude after 5 min; A10 = amplitude after 10 min; MCF = maximum clot firmness; PT = prothrombin time; APTT = activated partial thromboplastin time.
Conventional coagulation analyses showed increased fibrinogen (mean 7.2 g/L, SD 1.5 g/L), prolonged PT (15.3 s, SD 2.0 s), and prolonged APTT (34.7 s, SD 8.7 s). Mean platelet count was slightly increased (404 109/L, SD 154 109/L). The coagulation variables are displayed in Table 2.
Ten patients (21.3%) developed thromboembolic complications during ICU admission, all pulmonary embolisms. MCF in EXTEM was lower in COVID-19 patients with thromboembolic complications compared to patients without complications (75.0 vs. 77.9 mm, p = 0.047). COVID-19 patients with thromboembolic complications had an increased CFT compared to patients without complications in EXTEM (54.1 vs. 42.9 s, p = 0.001) and INTEM (53.3 vs. 43.5 s, p = 0.03). EXTEM clot firmness at 5 min (A5) was decreased in patients with thromboembolic complications compared to patients without complications (58.5 vs. 64.3 mm, p = 0.009).
The conventional coagulation variables showed no differences between the group with and without thromboembolic complications.
Our results show changes in ROTEM® variables supporting the presence of a hypercoagulable state in COVID-19 patients. ROTEM® profiles of COVID-19 patients showed a decreased CFT and increased MCF. The increased CT in EXTEM corresponds with prolonged PT and APTT in the conventional coagulation test. Standard coagulation variables showed higher fibrinogen levels in COVID-19 patients compared to normal, consistent with findings in other studies [2]. The influence of fibrinogen on clot firmness was confirmed by the high MCF in FIBTEM.
Visco-elastic coagulation testing, such as ROTEM®, is gaining popularity because of its ability to offer a rapid and detailed evaluation of the clotting process. Although mainly used in blood loss, ROTEM is also used to detect thrombosis [5]. ROTEM also manages to measure hypercoagulability, where it is not detected by conventional coagulation tests.
Severe hypercoagulability in COVID-19 patients has recently been demonstrated in other visco-elastic hemostatic assays, such as the Quantra and TEG [6,7]. The results of our study are consistent with the findings of two other studies on ROTEM® profiles showing a decreased CFT and higher MCF [8,9]. Only one study on visco-elastic coagulation testing in COVID-19 patients reported the incidence of thromboembolic complications in their study cohort [9].
Contrary to expectations, clot firmness was lower and CFT longer in patients with thromboembolic complications. The finding of more thromboembolic complications in patients with normal ROTEM® tracing might be explained by coincidence. Also, heparin could have an effect on ROTEM® outcomes and all patients had prophylactic anticoagulation at the time of ROTEM® measurements. More studies are required to study the ROTEM® profiles of COVID-19 patients with thromboembolic complications.
The development of thromboembolic complications in COVID-19 patients might be explained by inflammatory changes and coagulation abnormalities. However, specific ICU factors such as immobilization, sedation, fluid restriction, use of vasopressors, central venous catheters, and vascular damage can also increase the risk of thrombosis. The risk on thromboembolic complications should be considered in light of these confounders.
Hypercoagulability might be associated with other clinical characteristics besides COVID-19. An increased MCF with ROTEM® analysis was also found in patients with severe sepsis and shock [5]. A larger MCF in EXTEM and INTEM can also be found in obese patients. Central obesity was found to be a predictor of a hypercoagulable state evaluated by ROTEM® analysis [10].
There were several limitations. First, the sample size of this observational study was rather small, increasing the vulnerability to confounding factors. Second, screening for thromboembolic complications was not standardized, and imaging was based on clinical suspicion. Therefore, only a minority of the patients underwent a CT-scan. Last, ROTEM® analysis was not performed on a pre-specified day after ICU admission. However, hypercoagulability in COVID-patients seems to persist over time [9].
In this study, ROTEM® analysis confirmed the hypercoagulable state of COVID-19 patients admitted to the ICU. However, our study does not support the use of ROTEM® in identifying COVID-19 patients at risk for developing thromboembolic complications. Further study into the hypercoagulability in COVID-19 patients admitted to the ICU is needed to confirm our findings and to define the clinical value in subgroup of patients. Also, future studies may investigate the effectiveness of ROTEM®-guided, individualized anticoagulation dosing in COVID-19 patients admitted to the ICU.
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of the article.
Outside the submitted work, Thomas Scheeren received research grants and honoraria from Edwards Lifesciences (Irvine, CA, USA) and Masimo Inc. (Irvine, CA, USA) for consulting and lecturing and from Pulsion Medical Systems SE (Feldkirchen, Germany) for lecturing. TWLS is chair of the Cardiovascular Dynamics section of the European Society of Intensive Care Medicine (ESICM). Not related to the submitted work, Karina Meijer received research grants from Pfizer, and research grants from Bayer and Sanquin and for lecturing, and from Boehringer Ingelheim, BMS and Aspen for lecturing, and from Uniqure for consulting.
References
- 1.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. Coronavirus Disease (COVID-2019) Weekly Epidemiological Update 23 August 2020.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available at. [Google Scholar]
- 2.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helms J., Tacquard C., Severac F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akay O.M. The double hazard of bleeding and thrombosis in hemostasis from a clinical point of view: a global assessment by rotational thromboelastometry (ROTEM) Clin. Appl. Thromb. Hemost. 2018;24:850–858. doi: 10.1177/1076029618772336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranucci M., Ballotta A., Di Dedda U. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14854. Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panigada M., Bottino N., Tagliabue P. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14850. Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiezia L., Boscolo A., Poletto F., Cerruti L., Tiberio I., Campello E., Navalesi P., Simioni P. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb. Haemost. 2020;120:998–1000. doi: 10.1055/s-0040-1710018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pavoni V., Gianesello L., Pazzi M. Evaluation of coagulation function by rotation thromboelastometry in critically ill patients with severe COVID-19 pneumonia. J. Thromb. Thrombolysis. 2020;11:1–6. doi: 10.1007/s11239-020-02130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campello E., Spiezia L., Zabeo E. Hypercoagulability detected by whole blood thromboelastometry (ROTEM®) and impedance Aggregometry (MULTIPLATE®) in obese patients. Thromb. Res. 2015;135:548–553. doi: 10.1016/j.thromres.2015.01.003. [DOI] [PubMed] [Google Scholar]