Abstract
Background
A prospective study was conducted during the second phase of the coronavirus disease 2019 (COVID-19) pandemic in India to assess the prevalence of anxiety and depressive symptoms among healthcare workers (HCWs) and factors that influence the outcome.
Methods
A self-administered questionnaire was completed by 1124 HCWs during the COVID-19 pandemic (March 30, 2020, to April 2, 2020). Demographic data, questions on COVID-19 and scores of the Hospital Anxiety and Depression Scale were analysed using the chi-square test (Bonferroni correction) and binary logistic regression.
Results
The study consists of 1124 HCWs, including 749 doctors, 207 nurses, 135 paramedics, 23 administrators and ten supporting staff members. The prevalence of anxiety and depressive symptoms were reported as 37.2% and 31.4%, respectively. The risk factors for anxiety were female gender (30.6% vs 45.5%), age group (20–35 years) (50.4% vs 61.2%), unmarried (21.2% vs 30.6%) and job profile (nurse) (14.7% vs 26.4%). The protective factor was having service of more than 20 years (23.4% vs 14.8%). The risk factors for depression were age group (20-35 years) (51.3% vs 61.3%) and employed at a primary care hospital (16.2% vs 23.4%). The protective factors were job profile (doctor) (69.9% vs 59.6%) and having service of more than 20 years (22.3% vs 15.5%).
Conclusion
Approximately one-third of the HCWs reported anxiety and depressive symptoms. The risk factors for anxiety symptoms were female gender, younger age and job profile (nurse) and for depressive symptoms were younger age and working at a primary care hospital. Future research studies should identify strategies for providing a safer and supportive work environment for HCWs to face epidemics/pandemics.
Keywords: COVID-19, Healthcare workers, Hospital anxiety, Depression score
Introduction
The world is presently scuffling with the coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS CoV-2).1,2 Cases and death are progressively increasing as the days are passing by.3 With the attack rate of 1.4–4.04,5 and no treatment or vaccine available in the near future, the COVID-19 pandemic has caused a substantial degree of panic, worry, fear and apprehension.
From March 23 midnight, India has gone in complete lockdown, with only essential services being functional. Most of the companies have encouraged their employees to “work from home”; however, no such provisions were offered to healthcare workers (HCWs). As a result, HCWs are encountering occupational hazards owing to high risk of exposure to coronavirus infection.6 It requires a lot of courage to work in potentially infectious environments that can impact the psychological health of HCWs. Among physicians, the primary risk factors of work-related conditions, lifestyles and physical health account for their anxiety and depressive symptoms.
A recent Chinese study reported symptoms of depression (50.4%) and anxiety (44.6%) among HCWs during the COVID-19 pandemic.7 Studies on mental health outcomes among HCWs involved in the SARS crisis showed the importance of specialised preparedness, working in “high-risk” environment, job-related stress and being quarantined as a staff member, all of which appeared to have a negative psychological impact.8 Almost everyone experiences health-related anxiety to some degree during epidemics, and high levels of health anxiety can be detrimental.9
This is an early study dealing with the emotional health of HCWs as India faces the second phase of the COVID-19 pandemic.10 The survey was conducted over four days from March 30, 2020, to April 2, 2020. The study aimed to investigate the prevalence of anxiety and depressive symptoms among HCWs during the COVID-19 pandemic across India.
Material & methods
Sample
A self-administered questionnaire link was sent to all personnel to their WhatsApp accounts or email, who were involved in health care and were known to the investigators or their contacts (quota sampling). They were followed up for responses. It was a multisite study. As data collection was performed during the period of lockdown, Google Forms was used to send the questionnaire across to the respondents. One thousand one hundred fifty-two responses were received over four days from March 30, 2020, to April 2, 2020. Twenty-eight responses were rejected because of duplicity: 8 filled in the questionnaire twice and submitted the same and 20 responses were incomplete, hence were not amenable to analysis.
Participants
The sample size was determined by using the following formula: N = Zα2 P (1−P)/d2. In this, α was 0.05, Zα was 1.96 (at the 95% confidence level) and the estimated acceptable margin of error for proportion d was 0.05. Based on the previous study on the SARS outbreak,11, 12, 13 the prevalence of psychological comorbidities was estimated to be around 32%. Based on the aforementioned formula, the sample size was estimated to be a minimum of 335 respondents.
Instruments of measurement
Demographic details
The demographic details of the respondents included age, gender, education level, job profile, duration of service and working place.
Questions pertaining to the COVID-19 pandemic
These questions were pertaining to feeling concerned regarding hospital-acquired infection, receiving adequate guidance on COVID-19, ensuring the use of personal protective equipment (PPE), dealing with patients with suspected/confirmed COVID-19, the sufficiency of existing healthcare facilities to cope with COVID-19, information on several media platforms regarding COVID-19 and difficulty in managing the spread of COVID-19.
Hospital Anxiety and Depression Scale
A self-administered scale was used to assess the present emotional health (anxiety and depression) among HCWs during the COVID-19 pandemic. It is a 14-item scale (7 each for anxiety and depression) with a score ranging from 0 to 21. The score of 0–7 was taken as normal and 8–21 were taken as abnormal for both symptoms. The Hospital Anxiety and Depression Scale (HADS) scale14 was initially designed for use in hospital practice. However, the scale has been frequently validated for use in community settings as well. Cronbach's alpha of the anxiety and depression subscale is 0.83 and 0.82, respectively, with a mean correlation of 0.56.15 The tool was not intended to be used for clinical purposes. It is not a scale for the diagnosis of anxiety or depression but to evaluate the emotional state of patients/others.
Statistical analysis
The data were analysed using IBM SPSS Statistics 23 for windows (version 23.0; IBM Corp., NY, USA). The chi-square test was used to analyse the descriptive statistics. The chi-square test was used to compare characteristics between the group with and the group without psychological abnormality. Post hoc testing was carried out after choosing the Bonferroni-corrected p-value for each contingency table. Subsequently, the p-value was calculated for each cell, and only cells with a p-value lower than the Bonferroni-corrected p-value were reported significant. Subgroup analysis of demographic variables and COVID-19–pertaining questions was carried out using binary logistic regression. A p-value of less than 0.05 was considered to be statistically significant.
Ethical considerations
The study was approved by the ethical review committee of a large tertiary care hospital. Informed consent was taken from all the respondents before filling in the questionnaire.
Result
Of 1450 Google Forms sent, responses were received from 1152 HCWs, making the response rate 79.44%. Of these, 28 responses were discarded owing to duplicity or incomplete responses. The final sample for the study consisted of 1124 respondents, comprising of 749 (66.6%) doctors, 207 (18.4%) nurses, 135 (12.0%) paramedics, 23 (2.0%) administrators and 10 (0.9%) supporting staff members. The respondents were divided into two groups: non-anxiety (total score <8) and anxiety group (total score ≥8) (Table 1).
Table 1.
Comparison of non-anxiety and anxiety symptoms with demographic data using the chi-square test.
| Variables | Total |
Non-anxiety group, n (%) |
Anxiety group, n (%) |
p |
|---|---|---|---|---|
| (n = 1124) | 706 (62.8) | 418 (37.2) | ||
| Age group (years) | ||||
| 20–35a | 612 (54.4) | 356 (50.4) | 256 (61.2) | 0.000 |
| 36–50 | 307 (27.3) | 198 (28.6) | 109 (26.1) | |
| 51–65 | 184 (16.4) | 134 (11.9) | 50 (12.0) | |
| >65 | 21 (1.9) | 18 (2.5) | 3 (0.7) | |
| Gender | ||||
| Femalea | 406 (36.1) | 216 (30.6) | 190 (45.5) | 0.000 |
| Male | 718 (63.9) | 490 (69.4) | 228 (54.5) | |
| Marital status | ||||
| Unmarrieda | 278 (24.7) | 150 (21.2) | 128 (30.6) | 0.001 |
| Married | 826 (73.5) | 540 (76.5) | 286 (68.4) | |
| Separated | 20 (1.8) | 16 (2.3) | 4 (1.0) | |
| Other | ||||
| Education level | ||||
| High school or lower | 17 (1.5) | 10 (1.4) | 7 (1.7) | 0.071 |
| Bachelor's degree | 439 (39.1) | 262 (37.1) | 177 (42.3) | |
| Master's degree | 402 (35.8) | 273 (38.7) | 129 (30.9) | |
| Doctoral degree | 266 (23.7) | 161 (22.8) | 10.05 (25.1) | |
| Job profile | ||||
| Doctor | 749 (66.6) | 485 (68.7) | 264 (63.2) | 0.001 |
| Nursea | 207 (18.4) | 104 (14.7) | 103 (26.4) | |
| Paramedics | 135 (12.0) | 94 (13.3) | 41 (9.8) | |
| Administrators | 23 (2.0) | 17 (2.4) | 6 (1.4) | |
| Supporting staff | 10 (0.9) | 6 (0.8) | 4 (1.0) | |
| Duration of service (years) | ||||
| 0–10 | 573 (51.0) | 335 (47.5) | 238 (56.9) | 0.001 |
| 11–20 | 324 (28.8) | 206 (29.2) | 118 (28.2) | |
| >20a | 227 (20.2) | 165 (23.4) | 62 (14.8) | |
| Working place | ||||
| Primary care hospital | 208 (18.5) | 116 (16.4) | 92 (22.0) | 0.057 |
| Secondary care hospital | 269 (23.9) | 177 (25.1) | 92 (22.0) | |
| Tertiary care hospital | 647 (57.6) | 413 (58.5) | 234 (56.0) | |
| Others | ||||
| Current working department | ||||
| OPD | 381 (33.9) | 249 (35.3) | 132 (31.6) | 0.117 |
| Flu clinic | 68 (6.0) | 47 (6.7) | 21 (5.0) | |
| Isolation ward | 60 (5.3) | 37 (5.2) | 23 (5.5) | |
| Intensive care unit (ICU) | 154 (13.7) | 82 (11.6) | 72 (17.2) | |
| Work from home | 78 (6.9) | 52 (7.4) | 26 (6.2) | |
| Other | 383 (34.1) | 239 (33.9) | 144 (34.4) | |
Bonferroni correction.
As per the HADS score, 418 (37.2%) respondents were having anxiety symptoms. The results of the chi-square test showed significant associations in the variables of age, gender, marital status, job profile and duration of service. Among the study population, those in the 20- to 35-year age group, women, nurses and those with less than 10 years of service were more likely to suffer from anxiety symptoms. The married population was less likely to be anxious. Other demographic parameters appeared to have no impact on the presence of anxiety symptoms (Table 1). Subsequently adjusted p-value was calculated using Bonferroni correction for each contingency table. Significant differences were found in the age group of 20–35 years (50.4% vs 61.2%), female gender (30.6% vs 45.5%), unmarried (21.2% vs 30.6%) and nurses (14.7% vs 26.4%), with more percentage of individuals in the anxiety group. Duration of service for more than 20 years (23.4% vs 14.8%) was found to be a protective factor, with more percentage of individuals in the non-anxiety group (Table 1).
The results of the chi-square test showed that more percentage of individuals in the anxiety group were concerned regarding hospital-acquired infection (48.9% vs 78.5%), were dealing with patients with suspected/confirmed COVID-19 (56.1% vs 68.5%), felt existing healthcare facilities have insufficient medical arrangements to cope with COVID-19 (43.2% vs 58.9%) and thought that it is difficult to manage the spread of COVID-19 (45.8% vs 69.1%). More percentage of individuals in the non-anxiety group received adequate guidance on COVID-19 (59.3% vs 46.7%) and ensured the use of PPE (66.9% vs 57.4%) (Fig. 1, Supplementary Table 1).
Fig. 1.
Comparison of non-anxiety and anxiety symptoms with questions pertaining to the COVID-19 pandemic using the chi-square test. ∗, Significant after Bonferroni correction. COVID-19 = coronavirus disease 2019; PPE = personal protective equipment.
Binary logistic regression analysis of anxiety-related factors is shown in Supplementary Table 3. Significance was found in demographic factors of female gender (odds ratio [OR] = 1.85, 95% confidence interval [CI] = 1.29–2.66), unmarried (OR = 3.63, 95% CI = 1.02–12.92), bachelor's degree (OR = 0.62, 95% CI = 0.40–0.97), master's degree (OR = 0.63, 95% CI = 0.43–0.93), working at a primary care hospital (OR = 1.83, 95% CI = 1.24–2.72), out patient department (OPD) (OR = 0.56, 95% CI = 0.41–0.88) and flu clinic (OR = 0.41, 95% CI = 0.21–0.82). With regard to questions pertaining to COVID-19, significance was found in individuals feeling concerned about hospital-acquired infection (yes) (OR = 4.45, 95% CI = 2.78–7.11), feeling concerned about adequate guidance on COVID-19 (no) (OR = 0.63, 95% CI = 0.45–0.89) and who think that it is difficult to manage the spread of COVID-19 (yes) (OR = 2.70, 95% CI = 1.91–3.81) (Supplementary Table 4).
The results of the HADS score (total score ≥8) showed 354 (31.4%) respondents were having depressive symptoms (Table 2).
Table 2.
Comparison of non-depressive and depressive symptoms with demographic data using the chi-square test.
| Total | n (%) |
Non-depression, n (%) |
Depression, n (%) |
P |
|---|---|---|---|---|
| 1124 (100) | 770 (68.6) | 354 (31.4) | ||
| Age group (years) | ||||
| 20–35a | 612 (54.4) | 395 (51.3) | 219 (61.3) | 0.009 |
| 36–50 | 307 (27.3) | 223 (29.0) | 84 (23.7) | |
| 51–65 | 184 (16.4) | 139 (18.1) | 45 (12.7) | |
| >65 | 21 (1.9) | 13 (1.7) | 08 (2.3) | |
| Gender | ||||
| Female | 406 (36.1) | 275 (35.7) | 131 (37.0) | 0.675 |
| Male | 718 (63.9) | 495 (64.3) | 223 (63.0) | |
| Marital status | ||||
| Unmarried | 278 (24.7) | 178 (23.1) | 100 (28.2) | 0.180 |
| Married | 826 (73.5) | 578 (75.1) | 248 (70.1) | |
| Separated | 20 (1.8) | 14 (1.8) | 06 (1.7) | |
| Education level | ||||
| High school or lower | 17 (1.5) | 12 (1.6) | 05 (1.4) | 0.470 |
| Bachelor's degree | 439 (39.1) | 293 (38.1) | 146 (41.2) | |
| Master's degree | 402 (35.8) | 287 (37.3) | 115 (32.5) | |
| Doctoral degree | 266 (23.7) | 178 (23.1) | 88 (24.9) | |
| Job profile | ||||
| Doctora | 749 (66.6) | 538 (69.9) | 211 (59.6) | 0.010 |
| Nurse | 207 (18.4) | 127 (16.5) | 80 (22.6) | |
| Paramedical staff | 135 (12.0) | 84 (10.9) | 51 (14.4) | |
| Hospital administration | 23 (2.0) | 13 (1.7) | 10 (2.8) | |
| Supporting staff | 10 (0.9) | 08 (1.0) | 02 (0.6) | |
| Duration of service (years) | ||||
| 0–10 | 573 (51.0) | 378 (49.1) | 195 (55.1) | 0.026 |
| 11–20 | 324 (28.8) | 220 (28.6) | 104 (29.4) | |
| >21a | 227 (20.2) | 172 (22.3) | 55 (15.5) | |
| Working place | ||||
| Primary care hospitala | 208 (18.5) | 125 (16.2) | 83 (23.4) | 0.012 |
| Secondary care hospital | 269 (23.9) | 194 (25.2) | 75 (21.2) | |
| Tertiary care hospital | 647 (57.6) | 451 (58.6) | 196 (55.4) | |
| Current working department | ||||
| OPD | 381 (33.9) | 261 (33.9) | 120 (33.9) | 0.902 |
| Flu clinic | 68 (6.0) | 50 (6.5) | 18 (5.1) | |
| Isolation ward | 60 (5.3) | 43 (5.6) | 17 (4.8) | |
| Intensive care unit (ICU) | 154 (13.7) | 106 (13.8) | 48 (13.6) | |
| Work from home | 78 (6.9) | 54 (7.0) | 24 (6.8) | |
| Others | 383 (34.1) | 256 (33.2) | 127 (35.9) | |
Bonferroni correction.
The chi-square test showed significant associations in the variables of age, job profile, duration of service and working place. Among the study population, those in the 20- to 35-year age group, those working at a primary care hospital and personnel with service less than 10 years were found to have more depressive symptoms. Among different HCWs, doctors were least likely to suffer from depressive symptoms. Other demographic parameters appeared to have no impact on the presence of depressive symptoms. The post hoc analyses showed significant differences in the age group of 20–35 years (51.3% vs 61.3%) and working at a primary care hospital (16.2% vs 23.4%), with more percentage of individuals in the depressive group. Doctors (69.9% vs 59.6%) and personnel with duration of service more than 20 years (22.3% vs 15.5%) were protective factors, with more percentage of individuals in the non-depressive group (Table 2).
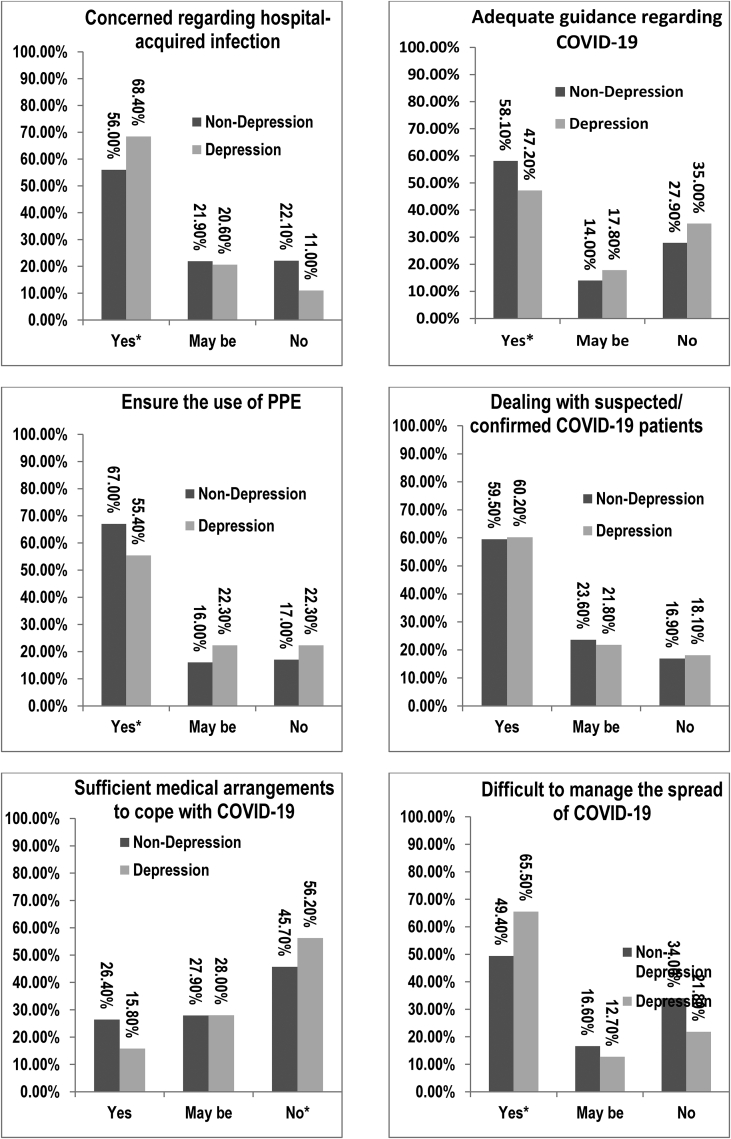
In the questionnaire related to COVID-19, higher levels of depressive symptoms were found in HCWs who were concerned about hospital-acquired infection (yes), who were concerned about current healthcare facilities to cope with COVID-19 (no) and who thought that it would be difficult to manage the spread of COVID-19 (yes). The protective factors for depressive symptoms were receiving adequate guidance regarding COVID-19 (yes) and ensuring the use of PPE (yes) (Fig. 2; Supplementary Table 2).
Fig. 2.
Comparisons between non-depressive and depressive symptoms in HCWs using the chi-square tests on questions pertaining to the COVID-19 pandemic. ∗, Significant after Bonferroni correction. COVID-19 = coronavirus disease 2019; PPE = personal protective equipment.
In binary logistic regression, significance was found among groups of education level (bachelor degree: OR = 0.52, 95% CI = 0.34–0.82; master degree: OR = 0.61, 95% CI = 0.41–0.88) and working at a primary care hospital (OR = 1.59, 95% CI = 1.09–2.31) (Supplementary Table 3). Regarding questions pertaining to COVID-19, significance was found among individuals who were concerned about hospital-acquired infection (yes) (OR = 2.04, 95% CI = 1.32–3.16), were concerned about existing healthcare facilities for the management of COVID-19 (yes) (OR = 0.62, 95% CI = 0.40–0.94) and thought that it is difficult to manage the spread of COVID-19 (yes) (OR = 1.95, 95% CI = 1.39–2.73) (Supplementary Table 4).
Discussion
To the best of our knowledge, this is the first study conducted on the emotional health of HCWs during the COVID-19 pandemic in India. As India is facing lockdown during stage II (local transmission) of the COVID-19 pandemic,6 it is essential to study the psychological impact among HCWs.
Among 1124 HCWs, anxiety symptoms were reported by 37.2% and depressive symptoms were reported by 31.4%. A recent study conducted on Chinese HCWs shows 44.6% with anxiety symptoms and 50.4% with depressive symptoms.7 However, this study was conducted during the acute phase of the COVID-19 pandemic, which could account for the increase in anxiety and depressive symptoms in the population. A similar study reported a prevalence of 25.3% of psychological morbidity among HCWs at a screening centre during the H1N1 pandemic in Singapore.16 The less prevalence could be attributed to the centre-only screening for H1N1.
The demographic risk factors for anxiety were female gender, young age group (20–35 years), unmarried status and job profile of nurses. The protective factor was HCWs having service for more than 20 years. The risk factor for anxiety among female HCWs was 1.8 times higher than among male HCWs. A longitudinal survey conducted in India reported that prevalence of anxiety symptoms is more (21.7%) in female gender than in male gender (16.2%).17
In the present study, levels of anxiety symptoms were reported to be higher in the young age group (20–35 years) and unmarried status. Unmarried HCWs had 3.6 times more risk of having anxiety symptoms than the separated group. This could be as younger HCWs are generally employed as front-line workers and are more prone to contact with infected patients.18 Moreover, inexperience of younger HCWs adds to the anxiety symptoms.
The result of binary logistic regression also showed an inverse relationship between education and anxiety symptoms (bachelor's degree: OR = 0.62, p = 0.04; master's degree: OR = 0.63, p = 0.02). Moreover, HCWs having a service of more than 20 years reported fewer anxiety symptoms. This is probably attributable to their knowledge, experience and maturity. In addition, they do not generally serve as front-line HCWs, so the chances of acquiring the first-hand infection are less.19
The levels of anxiety symptoms of nurses were reported to be higher than those of doctors and other HCWs. A possible explanation for this can be the nurse's longer duration of exposure with patients. Similarly, nurses reported the highest level of distress compared with doctors and other HCWs during the SARS outbreak.20 In another study assessing the association between distress and job role, nurses were found to have higher distress levels during the SARS outbreak.21 In a study during the COVID-19 pandemic, nurses were found to have a higher level of anxiety and distress probably due to longer working hours and frequent patient exposure.22 As nurses in the present study are all women, they are more likely to have anxiety symptoms. The contribution of anxiety and depression to the total of disability-adjusted life years was considerably higher in female gender than in male gender.17
HCWs working at primary care hospitals have a higher risk of anxiety and depressive symptoms than those working at a tertiary care hospital as they generally work in screening centres with a large load of patients. Moreover, primary care hospitals in India are quite ill-equipped as compared with tertiary care hospitals. In the present study, significant anxiety symptoms were reported by HCWs who were concerned about acquiring COVID-19 from the hospital while dealing with patients with suspected/confirmed COVID-19, did not feel existing healthcare facilities are sufficient to cope with COVID-19 and thought that it is difficult to control the spread of COVID-19.
Fear for health and job stress of HCWs accounted for major psychological impact during the SARS outbreak.23 The health of self, the spread of virus and changes in routine work accounted for major stressors among HCWs during the SARS outbreak.20 One of the risk factors for depression was age group (20–35 years) as younger HCWs are generally inexperienced, employed in front-line jobs and more prone to exposure to infected patients.18 The protective factors for depression were the job profile of doctors, education level (bachelor's or master's degree) and service of more than 20 years. This is probably attributable to their knowledge, experience and maturity. The level of depressive symptoms did not depend on the gender, marital status and current working department.24
The present study shows working at primary care hospitals has a 1.5 times higher risk of having depressive symptoms than working at tertiary care hospitals. Similar findings were reported in an Egyptian study, with higher levels of depressive symptoms among HCWs working at primary healthcare centres (71.4%) than among those working at tertiary care hospitals (59%).25 HCWs, who felt concerned regarding hospital-acquired infection or thought it is difficult to manage the spread of COVID-19, showed twice the probability of having depressive symptoms. In addition, HCWs reporting insufficiency of healthcare facilities were more prone to depressive symptoms. Similarly, higher levels of stress were detected among nurses who had direct patient exposure during the SARS epidemic and had negative emotions.12
Limitations
One of the limitations of the study was that the self-administered questionnaire (HADS) could not be validated with mental status examination as the study was conducted during the lockdown period in India. In addition, the survey was conducted rapidly to assess the emotional health of HCWs during the outbreak of COVID-19. To measure the impact of the COVID-19 pandemic on HCWs, a self-designed questionnaire was used as no standardised questionnaire could be traced for studying the impact of epidemics and pandemics. Another limitation could be the confounding effect of HCWs in government and private institutions, which was not included in the demographics. However, the biggest limitation of this survey is that it has been carried out relatively early during the pandemic in India; therefore, the findings may change with time.
Conclusion
The present study denotes approximately one-third of HCWs have symptoms of anxiety and depression. The risk factors for anxiety symptoms are female gender, young age group and the job profile of nurses and for depressive symptoms are younger age group and the working place of the primary care hospital. Higher levels of anxiety and depressive symptoms were found in HCWs with concern regarding acquiring COVID-19 infection, thinking healthcare facilities are inadequate to cope with COVID-19 or thinking it is difficult to manage the spread of COVID-19. Healthcare facilities should provide a structured and safer work environment to HCWs to cope with future biodisasters. Future research studies should be triggered to identify interventions and strategies required for supporting the emotional health of HCWs in the face of epidemics or pandemics.
Disclosure of competing interest
The authors have none to declare.
Acknowledgements
The authors would like to thank the following people:
Rajat Shukla, S Karthik, Ravi K Anadure, Anindya Kumar Gupta, Harshit Khurana and Parthasarthi Ghana.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mjafi.2020.07.006.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/nejmoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus Disease (COVID-19) - Events as They Happen [Internet]. [cited 2020 Jul 30]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
- 3.Coronavirus Disease (COVID-19) Situation Reports [Internet]. World Health Organization; [cited 2020 Jul 30]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 4.Statement on the Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus 2019 (n-CoV) on 23 January 2020 [Internet]. World Health Organization; [cited 2020 Jul 30]. Available from: https://www.who.int/news-room/detail/23-01-2020-statement-on-the-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov).
- 5.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Circulars for Covid-19: Ministry of Home Affairs [Internet]. Ministry of Home Affairs | GoI. [cited 2020 Jul 30]. Available from: https://www.mha.gov.in/notifications/circulars-covid-19.
- 7.Zhang C., Yang L., Liu S. Survey of insomnia and related social psychological factors among medical staffs involved with the 2019 novel coronavirus disease outbreak. SSRN Electr J. 2020 doi: 10.3389/fpsyt.2020.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boer J.C.D., Lok A., Verlaat E.V.T., Duivenvoorden H.J., Bakker A.B., Smit B.J. Work-related critical incidents in hospital-based health care providers and the risk of post-traumatic stress symptoms, anxiety, and depression: a meta-analysis. Soc Sci Med. 2011;73(2):316–326. doi: 10.1016/j.socscimed.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asmundson G.J.G., Abramowitz J.S., Richter A.A., Whedon M. Health anxiety: current perspectives and future directions. Curr Psychiatr Rep. 2010 Dec;12(4):306–312. doi: 10.1007/s11920-010-0123-9. [DOI] [PubMed] [Google Scholar]
- 10.Delhi ICMRN. [Internet]. Indian Council of Medical Research, New Delhi. [cited 2020 Jul 30]. Available from: https://www.icmr.gov.in/index.html.
- 11.Nickell L.A., Crighton E.J., Tracy C.S. CMAJ; 2004. Psychosocial Effects of SARS on Hospital Staff: A Survey of a Large Tertiary Care Institution.https://www.cmaj.ca/content/170/5/793 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su TP, Lien TC, Yang CY Prevalence of psychiatric morbidity and psychological adaptation of the nurses in a structured SARS caring unit during outbreak: a prospective and periodic assessment study in Taiwan. J Psychiat Res. 2007 Jan 1;41(1-2):119–130. doi: 10.1016/j.jpsychires.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee A.M., Wong J.G., Mcalonan G.M. Stress and psychological distress among SARS survivors 1 year after the outbreak. Can J Psychiatr. 2007;52:233–240. doi: 10.1177/070674370705200405. [DOI] [PubMed] [Google Scholar]
- 14.Zigmond A.S., Snaith R.P. Wiley Online Library. John Wiley & Sons, Ltd; 2007. The Hospital Anxiety and Depression Scale.https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0447.1983.tb09716.x [Internet] Available from: [Google Scholar]
- 15.Bocéréan C., Dupret E. A validation study of the Hospital Anxiety and Depression Scale (HADS) in a large sample of French employees. BMC Psychiatr. 2014;14 doi: 10.1186/s12888-014-0354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phua D. Coping responses of emergency physicians and nurses to the 2003 severe acute respiratory syndrome outbreak. Acad Emerg Med. 2005;12:322–328. doi: 10.1197/j.aem.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Sagar R., Dandona R., Gururaj G. The burden of mental disorders across the states of India: the Global Burden of Disease Study 1990–2017. Lancet Psychiatr. 2020;7:148–161. doi: 10.1016/s2215-0366(19)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chua S.E., Cheung V., Cheung C. Psychological effects of the SARS outbreak in Hong Kong on high-risk health care workers. Can J Psychiatr. 2004;49:391–393. doi: 10.1177/070.674370404900609. [DOI] [PubMed] [Google Scholar]
- 19.Cheng C., Cheung M.W.L. Psychological responses to the outbreak of severe acute respiratory syndrome: a prospective, multiple time-point study. J Pers. 2005;73:261–285. doi: 10.1111/j.1467-6494.2004.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong T.W., Yau J.K., Chan C.L. The psychological impact of severe acute respiratory syndrome outbreak on healthcare workers in emergency departments and how they cope. Eur J Emerg Med. 2005;12:13–18. doi: 10.1097/00063110-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Maunder R., Lancee W., Balderson K. Long-term psychological and occupational effects of providing hospital healthcare during SARS outbreak. Emerg Infect Dis. 2006;12:1924–1932. doi: 10.3201/eid1212.060584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai J., Ma S., Wang Y. Factors associated with mental health outcomes among health care workers exposed to coronavirus Disease 2019. JAMA Netw Open. 2020:3. doi: 10.1001/jamanetworkopen.2020.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Styra R., Hawryluck L., Robinson S., Kasapinovic S., Fones C., Gold W.L. Impact on health care workers employed in high-risk areas during the Toronto SARS outbreak. J Psychosom Res. 2008;64:177–183. doi: 10.1016/j.jpsychores.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mcalonan G.M., Lee A.M., Cheung V. Immediate and sustained psychological impact of an emerging infectious Disease outbreak on health care workers. Can J Psychiatr. 2007;52:241–247. doi: 10.1177/070674370705200406. [DOI] [PubMed] [Google Scholar]
- 25.El-Hamrawya L.G., Hegazy N.N., El-Halawany S.M. Prevalence of depressive symptoms among healthcare providers in Shibin El-Kom city in Menoufia governorate. Menoufia Med J. 2018;31:708–715. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.