Abstract
Background
Medication for opioid use disorder (MOUD) can decrease the risk of opioid overdose (OOD) in individuals with opioid use disorder. Peer recovery support services (PRSS) are increasingly used to promote MOUD engagement but evidence of their efficacy is limited. This study’s objective was to evaluate a single 20-minute telephone-delivered PRSS intervention for increasing MOUD enrollment and decreasing recurring OODs.
Method
This single-site, randomized controlled pilot trial enrolled adults, primarily recruited from a syringe service program, with an opioid-positive urine drug screen (UDS) reporting having been treated for an OOD within the past 6 months. Participants (N = 80) were randomized to PRSS (n = 40) or Control (n = 40) condition with all participants receiving personally-tailored OOD education and naloxone. Outcome measures obtained at 3 (n = 66), 6 (n = 58), and 12 (n = 44) months post-randomization included verified MOUD enrollment (primary), self-reported OOD, and opioid use assessed by self-report and UDS.
Results
Through 12-month follow-up, 32.5 % of PRSS, compared to 17.5 % of Control participants enrolled in MOUD (X2 = 2.4, p = 0.12; odds ratio = 2.27 (0.79–6.49)). PRSS participants were significantly less likely to have experienced an OOD through 12-month follow-up (12.5 % of PRSS participants, 32.5 % of Control, p = 0.03). No significant treatment effect was found for opioid use through 12-month follow-up as measured by either opioid-positive UDSs or self-reported past month opioid use days. Based on self-report, PRSS had good acceptability for both the interventionists and participants.
Conclusions
The results suggest that further development and testing of this PRSS telephone intervention to encourage MOUD enrollment and reduce OOD may be warranted.
Keywords: Peer recovery support services, Opioid, Overdose, Medication for opioid use disorder
1. Introduction
In recent years, the U.S. has experienced a growing opioid-use epidemic accompanied by a dramatic rise in opioid overdoses (Scholl et al., 2018; Straus et al., 2013). Research suggests that opioid overdose prevention education and naloxone distribution can reduce the rate of opioid overdose fatalities (Clark et al., 2014; Naumann et al., 2019; Walley et al., 2013). However, the only effective method for preventing opioid overdose is successful treatment of the underlying opioid use disorder (OUD). Receiving medication for OUD (MOUD; e.g., methadone- or buprenorphine-maintenance) significantly reduces the likelihood of opioid overdose (Larochelle et al., 2019; Sordo et al., 2017). MOUD enrollment is particularly important for individuals at heightened risk for opioid overdose, including those who have survived an overdose (Hasegawa et al., 2014; Weiner et al., 2020) but MOUD is widely underutilized (Volkow and Wargo, 2018). In addition to the barriers of waiting lists and the costs of MOUD, inaccurate perceptions of MOUD – including myths about its side effects and lack of efficacy – also prevent some individuals with OUD from entering treatment (Peterson et al., 2010; Uebelacker et al., 2016; Zaller et al., 2009).
To help address the opioid overdose crisis while also combatting negative perceptions of MOUD, our team created a personally-tailored opioid overdose prevention education and naloxone distribution (PTOEND) intervention. The PTOEND intervention provides information about the individual’s personal risk factors for opioid overdose as well as education about overdose and MOUD. Our team also created a single-session Peer Recovery Support Service (PRSS) telephone intervention to encourage MOUD enrollment. The use of PRSSs to engage individuals in MOUD and other recovery services has increased dramatically in recent years but the evidence that these services are effective is limited (Eddie et al., 2019; McGuire et al., 2020). The present study evaluated the PRSS intervention, relative to the control condition of PTOEND without PRSS. We hypothesized that, over the 12-month follow-up period, the PRSS would increase MOUD enrollment (primary outcome measure) and decrease recurring opioid overdose and illicit opioid use (secondary outcome measures) in individuals with a prior overdose receiving PTOEND.
2. Method
2.1. Design
This pilot study was a randomized controlled intent-to-treat (ITT) clinical trial. Eligible participants were randomized in a 1:1 ratio to the PRSS or Control condition. The randomization sequence was created by the project statistician (D.L.), using a randomized block design (blocks of size 2 or 4); the randomization sequence was unknown to the research staff performing the study procedures. All participants were scheduled to complete a follow-up phone call approximately 3-weeks post-randomization, and in-person visits at approximately 3, 6, and 12months following enrollment. Study assessments were administered by an RA who was not blinded to the participant’s study arm.
A pilot study necessitates a limited sample size, and is more useful for showing feasibility than providing an effect size estimate. The proportion of patients who would enroll in MOUD (primary outcome) was unknown for both the PRSS and control group. We based the estimated proportions for the a-priori power analysis on a randomized trial comparing buprenorphine administration during medical hospitalization and linkage to office-based buprenorphine post-discharge to buprenorphine detoxification (Liebschutz et al., 2014). In that study, the linkage patients were more likely to enter office-based buprenorphine treatment (72.2 %) compared to the detox group (11.9 %). The linkage condition was much more intensive than PRSS and, thus, it was expected that the PRSS enrollment rate would be considerably less than that observed for the linkage group. Assuming that 12 % of the Control group enrolled in MOUD, and a total sample size of 80 yielded 80 % power using a two-tailed test and α = .05 to detect a PRSS effect for a MOUD enrollment rate of ≥ 39 % with no missing data. Power to detect a significant effect would diminish accordingly with the level of missing data.
2.2. Participants
Potential participants were recruited through various methods including advertisements, flyers, and word-of-mouth in Cincinnati, Ohio. All participants were given a thorough explanation of the study and signed an informed consent form that was approved by the University of Cincinnati Institutional Review Board. Eligible participants were at least 18 years of age and reported being treated for an opioid overdose within the past 6 months. To be eligible, participants were required to have an opioid-positive urine drug screen (UDS), score as “high risk” for heroin and/or non-medical use of prescription opioids on the NIDA modified ASSIST (i.e., ≥ 27), be willing to have their intervention audio recorded and rated if randomized to the PRSS arm, and have access to a phone. Participants were excluded from the study if they self-reported being currently engaged in substance use disorder treatment or unlikely to complete the study (e.g., probable incarceration, residence > 40 miles from site, unable to provide reliable locators). Study data were collected from January 2018 through July 2019.
2.3. PRSS interventionists
Eligible PRSS Interventionists were at least 18 years of age, able to provide informed consent in English, enrolled in a MOUD program for at least one year, currently enrolled in treatment at a University of Cincinnati (UC)-affiliated MOUD program, reported being abstinent from illicit opioids for at least one year, and had experienced, witnessed, and/or lost a family member or friend to an overdose. Potential PRSS Interventionists were excluded if: 1) they were unwilling to sign a release of information to allow research staff to confirm pertinent eligibility criteria and to monitor clinical status with UC-MOUD staff; 2) treatment program staff had significant clinical concerns about their participation; 3) they were unwilling to have their sessions audio recorded and assessed by an intervention trainer; or 4) they had specific plans to leave the UC-MOUD program within the next 6 months. All PRSS Interventionists successfully completed intervention training. At the end of their participation, the PRSS Interventionists were asked to rate their satisfaction with being an Interventionist on a 5-point Likert scale (1=Very Dissatisfied to 5=Very Satisfied).
2.4. Study treatments
2.4.1. Personally-tailored OEND (PTOEND)
All randomized participants received a Narcan® Nasal Spray kit and PTOEND which included an information packet with three reports that a research assistant (RA) reviewed with the participant. The reports were generated from the participant’s responses to two surveys: the Personal Opioid-Overdose Risk Survey (PORS) and the Opioid Overdose and Treatment Awareness Survey (OOTAS); detailed information about the reports and surveys are published elsewhere (Winhusen et al., 2016). The “Personal Overdose Risk Factors Report” was generated from the participant’s responses to the PORS, which assesses an individual’s opioid overdose risk factors. The PORS only includes risk factors for which there is documented evidence and scoring for each item is based on the strength of the evidence that the factor increases risk (Winhusen et al., 2016). The “Opioid Overdose Information Report” and the “Medication Assisted Treatment (MAT) Report” were generated from the OOTAS, which assesses knowledge about opioid overdose and MOUD. The OOTAS is comprised of four sections: 1) opioid overdose risk factors; 2) signs of an opioid overdose; 3) how to respond to an opioid overdose; and 4) myths about MOUD (Winhusen et al., 2016). The first three sections include only evidence-based items supported by a literature review, while items for the fourth section were based on both a literature review and on input from the medical staff of the UC-affiliated methadone program. The reports generated from the OOTAS provide feedback on the questions answered incorrectly by the participant to provide targeted knowledge enhancement, including the correction of myths about MOUD. All participants also received information about local MOUD providers and standard information about overdose and MOUD including 1) SAMHSA’s “Opioid Overdose Prevention Toolkit: Safety Advice for Patients and Family Members”(Substance Abuse and Mental Health Services Administration, 2016b) and “Recovering from Opioid Overdose” (Substance Abuse and Mental Health Services Administration, 2016a) (Substance Abuse and Mental Health Services Administration, 2011); and 2) SAMHSA’s “Medication-Assisted Treatment for Opioid Addiction: Facts for Families and Friends”.
2.4.2. PRSS
2.4.2.1. Intervention
The participants randomized to the PRSS arm received the 20-minute telephone intervention in addition to PTOEND. The PRSS Interventionist was provided with the results of the participant’s three PTOEND reports so that the Interventionist could answer participant questions during the call. The primary goal of the call was to encourage the participant to enroll in MOUD; however, PRSS Interventionists were reminded that it was important to be accepting and supportive if the participant was not currently interested in MOUD. The call was unscripted but the PRSS Interventionists were provided with guidelines during the initial training and certification process as well as through ongoing monitoring of the recorded phone conversations. PRSS Interventionists were provided with study cell phones for use in delivering the intervention and for staying in contact with research staff regarding scheduled and completed interventions.
2.4.2.2. Training
A comprehensive PRSS Interventionist training package was developed and utilized for the trial (Table 1 ). PRSS Interventionists completed 5 training requirements: 1) reading the training manual; 2) attending a training session on the intervention; 3) completing a practice intervention with the assigned trainer taking the role of a participant; 4) mastering knowledge of MOUD and opioid overdose, as demonstrated by scoring at least 90 % on the OOTAS; and 5) passing a scored mock intervention in which the intervention is provided to a person who is not a patient. All four PRSS Interventionists successfully completed the training and certification process within the target 4 -h timeframe.
Table 1.
Peer Recovery Support Service Training Package.
| Item | Description |
|---|---|
| Peer Interventionist Training Manual | General instruction for the intervention, includes these sections: a) Introduction and Overview b) Treatment Engagement Phone Call: general guidelines, basic listening strategies, a sample introduction, instruction on how to ask about the participant feedback reports, talking about treatment engagement, managing participant questions, and maintaining confidentiality c) Participant Reports: an explanation of each item on the feedback reports, plus anticipated questions and standard responses to use during the call. The interventionist must understand this information about overdose risk and medication assisted treatment in order to manage participant questions and clarify misconceptions during the intervention. d) Becoming a Certified PRSS Interventionist: information about the requirements and process for becoming certified e) Sample Reports: this appendix provides sample reports as they would appear to the participant |
| Training Slides | Full-color deck of 35 PowerPoint slides for use in live training situations. The slides cover the information in the Training Manual, plus additional guidance in each area and the opportunity to role-play various elements of the intervention. |
| Opioid Overdose Knowledge Check | Trainees are provided with the Opioid Overdose and Treatment Awareness Survey (OOTAS) as a knowledge check and demonstration of content competency; a score of 90 % is required for certification |
| Mock Video Demonstration | 7-minute video provides brief role-plays of each element of the telephone intervention. The video provides both good and bad examples for delivering the intervention. |
| Mock Audio Demonstrations | 3 brief (scripted) audio files demonstrate how the intervention might be provided with a variety of patient responses. These files can be provided to the interventionists as “refresher” demonstrations to assist them in adhering to the intervention post-certification. |
| Actual audio recordings | 4 audio recordings of “actual” intervention sessions from the Training Phase of the TTIP-PRO study. These recordings are valuable for sharing various intervention delivery styles. |
| Administrative forms | a) Interventionist Agreement: provides guidelines for serving as an interventionist, including management of audio equipment and confidentiality b) Equipment Form: provides a record of audio equipment provided to and returned by the PRSS Interventionist c) Audio File Log: provides a standard form for the PRSS Interventionist to log completed calls and audio files |
2.4.2.3. Treatment integrity
During the study, PRSS Interventionist adherence and competence were rated on 6 dimensions: 1) Ability to provide information while maintaining a conversational tone; 2) Ability to successfully complete the intervention within 20 min; 3) Ability to listen; 4) Sufficient familiarity with correct information about opioid overdose and MOUD to answer the participant’s questions; 5) Ability to remain non-judgmental and encouraging; and 6) Ability to avoid confrontation. Each dimension was rated as 1 – Meets Expectation, 2 – Needs Improvement, or 3 – Expectations Not Met/Additional Training Required. If PRSS Interventionists scored below 1 on more than 1 dimension for a given intervention, they were required to receive additional individualized training. Prior to the start of the randomized trial, two trainers rated training tapes to determine their inter-rater reliability via intraclass correlation coefficients (ICCs) (Hallgren, 2012; Shrout and Fleiss, 1979). The ICC of the two raters was 0.89, with an ICC of 0.75–1.0 reflecting excellent agreement (Hallgren, 2012). With the exception of 4 sessions that were not audiotaped due to difficulties with the recording equipment, all PRSS calls were rated by one of the trainers for which inter-rater reliability was established. There were no instances in which a PRSS Interventionist scored less than 1 on more than 1 dimension and, thus, no additional training was required during the study.
2.4.2.4. Intervention costs
PRSS interventionist training and supervision was paid for with approximately 3% full-time equivalent salary support of a master’s level therapist/trainer over the course of the trial. PRSS interventionists were provided a basic flip-style phone with a pre-paid year’s supply of activation and minutes, costing approximately $130 each. An external digital voice recorder with telephone pickup microphone cost an additional $82 each. Printed materials, including the 34-page training manual and additional administrative forms, cost less than $1 per PRSS interventionist. PRSS Interventionists were compensated $40 for completing training/certification and $20 for each participant for whom they provided the intervention.
2.5. Procedures
Study data collection and randomization were performed using REDCap (Harris et al., 2019).
After signing the informed consent form, the study candidate completed screening and baseline assessments. All eligible participants were randomized to PRSS or Control and received the PTOEND intervention during the randomization visit. Participants in the PRSS arm were scheduled to complete the telephone intervention within 2 weeks of randomization. Study participants completing screening and attending all in-person study visits were reimbursed $200 for their time and travel. Participants completing the telephone visit 3 weeks post-randomization received $20 reimbursement.
2.6. Participant measures
The a-priori primary outcome measure was enrollment in MOUD (Yes/No) within the 12-month follow-up period. A release of information was obtained for participants reporting entry into MOUD to allow verification of treatment entry. The a-priori secondary outcome measures were experiencing an opioid overdose (Yes/No) within the 12-month follow-up and change in opioid use, as measured by the Timeline Follow-back (TLFB) procedure and urine drug screens (UDS), between baseline and 12-month follow-up. Consistent with other research (Connery et al., 2019), an opioid overdose was defined as a self-report of an overdose in which the participant was not able to respond to others or breathe adequately, resulting in Narcan/naloxone rescue and/or emergency medical care. Urine samples were collected using temperature monitoring and the validity of urine samples was checked with the use of a commercially available adulterant test. Urine samples were tested for the following opioids: buprenorphine, fentanyl, opiates, methadone, and oxycodone; a positive buprenorphine/ methadone result was not scored as illicit opioid use for individuals with verified MOUD enrollment. Self-report of past month opioid use was assessed using the Timeline Follow-back (TLFB) method (Fals-Stewart et al., 2000), which is a widely employed and well-validated method. The MOUD enrollment, opioid overdose, and opioid use outcome measures were collected at each research visit.
The acceptability of the intervention was assessed during the telephone visit 3 weeks post-randomization. The assessment used, the Helpfulness of Peer Intervention (HOPI), was created by the authors to assess the degree to which the intervention was seen as personally relevant and credible. The HOPI was created after a review of the literature revealed no existing measure; given that peers have similar life experiences to the participants and are generally viewed to be credible (Solomon, 2004) it was predicted that the PRSS participants would rate the intervention highly on both relevance and credibility. The HOPI is a 12-item assessment in which each statement is rated on a 5-point Likert scale (1=strongly disagree; 5=strongly agree). The HOPI has “face validity” but has not otherwise undergone validity or reliability testing.
2.7. Data analysis
All analyses were completed on the intention-to-treat sample using SAS (SAS Institute, Inc.). For MOUD enrollment and opioid overdose, status at a missed visit was assumed to be no MOUD entry / no overdose. However, MOUD enrollment and opioid overdose measures were cumulative over the entire time period and, thus, participants were scored as enrolled in MOUD / having had an opioid overdose within the 12-month follow-up period if they met criteria at any of the completed visits (i.e., the 12-month values also included any MOUD enrollment or overdose previously reported at Month 3 or 6). Statistical tests were conducted at a 5 % Type I error rate (two-sided) for all measures. Odds ratios with 95 % confidence intervals were computed where appropriate. Pearson's chi-squared tests, Fisher's exact tests, and Cochran-Armitage tests were used for categorical data, depending on the nature of the data. Wilcoxon rank-sum tests and Student's t-tests were used for numeric data, depending on which test's assumptions were more appropriate.
3. Results
3.1. PRSS interventionists
The four PRSS Interventionists for the trial included three women (75 %). All of the PRSS Interventionists were White and non-Hispanic. Their average age was 44.3 (SD = 8.4). Three of the PRSS Interventionists were enrolled in a methadone program (75 %) and one in a buprenorphine program (25 %). All of the PRSS Interventionists completed the study and rated their satisfaction with being an Interventionist as “satisfied” or “very satisfied”.
3.2. Participants and disposition
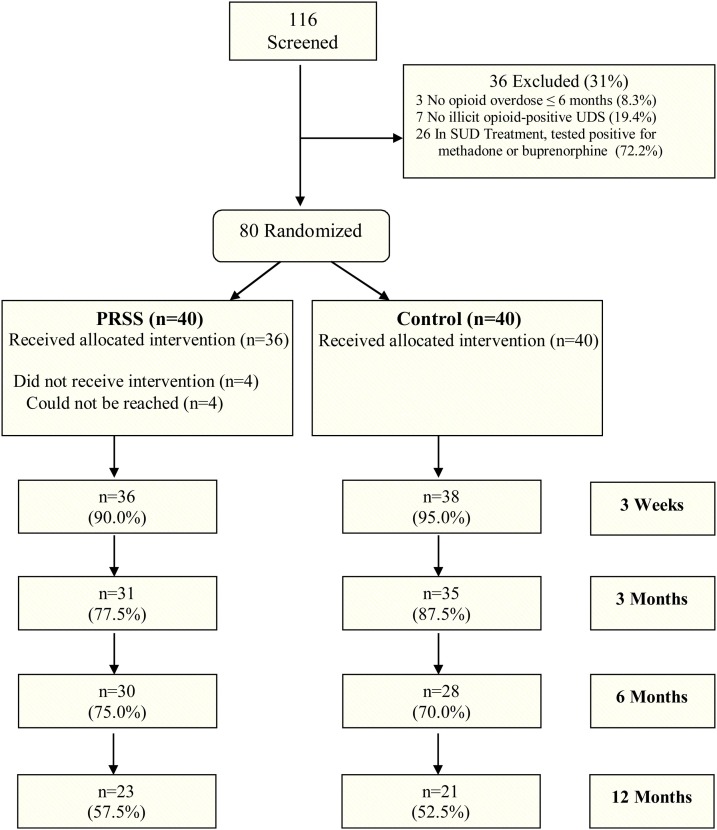
As shown in Fig. 1 , of 116 potential participants consented and screened, 80 were randomized to PRSS (n = 40) or Control (n = 40). Over 90 % of participants were recruited from flyers handed out at a syringe exchange program. The participants were recruited over the course of 7 months, yielding a recruitment rate of 11.4 randomizations per month. Approximately 55 % of participants completed the 12-month follow-up visit, with no group differences on completion rate or reasons for non-completion. Approximately 23 % of non-completers could not complete the study due to being incarcerated, one participant withdrew consent, and the rest of the non-completers were individuals who did not return for the research visit and could not be contacted. Demographic and baseline characteristics did not differ significantly between groups. The sample was 55 % male and 12.5 % African American; participants were 39 years of age on average (Table 2 ).
Fig. 1.
CONSORT chart of participant flow from pre-screening to 12-month follow-up.
Table 2.
Participant demographic and baseline characteristics as a function of treatment group.
| PRSSa (N = 40) | Control (N = 40) | Treatment Group Test Statistic | |
|---|---|---|---|
| Age, mean (std. dev.) | 40.3 (12.5) | 38.0 (10.3) | Wb = -0.5 |
| Gender, male, n (%) | 26 (65.0 %) | 18 (45.0 %) | X2 (1) = 3.2 |
| Race, n (%) | Fc = 0.09 | ||
| African-American | 5 (12.5 %) | 5 (12.5 %) | |
| Caucasian | 32 (80.0 %) | 32 (80.0 %) | |
| Other/mixed | 3 (7.5 %) | 3 (7.5 %) | |
| Ethnicity, Hispanic, n (%) | 1 (2.5 %) | 1 (2.5 %) | F = 0.51 |
| Years of opioid use, mean (std. dev.) | 15.9 (12.2) | 12.8 (10.5) | W = -1.2 |
| Number of overdoses, mean (std. dev.) | 7.5 (8.2) | 6.0 (5.9) | td (70.9) = -0.9 |
| Intravenous opioid use, n (%) | 36 (90.0 %) | 37 (92.5 %) | F = 0.28 |
| Opioid use, days in past 30, mean (std. dev.) | 26.8 (3.3) | 26.3 (5.1) | t (66.4) = -0.5 |
| Type of opioid used | X2 (1) = 1.4 | ||
| Heroin only n (%) | 17 (42.5 %) | 12 (30.0 %) | |
| Prescription only n (%) | 0 (0.0 %) | 0 (0.0 %) | |
| Prescription and heroin n (%) | 23 (57.5 %) | 28 (70.0 %) |
Peer Recovery Support Services; bW = Wilcoxon; cF = Fisher’s Exact; dt = Student’s t-test.
3.3. Treatment exposure
Of the 40 participants randomized to the PRSS arm, 36 (90 %) received the intervention. The other four participants could not be reached within the two-week window allotted for the administration of the intervention. Per the intention-to-treat principle, the analysis was based on the randomized treatment assignment, not on the intervention actually received.
3.4. MOUD entry
At 12-month follow-up, 32.5 % of PRSS, compared to 17.5 % of Control participants had verified MOUD entry (Table 3 ), although the effect was not statistically significant (X2 = 2.4, p = 0.12; odds ratio = 2.27 (95 % confidence interval: 0.79–6.49)).
Table 3.
MOUD entry, opioid overdose, and opioid use as a function of treatment arm and time.
| PRSSa (Nrand. = 40) | Control (Nrand. = 40) | Treatment Group Test Statistic | |
|---|---|---|---|
| Verified MOUDb Treatment Entry | |||
| 3-month follow-up, n (%) | 7 (17.5 %) | 4 (10.0 %) | X2(1) = 0.9, p = 0.33 |
| 6-month follow-up, n (%) | 13 (32.5 %) | 7 (17.5 %) | X2(1) = 2.4, p = 0.12 |
| 12-month follow-up, n (%) | 13 (32.5 %) | 7 (17.5 %) | X2(1) = 2.4, p = 0.12 |
| Self-reported Opioid Overdose | |||
| 3-month follow-up, n (%) | 1 (2.5 %) | 6 (15.0 %) | Fc = 0.048, p = 0.11 |
| 6-month follow-up, n (%) | 4 (10.0 %) | 12 (30.0 %) | X2(1) = 5.0, p = 0.03 |
| 12-month follow-up, n (%) | 5 (12.5 %) | 13 (32.5 %) | X2(1) = 4.6, p = 0.03 |
| Opioid-positive Urine Drug Screensd | |||
| 3-month follow-up, n (%) | 28 (93.3 %) | 30 (88.2 %) | CAe = 0.70, p = 0.68 |
| 6-month follow-up, n (%) | 29 (100.0 %) | 24 (100.0 %) | – |
| 12-month follow-up, n (%) | 20 (87.0 %) | 18 (85.7 %) | CA = 0.12, p = 1.00 |
| Self-reported Past Month Opioid Use Days | |||
| 3-month follow-up, n (%) | 21.6 (10.9) | 17.3 (12.1) | Wf = 1.4, p = 0.18 |
| 6-month follow-up, n (%) | 19.1 (10.5) | 19.3 (3.3) | W = 0.0, p = 0.99 |
| 12-month follow-up, n (%) | 17.7 (12.5) | 15.7 (13.5) | W = -0.3, p = 0.73 |
Peer Recovery Support Services; bMedication for Opioid Use Disorder; cF = Fisher’s Exact; dMethadone and buprenorphine excluded for participants enrolled in MOUD; eCA = Cochran-Armitage; fW = Wilcoxon.
3.5. Opioid overdose
As can be seen in Table 3, a significantly lower proportion of PRSS participants experienced an opioid overdose by 12-month follow-up (12.5 % of PRSS participants, 32.5 % of Control, p = 0.03). While the difference in overdose proportion was significant at both the 12-month and 6-month follow-ups, the difference was somewhat larger at 6-month follow-up.
3.6. Opioid use
There were no significant group differences on opioid-positive UDS, nor were there significant group differences on self-reported days of illicit opioid use (Table 3). Opioid-positive UDS results remained high (≥85 %) throughout the study.
3.7. Intervention acceptability
As can be seen in Table 4 , the PRSS participants completing the HOPI (n = 35) generally reported that the intervention was both relevant and credible. On all 12 items of the HOPI, ≥ 80 % of the respondents strongly agreed or agreed somewhat with the relevance and credibility statements.
Table 4.
Ratings of intervention relevance and credibility from Peer recovery support services (PRSS) participants.
| 1: Strongly Disagree | 2: Disagree Somewhat | 3: Neither Agree nor Disagree | 4: Agree Somewhat | 5: Strongly Agree | |
|---|---|---|---|---|---|
| Relevance Scores, n (%) | |||||
| Learned new helpful things | 0 (0.0 %) | 1 (2.9 %) | 5 (14.3 %) | 10 (28.6 %) | 19 (54.3 %) |
| Learned things to prevent overdose | 1 (2.9 %) | 3 (8.6 %) | 3 (8.6 %) | 9 (25.7 %) | 19 (54.3 %) |
| Learned things about substance abuse treatment | 0 (0.0 %) | 1 (2.9 %) | 1 (2.9 %) | 7 (20.0 %) | 26 (74.3 %) |
| Plan to make some changes based on learning | 0 (0.0 %) | 1 (2.9 %) | 3 (8.6 %) | 18 (51.4 %) | 13 (37.1 %) |
| Information applies to me | 3 (8.6 %) | 0 (0.0 %) | 1 (2.9 %) | 8 (22.9 %) | 23 (65.7 %) |
| Information is important and should be shared with those who overdose | 0 (0.0 %) | 0 (0.0 %) | 1 (2.9 %) | 5 (14.3 %) | 29 (82.9 %) |
| Credibility Scores, n (%) | |||||
| Peer’s advice was helpful | 0 (0.0 %) | 1 (2.9 %) | 1 (2.9 %) | 7 (20.0 %) | 26 (74.3 %) |
| Peer was knowledgeable | 0 (0.0 %) | 0 (0.0 %) | 1 (2.9 %) | 11 (31.4 %) | 23 (65.7 %) |
| Believed what the peer said | 2 (5.7 %) | 1 (2.9 %) | 0 (0.0 %) | 10 (28.6 %) | 22 (62.9 %) |
| Peer understood my point-of-view | 0 (0.0 %) | 0 (0.0 %) | 0 (0.0 %) | 11 (31.4 %) | 24 (68.6 %) |
| Enjoyed talking with peer | 0 (0.0 %) | 1 (2.9 %) | 0 (0.0 %) | 11 (31.4 %) | 23 (65.7 %) |
| Understood what peer was trying to tell me | 2 (5.7 %) | 1 (2.9 %) | 0 (0.0 %) | 8 (22.9 %) | 24 (68.6 %) |
Table only includes PRSS participants who completed the Week 3 telephone visit and reported engagement with the intervention. Items are 1–5 Likert Scale values (1=Strongly Disagree; 5=Strongly Agree).
4. Discussion
There has been a dramatic increase in the use of PRSS to provide opioid overdose education and promote MOUD engagement, due in part to the increasing availability of reimbursement for these services (McGuire et al., 2020; Medicaid and CHIP Payment and Access Commission, 2019) but their efficacy has not been established in the research literature. The present randomized pilot trial found that a significantly lower proportion of participants with a prior opioid overdose who received a single 20-min telephone-delivered PRSS, relative to Control, experienced another overdose by 12-month follow-up. Participants receiving the PRSS intervention also doubled their rate of verified MOUD entry by 12-month follow-up, but the difference did not reach statistical significance. There was no significant treatment effect observed for illicit opioid use, which is perhaps not surprising since the goal of the present PRSS was to encourage enrollment in MOUD and illicit opioid use was not directly addressed. Peer interventionist and PRSS participant ratings suggest that the intervention had good acceptability.
The results from the present study suggest that brief PRSS interventions may be efficacious for reducing recurring opioid overdoses and may also have promise for encouraging MOUD enrollment. The PRSS evaluated in the present trial was a single 20-minute telephone-delivered intervention, which is much less intensive than many of the PRSS interventions being developed and evaluated (Eddie et al., 2019; McGuire et al., 2020). When the present PRSS intervention was initially being developed in 2014, PRSSs were not being utilized as widely and were not as well reimbursed and, hence the goal was to develop an intervention that was of sufficiently low cost to be sustainable regardless of insurance reimbursement. The present study found that the Peer Interventionists were able to be trained and certified within 4 h, were satisfied with their roles as Interventionists, and the intervention was successfully implemented with relatively low cost. In the current era of COVID-19, it is notable that this intervention was designed to be implemented by phone, thus eliminating the need for in-person contact.
The finding of a significant reduction in opioid overdose and a clinically meaningful, albeit not statistically significant, impact on MOUD enrollment suggests that this low cost, easy to train and deliver intervention is promising and may be worthy of further development to strengthen the treatment effect. For both MOUD entry and overdose, the PRSS intervention reached its maximum impact by 6-month follow-up and retention was still adequate at 6 months, with 72 % of the sample completing the 6-month visit. Therefore, the impact of the intervention might be amplified, for example, by including a booster call at the 6-month follow-up. The booster phone call could be designed to encourage those who have not entered treatment to do so and to provide encouragement for those enrolled in MOUD to stay in treatment.
The results from the present pilot trial should be considered in light of several limitations. First, the sample sizes were relatively small, with 40 per group, and the study was conducted at a single site, thus the degree to which the findings are generalizable is unclear. Second, while our definition of opioid overdose is consistent with the definition used in other research (Connery et al., 2019), it is still a self-report measure and, thus open to response biases. Third, because all participants in the present trial received PTOEND we do not know whether the PRSS intervention would have a similar impact in the absence of PTOEND. Finally, it is important to note the relatively low level of study visit attendance at Month 12 (55 %). This level of missing data likely leads to underestimation of both MOUD enrollment and opioid overdose for the full 12-month study period, but attendance rates were not substantially different between the two randomized treatment arms. The a-priori power analysis suggested sufficient power (i.e., 80 %) to detect a significant difference in MOUD enrollment with 80 participants assuming 12 % of Control and ≥39 % of PRSS participants enrolled in MOUD with power decreasing as missing data increased; thus, the failure to detect a significant treatment effect for MOUD enrollment was likely due to MOUD entry being higher in the Control (17.5 %) and lower in PRSS (32.5 %) groups than anticipated exacerbated by the 45 % missing data at Month 12. A sensitivity analysis using varying assumptions about missing data would be under-powered in this small pilot trial, which was designed only to assess whether the treatment has sufficient promise to warrant further investigation. Future trials of the intervention will be powered to more definitively demonstrate a treatment effect, and the inferences from such a trial will be subject to greater examination of sensitivity to missing data assumptions. Given that 23 % of missing data were due to participants being incarcerated, future research might mitigate missing data by securing data collection from incarcerated participants.
The present trial also had several strengths. In a recent review of the PRSS research literature, Eddie and colleagues (Eddie et al., 2019) outlined a number of methodological limitations of the existing PRSS research including: 1) limited use of the randomized controlled trial (RCT) design; 2) lack of information about peer training protocols; and 3) inability to isolate the effect of PRSS due to studies which combined PRSS with clinician-administered interventions. The present study addressed these limitations by utilizing a RCT design, utilizing a comprehensive training package, and evaluating the PRSS intervention without an additional clinician-delivered intervention. The independent verification of MOUD entry is another study strength.
In conclusion, the results from this randomized pilot trial suggest that a brief, telephone-delivered PRSS intervention has promise for increasing MOUD enrollment and decreasing recurring opioid overdoses in individuals surviving an opioid overdose. Further development and testing of this PRSS intervention, particularly in light of the current U.S. opioid epidemic, seems warranted.
Contributors
Dr. Winhusen was the principal investigator for this study, conceptualized the intervention and the study design, contributed to the analysis and interpretation of the data, and led the drafting of the manuscript. Drs. Lyons and Wilder contributed to the study design, contributed to interpretation of the data, and critically reviewed the manuscript. Ms. Kropp developed the training package, contributed to the interpretation of the data, and critically reviewed the manuscript. Mr. Theobald contributed to the interpretation of the data and critically reviewed the manuscript. Mr. Lewis conducted analyses, contributed to the interpretation of the data, and critically reviewed the manuscript. All authors contributed to and have approved the final manuscript.
Role of funding source
This study was supported by grant R34DA040862 from the National Institute on Drug Abuse (NIDA), which had no further role in study design, collection and analysis of data, the writing of this manuscript, or in the decision to submit this manuscript for publication.
Declaration of Competing Interest
The authors have no potential conflicts of interest to report.
References
- Clark A.K., Wilder C.M., Winstanley E.L. A systematic review of community opioid overdose prevention and naloxone distribution programs. J. Addict. Med. 2014;8(3):153–163. doi: 10.1097/ADM.0000000000000034. [DOI] [PubMed] [Google Scholar]
- Connery H.S., Taghian N., Kim J., Griffin M., Rockett I.R.H., Weiss R.D., Kathryn McHugh R. Suicidal motivations reported by opioid overdose survivors: a cross-sectional study of adults with opioid use disorder. Drug Alcohol Depend. 2019;205 doi: 10.1016/j.drugalcdep.2019.107612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddie D., Hoffman L., Vilsaint C., Abry A., Bergman B., Hoeppner B., Weinstein C., Kelly J.F. Lived experience in new models of care for substance use disorder: a systematic review of peer recovery support services and recovery coaching. Front Psychol. 2019;10:1052. doi: 10.3389/fpsyg.2019.01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fals-Stewart W., O’Farrell T.J., Freitas T.T., McFarlin S.K., Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J. Consult. Clin. Psychol. 2000;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Hallgren K.A. Computing inter-rater reliability for observational data: an overview and tutorial. Tutor. Quant. Methods Psychol. 2012;8(1):23–34. doi: 10.20982/tqmp.08.1.p023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., McLeod L., Delacqua G., Delacqua F., Kirby J., Duda S.N., Consortium R.E. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inf. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Brown D.F., Tsugawa Y., Camargo C.A., Jr. Epidemiology of emergency department visits for opioid overdose: a population-based study. Mayo Clin. Proc. 2014;89(4):462–471. doi: 10.1016/j.mayocp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Larochelle M.R., Stopka T.J., Xuan Z., Liebschutz J.M., Walley A.Y. Medication for opioid use disorder after nonfatal opioid overdose and mortality. Ann. Intern. Med. 2019;170(6):430–431. doi: 10.7326/L18-0685. [DOI] [PubMed] [Google Scholar]
- Liebschutz J.M., Crooks D., Herman D., Anderson B., Tsui J., Meshesha L.Z., Dossabhoy S., Stein M. Buprenorphine treatment for hospitalized, opioid-dependent patients: a randomized clinical trial. JAMA Intern. Med. 2014;174(8):1369–1376. doi: 10.1001/jamainternmed.2014.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire A.B., Powell K.G., Treitler P.C., Wagner K.D., Smith K.P., Cooperman N., Robinson L., Carter J., Ray B., Watson D.P. Emergency department-based peer support for opioid use disorder: emergent functions and forms. J. Subst. Abuse Treat. 2020;108:82–87. doi: 10.1016/j.jsat.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicaid and CHIP Payment and Access Commission . 2019. Recovery Support Services for Medicaid Beneficiaries with Substance Use Disorder. Washington, DC. [Google Scholar]
- Naumann R.B., Durrance C.P., Ranapurwala S.I., Austin A.E., Proescholdbell S., Childs R., Marshall S.W., Kansagra S., Shanahan M.E. Impact of a community-based naloxone distribution program on opioid overdose death rates. Drug Alcohol Depend. 2019;204 doi: 10.1016/j.drugalcdep.2019.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J.A., Schwartz R.P., Mitchell S.G., Reisinger H.S., Kelly S.M., O’Grady K.E., Brown B.S., Agar M.H. Why don’t out-of-treatment individuals enter methadone treatment programmes? Int. J. Drug Policy. 2010;21(1):36–42. doi: 10.1016/j.drugpo.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L., Seth P., Kariisa M., Wilson N., Baldwin G. Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR. Morb. Mortal. Wkly Rep. 2018;67(5152):1419–1427. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Solomon P. Peer support/peer provided services underlying processes, benefits, and critical ingredients. Psychiatr. Rehabil. J. 2004;27(4):392–401. doi: 10.2975/27.2004.392.401. [DOI] [PubMed] [Google Scholar]
- Sordo L., Barrio G., Bravo M.J., Indave B.I., Degenhardt L., Wiessing L., Ferri M., Pastor-Barriuso R. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus M.M., Ghitza U.E., Tai B. Preventing deaths from rising opioid overdose in the US - the promise of naloxone antidote in community-based naloxone take-home programs. Subst. Abuse Rehabil. 2013;2013(4) doi: 10.2147/SAR.S47463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Substance Abuse and Mental Health Services Administration. 2011. Medication-assisted treatment for opioid addiction: facts for families and Friends. HHS publication No. (SMA) 09-4443. (Ed.). Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Substance Abuse and Mental Health Services Administration. 2016. SAMHSA opioid overdose prevention toolkit: recovering from opioid overdose. HHS publication No. (SMA) 16-4742. (Ed.). Rockville, MD. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Substance Abuse and Mental Health Services Administration. 2016. SAMHSA opioid overdose prevention toolkit: safety advice for patients & family members. HHS publication No. (SMA) 16-4742. (Ed.). Rockville, MD. [Google Scholar]
- Uebelacker L.A., Bailey G., Herman D., Anderson B., Stein M. Patients’ beliefs about medications are associated with stated preference for methadone, buprenorphine, naltrexone, or no medication-assisted therapy following inpatient opioid detoxification. J. Subst. Abuse Treat. 2016;66:48–53. doi: 10.1016/j.jsat.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wargo E.M. Overdose prevention through medical treatment of opioid use disorders. Ann. Intern. Med. 2018;169(3):190–192. doi: 10.7326/M18-1397. [DOI] [PubMed] [Google Scholar]
- Walley A.Y., Xuan Z., Hackman H.H., Quinn E., Doe-Simkins M., Sorensen-Alawad A., Ruiz S., Ozonoff A. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174. doi: 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner S.G., Baker O., Bernson D., Schuur J.D. One-year mortality of patients after emergency department treatment for nonfatal opioid overdose. Ann. Emerg. Med. 2020;75(1):13–17. doi: 10.1016/j.annemergmed.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T., Theobald J., Lewis D., Wilder C.M., Lyons M.S. Development and initial testing of a tailored telephone intervention delivered by peers to prevent recurring opioid-overdoses (TTIP-PRO) Health Educ. Res. 2016;31(2):146–160. doi: 10.1093/her/cyw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaller N.D., Bazazi A.R., Velazquez L., Rich J.D. Attitudes toward methadone among out-of-treatment minority injection drug users: implications for health disparities. Int. J. Environ. Res. Public Health. 2009;6(2):787–797. doi: 10.3390/ijerph6020787. [DOI] [PMC free article] [PubMed] [Google Scholar]