Abstract
Coronavirus disease 2019 (COVID-19) pandemic poses great challenge on public health globally. To clarify the impact of COVID-19 pandemic on in-hospital management and outcomes for ST-segment elevation myocardial infarction (STEMI) patients in the nonepicenter. We enrolled consecutive STEMI patients who visited Fuwai Hospital from January to March, 2020 (N = 73) and also established a historical control including all consecutive STEMI patients in the same period of 2019 (N = 95). The primary outcome was defined as a composite endpoint of all-cause death, heart failure, cardiac shock, and cardiac arrest during hospitalization. Emergency response for COVID-19 resulted in a significant 77.6% reduction in the number of primary percutaneous coronary intervention, and a trend toward higher rate of primary composite endpoint (15.1% vs 11.6%, P = 0.51). COVID-19 pandemic results in a significant reduction in emergent reperfusion therapy, and a trend toward higher in-hospital adverse events risk.
Introduction
Since December 2019, the outbreak of coronavirus disease 2019 (COVID-19) has rapidly become a public concern, with more than 13,287,651 confirmed cases and 577,954 deaths as of July 15, 2020.1 Coexistence of COVID-19 and cardiovascular diseases (CVD) is often associated with poor prognosis.2 Once myocardial injury occurs, patients infected with COVID-19 are at high risk for severe conditions and admission to intensive care unit.3 In addition, patients with CVD account for a large proportion of deaths from COVID-19.4 Patients with acute coronary syndrome are more likely to suffer sudden deterioration in medical condition with concomitant COVID-19 due to reduced cardiac function caused by myocardial ischemia or necrosis.5
ST-segment elevation myocardial infarction (STEMI) is an emergent CVD and requires timely primary reperfusion therapy (mainly primary percutaneous coronary intervention [PCI]).6, 7, 8 The public health emergency response for COVID-19 had significant impact on the healthcare system for STEMI globally. Nearly 27% decline of STEMI admissions was reported in Italy9 and Australia,10 as well as a 38% reduction in US cardiac catheterization laboratory STEMI activations.11 In China, hospitals are divided into designated hospitals for treating patients with diagnosed COVID-19, and nondesignated hospitals for those without COVID-19 infection. In nondesignated hospitals, to minimize infection risk among medical staffs and nosocomial transmission, cutting down on the number of primary PCI and choosing a more conservative approach is unavoidable. The present study aimed to clarify the impact of public health emergency response for COVID-19 on in-hospital management and outcomes for STEMI patients.
Methods
Study Population
We enrolled all the STEMI patients with ischemic symptoms duration ≤48 hours at presentation to the emergency department at Fuwai Hospital from January 24 to March 31 during the COVID-19 epidemic in Beijing, China. We also included all consecutive STEMI patients who were treated at Fuwai Hospital between January 24 and March 31 in 2019 as historical control. This study was approved by the ethics committee of Fuwai Hospital.
Treatment Principles for STEMI During COVID-19 Epidemic
Detailed classification criteria and corresponding management protocols for STEMI patients are described in the Supplementary Appendix. In brief, patients with confirmed or suspected COVID-19 according to the COVID-19 Diagnosis and Treatment (7th edition)12 should be transferred to COVID-19-designated hospitals and receive medical therapy as soon as possible. Patients in whom COVID-19 cannot be ruled out temporarily , defined as the absence of epidemiological history of COVID-19, with 1-2 clinical manifestations of COVID-19, but not fulfilling diagnostic criteria for COVID-19, should be transferred to designated clinics and treated with medical therapy, and at the same time screened for COVID-19 and transferred to designated hospitals if test is positive.13 Excluded patients are defined as having a clinical very small risk of COVID-19 infection, which included the absence of fever, respiratory symptoms, decreased WBC count, and epidemiological exposure to other COVID-19 cases or areas with cluster transmission.
Algorithm for Management of STEMI Patients
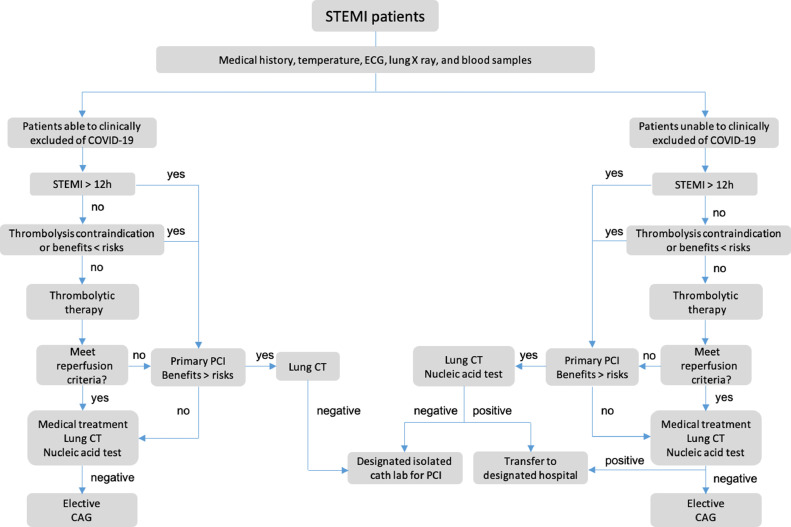
Detailed management algorithm of STEMI patients during COVID-19 pandemic in our institution are described in the supplemental material (Fig 1 ). In brief, medical staffs firstly assessed whether COVID-19 could be ruled out clinically. For patients who can be ruled out for COVID-19 infection and within 12 hours after symptom onset, with no contraindications and would benefit from thrombolysis, thrombolytic therapy should be initiated immediately. For patients with thrombolytic contraindications, failed thrombolysis, who would not benefit from thrombolysis or presenting >12 hours after symptom onset, a comprehensive benefit-risk evaluation of primary PCI is required. For patients who cannot be ruled out for COVID-19 infection, all medical activity should start in designated screening room.
FIG 1.
Algorithm for management of STEMI patients for nondesignated hospital during COVID-19 epidemic. Medical staff should first evaluate whether COVID-19 can be excluded. For patients with a clinical small risk of COVID-19 infection, within 12 hours after symptom onset, with no contradiction and will possibly gain benefit, thrombolysis should be initiated immediately. For patients with thrombolytic contraindications or failed thrombolysis, a comprehensive benefit-risk assessment should be performed, and primary PCI should be started immediately when appropriate. For patients who cannot be ruled out for COVID-19 infection temporarily, all medical practice and COVID-19 screening should be conducted simultaneously. CAG, coronary angiography; COVID-19, coronavirus disease 2019; CT, computed tomography; ECG, electrocardiogram; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
Data Collection and Adverse Clinical Event Definitions
All data were obtained by screening patient medical document. The primary outcome was defined as a composite endpoint of all-cause death, cardiac shock, cardiac arrest, and heart failure during hospitalization. Diagnostic criteria for recurrent myocardial infarction were in accordance with the fourth universal definition of myocardial infarction, when cardiac troponin (cTn) value is above the 99th percentile upper reference limit and at least one of the following characteristics: (1) presentation of myocardial symptoms; (2) new ischemic ECG changes or development of pathological Q waves.14 We also studied each individual component of the primary outcome and other 2 clinical outcomes including mechanical complication and arrhythmia. Arrhythmia included atrial fibrillation, atrial flutter, ventricular tachycardia, atrioventricular block, and sinus arrest. Mechanical complication was defined as rupture of ventricular wall, ventricular septum, or papillary muscle. Two cardiologists independently adjudicated all events by using original source documents.
Statistical Analysis
Continuous data are expressed as mean ± SD or median (interquartile range) and categorical variables are presented as counts and percentages. The continuous variables were compared using the Student t test or the Wilcoxon signed-rank test, as appropriate. The categorical variables were compared by the likelihood ratio chi-square or the Fisher exact test. Logistic regression model was used to calculate odds ratio and 95% confidence intervals (CI) for clinical adverse events according to the year of hospitalization. A total of 3 multivariate models with different level of adjustment were used: Model 1 was adjusted for age and sex, model 2 was additionally adjusted for previous myocardial infarction and previous renal insufficiency. Model 3 was further adjusted for time from symptom to hospital. All statistical analyses were performed with SAS 9.4 (SAS Institute, Cary, NC).
Results
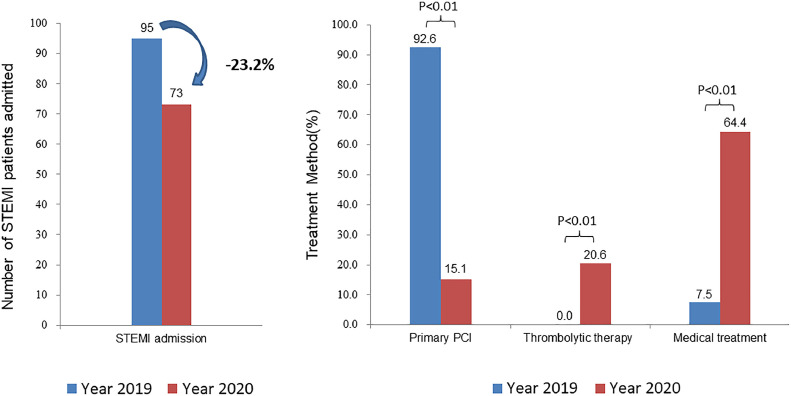
During the COVID-19 epidemic in China from January 24 to March 31, 2020, a total of 73 consecutive patients presenting to the emergency department at Fuwai Hospital within 48 hours after ischemia symptoms onset were diagnosed with STEMI. During the same period in 2019, a total of 95 consecutive STEMI patients arrived at the emergency department at Fuwai Hospital within 48 hours after symptoms onset. As expected, compared with year 2019, the proportion of patients receiving primary PCI significantly reduced (77.6% reduction, 95% CI: 66.6%-88.5%, P < 0.01), and the proportion of thrombolytic therapy (20.6% increase, 95% CI: 10.1%-31.0%, P < 0.01) and medical therapy (57.0% increase, 95% CI: 43.6%-70.4%, P < 0.01) increased (Fig 2 ). Detailed reasons for not receiving thrombolysis and primary PCI for STEMI patients in 2020 are described in the Supplementary Appendix. In brief, for patients within 12 hours after symptom onset, the primary reasons were improvement in symptoms (>50% ) and ST-segment return on ECG, advanced age, relative contradiction of thrombolysis or refusal by patients’ family members after learning about potential related risks. For patients >12 hours after symptom onset, the main reasons were symptoms improved and ST-segment return on ECG, or refusal by patients’ family members after consultation with the medical staff about the risk and benefit of PCI.
FIG 2.
Impact of public health emergency response for COVID-19 on in-hospital outcome and treatment strategy. During COVID-19 pandemic, there was a 23.2% reduction in STEMI admission and 77.6% reduction in the number of primary PCI compared with historic control. PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
Other baseline and clinical characteristics of the total study population are shown in Table 1 . There were no significant differences in baseline and clinical characteristics between groups.
TABLE 1.
Baseline and clinical characteristics of STEMI patients according to the year of hospitalization
| Variable | Year 2020 (N = 73) | Year 2019 (N = 95) | Difference (95% CI) | P value |
|---|---|---|---|---|
| Age (y) | 61.6 ± 13.1 | 60.6 ± 13.9 | 1.0 (−3.2, 5.1) | 0.65 |
| Female | 14 (19.2) | 27 (28.4) | −9.5 (−23.3, 4.8) | 0.17 |
| Hypertension | 43 (58.9) | 56 (59.0) | −0.04 (−16.3, 16.2) | 1.00 |
| Hyperlipidemia | 39 (53.4) | 51 (53.7) | −0.3 (−16.7, 16.2) | 0.97 |
| Current smoking | 36 (49.3) | 55 (57.9) | −8.6 (−25.0, 7.8) | 0.27 |
| Diabetes mellitus | 16 (21.9) | 22 (23.2) | −1.2 (−15.2, 12.7) | 0.85 |
| Previous MI | 7 (9.6) | 5 (5.3) | 4.3 (−5.0, 13.7) | 0.28 |
| Previous PCI | 7 (9.6) | 9 (9.5) | 0.1 (−10.1, 10.3) | 0.98 |
| Previous CABG | 2 (2.7) | 2 (2.1) | 0.6 (−5.3, 6.6) | 1.00 |
| Previous stroke | 10 (13.7) | 12 (12.6) | 1.1 (−10.5, 12.6) | 0.84 |
| Previous heart failure | 2 (2.7) | 2 (2.1) | 0.6 (−5.3, 6.6) | 1.00 |
| Previous renal insufficiency | 0 | 5 (5.3) | −5.3 (−11.0, 0.4) | 0.07 |
| Heart rate | 76.0 ± 19.9 | 75.8 ± 18.4 | 0.2 (−5.8, 6.2) | 0.95 |
| Systolic BP (mm Hg) | 134.3 ± 23.4 | 131.3 ± 23.7 | 3.0 (−4.4, 10.3) | 0.43 |
| Diastolic BP (mm Hg) | 81.6 ± 15.9 | 78.2 ± 16.7 | 3.3 (−1.8, 8.4) | 0.20 |
| LV (mm) | 50.0 ± 5.2 | 49.2 ± 5.1 | 0.8 (−0.8, 2.4) | 0.34 |
| Ejection fraction (%) | 51.4 ± 7.7 | 52.3 ± 7.6 | −0.9 (−3.3, 1.5) | 0.45 |
| Primary value TNI (ng/mL) | 0.2 (0.04, 1.5) | 0.2 (0.05, 3.2) | 0.003 (−0.07, 0.11) | 0.88 |
| Peak value TNI (ng/mL) | 22.6 (8.4, 44.0) | 23.0 (11.8,44.7) | −2.9 (−8.6, 3.1) | 0.33 |
| Primary value NT-proBNP (pg/fL) | 199.0 (61.4, 553.0) | 153.2 (40.7, 723.0) | −1.0 (−70.5, 56.4) | 0.96 |
| Peak value NT-proBNP (pg/fL) | 1927.0 (1074.0, 3511.0) | 1957.0 (921.6, 4014.0) | −74.8 (−586.6, 456.8) | 0.78 |
| D-B time of primary PCI (min) | 122.5 (78.5, 187.5) | 106.0 (80.0, 138.0) | 16.0 (−7.0, 46.0) | 0.15 |
| Killip classification | ||||
| I | 59 (80.8) | 82 (86.3) | −5.5 (−18.1, 7.1) | 0.34 |
| II | 8 (11.0) | 10 (10.5) | 0.4 (−10.2, 11.1) | 0.93 |
| III | 2 (2.7) | 1 (1.1) | 1.7 (−3.8, 7.2) | 0.58 |
| IV | 4 (5.5) | 2 (2.1) | 3.4 (−3.8, 10.6) | 0.41 |
| Hours after symptom onset | ||||
| 0-12h | 56 (76.7) | 70 (73.7) | 3.0 (−11.3, 17.4) | 0.65 |
| 12h-24h | 10 (13.7) | 17 (17.9) | −4.2 (−16.4, 8.0) | 0.46 |
| 24h-48h | 6 (8.2) | 6 (6.3) | 1.9 (−7.3, 11.1) | 0.63 |
| Type of AMI | ||||
| Extensive anterior wall | 13 (17.8) | 22 (23.2) | −5.4 (−18.8, 8.1) | 0.40 |
| Anterior wall | 19 (26.0) | 20 (21.1) | 5.0 (−9.2, 19.2) | 0.45 |
| Inferior wall | 41 (56.2) | 47 (49.5) | 6.7 (−9.7, 23.1) | 0.39 |
| High lateral wall | 0 | 6 (6.3) | −6.3 (−12.4, −0.2) | 0.04 |
| Clinical therapy | ||||
| Primary PCI | 11 (15.1) | 88 (92.6) | −77.6 (−88.5, −66.6) | <0.01 |
| Thrombolytic therapy | 15 (20.6) | 0 | 20.6 (10.1, 31.0) | <0.01 |
| Medical treatment | 47 (64.4) | 7 (7.4) | 57.0 (43.6, 70.4) | <0.01 |
Data are presented as n (%), mean + SD, or median (interquartile range). AMI, acute myocardial infarction; BP, blood pressure; CABG, coronary artery bypass grafting; D-B, door to balloon; LV, left ventricle; MI, myocardial infarction; PCI, percutaneous coronary intervention; TNI, troponin I.
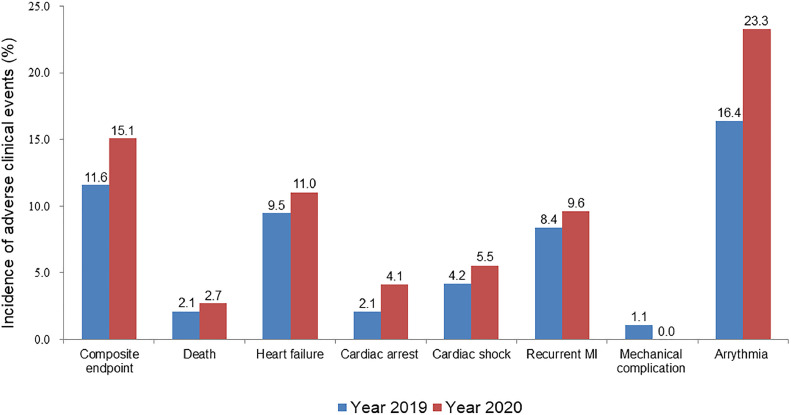
A comparison of adverse clinical events during hospitalization between patients in the 2 groups is shown in Table 2 . Patients enrolled during COVID-19 pandemic had a trend toward higher rate of composite endpoint (15.1% vs 11.6%, rate difference 3.5% [95% CI: −8.2%, 15.1%], P = 0.51), and higher rate of most other adverse events than historic control (Fig 3 ). In-hospital adverse clinical outcomes after primary PCI in the 2 groups are shown in the Supplementary Appendix Table S1. Angiographic characteristics and TIMI flow grade in patients enrolled in 2020 are shown in the Supplementary Appendix Table S2.
TABLE 2.
In-hospital clinical adverse events of patients according to the year of hospitalization
| Adverse event | Year 2020 (N = 73) | Year 2019 (N = 95) | Difference (95% CI) (%) | P value |
|---|---|---|---|---|
| Composite endpoint | 11 (15.1) | 11 (11.6) | 3.5 (−8.2, 15.1) | 0.51 |
| Death | 2 (2.7) | 2 (2.1) | 0.6 (−5.3, 6.6) | 1.00 |
| Heart failure | 8 (11.0) | 9 (9.5) | 1.5 (−9.0, 12.0) | 0.75 |
| Cardiac arrest | 3 (4.1) | 2 (2.1) | 2.0 (−4.6, 8.6) | 0.65 |
| Cardiac shock | 4 (5.5) | 4 (4.2) | 1.3 (−6.5, 9.1) | 0.73 |
| Recurrent MI | 7 (9.6) | 8 (8.4) | 1.2 (−8.8, 11.1) | 0.79 |
| Mechanical complication | 0 | 1 (1.1) | −1.1 (−4.3, 2.2) | 1.00 |
| Arrhythmia | 17 (23.3) | 16.8 | 6.5 (−7.0, 19.9) | 0.30 |
Data are presented as n (%). MI, myocardial infarction.
FIG 3.
Rate of adverse clinical events according to the year of hospitalization. MI, myocardial infarction.
Table 3 shows the results of univariate and multivariate logistic regression analyses. Hospitalization during COVID-19 pandemic was associated with trend toward higher risk of composite endpoint (odds ratio: 1.35 95% CI: 0.52, 3.51) compared with historic control, after adjustment of age, sex, previous MI, and renal insufficiency, and time from symptom to hospital. Similarly, there was a trend toward higher risk of each in-hospital individual adverse events during COVID-19 pandemic than historic control. Table 4 shows the comparison of baseline and clinical characteristics between nonreperfusion and reperfusion patients in STEMI cases enrolled in 2020. More extensive anterior acute myocardial infarction (AMI), was found in reperfusion patients (8.5% vs 34.6%, P = 0.01). Clinical outcomes between nonreperfusion and reperfusion STEMI patients hospitalized in 2020 are shown in Table 5 . Compared with reperfusion group, patients who did not receive emergent reperfusion therapy had a trend toward higher rate of composite endpoint (19.2% vs 7.7%, rate difference: 11.5% [95%CI: −6.7%, 29.7%], P = 0.31) and other adverse events during hospitalization.
TABLE 3.
Association between the year of hospitalization with adverse clinical events
| Adverse events | Univariate model | Multivariate model |
||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Composite endpoint | 1.36 (0.55, 3.33) | 1.37 (0.55, 3.41) | 1.35 (0.52, 3.51) | 1.35 (0.52, 3.51) |
| Death | 1.31 (0.18, 9.53) | 1.21 (0.16, 9.07) | 2.10 (0.17, 26.61) | 2.25 (0.18, 28.18) |
| Heart failure | 1.18 (0.43, 3.21) | 1.23 (0.43, 3.52) | 1.16 (0.37, 3.58) | 1.10 (0.35, 3.46) |
| Cardiac arrest | 1.99 (0.32, 12.25) | 1.98 (0.31, 12.53) | 4.68 (0.43, 50.58) | 4.40 (0.40, 48.70) |
| Cardiac shock | 1.32 (0.32, 5.46) | 1.20 (0.29, 5.05) | 1.51 (0.32, 7.17) | 1.53 (0.32, 7.22) |
| Recurrent MI | 1.15 (0.40, 3.34) | 1.35 (0.45, 4.09) | 1.32 (0.43, 4.01) | 1.31 (0.43, 4.02) |
| Mechanical complication | NA | NA | NA | NA |
| Arrhythmia | 1.50 (0.70, 3.22) | 1.44 (0.66, 3.14) | 1.44 (0.65, 3.18) | 1.44 (0.65, 3.19) |
Data are presented as n (%). MI, myocardial infarction; NA, not available.
Model 1: adjusted for age and sex.
Model 2: adjusted for age, sex, previous myocardial infarction, and previous renal insufficiency.
Model 3: adjusted for age, sex, previous myocardial infarction, and previous renal insufficiency and time from symptom to hospital.
TABLE 4.
Baseline and clinical characteristics of patients enrolled in 2020 according to reperfusion treatment
| Variable | Nonreperfusion group (N = 47) | Reperfusion group (N = 26) | Difference (95% CI) | P value |
|---|---|---|---|---|
| Age (y) | 63.1 ± 13.9 | 58.8 ± 11.2 | 4.3 (−2.0, 10.7) | 0.18 |
| Female | 9 (19.2) | 5 (19.2) | −0.1 (−21.9, 21.8) | 1.00 |
| Hypertension | 29 (61.7) | 14 (53.9) | 7.9 (−18.8, 34.5) | 0.51 |
| Hyperlipidemia | 26 (55.3) | 13 (50.0) | 5.3 (−21.6, 32.2) | 0.66 |
| Current smoking | 22 (46.8) | 14 (53.9) | −7.0 (−33.9, 19.8) | 0.56 |
| Diabetes mellitus | 13 (27.7) | 3 (11.5) | 16.1 (−4.6, 36.8) | 0.11 |
| Previous MI | 4 (8.5) | 3 (11.5) | −3.0 (−20.7, 14.6) | 0.69 |
| Previous PCI | 4 (8.5) | 3 (11.5) | −3.0 (−20.7, 14.6) | 0.69 |
| Previous CABG | 2 (4.3) | 0 | 4.3 (−4.5, 13.0) | 0.54 |
| Previous stroke | 5 (10.6) | 5 (19.2) | −8.6 (−29.1, 11.9) | 0.31 |
| Previous heart failure | 2 (4.3) | 0 | 4.3 (−4.5, 13.0) | 0.54 |
| Previous renal insufficiency | 0 | 0 | 0 | |
| Heart rate | 74.9 ± 20.2 | 77.8 ± 19.5 | −2.9 (−12.7, 6.8) | 0.55 |
| Systolic BP (mm Hg) | 135.9 ± 23.6 | 131.3 ± 23.3 | 4.6 (−6.8, 16.1) | 0.42 |
| Diastolic BP (mm Hg) | 81.2 ± 16.1 | 82.3 ± 15.9 | −1.1 (−8.9, 6.7) | 0.78 |
| Initial LV (mm) | 50.3 ± 6.1 | 49.4 ± 3.3 | 0.92 (−1.3, 3.1) | 0.40 |
| Last LV (mm) | 51.4 ± 5.3 | 50.7 ± 3.8 | 0.68 (−1.7 ± 3.1) | 0.55 |
| Initial ejection fraction (%) | 51.4 ± 7.6 | 51.4 ± 7.9 | 0.04 (−3.7, 3.8) | 0.98 |
| Last ejection fraction (%) | 52.0 ± 10.8 | 52.5 ± 5.5 | −0.5 (−4.5, 3.6) | 0.81 |
| Primary value TNI (ng/mL) | 0.4 (0.04,1.6) | 0.2 (0.04, 1.28) | 0.03 (−0.1, 0.48) | 0.58 |
| Peak value TNI (ng/mL) | 15.8 (6.0, 27.1) | 44.5 (24.9, 50.0) | −20.3 (−29.8, −11.3) | <0.01 |
| Primary value NT-proBNP (pg/fL) | 258.0 (79.7, 672.0) | 97.9 (28.7, 244.9) | 94.2 (−2.3, 275.2) | 0.06 |
| Peak value NT-proBNP (pg/fL) | 1993.0 (795.8, 4434.0) | 1862.0 (1448.0, 2929.0) | 125.5 (−709.7, 1064.0) | 0.69 |
| Hours after symptom onset | ||||
| 0-12h | 31 (66.0) | 25 (96.2) | −30.2 (−48.6, −11.8) | <0.01 |
| 12h-24h | 9 (19.2) | 1 (3.9) | 15.3 (−1.1, 31.8) | 0.09 |
| 24h-48h | 6 (12.8) | 0 | 12.8 (0.002, 0.25) | 0.08 |
| Type of AMI | ||||
| Extensive anterior wall | 4 (8.5) | 9 (34.6) | −26.1 (−49.0, −3.2) | 0.01 |
| Anterior wall | 14 (29.8) | 5 (19.2) | 10.6 (−12.4, 33.6) | 0.32 |
| Inferior wall | 29 (61.7) | 12 (46.2) | 15.6 (−11.1, 42.2) | 0.20 |
| Lateral wall | 0 (0) | 0 (0) | 0 | NA |
| Killip classification | ||||
| I | 36 (76.6) | 23 (88.5) | −11.9 (−32.1, 8.4) | 0.35 |
| II | 7 (14.9) | 1 (3.9) | 11.1 (−4.5, 26.6) | 0.25 |
| III | 2 (4.3) | 0 | 4.3 (−4.5, 13.0) | 0.54 |
| IV | 2 (4.3) | 2 (7.7) | −3.4 (−18.2, 11.3) | 0.61 |
Data are presented as n (%), mean ± SD, or median (interquartile range). AMI, acute myocardial infarction; BP, blood pressure; CABG, coronary artery bypass grafting; D-B, door to balloon; LV, left ventricle; MI, myocardial infarction; PCI, percutaneous coronary intervention; TNI, troponin I.
TABLE 5.
Clinical adverse events during hospitalization of patients enrolled in 2020 according to reperfusion treatment
| Adverse event | Nonreperfusion group (N = 47) | Reperfusion group (N = 26) | Difference (95% CI) | P value |
|---|---|---|---|---|
| Composite endpoint | 9 (19.2) | 2 (7.7) | 11.5 (−6.7, 29.7) | 0.31 |
| Death | 2 (4.3) | 0 | 4.3 (−4.5, 13.0) | 0.54 |
| Heart failure | 7 (14.9) | 1 (3.9) | 11.1 (−4.5, 26.6) | 0.25 |
| Cardiac arrest | 2 (4.3) | 1 (3.9) | 0.4 (−12.0, 12.8) | 1.00 |
| Cardiac shock | 4 (8.5) | 0 | 8.5 (−2.5, 19.5) | 0.29 |
| Recurrent MI | 6 (12.8) | 1 (3.9) | 8.9 (−6.1, 24.0) | 0.41 |
| Mechanical complication | 0 | 0 | 0 | |
| Arrhythmia | 16 (34.0) | 1 (3.9) | 30.2 (11.8, 48.6) | 0.004 |
Data are presented as n (%). MI, myocardial infarction.
Discussion
The present study reviewed clinical characteristics and in-hospital outcomes of the 73 STEMI patients at our hospital who received relatively conservative therapy during the peak of COVID-19 outbreak. Main findings were a significant reduction in the number of emergent reperfusion therapy and corresponding increase in conservative medical treatment during COVID-19 outbreak. Patients enrolled during COVID-19 pandemic had a trend toward higher risk of most clinical adverse events compared with historic control, particular for those who did not receive timely reperfusion therapy.
It has been well established that timely reperfusion therapy (mainly primary PCI) is the cornerstone of STEMI therapy.6 , 8 However, it is inevitable for nondesignated hospitals to adopt relatively conservative STEMI management strategy during COVID epidemic to avoid and limit nosocomial transmission. In fact, it was estimated that there was a 38% reduction in US cardiac catheterization laboratory STEMI activations.11 Our study also found a 77.6% reduction in the number of primary PCI, and a corresponding 20.6% increase in thrombolytic therapy and a 57% increase in conservative medication therapy. There was a trend towards risk of most in-hospital clinical adverse events during COVID-19 pandemic. Our findings highlighted the potential unignorable adverse impact of COVID-19 pandemic on the efficient treatment of AMI. Failure of timely reperfusion not only negatively affects patients’ in-hospital outcome, but also long-term prognosis due to larger infarction size and subsequent left ventricle dysfunction.15 In addition, a 23% reduction in STEMI admission was noticed. Patients failed to reach out to hospital were probably had subsequent higher mortality risk.
Although we observed an increasing trend in the rate of adverse events during COVID-19 pandemic, the increase was less significant than that of other studies. In Italy, case fatality rate of AMI increased from 2.8% in 2019 to 9.7% during COVID-19 pandemic.9 In Hong Kong, in-hospital mortality rate of AMI increased from 5.9% in 2019 to 12.5% during the pandemic.16 Main reasons included: (1) We performed timely reperfusion therapy, mainly thrombolytic treatment, in patients who may benefit the most from revascularization, with a short time to reperfusion and high successful thrombolysis rate. Another advantage associated with this strategy is the decreased need for repeated usage of cath lab for emergency procedure, which lowers the risk of nosocomial transmission and medical overuse. (2) The proportion of (extensive) anterior wall AMI was high in participants who received reperfusion therapy in 2020, which was higher than nonreperfusion group or historic control. Therefore, although the overall reperfusion rate in 2020 was lower than that in 2019, no significant difference in major adverse cardiac events (MACE) was found between 2 years. (3) Most of the patients who did not receive thrombolysis or PCI had clinical signs of thrombus autolysis, or were unlikely to benefit from revascularization (see details in supplement). More importantly, the majority of these patients received revascularization during hospitalization.
Of note, not a single case of in-hospital infection occurred throughout the process of treatment among all STEMI patients at our hospital. Besides the low severity of COVID-19 epidemics in Beijing, the strict protective measures were the key contributor to the “zero nosocomial infection” rate. During COVID-19 pandemic, it is essential to be prepared in advance and set priorities for cath labs.17 The key points of our institution's management algorithm include: (1) Timely initiation of reperfusion therapy under the premise of minimizing the risk of nosocomial transmission. (2) As for methods of revascularization, thrombolysis should be considered as the first choice and rescue PCI as an adjunctive. (3) All patients who cannot be ruled out for COVID-19 infection should be quarantined in a designated screening room, closely treated and screened. (4) All patients with confirmed or suspected COVID-19 should be transferred as quickly as possible and concurrently receive medical therapy.
Limitations
The present study may be subject to the bias inherent to its retrospective nonrandomized design. However, baseline characteristics were comparable between study and historical control groups. The sample size was relatively small and all data were derived from a large single center. Finally, the current study investigated in-hospital adverse events with short-term (30-day) follow-up period. Whether this modified strategy affects patients’ long-term outcome remains unclear.
Conclusions
Our preliminary data demonstrate that the public health emergency response for COVID-19 leads to a significant reduction in emergent reperfusion, and a corresponding trend toward higher risk of most adverse events during hospitalization, particular for patients who did not receive timely reperfusion therapy. How to better balance the risks and benefits from STEMI management, under the premise of prevention and control of COVID-19 transmission, remains an unprecedented challenge and urgently requires future research action.
Data Availability
Data are available based on reasonable request to the corresponding authors.
Acknowledgment
We acknowledged Changdong Guan for her kind help in manuscript editing.
Footnotes
Disclosures: All authors declared no conflict of interest.
Ethics: The current study was approved by the institutional review board central committee at Fuwai Hospital, National Center for Cardiovascular Diseases, China (2012-431).
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cpcardiol.2020.100693.
Appendix. Supplementary materials
References
- 1.https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed July 15, 2020.
- 2.Han Y. Initial COVID-19 affecting cardiac patients in China. Eur Heart J. 2020;41:1719. doi: 10.1093/eurheartj/ehaa257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng YY, Ma YT, Zhang JY, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett R. Acute myocardial infarction. Lancet. 2019;393:2580. doi: 10.1016/S0140-6736(19)31419-9. [DOI] [PubMed] [Google Scholar]
- 7.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J Am Coll Cardiol. 2016;67:1235–1250. doi: 10.1016/j.jacc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 9.De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the COVID-19 era. Eur Heart J. 2020;41:2083–2088. doi: 10.1093/eurheartj/ehaa409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Metzler B, Siostrzonek P, Binder RK, et al. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia S, Albaghdadi MS, Meraj PM, et al. Reduction in ST-segment elevation cardiac catheterization laboratory activations in the United States during COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2871–2872. doi: 10.1016/j.jacc.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chinese Clinical Guideline for COVID-19 Diagnosis and Treatment. 7th ed.China, National Health Committee. http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. Accessed March 4, 2020.
- 13.Han Y, Zeng H, Jiang H, et al. CSC expert consensus on principles of clinical management of patients with severe emergent cardiovascular diseases during the COVID-19 epidemic. Circulation. 2020;141:e810–e816. doi: 10.1161/CIRCULATIONAHA.120.047011. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, et al. Fourth universal definition of myocardial infarction (2018) J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 15.Orn S, Manhenke C, Anand IS, et al. Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. Am J Cardiol. 2007;99:1109–1114. doi: 10.1016/j.amjcard.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 16.Tam CF, Cheung KS, Lam S, et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on outcome of myocardial infarction in Hong Kong, China. Catheter Cardiovasc Interv, 10.1002/ccd.28943. [online ahead of print]. [DOI] [PMC free article] [PubMed]
- 17.Campo G, Rapezzi C, Tavazzi L, et al. Priorities for Cath labs in the COVID-19 tsunami. Eur Heart J. 2020;41:1784–1785. doi: 10.1093/eurheartj/ehaa308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available based on reasonable request to the corresponding authors.