Abstract
The coronavirus disease 2019 (COVID-19) pandemic remains threatening to women and children, but clinical evidence regarding women during pregnancy, puerperium and lactation is limited. We assessed clinical and immunologic features of and breastfeeding advice provided to mother–infant pairs. This observational analysis was conducted in a tertiary-care centre in Wuhan, China. Pregnant patients with laboratory-confirmed COVID-19 who delivered during hospitalization were enrolled. Clinical characteristics and serial specimens of the mother–infant pairs were examined, supplemented with follow-ups regarding recovery and breastfeeding. Fourteen pregnant patients had live births and recovered well; four patients continued breastfeeding while taking precautions. No neonatal infections were observed. No infants developed COVID-19 during breastfeeding. Common maternal symptoms were fever (11/14, 78.1%) and cough (6/14, 42.9%). A pregnancy-specific symptom was abnormal foetal movement, which was noticed by three patients (21.4%). The mean virus shedding time was 9 days (standard deviation, 6 days; range, 1–22 days). The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome was not detected in breast milk or maternal vaginal secretions. Immunologic assay revealed seroconversion of IgM on day 8 after onset and IgG on day 28. Both IgM and IgG antibodies to SARS-CoV-2 were detected in breast milk, cord blood and neonatal serum. The study results suggest that passive acquisition of antibodies against SARS-CoV-2 is available by ingesting breast milk. Breastfeeding has a low risk of transmitting SARS-CoV-2 or escalating maternal disease, so continuing breastfeeding with prudent precautions is encouraged.
Keywords: Breastfeeding, COVID-19, infectious disease, lactation period, passive immunity, pregnancy
Introduction
Severe acute respiratory virus 2 (SARS-CoV-2), first identified in December 2019 in Wuhan, China, has continued to spread rapidly, causing the ongoing pandemic [1]. As coronavirus disease 2019 (COVID-19) unfolded, various investigations have been launched to explore the pathophysiology of SARS-CoV-2. To date, the virus's genome has been detected in many different kinds of body fluids, including upper respiratory droplets, bronchoalveolar lavage, saliva, tears, conjunctival secretions, faeces, urine, blood and cerebrospinal fluid [[2], [3], [4]]. Breast milk, as a well-studied body fluid that delivers immunologic, nutritional and cognitive benefits and that provides maternal–foetal emotional effects [5], has not yet been fully characterized in terms of the novel coronavirus. Clinical data are also limited. Chen et al. [6] described a failure to detect the SARS-CoV-2 genome in the breast milk of six affected women in the third trimester of pregnancy. However, with more women in the early pregnancy, puerperium and lactation periods affected by and recovering from COVID-19, it is important to learn whether breastfeeding is safe, whether it plays a role in transmitting SARS-CoV-2, what precautions ought to be taken and whether breastfeeding will affect the mother's disease course.
We assessed the clinical and immunologic features of COVID-19–affected mother–infant pairs; specifically tested breast milk for pathogens, SARS-CoV-2 neutralizing antibodies and immunologic components; and explored the feasibility of breastfeeding and related transmission possibilities.
Patients and methods
Study design and participants
The ambispective observational clinical analysis was conducted in a single tertiary-care centre, Tongji Hospital, affiliated with the Huazhong University of Science and Technology, located in Wuhan, China. The study retrospectively collected data of seven pregnant patients with laboratory-confirmed COVID-19 treated between 19 January and 7 February 2020, while 13 patients in various stages of pregnancy who were later diagnosed consented to the study from its baseline. Follow-up investigation focused on mothers' and infants' outcomes, breastfeeding and recovery. The last follow-up date was 5 April 2020. Patients who were enrolled met the following criteria [7]: positive real-time reverse transcription PCR (RT-PCR) test results of SARS-CoV-2 in oropharyngeal or nasopharyngeal swabs; tested positive by the IgM-IgG combined antibody test for SARS-CoV-2 1 week after disease onset, combined with epidemiology exposure and suspected symptoms; and agreed to and were compliant with testing and treatment protocols.
Data and specimen collection
The collection and analysis of human clinical specimens was approved by the health and ethnic boards of Tongji Hospital (approval TJ-IRB20200222). Written informed consent was provided by patients and the neonates' adult proxy.
Demographic information and clinical characteristics of the enrolled patients were extracted from the electronic medical system, supplemented with telephone follow-up. Within 7 days after giving birth, breast milk was self-pumped after hand sanitization, then placed in containers. Mother–child isolation was performed immediately after cord clamping. Neonates were either observed in neonatal intensive care units (NICU) or kept in a quarantine center as close contacts with family member's accompany. Neonates in the NICU were sampled by nasal or oropharyngeal swabbing for SARS-CoV-2 two times in a row, 24 hours apart. Neonatal faeces were sampled after meconium passed. All clinical specimens underwent RT-PCR assays for SARS-CoV-2 ORF1ab/N genes via detection kits [8], as authorized by the Chinese centre for disease control and prevention. SARS-CoV-2 IgM-IgG combined antibody tests [9] and fully automated chemiluminescence immunoassay were performed of breast milk and of maternal and neonatal sera after the test's introduction on 26 February 2020. IgM or IgG titres of more than 10 arbitrary unit (AU)/mL were considered positive; other results were considered negative.
Statistical analysis
Statistical analysis was performed by SPSS 20.0 software (IBM, Armonk, NY, USA). Continuous variables were presented as mean and standard deviations (SDs), or medians and interquartile ranges. Categorical variables were expressed as counts and percentages.
Results
Clinical characteristics and perinatal outcomes
Demographic information and clinical features of 14 mothers with confirmed COVID-19 are presented in Table 1, where data of four patients who were cotested with immunologic assays of SARS-CoV-2 are detailed.
Table 1.
Baseline information of patients with COVID-19 during pregnancy
| Characteristic | Patients with COVID-19 during pregnancy (n = 14) | Patients who developed SARS-CoV-2–neutralized IgM/IgG antibodies after delivery |
|||
|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | ||
| Age (years), mean (SD, range) | 31 (2.4, 27–35) | 30 | 33 | 27 | 30 |
| Mean GA at disease onset (weeks) | 36+3 | 32 | 37 | 41 | 32+3 |
| Mean GA at admission (weeks) | 38 | 33+3 | 39+1 | 41+2 | 39+3 |
| Epidemiology exposure | |||||
| Wuhan or Hubei travel history | 14 (100) | Yes | Yes | Yes | Yes |
| Healthcare worker | 2 (14.2) | Yes | No | No | Yes |
| Contact with confirmed or suspected case | 3 (21.4) | Yes | No | No | Yes |
| Family aggregation occurrence | 2 (14.2) | No | No | No | Yes |
| Gestational comorbidity | |||||
| ICP | 2 (14.2) | No | No | No | No |
| Gestational diabetes | 1 (7.1) | No | No | No | No |
| Thyroid dysfunction | 3 (21.4) | No | No | Yes | No |
| Congenital heart disease | 1 (7.1) | No | No | Yes | No |
| Chronic HBV infection | 1 (7.1) | Yes | No | No | No |
| Tuberculosis | 1 (7.1) | No | No | No | No |
| Signs and symptoms | |||||
| Fever | 11 (78.6) | Yes | Yes | No | No |
| Fever during pregnancy | 8 (57.1) | Yes | Yes | No | No |
| Postpartum fever | 8 (57.1) | No | No | No | No |
| Malaise | 2 (14.2) | No | No | No | No |
| Myalgia | 2 (14.2) | Yes | No | No | No |
| Headache | 1 (7.1) | No | No | No | No |
| Sore throat | 2 (14.2) | No | No | No | Yes |
| Cough | 6 (42.9) | Yes | No | No | Yes |
| Chest fullness | 2 (14.2) | No | No | No | No |
| Chest pain | 2 (14.2) | No | No | No | No |
| Diarrhoea | 1 (7.1) | No | No | No | No |
| Vomiting | 1 (7.1) | No | No | No | No |
| Anorexia | 3 (21.4) | Yes | No | No | No |
| Abnormal foetal movement | 3 (21.4) | No | No | No | No |
| Respiratory support | 8 (57.1) | Yes | No | No | No |
| Laboratory results, reference value, mean (SD, range) | |||||
| Chest CT abnormalities | 13 (92.9) | Yes | Yes | Yes | Yes |
| WBC, 3.50–9.50 × 109/L | 8.6 (2.9, 5.0–16.4) | 5.7 | 9.2 | 8.5 | NA |
| Neutrophils, 1.80–6.30 × 109/L | 6.9 (2.5, 3.0–13.59) | 4.8 | 7.9 | 6.3 | NA |
| Lymphocytes, 1.10–3.20 × 109/L | 1.2 (0.4, 0.6–1.9) | 0.7 | 0.8 | 1.7 | NA |
| Platelets, 125–350 × 109/L | 199 (64, 121–318) | 214 | 313 | 236 | NA |
| Haemoglobulin, 115–50 g/L | 123 (15.2, 100–55) | 113 | 109 | 100 | NA |
| CRP, <1 mg/L (n = 7) | 27.5 (22.1, 1.1–0.5) | 70.5 | 1.1 | NA | NA |
| ESR, 0–20 mm/h (n = 3) | 50 (24.6, 3–5) | 95 | NA | NA | NA |
| PCT, 0.02–0.05 ng/L (n = 6) | 0.4 (0.7, 0.04–0.05) | 0.32 | 0.06 | NA | NA |
| ALT, <33 U/L | 105 (226, 5–82) | 18 | 7 | 5 | NA |
| AST, <32 U/L | 126 (220, 13–83) | 38 | 13 | 14 | NA |
| LDH, 135–14 U/L (n = 10) | 283.9 (159.4, 141–86) | 304 | 167 | NA | NA |
| D-D, <0.5 μg/mL (n = 11) | 2 (0.8, 0.6–3.2) | 1.7 | 0.65 | NA | NA |
| Coinfection | 5 (35.7) | Yesa | No | No | No |
Data are presented as n (%) unless otherwise indicated. AST, aspartate aminotransferase; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; D-D, d-dimer; ESR, erythrocyte sedimentation rate; GA, gestational age; HBV, hepatitis B virus; ICP, intrahepatic cholestasis of pregnancy; LDH, lactate dehydrogenase; LT, alanine transaminase; NA, not applicable; PCT, procalcitonin; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; WBC, white blood cell.
GA, gestational weeks, the superior symbol means day exceeds the weeks.
Coinfected with influenza A.
In this cohort, mean maternal age was 31 years (SD, 2.4 years; range, 27–35 years). All had a singleton pregnancy; four (28.5%) were the mothers' first babies and ten were not. All patients were Wuhan residents, two (14.2%) were healthcare workers, three (21.4%) had contact with confirmed or suspected cases and two (14.2%) had family aggregation occurrence. The most common symptoms were fever (11/14, 78.1%) and cough (6/14, 42.9%). A pregnancy-specific symptom, abnormal foetal movement, occurred in three patients (21.4%), with one feeling evidently increased foetal movement and two decreased movement. All patients had abnormalities found by chest computed tomography (CT). Typical findings were ground-glass opacities, multiple patches in lung fields and subpleural adhesions. Laboratory results showed lymphopenia (lymphocytes <1.1 × 1012) in seven patients (50%); five patients had abnormal liver functions, two of whom had coexisting intrahepatic cholestasis of pregnancy. We also performed immunoserologic testing of other common respiratory pathogens, including respiratory syncytial virus, adenovirus, influenza A, influenza B, parainfluenza virus, Mycoplasma pneumoniae and Legionella pneumophila. Four patients (26.1%) tested positive for influenza A IgM and one (4.3%) tested positive for Mycoplasma pneumoniae IgM.
Respiratory support was applied for 4 hours after surgery for those who underwent caesarean sections and for those whose blood oxygen saturation dropped below 93%. Eight patients (57.1%) received oxygen via nasal catheter; no respirator or mechanical ventilation was indicated.
All patients underwent successful term delivery with no severe complications or admission to the intensive care unit. Perinatal outcomes and neonate baseline information are shown in Table 2. The mean interval from onset of disease to delivery was 5.4 days (SD, 6.3 days; range, 1–46 days). Two patients (14.2%) had foetal distress, which was marked by variable decelerations observed on foetal heart tracing and third-degree amniotic foetal meconium pollution. Twelve women (85.8%) chose to deliver via caesarean section; two patients (14.2%) gave birth vaginally without mechanical assistance. The surgeries were performed in an isolated surgical suite with continuous lumbar epidural analgesia. The mean birth weight of neonates was 3224 g (SD, 421 g; range, 2700–4120 g). One neonate, born to a mother with complications related to intrahepatic cholestasis of pregnancy, had mild asphyxia at birth. One neonate had transient fever (anal temperature 37.9°C) at 30 hours after birth.
Table 2.
Perinatal outcomes of patients with COVID-19 during pregnancy
| Outcome | Patients with COVID-19 during pregnancy (n = 14) | Patients who developed SARS-CoV-2 neutralized IgM/IgG antibodies after delivery |
|||
|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | ||
| GA of delivery (weeks), mean (range) | 38 + 3 | 39 | 39 + 1 | 41 + 2 | 39 + 5 |
| Time from disease onset to delivery (days), mean (SD, range) | 5.4 (6.3, 1–21) | 46 | 15 | 2 | 43 |
| PROM | 2 (14.2) | No | No | No | No |
| Foetal distress | 2 (14.2) | No | No | No | No |
| Caesarean section | 12 (85.7) | Yes | Yes | Yes | No |
| Amniotic fluid pollution | 6 (42.8) | No | No | No | No |
| Live birth | 14 (100) | Yes | Yes | Yes | Yes |
| Hospital stay (days), mean (SD, range) | 19 (7.9, 9–9) | 39 | 16 | 10 | 9 |
| Neonate baseline characters | |||||
| Birth weight (g), mean (SD, range) | 3224 (421, 2700–120) | 2700 | 3930 | 4120 | 2900 |
| NICU admission | 7 (50) | Yes | Yes | Yes | No |
| Neonatal asphyxia | 1 (7.1) | No | No | No | No |
| Neonatal jaundice | 5 (35.7) | Yes | Yes | No | No |
| Fever | 1 (7.1) | Yes | No | No | No |
| Anaemia | 1 (7.1) | No | No | No | No |
Data are presented as n (%) unless otherwise indicated. COVID-19, coronavirus disease 2019; GA, gestational age; NICU, neonatal intensive care unit; PROM, premature rupture of membrane; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Maternal and neonatal RT-PCR SARS-CoV-2 detection results are shown in Table 3. Samples were taken by oropharyngeal or nasopharyngeal swabbing every 3 days during hospitalization. SARS-CoV-2 virus shedding days were described as the first positive RT-PCR result to the first continuously negative RT-PCR result. The mean maternal virus shedding time was 9 days (SD, 6 days; range, 1–22 days). SARS-CoV-2 nucleic acids were not detected in maternal breast milk (n = 12), vaginal secretions (n = 10), neonatal oropharyngeal swabs (n = 12) or meconium specimens (n = 6).
Table 3.
Detection of SARS-CoV-2 nucleic acids in confirmed mother–infant pairs in early 2020
| Characteristic | Pair no. |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| Delivery date | 19 Jan | 22 Jan | 24 Jan | 30 Jan | 31 Jan | 31 Jan | 3 Feb | 5 Feb | 9 Feb | 9 Feb | 20 Feb | 29 Feb | 5 Mar | 16 Mar |
| Gestational age at delivery (weeks) | 36+4 | 38+4 | 38+1 | 36+5 | 37 | 38+2 | 40+5 | 38+4 | 39+1 | 39 | 41+1 | 39 | 38 | 39+5 |
| Caesarean section | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No |
| Maternal SARS-CoV-2 nucleic acid detection | ||||||||||||||
| Positive throat swab date | 31 Jan | 5 Feb | 28 Jan | 8 Feb | 31 Jan | 31 Jan | 6 Feb | 5 Feb | 10 Feb | 9 Feb | NA | NA | 2 Feb | 4 Feb |
| Negative throat swab date 1 | 18 Feb | 17 Feb | 31 Jan | 9 Feb | 8 Feb | 22 Feb | 9 Feb | 13 Feb | 21 Feb | 12 Feb | 17 Feb | 3 Feb | 12 Feb | 12 Feb |
| Negative throat swab date 2 | 22 Feb | 19 Feb | NA | 11 Feb | 13 Feb | 24 Feb | NA | 16 Feb | 24 Feb | 14 Feb | 19 Feb | 1 Mar | 4 Mar | 14 Feb |
| SARS-CoV-2 shedding time (days) | 18 | 12 | 3 | 1 | 8 | 22 | 3 | 8 | 11 | 3 | NA | NA | 10 | 8 |
| Breast milk (n = 12) | NA | NA | — | — | — | — | — | — | — | — | — | — | — | — |
| Vaginal secretion (n = 10) | NA | NA | NA | — | NA | — | — | — | — | — | — | — | — | — |
| Anal swab (n = 12) | NA | — | NA | — | — | — | — | — | — | — | — | — | — | — |
| Neonatal SARS-CoV-2 nucleic acids detection | ||||||||||||||
| Throat swab (n = 12) | — | — | NA | — | NA | — | — | — | — | — | — | — | — | — |
| Meconium (n = 6) | NA | NA | NA | NA | NA | NA | NA | — | — | — | — | — | — | NA |
Maternal negative throat swab dates were presented as two continuous dates separated by at least 48 hours. SARS-CoV-2 shedding time was counted as duration from first positive date to first consecutive negative date. Disease of mothers 11 and 12 was confirmed by epidemiologic exposure, characteristic symptoms and positive SARS-CoV-2 combined IgM/IgG tests; their throat swab results remained negative two separate times.
Dash indicates negative results. COVID-19, coronavirus disease 2019; NA, not available; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
SARS-CoV-2 immunoassay in breast milk and in maternal and neonatal sera
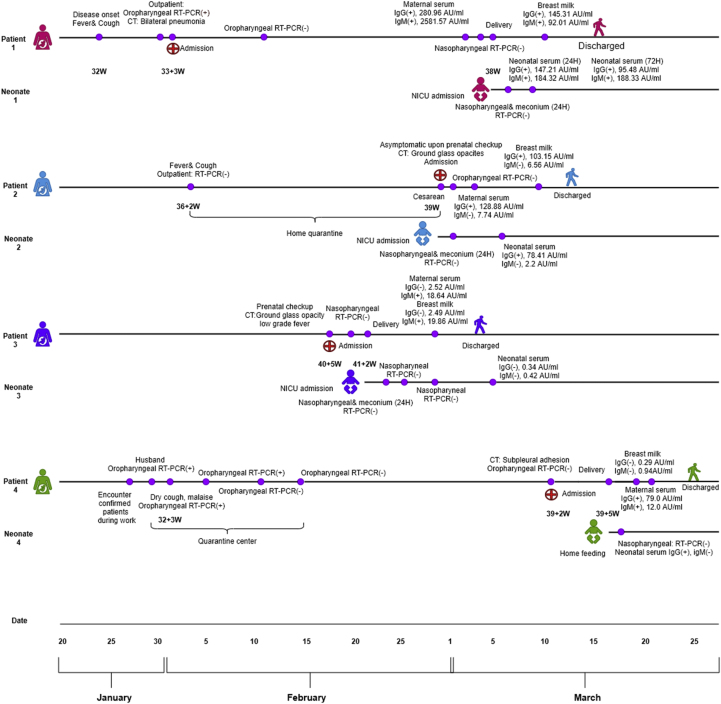
Immunologic features and the disease courses of four mother–infant pairs are presented in Fig. 1. Maternal seroconversion of IgM was observed on day 8 after disease onset, and IgG on day 28. Three breast milk samples tested positive for SARS-CoV-2 IgM or IgG. Three neonates tested positive for SARS-CoV-2 IgG. One neonate tested positive for IgM within 24 hours of birth. IgG was detected in one cord blood sample.
Fig. 1.
Timeline and immunologic findings in breast milk and sera among four mother–infant pairs.
Patient 1, a healthcare worker, had fever and cough on 24 January at 32 weeks of pregnancy. She tested positive from a oropharyngeal swab sample on 1 February. Chest CT revealed bilateral pneumonia (Fig. 2). She was admitted and received oxygen and other supportive treatment. She had two consecutive negative results on 12 and 14 February, but she still complained of persistent dry cough and malaise. On 3 March, at 37 + 5 weeks of pregnancy, her SARS-CoV-2 antibody assay showed evidently increased antibodies, with IgG 280.96 AU/mL and IgM 2581.57 AU/mL, while the nasopharyngeal swab test results were negative. An elective caesarean section was planned to avoid maternal exhaustion. A boy was born, with an Apgar score of 1 minute after birth is 8 and 9 for 5 minutes; birth weight was 2700 g. At testing within 24 hours of birth, the neonatal SARS-CoV-2 immunologic assays showed positive titres (IgG 147.21 AU/mL, IgM 184.3 AU/mL). At 72 hours after birth, IgG titre was 95.48 AU/mL and IgM 188.33 AU/mL. SARS-CoV-2 nucleic acids were not detected in neonatal oropharyngeal swab or meconium samples. Breast milk was examined on postpartum day 6; IgG titre was 145.31 AU/mL and IgM 92.01 AU/mL. The immunologic components (IgA, IgM, IgG, C3, C4) of breast milk were within normal limit. During isolation, the neonate was fed formula only. The mother resumed breastfeeding when discharged with bottle feeding of expressed breast milk.
Fig. 2.
Chest computed tomographic (CT) image of pregnant patients diagnosed with coronavirus disease 2019 (COVID-19). (A) CT image of patient 1 on 6 February 2020 (day 13 after disease onset) showing bilateral lung multiple patches and ground-glass opacities with ragged edges. (B) CT image of patient 1 on 9 March 2020 (postpartum day 4, or 45 days after disease onset) showing evident absorption and improvement, with partial stranding in left lung subpleural area.
Patient 2 was asymptomatic when admitted on 29 February at 39 weeks of pregnancy. Her nasopharyngeal swab results at admission were negative. She reported fever and cough during 36 weeks of pregnancy and was advised to quarantine at home, where the symptoms self-resolved. She underwent an elective caesarean section as a result of a scarred uterus. The surgery was uncomplicated; a 3930 g boy was born. Antibody testing of serum and breast milk was performed on postpartum day 3; results indicated markedly increased IgG titres and was negative for IgM. Breast milk level of IgG was 103.15 AU/mL (an immunologic component in the normal range). The neonate was tested within 24 hours of birth; increased IgG (78.41 AU/mL) and negative IgM were noted. Patient 2 continued breastfeeding with expressed breast milk. The neonate was well appearing, with normal weight gain according to the father during follow-up.
Patient 3 was admitted on 20 February with low-grade fever (temperature 37.3°C) at 41 weeks of pregnancy. Outpatient chest CT scan obtained 2 days before revealed bilateral ground-glass opacities. Elective caesarean section was performed, and the 4120 g female neonate was transferred to the NICU for observation. Breast milk and maternal serum were tested for antibodies on postpartum day 6, showing increased IgM antibody titres and negative IgG. Breast milk IgM level was 19.86 AU/mL, and maternal serum IgM was 18.64 AU/mL. Neonatal serum antibody test at 14-day checkup was negative for both IgM and IgG. The neonate was fed formula in the NICU and breastfed after isolation finished. During follow-up, the mother reported bottle feeding her baby with breast milk on most occasions. She was advised to wear a mask during direct breastfeeding.
Patient 4 was a nurse in outpatient triage centre. She encountered patients with confirmed COVID-19 on 27 January; her husband was also confirmed to have COVID-19. She had dry cough and malaise on 2 February, at 32 + 3 weeks of pregnancy, and subsequently tested positive. She was admitted to the quarantine centre for observation with no further treatment. Her symptoms spontaneously resolved 1 week later. Testing of oropharyngeal swabs on 12 and 14 February had negative results, and the patient was discharged. At a prenatal checkup on 11 March, her nasopharyngeal swab test results remained negative; CT revealed clear lung fields with subpleural adhesions. Testing results for SARS-CoV-2 antibodies were positive for IgG and negative for IgM. She was considered cured and admitted to the regular obstetrics ward. On 16 March, at 39 + 5 weeks of pregnancy, she vaginally delivered a baby girl (Apgar score 8–9, weight 2900 g). The cord blood tested positive for SARS-CoV-2 IgG and negative for IgM. Breast milk was tested on postpartum day 3, with negative SARS-CoV-2 antibody components; maternal serum samples taken the same day had IgG 79.0 AU/mL and IgM 12.0 AU/mL. She did not isolate with her baby and breastfed directly without a mask. At birth, the neonate tested positive for SARS-CoV-2 IgG and negative for IgM, and negative nasopharyngeal swab test results were found on the day 14 checkup.
Discussion
The study enrolled 14 patients who delivered during their hospitalization with COVID-19 from 32 weeks of pregnancy to postpartum day 2 in Wuhan, China. All of them experienced good recovery, with no reported COVID-19–related sequelae. No infection of the neonate was noticed. SARS-CoV-2 nucleic acids were not detected in 12 breast milk samples of mothers with COVID-19 in various stages of disease. In contrast, neutralized SARS-CoV-2 antibodies were identified in three breast milk samples. Among the symptomatic breastfeeding mothers, extreme precautions were taken to avoid postnatal infection. For recovered or cured mothers, direct breastfeeding did not result in neonatal infection.
Breastfeeding has been proven to have many benefits to both mother and child [10]. With the ongoing COVID-19 pandemic, the general population is overall vulnerable to the novel coronavirus. Women in pregnant and puerperium states are believed to be particularly susceptible to infection, as they are in other coronavirus diseases, such as SARS-CoV and Middle East respiratory syndrome coronavirus [11,12], in which poorer clinical outcomes and higher morbidity and mortality were reported. So far, COVID-19 during pregnancy has been reported to be mostly mild in presentation and has a good prognosis [13], but neonatal infection was reported during the Wuhan epidemic [14], thus focusing discussion on postnatal management and breastfeeding advice.
Known pathogens that can be transmitted during breastfeeding include HIV-1, human T-lymphotropic virus 1 and cytomegalovirus; the pathogen can be isolated from breast milk and causes corresponding illness in neonates. On other occasions, breast lesions can serve as an infection source while the neonate sucks; such lesions may be caused by tuberculosis or varicella zoster virus [15]. In our findings, SARS-CoV-2 was not detected in breast milk, and the mothers who continued breastfeeding had no breast lesions. Direct breastfeeding does carry certain risks; for example, maternal respiratory droplets are considered contagious, especially during the acute symptomatic stage, and they have an exceedingly high virus load [16]. SARS-CoV-2's viraemia provides an opportunity for breast lesions to transmit the virus to the neonate during sucking. Neonates also have weaker immune systems and inadequate protection against pathogens, which makes them vulnerable to the overall environment during breastfeeding. However, those risks are avoidable if certain measures are taken. Wearing a surgical mask during breastfeeding, handwashing before and after pumping milk and thoroughly sanitizing milk-expressing devices can help minimize the risk of virus transmission.
The effect of passive acquisition of antibody to SARS-CoV-2 from breast milk and maternal serum remains to be addressed. Zeng et al. [17] described neonatal acquisition of both IgM and IgG antibodies from a COVID-19–affected mother. In our findings, antibodies were also identified in cord blood and breast milk samples, indicating passive acquisition via the placenta and through breast milk. A prospective controlled study discussing the effect of anticholera antibodies in breast milk [18] showed that breast milk ingestion did not stop the colonization of Vibrio cholerae. However, colonized infants had a milder disease presentation and a shorter disease course. For Enterovirus infections, one study reported that breast milk antibodies had specific protective effects [19]. During combat with COVID-19, convalescent plasma (CP) therapy has shown promising effects. Chen et al. [20] described five critically ill patients who experienced remission after CP therapy, concluding that CP contributed to disease remission and virus clearance. However, concerns regarding transfusion-related allergy and possible lethal hyperimmunity attacks of CP must be addressed. Breast milk from recovered mothers possesses similar neutralization antibody components, is nonallergenic, is easy to acquire and is delivered orally. In addition, breast milk contains a wide variety of cell-rich components, including macrophages, immunoglobulin, complement bodies and cytokines, which have wide-spectrum antiviral effects.
On the basis of current information, we hypothesize that breastfeeding's role in transmission is low, and passive acquisition of antibodies to SARS-CoV-2 is beneficial to the infants. However, because there are other possible infection routes during mother–infant contact, prudent precautions should be taken.
Further investigation should focus on creating regulations for COVID-19 mother–infant contact that both prevent neonatal infection and permit emotional bonding. The SARS-CoV-2 mother–infant immunologic trends require long-term follow-up regarding antibody classes, duration and titre changes. Moreover, prospective studies of infants with or without passive acquisition of antibodies to SARS-CoV-2 should be conducted to evaluate the immunity effects.
Conclusions
Breastfeeding has a low risk of transmitting SARS-CoV-2 or escalating maternal disease; mothers should continue to breastfeed but should take prudent precautions. Infants can additionally benefit from direct acquisition of antibodies against SARS-CoV-2 through breast milk.
Conflict of interest
None declared.
Acknowledgements
Funded by Huazhong University of Science and Technology COVID-19 Rapid Response Call (2020kfyXGYJ00) and National Developmental Key Project “Epidemiology and Prevention of SARS-CoV-2 Strategy Evaluation Research” (2020YFC0846300). We are grateful to all the participating patients and their families, who showed great understanding, strength and inspiring optimism during this difficult time. We thank those who donated various supporting goods to Wuhan and Tongji hospital.
Contributor Information
Z. Sun, Email: zysun@tjh.tjmu.edu.cn.
L. Feng, Email: fltj007@163.com.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J.C., Wang S.B., Xue Y.D. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J Med Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence R.M., Lawrence R.A. Breast milk and infection. Clin Perinatol. 2004;31:501–528. doi: 10.1016/j.clp.2004.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinese Center for Disease Control and Prevention; National Institute for Viral Disease Control and Prevention Specific primers and probes for detection 2019 novel coronavirus. http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html
- 9.Xu W.Z., Li J., He X.Y., Zhang C.Q., Mei S.Q., Li C.R. Application of chemiluminescence immunoassay in SARS-CoV-2 IgM-IgG tests. Chin Lab Med. 2020 doi: 10.3760/cma.j.cn114452-20200223-00109. [DOI] [Google Scholar]
- 10.Brock E.G., Long L. Breast feeding. Obst Gynaecol Reprod Med, Churchill Livingstone. 2019 May 1 doi: 10.1016/j.ogrm.2019.02.003. [DOI] [Google Scholar]
- 11.Wong S.F., Chow K.M., Leung T.N., Ng W.F., Ng T.K., Shek C.C. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assiri A., Abedi G.R., Al Masri M., Bin Saeed A., Gerber S.I., Watson J.T. Middle East respiratory syndrome coronavirus infection during pregnancy: a report of 5 cases from Saudi Arabia. Clin Infect Dis. 2016;63:951–953. doi: 10.1093/cid/ciw412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L., Jiang Y., Wei M., Cheng B.H., Zhou X.C., Li J. Analysis of pregnancy outcomes in pregnant women with COVID-19 in Hubei province. Zhonghua Fu Chan Ke Za Zhi. 2020;55:166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 14.Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J. A case report of neonatal COVID-19 infection in China. Clin Infect Dis. 2020;71:853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanari M., Sogno Valin P., Natale F., Capretti M.G., Serra L. Human milk, a concrete risk for infection. J Matern Neonatal Med. 2012;25(Suppl. 4):67–69. doi: 10.3109/14767058.2012.715009. [DOI] [PubMed] [Google Scholar]
- 16.Woelfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Mueller M.A. 8 March 2020. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. medRxiv. [DOI] [Google Scholar]
- 17.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glass R.I., Svennerholm A.M., Stoll B.J., Khan M.R., Hossain K.M.B., Hug M.I. Protection against cholera in breast-fed children by antibodies in breast milk. N Engl J Med. 1983;308:1389–1392. doi: 10.1056/NEJM198306093082304. [DOI] [PubMed] [Google Scholar]
- 19.Sadeharju K., Knip M., Virtanen S.M., Savilahti E., Tauriainen S., Koskela P. Maternal antibodies in breast milk protect the child from enterovirus infections. Pediatrics. 2007;119:941–946. doi: 10.1542/peds.2006-0780. [DOI] [PubMed] [Google Scholar]
- 20.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]