Abstract
The increased risk of cardiovascular morbidity and mortality in rheumatoid arthritis and gout has been increasingly acknowledged in past decades, with accumulating evidence that gout, just as with rheumatoid arthritis, is an independent cardiovascular risk factor. Although both diseases have a completely different pathogenesis, the underlying pathophysiological mechanisms in systemic inflammation overlap to some extent. Following the recognition that systemic inflammation has an important causative role in cardiovascular disease, anti-inflammatory therapy in both conditions and urate-lowering therapies in gout are expected to lower the cardiovascular burden of patients. Unfortunately, much of the existing data showing that urate-lowering therapy has consistent beneficial effects on cardiovascular outcomes in patients with gout are of low quality and contradictory. We will discuss the latest evidence in this respect. Cardiovascular disease risk management for patients with rheumatoid arthritis and gout is essential. Clinical guidelines and implementation of cardiovascular risk management in daily clinical practice, as well as unmet needs and areas for further investigation, will be discussed.
Introduction
Cardiovascular disease is the most frequent cause of death worldwide. The 2017 Global Burden of Disease Study showed that 17·8 million people died of cardiovascular disease globally, accounting for 21% of all deaths.1 Well established, traditional risk factors for cardiovascular disease comprise age, sex, race, hypertension, diabetes, smoking, and hyperlipidaemia, all of which are included in various prediction models. However, over the past 20 years several non-traditional risk factors, such as chronic inflammation, have emerged as amplifiers of cardiovascular disease risk.2
Rheumatoid arthritis is the most common autoimmune arthritis, with a prevalence of up to 1%,2 and is characterised by a symmetrical polyarthritis with possible systemic manifestations. Rheumatoid arthritis is an accepted independent risk factor for cardiovascular disease, driven by the underlying chronic inflammatory process. However, traditional cardiovascular risk factors remain important.3
Gout is the most common crystal-induced, autoinflammatory joint disease with a prevalence of between 0·1% and 10·0%.4 Gout occurs when monosodium urate crystals are deposited in joints and soft tissues. Hyperuricaemia—defined by a serum urate concentration above the saturation point (ie, ≥0·41 mmol/L [≥6·8 mg/dL])—results predominantly from reduced renal excretion of uric acid, which is a consequence of genetics, comorbidities, and therapies. A continuum has been suggested, from asymptomatic hyperuricaemia to asymptomatic subclinical crystal deposition detectable only by ultrasound or dual-energy CT, to the clinical inflammatory state of gout flares, to chronic gouty arthritis with tophi and gouty bone erosions.5 If not treated adequately, gout is a debilitating disease with systemic manifestations, such as monosodium urate crystal deposition in organs and worsening of cardiorenal function.6, 7 In addition to gout flares, patients with gout frequently have a high burden of cardiovascular comorbidities, which might explain, in part, the high cardiovascular mortality when compared with the general population.8 During the past 20 years, gout has been shown to be an independent cardiovascular risk factor, with higher cardiovascular mortality than in the general population.9
In this Review, we will discuss epidemiological data on cardiovascular disease in rheumatoid arthritis and gout, not only for atherosclerotic disease but also for venous thrombotic disease and heart failure, as clinical and subclinical prevalence of the two diseases is higher than previously thought. The underlying pathophysiology of increased cardiovascular risk relevant to inflammatory arthritis, as well as the observed effect of anti-inflammatory and disease modifying treatments such as urate-lowering therapies in gout, will be reviewed and discussed. Increased cardiovascular risk in patients with inflammatory arthritis necessitates cardiovascular risk assessment and current management guidelines and their practical implications will be discussed. Finally, we consider topics that need further research with the aim to decrease the cardiovascular burden of patients.
Epidemiology
Rheumatoid arthritis
Patients with rheumatoid arthritis have up to a two-times higher risk of developing atherosclerotic cardiovascular disease than the general population, similar to patients with diabetes.10 The risk of ischaemic heart disease is increased in patients with early rheumatoid arthritis and symptom duration of less than 1 year, and probably even in the subclinical stage.11 The risk of cerebrovascular incidents is increased by about 50% (relative risk 1·48, 95% CI 0·70–3·12), whereas the risk of myocardial infarction is doubled (relative risk 2·00, 1·23–3·29).11 Moreover, patients with rheumatoid arthritis have almost twice the risk of developing congestive heart failure (rate ratio 1·7, 95% CI 1·3–2·1), including both heart failure with preserved ejection fraction and heart failure with reduced ejection fraction.12
Several factors contribute to increased cardiovascular risk, including comorbidities such as diabetes, dyslipidaemia, and hypertension;13 albeit the data for hypertension are somewhat conflicting.14 Lipids seem to have paradoxical associations with cardiovascular risk in rheumatoid arthritis. During active disease, low total cholesterol and LDL cholesterol are associated with increased cardiovascular risk (the so-called lipid paradox).15 Effective antirheumatic therapies resulting in reduced disease activity of rheumatoid arthritis reverse the cholesterol reduction, thus leading to increased lipid concentrations,16 and lipid concentrations in well controlled rheumatoid arthritis are generally stable and similar to those in the general population.11, 15, 17 Furthermore, patients with rheumatoid arthritis also have more than a two-times increased risk of venous thrombotic disease compared with the general population (cumulative incidence of 6·7% [SE 1·7] vs 2·8% [1·1], p=0·005).18
Studies show that all-cause mortality among patients with rheumatoid arthritis was 54% higher than in the general population, primarily because of cardiovascular disease (32%),19 with a median shortened life expectancy of 6–7 years.20 In one study, the estimated standardised cardiovascular-mortality ratio was 1·2 (95% CI 1·05–1·43),21 which is substantially less than reported in earlier studies. The improvement in cardiovascular mortality in patients with rheumatoid arthritis over the past 20 years could be attributed to the early initiation of more effective antirheumatic treatments (conventional synthetic and biological disease-modifying antirheumatic drugs [DMARDs]).22 Decreased disease activity following effective therapy is associated with a lower cardiovascular risk, and vice versa, whereas cardiovascular risk remains unchanged in patients who do not respond to biological DMARDs.23 However, it should be noted that about 50% of increased cardiovascular disease risk in patients with rheumatoid arthritis is associated with traditional cardiovascular risk factors.24
Gout
A large retrospective database study in the UK (8386 patients with gout vs 39 766 without gout) showed that the prevalence of hypertension in patients with gout at baseline was twice as high compared with the control group (36% vs 17%). Patients were also more often obese (60% vs 44%) and hyperlipidaemic (6% vs 3%) with more prevalent use of statins (34% vs 26%).25 A high prevalence of these traditional risk factors was also seen in the large National Health and Nutrition Examination Survey 2007–08, including 7·7 million US patients with gout. Moreover, the National Health and Nutrition Examination Survey showed that 26% of patients with gout had diabetes compared with almost 8% in the non-gout population (OR 2·36, 95% CI 1·5–3·7).26
In a large retrospective Dutch cohort of primary care patients with gout, 796 (30%) of 2655 patients already had established cardiovascular disease at cohort entry compared with 1557 (20%) of 7891 in the non-gout control group. After 3 years of follow-up, 154 (8%) of 1859 patients with gout had developed cardiovascular disease, compared with 318 (5%) of 6334 in the non-gout control group.13 There was an even higher incidence of cardiovascular disease (47%) in patients managed by rheumatologists, possibly explained by a higher portion of severe gout seen in this setting.8
Gout is an independent risk factor for cardiovascular disease; however, the strength of the association of hyperuricaemia and gout with other traditional cardiovascular risk factors makes distinguishing the isolated influence of gout on that risk difficult.27 Although atherosclerotic disease, hypertension, and chronic kidney disease are associated with elevated serum urate concentrations, the causal relationship and direction of association between urate and these disorders is still under debate.28 An increase in serum urate concentration leads to an increased risk of hypertension (odds ratio [OR] 1·16 per 0·06 mmol/L increase [1 mg/dL increase], 95% CI 1·07–1·24) and increased LDL cholesterol (men: OR 1·16 per 0·06 mmol/L increase [1 mg/dL increase], 1·01–1·33; women: OR 1·22 per 0·06 mmol/L increase [1 mg/dL increase], 1·06–1·39).29, 30
In the past decade, several observational studies have suggested an association between hyperuricaemia, gout, and congestive heart failure. A cross-sectional study of 15 722 patients in the USA found the prevalence of congestive heart failure to be 10% in patients with gout versus 2% in patients without gout.31 Another study in patients with coronary artery disease showed that patients with gout had a higher prevalence of congestive heart failure (500 [36%] of 1406) than those without gout (3847 [25%] of 15 795).32 Furthermore, individuals with asymptomatic hyperuricaemia already have an increased risk of congestive heart failure,33, 34 and there appears to be a linear relationship with serum urate concentrations.
Gout increases the risk of mortality from cardiovascular disease (hazard ratio [HR] 1·29, 95% CI 1·14–1·44).35 During a mean follow-up of 4 years among 706 patients with gout, 38 (59%) of 64 deaths had a cardiovascular attribution.36 Looking for trends and possible improvement of cardiovascular mortality in gout, analysis of a medical record database representative of the UK compared cumulative mortality rates of individuals with gout versus non-gout between 1999–2006 and 2007–14. The investigators found that the high mortality in patients with gout remained unchanged, whereas mortality rates for rheumatoid arthritis have improved over the same time period. This mortality gap might be related to suboptimal gout care (insufficient allocation of medication, insufficient treatment, and low drug adherence), as well as insufficient management of cardiovascular comorbidities. Gout specific characteristics that are associated with high cardiovascular risk are elevated serum urate concentrations (>0·55 mmol/L [>9·1 mg/dL]), longer disease duration (≥2 years), oligoarticular or polyarticular disease, and joint damage and tophi, which all reflect a more severe disease state.8
Pathophysiology
Although rheumatoid arthritis and gout have different pathogenic stimuli (not known for rheumatoid arthritis; monosodium urate crystal deposition for gout), different immune pathways (eg, antibody-mediated in rheumatoid arthritis; interleukin [IL]-1 driven in gout flares), and in most cases different clinical presentation (more chronic symmetric synovitis in rheumatoid arthritis; mostly monoarthritic gout flares with asymptomatic periods), imaging studies have shown that in gout, subclinical inflammation and synovitis are present, even during asymptomatic periods.37 Importantly, persistent inflammation is known to be a key mechanism in the development of cardiovascular disease.38, 39 Cardiovascular disease in patients with gout and rheumatoid arthritis can be divided into three main categories: atherosclerosis, thromboembolism, and cardiac dysfunction (figure 1 ). The pathophysiological mechanisms of these diseases are different. However, the inflammatory processes of gout and rheumatoid arthritis in the development of cardiovascular diseases partly overlap.
Figure 1.
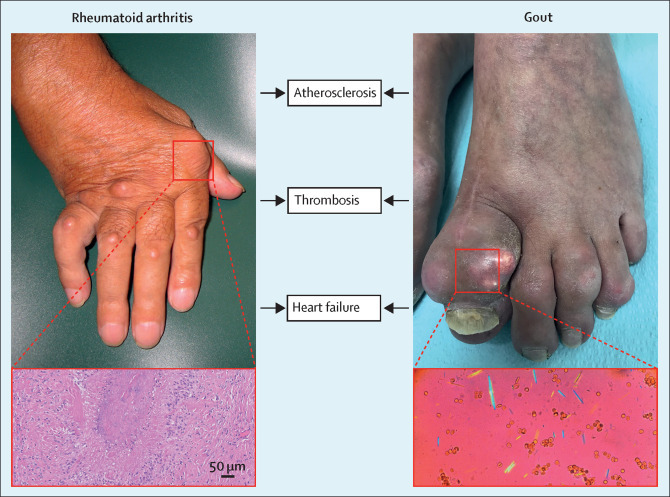
Rheumatoid arthritis, gout, and their shared pathophysiological mechanisms behind cardiovascular comorbidities
The histological image (left) shows synovitis in rheumatoid arthritis. The polarisation microscopy image (right) illustrates monosodium urate crystals in synovial fluid during a gout flare.
Atherosclerosis
Postulated shared mechanisms of rheumatoid arthritis and gout are systemic inflammation, reactive oxygen species (ROS)-induced oxidative stress, and endothelial dysfunction, all of which lead to atherosclerosis (figure 2 ).
Figure 2.
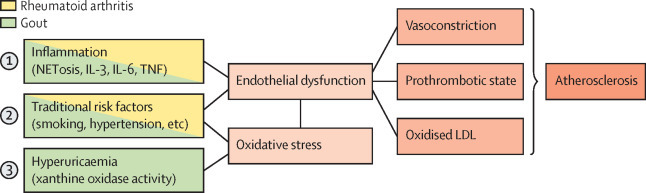
Summary of shared mechanisms in the development of cardiovascular disease in patients with rheumatoid arthritis and gout
The left column describes gout and rheumatoid arthritis-related mechanisms inducing atherosclerosis. NET=neutrophil extracellular trap.
The pathways of inflammation in rheumatoid arthritis and gout share some components of the innate and adaptive immune system, including activated neutrophils. Exaggerated neutrophil activation has been linked to autoimmunity and autoinflammation in rheumatoid arthritis and gout. One of the supposed links is the formation of neutrophil extracellular traps (NETs). NETosis involves a neutrophil cell death process in which DNA is extruded, together with cytoplasmic and granular contents, to trap and eliminate extracellular pathogens and neutralise inflammatory cytokines. This process, which was first described in gout by Schauer and colleagues,40 could explain the self-limiting character of gout flares. Extracellular DNA exerts cytotoxic and prothrombotic effects and might be a causal link between inflammation and coagulation. Moreover, complexes of presumably NET-borne DNA stimulate vascular plasmacytoid dendritic cells, with a strong interferon type 1 response, promoting atherogenesis.41 NETs might also directly cause endothelial dysfunction by activation and induction of damage to endothelial cells, illustrated by the establishment of NETs in atherosclerotic lesions and arterial thrombi in humans.42
In addition to neutrophils, different proinflammatory cytokines play a central role in the pathogenesis of rheumatoid arthritis and gout. In rheumatoid arthritis, the reason for immune system activation has not been completely elucidated. However, proinflammatory cytokines, such as tumour necrosis factor (TNF), IL-6, and IL-1, are important in the inflammatory process. In gout, the central inflammatory pathogenic process is assumed to be hyperuricaemia, leading to monosodium urate crystal deposition.43 These crystals provoke a host response, resulting in recurrent gout flares by acting as a danger signal for the innate immune system through activation of the NLRP3 inflammasome with the immediate release of mature IL-1β by resident macrophages, as well the release of other cytokines.44 The NLRP3 inflammasome has an important role in the initiation of a gout flare. Inflammasome activity causes oxidative stress, mitochondrial dysfunction, endoplasmic reticulum stress, and lysosome rupture, which are all important events in atherogenesis and could lead to atherosclerotic plaque instability and ultimately rupture.42
ROS are a group of small reactive species that play crucial roles in the regulation of biocellular processes. The balance between ROS and antioxidant species is essential to maintain cellular homoeostasis. Hence, an imbalance in oxidant and antioxidant mechanisms leads to a state of oxidative stress. In several chronic diseases, including rheumatoid arthritis, there is abundant oxidative stress.45 Although essential for vascular homoeostasis, an excess of ROS might lead to vascular injury. Vascular injury being the result of a complex cascade, including oxidative modification of lipoproteins, endothelial activation, and leucocyte migration and differentiation resulting in acceleration of atherogenesis.46 Oxidative stress might also link gout with cardiovascular disease. Although soluble urate has antioxidant properties, during urate generation the catalytic enzyme xanthine oxidase produces substantial amounts of ROS that affect biocellular mechanisms. There is some experimental evidence that in patients with gout, higher activity of xanthine oxidoreductase (XO) is associated with larger amounts of ROS. Indirectly, it was shown that inhibiting endothelium-bound XO resulted in reduced ROS with favourable effects on vascular function.47, 48
Endothelial dysfunction is a maladaptive state with disruption of vascular homoeostasis resulting from a proinflammatory state. By expressing adhesion molecules and inflammatory cytokines, endothelial cells amplify an inflammatory cascade facilitating monocyte infiltration in the subendothelial layer. LDL cholesterol accumulates in the subendothelial space and is oxidised, which is essential for the development of atherosclerotic plaques.46 Endothelial dysfunction is the earliest phenomenon in the development of atherosclerosis and was first described in rheumatoid arthritis in 2002;49 it has been observed in patients with early rheumatoid arthritis without traditional risk factors and in those with long-standing disease. In rheumatoid arthritis, systemic inflammation with proinflammatory mediators, such as IL-1β and TNF, is assumed to play a role in the development of endothelial dysfunction, as shown in rodent models.50 Impaired endothelium-dependent arterial responsiveness has been observed in patients with untreated gout, compared with individuals without gout, with the degree of impairment related to both serum urate and high sensitive C-reactive protein concentrations.51
There are published case series and case reports of urate crystal deposition in the spine and solid organs in histological samples fixed with alcohol (no dissolution of crystals).52 A current observational study investigated alcohol-fixed specimens of coronary arteries from explanted hearts with polarisation microscopy and detected strongly birefringent urate crystals in about 10% of coronary arteries.53 Whether or not crystal deposition in the vessels with subsequent local inflammation contributes to a higher cardiovascular risk in these patients is not known.
Venous thromboembolism
The immune system and coagulation system are linked to increased activity of the fibrinolytic system in patients with inflammatory joint diseases. Tissue factor initiates the extrinsic coagulation cascade and is found on extravascular cells, such as monocytes and neutrophils. C-reactive protein, TNF, IL-6, and complement activation might amplify tissue factor synthesis in monocytes and endothelial cells, and high concentrations of tissue factor have been found in patients with rheumatoid arthritis, particularly in those with a high disease activity.54 Consequently, increased concentrations of coagulation factors were shown in patients with rheumatoid arthritis.54 The role for TNF seems plausible, as in the general population this cytokine might induce an imbalance between clotting and fibrinolysis, resulting in a hypercoagulable state. Furthermore, a role for IL-6 was suggested by a randomised trial in patients with rheumatoid arthritis that showed that IL-6 receptor blockade reduced coagulation activation parameters by more than 40% compared with placebo.55 To date, data on the role of cytokines in the development of thromboembolism in gout are not available and observational data suggests that gout is an independent risk factor for the development of deep vein thrombosis and pulmonary embolism.56 In addition to extensive neutrophil activation in gout, increased platelet reactivity has been detected. This finding might serve as one explanation for a prothrombotic state and association with thromboembolism in gout.57
Cardiac dysfunction
Initially, cardiac dysfunction in patients with rheumatoid arthritis was assumed to be secondary to ischaemic heart disease. However, the incidence of congestive heart failure (heart failure with preserved ejection fraction and heart failure with reduced ejection fraction) cannot be explained by atherosclerotic disease alone. A case-control study showed that patients with rheumatoid arthritis had a rapidly increasing risk of developing non-ischaemic congestive heart failure after clinical onset.58 Congestive heart failure was seen more frequently in patients with rheumatoid arthritis compared with patients with osteoarthritis (adjusted risk 3·9% [95% CI 3·4–4·3] for rheumatoid arthritis vs 2·3% [1·6–3·3] for osteoarthritis).59 Patients with rheumatoid arthritis treated with TNF-blocking agents had significantly less congestive heart failure than those who were not receiving anti-TNF treatment.59 Another study found an increased prevalence of heart failure with preserved ejection fraction in patients with rheumatoid arthritis, which correlated primarily with disease activity and with anti-inflammatory treatments (ie, conventional synthetic DMARDs and biological DMARDs).60 In addition, patients with rheumatoid arthritis are susceptible to more rapid subclinical changes in diastolic function than are the general population.61 Altogether, these studies suggest that systemic inflammatory activity and cardiac dysfunction cannot be attributed to atherosclerosis alone.
Chronic systemic inflammation has two distinct pathways in the development of cardiac dysfunction (figure 3 ). First, chronic systemic inflammation leads to myocardial remodelling, and, specifically, to diastolic dysfunction. This process starts with inflammation-induced microvascular dysfunction,63 leading to deposition of collagen with subsequent stiffness and hypertrophy of cardiomyocytes and a decreased ability of the myocardium to contract and relax, which might evolve into heart failure with preserved ejection fraction.64 Second, systemic inflammation and traditional cardiovascular risk factor-induced coronary microvascular dysfunction and activation lead to atherosclerosis with ischaemic heart disease. This ischaemia leads to autophagy, apoptosis and necrosis of cardiomyocytes, and collagen deposition in the interstitial space giving rise to systolic ventricular dysfunction. In severe cases, heart failure with reduced ejection fraction might develop.62 Several studies have assessed the effect of anti-inflammatory therapy on cardiac function in rheumatoid arthritis, and a systematic review revealed that biological DMARD therapy probably has favourable effects on cardiac dysfunction in rheumatoid arthritis.65
Figure 3.
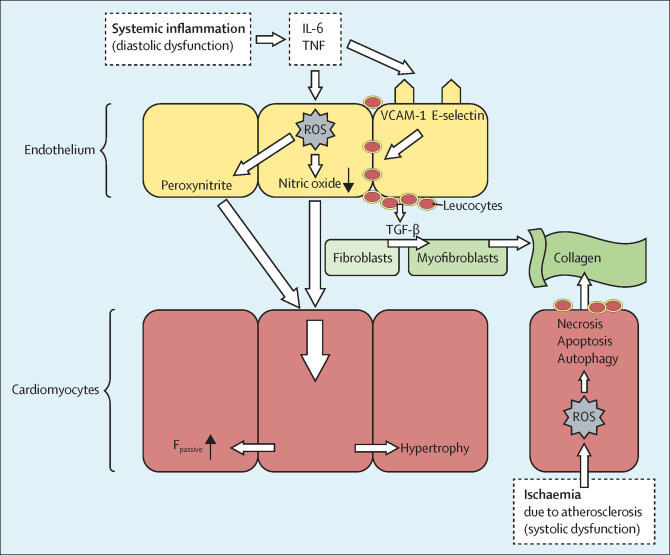
Shared mechanisms in the development of heart failure with preserved or reduced ejection fraction in patients with rheumatoid arthritis and gout
Systemic inflammation with elevated concentrations of circulating cytokines, such as IL-6 and TNF, induce oxidative stress and endothelial activation. Consequently, presentation of adhesion molecules (VCAM-1 and E-selectin) by endothelial cells leads to monocyte infiltration in the myocardium. These monocytes produce TGF-β, resulting in the differentiation of fibroblasts into myofibroblasts, with subsequent deposition of collagen in the interstitial space. In addition, intracellular oxidative stress results in disrupted crosstalk between endothelial cells and cardiomyocytes, leading to stiffness and hypertrophy of cardiomyocytes with a subsequent decreased ability to contract and relax. These processes ultimately lead to preclinical diastolic ventricular dysfunction, which might evolve into heart failure with preserved ejection fraction. Ischaemia, mostly secondary to atherosclerosis, leads to autophagy, apoptosis, and necrosis of cardiomyocytes, and deposition of collagen in the interstitial space. This condition can give rise to systolic ventricular dysfunction, which in severe cases can lead to heart failure with reduced ejection fraction. Adapted from Paulus and Tschöpe,62 by permission of Elsevier. ROS=reactive oxygen species.
Remarkably, the pathogenesis of cardiac dysfunction in hyperuricaemia and gout has not been adequately investigated to date.66 Previous studies showed an association between serum urate concentration and inflammation, thus suggesting a comparable pathogenesis with rheumatoid arthritis.67 Furthermore, increased serum urate concentration and higher activity of ROS-generating XO in gout might lead to endothelial dysfunction with decreased nitric oxide production.68 Hypertension might also have a causal role because gout and hyperuricaemia are independent predictors of developing hypertension. One interventional study assessing urate-lowering therapy in patients with heart failure did not find significant beneficial effects of allopurinol, probably because there was already advanced structural myocardial damage and multimorbidity in the patient population.69 Studies with early urate-lowering interventions are needed to answer the question of whether, and to what extent, urate concentration and the associated inflammation in gout constitutes an independent risk factor for cardiac dysfunction.70
Cardiovascular effects of drug treatment
Rheumatoid arthritis
Most non-steroidal anti-inflammatory drugs (NSAIDs), including the cyclooxygenase inhibitors, are associated with about a two-times increased cardiovascular risk.71 The exception is probably naproxen, although the published literature is conflicting in this respect.72 Cardiovascular risk is associated with COX-2 selectivity, and the lower COX-2 selectivity of naproxen than of other NSAIDs (eg, cyclooxygenase inhibitors) is assumed to translate into a lower cardiovascular risk.73 In clinical practice, weighing the benefits of these drugs against cardiovascular risk is often difficult. The first risk model that could aid in clinical decision making regarding the use of these drugs was published in 2019.74 This model was based on the Precision trial,75 a large randomised controlled trial in which patients with osteoarthritis or rheumatoid arthritis were randomised to naproxen, ibuprofen, or celecoxib.75 A major toxicity risk calculator was developed from easily accessible cardiovascular risk factors (ie, age, gender, diabetes, cardiovascular disease, hypertension, current smoking, statin use, serum creatinine, rheumatoid arthritis, and haematocrit) to calculate a 1-year risk score yielding three risk categories: low (<1%), intermediate (1–4%), or high risk (>4%). However, external validation is necessary before such a calculator can be implemented in daily clinical practice.
There has been a long-standing debate on whether steroids (eg, low dose of prednisolone, <10 mg daily) have unfavourable cardiovascular effects in an inflammatory situation. This effect is suggested by observational studies, but these data are confounded by indication because of high disease activity and should therefore be interpreted with caution.76 Hopefully this debate can be settled by the Gloria trial (NCT02585258), a multicentre, 2-year trial assessing the safety and effectiveness of a daily 5 mg dose of prednisolone versus a matching placebo added to standard of care in older patients (aged ≥65 years) with rheumatoid arthritis.
One of the first studies suggesting that cardiovascular mortality risk in rheumatoid arthritis could be reduced by methotrexate was published in 2002.77 Of 1240 patients with rheumatoid arthritis with a mean follow-up of 6 years, 191 (15%) patients had died, 84 (44%) of which were cardiovascular deaths. Methotrexate use was associated with a 70% reduction in cardiovascular mortality (HR 0·3, 95% CI 0·2–0·7). Methotrexate also has favourable effects on non-fatal cardiovascular events, with meta-analysis showing a risk reduction of 28% in comparison with treatment without methotrexate.78 Other conventional synthetic DMARDs, such as sulfasalazine, might also have favourable cardiovascular effects.79 In addition to beneficial effects on the lipid profile, hydroxychloroquine has been associated with a reduction in cardiovascular events in patients with rheumatoid arthritis.80 The recent association of high-dose hydroxychloroquine with QT prolongation in patients with COVID-19 has raised questions about the use of hydroxychloroquine in patients with rheumatoid arthritis.81 As such, it is important to note that disease activity of rheumatoid arthritis itself is associated with QT prolongation.82 Furthermore, there have been no published reports indicating that the use of hydroxychloquine in patients with rheumatoid arthritis is associated with an increased risk for QT prolongation. Hence, there is no indication to restrict the use of hydroxychloroquine in people with rheumatoid arthritis to require baseline electrocardiograms.
The first large-scale investigation that compared the effect of anti-TNF agents versus conventional synthetic DMARDs on cardiovascular mortality in patients with rheumatoid arthritis found an adjusted HR for death of 0·65 (95% CI 0·46–0·93).83 The risk of acute coronary syndrome in rheumatoid arthritis was studied in 7704 patients from a Swedish registry (32 621 patient-years) compared with the general population;84 the HR for acute coronary syndrome was 2·0 (95% CI 1·8–2·3) for patients who were previously untreated with biologics and 1·6 (1·4–1·9) for patients using TNF inhibitors.
A meta-analysis of 28 studies with 236 525 patients with rheumatoid arthritis investigated the reduction in cardiovascular events with methotrexate and TNF inhibitors in comparison with patients previously untreated with these agents.85 5410 cardiovascular events were observed and the relative risk was 0·72 (95% CI 0·57–0·91) for methotrexate and 0·70 (0·54–0·90) for TNF inhibitors. Hence, favourable cardiovascular effects might be mediated through an overall reduction in inflammation and are not drug specific.
In 2017, Janus kinase (JAK) inhibitors were suggested to be associated with an increased risk of thromboembolic disease86 and concerns have been reported by the US Food and Drug Administration (FDA) about the safety of tofacitinib. These concerns emerged from a postmarketing trial evaluating the safety of tofacitinib at two doses (5 mg twice daily and 10 mg dose twice daily) versus a control group given a TNF inhibitor.87 There was an increased incidence of pulmonary embolism and an increased overall mortality in patients taking 10 mg tofacitinib twice daily.88 Subsequently, a warning was issued indicating that tofacitinib 10 mg twice daily is contraindicated in patients who have an increased thromboembolic risk. In view of these events, it is important that the prescribed dose for the treatment of rheumatoid arthritis is restricted to 5 mg twice daily. The trial programme for baricitinib, the other JAK inhibitor currently licensed for rheumatoid arthritis, identified more thromboembolic events in the 4 mg group in comparison with the placebo group,89 and as a result the 4 mg dose was not approved in the USA. By contrast, a warning was issued in Europe, indicating that the 4 mg dose should not be used in patients with rheumatoid arthritis with an increased risk of venous thromboembolism. Further studies are needed to investigate and to differentiate between the potential adverse effect of JAK inhibitors and the hypercoagulable character of rheumatoid arthritis.
Gout
First-line treatment of gout flares is usually with an NSAID or cyclooxygenase inhibitor. The duration of treatment, in general, is short, as gout flares in the early stages of disease are often limited to a few days. Whether or not this treatment increases cardiovascular risk is not known. Furthermore, there are no data on the cumulative yearly dose of NSAID or cyclooxygenase inhibitor intake for patients with recurrent gout flares, as these drugs are often available without prescription. In advanced gout in particular, chronic use of these drugs might be relevant with respect to adverse cardiovascular effects.
Colchicine is frequently used for treatment of acute gout flares and is also given as a low-dose regimen to prevent gout flares during initiation of urate-lowering therapy. Colchicine was first used as an anti-inflammatory treatment in cardiovascular disease. A 2018 narrative review showed that colchicine has beneficial effects on atherosclerosis with plaque stabilisation, reduction of cardiovascular damage, and reduction in recurrence of acute coronary syndromes.90 Colchicine might also have protective effects in stable coronary artery disease.91 Two retrospective cohort studies in patients with gout showed a lower incidence of combined cardiovascular outcomes in patients treated with colchicine.92, 93 By contrast, a trial showed that short-term low-dose colchicine does not improve endothelial function in patients with coronary artery disease.94 However, an exploratory analysis indicated that endothelial function was significantly improved in the subgroup of patients with leucocyte activation, further indicating the important role of inflammation in atherosclerosis.94 Ongoing trials, such as the COLCOT trial (NCT02551094), will hopefully settle the debate about the efficacy of low-dose colchicine for secondary prevention in patients with coronary disease. In patients with gout, more research is needed to establish the effects of colchicine on cardiovascular risk.
Gout flares that do not respond to NSAIDs, cyclooxygenase inhibitors, or colchicine are frequently treated with steroids, either systemically or by intra-articular injection. As data on patients with gout are scarce, one can only speculate as to whether the typically short treatment duration has any unfavourable cardiovascular effects.
In 2013, the anti-IL-1β antibody canakinumab was approved by the FDA and the European Medicines Agency for gout inflammation refractory to conventional anti-inflammatory treatment.95 A 2019 trial of the IL-1 receptor antagonist, anakinra, showed efficacy in controlling inflammation during gout flare without cardiovascular safety issues.96 The CANTOS trial,97 which investigated anti-inflammatory therapy with canakinumab for secondary cardiovascular prevention in patients with myocardial infarction, showed only a modest decrease in recurrent cardiovascular events (about 15%) compared with placebo, and this result was independent of lipid-lowering effects. Whether patients with gout had a cardiovascular benefit from IL-1β-blocking therapy was not answered by this trial.
In patients with diagnosed gout, urate-lowering therapy with a treat-to-target strategy to reduce serum urate below 0·36 mmol/L (6 mg/dL) is an essential therapeutic intervention recommended by current gout guidelines.98 The efficacy of a treat-to-target approach on regression of tophi, frequency of gout flares, and MRI-detected synovitis has been shown.99 To reach the serum urate target, patients could be treated with urate-lowering drugs such as XO inhibitors (eg, allopurinol), uricosurics (and combination with XO inhibitors—eg, benzbromaron), or a recombinant uricase (eg, rasburicase). Urate-lowering therapy should, in theory, reduce the risk of cardiovascular disease in gout. This therapy could decrease the risk by directly lowering urate concentration or indirectly through XO inhibition, with a subsequent reduction in oxidative stress and improvements in endothelial function.
A systematic review and meta-analysis of randomised controlled trials100 revealed that urate-lowering therapy with allopurinol, a purine XO inhibitor, lowered the incidence of major cardiovascular events (OR 0·65, 95% CI 0·41–1·05), total cardiovascular events (OR 0·57, 0·46–0·72), myocardial infarction or the need of urgent revascularisation (OR 0·38, 0·17–0·83), and new or worsening hypertension (OR 0·32, 0·18–0·58) in patients with various cardiovascular conditions versus the control group, but did not affect overall or cardiovascular mortality. These effects were not observed with non-purine-like XO inhibitors, such as febuxostat.100 However, febuxostat lowered the composite event rate (cerebral, cardiovascular, and renal events, as well as all deaths) in patients with hyperuricaemia who were at risk of cerebral, cardiovascular, or renal disease compared with a non-febuxostat group (HR 0·75, 95% CI 0·59–0·95).101
In a systematic review and meta-analysis in patients with gout urate-lowering therapy with an XO inhibitor was not shown to reduce cardiovascular events compared with placebo.102 In addition, another systematic review and meta-analysis showed that there was no significant association between all-cause mortality and allopurinol use in patients with gout, albeit the number of studies was small.103 Finally, a trial of nurse-led gout care in a primary care setting consisting of extensive patient education and involvement, as well as a treat-to-target strategy, was shown to be superior in reaching a serum urate concentration of less than 0·36 mmol/L (6 mg/dL) compared with standard care (95% vs 30%, risk ratio 3·18, 95% CI 2·42–4·18, p<0·0001).104 Fewer deaths were observed in the nurse-led care group, but numbers were too small to draw firm conclusions. To date, data regarding the effect of uricosurics or uricase treatment on cardiovascular risk in gout are absent. In general, available evidence is not sufficient to draw definite conclusions and further studies are needed.
In 2019, the FDA issued a public safety alert with a warning of increased risk of death with febuxostat compared with allopurinol.105 This warning was based on results from the CARES trial. Although febuxostat was shown to be non-inferior to allopurinol for the primary outcome (a composite of death and major cardiovascular events; HR 1·03, upper limit of the one-sided 98·5% CI 1·23, p=0·002 for non-inferiority), the incidence of secondary outcome measures, including death from any cause (HR 1·22, 95% CI 1·01–1·47) and cardiovascular death (HR 1·34, 1·03–1·73), was significantly higher with febuxostat than with allopurinol.106 Definite conclusions from this trial are difficult to draw: first, no significant difference was observed in the primary outcome of the trial, second, there was a very high rate of discontinuation of therapy, with the majority of deaths occurring after stopping the drug, and finally, a control group without a XO inhibitor was absent.106 The FDA therefore first mandated febuxostat not to be used in patients with cardiovascular disease but thereafter restricted the approved use to patients who could not be treated effectively or had severe side-effects with allopurinol. Important data will come from the FAST trial, comparing the cardiovascular safety of allopurinol and febuxostat in the management of symptomatic hyperuricaemia (EUDRACT number: 2011–001883–23, ISRCTN72443728).
Management of cardiovascular risk
The recognition of an increased cardiovascular disease risk in arthritis prompted the European League Against Rheumatism (EULAR) to set out evidence-based recommendations for the management of cardiovascular disease risk in inflammatory arthritis, and these guidelines were updated in 2017.107 Rheumatoid arthritis is also now accepted as an independent cardiovascular disease risk factor by the European Society of Cardiology guidelines.108 To reduce the risk of cardiovascular disease in patients with rheumatoid arthritis, optimal disease control is necessary, and cardiovascular disease management should be the responsibility of the rheumatologist. Risk assessment should be done at least once every 5 years, and should be reassessed following major changes in DMARD therapy. Cardiovascular risk management is also important for patients with gout, since gout is associated with a high rate of traditional cardiovascular risk factors and is also considered an independent risk factor for cardiovascular disease and associated mortality. In the 2016 update of EULAR's evidence-based recommendations for the management of gout, two overarching principles were that every patient with gout should receive advice regarding lifestyle and that they should be screened for cardiovascular comorbidities and cardiovascular risk factors. However, no specific recommendations for cardiovascular risk management were given.109 This omission was one of the main reasons for the assembly of a EULAR taskforce to develop recommendations for the management of atherosclerotic cardiovascular risk in rheumatic and musculoskeletal diseases, including systemic lupus erythematosus, antiphospholipid syndrome, vasculitis, and gout.110
Cardiovascular risk prediction models
In Europe, the Systematic Coronary Risk Evaluation calculator is used for cardiovascular disease risk prediction. This model estimates the 10-year risk of cardiovascular mortality and includes age, gender, smoking status, total cholesterol to HDL cholesterol ratio, and systolic blood pressure. However, Systematic Coronary Risk Evaluation was developed solely for the general population and therefore underestimates the cardiovascular risk in patients with rheumatoid arthritis. To correct for this underestimation, EULAR recommends use of a multiplication factor of 1·5.107 Importantly, EULAR advises screening for cardiovascular disease risk in all patients with inflammatory arthritis and states that the rheumatologist is responsible for its initiation. Subsequently, several prediction models for cardiovascular disease risk, such as QRISK3, have incorporated rheumatoid arthritis in their risk model.111 The Expanded Risk Score in rheumatoid arthritis is derived from the CORRONA registry and includes rheumatoid arthritis specific features (laboratory data are not needed).74 This risk score appears to perform as well as QRISK3.112 The The table shows the characteristics of these algorithms. Thus far, no gout-specific cardiovascular risk scores have been developed, probably because of an absence of consistent data on cardiovascular outcomes.
Table.
Characteristics of different cardiovascular prediction models
| Age (years) | Prediction | Risk factors and variables | Comments | |
|---|---|---|---|---|
| Systematic Coronary Risk Evaluation | 40–70 | 10-year risk for cardiovascular mortality | Age, gender, smoking habits, total cholesterol to HDL cholesterol ratio, systolic blood pressure | Multiplication by 1·5 to correct for underestimation |
| QRISK3 | 24–80 | 10-year risk for cardiovascular morbidity | Established risk factors and additional factors, such as the stage of chronic kidney disease (assessed in 5 grades), a measure of systolic blood pressure variability, migraine, corticosteroids, systemic lupus erythematosus, atypical antipsychotics, severe mental illness, HIV/AIDS), and erectile dysfunction in men | Helps identifying patients with highest cardiovascular risk |
| Expanded Risk Score in rheumatoid arthritis | All | 10-year risk for cardiovascular morbidity | Rheumatoid arthritis specific factors: disease duration, disease activity, disability, and use of corticosteroids | Laboratory data are not needed; internally validated in the CORRONA registry;74 not yet externally validated |
Antihypertensive agents and statins in rheumatoid arthritis and gout
Statins reduce cholesterol concentrations and cardiovascular disease in patients with rheumatoid arthritis to a similar extent as in the general population. Furthermore, statins are not associated with more adverse events in comparison to the general population. The TRACE RA study compared the effect of 40 mg atorvastatin with placebo in patients with rheumatoid arthritis and found a significant reduction in LDL cholesterol in the atorvastatin group.113 In this group, LDL concentrations were similar to those observed in the general population. A risk reduction of 34% in major cardiovascular events compared with placebo was observed, although this difference did not reach statistical significance possibly due to a lower than expected event rate. Furthermore, statins have anti-inflammatory properties that might translate into further reductions in cardiovascular disease risk as well as a modest reduction in rheumatic disease. A meta-analysis has shown the pleiotropic effects of statins, with a decline in inflammation markers (oestrogen receptor, C-reactive protein), pro-inflammatory cytokines (TNF, IL-1, and IL-6), and fewer tender and swollen joints.114
The EULAR recommendations for gout advise systematic screening and care in terms of cardiovascular diseases and risk factors.98 This strategy can be complicated because many drugs targeting comorbidities can aggravate hyperuricaemia and gout by lowering renal excretion of uric acid—eg, antihypertensive drugs such as β blockers, non-losartan angiotensin 2 receptor blockers, thiazides, and loop diuretics.115 This is also true for low-dose aspirin, but as the effect is very modest, use of low-dose aspirin for cardioprotective reasons should not be precluded in high-risk patients with gout.116 Statins (atorvastatin and simvastatin), and also fenofibrate, have urate-lowering effects by increasing renal-urate excretion.117, 118 Importantly, initiation of statins lowers the risk of all-cause mortality in gout, and might have greater benefits amongst those without previous circulatory disease.119
Lifestyle
The effects of dietary measures in rheumatoid arthritis are still uncertain because of an absence of adequately powered studies. Observational studies show that diet, exercise, and stress are associated with outcomes such as inflammation or disease activity. Therefore, EULAR recommends a general healthy lifestyle for all patients with rheumatoid arthritis, defined as a healthy diet (Mediterranean), regular exercise, and smoking cessation. Exercise improves cardiorespiratory fitness and decreases cardiovascular risk in patients with rheumatoid arthritis.120
There is low-to-moderate quality evidence for beneficial effects of weight loss with respect to lowering serum uric acid, achieving serum uric acid targets, and preventing flares in patients with gout.121 The EULAR guideline recommends that patients with gout receive advice regarding lifestyle that includes avoidance of alcohol and sugar sweetened drinks, and discourages excessive intake of meat and seafood (purine-low diet). In addition to purines, the intake of fructose is also associated with an increase in uric acid production and should be avoided.
Guidance on smoking cessation is similar to the general population as smoking has multiple adverse effects, such as increasing the risk of cardiovascular disease, respiratory disease, and cancer. Smoking also seems to have a pathogenic role in rheumatoid arthritis, promoting disease activity and reducing response to antirheumatic therapy. In contrast to rheumatoid arthritis, a relationship between smoking and the development of gout has not been found.122
Cardiovascular risk management in daily clinical practice
Many studies have indicated poor adherence to cardiovascular disease risk, management in rheumatoid arthritis. For example, in a cross-sectional study 282 (71%) of 400 patients with rheumatoid arthritis had hypertension, of which 171 (61%) were treated with antihypertensive medications. Moreover, despite treatment, many of these patients had suboptimal blood pressure.123 Also, hypercholesterolaemia is underdiagnosed and undertreated in patients with rheumatoid arthritis.124
Notwithstanding guidelines for the implementation of cardiovascular risk, management in clinical practice is often difficult.125 Nevertheless, successful implementation of cardiovascular disease risk management was shown in a Dutch programme for cardiovascular disease care.126 This programme included a collaboration between primary care and secondary care professionals with a shared laboratory system for primary care physicians and rheumatologists, in which primary care physicians received reimbursement for additional costs from health-care insurance companies. Lipid screening (as a proxy for cardiovascular disease risk management) was done in 454 (72%) of 600 patients with rheumatoid arthritis and increased to almost 90% after primary care physicians were sent reminder letters. Implementation of cardiovascular risk management for patients with gout has not been systematically studied and to what extent it has been applied in either primary or secondary care is unknown.
Research agenda
Unfortunately, despite several guidelines for cardiovascular disease risk management, implementation in daily clinical practice is still poor. A study in the UK confirmed this in a group of 673 patients with gout in primary care. Monitoring of lipids (34 [5%]), blood pressure (178 [26%]), and glucose (43 [6%]) within 1 month after their first gout consultation was infrequently recorded.127 This finding indicates the urgent unmet need for optimisation of cardiovascular risk management in these patients. At the same time, we should not forget that patients often also have other comorbid conditions. Optimal preventive treatment requires attention to these comorbidities, which are commonly seen in inflammatory arthritis and include osteoporosis, fatigue, and depression. The rationale is that the underlying mechanisms of these common comorbidities, particularly in rheumatoid arthritis, overlap. In our view, such a multimorbidity, holistic approach is the optimal way to achieve substantial improvement in the quality of life of these patients. Lastly, further randomised controlled trials with cardiovascular endpoints are needed, especially in gout, to ascertain optimal serum urate concentrations to improve the cardiovascular-risk profile. Another relevant question is whether early urate-lowering therapy—in case of asymptomatic hyperuricaemia and evidence of crystal deposition by ultrasound or dual-energy CT—has favourable cardiovascular effects, as shown for early treatment intervention in patients with rheumatoid arthritis.
Conclusions and future research
Rheumatoid arthritis and gout—two inflammatory joint diseases with different underlying causes—are associated with about a 50–70% increased risk of cardiovascular disease compared with the general population. These patients not only have inflammation of joints but also experience systemic effects, including cardiovascular and haematological manifestations. Different underlying pathophysiological mechanisms, such as systemic inflammation, elevated oxidative stress, endothelial dysfunction, and changes in lipid profiles, might contribute to a substantially higher cardiovascular risk in these patients. The increased cardiovascular risk includes not only a higher rate of ischaemic cardiovascular disease but also subclinical heart failure, which seems far more prevalent than previously thought. Early therapeutic intervention with current antirheumatic treatment in rheumatoid arthritis has shown favourable effects on cardiovascular disease risk. Unfortunately, in gout, evidence that urate-lowering therapy has consistent beneficial effects on cardiovascular outcomes is still scarce. Following the recognition that inflammation has an important causative role for cardiovascular risk in gout, anti-inflammatory therapy and urate-lowering therapies are expected to reduce the cardiovascular burden of these patients. Therefore, optimal anti-inflammatory control of patients with rheumatoid arthritis and effective urate-lowering therapy in patients with gout are the main treatment goals to date. In addition to adequate control of disease activity, attention of the treating physician should be given to the treatment of concomitant cardiovascular diseases and management of risk factors—eg, adequate control of hypertension and dislipidaemia. For individual cardiovascular risk estimation in patients with rheumatoid arthritis and gout, the use of established cardiovascular risk scores (eg, Systematic Coronary Risk Evaluation) could be implemented in daily assessments. The inclusion of a multiplication factor for gout in cardiovascular risk scores, as already accepted for rheumatoid arthritis, should be considered.
Search strategy and selection criteria
We followed the main steps in writing a narrative review, and MTN formulated cardiovascular research questions addressing epidemiological and pathophysiological aspects of rheumatoid arthritis and gout, cardiovascular effects of drug treatment, and management of cardiovascular risk. References for this Review were identified through searches of PubMed with the terms “rheumatoid arthritis”, “gout”, “hyperuricemia”, “gouty”, “arthritis, “gouty”, “atherosclerosis”, “cardiovascular disease”, “cardiovascular event”, “myocardial ischemia”, “cerebrovascular disease”, “stroke”, “angina pectoris”, “coronary disease”, “coronary artery disease”, “coronary arterial disease”, “peripheral artery disease”, “peripheral arterial disease”, “congestive heart failure”, “cardiac dysfunction”, and “cardiovascular risk management” from Jan 1, 1998, to April 1, 2019. Articles were also identified through searches of the authors' own files. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this Review.
Contributors
All authors searched and screened references. The figures were constructed by MB and DV. The drafts were co-written by RH, DV, MB, A-KT, MG, and MTN. All authors contributed to the final writing and editing of the submitted manuscript.
Declaration of interests
DV has received speaker fees from Novartis. A-KT has received speaker fees from Berlin-Chemie Menarini, Novartis, and Pfizer; personal fees and non-financial support from AstraZeneca; and personal fees from Grünenthal, outside the submitted work. MG has received speaker fees from Sobi and Roche, and research support from Berlin-Chemie Menarini and Grünenthal. MTN received consulting fees from AbbVie, Celgene, Celltrion, Eli Lilly, Janssen, and Sanofi; speakers fees from AbbVie, Bristol-Myers Squibb, Eli Lilly, Roche, and Sanofi; and research funding from AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, MSD, Mundipharma, Novartis, Pfizer, Roche, and Sanofi. All other authors declare no competing interests.
References
- 1.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018;32:174–187. doi: 10.1016/j.berh.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Liao KP. Cardiovascular disease in patients with rheumatoid arthritis. Trends Cardiovasc Med. 2017;27:136–140. doi: 10.1016/j.tcm.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo CF, Grainge MJ, Zhang W, Doherty M. Global epidemiology of gout: prevalence, incidence and risk factors. Nat Rev Rheumatol. 2015;11:649–662. doi: 10.1038/nrrheum.2015.91. [DOI] [PubMed] [Google Scholar]
- 5.Dalbeth N, Stamp L. Hyperuricaemia and gout: time for a new staging system? Ann Rheum Dis. 2014;73:1598–1600. doi: 10.1136/annrheumdis-2014-205304. [DOI] [PubMed] [Google Scholar]
- 6.Tausche AK, Manger B, Müller-Ladner U, Schmidt B. Gout as a systemic disease. Manifestations, complications and comorbidities of hyperuricaemia. Z Rheumatol. 2012;71:224–230. doi: 10.1007/s00393-011-0953-9. [DOI] [PubMed] [Google Scholar]
- 7.Bursill D, Taylor WJ, Terkeltaub R, et al. Gout, Hyperuricaemia and Crystal-Associated Disease Network (G-CAN) consensus statement regarding labels and definitions of disease states of gout. Ann Rheum Dis. 2019;78:1592–1600. doi: 10.1136/annrheumdis-2019-215933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Disveld IJM, Fransen J, Rongen GA, et al. Crystal-proven gout and characteristic gout severity factors are associated with cardiovascular disease. J Rheumatol. 2018;45:858–863. doi: 10.3899/jrheum.170555. [DOI] [PubMed] [Google Scholar]
- 9.Lottmann K, Chen X, Schädlich PK. Association between gout and all-cause as well as cardiovascular mortality: a systematic review. Curr Rheumatol Rep. 2012;14:195–203. doi: 10.1007/s11926-011-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the CARRE Investigation. Ann Rheum Dis. 2009;68:1395–1400. doi: 10.1136/ard.2008.094151. [DOI] [PubMed] [Google Scholar]
- 11.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 12.Nicola PJ, Maradit-Kremers H, Roger VL, et al. The risk of congestive heart failure in rheumatoid arthritis: a population-based study over 46 years. Arthritis Rheum. 2005;52:412–420. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 13.Janssens HJ, Arts PG, Schalk BW, Biermans MC. Gout and rheumatoid arthritis, both to keep in mind in cardiovascular risk management: a primary care retrospective cohort study. Joint Bone Spine. 2017;84:59–64. doi: 10.1016/j.jbspin.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Panoulas VF, Metsios GS, Pace AV, et al. Hypertension in rheumatoid arthritis. Rheumatology (Oxford) 2008;47:1286–1298. doi: 10.1093/rheumatology/ken159. [DOI] [PubMed] [Google Scholar]
- 15.Robertson J, Peters MJ, McInnes IB, Sattar N. Changes in lipid levels with inflammation and therapy in RA: a maturing paradigm. Nat Rev Rheumatol. 2013;9:513–523. doi: 10.1038/nrrheum.2013.91. [DOI] [PubMed] [Google Scholar]
- 16.Myasoedova E, Crowson CS, Kremers HM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. 2011;70:482–487. doi: 10.1136/ard.2010.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boers M, Nurmohamed MT, Doelman CJ, et al. Influence of glucocorticoids and disease activity on total and high density lipoprotein cholesterol in patients with rheumatoid arthritis. Ann Rheum Dis. 2003;62:842–845. doi: 10.1136/ard.62.9.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum. 2012;64:53–61. doi: 10.1002/art.33322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Hoek J, Boshuizen HC, Roorda LD, et al. Mortality in patients with rheumatoid arthritis: a 15-year prospective cohort study. Rheumatol Int. 2017;37:487–493. doi: 10.1007/s00296-016-3638-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lassere MN, Rappo J, Portek IJ, Sturgess A, Edmonds JP. How many life years are lost in patients with rheumatoid arthritis? Secular cause-specific and all-cause mortality in rheumatoid arthritis, and their predictors in a long-term Australian cohort study. Intern Med J. 2013;43:66–72. doi: 10.1111/j.1445-5994.2012.02727.x. [DOI] [PubMed] [Google Scholar]
- 21.van den Hoek J, Roorda LD, Boshuizen HC, et al. Trend in and predictors for cardiovascular mortality in patients with rheumatoid arthritis over a period of 15 years: a prospective cohort study. Clin Exp Rheumatol. 2016;34:813–819. [PubMed] [Google Scholar]
- 22.Myasoedova E, Gabriel SE, Matteson EL, Davis JM, 3rd, Therneau TM, Crowson CS. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (RA) in recent years: dawn of a new era in cardiovascular disease in RA? J Rheumatol. 2017;44:732–739. doi: 10.3899/jrheum.161154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixon WG, Watson KD, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti-tumor necrosis factor alpha therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2007;56:2905–2912. doi: 10.1002/art.22809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowson CS, Rollefstad S, Ikdahl E, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. 2018;77:48–54. doi: 10.1136/annrheumdis-2017-211735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clarson LE, Hider SL, Belcher J, Heneghan C, Roddy E, Mallen CD. Increased risk of vascular disease associated with gout: a retrospective, matched cohort study in the UK clinical practice research datalink. Ann Rheum Dis. 2015;74:642–647. doi: 10.1136/annrheumdis-2014-205252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125:679–687. doi: 10.1016/j.amjmed.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 27.Kleber ME, Delgado G, Grammer TB, et al. Uric acid and cardiovascular events: a mendelian randomization study. J Am Soc Nephrol. 2015;26:2831–2838. doi: 10.1681/ASN.2014070660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan DM, Choi HK, Verbanck M, et al. No causal effects of serum urate levels on the risk of chronic kidney disease: a mendelian randomization study. PLoS Med. 2019;16 doi: 10.1371/journal.pmed.1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuwabara M, Hisatome I, Niwa K, et al. Uric acid is a strong risk marker for developing hypertension from prehypertension: a 5-year Japanese cohort study. Hypertension. 2018;71:78–86. doi: 10.1161/HYPERTENSIONAHA.117.10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuwabara M, Borghi C, Cicero AFG, et al. Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: a five-year cohort study in Japan. Int J Cardiol. 2018;261:183–188. doi: 10.1016/j.ijcard.2018.03.045. [DOI] [PubMed] [Google Scholar]
- 31.Stack AG, Hanley A, Casserly LF, et al. Independent and conjoint associations of gout and hyperuricaemia with total and cardiovascular mortality. QJM. 2013;106:647–658. doi: 10.1093/qjmed/hct083. [DOI] [PubMed] [Google Scholar]
- 32.Pagidipati NJ, Clare RM, Keenan RT, Chiswell K, Roe MT, Hess CN. Association of gout with long-term cardiovascular outcomes among patients with obstructive coronary artery disease. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H, Huang B, Li Y, et al. Uric acid and risk of heart failure: a systematic review and meta-analysis. Eur J Heart Fail. 2014;16:15–24. doi: 10.1093/eurjhf/hft132. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan E. Gout and the risk for incident heart failure and systolic dysfunction. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2011-000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarson LE, Chandratre P, Hider SL, et al. Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J Prev Cardiol. 2015;22:335–343. doi: 10.1177/2047487313514895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perez-Ruiz F, Martínez-Indart L, Carmona L, Herrero-Beites AM, Pijoan JI, Krishnan E. Tophaceous gout and high level of hyperuricaemia are both associated with increased risk of mortality in patients with gout. Ann Rheum Dis. 2014;73:177–182. doi: 10.1136/annrheumdis-2012-202421. [DOI] [PubMed] [Google Scholar]
- 37.Chowalloor P, Raymond WD, Cheah P, Keen H. The burden of subclinical intra-articular inflammation in gout. Int J Rheum Dis. 2020;23:661–668. doi: 10.1111/1756-185X.13811. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan E. Inflammation, oxidative stress and lipids: the risk triad for atherosclerosis in gout. Rheumatology (Oxford) 2010;49:1229–1238. doi: 10.1093/rheumatology/keq037. [DOI] [PubMed] [Google Scholar]
- 39.Urman A, Taklalsingh N, Sorrento C, McFarlane IM. Inflammation beyond the joints: rheumatoid arthritis and cardiovascular disease. Scifed J Cardiol. 2018;2 [Google Scholar]
- 40.Schauer C, Janko C, Munoz LE, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 41.Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120:736–743. doi: 10.1161/CIRCRESAHA.116.309692. [DOI] [PubMed] [Google Scholar]
- 42.Megens RT, Vijayan S, Lievens D, et al. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb Haemost. 2012;107:597–598. doi: 10.1160/TH11-09-0650. [DOI] [PubMed] [Google Scholar]
- 43.Chatfield SM, Grebe K, Whitehead LW, et al. Monosodium urate crystals generate nuclease-resistant neutrophil extracellular traps via a distinct molecular pathway. J Immunol. 2018;200:1802–1816. doi: 10.4049/jimmunol.1701382. [DOI] [PubMed] [Google Scholar]
- 44.Kienhorst LB, van Lochem E, Kievit W, et al. Gout is a chronic inflammatory disease in which high levels of interleukin-8 (CXCL8), myeloid-related protein 8/myeloid-related protein 14 complex, and an altered proteome are associated with diabetes mellitus and cardiovascular disease. Arthritis Rheumatol. 2015;67:3303–3313. doi: 10.1002/art.39318. [DOI] [PubMed] [Google Scholar]
- 45.Mateen S, Moin S, Khan AQ, Zafar A, Fatima N. Increased reactive oxygen species formation and oxidative stress in rheumatoid arthritis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19:42. doi: 10.1007/s11883-017-0678-6. [DOI] [PubMed] [Google Scholar]
- 47.Dai Y, Cao Y, Zhang Z, Vallurupalli S, Mehta JL. Xanthine oxidase induces foam cell formation through LOX-1 and NLRP3 activation. Cardiovasc Drugs Ther. 2017;31:19–27. doi: 10.1007/s10557-016-6706-x. [DOI] [PubMed] [Google Scholar]
- 48.Zamudio-Cuevas Y, Martínez-Flores K, Fernández-Torres J, et al. Monosodium urate crystals induce oxidative stress in human synoviocytes. Arthritis Res Ther. 2016;18:117. doi: 10.1186/s13075-016-1012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergholm R, Leirisalo-Repo M, Vehkavaara S, Mäkimattila S, Taskinen MR, Yki-Järvinen H. Impaired responsiveness to NO in newly diagnosed patients with rheumatoid arthritis. Arterioscler Thromb Vasc Biol. 2002;22:1637–1641. doi: 10.1161/01.atv.0000033516.73864.4e. [DOI] [PubMed] [Google Scholar]
- 50.Totoson P, Maguin-Gaté K, Nappey M, Wendling D, Demougeot C. Endothelial dysfunction in rheumatoid arthritis: mechanistic insights and correlation with circulating markers of systemic inflammation. PLoS One. 2016;11 doi: 10.1371/journal.pone.0146744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krasnokutsky S, Romero AG, Bang D, et al. Impaired arterial responsiveness in untreated gout patients compared with healthy non-gout controls: association with serum urate and C-reactive protein. Clin Rheumatol. 2018;37:1903–1911. doi: 10.1007/s10067-018-4029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20:140. doi: 10.1186/s12891-019-2519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park JJ, Roudier MP, Soman D, Mokadam NA, Simkin PA. Prevalence of birefringent crystals in cardiac and prostatic tissues, an observational study. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-005308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van den Oever IA, Sattar N, Nurmohamed MT. Thromboembolic and cardiovascular risk in rheumatoid arthritis: role of the haemostatic system. Ann Rheum Dis. 2014;73:954–957. doi: 10.1136/annrheumdis-2013-204767. [DOI] [PubMed] [Google Scholar]
- 55.McInnes IB, Thompson L, Giles JT, et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann Rheum Dis. 2015;74:694–702. doi: 10.1136/annrheumdis-2013-204345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li L, McCormick N, Sayre EC, et al. Trends of venous thromboembolism risk before and after diagnosis of gout: a general population-based study. Rheumatology (Oxford) 2020;59:1099–1107. doi: 10.1093/rheumatology/kez398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conway R, Murphy CL, Madigan A, et al. Increased platelet reactivity as measured by plasma glycoprotein VI in gout. Platelets. 2018;29:821–826. doi: 10.1080/09537104.2017.1366974. [DOI] [PubMed] [Google Scholar]
- 58.Mantel Ä, Holmqvist M, Andersson DC, Lund LH, Askling J. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017;69:1275–1285. doi: 10.1016/j.jacc.2016.12.033. [DOI] [PubMed] [Google Scholar]
- 59.Wolfe F, Michaud K. Heart failure in rheumatoid arthritis: rates, predictors, and the effect of anti-tumor necrosis factor therapy. Am J Med. 2004;116:305–311. doi: 10.1016/j.amjmed.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 60.Schau T, Gottwald M, Arbach O, et al. Increased prevalence of diastolic heart failure in patients with rheumatoid arthritis correlates with active disease, but not with treatment type. J Rheumatol. 2015;42:2029–2037. doi: 10.3899/jrheum.141647. [DOI] [PubMed] [Google Scholar]
- 61.Davis JM, 3rd, Lin G, Oh JK, et al. Five-year changes in cardiac structure and function in patients with rheumatoid arthritis compared with the general population. Int J Cardiol. 2017;240:379–385. doi: 10.1016/j.ijcard.2017.03.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 63.Mygind ND, Michelsen MM, Pena A, et al. Coronary microvascular function and cardiovascular risk factors in women with angina pectoris and no obstructive coronary artery disease: the iPOWER study. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Westermann D, Lindner D, Kasner M, et al. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 65.Baniaamam M, Paulus WJ, Blanken AB, Nurmohamed MT. The effect of biological DMARDs on the risk of congestive heart failure in rheumatoid arthritis: a systematic review. Expert Opin Biol Ther. 2018;18:585–594. doi: 10.1080/14712598.2018.1462794. [DOI] [PubMed] [Google Scholar]
- 66.Bardin T, Richette P. Impact of comorbidities on gout and hyperuricaemia: an update on prevalence and treatment options. BMC Med. 2017;15:123. doi: 10.1186/s12916-017-0890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leyva F, Anker SD, Godsland IF, et al. Uric acid in chronic heart failure: a marker of chronic inflammation. Eur Heart J. 1998;19:1814–1822. doi: 10.1053/euhj.1998.1188. [DOI] [PubMed] [Google Scholar]
- 68.Kang DH, Park SK, Lee IK, Johnson RJ. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol. 2005;16:3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 69.Givertz MM, Anstrom KJ, Redfield MM, et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the Xanthine Oxidase Inhibition for Hyperuricemic Heart Failure Patients (EXACT-HF) study. Circulation. 2015;131:1763–1771. doi: 10.1161/CIRCULATIONAHA.114.014536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pontremoli R. The role of urate-lowering treatment on cardiovascular and renal disease: evidence from CARES, FAST, ALL-HEART, and FEATHER studies. Curr Med Res Opin. 2017;33(suppl 3):27–32. doi: 10.1080/03007995.2017.1378523. [DOI] [PubMed] [Google Scholar]
- 71.Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342 doi: 10.1136/bmj.c7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bally M, Dendukuri N, Rich B, et al. Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ. 2017;357 doi: 10.1136/bmj.j1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Angiolillo DJ, Weisman SM. Clinical pharmacology and cardiovascular safety of naproxen. Am J Cardiovasc Drugs. 2017;17:97–107. doi: 10.1007/s40256-016-0200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Solomon DH, Shao M, Wolski K, Nissen S, Husni ME, Paynter N. Derivation and validation of a major toxicity risk score among nonsteroidal antiinflammatory drug users based on data grom a randomized controlled trial. Arthritis Rheumatol. 2019;71:1225–1231. doi: 10.1002/art.40870. [DOI] [PubMed] [Google Scholar]
- 75.Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375:2519–2529. doi: 10.1056/NEJMoa1611593. [DOI] [PubMed] [Google Scholar]
- 76.van Sijl AM, Boers M, Voskuyl AE, Nurmohamed MT. Confounding by indication probably distorts the relationship between steroid use and cardiovascular disease in rheumatoid arthritis: results from a prospective cohort study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi HK, Hernán MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 78.De Vecchis R, Baldi C, Palmisani L. Protective effects of methotrexate against ischemic cardiovascular disorders in patients treated for rheumatoid arthritis or psoriasis: novel therapeutic insights coming from a meta-analysis of the literature data. Anatol J Cardiol. 2016;16:2–9. doi: 10.5152/akd.2015.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Halm VP, Nurmohamed MT, Twisk JW, Dijkmans BA, Voskuyl AE. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther. 2006;8:R151. doi: 10.1186/ar2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2018;77:98–103. doi: 10.1136/annrheumdis-2017-211836. [DOI] [PubMed] [Google Scholar]
- 81.Jankelson L, Karam G, Becker ML, Chinitz LA, Tsai MC. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: a systematic review. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.05.008. published online May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lazzerini PE, Capecchi PL, Bertolozzi I, et al. Marked QTc prolongation and torsades de pointes in patients with chronic inflammatory arthritis. Front Cardiovasc Med. 2016;3:3. doi: 10.3389/fcvm.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jacobsson LT, Turesson C, Nilsson JA, et al. Treatment with TNF blockers and mortality risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:670–675. doi: 10.1136/ard.2006.062497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ljung L, Askling J, Rantapää-Dahlqvist S, Jacobsson L, ARTIS Study Group The risk of acute coronary syndrome in rheumatoid arthritis in relation to tumour necrosis factor inhibitors and the risk in the general population: a national cohort study. Arthritis Res Ther. 2014;16:R127. doi: 10.1186/ar4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.EP Vantage Olumiant clot signal echoes Xeljanz experience. July 26, 2017. https://seekingalpha.com/article/4090841-olumiant-clot-signal-echoes-xeljanz-experience
- 87.US FDA FDA responds to safety signal reported in required postmarketing trial for Xeljanz. Feb 25, 2019. https://www.fda.gov/news-events/fda-brief/fda-brief-fda-responds-safety-signal-reported-required-postmarketing-trial-xeljanz
- 88.European Medicines Agency Restrictions in use of Xeljanz while EMA reviews risk of blood clots in lungs. May 17, 2019. https://www.ema.europa.eu/en/news/restrictions-use-xeljanz-while-ema-reviews-risk-blood-clots-lungs
- 89.Taylor PC, Weinblatt ME, Burmester GR, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol. 2019;71:1042–1055. doi: 10.1002/art.40841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Spartalis M, Spartalis E, Tzatzaki E, et al. The beneficial therapy with colchicine for atherosclerosis via anti-inflammation and decrease in hypertriglyceridemia. Cardiovasc Hematol Agents Med Chem. 2018;16:74–80. doi: 10.2174/1871525717666181211110332. [DOI] [PubMed] [Google Scholar]
- 91.Fiolet ATL, Nidorf SM, Mosterd A, Cornel JH. Colchicine in stable coronary artery disease. Clin Ther. 2019;41:30–40. doi: 10.1016/j.clinthera.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 92.Crittenden DB, Lehmann RA, Schneck L, et al. Colchicine use is associated with decreased prevalence of myocardial infarction in patients with gout. J Rheumatol. 2012;39:1458–1464. doi: 10.3899/jrheum.111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solomon DH, Liu CC, Kuo IH, Zak A, Kim SC. Effects of colchicine on risk of cardiovascular events and mortality among patients with gout: a cohort study using electronic medical records linked with Medicare claims. Ann Rheum Dis. 2016;75:1674–1679. doi: 10.1136/annrheumdis-2015-207984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kajikawa M, Higashi Y, Tomiyama H, et al. Effect of short-term colchicine treatment on endothelial function in patients with coronary artery disease. Int J Cardiol. 2019;281:35–39. doi: 10.1016/j.ijcard.2019.01.054. [DOI] [PubMed] [Google Scholar]
- 95.Bardin T. Canakinumab for the patient with difficult-to-treat gouty arthritis: review of the clinical evidence. Joint Bone Spine. 2015;82(suppl 1):eS9–eS16. doi: 10.1016/S1297-319X(15)30003-8. [DOI] [PubMed] [Google Scholar]
- 96.Janssen CA, Oude Voshaar MAH, Vonkeman HE, et al. Anakinra for the treatment of acute gout flares: a randomized, double-blind, placebo-controlled, active-comparator, non-inferiority trial. Rheumatology (Oxford) 2019;58:1433–1452. doi: 10.1093/rheumatology/key402. [DOI] [PubMed] [Google Scholar]
- 97.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 98.Richette P, Doherty M, Pascual E, et al. 2018 updated European League Against Rheumatism evidence-based recommendations for the diagnosis of gout. Ann Rheum Dis. 2020;79:31–38. doi: 10.1136/annrheumdis-2019-215315. [DOI] [PubMed] [Google Scholar]
- 99.Bursill D, Dalbeth N. What is the evidence for treat-to-target serum urate in gout? Curr Rheumatol Rep. 2018;20:11. doi: 10.1007/s11926-018-0719-3. [DOI] [PubMed] [Google Scholar]
- 100.Bredemeier M, Lopes LM, Eisenreich MA, et al. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2018;18:24. doi: 10.1186/s12872-018-0757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kojima S, Matsui K, Hiramitsu S, et al. Febuxostat for Cerebral and cardiorenovascular events prevention study. Eur Heart J. 2019;40:1778–1786. doi: 10.1093/eurheartj/ehz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang T, Pope JE. Cardiovascular effects of urate-lowering therapies in patients with chronic gout: a systematic review and meta-analysis. Rheumatology (Oxford) 2017;56:1144–1153. doi: 10.1093/rheumatology/kex065. [DOI] [PubMed] [Google Scholar]
- 103.Hay CA, Prior JA, Belcher J, Mallen CD, Roddy E. Mortality in patients with gout treated with allopurinol: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 2020 doi: 10.1002/acr.24205. published online April 14. [DOI] [PubMed] [Google Scholar]
- 104.Doherty M, Jenkins W, Richardson H, et al. Efficacy and cost-effectiveness of nurse-led care involving education and engagement of patients and a treat-to-target urate-lowering strategy versus usual care for gout: a randomised controlled trial. Lancet. 2018;392:1403–1412. doi: 10.1016/S0140-6736(18)32158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.US FDA FDA adds boxed warning for increased risk of death with gout medicine uloric (febuxostat) Feb 21, 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-increased-risk-death-gout-medicine-uloric-febuxostat
- 106.White WB, Saag KG, Becker MA, et al. cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. 2018;378:1200–1210. doi: 10.1056/NEJMoa1710895. [DOI] [PubMed] [Google Scholar]
- 107.Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. 2017;76:17–28. doi: 10.1136/annrheumdis-2016-209775. [DOI] [PubMed] [Google Scholar]
- 108.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth Joint Task Force of the European Society of Cardiology and other societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR) Eur Heart J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 110.European League Against Rheumatism EULAR recommendations for cardiovascular risk management in rheumatic and musculoskeletal diseases (including SLE and the antiphospholipid syndrome) 2019. https://www.eular.org/clinical_affairs_initiatives.cfm
- 111.Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357 doi: 10.1136/bmj.j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Salaffi F, Carotti M, Di Carlo M, et al. The Expanded Risk Score in Rheumatoid Arthritis (ERS-RA): performance of a disease-specific calculator in comparison with the traditional prediction scores in the assessment of the 10-year risk of cardiovascular disease in patients with rheumatoid arthrit. Swiss Med Wkly. 2018;148 doi: 10.4414/smw.2018.14656. [DOI] [PubMed] [Google Scholar]
- 113.Kitas GD, Nightingale P, Armitage J, et al. A Multicenter, randomized, placebo-controlled trial of atorvastatin for the primary prevention of cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheumatol. 2019;71:1437–1449. doi: 10.1002/art.40892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lv S, Liu Y, Zou Z, et al. The impact of statins therapy on disease activity and inflammatory factor in patients with rheumatoid arthritis: a meta-analysis. Clin Exp Rheumatol. 2015;33:69–76. [PubMed] [Google Scholar]
- 115.Choi HK, Soriano LC, Zhang Y, Rodríguez LA. Antihypertensive drugs and risk of incident gout among patients with hypertension: population based case-control study. BMJ. 2012;344 doi: 10.1136/bmj.d8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y, Neogi T, Chen C, Chaisson C, Hunter DJ, Choi H. Low-dose aspirin use and recurrent gout attacks. Ann Rheum Dis. 2014;73:385–390. doi: 10.1136/annrheumdis-2012-202589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Waldman B, Ansquer JC, Sullivan DR, et al. Effect of fenofibrate on uric acid and gout in type 2 diabetes: a post-hoc analysis of the randomised, controlled FIELD study. Lancet Diabetes Endocrinol. 2018;6:310–318. doi: 10.1016/S2213-8587(18)30029-9. [DOI] [PubMed] [Google Scholar]
- 118.Derosa G, Maffioli P, Reiner Ž, Simental-Mendía LE, Sahebkar A. Impact of statin therapy on plasma uric acid concentrations: a systematic review and meta-analysis. Drugs. 2016;76:947–956. doi: 10.1007/s40265-016-0591-2. [DOI] [PubMed] [Google Scholar]
- 119.Keller SF, Rai SK, Lu N, et al. Statin use and mortality in gout: a general population-based cohort study. Semin Arthritis Rheum. 2018;48:449–455. doi: 10.1016/j.semarthrit.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 120.Stavropoulos-Kalinoglou A, Metsios GS, Veldhuijzen van Zanten JJ, Nightingale P, Kitas GD, Koutedakis Y. Individualised aerobic and resistance exercise training improves cardiorespiratory fitness and reduces cardiovascular risk in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1819–1825. doi: 10.1136/annrheumdis-2012-202075. [DOI] [PubMed] [Google Scholar]
- 121.Nielsen SM, Bartels EM, Henriksen M, et al. Weight loss for overweight and obese individuals with gout: a systematic review of longitudinal studies. Ann Rheum Dis. 2017;76:1870–1882. doi: 10.1136/annrheumdis-2017-211472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jee Y, Jeon C, Sull JW, Go E, Cho SK. Association between smoking and gout: a meta-analysis. Clin Rheumatol. 2018;37:1895–1902. doi: 10.1007/s10067-018-4118-y. [DOI] [PubMed] [Google Scholar]
- 123.Panoulas VF, Douglas KM, Milionis HJ, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 2007;46:1477–1482. doi: 10.1093/rheumatology/kem169. [DOI] [PubMed] [Google Scholar]
- 124.van Breukelen-van der Stoep DF, van Zeben D, Klop B, et al. Marked underdiagnosis and undertreatment of hypertension and hypercholesterolaemia in rheumatoid arthritis. Rheumatology (Oxford) 2016;55:1210–1216. doi: 10.1093/rheumatology/kew039. [DOI] [PubMed] [Google Scholar]
- 125.Heslinga M, Van Den Oever I, Jonker DL, et al. Suboptimal cardiovascular risk management in rheumatoid arthritis patients despite an explicit cardiovascular risk screening programme. Scand J Rheumatol. 2019;48:345–352. doi: 10.1080/03009742.2019.1600718. [DOI] [PubMed] [Google Scholar]
- 126.Weijers JM, Rongen-van Dartel SAA, Hoevenaars DMGMF, Rubens M, Hulscher MEJL, van Riel PLCM. Implementation of the EULAR cardiovascular risk management guideline in patients with rheumatoid arthritis: results of a successful collaboration between primary and secondary care. Ann Rheum Dis. 2018;77:480–483. doi: 10.1136/annrheumdis-2017-212392. [DOI] [PubMed] [Google Scholar]
- 127.Roddy E, Mallen CD, Hider SL, Jordan KP. Prescription and comorbidity screening following consultation for acute gout in primary care. Rheumatology (Oxford) 2010;49:105–111. doi: 10.1093/rheumatology/kep332. [DOI] [PubMed] [Google Scholar]


