Abstract
Coronavirus disease 2019 (COVID-19) is a novel, highly transmittable and severe strain disease, which has rapidly spread worldwide. Despite epidemiological evidence linking COVID-19 with cardiovascular diseases, little is known about whether and how COVID-19 influences atrial fibrillation (AF), the most prevalent arrhythmia in clinical practice. Here, we review the available evidence for prevalence and incidence of AF in patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and discuss disease management approaches and potential treatment options for COVID-19 infected AF patients.
Keywords: Anticoagulation, Arrhythmia, Inflammasome, Remote monitoring, Thromboembolic risk
1. Introduction
Coronavirus disease 2019 (COVID-19) is a novel, highly transmittable and severe strain disease, which has rapidly spread worldwide. Currently, almost 11 million cases have been diagnosed and more than 500,000 infected people have died [1]. However, the true prevalence is likely much higher, as many individuals are asymptomatic and therefore never tested. Some reports show that up to 80% of infected individuals have mild to moderate symptoms and, in theory, represent a group that might not seek medical care and thus do not contribute to the estimated prevalence biasing the calculation of real infection rate. [2], [3], [4] Despite the fact that the pandemic is decelerating in most countries, the question remains whether an asymptomatic infection can affect and facilitate, like a “Trojan horse”, the development of other diseases in the near future.
Although COVID-19 is mostly characterized by symptoms in the respiratory tract, cardiovascular diseases and complications frequently accompany COVID-19 infections increasing morbidity and mortality of COVID-19 patients [5]. Arrhythmias are frequently reported in COVID-19 patients, with atrial fibrillation (AF) being the most common form. Although electrical, calcium handling, and structural remodeling plays a key role in AF pathophysiology [6], [7], [8], [9], the clinical presentation of AF is diverse and the precise mechanisms of AF remain unclear in a large proportion of patients [10]. The underlying causes of AF in COVID-19 patients are largely unknown. Here, we review the available evidence for prevalence and incidence of AF in patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and discuss disease management approaches and potential treatment options for COVID-19 infected AF patients.
2. Prevalence of AF in patients with COVID-19
There are no specific reports on the occurrence of AF during COVID-19 infection. Based on available literature, among COVID-19 patients, AF was detected in 19% to 21% of all cases. [11], [12] One study reported a prevalence of 36% in patients with cardiovascular diseases, with AF being observed in 42% of patients who did not survive [11]. In a small report, up to 75% of hospitalized COVID-19 geriatric patients had a past history of AF [13]. The most recent statistics by the COVID-19 Task Force of Italian National Institute of Health showed that 24.5% of 355 non-surviving COVID-19 patients (mean age 79.5 years, 70% men) presented with AF before the SARS-CoV-2 infection [14].
In patients with severe pneumonia, acute respiratory distress syndrome (ARDS) and sepsis, the incidence of AF during hospitalization is usually high. [15], [16], [17] For instance, 23–33% of critically ill patients with sepsis or ARDS have AF recurrences and 10% develop new-onset AF. [18]. However, reliable data regarding first-diagnosed AF in patients with COVID-19 are limited. Based on case reports [19], [20] and small clinical studies [21], [22], [23], [24], new-onset AF varies between 3.6% and 6.7% in patients with COVID-19.
According to the Danish nationwide registry, new-onset AF decreased by 47% during the first three weeks of the national lockdown compared with the same period of previous year. During lockdown, 30 patients (5.3%) with new-onset AF suffered from ischemic stroke and 15 patients (2.7%) died, compared with 45 and 14 patients (4.3% and 1.3%, respectively) during the corresponding 2019 period. The adjusted odds ratio of a related event (ischemic stroke or all-cause death) during lockdown compared with the corresponding weeks was 1.41 (95% confidence interval 0.93–2.12) [25]. These results likely reflect the fact that the majority of patients with first symptoms of AF were delaying or refusing care. Perhaps they were afraid of contact with medical services due to the pandemic, thereby postponing the initiation of anticoagulation and increasing their risk of thromboembolic complications. It is very likely that only those who consequently did suffer from those complications were eventually hospitalized.
3. Mechanisms of AF in COVID-19 patients
The COVID-19 infection is an acute disease with incubation period on average of five to six days, in some cases up to 14 days [26]. This relatively short time period is not sufficient to increase the risk of AF by for instance causing fibrosis, which usually requires weeks to months to develop. While atrial structural remodeling is important in providing the AF-maintaining substrate, AF onset and its paroxysms are often temporally related to acute COVID-19 infections. Of note, COVID-19 patients developing AF were older and most of them had at least one preexisting risk factor, including hypertension [19], [23], while some did not report any illness. [19], [20], [27] Older age and occurrence of heart failure were also associated with greater likelihood of incident AF during the COVID-19 infection [21]. Therefore, COVID-19 patients with newly diagnosed AF may have a preexisting substrate for AF and the acute COVID-19 infection may provide the trigger for AF initiation, which is consistent with the temporal relationship between new-onset AF and the COVID-19 infection.
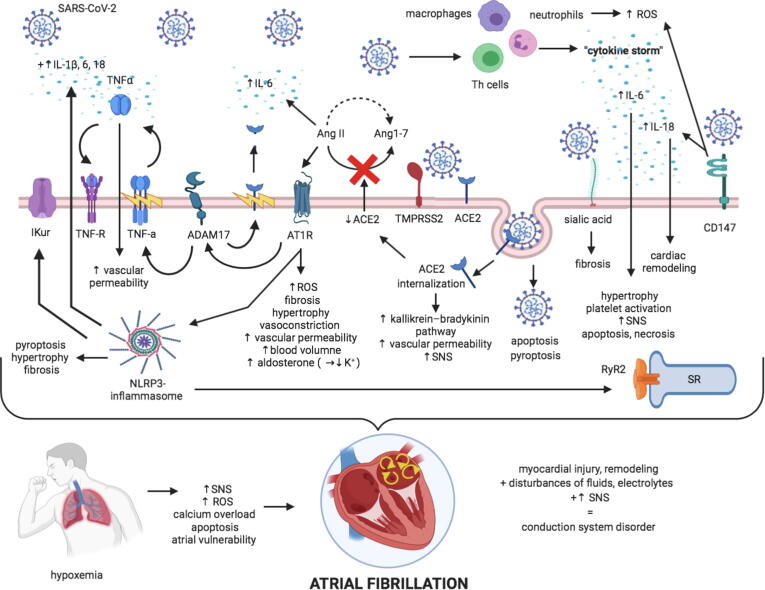
The pathophysiology of COVID-19 related AF is not well understood and proposed putative mechanisms include a reduction in angiotensin-converting enzyme 2 (ACE2) receptor availability, CD147- and sialic acid-spike protein interaction, enhanced inflammatory signalling eventually culmination in inflammatory cytokine storm, direct viral endothelial damage, electrolytes and acid-base balance abnormalities in the acute phase of severe illness and increased adrenergic drive [28]. (Fig. 1).
Fig. 1.
Putative mechanisms of AF in COVID-19 patients. Infection by SARS-CoV-2, facilitated by TMPRSS2, is manifested by the progression of immune cell over-activation leading to “cytokine storm”, ACE2 internalization and loss of both ACE2-mediated cardiovascular protection and fluid-electrolyte homeostasis, atrial structural changes via CD147- and sialic acid-spike protein, and hypoxemia. Subsequently, all abovementioned mechanisms result in myocardial injury and remodelling, sympathetic nervous system activation that coupled with electrolytes and fluids disturbances lead to conduction system disorder, hence atrial fibrillation susceptibility.
3.1. ACE2-related signalling pathways
ACE2, a membrane-bound protease, has been identified as a functional receptor for coronaviruses [29]. Although other receptors/facilitators on the surface of human cells such as sialic acid [30] and extracellular matrix metalloproteinase inducer (CD147) [31] have been also shown to mediate the entry of SARS-CoV-2, ACE2 appears to be the major and most studied entry pathway. Upon cleavage (priming) of the viral spike protein of SARS-CoV-2 by the transmembrane protease serine 2 (TMPRSS2), SARS-CoV-2 uses ACE2 to enter and infect multiple types of host cells such as pneumocytes, macrophages, endothelial cells, pericytes, and cardiomyocytes, among others.
ACE2 internalization after binding with SARS-CoV-2 results in a reduction of ACE2 at the cell surface suppressing a key pathway for angiotensin II (AngII) degradation to cardioprotective Ang1–7. The subsequent increase in AngII:Ang1–7 ratio following ACE2 internalization shifts the balance to AngII thereby promoting cardiac hypertrophy, vasoconstriction, tissue fibrosis and oxidative stress [32], potentially increasing the susceptibility to AF. AngII increases the activity of a disintegrin and metalloproteinase 17 (ADAM17) that cleaves its primary substrate to release soluble tumor necrosis factor-α (TNF-α) into the extracellular space where it exerts auto- and paracrine functions, along with ACE2 shedding from the cell membrane. Loss of ACE2 in the brain may also increase the sympathetic drive and impair the baroreflex, potentially promoting AF. [32], [33], [34] In addition, reduced expression of ACE2 in the vasculature may cause an activation of the kallikrein–bradykinin system, thereby increasing vascular permeability, promoting endothelial dysfunction and inflammation and exacerbating existing atherosclerosis and diabetes [35], two common risk factors of AF. Decreases in cell surface ACE2 in the tubular epithelium of the kidney may also alter sodium transport causing kidney injury and hypertension [36], also potentially promoting AF. Finally, loss of ACE2 promotes epicardial adipose tissue inflammation [37], pericarditis and the development of pericardial effusion [22], all of which may predispose to the development of AF, as epicardial fat has been linked to atrial electrical remodeling, progression and outcome of ablation AF [38], [39].
3.2. CD147- and sialic acid-spike protein interaction
CD147, also known as basigin or extracellular matrix metalloproteinase inducer (EMMPRIN), is a transmembrane glycoprotein that belongs to the immunoglobulin superfamily and plays a functional role in facilitating SARS-CoV-2 invasion for host cells including cardiomyocytes [40], [41], [42], upregulates the expression of several cytokines, promotes oxidative stress in cardiomyocytes and causes negative ionotropic effects. [42], [43] CD147 is a potent inducer of IL-18 mRNA and protein expression in adult mouse cardiomyocytes [42]. IL-18 activates metalloproteinases and increases extracellular matrix components degradation causing cardiac remodeling [42]. and circulating IL-18 levels positively correlates with AF development. [44], [45], [46] However, whether and how IL-18 causes AF requires further extensive investigation.
The spike proteins of a number of coronaviruses are able to bind to sialic acids present on the cell surface. N-acetylneuraminic acid is the predominant sialic acid found in human glycoproteins and gangliosides. [47] As N-acetylneuraminic acid plays a key role in severe coronary artery diseases, involving RhoA signaling pathway activation, which is critically involved in cardiac fibrosis, N-acetylneuraminic acid may contribute AF pathophysiology [48]. Future work should test this hypothesis.
3.3. Cytokine storm
Infection by SARS-CoV-2 is manifested by the evolution of systemic inflammation and immune cell overactivation, leading to a ‘cytokine storm’ triggered by an imbalance between T–helper–1 (Th1) and Th2 cells, which results in an elevated levels of cytokines such as interleukin (IL)-1β, IL-2, IL-6, IL-7, interferon gamma (IFN-γ), IFN-inducible protein (IP)-10, TNF-α, monocyte chemoattractant protein (MCP)-1, and macrophage inflammatory protein (MIP)-1A) [49], among others. The strong release of proinflammatory cytokines can lead to apoptosis or necrosis of myocardial cells, which may produce intra-atrial repolarization and conduction disturbances. Some cytokines, such as IL-6, have direct proatherogenic effects including stimulation of vascular smooth muscle proliferation, endothelial cell activation and platelet activation. Of note, in the state of hyperinflammatory response, coronary atherosclerotic plaques are prone to rupture, causing acute cardiac injury and increasing the susceptibility for arrhythmias [50]. A multicentric study on COVID-19 patients showed elevated IL-6 levels in non-survivors compared to survivors [51] suggesting that the increased mortality might be mediated by virally driven hyperinflammation and increased susceptibility to lethal arrhythmias. Interestingly, SARS-CoV-2 may also induce the formation of the Nod-like receptor family, pyrin domains-containing 3 (NLRP3) inflammasome through its binding to ACE2 cardiomyocytes [52], to purinergic P2RX7 receptors in macrophages [52], [53], components of the complement-mediated pathway [53] and AngII type 1 receptor (AT1R) activation via elevated levels of AngII [52]. Activation of the NLRP3 inflammasome triggers an immune response via intracellular caspase-1, which leads to further release of proinflammatory cytokines (IL-1β, IL-6, IL-18) [52], inflammatory cell death (pyroptosis) and AngII-mediated vascular smooth muscle cell proliferation and remodeling which may lead to hypertension [53]. There is a causal link between activation of the NLRP3 inflammasome in atrial cardiomyocytes and AF development [54]. The mechanisms underlying the proarrhythmic effects of NLRP3-inflammasome activation in the atria include 1) abnormal diastolic ryanodine receptor type-2 (RyR2)-mediated sarcoplasmic reticulum Ca2+ releases with subsequent generation of potentially proarrhythmic delayed afterdepolarizations [55], 2) enhanced function of ultra-rapid delayed-rectifier K+ current (IKur) along with reentry-promoting action potential abbreviation, 3) and atrial structural remodeling including hypertrophy and fibrosis [56].
3.4. Endothelial dysfunction and vascular leakage
Endothelial dysfunction in patients with severe COVID-19 is caused by multiple mechanisms. First, the downregulation of ACE2 at cell membrane activates the kallikrein–bradykinin system, increasing vascular permeability [57]. Activated neutrophils, recruited to endothelial cells, produce histotoxic mediators including reactive oxygen species [58]. Then immune cells, inflammatory cytokines (Il-6, IL-8, TNFα) and vasoactive molecules (thrombin, histamine, bradykinin, thromboxane A2, vascular endothelial growth factor) lead to enhanced endothelial cells contractility and the loosening of inter-endothelial junctions. [57], [58] The cytokines IL-1β and TNFα activate glucuronidases that degrade the glycocalyx and upregulate hyaluronic acid synthase type-2, leading to increased deposition of hyaluronic acid in the extracellular matrix promoting fluid retention [58]. Together, these mechanisms lead to increased vascular permeability and vascular leakage. Finally, the virus can directly (via apoptosis and pyroptosis) impair endothelial cell function, because SARS-CoV-2-infected endothelial cells were detected in several organs of deceased patients [59].
Endothelial dysfunction increases oxidative stress, increases the formation proinflammatory cytokines and impairs nitric oxide-dependent vasorelaxation. Excessive production of reactive oxygen species is likely involved in the atrial oxidative injury, and the structural and electrical remodeling, contributing to AF [60].
3.5. Disturbances of fluids, electrolytes and acid-base balance
Hypokalemia is prevailing in patients with COVID-19 [61] and occurs in up to 61% of hospitalized patients [61]. This is assumed to be due to increased urinary and/or gastrointestinal loss of potassium. SARS-CoV-2 binds ACE2 and enhances the degradation of ACE2, and thus decreases the countering effects of ACE2 on the renin-AngII system. The final effect is to increase reabsorption of sodium and water, and thereafter increase blood pressure and excretion of potassium. Besides, patients with COVID-19 often have gastrointestinal symptoms such as diarrhea and vomiting, lowering potassium resources in the human body. [61], [62] Subsequently, hypokalemia results in cellular hyperpolarity, increases resting membrane potential and hastens depolarization in cardiac cells that predispose to AF [63].
3.6. Hypoxemia
In patients with severe SARS-CoV-2 infection, pneumonia may cause significant impairment in gas exchange and airway obstructions, leading to hypoxemia, which substantially reduces the energy supply by cell metabolism, and increases anaerobic fermentation, causing intracellular acidosis and oxygen free radicals which may destroy the phospholipid layer of cell membrane. Hypoxia-induced influx of calcium ions also leads to injury and apoptosis of cardiomyocytes [64]. During a COVID-19 infection, airflow limitation and dynamic hyperinflation result in changes in blood gases along with transmural pressure gradients. Consequently, increased pulmonary pressure leads to tricuspid regurgitation which particularly impairs the right atrium. In a sheep model, intermittent deoxygenation and reoxygenation rather than sustained hypoxemia is characterized by increased atrial vulnerability due to a differential recovery of right atrial conduction properties and refractoriness. Additionally, intrathoracic pressure swings, accompanying dynamic hyperinflation and obstructive respiratory events, have been shown to transiently shorten right atrial effective refractory period, right atrial action potential duration and increase AF inducibility by parasympathetic mediated mechanisms in a spontaneously breathing pig model. Repetitive obstructive respiratory events as observed in pig models manifest also by an increase in the number of spontaneous premature atrial beats, activation of the renin-AngII system with accompanying atrial oxidative stress and elevation in expression of connective tissue growth factor that may result in atrial tissue fibrosis [65]. Combined these processes may create an arrhythmogenic substrate which transiently increases risk of during the SARs-CoV-2 infection. [66], [67], [68]
3.6.1. Activation of the sympathetic nervous system
Severe infections activate the sympathetic nervous system (SNS), and there is also a relationship between SNS activity and AF. [69], [70] The putative underlying mechanisms likely involves a SNS-mediated increase in calcium influx into the cardiomyocytes [71], with subsequent calcium overload of the sarcoplasmic reticulum, thereby increasing the frequency of spontaneous diastolic calcium releases via ryanodine receptor channels with subsequent generation of delayed afterdepolarizations and triggered action potentials, increasing the likelihood of AF induction [72].
It has been also demonstrated that inflammatory cytokines, particularly IL-6, can cause SNS hyperactivation, via both central hypothalamus-mediated (inflammatory reflex) and a peripheral (left stellate ganglia activation) pathways [73]. In some COVID-19 patients, anxiety may also cause a SNS hyperactivation with subsequent promotion of arrhythmias [28].
4. AF management in COVID-19 patients
4.1. Rhythm and rate control
Contemporary therapy of AF with antiarrhythmic drugs and anticoagulants is complex and suboptimal and is associated with substantial side effects. [74], [75] Little data are available on the value of rhythm and rate control strategies in AF patients with COVID-19 patients. Intensified treatment of the underlying hypoxemia, inflammation and other reversible triggers (i.e. hypokalemia, hypomagnesaemia, acidosis) appears the empiric basis for treatment.
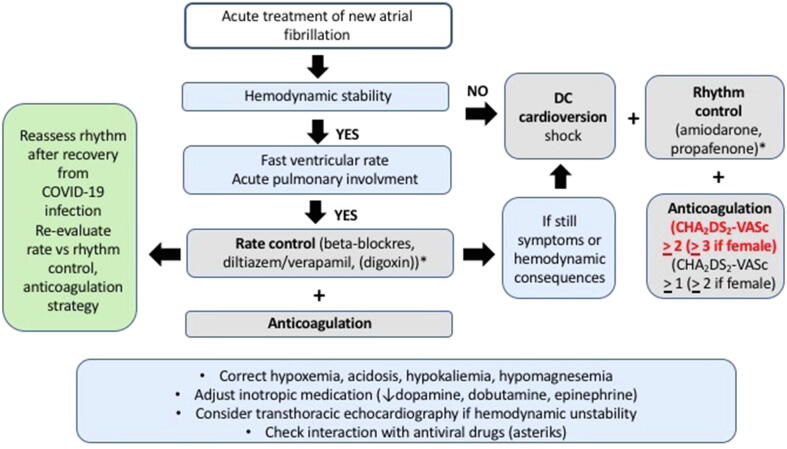
Urgent cardioversion (performed within days) should be considered in hemodynamically instable patients (also in case of acute myocardial infarction or acute heart failure) due to new-onset AF or in whom AF may be a participating factor”. In the rest of patients, not in need of urgent cardioversion, the need for cardioversion should be balanced against the need for more equipment and personnel at the side of the patients, and the possible need for intubation (with the risk of increased viral aerosol creation). In critically ill patients with hemodynamic instability due to new-onset AF intravenous amiodarone is the antiarrhythmic drug of choice for rhythm control [76]. Notably, intravenous amiodarone can cause acute pulmonary toxicity and severe hypotension [76], [77], therefore used with caution by clinicians. Sufficient rate control may be also achieved in critically ill patients using intravenous diltiazem [78]. Transthoracic echocardiography should be performed if signs of heart failure, hemodynamic instability, unexplained deterioration of the clinical status, if cardiac dysfunction is suspected. Transesophageal echocardiography should be obviated by early start of anticoagulation in new-onset AF, or continuation in newly admitted COVID-19 patients with documented AF [18]. Cardiac computed tomography can be considered instead of transesophageal echocardiography in stable patients for evaluation of left atrial appendage and intracardiac thrombus prior to cardioversion [79].
In hospitalized patients under antiviral treatment with new-onset or recurrent AF but without hemodynamic instability, discontinuation of antiarrhythmic drugs is preferred and initiation of rate control therapy with beta-blockers (or non-dihydropyridine calcium channel blockers, unless contraindicated, with or without digoxin) is preferred to allow safe antiviral medication without the risk of QT prolongation [18]. During COVID-19 infection, the QT-related risk may be amplified by concomitant use of QT-prolonging medications (e.g. hydroxychloroquine, azithromycin, lopinavir/ ritonavir), myocardial inflammation and/or electrolyte imbalances (hypokalemia, hypomagnesaemia and/or hypocalcemia) [18]. Drug-drug interactions including antiviral and antiarrhythmic drugs should be considered before start of therapy (Table S1; supplementary material) and several web pages (e.g. https://www.crediblemeds.org; www.covid19-druginteractions.org) provide a clinically useful, up-to-date, drug-drug interaction resource, freely available to healthcare workers, patients and researchers. Possible workflow of acute AF management is shown in Fig. 2.
Fig. 2.
Flowchart of acute AF management in COVID-19 patients.
All AF ablation procedures should be postponed at least for three months, expect cases of AF causing severe symptoms such as in heart failure related AF. Medically refractory AF with repeated emergency room visits should be performed within less than three months [18].
5. Thromboprophylaxis
5.1. Impairment of coagulation system by SARS-CoV-2
The first experiences in the Wuhan province in China and then in other parts of the world allowed to estimate the putative incidence of thromboembolic complications ranging from around 15% to 85% in hospitalized COVID-19 patients [80]. Of note, thromboembolic risk is influenced by race and ethnicity, and is significantly lower in Chinese compared to Caucasian individuals [81]. It is also gender-dependent: men have a worse outcome compared to women, perhaps because the activation of endothelial estrogen receptors increases nitrogen oxide and decreases reactive oxygen species, protecting the vascular system from AngII-mediated vasoconstriction, inflammation, endothelial dysfunction and associated coagulation response.
Impairment of the coagulation system, caused by SARS-CoV-2 infection, seems to increase the risk of thromboembolism in patients with AF, although this requires further investigation and validation.
Activation of endothelial cells by SARS-CoV-2 initiates the coagulation cascade by upregulation expression of von Willebrand factor and P-selectin, allowing the recruitment platelets and adhesion molecules including intercellular adhesion molecule-1, vascular cell adhesion protein-1 and E-selectin, promoting leukocyte adhesion. The activation of endothelial cells also triggers the expression of tissue factor (TF) activating the extrinsic coagulation pathway, with α-thrombin formation leading to fibrin generation and platelet activation. The effects of α-thrombin are amplified by the reduction of endothelial antithrombotic actions of molecules such as thrombomodulin, activated protein C pathway and TF-pathway inhibitor (TFPI) [82]. Thrombin generation through TF (extrinsic) and contact pathways lead to clot formation and microvascular occlusion. Activation of the complement system may also contribute to microvascular injury and thrombosis accompanying the evolution of severe COVID-19 [83]. There is also evidence for a putative role of antiphospholipid antibodies (anticardiolipin, anti-β2 glycoprotein-1) in COVID-19-related thrombotic events [84]. Noteworthy, in injured vessels, the production of nitric oxide is impaired, also promoting thrombus formation [85].
5.2. Anticoagulation
According to current guidelines [86] anticoagulation is recommended in AF patients with CHA2DS2-VASc score of 2 or more (3 or more in women) and should be considered in those with CHA2DS2-VASc score of 1 or more (2ormoreinwomen). Since hospitalized COVID-19 patients are usually older than 65 years and present in approximately 70% of cases with two or more comorbidities, most of AF patients require long-term anticoagulation [87]. Hemodynamically stable, hospitalized COVID-19 patients with AF may be treated with either unfractionated heparin, low molecular weight heparin (LMWH) or direct oral anticoagulants (DOACs), depending on the possibility of oral treatment, renal function and other clinical conditions. Of note, some of investigational drugs for COVID-19 may have relevant interactions with DOACs. In particular, this may be the case for lopinavir/ritonavir via cytochrome P450 CYP3A4 interaction and for antimalarial drugs via P-glycoprotein inhibition. In such cases, bleeding risk may be increased and DOACs should be avoided. DOACs are preferred over vitamin K antagonists (VKAs) owing to their better safety profile and fixed dosing.
Given that during treatment with VKAs regular international normalized ratio monitoring is necessary, which may contribute to spreading of the infection, VKAs should be considered in specific patient populations such as those with mechanical prosthetic valves or antiphospholipid syndrome. Although data about the effects of VKAs in patients with COVID-19 are lacking, deficiency of vitamin K might be suspected to be associated with worse COVID-19 outcome. Based on pleiotropic effects of various vitamin K-dependent anticoagulant factors (proteins C and S) as well as a protein outside the coagulation cascade (matrix Gla protein, MGP), it is likely that vitamin K might play a key role in the pathogenesis of COVID-19. Recently, a Dutch study discovered that low vitamin K levels, assessed by measuring desphospho-uncarboxylated MGP (dp-ucMGP; inversely related to vitamin K status), are more often observed in patients with COVID-19 infection (vs healthy controls), and higher dp-ucMGP levels are found in COVID-19 patients who needed mechanical ventilation or died (vs patients without need of invasive ventilation) [88]. Idiopathic pulmonary fibrosis (IPF) shows similarities with pulmonary COVID-19 infection, therefore the effects of VKA therapy in IPF patients that have been studied in two prospective clinical trials [89], [90] could be similar in COVID-19 patients. In contrast to the first study which showed a favorable effect of anticoagulants (LMWH followed by VKA therapy) on mortality related to IPF [89], the second study demonstrated an increased mortality in patients treated with VKA alone [90]. It is important to bear in mind that vitamin K deficiency induced by administration of VKAs causes both elastic fiber degradation and calcification in an animal model [91], which could contribute to the worse prognosis in IPF patients.
As heparins are not expected to interact with drugs used for COVID-19 treatment, they may be considered a safe and appealing alternative to oral anticoagulants for stroke prevention in AF patients hospitalized for COVID-19. Interestingly, in addition to the antithrombotic effect, the anti-inflammatory actions of heparin might be also relevant in this setting. Heparan sulfate proteoglycans bind to SARS-CoV2 spike proteins and may decrease binding ability to host protein, and reduce the proinflammatory activities of damage associated molecular patterns, chemokines, neutrophil chemotaxis and leukocyte migration [18]. Consistent with this notion, LMWH therapy was associated with lower 28-day mortality in SARS-CoV-2-infected patients with signs of coagulopathy [92]. After recovery from the COVID-19 infection, the long-term anticoagulation should be continued based on the CHA2DS2-VASc score.
6. Long-term management of AF patients with COVID-19 infection
To protect vulnerable patients with AF from being infected by COVID-19, social distancing was implemented in vast majority of healthcare centers to prevent extensive spread of the virus between healthcare staff and patients, and face-to-face outpatient appointments were transferred into teleconsultations. Some of the centers moved forward and combined teleconsultations with remote rhythm and rate monitoring, enabling comprehensive AF management. An example of such approach is TeleCheck-AF project, integrated, so far, in 37 European centers. [93], [94] Further, this approach will change the current standard of care by reducing the number of hospital visits (planned and unplanned) and thereby reduce healthcare costs while maintaining appropriate AF treatment. Additionally, it may represent a good strategy to prepare for future crisis, when attendance of outpatient clinics or travelling to the hospital is not possible or undesirable.
7. Conclusions
Acute SARS-CoV-2 infection may increase the susceptibility to AF and promote the evolution of a prothrombotic state. The potential development of long-term complications including development of cardiac arrhythmias in COVID-19 survivors remains to be established, especially as COVID-19 survivors are unlikely to produce long-lasting protective antibodies against this virus [95], [96], hence may be susceptible to reinfection within weeks or months. As in the acute phase of COVID-19 infection, the susceptibility to AF is increased and a worsening of existing AF likely, utilization of personal electrocardiogram devices as well as remote monitoring (teleconsultations) could optimize care of patients with AF and those with a high risk for developing AF.
8. Sources of funding
D.D. is supported by the National Institutes of Health (R01-HL131517, R01-HL136389, and R01-HL0)
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100631.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Coronavirus disease 2019 (COVID-19) Situation Report – 165 [Accessed: Jul 3, https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200703-covid-19-sitrep-165.pdf?sfvrsn=b27a772e_2 Af.
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. Coronavirus Disease 2019 (COVID-19) Situation Summary. Centers for Disease Control and Prevention. Accessed on Jul 3 Afhwcgc-n.
- 4.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Biondi-Zoccai G. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwenandar F., Japar K.V., Damay V., Hariyanto T.I., Tanaka M., Lugito N.P.H. Coronavirus disease 2019 and cardiovascular system: A narrative review. Int J Cardiol Heart Vasc. 2020;29:100557. doi: 10.1016/j.ijcha.2020.100557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobrev D., Aguilar M., Heijman J., Guichard J.B., Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat. Rev. Cardiol. 2019;16(7):417–436. doi: 10.1038/s41569-019-0166-5. [DOI] [PubMed] [Google Scholar]
- 7.Molina C.E., Abu-Taha I.H., Wang Q., Rosello-Diez E., Kamler M., Nattel S. Profibrotic, Electrical, and Calcium-Handling Remodeling of the Atria in Heart Failure Patients With and Without Atrial Fibrillation. Front. Physiol. 2018;9:1383. doi: 10.3389/fphys.2018.01383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nattel S., Dobrev D. Electrophysiological and molecular mechanisms of paroxysmal atrial fibrillation. Nat. Rev. Cardiol. 2016;13(10):575–590. doi: 10.1038/nrcardio.2016.118. [DOI] [PubMed] [Google Scholar]
- 9.Nattel S., Heijman J., Zhou L., Dobrev D. Molecular Basis of Atrial Fibrillation Pathophysiology and Therapy. Circ. Res. 2020;127(1):51–72. doi: 10.1161/CIRCRESAHA.120.316363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrade J., Khairy P., Dobrev D., Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res. 2014;114(9):1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 11.Inciardi R.M., Adamo M., Lupi L., Cani D.S., Di Pasquale M., Tomasoni D. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopinathannair R., Merchant F.M., Lakkireddy D.R., Etheridge S.P., Feigofsky S., Han J.K. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020 doi: 10.1007/s10840-020-00789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fumagalli S., Salani B., Gabbani L., Mossello E., Ungar A. Covid-19 cases in a no-Covid-19 geriatric acute care setting. A sporadic occurrence? Eur J Intern Med. 2020;77:141–142. doi: 10.1016/j.ejim.2020.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onder G., Rezza G., Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 15.Ambrus D.B., Benjamin E.J., Bajwa E.K., Hibbert K.A., Walkey A.J. Risk factors and outcomes associated with new-onset atrial fibrillation during acute respiratory distress syndrome. J. Crit. Care. 2015;30(5):994–997. doi: 10.1016/j.jcrc.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein Klouwenberg P.M., Frencken J.F., Kuipers S., Ong D.S., Peelen L.M., van Vught L.A. Incidence, Predictors, and Outcomes of New-Onset Atrial Fibrillation in Critically Ill Patients with Sepsis. A Cohort Study. Am. J. Respir. Crit. Care Med. 2017;195(2):205–211. doi: 10.1164/rccm.201603-0618OC. [DOI] [PubMed] [Google Scholar]
- 17.Walkey A.J., Hammill B.G., Curtis L.H., Benjamin E.J. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146(5):1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance. (Last update: 10 June 2020). TESfCEGftDaMoCDdtC-P.
- 19.Taha M.E., Alsafi W., Taha M., Eljack A., Ibrahim H. Coronavirus Disease and New-Onset Atrial Fibrillation: Two Cases. Cureus. 2020;12(5) doi: 10.7759/cureus.8066. e8066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seecheran R., Narayansingh R., Giddings S., Rampaul M., Furlonge K., Abdool K. Atrial Arrhythmias in a Patient Presenting With Coronavirus Disease-2019 (COVID-19) Infection. J Investig Med High Impact Case Rep. 2020;8 doi: 10.1177/2324709620925571. 2324709620925571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatla A., Mayer M.M., Adusumalli S., Hyman M.C., Oh E., Tierney A. COVID-19 and Cardiac Arrhythmias. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angeli F., Spanevello A., De Ponti R., Visca D., Marazzato J., Palmiotto G. Electrocardiographic features of patients with COVID-19 pneumonia. Eur J Intern Med. 2020 doi: 10.1016/j.ejim.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sala S., Peretto G., De Luca G., Farina N., Campochiaro C., Tresoldi M. Low prevalence of arrhythmias in clinically stable COVID-19 patients. Pacing Clin. Electrophysiol. 2020 doi: 10.1111/pace.13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q., Xu L., Dai Y., Ling Y., Mao J., Qian J. Cardiovascular manifestations in severe and critical patients with COVID-19. Clin. Cardiol. 2020 doi: 10.1002/clc.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holt A., Gislason G.H., Schou M., Zareini B., Biering-Sorensen T., Phelps M. New-onset atrial fibrillation: incidence, characteristics, and related events following a national COVID-19 lockdown of 5.6 million people. Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200402-sitrep-73-covid-19.pdf?sfvrsn=5ae25bc7_4 CdC-SRAJ.
- 27.Kochav S.M., Coromilas E., Nalbandian A., Ranard L.S., Gupta A., Chung M.K. Cardiac Arrhythmias in COVID-19 Infection. Circ Arrhythm Electrophysiol. 2020;13(6) doi: 10.1161/CIRCEP.120.008719. e008719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kochi A.N., Tagliari A.P., Forleo G.B., Fassini G.M., Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 2020;31(5):1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25(6):291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tortorici M.A., Walls A.C., Lang Y., Wang C., Li Z., Koerhuis D. Structural basis for human coronavirus attachment to sialic acid receptors. Nat. Struct. Mol. Biol. 2019;26(6):481–489. doi: 10.1038/s41594-019-0233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z., Mi L., Xu J., Yu J., Wang X., Jiang J. Function of HAb18G/CD147 in invasion of host cells by severe acute respiratory syndrome coronavirus. J. Infect. Dis. 2005;191(5):755–760. doi: 10.1086/427811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.South A.M., Diz D.I., Chappell M.C. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084–H1090. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diz D.I., Garcia-Espinosa M.A., Gegick S., Tommasi E.N., Ferrario C.M., Ann Tallant E. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp. Physiol. 2008;93(5):694–700. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu P., Sriramula S., Lazartigues E. ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300(4):R804–R817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahara M., Ikutomi M., Morita T., Minami Y., Nakajima T., Hirata Y. Deletion of angiotensin-converting enzyme 2 promotes the development of atherosclerosis and arterial neointima formation. Cardiovasc. Res. 2014;101(2):236–246. doi: 10.1093/cvr/cvt245. [DOI] [PubMed] [Google Scholar]
- 36.Simoes E.S.A.C., Teixeira M.M. ACE inhibition, ACE2 and angiotensin-(1–7) axis in kidney and cardiac inflammation and fibrosis. Pharmacol. Res. 2016;107:154–162. doi: 10.1016/j.phrs.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Patel VB, Oudit GY. Response to Comment on Patel et al. ACE2 Deficiency Worsens Epicardial Adipose Tissue Inflammation and Cardiac Dysfunction in Response to Diet-Induced Obesity. Diabetes 2016;65:85-95. Diabetes. 2016;65(2):e3-4. [DOI] [PMC free article] [PubMed]
- 38.Thanassoulis G., Massaro J.M., O'Donnell C.J., Hoffmann U., Levy D., Ellinor P.T. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ Arrhythm Electrophysiol. 2010;3(4):345–350. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sepehri Shamloo A.D.N., Dinov B. Is epicardial fat tissue associated with atrial fibrillation recurrence after ablation? A systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2019;22:132–138. doi: 10.1016/j.ijcha.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seizer P., Gawaz M., May A.E. Cyclophilin A and EMMPRIN (CD147) in cardiovascular diseases. Cardiovasc. Res. 2014;102(1):17–23. doi: 10.1093/cvr/cvu035. [DOI] [PubMed] [Google Scholar]
- 41.Siwik D.A., Kuster G.M., Brahmbhatt J.V., Zaidi Z., Malik J., Ooi H. EMMPRIN mediates beta-adrenergic receptor-stimulated matrix metalloproteinase activity in cardiac myocytes. J. Mol. Cell. Cardiol. 2008;44(1):210–217. doi: 10.1016/j.yjmcc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 42.Venkatesan B., Valente A.J., Prabhu S.D., Shanmugam P., Delafontaine P., Chandrasekar B. EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB andMKK7/JNK/AP-1 signaling. J. Mol. Cell. Cardiol. 2010;49(4):655–663. doi: 10.1016/j.yjmcc.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt R., Bultmann A., Fischel S., Gillitzer A., Cullen P., Walch A. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circ. Res. 2008;102(3):302–309. doi: 10.1161/CIRCRESAHA.107.157990. [DOI] [PubMed] [Google Scholar]
- 44.Luan Y., Guo Y., Li S., Yu B., Zhu S., Li S. Interleukin-18 among atrial fibrillation patients in the absence of structural heart disease. Europace. 2010;12(12):1713–1718. doi: 10.1093/europace/euq321. [DOI] [PubMed] [Google Scholar]
- 45.Racca V., Torri A., Grati P., Panzarino C., Marventano I., Saresella M. Inflammatory Cytokines During Cardiac Rehabilitation After Heart Surgery and Their Association to Postoperative Atrial Fibrillation. Sci. Rep. 2020;10(1):8618. doi: 10.1038/s41598-020-65581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan S., Lin A., He Q.Q., Burgess S., Larsson S.C. Circulating interleukins in relation to coronary artery disease, atrial fibrillation and ischemic stroke and its subtypes: A two-sample Mendelian randomization study. Int. J. Cardiol. 2020;313:99–104. doi: 10.1016/j.ijcard.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fantini J., Di Scala C., Chahinian H., Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents. 2020;55(5) doi: 10.1016/j.ijantimicag.2020.105960. 105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu W., Xie J., Zhu T., Meng G., Wang M., Zhou Z. Serum N-Acetylneuraminic Acid Is Associated with Atrial Fibrillation and Left Atrial Enlargement. Cardiol Res Pract. 2020;2020:1358098. doi: 10.1155/2020/1358098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madjid M., Vela D., Khalili-Tabrizi H., Casscells S.W., Litovsky S. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries: clues to the triggering effect of acute infections on acute coronary syndromes. Tex. Heart Inst. J. 2007;34(1):11–18. [PMC free article] [PubMed] [Google Scholar]
- 51.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ratajczak M.Z., Kucia M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia. 2020;34(7):1726–1729. doi: 10.1038/s41375-020-0887-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman TL STTtNIiSC-FIPJdf.
- 54.Yao C., Veleva T., Scott L., Jr., Cao S., Li L., Chen G. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation. 2018;138(20):2227–2242. doi: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dobrev D., Wehrens X.H. Role of RyR2 phosphorylation in heart failure and arrhythmias: Controversies around ryanodine receptor phosphorylation in cardiac disease. Circ. Res. 2014;114(8):1311–1319. doi: 10.1161/CIRCRESAHA.114.300568. discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott L., Jr., Li N., Dobrev D. Role of inflammatory signaling in atrial fibrillation. Int. J. Cardiol. 2019;287:195–200. doi: 10.1016/j.ijcard.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007;7(10):803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 58.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guazzi M., Arena R. Endothelial dysfunction and pathophysiological correlates in atrial fibrillation. Heart. 2009;95(2):102–106. doi: 10.1136/hrt.2007.135277. [DOI] [PubMed] [Google Scholar]
- 61.Chen D., Li X., Song Q., Hu C., Su F., Dai J. Assessment of Hypokalemia and Clinical Characteristics in Patients With Coronavirus Disease 2019 in Wenzhou, China. JAMA Netw Open. 2020;3(6) doi: 10.1001/jamanetworkopen.2020.11122. e2011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krijthe B.P., Heeringa J., Kors J.A., Hofman A., Franco O.H., Witteman J.C. Serum potassium levels and the risk of atrial fibrillation: the Rotterdam Study. Int. J. Cardiol. 2013;168(6):5411–5415. doi: 10.1016/j.ijcard.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 64.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Linz D., Hohl M., Nickel A., Mahfoud F., Wagner M., Ewen S. Effect of renal denervation on neurohumoral activation triggering atrial fibrillation in obstructive sleep apnea. Hypertension. 2013;62(4):767–774. doi: 10.1161/HYPERTENSIONAHA.113.01728. [DOI] [PubMed] [Google Scholar]
- 66.Stevenson I.H., Roberts-Thomson K.C., Kistler P.M., Edwards G.A., Spence S., Sanders P. Atrial electrophysiology is altered by acute hypercapnia but not hypoxemia: implications for promotion of atrial fibrillation in pulmonary disease and sleep apnea. Heart Rhythm. 2010;7(9):1263–1270. doi: 10.1016/j.hrthm.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 67.Linz D., Schotten U., Neuberger H.R., Bohm M., Wirth K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm. 2011;8(9):1436–1443. doi: 10.1016/j.hrthm.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 68.Linz D., Hohl M., Ukena C., Mahfoud F., Wirth K., Neuberger H.R. Obstructive respiratory events and premature atrial contractions after cardioversion. Eur. Respir. J. 2015;45(5):1332–1340. doi: 10.1183/09031936.00175714. [DOI] [PubMed] [Google Scholar]
- 69.Otake H., Suzuki H., Honda T., Maruyama Y. Influences of autonomic nervous system on atrial arrhythmogenic substrates and the incidence of atrial fibrillation in diabetic heart. Int Heart J. 2009;50(5):627–641. doi: 10.1536/ihj.50.627. [DOI] [PubMed] [Google Scholar]
- 70.Linz D., Elliott A.D., Hohl M., Malik V., Schotten U., Dobrev D. Role of autonomic nervous system in atrial fibrillation. Int. J. Cardiol. 2019;287:181–188. doi: 10.1016/j.ijcard.2018.11.091. [DOI] [PubMed] [Google Scholar]
- 71.Bers D.M. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 72.Denham N.C., Pearman C.M., Caldwell J.L., Madders G.W.P., Eisner D.A., Trafford A.W. Calcium in the Pathophysiology of Atrial Fibrillation and Heart Failure. Front. Physiol. 2018;9:1380. doi: 10.3389/fphys.2018.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, Arrhythmic Risk and Inflammation: Mind the Gap! Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 74.Dan G.A., Dobrev D. Antiarrhythmic drugs for atrial fibrillation: Imminent impulses are emerging. Int J Cardiol Heart Vasc. 2018;21:11–15. doi: 10.1016/j.ijcha.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang T.Y.L.J., Chao T.F. Oral anticoagulant use for stroke prevention in atrial fibrillation patients with difficult scenarios. Int. J. Cardiol. Heart Vasc. 2018;20:56–62. doi: 10.1016/j.ijcha.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mujovic N., Dobrev D., Marinkovic M., Russo V., Potpara T.S. The role of amiodarone in contemporary management of complex cardiac arrhythmias. Pharmacol. Res. 2020;151 doi: 10.1016/j.phrs.2019.104521. 104521. [DOI] [PubMed] [Google Scholar]
- 77.Goldschlager N., Epstein A.E., Naccarelli G.V., Olshansky B., Singh B., Collard H.R. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4(9):1250–1259. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 78.Delle Karth G., Geppert A., Neunteufl T., Priglinger U., Haumer M., Gschwandtner M. Amiodarone versus diltiazem for rate control in critically ill patients with atrial tachyarrhythmias. Crit. Care Med. 2001;29(6):1149–1153. doi: 10.1097/00003246-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 79.Ganatra S., Dani S.S., Shah S., Asnani A., Neilan T.G., Lenihan D. Management of Cardiovascular Disease During Coronavirus Disease (COVID-19) Pandemic. Trends Cardiovasc. Med. 2020;30(6):315–325. doi: 10.1016/j.tcm.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ribes A.V.-B.F., Mémier V., Poette M., Au-Duong J., Garcia C. Thromboembolic events and Covid-19. Advances in Biological. Regulation. 2020;77 doi: 10.1016/j.jbior.2020.100735. 100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P. More on COVID-19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020;189(6):1060–1061. doi: 10.1111/bjh.16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iba T., Levy J.H., Wada H., Thachil J., Warkentin T.E., Levi M. Differential diagnoses for sepsis-induced disseminated intravascular coagulation: communication from the SSC of the ISTH. J. Thromb. Haemost. 2019;17(2):415–419. doi: 10.1111/jth.14354. [DOI] [PubMed] [Google Scholar]
- 83.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N. Engl. J. Med. 2020;382(17) doi: 10.1056/NEJMc2007575. e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Green S.J. Covid-19 accelerates endothelial dysfunction and nitric oxide deficiency. Microbes Infect. 2020;22(4–5):149–150. doi: 10.1016/j.micinf.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18(11):1609–1678. doi: 10.1093/europace/euw295. [DOI] [PubMed] [Google Scholar]
- 87.Russo V., Rago A., Carbone A., Bottino R., Ammendola E., Della Cioppa N. Atrial Fibrillation in COVID-19: From epidemiological association to pharmacological implications. J. Cardiovasc. Pharmacol. 2020 doi: 10.1097/FJC.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 88.Dofferhoff A.S.P.I., Schurgers L.J., Walk J., van den Ouweland J.M., Hackeng T.M. Reduced Vitamin K Status as A Potentially Modifiable Prognostic Risk Factor in COVID-19. Preprints. 2020;2020040457 [Google Scholar]
- 89.Kubo H., Nakayama K., Yanai M., Suzuki T., Yamaya M., Watanabe M. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128(3):1475–1482. doi: 10.1378/chest.128.3.1475. [DOI] [PubMed] [Google Scholar]
- 90.Noth I., Anstrom K.J., Calvert S.B., de Andrade J., Flaherty K.R., Glazer C. A placebo-controlled randomized trial of warfarin in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2012;186(1):88–95. doi: 10.1164/rccm.201202-0314OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bouvet C., Moreau S., Blanchette J., de Blois D., Moreau P. Sequential activation of matrix metalloproteinase 9 and transforming growth factor beta in arterial elastocalcinosis. Arterioscler. Thromb. Vasc. Biol. 2008;28(5):856–862. doi: 10.1161/ATVBAHA.107.153056. [DOI] [PubMed] [Google Scholar]
- 92.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pluymaekers N., Hermans A.N.L., van der Velden R.M.J., den Uijl D.W., Vorstermans B., Buskes S. On-demand app-based rate and rhythm monitoring to manage atrial fibrillation through tele-consultations during COVID-19. Int J Cardiol Heart Vasc. 2020;100533 doi: 10.1016/j.ijcha.2020.100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Linz D., Pluymaekers N., Hendriks J.M. TeleCheck-AF for COVID-19. Eur. Heart J. 2020;41(21):1954–1955. doi: 10.1093/eurheartj/ehaa404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Long Q.X., Tang X.J., Shi Q.L., Li Q., Deng H.J., Yuan J. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020 doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 96.Liu T., Wu S., Tao H., Zeng G., Zhou F., Guo F. Prevalence of IgG antibodies to SARS-CoV-2 in Wuhan - implications for the ability to produce long-lasting protective antibodies against SARS-CoV-2. medRxiv. 2020 2020.06.13.20130252. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.