Abstract
Objective
Lung segmentation using volumetric quantitative computed tomography (CT) analysis may help predict outcomes of patients with coronavirus disease (COVID-19). The aim of this study was to investigate the relationship between CT volumetric quantitative analysis and prognosis in patients with COVID-19.
Materials and Methods
CT images from patients diagnosed with COVID-19 from February 18 to April 15, 2020 were retrospectively analyzed. CT with a negative finding, failure of quantitative analysis, or poor image quality was excluded. CT volumetric quantitative analysis was performed by automated volumetric methods. Patients were stratified into two risk groups according to CURB-65: mild (score of 0–1) and severe (2–5) pneumonia. Outcomes were evaluated according to the critical event-free survival (CEFS). The critical events were defined as mechanical ventilator care, ICU admission, or death. Multivariable Cox proportional hazards analyses were used to evaluate the relationship between the variables and prognosis.
Results
Eighty-two patients (mean age, 63.1 ± 14.5 years; 42 females) were included. In the total cohort, male sex (hazard ratio [HR], 9.264; 95% confidence interval [CI], 2.021–42.457; p = 0.004), C-reactive protein (CRP) (HR, 1.080 per mg/dL; 95% CI, 1.010–1.156; p = 0.025), and COVID-affected lung proportion (CALP) (HR, 1.067 per percentage; 95% CI, 1.033–1.101; p < 0.001) were significantly associated with CEFS. CRP (HR, 1.164 per mg/dL; 95% CI, 1.006–1.347; p = 0.041) was independently associated with CEFS in the mild pneumonia group (n = 54). Normally aerated lung proportion (NALP) (HR, 0.872 per percentage; 95% CI, 0.794–0.957; p = 0.004) and NALP volume (NALPV) (HR, 1.002 per mL; 95% CI, 1.000–1.004; p = 0.019) were associated with a lower risk of critical events in the severe pneumonia group (n = 28).
Conclusion
CRP in the mild pneumonia group; NALP and NALPV in the severe pneumonia group; and sex, CRP, and CALP in the total cohort were independently associated with CEFS in patients with COVID-19.
Keywords: Volumetric quantitative analysis, Severe acute respiratory syndrome coronavirus 2, Coronavirus disease, Prognostic implication
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that causes coronavirus disease (COVID-19) that was initially identified in Wuhan, China. The clinical manifestation of COVID-19 ranges from asymptomatic to severe respiratory syndrome that causes multiple organ failure, requiring mechanical ventilation and support from the intensive care unit (ICU) (1). About 80% of patients with COVID-19 experience mild disease with mild respiratory symptoms and radiologic abnormalities, while the other 20% suffer severe illness (2). Up to 14% of patients with COVID-19 pneumonia require mechanical ventilation (3). Moreover, COVID-19 pneumonia has a mortality rate ranging from 11% to 15% (4,5). Elderly patients and patients with coexisting diseases are more prone to severe illness, acute respiratory distress syndrome (ARDS), or death (1,2,6,7).
Previous studies reported that the possible pathologic manifestations of COVID-19 are diffuse alveolar hemorrhage and inflammatory exudation, which is similar to histologic findings seen in ARDS (8). Since the pathologic evaluation during the disease course of COVID-19 has not been established, computed tomography (CT) can reveal ground glass opacity and consolidation, which may reflect pathologic changes in these patients. Estimation of the volumetric quantification of chest CT images has been used in patients with various lung diseases including asthma, chronic obstructive pulmonary disease, interstitial lung disease, and oncological disease (9,10,11). Several studies have shown a potential role of chest CT volumetric quantification in predicting the mortality of ARDS (12,13,14,15).
Recent studies reported the use of well-aerated lung volume in patients with ARDS (14) and COVID-19 as a potential imaging biomarker to predict mortality (15). We hypothesized that quantitative volumetric CT image analysis would reflect a variable prognosis for patients with COVID-19. The purpose of this study was to investigate the relationship between quantitative volumetric analysis and prognosis in patients with COVID-19 according to pneumonia severity.
MATERIALS AND METHODS
The Institutional Review Board of participating hospitals approved this retrospective study, and patient consent was waived.
Study Population and CT Acquisition
We retrospectively reviewed 99 chest CT scans of COVID-19 patients obtained in four tertiary hospitals, Daegu, Korea, from February 18 to April 15, 2020. All the patients performed reverse-transcription polymerase chain reaction test for SARS-CoV-2 using nasal-pharyngeal swabs and CT images were performed within 5 days from diagnosis. Seventeen chest CT scans were excluded from our study due to following reasons: 1) negative CT scans (n = 7); 2) failure of quantitative analysis (n = 5); and 3) poor image quality (n = 5). Finally, 82 chest CT images from 82 patients (mean age, 63.1 ± 14.5 years; 40 males and 42 females) were included in this study. All the CT scans were performed using one of the following 8 multi-detector CT scanners: Somatom Sensation 64, Somatom Definition AS, Somatom Definition Flash, and Somatom Perspective (Siemens Healthineers); Optima CT660, LightSpeed 16, and Revolution EVO (GE Healthcare); Aquilion PRIME (Toshiba Medical Systems). CT examinations were obtained in the supine position, in full inspiration without contrast media, with the following CT parameters: a tube voltage of 100 or 120 kVp; a tube current of 58–192 mAs with a volume CT dose index of 3.97–13.77 mGy. Axial images were reconstructed with a sharp or standard reconstruction kernel at a 1.0–3.0-mm slice thickness.
Clinical Parameters
The following clinical and laboratory data were collected from medical records for each patient at admission: age, sex, underlying disease including hypertension (HTN) and diabetes mellitus (DM), white blood cell count (WBC), erythrocyte sedimentation rate (ESR), blood urea nitrogen, respiratory rate; blood pressure, and C-reactive protein (CRP). The CURB-65 is a six-point system based on clinical parameters including confusion, serum urea > 7 mmol/L, respiratory rate ≥ 30/min, low blood pressure, and age ≥ 65 years for evaluating patients. For each patient, the CURB-65 was calculated from the first recorded set of observations after hospital admission. Patients were subsequently classified into two groups according to the CURB-65 score, mild pneumonia group (score of 0–1) and severe pneumonia group (score ≥ 2) (16). We collected the occurrences of clinical critical events including mechanical ventilator care, ICU admission, or death. Then, the time from the diagnosis to the events or the last follow-up were measured.
Volumetric Quantitative CT Image Analysis
Volumetric quantitative analysis of CT images was performed by using the Pulmo 3D Workspace of the Syngo Via (Siemens Healthineers). A chest radiologist (with 5 years of chest CT interpretation) unaware of the patients' other data evaluated the acquired images. The CT images were loaded manually into the integrated tool Pulmo 3D for densitometry. The mediastinal and hilar pulmonary structures, tracheobronchial tree, pulmonary vascular structure and pleural effusion, were automatically eliminated. All segmented images were reviewed by another chest radiologist (with 13 years of chest CT interpretation) to certify the accuracy of segmentation. Further adjustment was supplemented by modifying the segmentation and excluding parenchymal lesions other than COVID-19 such as honeycombing, tuberculous sequelae, bronchiectasis, dependent densities, pleural effusions, and areas of motion artifacts. Total lung volume was calculated and lung regions were classified into 3 categories by CT attenuation value: 1) emphysema %, proportion of density between −1000 and −951 Hounsfield units (HU); 2) normally aerated lung proportion (NALP), proportion of density between −950 and −701 HU; and 3) COVID-affected lung proportion (CALP) was calculated by using the commercially available segmentation software (MEDIP COVID19 v1.2.0.0, MEDICALIP, Co. Ltd.) as previously described (17). Emphysema volume, NALP volume (NALPV), and CALP volume (CALPV) were calculated by multiplying each parameter with total lung volume.
Statistical Analysis
All numeric values are expressed as mean (± standard deviation). After confirming that the parameters were normally distributed, all group comparisons were performed using the Student t test and the Mann-Whitney U test as appropriate. The categorical data were compared using chi-squared test. Clinical parameters and volumetric quantitative CT data were compared between groups according to the CURB-65. Adjusted hazard ratio (HR) and 95% confidence interval (CI) was estimated using multivariable Cox proportional hazards regression to identify factors independently associated with critical event-free survival (CEFS). Variables with a p value < 0.2 on univariable analysis were included as input variables for multivariable Cox regression analysis using backward stepwise selection. For all statistical analyses, the level of significance was set at values of p < 0.05. All statistical analyses were performed using SPSS statistical software (version 22.0; IBM Corp.) and R software package (R version 3.6.3, The R Foundation for Statistical Computing).
RESULTS
Demographics, Clinical Parameters, and Quantitative CT Analysis
The demographics, clinical parameters, and results of the quantitative CT analysis are shown in Table 1. The patients were classified into two groups, the mild pneumonia group (n = 54) and the severe pneumonia group (n = 28) based on CURB-65. Representative examples of CT volumetric quantitative analysis in the mild and severe pneumonia groups were shown in Figures 1 and 2, respectively.
Table 1. Clinical Parameter, Demographics, and Volumetric Quantitative CT Analysis according to Pneumonia Severity.
| Total Cohort (n = 82) | Mild Pneumonia Group (n = 54) | Severe Pneumonia Group (n = 28) | P | |
|---|---|---|---|---|
| Age (yr) | 63.1 ± 14.5 | 59.6 ± 14.4 | 69.7 ± 12.2 | 0.002 |
| Sex | 0.391 | |||
| Female | 42 (51.2) | 30 (55.6) | 12 (42.9) | |
| Male | 40 (48.8) | 24 (44.4) | 16 (57.1) | |
| Hypertension | 28 (34.1) | 12 (22.2) | 16 (57.1) | 0.004 |
| DM | 24 (29.3) | 12 (22.2) | 12 (42.9) | 0.091 |
| ESR (mm/hr) | 42.2 ± 21.7 | 38.5 ± 21.6 | 46.4 ± 21.6 | 0.255 |
| CRP (mg/dL) | 4.9 ± 6.0 | 2.7 ± 4.2 | 8.3 ± 7.0 | < 0.001 |
| WBC (cells/μL) | 5584.8 ± 2997.5 | 4929.7 ± 2828.6 | 6637.7 ± 3009.5 | 0.017 |
| Critical event | 15 (18.3) | 3 (5.6) | 12 (42.9) | < 0.001 |
| Death | 8 (9.8) | 2 (3.7) | 6 (21.4) | 0.030 |
| Total lung volume (mL) | 3457.1 ± 972.1 | 3561.1± 930.9 | 3256.5 ± 1034.7 | 0.180 |
| Emphysema % | 3.1 ± 3.2 | 3.7 ± 3.5 | 1.8 ± 1.9 | 0.001 |
| NALP (%) | 73.4 ± 14.2 | 79.7 ± 5.5 | 61.3 ± 17.6 | < 0.001 |
| CALP (%) | 8.8 ± 12.0 | 3.7 ± 4.1 | 18.6 ± 15.7 | < 0.001 |
| Emphysema volume (mL) | 123.2 ± 144.7 | 154.4 ± 160.9 | 63.0 ± 79.2 | 0.001 |
| NALPV (mL) | 2627.2 ± 985.5 | 3004.4 ± 723.3 | 1899.8 ± 1026.3 | < 0.001 |
| CALPV (mL) | 232.5 ± 229.1 | 134.0 ± 144.7 | 422.4 ± 244.2 | < 0.001 |
| F/U periods (day) | 24.3 ± 15.4 | 28.4 ± 14.7 | 16.3 ± 13.6 | < 0.001 |
Continuous variables are shown as mean ± SD and categorical variables are expressed as counts and percentage in parenthesis. CALP= COVID-affected lung proportion, CALPV = CALP volume, CRP = C-reactive protein, CT = computed tomography, DM = diabetes mellitus, ESR = erythrocyte sedimentation rate, F/U = follow-up, NALP = normally aerated lung proportion, NALPV = NALP volume, SD = standard deviation, WBC = white blood cells
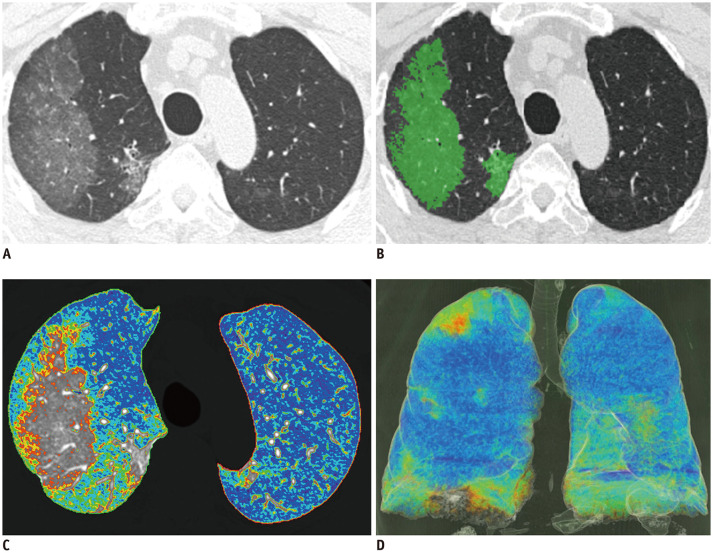
Fig. 1. COVID-19 infected 68-year-old male patient with CURB-65 score of 1.
(A) Non-enhanced axial chest CT, (B) CALP with green color-coded axial image, (C) color-coded axial image, (D) color-coded 3D reconstructed image. Color-coded regions of (C) and (D) were identified by HU according to following; purple, −1000 to −951 HU; blue, −950 to −901 HU; sky blue, −900 to −851 HU; green, −850 to −801 HU; yellow, −800 to −751 HU; and red, −750 to −701 HU. Total lung volume was 4318.0 mL, emphysema %; 10.3%, NALP 77.0%, emphysema volume 444.8 mL, NAPLV 3324.9 mL, and CALPV 168.4 mL. Patient was discharged alive without any ICU admission or mechanical ventilator treatment. CALP = COVID-affected lung proportion, CALPV = CALP volume, CEFS = critical event-free survival, HU = Hounsfield unit, ICU = intensive care unit, NALP = normally aerated lung proportion, NALPV = NALP volume
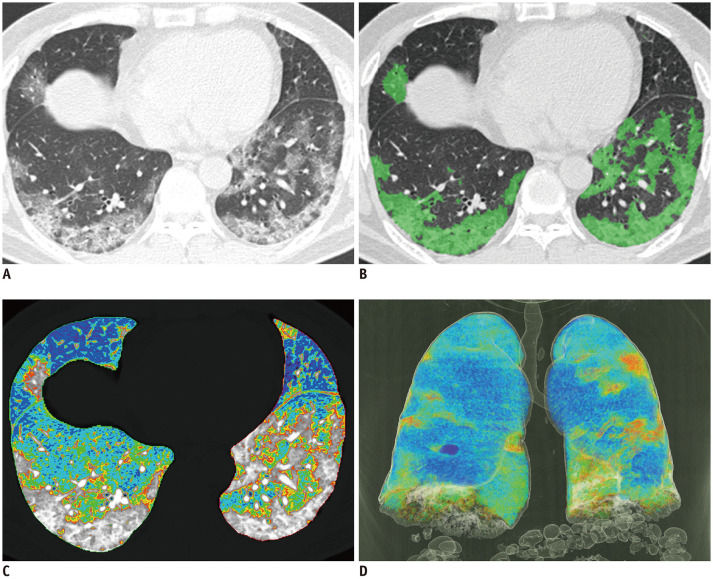
Fig. 2. COVID-19 infected 63-year-old male patient with CURB-65 score of 3.
(A) Non-enhanced axial chest CT, (B) CALP with green color-coded axial image, (C) color-coded axial image, (D) color-coded 3D reconstructed image. Color-coded regions of (C) and (D) were identified by HU according to following: purple, −1000 to −951 HU; blue, −950 to −901 HU; sky blue, −900 to −851 HU; green, −850 to −801 HU; yellow, −800 to −751 HU; and red, −750 to −701 HU. Total lung volume was 3351.0 mL, emphysema % 2.3%, NALP 65.9%, emphysema volume 77.1 mL, NAPLV 2208.3 mL, and CALPV 656.8 mL. Patient was admitted to ICU and was placed on mechanical ventilator care; however, patient died.
Age (59.6 ± 14.4 years vs. 69.7 ± 12.2 years, p = 0.002), HTN (22.2% vs. 57.1%, p = 0.004), CRP (2.7 ± 4.2 mg/dL vs. 8.3 ± 7.0 mg/dL, p < 0.001), and WBC (4929.7 ± 2828.6 vs. 6637.7 ± 3009.5, p = 0.017) were significantly lower in the mild pneumonia group than the severe pneumonia group. The clinically critical events (5.6% vs. 42.9%, p < 0.001) and mortality rate (3.7% vs. 21.4%, p = 0.030) were significantly higher in the severe pneumonia group than mild pneumonia group. Emphysema % (3.7 ± 3.5% vs. 1.8 ± 1.9%, p = 0.001), NALP (79.7 ± 5.5% vs. 61.3 ± 17.6%, p < 0.001), emphysema volume (154.4 ± 160.9 mL vs. 63.0 ± 79.2 mL, p = 0.001), and NALPV (3004.4 ± 723.3 mL vs. 1899.8 ± 1026.3 mL, p < 0.001) were significantly higher in the mild pneumonia group than severe pneumonia group. CALP (3.7 ± 4.1% vs. 18.6 ± 15.7%, p < 0.001) and CALPV (134.0 ± 144.7 mL vs. 422.4 ± 244.2 mL, p < 0.001) were significantly higher in severe pneumonia group than mild pneumonia group.
Factors Associated with CEFS in the Total Cohort
Univariable and multivariable analysis results for CEFS in the total cohort are shown in Table 2. In univariable analysis, the following factors were significantly associated with CEFS: male (HR, 4.778; 95% CI, 1.347–16.945; p = 0.015), HTN (HR, 4.499; 95% CI, 1.530–13.229; p = 0.006), CRP (HR, 1.135 per mg/dL; 95% CI, 1.072–1.201; p < 0.001), WBC (HR, 1.000 per cells/µL; 95% CI, 1.000–1.000; p = 0.002), total lung volume (HR, 0.999 per mL; 95% CI, 0.999–1.000; p = 0.033), NALP (HR, 0.936 per percentage; 95% CI, 0.913–0.960; p < 0.001), CALP (HR, 1.066 per percentage; 95% CI, 1.040–1.093; p < 0.001), NALPV (HR, 0.999 per mL; 95% CI, 0.999–1.000; p = 0.001), and CALPV (HR, 1.005 per mL; 95% CI, 1.003–1.007; p < 0.001). Variables including age, sex, HTN, DM, CRP, WBC, total lung volume, NALP, CALP, NALPV, and CALPV were included in the multivariable analysis using backward stepwise selection. In the multivariable analysis, male sex (HR, 9.264; 95% CI, 2.021–42.457; p = 0.004), CRP (HR, 1.080 per mg/dL; 95% CI, 1.010–1.156; p = 0.025), and CALP (HR, 1.067 per percentage; 95% CI, 1.033–1.101; p < 0.001) showed a significant independent association with CEFS.
Table 2. Univariable and Multivariable Cox Regression Analysis for CEFS in Total Cohort.
| Univariable Cox Regression Analysis | Multivariable Cox Regression Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.030 | 0.988–1.074 | 0.165* | Stepwise eliminated | ||
| Sex | 0.015* | 0.004 | ||||
| Male | 4.778 | 1.347–16.945 | 9.264 | 2.021–42.457 | ||
| Female | 1.000 | Reference | 1.000 | Reference | ||
| Hypertension | 0.006* | Stepwise eliminated | ||||
| Yes | 4.499 | 1.530–13.229 | ||||
| No | 1.000 | Reference | ||||
| DM | 0.174* | Stepwise eliminated | ||||
| Yes | 2.021 | 0.732–5.577 | ||||
| No | 1.000 | Reference | ||||
| ESR (mm/hr) | 0.996 | 0.971–1.022 | 0.767 | |||
| CRP (mg/dL) | 1.135 | 1.072–1.201 | < 0.001* | 1.080 | 1.010–1.156 | 0.025 |
| WBC (cells/μL) | 1.000 | 1.000–1.000 | 0.002* | Stepwise eliminated | ||
| Total lung volume (mL) | 0.999 | 0.999–1.000 | 0.033* | Stepwise eliminated | ||
| Emphysema % | 0.901 | 0.742–1.094 | 0.293 | |||
| NALP (%) | 0.936 | 0.913–0.960 | < 0.001* | Stepwise eliminated | ||
| CALP (%) | 1.066 | 1.040–1.093 | < 0.001* | 1.067 | 1.033–1.101 | < 0.001 |
| Emphysema volume (mL) | 0.998 | 0.993–1.002 | 0.321 | |||
| NALPV (mL) | 0.999 | 0.999–1.000 | 0.001* | Stepwise eliminated | ||
| CALPV (mL) | 1.005 | 1.003–1.007 | < 0.001* | Stepwise eliminated | ||
*Variables with p value < 0.2 on univariable analysis were included in multivariable analysis. CEFS = critical event-free survival, HR = hazard ratio, CI = confidence interval
Factors Associated with CEFS in Mild Pneumonia Group
Table 3 shows univariable and multivariable analysis results in the mild pneumonia group. In univariable analysis, CRP (HR, 1.204 per mg/dL; 95% CI, 1.033–1.403; p = 0.018) was significantly associated with CEFS. All patients with a critical event in this group were males with HTN. Therefore, these variables were excluded from the univariable analysis. Variables including CRP, NALPV, and CALPV were included in the multivariable analysis using backward stepwise selection. In multivariable analysis, CRP (HR, 1.164 per mg/dL; 95% CI, 1.006–1.347; p = 0.041) showed a significant independent association with CEFS.
Table 3. Univariable and Multivariable Cox Regression Analysis for CEFS in Mild Pneumonia Group.
| Univariable Cox Regression Analysis | Multivariable Cox Regression Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.032 | 0.919–1.160 | 0.594 | |||
| DM | 0.412 | |||||
| Yes | 3.195 | 0.199–51.228 | ||||
| No | 1.000 | Reference | ||||
| ESR (mm/hr) | 0.967 | 0.891–1.050 | 0.425 | |||
| CRP (mg/dL) | 1.204 | 1.033–1.403 | 0.018* | 1.164 | 1.006–1.347 | 0.041 |
| WBC (cells/μL) | 1.000 | 1.000–1.001 | 0.850 | |||
| Total lung volume (mL) | 0.999 | 0.998–1.001 | 0.273 | |||
| Emphysema % | 1.120 | 0.776–1.616 | 0.545 | |||
| NALP (%) | 0.954 | 0.747–1.219 | 0.706 | |||
| CALP (%) | 1.018 | 0.733–1.415 | 0.915 | |||
| Emphysema volume (mL) | 1.003 | 0.996–1.010 | 0.426 | |||
| NALPV (mL) | 1.001 | 1.000–1.003 | 0.155* | 1.001 | 0.999–1.004 | 0.295 |
| CALPV (mL) | 1.008 | 0.999–1.017 | 0.087* | Stepwise eliminated | ||
*Variables with p value < 0.2 on univariable analysis were included in multivariable analysis.
Factors Associated with CEFS in Severe Pneumonia Group
Table 4 shows univariable and multivariable analysis results in the severe pneumonia group. On univariable analysis, WBC (HR, 1.000 per cells/µL; 95% CI, 1.000–1.000; p = 0.034), NALP (HR, 0.957 per percentage; 95% CI, 0.926–0.989; p = 0.009), CALP (HR, 1.041 per percentage; 95% CI, 1.009–1.075; p = 0.012), and CALPV (HR, 1.040 per mL; 95% CI, 1.001–1.007; p = 0.006) were significantly associated with CEFS. Variables including age, sex, CRP, WBC, NALP, CALP, NALPV, and CALPV were included in the multivariable analysis using backward stepwise selection. In the multivariable analysis, NALP (HR, 0.872 per percentage; 95% CI, 0.794–0.957; p = 0.004) and NALPV (HR, 1.002 per mL; 95% CI, 1.000–1.004; p = 0.019) showed a significant independent association with CEFS.
Table 4. Univariable and Multivariable Cox Regression Analysis for CEFS in Severe Pneumonia Group.
| Univariable Cox Regression Analysis | Multivariable Cox Regression Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 0.972 | 0.931–1.015 | 0.194* | Stepwise eliminated | ||
| Sex | 0.097* | Stepwise eliminated | ||||
| Male | 3.033 | 0.819–11.238 | ||||
| Female | 1.000 | Reference | ||||
| Hypertension | 0.526 | |||||
| Yes | 1.475 | 0.443–4.909 | ||||
| No | 1.000 | Reference | ||||
| DM | 0.864 | |||||
| Yes | 1.105 | 0.356–3.432 | ||||
| No | 1.000 | Reference | ||||
| ESR (mm/hr) | 0.988 | 0.958–1.018 | 0.416 | |||
| CRP (mg/dL) | 1.069 | 0.996–1.147 | 0.063* | 1.071 | 0.984–1.164 | 0.112 |
| WBC (cells/μL) | 1.000 | 1.000–1.000 | 0.034* | Stepwise eliminated | ||
| Total lung volume (mL) | 1.000 | 1.000–1.001 | 0.554 | |||
| Emphysema % | 0.968 | 0.670–1.398 | 0.861 | |||
| NALP (%) | 0.957 | 0.926–0.989 | 0.009* | 0.872 | 0.794–0.957 | 0.004 |
| CALP (%) | 1.041 | 1.009–1.075 | 0.012* | Stepwise eliminated | ||
| Emphysema volume (mL) | 0.999 | 0.990–1.008 | 0.788 | |||
| NALPV (mL) | 0.999 | 0.999–1.000 | 0.111* | 1.002 | 1.000–1.004 | 0.019 |
| CALPV (mL) | 1.040 | 1.001–1.007 | 0.006* | Stepwise eliminated | ||
*Variables with p value < 0.2 on univariable analysis were included in multivariable analysis.
DISCUSSION
The main result of our study was that the male sex, high CALP on volumetric quantitative analysis, and high CRP level were independently associated with poor prognosis in patients with COVID-19. Patients were classified into two groups according to the CURB-65 score. Interestingly, CRP was independently associated in predicting the CEFS in the mild pneumonia group, whereas NALP and NALPV were independent predicting factors in the severe pneumonia group.
Females, low CALP, and low CRP level showed statistically better prognostic outcomes in the total cohort. Recently, several studies have indicated a close relationship between clinical and radiological severity in patients with COVID-19 (18,19). When the clinical severity was subdivided into mild and severe cases, the clinically severe group of COVID-19 patients showed more frequent bilateral distribution (2) and a wider extent of COVID-19 pneumonia than the clinically mild cases (7). Similarly, the CT score of the pneumonia burden had been reported as a risk factor for mortality in ARDS (20). Yang et al. (21) reported that CT severity score using semi-quantitative CT analysis might be a surrogate parameter in estimating the pneumonia burden of COVID-19. Yuan et al. (19) reported that the CT scores of COVID-19 pneumonia were much higher in the mortality group compared to those in the survival group on admission. The percent extent of COVID-19 pneumonia is a straightforward outcome indicating the radiologic severity, which was consistent with the main results of our study.
Our study showed that NALP and NALPV were independent predicting factors in the severe pneumonia group. Nishiyama et al. (14) reported that well-ventilated lung volume in CT volumetry could reflect a better prognosis in patients with ARDS. Colombi et al. (15) have reported that well-aerated lung volume is associated with better prognosis in patients with COVID-19, which was consistent with the subgroup analysis of our study. Unlike the previous study, our study analyzed emphysema as emphysema % separately from NALP and CALP and excluded pulmonary fibrosis. In addition, the authors thought a relatively large number of mild pneumonia could affect the results; hence, we compared the mild and severe pneumonia groups. Again, our NALP (−950 to −701 HU) reflects that the lungs are almost completely ventilated as compared to severely COVID-affected lung regions, and it suggests how well respiratory functions could be maintained in advanced pneumonia, especially in ARDS (9). The well-aerated lung region in severe COVID-19 pneumonia is substantially reduced in volume and might represent an important parameter for patients treated with a mechanical ventilator in ICU or those expired.
In practice, there are many technical difficulties in estimating the volumetric quantitative image analysis in patients with severe pneumonia. Patients with severe pneumonia or ARDS often have motion artifacts on CT due to acute respiratory failure or mechanical ventilator care, or it is often difficult to adequately obtain the CT image on full inspiration. On the other hand, the estimation of the normally aerated lung region is a relatively reliable and reproducible analysis. Therefore, the authors believed that the volume or proportion of normally aerated lung regions could be a potential image biomarker in patients with severe pneumonia or ARDS including patients with COVID-19.
Interestingly, we found that CRP was independently associated with predicting the CEFS in the mild pneumonia group. This result suggests that an increased level of CRP in the mild pneumonia group could predict CEFS. Tan et al. (22) reported that CRP and ESR levels were significantly elevated in the early stages in patients with severe COVID-19 pneumonia. Although initial CT images on admission are the main assessment tools to judge the disease severity, clinical parameters, such as CRP, would be more important to predict the clinical course of patients with COVID-19 pneumonia.
We found that the severity of patients with COVID-19 pneumonia could reflect the prognosis, which is similar to previous several studies (19,21). However, most patients included in this study (54/82, 65.8%) were in the mild pneumonia group, and CRP was the independent factor in predicting the prognosis in this group, rather than pneumonia severity. The authors believe that CRP would be a more important parameter for predicting the prognosis in patients with COVID-19. On the other hand, in the severe pneumonia group, NALP and NALPV were independent prognostic factors, which may mean that normally aerated lung parenchyma could be associated with better prognosis.
Several limitations exist in this study. First, because this present study is a retrospective analysis using data from four institutions, the management protocol was not standardized and it is difficult to assess risk factors for poor prognosis. Second, we used various CT scanners with different CT protocols, which could affect volumetric quantitative values. However, the authors believe that the impact was minimal because the patients with poor CT image quality were all excluded. Third, the number of patients with a critical event was relatively small. It is believed that a large cohort study will be needed in the future. Lastly, inter-reader agreement for the software-based quantitative analysis was not calculated but is expected to be high because this study was based on the consensus of two experienced thoracic radiologists.
In conclusion, CRP level was an independent predictor of ICU admission, ventilator care, or death in the mild pneumonia group, whereas the proportion of normally aerated lung parenchyma and normally aerated lung volume from volumetric quantitative CT image analysis were independent predictors of CEFS in the severe pneumonia group. Female sex, low CRP level, and low proportion of COVID-affected lung parenchyma showed a significantly better prognosis in COVID-19 patients.
Acknowledgments
This research was supported by Medicity Daegu funded by Daegu Metropolitan City.
Footnotes
This research was supported by Medicity Daegu funded by Daegu Metropolitan City.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in china: summary of a report of 72,314 cases from the Chinese center for disease control and prevention. JAMA. 2020 Feb 24; doi: 10.1001/jama.2020.2648. [Epub] [DOI] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol. 2020;21:494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen A, Karwoski RA, Gierada DS, Bartholmai BJ, Koo CW. Quantitative CT analysis of diffuse lung disease. Radiographics. 2020;40:28–43. doi: 10.1148/rg.2020190099. [DOI] [PubMed] [Google Scholar]
- 10.Chuang CC, Chou YH, Peng SL, Tai JE, Lee SC, Tyan YS, et al. Calculating air volume fractions from computed tomography images for chronic obstructive pulmonary disease diagnosis. PLoS One. 2020;15:e0231730. doi: 10.1371/journal.pone.0231730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Labaki WW, Martinez CH, Martinez FJ, Galbán CJ, Ross BD, Washko GR, et al. The role of chest computed tomography in the evaluation and management of the patient with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196:1372–1379. doi: 10.1164/rccm.201703-0451PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cereda M, Xin Y, Hamedani H, Bellani G, Kadlecek S, Clapp J, et al. Tidal changes on CT and progression of ARDS. Thorax. 2017;72:981–989. doi: 10.1136/thoraxjnl-2016-209833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Ruan Z, Zhang J, Jin W. The value of pulmonary contusion volume measurement with three-dimensional computed tomography in predicting acute respiratory distress syndrome development. Ann Thorac Surg. 2011;92:1977–1983. doi: 10.1016/j.athoracsur.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama A, Kawata N, Yokota H, Sugiura T, Matsumura Y, Higashide T, et al. A predictive factor for patients with acute respiratory distress syndrome: CT lung volumetry of the well-aerated region as an automated method. Eur J Radiol. 2020;122:108748. doi: 10.1016/j.ejrad.2019.108748. [DOI] [PubMed] [Google Scholar]
- 15.Colombi D, Bodini FC, Petrini M, Maffi G, Morelli N, Milanese G, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;201433 doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi H, Qi X, Yoon SH, Park SJ, Lee KH, Kim JY, et al. Extension of Coronavirus Disease 2019 (COVID-19) on chest CT and implications for chest radiograph interpretation. Radiol Cardiothorac Imaging. 2020;2:e200107. doi: 10.1148/ryct.2020204001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55:327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020;15:e0230548. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Chest. 2020;158:106–116. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang R, Li X, Liu H, Zhen Y, Zhang X, Xiong Q, et al. Chest CT severity score: an imaging tool for assessing severe covid-19. Radiol Cardiothorac Imaging. 2020;2:e200047. doi: 10.1148/ryct.2020200047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan C, Huang Y, Shi F, Tan K, Ma Q, Chen Y, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. 2020;92:856–862. doi: 10.1002/jmv.25871. [DOI] [PMC free article] [PubMed] [Google Scholar]