Abstract
Objective
We investigated the prevalence of pneumonia in novel coronavirus disease 2019 (COVID-19) patients using chest radiographs to identify the characteristics of those with initially negative chest radiographs, who were positive for pneumonia on follow-up.
Materials and Methods
Retrospective cohort data of 236 COVID-19 patients were reviewed. Chest radiography was performed on admission, with serial radiographs obtained until discharge. The ‘positive conversion group’ was defined as patients whose initial chest radiographs were negative but were positive for pneumonia during follow-up. Patients with initially positive chest radiographs were defined as the ‘initial pneumonia group.’ Patients with negative initial and follow-up chest radiographs were defined as the ‘non-pneumonia group.’ Clinical and laboratory findings were compared between groups, and predictors of positive conversion were investigated.
Results
Among 236 patients, 108 (45.8%) were in the non-pneumonia group, 69 (29.2%) were in the initial pneumonia group, and 59 (25%) were in the positive conversion group. The patients in the ‘initial pneumonia group’ and ‘positive conversion group’ were older, had higher C-reactive protein (CRP) and lactate dehydrogenase levels, and lower absolute lymphocyte counts than those in the ‘non-pneumonia group’ (all p < 0.001). Among patients with negative initial chest radiographs, age ≥ 45 years (odds ratio [OR]: 3.93, 95% confidence interval [CI]: 1.76–8.75, p = 0.001), absolute lymphocyte count < 1500 cells/µL (OR: 2.25, 95% CI: 1.03–4.89, p = 0.041), and CRP > 0.5 mg/dL (OR: 3.91, 95% CI: 1.54–9.91, p = 0.004) were independent predictors for future development of pneumonia.
Conclusion
More than a half of COVID-19 patients initially had normal chest radiographs; however, elderly patients (≥ 45 years of age) with abnormal laboratory findings (elevated CRP and low absolute lymphocyte counts) developed pneumonia on follow-up radiographs.
Keywords: Radiography, Coronavirus, COVID-19, Pneumonia, C-reactive protein, Lymphopenia, Lactate dehydrogenase
INTRODUCTION
In December 2019, the novel coronavirus disease 2019 (COVID-19) outbreak occurred in Wuhan, China. As of March 2020, approximately 80000 people were infected in China, with more than 3100 deaths reported from the infection (1). The disease spread rapidly to neighboring countries. In Korea, the first COVID-19 patient was diagnosed on January 20, 2020, and in February, the number of COVID-19 patients had increased rapidly, particularly among a certain religious group in Daegu city (2,3). On March 11, 2020, the World Health Organization (WHO) declared the COVID-19 outbreak a pandemic (4).
The Fleischner Society states that imaging is indicated in patients at risk of disease progression, with a worsening respiratory status (5). Therefore, it is important to identify the characteristics of those who develop pneumonia and need chest radiographs in a resource-constrained environment.
Several recent studies have reported that chest radiographs, or even computed tomography (CT), may appear normal in the early phases of COVID-19 infection (6,7). Interestingly, a few studies reported that some COVID-19 infected patients with normal CT results, eventually develop pneumonia on follow-up CT scans (7,8). The reported rates of mortality and receipt of intensive care are 4.6% and 26%, respectively, in patients who develop pneumonia (9). However, patient characteristics associated with the development of pneumonia among patients who initially had normal findings in the early stages, remains unclear.
In this emergent pandemic situation, all patients infected with COVID-19 cannot be admitted due to insufficient hospital beds. Thus, it is important to predict who will develop pneumonia and require treatment.
Therefore, this study aimed to investigate the prevalence of pneumonia in COVID-19 patients using chest radiographs, and identify the characteristics of those who had negative chest radiographs initially, who were then positive for pneumonia on follow-up chest radiographs.
MATERIALS AND METHODS
Patients
This single cohort retrospective study was approved by the Institutional Review Board of Keimyung University Dongsan Hospital (2020-04-065), and the requirement to obtain informed patient consent was waived. Our institution was designated a cohort hospital for admitting patients with COVID-19. A total of 236 patients (67 men, 169 women; 46.5 ± 15.3 years old) diagnosed with COVID-19 were admitted to the hospital between February 22, 2020 and February 27, 2020. Since it was a cohort hospital, and only COVID-19 patients were hospitalized, no patients were excluded. All patients were diagnosed with COVID-19 via laboratory testing with real-time reverse transcriptase-polymerase chain reaction of sputum or nasal secretions in other screening clinics such as public health centers. In this cohort, 142 patients (60.2%) belonged to a certain religious group, where a community infection was suspected. Most of the patients had mild symptoms such as fever, sore throat, myalgia, chills, headache, sputum, cough, rhinorrhea, and dyspnea, not requiring intensive care (such as ventilator use) at the time of admission. Twelve patients were asymptomatic (5.1%) and had been diagnosed using screening tests based on their contact history with infected persons. Three patients (1.3%) were not able to remember the exact date of symptom onset. For two of the patients (0.8%), it was not possible to specify symptom type because of severe communication difficulties.
Clinical Data Extraction
Patients' demographic data, underlying diseases, symptom types, and symptom onset dates were reviewed and collected. Initial laboratory results at the time of admission, including complete blood count with differential counts, C-reactive protein (CRP), and lactate dehydrogenase (LDH) levels were obtained. Data on clinical severity, such as the occurrence of severe pneumonia according to the WHO criteria, admission to the intensive care unit (ICU), and death due to worsening of COVID-19, were obtained.
Chest Radiograph
In our COVID-19 cohort hospital, the entire premises was designated as a contaminated isolation zone. The radiographers wore personal protective equipment to acquire chest radiographs of COVID-19 patients. A digital radiography (INNOVISION, DK Medical systems) system was used. All patients underwent initial chest radiography in the erect position (posterior-anterior projection) on the day of admission, and follow-up chest radiographs were taken every two or three days until discharge. All images were reviewed by two chest radiologists (with 9 and 16 years of experience, respectively), who were blinded to previous images and clinical information. In cases of inter-reader discrepancies, a consensus was reached through discussion.
Patients whose chest radiographs were initially negative on admission, but subsequently developed pneumonia during hospitalization, were classified as the ‘positive conversion group.’ Patients with pneumonia detected on the initial chest radiographs were classified as the ‘initial pneumonia group,’ and those patients with no evidence of pneumonia on initial and follow-up chest radiographs during hospitalization, were classified as the ‘non-pneumonia group.’ The presence of a lesion on a chest radiograph was recorded when opacities were seen. To avoid false positives in the pneumonia group, increased opacities on chest radiographs of 57 patients (44.5%) were confirmed as pneumonia on CT scans taken during hospitalization; in the remaining 71 patients (55.5%) pneumonia was determined through improvement, or new development, of opacities during hospitalization. The presence and location of pneumonia on the initial and all follow-up chest radiographs were analyzed, and the radiograph fields were divided into six zones: upper, middle, and lower lung zones on each side. The extent of pneumonia in each zone was scored from 0 to 4 (score 0: no evidence of pneumonia, score 1: 1–25% involvement of pneumonia, score 2: 26–50% involvement of pneumonia, score 3: 51–75% involvement of pneumonia, and score 4: > 75% involvement of pneumonia) (10). The scores of the 6 lung zones were added, with scores ranging 0 to 24.
Statistical Analysis
Categorical variables are presented as numbers with percentages, and continuous variables are presented as means and standard deviations. Differences between groups were analyzed using the chi-square or Fisher's exact tests for categorical variables and the ANOVA or Kruskal-Wallis test for continuous variables. Post-hoc analysis was performed using the Bonferroni method. The cut-off value for continuous variables was set based on receiver operating characteristic curve analysis for positive conversion. Logistic regression analyses were used to find clinical predictors of positive conversion of chest radiographs among patients with initially negative chest radiographs. Factors with p < 0.1 were entered into multivariable analysis. Spearman correlation analysis was performed to identify the correlation between the pneumonia scores on chest radiographs and the clinical variables. Statistical analyses were performed using SPSS version 25.0 (IBM Corp.). P values < 0.05 were considered statistically significant.
RESULTS
Patients
A total of 236 patients were included in the study and the entire follow-up period was 80 days. During hospitalization, 21 patients (8.9%) progressed to severe pneumonia and received inhalation oxygen support and 11 patients (4.7%) were admitted to the ICU. Among the latter, eight patients were eventually transferred to other tertiary hospitals due to lack of ICU space. One transferred patient (0.4%) died due to acute respiratory failure.
Initial and Follow-Up Chest Radiographs
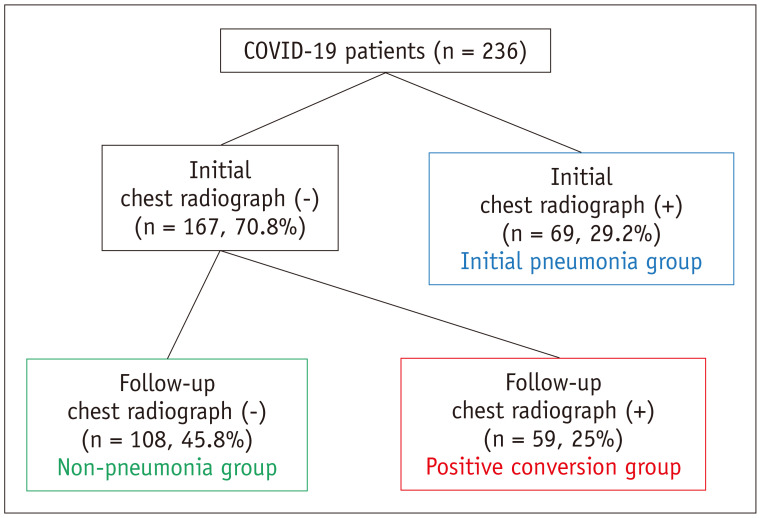
All COVID-19 patients had undergone chest radiography on the day of admission, or the following day. Among 236 patients, 108 (45.8%) were in the non-pneumonia group, 69 (29.2%) were in the initial pneumonia group, and 59 (25%) were in the positive conversion group (Fig. 1). The majority (11/12, 92%) of asymptomatic patients showed negative findings on the initial chest radiographs; however, three patients eventually showed pneumonia on follow-up chest radiographs, with symptom presentation. Among the total 236 initial chest radiographs, there were 11 discrepant cases between readers, and consensus was achieved by reviewing the cases in conjunction.
Fig. 1. Study population.
Among 236 patients, 108 (45.8%) were in non-pneumonia group, 69 (29.2%) were in initial pneumonia group, and 59 (25%) were in positive conversion group. N = number of patients, COVID-19 = novel coronavirus disease 2019
Clinical Features between Groups (Non-Pneumonia vs. Positive Conversion vs. Initial Pneumonia Groups)
The clinical characteristics of each group are summarized in Table 1. The mean age was significantly higher in the positive conversion and initial pneumonia groups than in the non-pneumonia group (mean, 51.5 years vs. 54.9 years vs. 38.5 years; p < 0.001). Chills were observed significantly more often in the initial pneumonia group than in the non-pneumonia group (p = 0.009). Myalgia was observed significantly more often in the positive conversion and initial pneumonia groups than in the non-pneumonia group (p = 0.010). Initial pneumonia and positive conversion groups had significantly more hypertension than the non-pneumonia group (both, p < 0.05). In all patients, blood tests were performed on the day of admission or the next day. Initial CRP (median, 1.3 mg/dL vs. 0.3 mg/dL vs. 0.1 mg/dL, p = 0.001) and LDH levels (median, 508 U/L vs. 448 U/L vs. 391 U/L, p < 0.001) were significantly higher and the absolute lymphocyte count (median 1260.4 cells/µL vs. 1489.0 cells/µL vs. 1645.7 cells/µL; p < 0.001) was significantly lower in the initial pneumonia and positive conversion groups compared to the non-pneumonia group. The number of patients who progressed to severe pneumonia were highest in the initial pneumonia group (7 patients, 10.1%) followed by the positive conversion group (3 patients, 5.1%). The number of patients admitted to the ICU was significantly higher in the initial pneumonia group (7 patients, 10.1%) and positive conversion group (4 patients, 6.8%) than in the non-pneumonia group (p = 0.001). One death (1.4%) occurred in the initial pneumonia group.
Table 1. Comparison of Clinical Features between Three Groups.
| Variables | Non-Pneumonia Group (n = 108) | Positive Conversion Group (n = 59) | Initial Pneumonia Group (n = 69) | P |
|---|---|---|---|---|
| Sex (male/female) | 32/76 | 16/43 | 19/50 | 0.947 |
| Age (years)*† | 38.5 ± 13.4 | 51.5 ± 14.7 | 54.9 ± 12.4 | < 0.001 |
| Vital sign at time of admission | ||||
| Body temperature (℃)*† | 37.0 ± 0.4 | 37.2 ± 0.6 | 37.4 ± 0.7 | < 0.001 |
| Pulse rate (bpm) | 82.5 ± 15.7 | 84.3 ± 15.8 | 87.0 ± 20.9 | 0.245 |
| Respiratory rate (bpm)* | 18.5 ± 1.7 | 18.7 ± 1.4 | 20.7 ± 10.4 | 0.031 |
| Systolic blood pressure (mm Hg)†‡ | 127.5 ± 17.3 | 140.9 ± 22.9 | 130.2 ± 18.5 | < 0.001 |
| Saturation (%)* | 98.1 ± 1.2 | 97.4 ± 1.3 | 95.7 ± 4.7 | 0.021 |
| Symptom at time of admission | ||||
| Asymptomatic | 8 (7.5) | 3 (5.1) | 1 (1.5) | 0.220 |
| Fever | 49 (45.4) | 31 (52.5) | 43 (62.3) | 0.061 |
| Chills* | 30 (27.8) | 25 (42.4) | 32 (46.4) | 0.019 |
| Cough | 57 (52.8) | 35 (59.3) | 41 (59.4) | 0.496 |
| Sputum | 49 (45.4) | 28 (47.5) | 33 (47.8) | 0.791 |
| Rhinorrhea | 26 (24.1) | 17 (28.8) | 14 (20.3) | 0.489 |
| Sore throat | 36 (33.3) | 21 (35.6) | 16 (23.2) | 0.228 |
| Myalgia*† | 38 (35.2) | 39 (56.5) | 33 (55.9) | 0.010 |
| Headache | 51 (47.2) | 30 (50.8) | 31 (44.9) | 0.738 |
| Diarrhea | 25 (23.1) | 19 (32.2) | 19 (29.0) | 0.353 |
| Dyspnea | 6 (5.6) | 7 (11.9) | 9 (13.2) | 0.175 |
| Chest pain | 7 (6.5) | 3 (5.1) | 5 (7.4) | 0.942 |
| Underlying disease | ||||
| Hypertension*† | 6 (5.6) | 11 (18.6) | 13 (19.1) | 0.009 |
| Diabetes | 3 (2.8) | 6 (10.2) | 7 (10.3) | 0.057 |
| Hyperlipidemia* | 3 (2.8) | 2 (3.4) | 9 (13.2) | 0.017 |
| Cardiovascular disease | 2 (1.9) | 2 (3.4) | 2 (2.9) | 0.767 |
| Cerebrovascular disease | 0 (0) | 0 (0) | 2 (2.9) | 0.145 |
| Chronic kidney disease | 0 (0) | 0 (0) | 0 (0) | NA |
| Malignancy | 1 (0.9) | 2 (3.4) | 3 (4.4) | 0.328 |
| Initial laboratory findings | ||||
| WBC count (× 103/µL) | 4442.0 ± 1340.9 | 4290.3 ± 1179.4 | 4441.0 ± 1531.7 | 0.762 |
| Absolute neutrophil count (µL) | 2065.0 (1370.0–2840.0) | 2150.0 (1720.0–2880.0) | 2440.0 (1700.0–3230.0) | 0.117 |
| Absolute lymphocyte count (cells/µL)*† | 1645.7 (1458.7–1959.4) | 1489.0 (1179.8–1771.3) | 1260.4 (1040.0–1568.8) | < 0.001 |
| CRP (mg/dL)*† | 0.1 (0.03–0.10) | 0.3 (0.20–0.70) | 1.3 (0.3–2.9) | 0.001 |
| LDH (U/L)*†‡ | 391.0 (349.5–464.5) | 448.0 (398.0–524.0) | 508.0 (425.0–622.0) | < 0.001 |
| Clinical severity | ||||
| Severe pneumonia* | 0 (0) | 3 (5.1) | 7 (10.1) | 0.005 |
| ICU admission*† | 0 (0) | 4 (6.8) | 7 (10.1) | 0.001 |
| Death | 0 (0) | 0 (0) | 1 (1.4) | 0.297 |
Data are presented as number of patients (percentage), median (25–75th percentile), or mean ± standard deviation. *Significantly different between ‘non-pneumonia’ and ‘initial pneumonia group,’ †Significantly different between ‘non-pneumonia’ and ‘positive conversion group,’ ‡Significantly different between ‘positive conversion group’ and ‘initial pneumonia group.’ Bpm = beats per minute, CRP = C-reactive protein, ICU = intensive care unit, LDH = lactate dehydrogenase, N = number of patients, NA = non-available, WBC = white blood cells
Predictors for Positive Conversion among the Patients with Initial Negative Chest Radiograph
Table 2 summarizes the results of univariable and multivariable analyses for finding predictors of positive conversion. On univariable analysis, age older than 45 years (odds ratio [OR]: 5.83, 95% confidence interval [CI]: 2.87–11.86, p < 0.001), underlying diseases including hypertension (OR: 3.74, 95% CI: 1.31–10.71, p = 0.014) and diabetes (OR: 3.82, 95% CI: 0.92-15.85, p = 0.065), absolute lymphocyte count of less than 1500 cells/µL (OR: 2.38, 95% CI: 1.24–4.55, p = 0.009), CRP levels higher than 0.5 mg/dL (OR: 11.23, 95% CI: 5.30–23.80, p < 0.001), and LDH levels higher than 430 U/L (OR: 2.19, 95 CI%: 1.15–4.17, p = 0.018) were significant predictors for positive conversion. Symptoms and hyperlipidemia were not significant predictors of positive conversion.
Table 2. Predictors of Positive Conversion in Patients with Initially Negative Chest Radiographs.
| Variates | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P | Adjusted OR (95% CI) | P | |
| Age ≥ 45 years old | 5.83 (2.87–11.86) | < 0.001 | 3.93 (1.76–8.75) | 0.001 |
| Hypertension | 3.74 (1.31–10.71) | 0.014 | 1.67 (0.48–5.75) | 0.418 |
| Diabetes | 3.82 (0.92–15.85) | 0.065 | 2.45 (0.48–12.53) | 0.281 |
| Hyperlipidemia | 1.18 (0.19–7.29) | 0.856 | ||
| Symptom | 1.55 (0.40–6.80) | 0.529 | ||
| Absolute lymphocyte count < 1500 cells/µL | 2.38 (1.24–4.55) | 0.009 | 2.25 (1.03–4.89) | 0.041 |
| CRP > 0.5 mg/dL | 11.23 (5.30–23.80) | < 0.001 | 3.91 (1.54–9.91) | 0.004 |
| LDH > 430 U/L | 2.19 (1.15–4.17) | 0.018 | 1.60 (0.73–3.51) | 0.244 |
CI = confidence interval, OR = odds ratio
On multivariable analysis, age older than 45 years (adjusted OR: 3.93, 95% CI: 1.76–8.75, p = 0.001), absolute lymphocyte counts of less than 1500 cells/µL (adjusted OR: 2.25, 95% CI: 1.03–4.89, p = 0.041), and CRP level higher than 0.5 mg/dL (adjusted OR: 3.91, 95% CI: 1.54–9.91, p = 0.004) were significant predictors for positive conversion (Fig. 2). However, hypertension, diabetes, and LDH levels were not significant predictors for positive conversion.
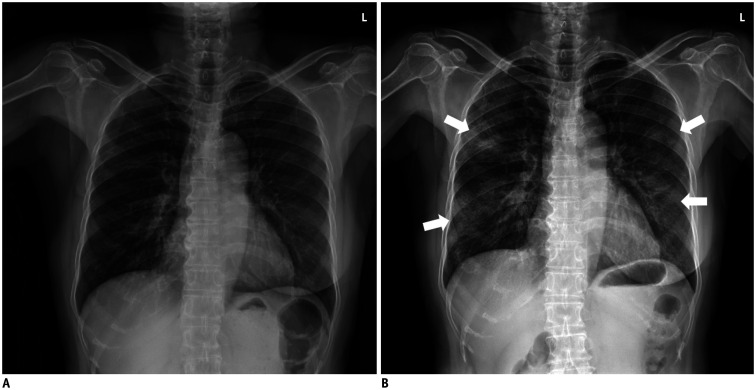
Fig. 2. Initial and follow-up chest radiographs in positive conversion group.
67-year-old woman with history of right mastectomy due to breast cancer.
A. Normal initial chest radiograph on day 5 after symptom onset. In laboratory tests, inflammatory indicators including C-reactive protein (19.8 mg/dL) and lactate dehydrogenase (906 U/L) were notably increased, and absolute lymphocyte count (591.6 cells/µL) was decreased. B. Follow-up chest radiograph demonstrated development of multifocal consolidations in both lungs (arrows) 3 days after initial chest radiograph.
Timing of Chest Radiograph after Symptom Onset
The time interval of chest radiographs taken after the initial symptom onset is summarized in Table 3. In the positive conversion group, the initial radiographs were obtained closer to symptom onset than that in patients who had pneumonia initially (positive conversion group; median, 5 days; 25–75th percentile; 3–7 days vs. initial pneumonia group; median, 6 days; 25–75th percentile; 4–9 days; p = 0.008). In the positive conversion group, follow-up chest radiographs showed pneumonia within a median of 4 days (25–75th percentile, 4–6 days) after the initial chest radiographs were obtained. As a result, the positive conversion group showed positive chest radiographs at a median of 10 days (25–75th percentile, 7–12 days) after symptom onset.
Table 3. Time Interval of Chest Radiographs between Groups.
| Non-Pneumonia Group (n = 108) | Positive Conversion Group (n = 59) | Initial Pneumonia Group (n = 66)* | P | |
|---|---|---|---|---|
| Symptom onset to initial radiograph (days)† | 5 (3–6) | 5 (3–7) | 6 (4–9) | 0.001 |
| Interval between initial negative chest radiograph to positive conversion (days) | NA | 4 (4–6) | NA | NA |
| Symptom onset to positive conversion (days) | NA | 10 (7–12) | NA | NA |
| All pneumonia group (n = 125) | ||||
| Symptom onset to positive chest radiograph (days) | NA | 8 (7–12) | NA | |
Data are presented as median (25–75th percentile). *Three patients not able to remember the date of symptom onset, †Significantly different between ‘initial pneumonia’ and ‘positive conversion group’ and between ‘initial pneumonia’ and ‘non-pneumonia group.’
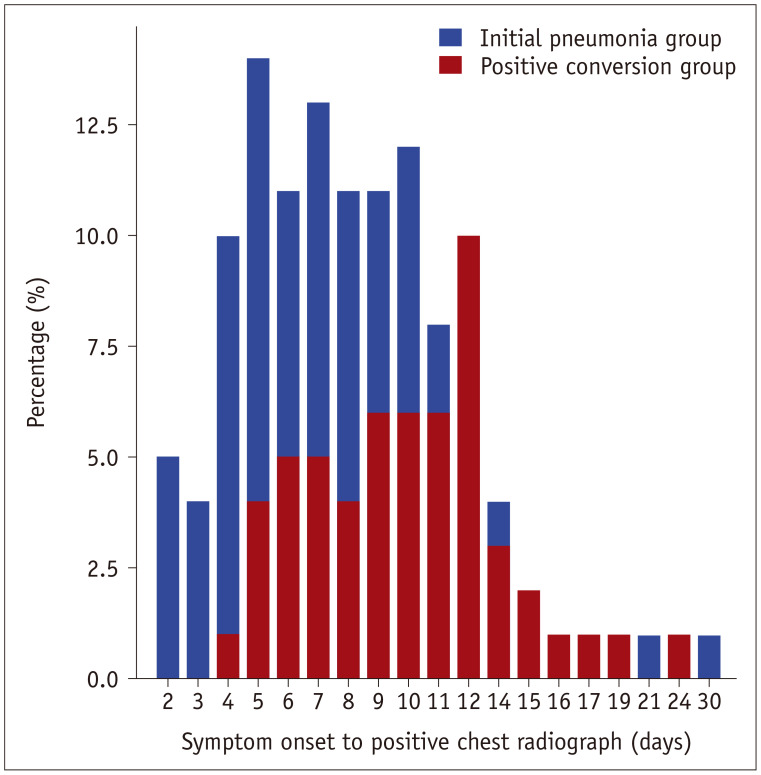
In all pneumonia groups (n = 125, both initial pneumonia and positive conversion groups), chest radiographs became positive at a median of 8 days (25–75th percentile, 7–12 days) after symptom onset (Fig. 3).
Fig. 3. Time interval between initial symptom onset to positive chest radiograph in patients with pneumonia (both initial pneumonia and positive conversion group, n = 125*).
Positive chest radiograph was seen in median of 8 days (25–75th percentile, 7–12 days) after symptom onset. *Three patients in initial pneumonia group not able to remember the date of symptom onset.
Chest Radiograph Features
The most common location of COVID-19 pneumonia was in both the lower zones (Table 4), and the median score of pneumonia was 3 (25–75th percentile score: 2–8) in the pneumonia groups (both, positive conversion and initial pneumonia groups). The radiographic pneumonia score was significantly associated with high CRP (rho = 0.447, p < 0.001), high LDH (rho = 0.377, p < 0.001), low lymphocyte counts (rho = 0.245, p = 0.006), and old age (rho = 0.225, p = 0.011). The median score of the initial pneumonia group was significantly higher than that of the positive conversion group (median 5 score, 25–75th percentile, 3–9.5 score vs. median 3 score, 25–75th percentile, 2–5 score, p = 0.001). The median score of the ICU admitted patients was 12 (25–75th percentile: 9–14). The score of one patient who died was 16.
Table 4. Location of Novel Coronavirus Disease 2019 Pneumonia on Chest Radiographs.
| Location of Pneumonia | n (%) |
|---|---|
| Left upper zone | 11 (8.6) |
| Left mid zone | 40 (31.3) |
| Left lower zone | 72 (56.3) |
| Right upper zone | 12 (9.4) |
| Right mid zone | 42 (32.8) |
| Right lower zone | 88 (68.8) |
| Involving both lower zones | 50 (39.1) |
Data are presented as number of patients (percentage).
DISCUSSION
Our study revealed that a more than half of patients (70.8%) with COVID-19 showed initial negative chest radiographs; however, some patients (25% of the entire cohort) eventually progressed to show COVID-19 pneumonia on follow-up radiographs within several days. In this positive conversion group, initial negative chest radiographs were associated with obtaining the images closer to symptom onset than in patients who showed pneumonia at the time of admission. Moreover, older age (≥ 45 years), high CRP (> 0.5 mg/dL), and low absolute lymphocyte count (< 1500 cells/µL) were the independent factors for future progression to pneumonia in patients with initial negative chest radiographs.
Several studies have shown that old age, underlying disease, elevated inflammatory indicators (CRP, LDH), and low absolute lymphocyte count are commonly related to poor prognosis in patients with COVID-19 (9,11,12,13). Indeed, age and inflammatory indicators at the time of admission were significantly related to the occurrence of later pneumonia when the initial chest radiographs were negative. Thus, an initial negative chest radiograph in a patient should not be considered conclusive for non-progression to pneumonia in the subsequent days or weeks.
In this study, the presence of pneumonia may have been dependent on the amount of time after symptom onset. In a recent study by Bernheim et al. (6), positive CT findings were related to symptom duration, with 56% of patients showing normal CT findings in the early (0–2 days) phase after symptom onset. Fang et al. (14) also reported that ground glass opacities (GGOs) were the main findings in the early stage of the disease as shown on a CT scan (0–4 days), and that abnormal CT findings were most common 10 days after the initial onset of symptoms. In our study, the patients in the positive conversion group had their initial chest radiographs taken earlier than those in the initial pneumonia group. Therefore, a negative chest radiograph at an early stage after symptom onset should not be concluded as the absence of pneumonia, especially in elderly patients with abnormal laboratory findings. Furthermore, in this study, the pneumonia presented at a median of 8 days after symptom onset in both pneumonia groups. Therefore, it is necessary to consider the patient's symptom onset day and clinical condition when performing imaging studies in resource constrained environments.
In our study, a follow-up chest radiograph became positive 4–6 days after an initial negative chest radiograph. Similarly, Pan et al. (7) and Yang et al. (15) also reported a positive CT scan 4 and 7 days following the initial negative scan, respectively. Therefore, we suggest follow-up chest radiographs should be taken within 1 week for older patients and those with increased CRP and decreased lymphocyte counts in order to monitor for future pneumonia development. In our study, 45.8% of patients continued to have no evidence of pneumonia on chest radiographs until discharge; this is similar to the findings of a large scale study from China, which reported that 45.8% of non-severe patients and 23.3% of severe patients had no abnormalities on chest radiographs (13). The non-pneumonia group was characterized by younger age, normal laboratory findings, and fewer underlying diseases.
Similar to the findings of previous reports, the most frequent radiographic findings of COVID-19 pneumonia in this study included bilateral consolidation and GGO with peripheral dominance (3,14,16). The radiographic score used in this study was significantly associated with age and abnormal laboratory findings.
There are several limitations to this study. First, this study was conducted using chest radiographs obtained in the erect position in the posterior-anterior direction; therefore, the results may not be applicable to chest radiographs obtained in the anterior-posterior direction and supine position. Second, this study may have a selection bias, as most of the patients had non-severe disease and were from a particular religious community. Third, not all the chest radiographs were compared with chest CTs; therefore, the possibility of false-negative cases may exist. Fourth, we did not detail morphological features of chest radiographs, such as consolidation or GGOs.
In conclusion, although a considerable proportion of patients with COVID-19 showed normal chest radiographs initially, older patients with abnormal laboratory findings progressed to demonstrate features of pneumonia on followup chest radiographs.
Footnotes
This research was supported by the Bisa Research Grant of Keimyung University in 2020.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020:200490. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Revel MP, Parkar AP, Prosch H, Silva M, Sverzellati N, Gleeson F, et al. COVID-19 patients and the radiology department - advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) Eur Radiol. 2020:1–7. doi: 10.1007/s00330-020-06865-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol. 2020;21:494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance 13 March 2020. [Accessed April 13, 2020]. Available at: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf.
- 5.Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the fleischner society. Chest. 2020;158:106–116. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang W, Yan F. Patients with RT-PCR-confirmed COVID-19 and normal chest CT. Radiology. 2020;295:E3. doi: 10.1148/radiol.2020200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oh MD, Park WB, Choe PG, Choi SJ, Kim JI, Chae J, et al. Viral load kinetics of MERS coronavirus infection. N Engl J Med. 2016;375:1303–1305. doi: 10.1056/NEJMc1511695. [DOI] [PubMed] [Google Scholar]
- 11.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang X, Zhao M, Li S, Yang L, Wu B. Changes of CT findings in a 2019 novel coronavirus (2019-nCoV) pneumonia patient. QJM. 2020;113:271–272. doi: 10.1093/qjmed/hcaa038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W, Cao Q, Qin L, Wang X, Cheng Z, Pan A, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388–393. doi: 10.1016/j.jinf.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei J, Xu H, Xiong J, Shen Q, Fan B, Ye C, et al. 2019 novel coronavirus (COVID-19) pneumonia: serial computed tomography findings. Korean J Radiol. 2020;21:501–504. doi: 10.3348/kjr.2020.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]