Graphical abstract
Abbreviations: AGREE II, Appraisal of Guidelines for Research and Evaluation II; COGS, Conference on Guideline Standardization; CPGs, Clinical practice guidelines; EQUATOR, Enhancing the QUAlity and Transparency Of health Research; GRADE, Grading of Recommendations Assessment Development and Evaluation; RIGHT, Reporting Items for practice Guidelines in Healthcare; RIGHT-TCM, RIGHT extension statement for traditional Chinese medicine; TCM, traditional Chinese medicine; PHEIC, Public Health Emergency of International Concern
Keywords: Traditional Chinese Medicine, Guidelines, RIGHT Statement, Reporting tool
Abstract
Nowadays, the number of traditional Chinese medicine (TCM) guidelines is constantly increasing, but its reporting quality remains unsatisfactory. One of the main reasons is that there is a lack of suitable reporting standard to guide it. In response to this long-standing problem, the Reporting Items for practice Guidelines in HealThcare (RIGHT) Working Group has invited a group of TCM clinical experts, methodologists and epidemiology, and developed the RIGHT Extension Statement for TCM (RIGHT-TCM) through a multi-staged development process, including systematic review, reporting quality evaluation and online Delphi expert consensus. The RIGHT-TCM extends two sections of the RIGHT Statement, includes basic information and recommendations section. Seven strong recommendation sub-items were added to RIGHT Statement and formed the final RIGHT-TCM. The group hopes that the RIGHT-TCM may assist TCM guideline developers in reporting guidelines, support journal editors and peer reviewers when considering TCM guideline reports, and help health care practitioners understand and implement a TCM guideline. This article will introduce its background, development, recommendations and explanation.
1. Background and development of the RIGHT-TCM
Reporting quality is a significant part of the study of clinical guidelines. Low-quality reports impede the presentation of the guidelines’ content even if the guidelines are well projected and developed, thereby hindering the user’s integration and evaluation of guidelines and even misleading clinical decisions [1,2]. High-quality reports better describe the guideline development process and provide useful and clear recommendations for readers. To solve the reporting problem of guidelines, researchers from different countries studied extensively and presented relevant reporting standards. In 1993, a nine-item reporting standard was developed for the summary of clinical practice guidelines (CPGs), which for the first time provided a template and basis for how to systematically and normatively report the information about the development and content of guidelines [3]. In 2003, the Conference on Guideline Standardization (COGS) Working Group was established to develop a reporting standard for CPGs [4]. The COGS standard consists of 18 items that cover the entire process of guideline development, but it is limited to the clinical field and has not been updated since 2003. Appraisal of Guidelines for Research and Evaluation II (AGREE II) has been widely used in the quality evaluation of guidelines since its launch in 2009 [[5], [6], [7]]. Although it is stated in the statement that it can also be used as a reporting standard, researchers generally use it as a quality evaluation tool. In 2016, the international RIGHT Working Group developed the RIGHT Statement as a reporting tool for practice guidelines [8]. Based on the World Health Organization guidelines and the items of the COGS and AGREE II, this international reporting tool was developed in strict accordance with existing framework and the Enhancing the QUAlity and Transparency Of health Research (EQUATOR) network approach. Consisting of 22 items across 7 domains, the RIGHT Statement has been widely applied to clinical practice, public health and health policy guidelines [[9], [10], [11]].
However, we found the RIGHT Statement checklist is not fully applicable to TCM guidelines and hard to reflect its major characteristics [12]. TCM is one of the oldest medical systems in the world whose theoretical system has unique characteristics of Chinese culture and philosophy, and has accumulated rich clinical experience [13]. For example, TCM is characterized by the concept of organic wholeness and treatment based on syndrome differentiation. Its guidelines also reflect its distinctive characteristics and Chinese traditional cultural characteristics. These factors may be ignored in the process of developing and reporting guidelines [14]. The reporting standard of the TCM guidelines should reflect the characteristics of Chinese medicine itself. This idea was endorsed by the RIGHT Working Group, and a multidisciplinary expert group was organized to develop the extended version.
The EQUATOR network approach, which was used as the methodological guidance for this research [15], included the following three steps: (1) establishment of research working group, (2) systematic review of the literature and items establishment, (3) expert consensus and items selection. Full search strategies are presented in Additional file 1. Conflict of interest and basic characteristics of the expert consensus group are presented in Additional file 2 and Additional file 3, respectively. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) grid rules were used to reach decisions when the consensus is elusive (Additional file 4) [16,17]. More details of material and methods is presented in Additional file 5.
2. Seven strong recommendation sub-items were determined as extension items into the RIGHT-TCM after two-round online Delphi expert consensus
2.1. Literature search and reporting quality evaluation
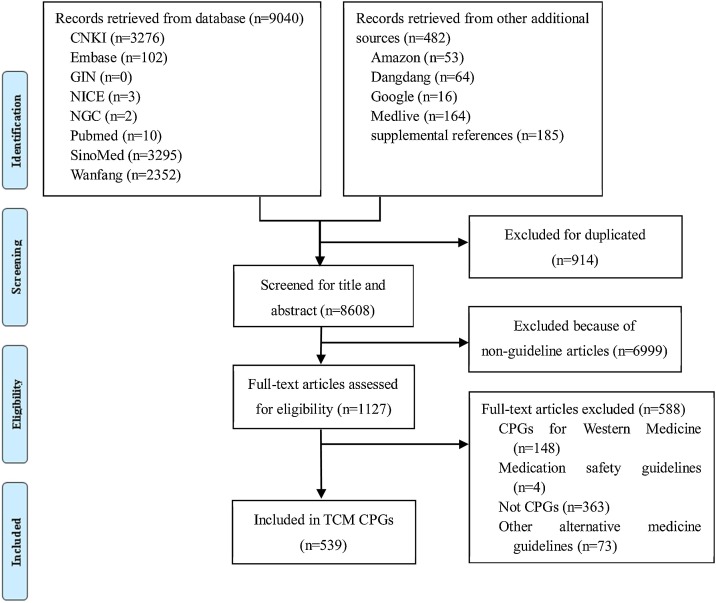
The diagram of the screening process is shown in Fig. 1 . After screened for potentially eligible CPGs, a total of 539 TCM CPGs were eventually included (Additional file 6). Our previous research results showed that the reporting quality of these 539 TCM CPGs was improving, but the overall quality remained suboptimal [12]. Some difficulties were encountered during the evaluation of the TCM guidelines when using the RIGHT Statement. For example, in the background section, TCM have their own unique historical evolution. In terms of epidemiology, there is a lack of statistics on the basic epidemiology of the health problem of TCM. In terms of recommendations, there is a lack of reporting content concerning principle-method-recipe-medicines of TCM. In terms of evidence, there is no uniform reporting standards for the quality of evidence of ancient classic theoretical Chinese medical case and famous experts experience. For these aspects, the existing RIGHT Statement items are not well applicable for TCM.
Fig. 1.
The diagram of the screening process.
2.2. Establishment of items
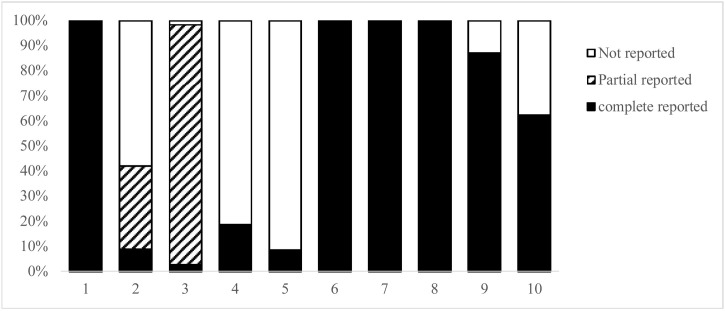
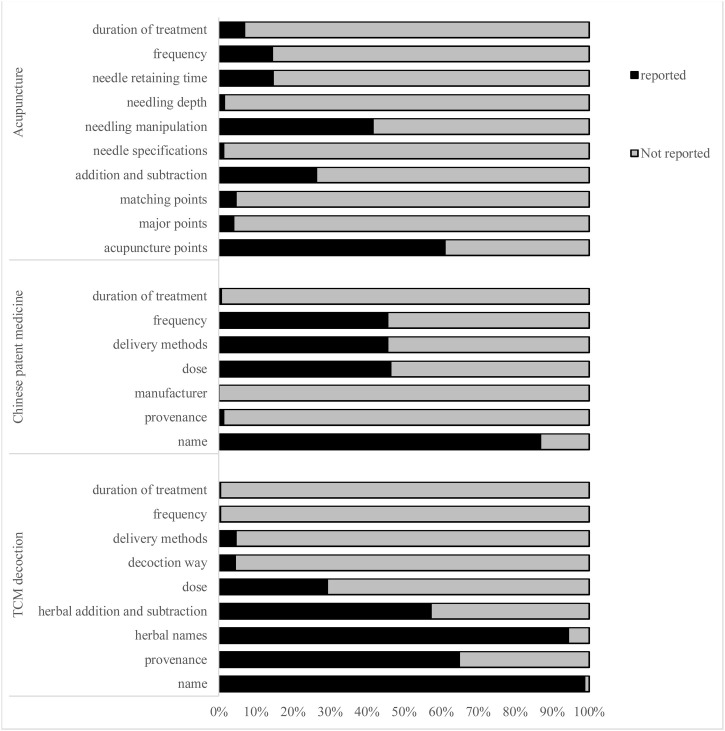
After extracting the information of these 539 TCM guidelines, the contents of 10 items were found not included in the RIGHT statement checklist. These items are reported at varying degrees in the TCM guidelines (Fig. 2 ). Among of them, items 8, 9 and 10 (traditional Chinese medicine decoction, traditional Chinese patent medicine and acupuncture) are the contents often mentioned in interventions section of TCM guidelines. As shown in Fig. 3 , specific reporting content of these three items were further analyzed. After discussion in the face-to-face meeting, the core working group determined 24 initial sub-items based on the results of Fig. 2, Fig. 3. At the same time, one sub-item was supplemented by brainstorming, and finally 25 initial sub-items were formed for expert consensus.
Fig. 2.
The reporting items of TCM guidelines not included in the RIGHT statement.
* 1: Identify as a clinical guideline for traditional Chinese medicine through the title.
2 : Describe the knowledge of disease based on biomedical theory and/or traditional Chinese medicine theory.
3: Describe the basis for diagnosing the disease based on biomedical theory and/or traditional Chinese medicine theory.
4: Describe the knowledge of disease pathogenesis in traditional Chinese medicine theory.
5: Describe the specific reasons for using traditional Chinese medicine to treat the disease.
6: Describe the principle and method of treatment for traditional Chinese medicine in the recommendations.
7: Describe whether to treat disease based on the syndrome differentiation of traditional Chinese medicine.
8: Provide clear and accurate description of traditional Chinese medicine decoction in the intervention.
9: Provide clear and accurate description of traditional Chinese patent medicine in interventions.
10: Provide clear and accurate description of the acupuncture in the intervention.
Fig. 3.
The specific reporting content in interventions section of TCM guidelines.
2.3. Expert Consensus
In two-round online consensus Delphi survey, 17 experts were invited into the expert consensus group to conduct consensus, selection and recommendation of items. With profound experience in guideline development, these experts from different research fields are regionally representative, and more than half of them have senior professional title (Additional file 3). All experts were asked to disclose any conflicts of interest before the Delphi technique-based consensus, and they declared no conflict of interest in this research (Additional file 2). The response rate for all 2 rounds of surveys was 100%. (1) First round Delphi consensus. Based on previous work, the 10 initial extension items included a total of 25 sub-items in the basic information, background and recommendations section. After the first round of expert consensus, there were 4 sub-items (item 7, 8, 10 and 10-1) with a consensus degree of ≥70% in “very important” option, which have reached the strong recommendation (Additional file 7). After discussion the first round Delphi consensus results, these 4 sub-items were considered to have reached consensus, and the other 21 items required into the second round of Delphi consensus. (2) Second round Delphi consensus. As shown in Additional file 8, there were 3 sub-items (item 1, 6, and 8-5) with a consensus degree of ≥70% in “very important” option, which have reached the strong recommendation. In addition, 16 sub-items were considered as weak recommendation, while 2 sub-items have been excluded because they have not reached any recommendations in the consensus.
2.4. Item formation
After two-round online Delphi consensus, there were a total of 7 strongly recommended sub-items (S1, S2, S3, S4, S4-1, S5 and S5-1) as extension items into the basic information and recommendations section of the RIGHT-TCM (Additional file 9). S1: Identify as a clinical guideline for traditional Chinese medicine through the title. S2: Describe the principle and method of treatment for traditional Chinese medicine in the recommendations. S3: Describe whether to treat disease based on the syndrome differentiation of traditional Chinese medicine. S4: Provide clear and accurate description of traditional Chinese medicine decoction in the intervention. S4-1: Describe the administration route (e.g., oral, topical), frequency of traditional Chinese medicine decoction. S5: Provide clear and accurate description of the acupuncture in the intervention. S5-1: Describe the acupuncture points, major points, matching points, and their addition and subtraction information. Besides, other 16 weak recommendation sub-items were not included, but these items could be served as a reference for future research (Table 1 ).
Table 1.
Results after two-round expert consensus.
| Number | Item | Recommended strength | Remarks |
|---|---|---|---|
| 1 | Identify as a clinical guideline for traditional Chinese medicine through the title. | Strong recommendation | Extension of RIGHT 1a item (basic information section) |
| 2 | Describe the knowledge of disease based on biomedical theory and/or traditional Chinese medicine theory. | Weak recommendation | Extension to the RIGHT background section |
| 3 | Describe the basis for diagnosing the disease based on biomedical theory and/or traditional Chinese medicine theory. | Weak recommendation | |
| 4 | Describe the knowledge of disease pathogenesis in traditional Chinese medicine theory. | Weak recommendation | |
| 5 | Describe the specific reasons for using traditional Chinese medicine to treat the disease. | Weak recommendation | |
| 6 | Describe the principle and method of treatment for traditional Chinese medicine in the recommendations. | Strong recommendation | Extension of RIGHT 13a item (recommendation section) |
| 7 | Describe whether to treat disease based on the syndrome differentiation of traditional Chinese medicine. | Strong recommendation | |
| 8 | Provide clear and accurate description of traditional Chinese medicine decoction in the intervention. | Strong recommendation | |
| 8-1 | Describe the name and provenance of traditional Chinese medicine decoction. | Weak recommendation | |
| 8-2 | Describe the herbal names, herbal addition and subtraction, dosage of traditional Chinese medicine decoction. | Weak recommendation | |
| 8-3 | Describe the composition principle, basis and interpretation of traditional Chinese medicine decoction. | No recommendation | |
| 8-4 | Describe the decocting method of traditional Chinese medicine decoction. | Weak recommendation | |
| 8-5 | Describe the administration route (e.g., oral, topical), frequency of traditional Chinese medicine decoction. | Strong recommendation | |
| 8-6 | Describe the duration of treatment of traditional Chinese medicine decoction. | Weak recommendation | |
| 9 | Provide clear and accurate description of traditional Chinese patent medicine in interventions. | Weak recommendation | |
| 9-1 | Describe the product name (e.g., the trade name), provenance and manufacturer of traditional Chinese patent medicine. | No recommendation | |
| 9-2 | Describe the dosage of traditional Chinese patent medicine. | Weak recommendation | |
| 9-3 | Describe the administration route (e.g., oral, topical), frequency of traditional Chinese patent medicine. | Weak recommendation | |
| 9-4 | Describe the duration of treatment of traditional Chinese patent medicines. | Weak recommendation | |
| 10 | Provide clear and accurate description of the acupuncture in the intervention. | Strong recommendation | |
| 10-1 | Describe the acupuncture points, major points, matching points, and their addition and subtraction information. | Strong recommendation | |
| 10-2 | Describe the specific information of the needling instrument used in the acupuncture process. | Weak recommendation | |
| 10-3 | Describe the needling manipulation, needling depth and needling retention time of treatment required for acupuncture. | Weak recommendation | |
| 10-4 | Describe the frequency of treatment required for acupuncture. | Weak recommendation | |
| 10-5 | Describe the duration of treatment required for acupuncture. | Weak recommendation |
3. Explanation and discussion about the RIGHT-TCM
The present paper describes a multi-staged development process of the RIGHT-TCM, including systematic review, reporting quality evaluation and online Delphi expert consensus. Seven strong recommendation sub-items were added on the basis of RIGHT Statement into the basic information and recommendations section. Consistent with the purpose of the RIGHT statement, the RIGHT-TCM may provide an instrument for improving the reporting quality of TCM guidelines and facilitate the target audience to quickly grasp the specific content of the guidelines. The RIGHT-TCM may not only improve the scientificity and transparency, but also reduce the risk of bias in the development process of the CPGs.
One of the purposes of a systematic evaluation is to reduce random errors and systematic errors as much as possible through comprehensive searches and rigorous review and to provide near-real scientific evidence for decision makers [18,19]. We have comprehensively collected the published CPGs for TCM and tried to use existing tools for evaluation. To begin with, we evaluated the reporting quality of TCM guidelines. The results show that the reporting quality of the TCM guidelines is poor, and the existing RIGHT Statement cannot be fully suitable for the TCM guidelines. There is a requirement to develop an extension of the RIGHT Statement to reflect the unique characteristics of TCM. In addition, we conducted a comprehensive analysis of the data from the TCM guidelines and condensed the data into initial items. We also used a brainstorming method to supplement the initial items, and we supplemented new items with such discussions to prevent omissions. Moreover, we used the Delphi method and GRADE grid rules in the consensus process. All the participating experts did not know each other, and they answered the questions raised without meeting each other and without discussion. This back-to-back anonymous approach has advantages and can avoid other influencing factors [20,21]. Finally, a total of 7 strongly recommended sub-items are determined as extension items into the RIGHT-TCM.
For item 1 (S1 in the checklist of the RIGHT-TCM), it is recommended that a clinical guideline for TCM could be identified by the title. This item is useful for rapid judgment, screening and classification for the TCM guidelines. For item 6 (S2 in the checklist of the RIGHT-TCM), it is recommended to describe the principle and method of TCM treatment in the recommendations section. These general principles and basic methods for treating diseases are based on the unique concept of holism and syndrome differentiation and have guiding significance for the specific treatment measures in traditional Chinese medicine [22]. For item 7 (S3 in the checklist of the RIGHT-TCM), it is recommended to describe whether to treat disease based on the syndrome differentiation of TCM. Syndrome differentiation is an important basic concept of TCM [[23], [24], [25]]. It is the process of applying “principle-method-recipe-medicines” to clinical practice. For item 8 (S4 in the checklist of the RIGHT-TCM), it is recommended to provide clear and accurate description of TCM decoction in the intervention. TCM decoction refers to a method consisting of herbs with relatively specific processing methods and usage. The decoction is the main prescription of TCM, so it is necessary to make a detailed report on the TCM decoction [26,27]. For item 8-5 (S4-1 in the checklist of the RIGHT-TCM), it is recommended to describe the administration route and frequency of TCM decoction. The administration route and frequency are also one of the ways to influence the efficacy of the medicine. The traditional administration route of TCM is mainly based on internal and external use (oral and dermatological drugs) [28,29]. In addition, there are many administration routes such as inhalation, sublingual administration, mucosal surface administration, rectal administration [30]. Currently, only 4% and 0.3% of the guidelines describe the route and frequency of administration, respectively. For item 10 (S5 in the checklist of the RIGHT-TCM), it is recommended to provide clear and accurate description of the acupuncture in the intervention. Acupuncture is an important component of TCM interventions. Acupuncture has been increasingly used as an integrative or complementary therapy and it is well-tolerated with little risk of serious adverse effects [[31], [32], [33], [34]]. For item 10-1 (S5-1in the checklist of the RIGHT-TCM), it is recommended to describe the acupuncture points, major points, matching points, and their addition and subtraction information. The acupoint is the area that the Qi in meridian flows into the body surface and is the area where the needle is stabbed [35].
After two-round online Delphi consensus, item 8-3 and item 9-1 have been excluded because they have not reached any recommendations. For item 8-3, the composition principle, basis and interpretation of TCM decoction may not the main focus point of the recommendations in the guidelines. For item 9-1, if the TCM guideline describe the product name and manufacturer of traditional Chinese patent medicine, it may involve more interest bias.
With the rapid development in recent years, TCM has been widely used in different area, and its guidelines and handbooks have also played an important role in Public Health Emergency of International Concern (PHEIC) [36]. Such as part of "TCM Classification Therapy to Improve Curative Efficacy" in the "Handbook of COVID-19 Prevention and Treatment" [37]. For PHEIC, the procedures and methodological requirements of the Rapid Advice Guideline should be adopted [[38], [39], [40]]. However, the current RIGHT-TCM reporting items for the Rapid Advice Guideline has yet to be developed, which may be a possible research direction in the future.
Although this study reflects the scalability of the RIGHT Statement, there are some limitations need to attention. In the current study, we only study the standardized reports of the most commonly TCM interventions, such as TCM decoction, traditional Chinese patent medicine and acupuncture. Interventions of TCM also included moxibustion, massage and cupping, etc. Therefore, we will revise the checklist in the future based on user feedback and evaluation results.
In summary, seven strong recommendation sub-items were added to RIGHT Statement and formed the final RIGHT-TCM. We hope that the RIGHT-TCM may assist TCM guideline developers in reporting guidelines and improving the reporting quality, while help the target audience understand and implement a guideline.
4. Declaration of Competing Interest
The authors have declared no conflict of interest.
Acknowledgments
The authors wish to express their gratitude to the following people who participated in the research and consensus process of the report specification: Hao Chen (Nanjing University of Chinese Medicine); Wei Chen (Center for Evidence-Based Medicine of Beijing University of Chinese Medicine); Wenjia Chen (The First Affiliated Hospital of Guangzhou University of Chinese Medicine); Yaolong Chen (Center for Evidence-based Medicine of Lanzhou University); Haibo Cheng (Nanjing University of Chinese Medicine); Jiayuan Hu (Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine); Yinghui Jin (Center for Evidence-Based and Translational Medicine, Zhongnan Hospital of Wuhan University); Bo Li (Beijing Institute of Traditional Chinese Medicine); Chengyu Li (Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine); Hui Li (The Second Affiliated Hospital of Guangzhou University of Chinese Medicine); Bin Ma (Evidence-Based Medicine Center, Lanzhou University); Xiaojia Ni (Guangdong Provincial Hospital of Chinese Medicine); Hongcai Shang (Beijing University of Chinese Medicine); Guihua Tian (Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine); Huimin Wang (Guangdong Provincial Hospital of Chinese Medicine); Yangyang Wang (Guangdong Provincial Hospital of Chinese Medicine); Wenjiang Wu (Shenzhen Hospital of Guangzhou University of Chinese Medicine); Yun Xia (Hainan Provincial Hospital of Traditional Chinese Medicine); Runsheng Xie (The Second Affiliated Hospital of Guangzhou University of Chinese Medicine); Xiuli Xie (The Second Affiliated Hospital of Guangzhou University of Chinese Medicine); Wenjie Xu (Beijing Hospital of Traditional Chinese Medicine); Chen Zhao (Hong Kong Baptist University); Qi Zhou (Evidence-Based Medicine Center, Lanzhou University); Zhao Zeng (Guangzhou University of Chinese Medicine).
Acknowledgments
Funding
The study was supported by The Specific Research Fund for TCM Science and Technology of Guangdong Provinc Hospital of Chinese Medicine (YN2015MS22, YN2019QL17).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.phrs.2020.105178.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Grilli R., Magrini N., Penna A., Mura G., Liberati A. Practice guidelines developed by specialty societies: the need for a critical appraisal. Lancet. 2000;355(9198):103–106. doi: 10.1016/S0140-6736(99)02171-6. [DOI] [PubMed] [Google Scholar]
- 2.von Elm E., Egger M. The scandal of poor epidemiological research. BMJ. 2004;329(7471):868–869. doi: 10.1136/bmj.329.7471.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayward R.S., Wilson M.C., Tunis S.R., Bass E.B., Rubin H.R., Haynes R.B. More informative abstracts of articles describing clinical practice guidelines. Ann Intern Med. 1993;118(9):731–737. doi: 10.7326/0003-4819-118-9-199305010-00012. [DOI] [PubMed] [Google Scholar]
- 4.Shiffman R.N., Shekelle P., Overhage J.M., Slutsky J., Grimshaw J., Deshpande A.M. Standardized reporting of clinical practice guidelines: a proposal from the Conference on Guideline Standardization. Ann Intern Med. 2003;139(6):493–498. doi: 10.7326/0003-4819-139-6-200309160-00013. [DOI] [PubMed] [Google Scholar]
- 5.Brouwers M.C., Kho M.E., Browman G.P., Burgers J.S., Cluzeau F., Feder G. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–42. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamás G., Abrantes C., Valadas A., Radics P., Albanese A., Tijssen M.A.J. Quality and reporting of guidelines on the diagnosis and management of dystonia. Eur J Neurol. 2018;25(2):275–283. doi: 10.1111/ene.13488. [DOI] [PubMed] [Google Scholar]
- 7.Brouwers M.C., Spithoff K., Kerkvliet K., Alonso-Coello P., Burgers J., Cluzeau F. Development and Validation of a Tool to Assess the Quality of Clinical Practice Guideline Recommendations. JAMA Netw Open. 2020;3(5) doi: 10.1001/jamanetworkopen.2020.5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Yang K., Marušic A., Qaseem A., Meerpohl J.J., Flottorp S. A Reporting Tool for Practice Guidelines in Health Care: The RIGHT Statement. Ann Intern Med. 2017;166(2):128–132. doi: 10.7326/M16-1565. [DOI] [PubMed] [Google Scholar]
- 9.Ke L.X., Wang J.J., Wang H., Xiao Y.J., Wang Z.J., Che G. Reporting quality evaluation of clinical practice guidelines published in journals of mainland China in 2016. Chin J Evid Based Pediat. 2018;13(3):194–199. doi: 10.3969/j.issn.1673-5501.2018.03.008. [DOI] [Google Scholar]
- 10.Lotfi T., Itani M.I., Howeiss P., Kilzar L., Rizk N.A., Akl E.A. Practice guidelines on migrants’ health: assessment of their quality and reporting. Health Qual Life Outcomes. 2020;18(1):125. doi: 10.1186/s12955-020-01363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao S., Cao J., Shi Q., Wang Z., Estill J., Lu S. A quality evaluation of guidelines on five different viruses causing public health emergencies of international concern. Ann Transl Med. 2020;8(7):500. doi: 10.21037/atm.2020.03.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun X., Yaolong C., Zhao Z., Qi Z., Yangyang W., Runshen X. Using the RIGHT Statement to evaluate the reporting quality of clinical practice guidelines in traditional Chinese medicine. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0207580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng C.W., Wu T.X., Shang H.C., Li Y.P., Altman D.G., Moher D. CONSORT Extension for Chinese Herbal Medicine Formulas 2017: Recommendations, Explanation, and Elaboration (Traditional Chinese Version) Ann Intern Med. 2017;167(2):W7–W20. doi: 10.7326/IsTranslatedFrom_M17-2977_1. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y., Wang C., Shang H., Yang K., Norris S.L. Clinical practice guidelines in China. BMJ. 2018;360:j5158. doi: 10.1136/bmj.j5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Schulz K.F., Simera I., Altman D.G. Guidance for developers of health research reporting guidelines. PLoS Med. 2010;7(2) doi: 10.1371/journal.pmed.1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt G.H., Oxman A.D., Schünemann H.J., Tugwell P., Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Jaeschke R., Guyatt G.H., Dellinger P., Schünemann H., Levy M.M., Regina Kunz. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive. BMJ. 2008;337:a744. doi: 10.1136/bmj.a744. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P.T., (editors) Green S. The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0.http://handbook.cochrane.org Available from. [Google Scholar]
- 19.Richardson P.E. David Sackett and the birth of Evidence Based Medicine: How to Practice and Teach EBM. BMJ. 2015;350:h3089. doi: 10.1136/bmj.h3089. [DOI] [PubMed] [Google Scholar]
- 20.Beattie E., Mackway-Jones K. A Delphi study to identify performance indicators for emergency medicine. Emerg Med J. 2004;21(1):47–50. doi: 10.1136/emj.2003.001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French S.D., Nielsen M., Hall L., Nicolson P.J.A., van Tulder M., Bennell K.L. Essential key messages about diagnosis, imaging, and self-care for people with low back pain: a modified Delphi study of consumer and expert opinions. Pain. 2019;160(12):2787–2797. doi: 10.1097/j.pain.0000000000001663. [DOI] [PubMed] [Google Scholar]
- 22.Yan E., Song J., Liu C., Hong W. A research on syndrome element differentiation based on phenomenology and mathematical method. Chin Med. 2017;12:19. doi: 10.1186/s13020-017-0141-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu J., Liu B. The basic theory, diagnostic, and therapeutic system of traditional Chinese medicine and the challenges they bring to statistics. Stat Med. 2012;31(7):602–605. doi: 10.1002/sim.4409. [DOI] [PubMed] [Google Scholar]
- 24.Gu Z., Qi X., Zhai X., Lang Q., Lu J., Ma C. Study on TCM Syndrome Differentiation of Primary Liver Cancer Based on the Analysis of Latent Structural Model. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/761565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H., Li L., Zhao H., Wang Y., Du J., Zhang P. A Large-Scale, Multi-Center Urine Biomarkers Identification of Coronary Heart Disease in TCM Syndrome Differentiation. J Proteome Res. 2019;18(5):1994–2003. doi: 10.1021/acs.jproteome.8b00799. [DOI] [PubMed] [Google Scholar]
- 26.Cai H., Li H., Zeng H., Xu D., Ouyang W., Lv A. Application evaluation of clinical practice guidelines for traditional Chinese medicine: a clinical analysis based on the analytic hierarchy process. BMC Complement Altern Med. 2019;19(1):277. doi: 10.1186/s12906-019-2683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng H., Xu J. Wendan decoction (Traditional Chinese medicine) for schizophrenia. Cochrane Database Syst Rev. 2017;6 doi: 10.1002/14651858.CD012217.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng W., Ao H., Peng C., Yan D. Gut microbiota, a new frontier to understand traditional Chinese medicines. Pharmacol Res. 2019;142:176–191. doi: 10.1016/j.phrs.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Williamson E.M., Lorenc A., Booker A., Robinson N. The rise of traditional Chinese medicine and its materia medica: a comparison of the frequency and safety of materials and species used in Europe and China. J Ethnopharmacol. 2013;149(2):453–462. doi: 10.1016/j.jep.2013.06.050. [DOI] [PubMed] [Google Scholar]
- 30.Henkin R.I. Inhaled insulin-intrapulmonary, intranasal, and other routes of administration: mechanisms of action. Nutrition. 2010;26(1):33–39. doi: 10.1016/j.nut.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Coutaux A. Non-pharmacological treatments for pain relief: TENS and acupuncture. Joint Bone Spine. 2017;84(6):657–661. doi: 10.1016/j.jbspin.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 32.McPhail P., Sandhu H., Dale J., Stewart-Brown S. Acupuncture in hospice settings: A qualitative exploration of patients’ experiences. Eur J Cancer Care (Engl). 2018;27(2) doi: 10.1111/ecc.12802. [DOI] [PubMed] [Google Scholar]
- 33.Chou P.C., Chu H.Y. Clinical Efficacy of Acupuncture on Rheumatoid Arthritis and Associated Mechanisms: A Systemic Review. Evid Based Complement Alternat Med. 2018;2018 doi: 10.1155/2018/8596918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly R.B., Willis J. Acupuncture for Pain. Am Fam Physician. 2019;100(2):89–96. [PubMed] [Google Scholar]
- 35.Yang Y., Ai F., Ma C.Y., Wan W.J., Li H.Y. Observation on clinical therapeutic effect of acupuncture treatment on functional dyspepsia based on syndrome differentiation. Chin J Integr Trad West Med. 2015;35(4):411–414. [PubMed] [Google Scholar]
- 36.Zhang D., Zhang B., Lv Jt, Sa Rn, Zhang Xm, Lin Zj. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacol Res. 2020;157 doi: 10.1016/j.phrs.2020.104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The First Affiliated Hospital of Zhejiang University School of Medicine . 2020. Handbook of COVID-19 Prevention and Treatment.https://gmcc.alibabadoctor.com/prevention-manual/detail?content_id=0 Available from. [Google Scholar]
- 38.Garritty C.M., Norris S.L., Moher D. Developing WHO rapid advice guidelines in the setting of a public health emergency. J Clin Epidemiol. 2017;82:47–60. doi: 10.1016/j.jclinepi.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization . 2nd ed. 2014. WHO handbook for guideline development.https://apps.who.int/iris/handle/10665/145714 Available from. [Google Scholar]
- 40.Norris S.L. Meeting public health needs in emergencies-World Health Organization guidelines. J Evid Based Med. 2018;11(3):133–135. doi: 10.1111/jebm.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.