Abstract
The COVID-19 pandemic is disrupting the provision of acute vascular surgery across the globe. Limited evidence of the impact of nosocomial infection on patient outcomes as well as concerns about critical care capacity will likely have an impact on surgical decision-making. Endovascular therapy offers a way by which perioperative risk can be reduced for vascular patients while also reducing the impact of acute surgery on intensive care unit capacity. This case report describes the management of a patient with complex aortoiliac occlusive disease by a hybrid endovascular approach in light of these constraints, with a successful outcome.
Keywords: Vascular surgery, CERAB, Endovascular, COVID-19, Coronavirus
The COVID-19 pandemic has significantly affected the provision of emergency surgery. Early data have highlighted the risks of morbidity and mortality in the event of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the postoperative period.1 Furthermore, critical care bed and overall hospital capacity will likely continue to have an impact on vascular surgery services. Minimally invasive techniques offer a way of mitigating these constraints while providing quality care with acceptable outcomes for patients.2,3 We report a case of a patient with acute-on-chronic TransAtlantic Inter-Society Consensus II (TASC II) D aortoiliac occlusive disease managed by covered endovascular reconstruction of the aortic bifurcation as an alternative to open surgery in the era of COVID-19. The patient provided written informed consent for the case details to be published.
Case report
A 57-year-old man presented to a tertiary level care unit with a 3-week history of left foot and calf pain at rest and a 4-day history of forefoot paresthesia. The patient denied right-sided symptoms. A history of hypertension, hyperlipidemia, and obesity was noted. The patient was an active smoker. He was taking rivaroxaban for a recently diagnosed left below-knee deep venous thrombosis, based on a duplex ultrasound scan reporting an isolated tibial vein that failed to be compressed normally. This in retrospect was likely an incorrect diagnosis that led to delayed referral. On examination, the left foot was pale, with a sensory deficit noted over the lateral foot. There was no tissue loss or ulceration and no motor deficit, and he had minimal calf tenderness. Lower limb pulses, including femoral pulses, were absent. A diagnosis of limb-threatening acute-on-chronic lower limb ischemia was made, and unfractionated heparin infusion was commenced. Left-sided toe waveforms were absent; ankle-brachial pressure index and toe-brachial index on the right were 0.64 and 0.81, respectively. Computed tomography angiography revealed extensive mural thrombus in the infrarenal aorta with >50% stenosis (Fig 1). Aneurysmal dilation of the right common iliac measuring up to 2.9 cm was observed, with extensive thrombus occluding >80% of the lumen proximally. There was complete occlusion of the left common iliac and external iliac artery. The left common femoral artery was recanalized through the left inferior epigastric artery. The left popliteal artery had further occlusion with tibials preserved.
Fig 1.

Three-dimensional reconstruction of lower limb computed tomography angiography image demonstrating abdominal aorta thrombus, right common iliac aneurysm with stenosis and left common iliac artery occlusion, left external iliac occlusion, and recanalization of the left common femoral artery through the left inferior epigastric artery.
The options of open aortobifemoral bypass and endovascular repair were discussed with the patient with input from the anesthesiology service. The decision was made to proceed with a hybrid covered endovascular reconstruction of the aortic bifurcation (CERAB) procedure. Given the absent femoral pulses bilaterally and the presence of a right common iliac aneurysm, it was anticipated that a femorofemoral bypass may be necessary in the case of an unsuccessful thromboembolectomy. Access was therefore through bilateral longitudinal groin incisions. A hydrophilic wire was passed retrogradely to the infrarenal aorta across a right proximal common iliac stenosis. The patient underwent an over-the-wire left iliac thromboembolectomy. The left internal iliac artery remained occluded. Given the presence of a left internal iliac artery occlusion and aneurysmal degeneration of the right common iliac artery, covered stents were used to complete the reconstruction. A BeGraft (Bentley, Hechingen, Germany) 14- × 59-mm covered, balloon-expandable stent was placed infrarenally through a 12F sheath in the right common femoral artery and the proximal portion molded with a 16-mm noncompliant balloon. A second BeGraft 14- × 49-mm stent was deployed more distally to within 2 cm of the aortic bifurcation. Next, two 10- × 59-mm Atrium stents (Maquet, Rastatt, Germany) were placed within the distal BeGraft. The right common iliac artery was stented with one more 10- × 38-mm Atrium stent. The left common and proximal external iliac arteries were stented with two 10- × 38-mm Atrium stents. A left common femoral endarterectomy was performed, with bovine pericardial patch closure (Fig 2). Completion angiography demonstrated bilateral internal iliac occlusion; the right internal iliac was unintentionally occluded although not covered by the most distal stent, whereas the left internal iliac had a pre-existing occlusion. A right superficial femoral artery embolectomy was necessary because of acute embolus, probably related to the large-volume right common iliac artery aneurysm thrombus, which may also have accounted for the unexpected right internal iliac occlusion. The popliteal occlusion was not revascularized but the left foot reperfused well. The patient did not require a critical care bed postoperatively. His rest pain resolved. He was discharged home on the third postoperative day. At 4 weeks, the patient's left-sided ankle-brachial pressure index was 0.54, with an absolute toe pressure of 85 mm Hg and a walking distance of 500 meters. Postoperative computed tomography angiography was satisfactory (Fig 3).
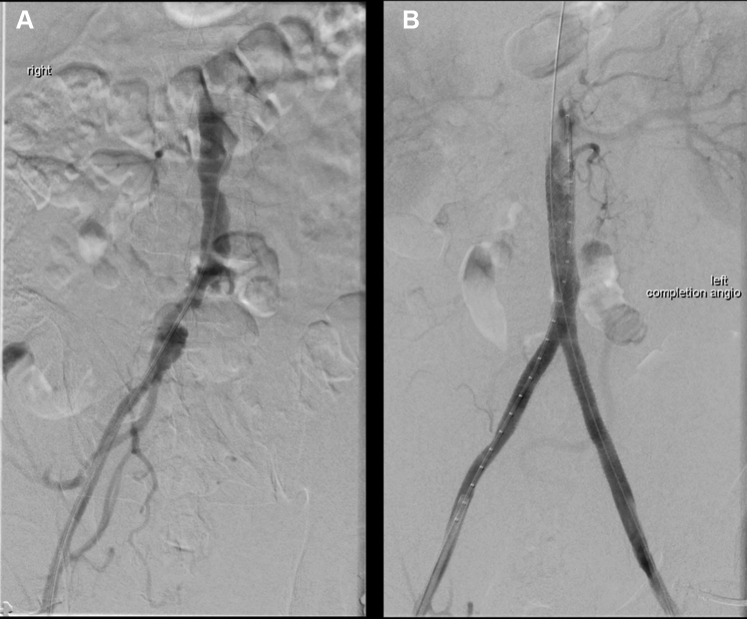
Fig 2.
Intraoperative digital subtraction angiography. A, Diagnostic angiogram demonstrating a right common iliac artery aneurysm and occlusion of the left common iliac artery. B, Completion angiogram after endovascular recanalization.
Fig 3.

Three-dimensional reconstruction of postoperative computed tomography angiography image showing covered endovascular stents in satisfactory position.
Discussion
This case report concerns a 57-year-old man with acute-on-chronic TASC II D aortoiliac occlusive disease and a favorable underlying risk profile. A hybrid endovascular approach negated the need for a critical care admission. In spite of bilateral internal iliac artery occlusion, the patient has no buttock claudication postoperatively and has maintained a reasonable walking distance. Open surgery with aortofemoral bypass remains the “gold standard” for the management of TASC II D aortoiliac occlusive disease.4 Patency rates of 75% to 80% at 10 years have yet to be matched by endovascular techniques.5,6 However, several studies have reported successful endovascular management of extensive aortoiliac disease in selected patients.7 Endovascular management carries a lower risk of perioperative morbidity at the expense of a higher reintervention rate and lower primary patency.8 Technical success rates of 95.1% have been recorded, with major complication rates of 1.9% and a short median hospital length of stay observed.9 Loss of primary patency after endovascular repair can often be managed by percutaneous techniques, with subsequent secondary patency rates of 80% to 98% reported in the literature.7 Whereas these studies demonstrate the safety and efficacy of an endovascular approach in severe disease, an open approach to TASC II D disease would normally be favored in this age group in our institution, given the higher long-term rates of primary patency.7 This case highlights the importance of proficiency in endovascular techniques to provide an individualized approach to patient care.
The coronavirus pandemic has had an impact on the management of vascular disease. Early data have highlighted the impact of SARS-CoV-2 infection on postoperative outcomes, with mortality rates as high as 40% in COVID-19-positive patients undergoing vascular surgery reported.10 The Vascular Society for Great Britain and Ireland has emphasized the importance of reducing inpatient length of stay and critical care bed dependency in a letter to members.11 Endovascular techniques are highlighted as a way by which this may be achieved to deliver acute care to patients requiring surgery while recognizing the complexities.11 In an international survey of 77 vascular surgeons, 92.2% of respondents were still performing all emergency vascular surgery.12 A flexible approach is therefore required to continue to provide quality care to this important cohort.
Conclusions
The COVD-19 pandemic has significantly affected the delivery of acute care vascular surgery. Concerns for postoperative mortality in the event of SARS-CoV-2 infection, critical care bed capacity, and inpatient length of stay will undoubtedly lead to a reimagining of the role of endovascular therapy in the management of complex aortoiliac occlusive disease. This case demonstrates the management of a TASC II D lesion with a hybrid endovascular approach, negating the need for a critical care bed and resulting in a successful outcome.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Lei S., Jiang F., Su W., Chen C., Chen J., Mei W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye W., Liu C.W., Ricco J.B., Mani K., Zeng R., Jiang J. Early and late outcomes of percutaneous treatment of TransAtlantic Inter-Society Consensus class C and D aorto-iliac lesions. J Vasc Surg. 2011;53:1728–1737. doi: 10.1016/j.jvs.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Conte M.S., Bradbury A.W., Kolh P., White J.V., Dick F., Fitridge R. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg. 2019;69:3S–125S.e40. doi: 10.1016/j.jvs.2019.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norgren L., Hiatt W.R., Dormandy J.A., Nehler M.R., Harris K.A., Fowkes F.G. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Brewster D.C. Clinical and anatomical considerations for surgery in aortoiliac disease and results of surgical treatment. Circulation. 1991;83(Suppl):I42–I52. [PubMed] [Google Scholar]
- 6.Pascarella L., Aboul Hosn M. Minimally invasive management of severe aortoiliac occlusive disease. J Laparoendosc Adv Surg Tech A. 2018;28:562–568. doi: 10.1089/lap.2017.0675. [DOI] [PubMed] [Google Scholar]
- 7.Jongkind V., Akkersdijk G.J., Yeung K.K., Wisselink W. A systematic review of endovascular treatment of extensive aortoiliac occlusive disease. J Vasc Surg. 2010;52:1376–1383. doi: 10.1016/j.jvs.2010.04.080. [DOI] [PubMed] [Google Scholar]
- 8.Mayor J., Branco B.C., Chung J., Montero-Baker M.F., Kougias P., Mills J.L., Sr. Outcome comparison between open and endovascular management of TASC II D aortoiliac occlusive disease. Ann Vasc Surg. 2019;61:65–71.e3. doi: 10.1016/j.avsg.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Grimme F.A., Goverde P.C., Verbruggen P.J., Zeebregts C.J., Reijnen M.M. Editor's choice—first results of the covered endovascular reconstruction of the aortic bifurcation (CERAB) technique for aortoiliac occlusive disease. Eur J Vasc Endovasc Surg. 2015;50:638–647. doi: 10.1016/j.ejvs.2015.06.112. [DOI] [PubMed] [Google Scholar]
- 10.Bellosta R., Luzzani L., Natalini G., Pegorer M.A., Attisani L., Cossu L.G. Acute limb ischemia in patients with COVID-19 pneumonia. J Vasc Surg. 2020 Apr 29 doi: 10.1016/j.jvs.2020.04.483. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imray C., Vascular Society for Great Britain and Ireland COVID-19 virus and vascular surgery. https://www.vascularsociety.org.uk/professionals/news/113/covid19_virus_and_vascular_surgery Available at: [DOI] [PubMed]
- 12.Ng J.J., Ho P., Dharmaraj R.B., Wong J.C., Choong A.M. The global impact of COVID-19 on vascular surgical services. J Vasc Surg. 2020;71:2182–2183.e1. doi: 10.1016/j.jvs.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]