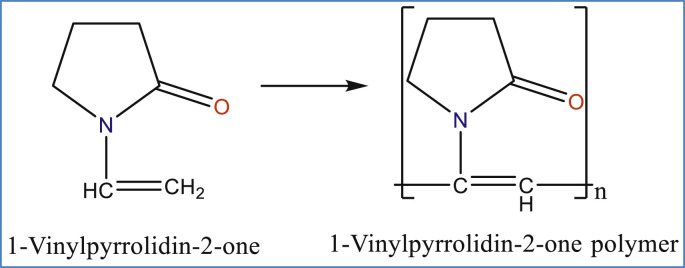
Abstract
Polyvinylpyrrolidone (PVP) is a water-soluble polymer obtained by polymerization of monomer N-vinylpyrrolidone. PVP is an inert, non-toxic, temperature-resistant, pH-stable, biocompatible, biodegradable polymer that helps to encapsulate and cater both hydrophilic and lipophilic drugs. These advantages enable PVP a versatile excipient in the formulation development of broad conventional to novel controlled delivery systems. PVP has tunable properties and can be used as a brace component for gene delivery, orthopedic implants, and tissue engineering applications. Based on different molecular weights and modified forms, PVP can lead to exceptional beneficial features with varying chemical properties. Graft copolymerization and other techniques assist PVP to conjugate with poorly soluble drugs that can inflate bioavailability and even introduces the desired swelling tract for their control or sustained release. The present review provides chemistry, mechanical, physicochemical properties, evaluation parameters, dewy preparation methods of PVP derivatives intended for designing conventional to controlled systems for drug, gene, and cosmetic delivery. The past and growing interest in PVP establishes it as a promising polymer to enhance the trait and performance of current generation pharmaceutical dosage forms. Furthermore, the scrutiny explores existing patents, marketed products, new and futuristic approaches of PVP that have been identified and scope for future development, characterization, and its use. The exploration spotlights the importance and role of PVP in the design of Povidone-iodine (PVP–I) and clinical trials to assess therapeutic efficacy against the COVID-19 in the current pandemic scenario.
Keywords: Polyvinylpyrrolidone, Povidone-iodine, Polymer, Excipient, Drug delivery, Conventional dosage forms, Controlled release, COVID 19
Graphical abstract
1. Introduction
Polyvinylpyrrolidone (PVP), also called as Povidone, is a synthetic polymer obtained by radical polymerization of the monomer, N-vinylpyrrolidone [1]. In 1939, German scientist Walter J. Reppe patented this process out of his acetylene chemistry research [1]. PVP is nontoxic, non-ionic, inert, temperature-resistant, pH-stable, biocompatible, and shows a complex affinity for both hydrophilic and hydrophobic drugs [[2], [3], [4]]. PVP is a water-soluble polymer available in different grades with varying molecular weight and viscosity [3]. In the 1940s, initially, PVP was used as a plasma volume expander [3]. In the 1950s, PVP entered into hair sprays market and replaced the shellac resin as a hair fixative agent [5]. Later PVP has gained its useful role in pharmaceutical [6,7], biomedical [4,7], cosmetics [5], and food industry [8]. With the unique properties and chemistry of PVP, there are advancements in the synthesis of PVP to obtain varied types like homopolymers of different molecular weights, copolymers, and crosslinked PVP [3].
2. Physicochemical properties
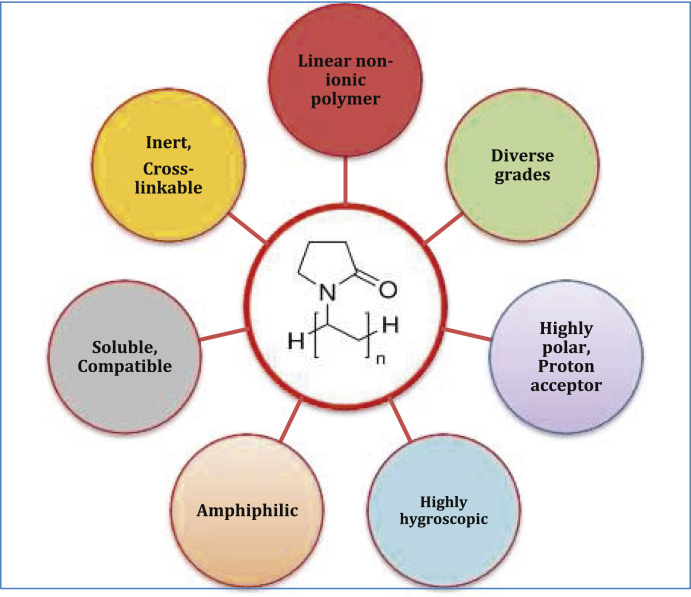
PVP has physicochemical properties making it suitable for usage in different fields like pharmaceutical, biomedical, cosmetic, and food industry. The physicochemical properties of PVP are given in Table 1 and specific properties are shown in Fig. 1 .
Table 1.
Physicochemical properties of PVP.
| S. No. | Property | Description |
|---|---|---|
| 1 | Descriptiona,b | Fine, white to off white odorless, veay hygroscopic, amorphous powder. |
| 2 | Molacalar formuaaa | (C6H9NO) n |
| 3 | Molecular weighta | 2500–30,00,000 Da |
| 4 | CAS Numbera | 9003-39-8 |
| 5 | Non-proprietary namea | Povidone (BP, USP, JP, PhEur) | |||
| 6 | Synonymsa | Povidone, PVP, Polyvidone, Plasdone, Kollidon, Poly[1-(2-oxo-1-pyrrolidinyl)ethylene], 1-vinyl-2-pyrrolidinone polymer, 2-pyrrolidinone-1-ethenyl- homopolymer. | |||
| 7 | IUPACa | 1-ethenylpyrrolidin-2-one | |||
| 8 | Melting pointa | Softens at 150 °C and decomposes after 180 °C. | |||
| 9 | pHa | 3-7 (varies with K-value and concentration of solution) |
| 10 | Solubilitya,c | Soluble in water, ethanol, methanol, chloroform, acids, and amines. Insoluble in ethers, hydrocarbons, some esters, some ketones, and mineral oil. |
| 11 | K-value rangea | 10–120 |
| 12 | Chemistrye, f, g, d | PVP polymer is comprised of functional groups C O, C–N, CH2 with a strong hydrophilic moiety – pyrrolidone and a strong hydrophobic moiety – alkyl group. The highest solubility of PVP in both water and non-aqueous solvents is attributed to the existence of highlyapolar amide moiety in pyrrolidone ring and apolar methylene and methine groups within the ring and along its backbone. The hydrophobic carbon chains show a steric hindrance effect. |
| 13 | Compatibilitya,h | Compatible in solution wjth a wide range of hydrophilic and hydrophobic, natural and synthetic resins; inorganic salts and other chemicals. PVP forms adducts in solution with sodium salicylate, salicylic acid, sulfathiazole, phenobarbital, tannin, and some other compounds. Due to the complex nature of thimerosal with povidone, the preservative action of the former agent is adversely affected. |
| 14 | Stability and storagee, f, g, d | Chemically stable in dry form. Can be stored in ordinary conditions but in a tightly closed container as it is highly hygroscopic |
| 15 | Relative viscosity in water (m.Pa.s)i | PVP-K12 (5%): 1.222–1.361 PVP-K17 (5%): 1.430–1.596 PVP-K25 (1%): 1.146–1.201 PVP-K30 (1%): 1.201–1.281 PVP-K90 (1%): 3.310–5.195 |
| 16 | Particle size distributionj | Kollidon 25/30: 90% > 50 μm, 50% > 100 μm, 5% > 200 μm; Kollidon 90: 90% > 200 μm, 95% > 250 μm. |
| 17 | Water sorptionh | As the relative humidity increases, the water sorption and weight of PVP increases. |
Fig. 1.
Specific properties of PVP.
3. Classification and marketed grades
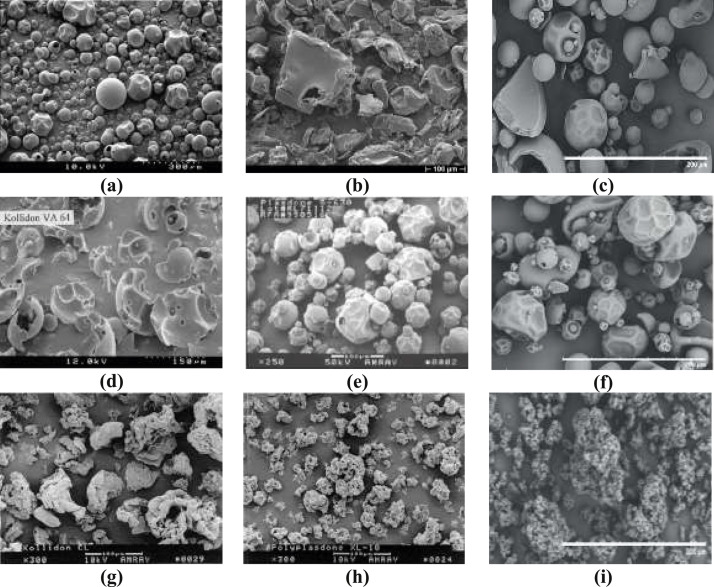
PVP is classified based on its chemistry, molecular weight, or its specific characteristic as shown in Table 2 . Scanning electron microscope (SEM) photographs of the different brands of povidone, copovidone, and crospovidone are shown in Fig. 2 .where, PF = Pyrogen free; LP = Low peroxide; D = Densified; M* = Milled; VA = Vinyl acetate; SR = Sustained release; CL = Crosslinked; XL = Crosslinked; M# = Micronized powder; F = Fine powder; SF = Superfine; Plasdone C grades = meant for parenterals use; Agrimer.
Table 2.
| Chemical form | Homopolymers | Copolymers | Cross-linked |
|---|---|---|---|
| Name | • Povidone | • Copovidone | • Crospovidone |
| Synthesis | • Free radical polymerization | • Copolymerization | • Popcorn polymerization |
| Main features | • Water-soluble • More hygroscopic • Types based on molecular weight |
• Water-soluble • Less hygroscopic • PVP-Vinyl acetate copolymer |
• Water-insoluble • Highly swellable • Crosslinked povidone |
| Synonyms | • Povidone • Povidonum • Poly(1-vinyl-2-pyrrolidone) • PVP |
• Copovidonum • Copolyvidone • Copovidon • PVP-Vac-copolymer |
• Crospovidone • Crosployvidone • Insoluble PVP • Crosslinked PVP • Polyvinyl-polypyrrolidone • PVPP |
| BASF marketed grades | Kollidon 12, 12 PF, 17 PF, 25, 30, 30LP, 90F | Kollidon VA 64, VA 64 Fine, Kollidon SR | Kollidon CL, CL-M#, CL-F, CL-SF |
| Ashland marketed grades | Agrimer 15, 30, 60L, 90, 120 | Agrimer VA 3E, 5E, 6, 64, 6E, 7E, 3I, 5I, 7I, 7W | Polyplasdone XL, XL-10, INF-10, Ultra, Ultra 10 |
| ISP marketed grades (acquired by Ashland) | Plasdone C-12, C-15, C-17, C-30, K-25, K-29/32, K-90, K-90D, K-90 M* | Plasdone S-630 | Polyplasdone XL, XL-10, INF-10, Ultra, Ultra 10 |
| JRS Pharma marketed grades | Vivapharm PVP K25, K 30 | Vivapharm PVP/VA 64 | Vivapham PVPP XL, XL-10 |
Fig. 2.
SEM photographs of povidone and its derivatives (a) Kollidon 30, (b) Kollidon 90F, (c) Vivapharm PVP K30, (d) Kollidon VA 64, (e) Plasdone S-630, (f) Vivapharm PVP/VA 64, (g) Kollidon CL, (h) Polyplasdone XL 10 and (i) Vivapharm PVPP [3,17,18,20,21].
Soluble PVP is available in different grades based on its average molecular weight, which can be determined by 3 different ways following different methods as shown in Table 3 .
Table 3.
Methods for determination of the molecular weight of PVP polymer [3].
| Type of representation of molecular weight | Method of determination |
|---|---|
| Weight average, Mw | Light scattering, ultracentrifuge |
| Number average, Mn | Osmometry, membrane filtration |
| Viscosity average, Mv | Viscosity |
The determination of the averages by specific methods is tedious. Different grades of PVP are represented by their K-values which represent the viscosity of a polymeric solution which in turn depends on the degree of polymerization and molecular weight of a polymer. Calculation of K-value for a PVP polymer is done using the Fikentscher's equation, as shown in equations (1), (2)F, following relative viscosity determination of the polymeric solution. The Fikentscher parameter is represented as lowercase, k whereas the grade of PVP is represented as uppercase, K. The reported nominal K-values of PVP are 1000 times the Fikentscher parameter, k that means reported K-value = 1000 k [9,22,23].
| (1) |
| (2) |
Where, z = relative viscosity of the solution of concentration, c K-value = 1000 k.
PVP exists in different grades based on its molecular weight as shown in Table 4 .
Table 4.
Approximate molecular weights of different grades of PVP [9].
| Grade with K-value | Approximate molecular weight (Daltons) |
|---|---|
| PVP K-12 | 2500 |
| PVP K-15 | 8000 |
| PVP K-17 | 10,000 |
| PVP K-25 | 30,000 |
| PVP K-30 | 50,000 |
| PVP K-60 | 4,00,000 |
| PVP K-90 | 10,00,000 |
| PVP K-120 | 30,00,000 |
4. Preparation methods of PVP and its derivatives
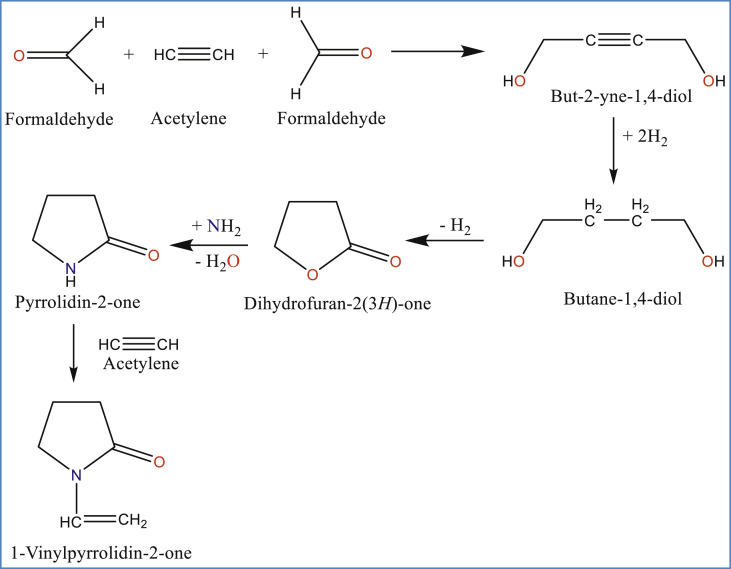
The synthesis of PVP is based on the Reppe Acetylene chemistry developed at BASF. Reppe process involves the primary synthesis of monomer, N-vinylpyrrolidone which further undergoes polymerization process to obtain the final product, PVP.
4.1. Synthesis of monomer, N-vinylpyrrolidone
Reppe process involves 5 step reaction, as shown in Fig. 3 , using acetylene as the starting material to obtain N-vinylpyrrolidone [1,3,9,24,25].
Fig. 3.
Synthesis of N-vinylpyrrolidone by following Reppe chemistry [1,9,24,25].
4.2. Polymerization of monomer
The monomer, N-vinylpyrrolidone obtained from the Reppe process is further treated to obtain PVP as shown in Fig. 4 .
Fig. 4.
There are 3 types of PVP as per synthetic procedure, namely, homopolymers (with different molecular weights), copolymers, and cross-linked PVP.
4.2.1. Homopolymer synthesis
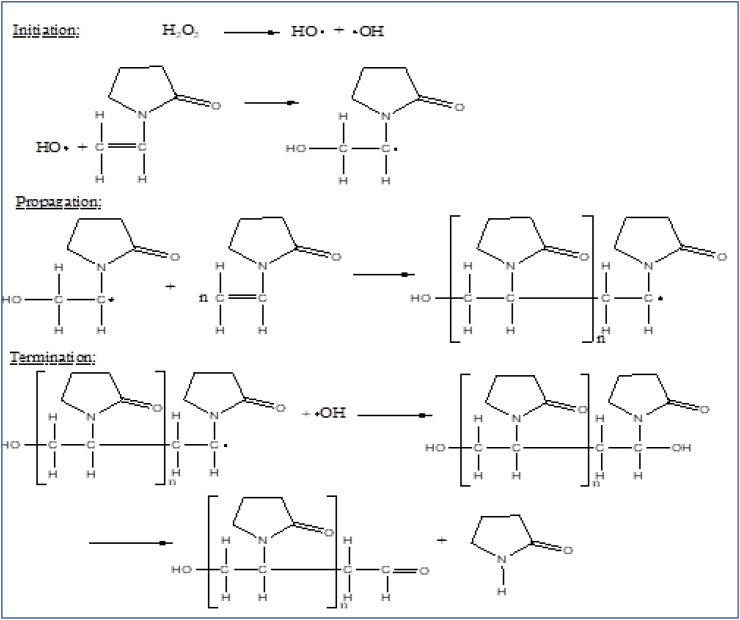
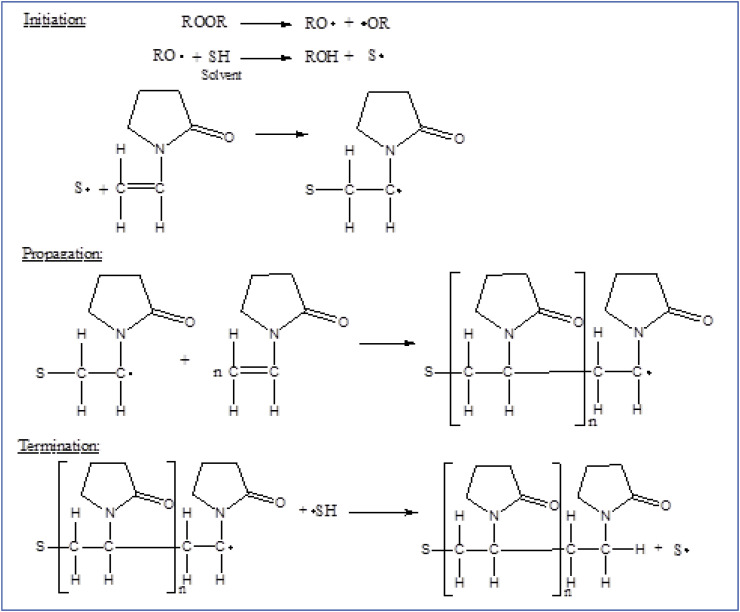
Free radical polymerization of N-vinylpyrrolidone either using aqueous media or organic solvent, a soluble polyvinylpyrrolidone having molecular weight range of 2500 to 1 million is produced which has high commercial value in different fields like biomedical, pharmacy, cosmetics and food industry [1,3]. The mechanism includes free radical polymerization of N-vinylpyrrolidone in water (aqueous solution) making use of hydrogen peroxide as initiator. By terminating the polymerization at any stage by regulating the concentration of hydrogen peroxide, the process can produce different molecular weights of soluble PVP. That's how PVP is available in different grades in the market. More the concentration of hydrogen peroxide smaller will be the molecular weight of PVP synthesized and vice versa. The mechanism of synthesis of PVP in aqueous solution is shown in Fig. 5 . In addition to this simple process performed in water, it is also possible to conduct the polymerization in organic solvents like alcohol (2-propanol), toluene, etc. To synthesize low molecular weight soluble PVP polymer meant for preparing injectables. The mechanism of synthesis of PVP in an organic solvent is shown in Fig. 6 . The spray drying process is followed for the production of pharmaceutical-grade PVP of low and medium molecular weights whereas the drum drying (roller drying) process is followed for the production of high molecular weight PVP grades [9].
Fig. 5.
Radical polymerization of N-vinylpyrrolidone in aqueous solution [1,3].
Fig. 6.
Solvent radical polymerization of N-vinylpyrrolidone in the organic solvent [1,3].
4.2.2. Copolymer synthesis
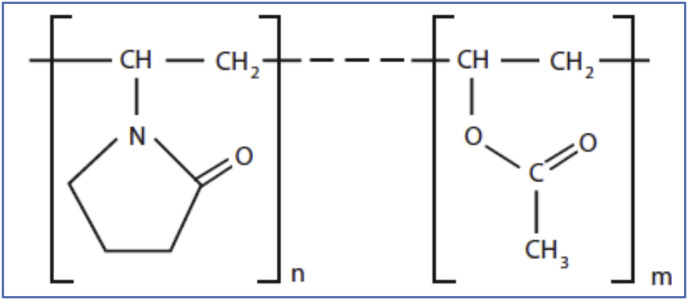
With the copolymerization process, the monomer is used in the synthesis of Copovidone (E.g., vinylpyrrolidone-vinyl acetate copolymer) [1,3]. Cationic copolymers have better adhesive properties suitable for cosmetics like hair curling agents. Anionic copolymers are soluble in oil and can be used as dispersing agents. Pictorial representations are given in Fig. 7 .
Fig. 7.
Structural formula of copovidone [3].
4.2.3. Cross-linked polymer synthesis
On the other hand, if the same monomer, N-vinylpyrrolidone is undergone popcorn polymerization either by using alkali hydroxide at >100 °C (produces in situ bifunctional monomer) or by direct addition of bifunctional monomer at ≤100 °C in water yields an insoluble crosslinked polyvinylpyrrolidone (Crospovidone) [1,3,[26], [27], [28]].
4.2.4. Advances in PVP synthesis
With versatile applications of PVP and its derivatives, more attention on their synthesis that can prepare the polymer with desired molecular weight, narrow polydispersity, and well-defined termination has been caught. In 2002 and 2004, Rimmer and Smith reported a method using 3-methyl butane-2-one as a chain transfer agent that produced PVPs with a polydispersity index (PDI) of <1.5, however it is producing low molecular weight polymers of around <10,000 [29,30]. In 2004, Yamango et al. reported the primary results of applying organostibine-mediated living radical polymerization (LRP) followed by the first successful complete application in 2006 by Ray et al. which produced PVP of high molecular weight (Mn > 10,000) and also its block copolymers. They obtained a PDI of <1.1 for low molecular weight PVP (Mn < 15,000), and a PDI of <1.3 for even high molecular weight PVP (Mn < 1,00, 000) [31]. The researchers also prepared copolymers of PVP in a controlled manner following this LRP process [31]. In 2007, Lu et al. reported the application of atom transfer radical polymerization (ATRP) in producing PVP of molecular weight around 8900 with PDI of 1.2–1.38 [32,33]. Advances in the procedures of controlled radical polymerization (CRP)/LRP [[34], [35], [36], [37], [38], [39], [40], [41], [42]], ATRPf [43], and reversible addition-fragmentation transfer radical polymerization (RAFT) [44,45] have created new avenues for better control on the synthesis of PVP and its derivatives.
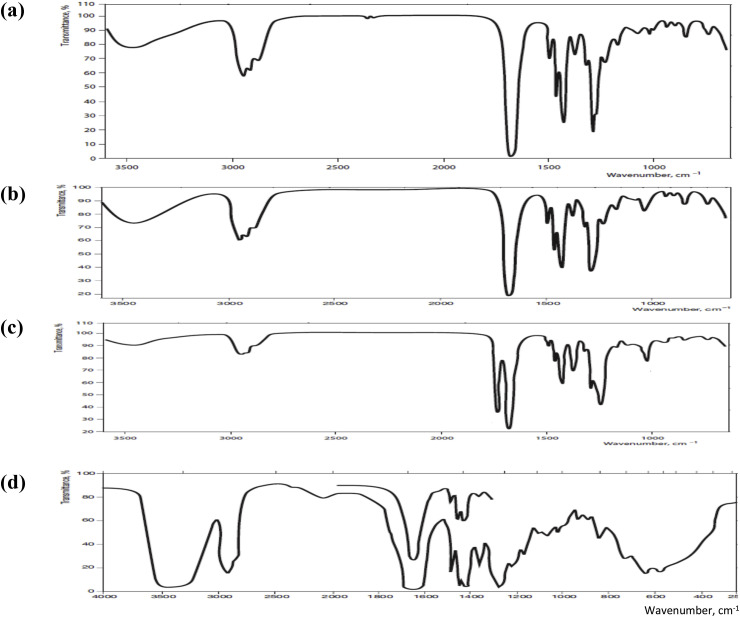
5. Characterization methods
PVP can be characterized and analytically determined by following several methods that are clearly described in the literature and monographs of different Pharmacopoeias [3]. Infrared spectroscopy is one important analytical method for the identification of PVP [46]. The infrared spectrum for different grades of PVP is shown in Fig. 8 . The specific type of PVP can further be identified based on other tests like solubility, morphology studies, particle size, molecular weight, and viscosity. Pharmacopeias have mentioned different chemical tests for the identification and qualitative estimation of PVP. For the quantitative determination of PVP, several methods are reported in the literature like photometry (using povidone-iodine complex) [47], vital red [48], turbidometry, pyrolytic gas chromatography [49]. Methods especially followed to differentiate the grades of PVP include IR spectral analysis (as shown in Fig. 8), thin-layer chromatography, paper chromatography, detection as the hydroxamic acid, and electrophoresis, nitrogen determination, gravimetric analysis, complexation capacity and solubility [3]. High-performance liquid chromatography (HPLC) and gas chromatography (GC) methods were clearly described in the literature and monographs of Pharmacopoeias for the determination of specific substances in PVP samples [3].
Fig. 8.
IR spectra of (a) PVP K 30, (b) PVP K 90, (c) Copovidone and (d) Crospovidone, in potassium bromide [3].
6. Pharmacokinetics, toxicology, and safety
After the introduction of PVP in polymer science, extensive research has been done in developing its pharmacokinetic and toxicological profile. Different animal models and humans have been tested to study the kinetic profiling of PVP [50]. Since PVP is proposed for different fields of interest, particularly the medical, pharmaceutical, and food industry, its safety profiling became a necessity and hence detailed information has been documented in the literature. Both the individual researchers and regulatory bodies have concluded that PVP usage is safe as both a food-grade additive and also as a pharmaceutical excipient. WHO has given a listing of its acceptable daily intake of 0–50 mg/kg body weight [8].
6.1. Pharmacokinetics
Upon several studies and reported detailed assessment [50], it was found that the absorption, distribution, metabolism, and excretion (ADME) of PVP is having a relationship with the molecular weight of the polymer, dosing frequency and route of administration. Several absorption studies revealed that there are no or very limited absorption of PVP in humans, rats [51,52], rabbits [53], and guinea pigs [54]. Minimal absorption has been found to result in the storage of PVP in mesenteric lymph nodes [51]. PVP is taken up by the reticuloendothelial system (RES) (mononuclear phagocyte system), following pinocytosis or phagocytosis mechanism [[55], [56], [57], [58]]. Ravin et al. reported that the retention of PVP in RES is proportional to its molecular weight, i.e., higher molecular weight PVP shown longer retention time [55]. Based on the studies performed in rats, dogs, and humans upon intravenous administration and verifying the samples of blood and urine, the researchers reported that there is no evidence of metabolism for the PVP in the biological system either in animals or humans [50,57]. The primary route of excretion for low molecular weight PVP (<25,000) is through kidneys upon intravenous and intraperitoneal administration [50,55,57]. Wessel et al. have reported the elimination of PVP with varying molecular weights based on then-existing literature [59].
6.2. Toxicology
A good number of reports claim that PVP is biologically inert and non-toxic with good tolerance [50,57,59]. A huge data concerning toxicological studies in animals like rats, pigs, dogs, and rabbits revealed that orally administered PVP shows no acute or subchronic or chronic toxicity [50,57,60]. In some cases, an extremely low effect like diarrhea is observed at high doses. The reason is the osmotic action shown by PVP at high doses which acts as a bulk purgative [50]. There is no evidence of toxicity in terms of clinical chemistry, histopathology, and hematology except diarrhea at high doses. A mild level of deposition of orally administered PVP in mesenteric lymph nodes is also of no toxicological importance. Intravenous and intraperitoneal routes of administration of high molecular weights of PVP shown storage in RES tissues. The reason might be due to lack of filtration of big size molecules at glomerulus. As per the reports, the LD50 value for oral administration of PVP (average molecular weight 40,000) is greater than 100 g/kg body weight in both rats and guinea pigs. Hence, PVP is claimed as neither a sensitizer nor an irritant. Even in humans, there are no reported adverse effects with oral administration of PVP [50]. Assessment of different types of toxicity of PVP has been listed in Table 5 .
Table 5.
| Toxicity | Claim/Evidence | Animal models/Humans |
|---|---|---|
| Teratogenicity, Prenataltoxicity | No | Rabbits, Rats |
| Embryo-toxicity | No | Rabbits embryos |
| Mutagenicity/Genotoxicity | No | Mice, In vitro cell lines |
| Carcinogenicity | No (earlier reports claimed tumors, but later found to be erroneous observations) | Rabbits, Rats |
| Oral toxicity | No | Rabbits, Rats, Dogs, Humans |
| Intravenous toxicity | Storage disease, Foam cells | Humans |
| Subcutaneous toxicity | Storage disease, Foam cells | Humans |
| Intramuscular toxicity | Subcutaneous granuloma | Humans |
| Inhalation toxicity | Thesaurosis reported (unproven) | Humans |
| Ocular toxicity | No | Humans |
| Dermal toxicity | No | Humans |
| Phototoxicity | No | Humans |
| Photoallergenicity | No | Humans |
6.3. Safety
Based on the toxicity studies conducted on a very extensive level it was confirmed that the PVP is a biologically inert substance. There are no or non-significant adverse effects on oral administration of PVP observed through acute, sub-chronic, and chronic toxicity studies. PVP is found to be non-toxic, non-irritant, non-sensitizer, and hence completely safe for use in animals and humans for both foods, medical and pharmaceutical applications. The accepted daily intake limit of 50 mg/kg/day proves the adequate margin of safety for PVP. PVP is found to be safe for oral and topical applications in any manner. PVP is also found to be safe for its use through ocular and dermal routes of administration based on the observations of literature reported studies with concern to molecular weight. For parenteral use, administration of PVP is warned in terms of high molecular weight, repeated dosing, and route of administration as the subcutaneous and intramuscular routes of administration shown subcutaneous granuloma [50,61,62]. Copovidone and crospovidone are also found to be safe with no toxicological reports and shown good tolerance [3].
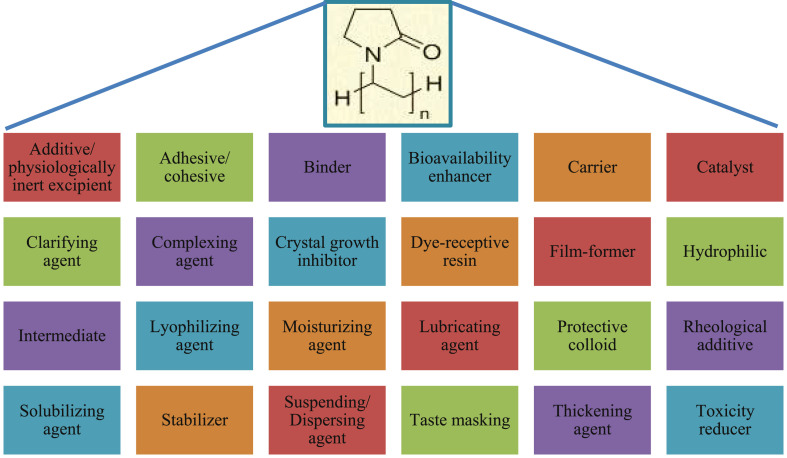
7. Pharmaceutical and other applications
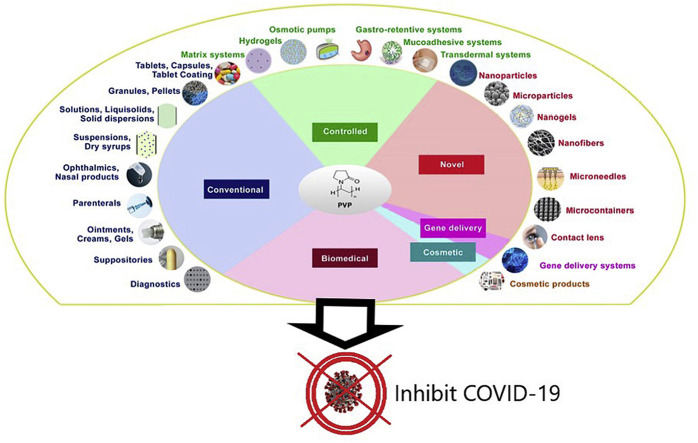
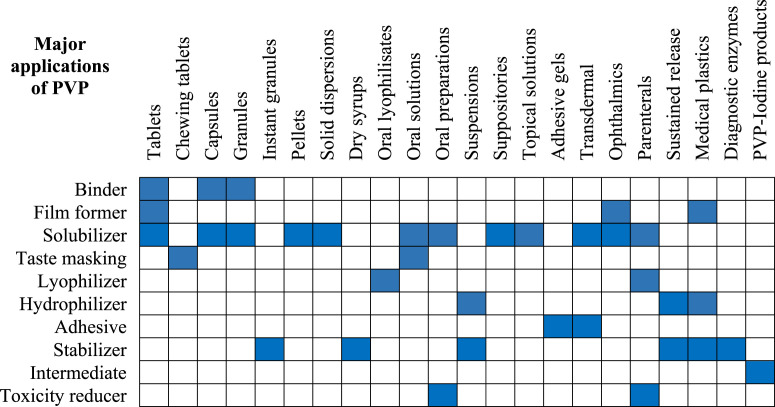
At the very early stage of the PVP introduction in the market, it was used as a blood plasma volume expander or substitute [3,55,63,64]. Later PVP has found vast applications in medicine, pharmacy, cosmetics, food, and industrial production. The wide range of applications of PVP is due to its unique properties as already discussed and also, its ability to interact with low molecular weight compounds. Various applications of PVP are listed in Fig. 9 . The diversity in applications of PVP is majorly due to the tailored properties based on its molecular weight and polymerization method. For example, the rate of dissolution is inversely proportional to the molecular weight whereas the viscosity, complexing nature, and adhesive power are directly proportional to the molecular weight of PVP. Even the biological elimination of PVP upon parenteral administration is also inversely proportional to its molecular weight. Hence, a specific molecular weight PVP that is suitable for the desired application has to be selected. Researchers have used PVP ranging from conventional dosage forms to controlled release systems. In the present review, a detailed note on the applications of PVP in both areas is presented.
Fig. 9.
Various pharmaceutical applications of PVP [3].
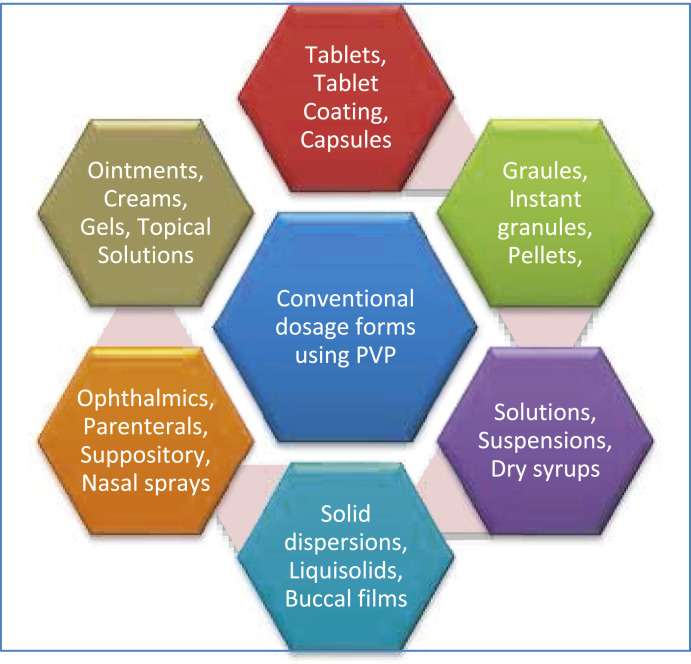
7.1. As an excipient in the development of conventional dosage forms
Several conventional dosage forms where PVP has been used is shown in Fig. 10 .
Fig. 10.
Schematic of conventional dosage forms with PVP application.
7.1.1. Oral solids
7.1.1.1. Tablets
Among several applications, the use of PVP in solid dosage forms (tablets, capsules, granules, pellets) is of traditional value. In tablets preparation, PVP solution is used as an excellent binder in wet granulation, either in water or alcohol or mixture, due to its adhesive properties. For effervescent tablets/granules, sometimes water may not be preferred due to hydrolysis problem and hence alcohol is suitable. In both the solvents, PVP is freely soluble. However, if the water is to be used as a solvent for the binder in the wet granulation method or the preparation of effervescent tablets, a fluidized bed granulator is preferred to avoid more contact time and hydrolysis. It can produce non-friable tablets with optimum hardness and a reliable rate of dissolution. PVP can also be used as a dry binder on mixing with powder blends in its dry form and granulated in site upon mixing of water/alcohol/hydroalcoholic solution. Since PVP is freely soluble in an aqueous environment like gastric fluid, the presence of PVP as binder doesn't influence the disintegration or dissolution rate of the drug from tablets even though it can help to formulate tablets with good hardness, which is not the case with other binders like gelatin or hydroxypropyl methylcellulose. Kimaro et al. (2019) formulated chewable albendazole tablets following a wet granulation method using PVP K30, intending to enhance the dissolution rate of the drug [65].
PVP is used as a binder in all the wet granulation modified methods, fluidized bed granulation [66,67], and extrusion spheronization [68,69]. PVP can also be used in combination with different grades or with other binders because of its good compatibility and to obtain any specific desired properties like plasticity. Examples of formulations include rifampicin, pyrazinamide, ranitidine effervescent, ascorbic acid effervescent tablets, etc. PVP polymers also gained a place in dry granulation [70] and direct compression [71] techniques. Direct compression is the simplest tableting process among all available methods. However, all drugs are not having a compressibility nature suitable for this method. In such a case, the concept of using auxiliaries available readymade in the market or by developing drug-binder mixtures suitable for direct compression is in use. PVP is also used as a binder in pre granulation forms of active substances meant for direct compression otherwise which are prone to hydrolysis in the wet granulation method. PVP is also used to develop direct compression auxiliaries (e.g., lactose granules for direct compression) and granulation of active ingredients (pre-granulated ascorbic acid granules with povidone) for direct compression [3].
7.1.1.2. Tablet coating
Because of its hydrophilic and film-forming nature, PVP avoids micro-cracks during the sugar-coating process and also aids for better adhesion of the applied coating layer over the hydrophobic core. Color solutions or suspensions also show homogenous distribution with the presence of PVP in a coating mixture representing its dispersing effect. PVP also maintains the stability of sugar suspension because of its crystal growth-inhibiting property. With the properties of film-forming, adhesiveness, an affinity for the hydrophobic surface, and dispersive, PVP is also used in film coating of tablets [72]. However, PVP is not used alone as a sole film former in coating mixtures because of its hygroscopicity. PVP is also used as an additive in the enteric coating of tablets along with enteric coating polymers [73].
7.1.1.3. Granules, pellets, capsules
PVP is used as a binder to prepare granules and pellets as final dosage form (e.g., effervescent, chewable, dry syrup, or instant for dispersion), or else they can be further used for tableting or filling into hard gelatin capsules or coating. Ascorbic acid granules prepared by dry granulation through roller compaction using PVP K30 as a dry binder is the typical example [74]. PVP is useful for preparing highly concentrating solutions (by enhancing the solubility of active ingredients with its hydrophilic nature) that can be filled in soft gelatin capsules [75].
7.1.1.4. Solid dispersions
PVP is used as a solubilizer to enhance the solubility, dissolution rate, and hence bioavailability of poorly soluble drugs in different formulations. The role of PVP in increasing the physical stability of amorphous drugs by preventing the crystallization in the solid-state and maintaining the drug supersaturation after dissolution has been proved in many studies [76,77]. PVP forms water-soluble complexes, because of its hydrophilic nature, with many drugs of low molecular weight, and also avoids crystallization of dissolved drugs. For example, the dissolution rate and hence the bioavailability of indomethacin, have been increased with the use of PVP [78]. Major factors that influence the magnitude of dissolution rate or bioavailability enhancement include the method of preparation (e.g., melting, coprecipitation, extrusion, solvent evaporation, physical mixing), the ratio of drug to PVP and molecular weight of PVP. Several drugs like phenytoin [79], sulfathiazole [80], hydrocortisone [80], disulfiram [81] have reported data with enhanced dissolution rate and/or bioavailability. Verma et al. prepared inclusion complexes of famotidine with cyclodextrin and PVP following spray drying technique to increase drug solubility [82]. They employed quality by design (QbD) approach using Plackett-Burman design as a systematic process. The results showed a promising increase in the solubility and dissolution rate of the selected model drug. Barmpalexis et al. investigated the influence of plasticizer, polyethylene glycol (PEG) molecular weight on solid dispersions of PVP prepared by melt mixing using tibolone as the model drug [83]. PVP blends with PEG 400 and PEG 600 were found to be completely miscible. All the prepared formulations showed long-term physical stability up to 18 months at ambient temperature. The studied dissolution parameters showed good prediction ability as per artificial neural networks. Frizon et al. prepared and evaluated solid dispersions of loratadine to enhance its dissolution rate by using PVP K30 as a hydrophilic carrier [84]. The investigation revealed that PVP based solid dispersions have increased the solubility of poorly soluble drugs prepared by following rotary evaporation and spray drying methods. PVP was used as a polymer to form amorphous solid dispersions with indomethacin within the tablet in situ by using microwave irradiation [85]. Dissolution rate enhancement of poorly soluble drugs like curcumin, silymarin, carbamazepine, nimodipine, β-lapachone, tadalafil, gliclazide, carvedilol were also reported using PVP. Supercritical antisolvent coprecipitation technique was followed to improve the solubility of poorly water soluble drugs like nimesulide and cefuroxime with the use of a hydrophilic polymer, PVP [86,87].
7.1.1.5. Liquisolid compacts
PVP has been used as an additive in the liquisolid technique for the enhancement of the dissolution rate of poorly soluble drugs. Molaei et al. has conducted a study to improve the dissolution rate of poorly water-soluble drug, ketoconazole by preparing liquid-solid compacts. They used microcrystalline cellulose as a carrier, colloidal silica as a coating material, PEG400 as a nonvolatile solvent and PVP as additive [77]. In another work, the presence of PVP as an additive in liquid-solid compacts has shown an increased dissolution rate of carbamazepine than the compacts prepared by direct compression [76]. It is claimed that PVP inhibits crystal growth by inhibiting the drug precipitation from supersaturated liquid medication and also it helps in providing increased exposed drug surface area against the dissolution medium due to adsorption on the carrier.
7.1.1.6. Buccal films
Elagamy et al. developed and evaluated rapidly dissolving buccal films using HPMC and PVP K30 for naftopidil [88]. Loading of the films was done with either pure form of drug or co-grinded form (with citric and/or tartaric acid) or as a self-micro emulsifying system (mixed with plurol oleique, labrasol, and tween 80). Naftopidil in the HPMC-PVP film developed a new crystalline structure. The crystalline nature got abolished in the presence of organic acids or self-micro emulsifying systems. In-vitro and in-vivo evaluation using the rabbit model revealed that there is a significant enhancement of the dissolution rate as well as bioavailability of the selected model drug administered as a buccal film.
7.1.2. Oral liquids
PVP polymer has wide applications in oral liquids (e.g., oral drops, solutions, suspensions, dispersions, emulsions) as solubility enhancers for several poorly soluble drugs like diclofenac [89]. With medium and high molecular weight PVP (acting as a thickening agent), desired viscosity of the liquid dosage form can be obtained which aids for constant drip rate, better appearance, dispersion, and physical stability. PVP is also reported for the taste masking effect [90,91] in products containing acetaminophen, trimethoprim, and sulfamethoxazole. PVP is used as a crystal growth inhibitor to avoid crystallization of product ingredients e.g., in syrups where cap locking is the problem [92].
7.1.2.1. Suspensions/emulsions/dispersions
PVP has applications like suspending, dispersing, stabilizing and viscosity-enhancing (thickening) agent in numerous oral liquids (dry syrups/suspensions/emulsions/dispersions/instant granules for reconstitution) [93,94]. PVP acts as a protective colloid and can effectively stabilize suspensions, emulsions, and dispersions by adsorbing as a thin molecular layer over the surface of the individual colloidal particles to avoid contact, aggregation or coalescence. This might also be attributed to hydrophilic nature rendered to the individual solid particles and letting them stay away from each other due to steric hindrance. As the viscosity is also enhanced by PVP, the sedimentation rate is reduced as per Stoke's law. Hence, better dispersibility is achieved and the sedimentation volume can be increased. The stability of suspensions can also be enhanced due to the addition of PVP as it reduces the zeta potential of substances like iron oxide pigments [95]. PVP is used as a binder for the preparation of instant granules or dry syrups meant for reconstitution as suspension. Medium and high molecular weight PVP grades are used in oral suspensions whereas low molecular endotoxin-free grades are used in suspensions meant for parenteral administration [96,97].
7.1.3. Ophthalmic
PVP is used to increase the contact time of eye products by enhancing the viscosity (thickness) of drug solutions and also acts as an effective lubricant for dry eyes [98,99]. In ophthalmics also, PVP can be used as a crystal growth inhibitor. PVP can also act as a solubilizer to obtain clear solutions meant for ophthalmic use and also aids for improved effect and bioavailability of active ingredients [33,100,101]. PVP also aids in reduced irritation caused by formulations [102]. Oxymetazoline irritant effect is reduced by using soluble PVP in ophthalmics [3]. The cleaning solutions for contact lenses are also prepared by PVP [103,104]. PVP is also used as a suspending, thickening or stabilizing agent for ophthalmic suspensions. Examples of drugs used in ophthalmics in combination with PVP include chloramphenicol, pilocarpine, timolol, prednisolone, etc. In the case of ophthalmics, low molecular weight grades are used for eye drops and suspensions whereas high molecular weight grades are preferred for contact lens solutions [105].
7.1.4. Parenteral
In parenteral, PVP polymers are used for solubilization or dispersion of drugs in injectable products for human or veterinary use. A low molecular weight endotoxin-free (pyrogen-free) PVP is easily eliminated upon parenteral administration without accumulation and hence PVP K12 or K17 are preferred than other grades with high molecular weight [50]. Injections of antibiotics, rifampicin, oxytetracycline are examples in which PVP is used as a solubilizer. PVP can also be successfully used in the lyophilization process of preparing injections for better solubility and stability [96,106,107]. PVP has been used to boost the viscosity of intracytoplasmic sperm injection [33,108].
7.1.5. Topical/transdermal preparations
In transdermal products like ointments, creams, and gels, PVP is used to develop hydrogels (that act as diffusion matrix) and as a crystal growth inhibitor in certain drug-adhesive matrix systems. Because of the excellent adhesive property shown by PVP in addition to its physiological safety and inertness, it is used as an adhesive in several mucoadhesive drug delivery systems [109,110] and transdermal systems [111] because of its properties like adhesiveness, crystal inhibition, solubilization, etc. Transdermal products include gels, patches, buccal tablets, meant for adhesion to the skin or mucous membrane [[112], [113], [114]]. Examples of drugs used along with PVP in transdermal products include captopril, chlorhexidine, bromhexine, isosorbide dinitrate, etc. PVP has found its valuable presence in the adhesive systems. Even it is also used in diagnostic applications like contact gel for electroencephalogram (EEG), electrocardiogram (ECG), and colostomy bags [115]. Rac et al. found that the addition of PVP to polyacrylic acid cryogels for transdermal drug delivery has shown decreased swelling, increased adhesivity, and compression moduli [113]. PVP is used as an adhesive or in adhesive formulations to fix medical electrode leads or ultrasound leads to the skin. It is also used for coating as a lubricating agent on medical devices like catheters. PVP is used as a pore-forming agent in membrane technology like haemodialysis membranes.
7.1.6. Nasal
PVP based nasal sprays and in-situ gel for nasal drug delivery are reported. A detailed report on the clinical study of liposomal povidone-iodine nasal spray has been published by those researchers [116]. In the latest pandemic coronavirus disease condition due to COVID-19, PVP-iodine nasal spray is proposed for avoiding or minimizing the cross-infection risk to the front line doctors or workers [116,117]. PVP can increase the mucociliary clearance timings of nasal applications [118].
7.1.7. Rectal
Suppositories for better drug release or sustained have been developed using PVP as an excipient along with other formulation ingredients. Liquid suppositories are also reported by PVP applications [112]. Saleem et al. reported the use of PVP along with agar for developing sustained release rectal suppositories of tramadol hydrochloride [119].
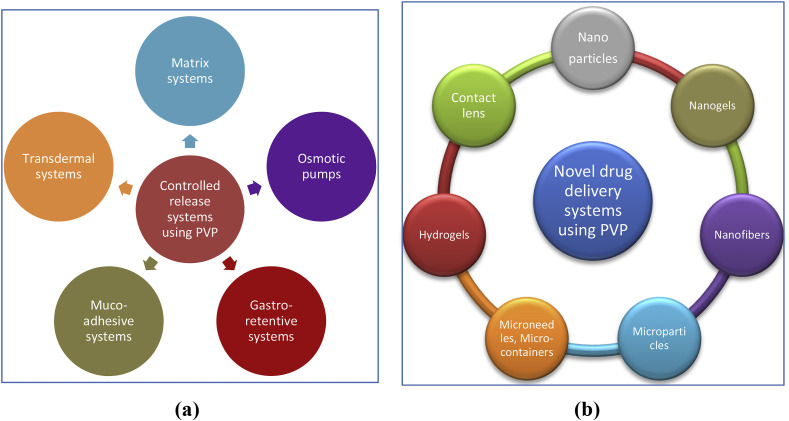
7.2. Designing controlled release systems
PVP has also been popularly used in designing controlled release systems. Herewith presented some of the prominent areas of research in controlled drug delivery as shown in Fig. 11 .
Fig. 11.
Schematic of PVP applications in (a) controlled release systems and (b) novel drug delivery systems.
7.2.1. Conventional controlled systems
7.2.1.1. Matrix tablets
Ian et al. developed HPMC matrix tablets of water-soluble drug, caffeine to modulate the drug release kinetics from linear to bi-modal pattern by using PVP K90 [120]. The concentrations of HPMC and PVP in the tablet were altered to obtain the modulated drug release kinetics. Bose et al. prepared sustained release matrix tablets of Itopride HCl following central composite design response surface methodology [121]. HPMC was used as release retardant, PVP K30 as a binder, and lactose as a filler. The optimized formulation has sustained the drug release for up to 12 h.
7.2.1.2. Osmotic pumps
Pandey et al. have developed elementary osmotic pump tablets using different gelling agents like cellulose derivatives, carbopol, and PVP K30 at different concentrations and different cosmogenic like mannitol, sodium chloride, potassium chloride and fructose followed by coating with cellulose acetate using spray coating technique [122]. Even after 24 h of exposure to the dissolution medium, the osmotic devices prepared with HPMC and PVP were found to be intact. Dasankoppa et al. developed ketorolac containing controlled porosity osmotic pump (tablet) for controlled release using PVP as a pore former, dextrose as osmogen, and ethyl cellulose as a coating material [123]. Studies revealed that the drug release was directly proportional to the concentration of pore former, PVP in the coating membrane, and inversely proportional to the membrane weight.
7.2.1.3. Gastroretentive and mucoadhesive systems
Chun et al. developed novel mucoadhesive floating granules of acetaminophen using the Carbopol-PVP interpolymer complex to achieve controlled drug release. Sodium bicarbonate was used as a gas-generating agent [109]. At a complex ratio of 1:1, the system has shown the maximum floating of 95% for 12 h. Hosmani et al. studied the application of interpolymer complex of Carbopol 971P with different grades of PVP like K25, K30, K90, VA/S-630 in the development of sustained-release systems using acyclovir as a model drug [124]. The optimized tablet has shown good control over the release of the drug. Yusif et al. developed ranitidine HCl gastro retentive matrix tablets following in situ interpolymer complexation of Carbopol with PVP (K25 and K90 grades) [125]. Tablets containing PVP K90 grade have shown better floating, mucoadhesion, and sustained drug release. X-ray imaging studies in human volunteers have also shown a mean gastric residence time of 6.8 h.
7.2.2. Novel drug delivery systems
Several novel carrier systems have been developed using PVP and its derivatives as shown in Fig. 11. Some of the promising works are discussed here.
7.2.2.1. Nanoparticles
Dong et al. prepared amorphous drug-polyelectrolyte nanoplex (nanoparticle complex) for the enhancement of solubility of poorly soluble drugs [126]. The existing nanoplexes suffer from long term storage instability due to the crystallization propensity of certain drugs. Hence, PVP is used as the crystal inhibitor for the drug-polyelectrolyte (ciprofloxacin-sodium dextran sulfate) nanoplex. The PVP stabilized nanoplexes shown better stability with an enhanced dissolution rate of up to 15–30% increase. Campardelli et al. developed hollow gold nanoshells (HGN) of rhodamine using biodegradable polymer, polylactic acid (PLA), and PVP as a stabilizer via galvanic replacement of cobalt nanoparticles [127]. The desired drug with irradiation has been reported. Guduru et al. developed magneto-electric nanoparticles (MENs) of paclitaxel using PVP as a component and used to enable field-controlled high-specificity for drug delivery to the ovarian cancer cells [128]. Javed et al. have shown that PVP and PEG capped CuO nanoparticles have shown antibacterial, antioxidant, antidiabetic, and cytotoxic effects than their uncapped nanoparticles [129]. They concluded that PVP doped nanoparticles are capable of use in biomedical applications like a carrier for drug and diagnostic molecules. Gaikwad et al. have prepared pectin-PVP curcumin particles using spray drying technique for better performance in terms of enhanced solubility, improved anticancer potential, and localized controlled drug release [130].
7.2.2.2. Nanogels
Dasankoppa et al. followed gamma radiation-induced polymerization for the preparation of nanogels (pH-sensitive hydrogel nanoparticles) with acrylic acid in an aqueous solution of PVP as a template polymer through crosslinking complexation mechanism and hydrogen bonding interaction [123]. PVP molecular weight was found to be one of the important parameters that influence the particle size and swelling ability of the nanogels. A novel method has been reported for the preparation of complex nanogels comprising of PVP and poly (2‐acrylamido‐2‐methylpropane sulfonic acid) (PAMPS) in PVP aqueous solution. These nanogels exhibited a pH-induced phase transition with the protonation effect on PAMPS chains. These nanogels are found to apply to the development of pH-controlled drug delivery systems [131]. Bueno et al. prepared PVP superabsorbent nanogels following Fenton reaction using PVP and the aqueous pool of hexadecyltrimethylammonium bromide reverse micelles. With tenfold swelling ratio, these are characterized as efficient superabsorbent nanogels and the swelling was proposed to be dependent on bound Fe3+ varied with pH and ionic strength [132]. A detailed review has been published by Kadlubowski on the radiation-induced synthesis of PVP nanogels [133].
7.2.2.3. Nanofibers
Yu et al. developed a core/sheath nanofibers of ketoprofen using PVP as the sheath polymer and ethyl cellulose as the core matrix following the coaxial electrospun method [134]. The system has shown a biphasic drug release profile with initial immediate release followed by sustained release. PVP has been studied in the presence of seven different solvents for the electrospinning technique to develop PVP nanofibers. The properties of the solvents like dielectric constant, viscosity, and surface tension has affected the electrospinnability, appearance, and size of the nanofibers produced. High dielectric constant, low surface tension and low viscosity are found to produce small and uniform nanofibers using PVP solutions confirming that PVP was useful as polymer carrier for the fabrication of nanofibers [135]. Albetran et al. developed TiO2/PVP nanofibers following the electrospinning technique based on the Taguchi design of the experiment. The formulation and fabrication characteristics were optimized to obtain the desired nanofibers [136].
7.2.2.4. Microspheres/microcapsules
Mennini et al. used PVP for the preparation of a ternary complexation system of the drug, celecoxib with hydroxypropyl-β-cyclodextrin and PVP K30 to enhance the solubility of the drug and for better vectorization during the development of chitosan-calcium alginate microspheres [137]. Results revealed that the ternary complexation system has shown better drug dissolution rate than the binary system of drug-cyclodextrin in turn which is better than that of pure drug alone. Kim and Park prepared and evaluated porous microcapsules of poly(ε-caprolactone) (PCL)-Eudragit RS100 using tulobuterol as model drug following solvent evaporation method in which drug and PVP were dissolved in dichloromethane along with polyvinyl alcohol to obtain pre-emulsion [138]. Several researchers have developed microspheres/microparticles for the enhancement of the dissolution rate by following different techniques [[139], [140], [141]].
7.2.2.5. Micro containers
Marizza et al. developed a technique for the effective loading of poorly soluble drugs in micro containers [142]. They combined the concepts of inkjet printing and supercritical fluid impregnation. Ketoprofen was used as a model drug and PVP as a polymer. PVP solutions were dispensed into the microcontainers with a quasi-no-waste performance by inkjet printing whereas drug is impregnated in the polymer matrix following supercritical fluid technology using supercritical fluid carbon dioxide as loading medium. The dissolution rate with the reproducibility of such drug-loaded polymer filled micro containers was found to be better than that from solid dispersions prepared with the same ingredients. The blend of these loading techniques has shown feasibility for the rapid fabrication of microdevices suitable as oral drug delivery systems with a safe and solvent-free solution.
7.2.2.6. Microneedles
Aung et al. developed dissolving microneedles using the combination of HPMC and PVP for the transdermal administration of alpha-arbutin, a skin lightener agent [143]. The dissolving microneedles showed the better delivery of an agent into the skin than with commercial cream or the microneedles done with HPMC alone. In vivo studies also revealed the same level of results. Liu et al. demonstrated that the insulin loaded calcium carbonate-PVP microneedles have shown 98.2% relative pharmacological availability and 96.6% relative bioavailability in diabetic rats for the regulation of glucose [144]. These systems have shown high efficiency and constant release in comparison with the traditional subcutaneous injection of insulin and are found as a promising drug delivery device. Moga et al. developed microneedles following adapted PRINT process using PVP films of low molecular weight, 10 kDa [145]. Rhodamine B dye was used as the drug surrogate. PVP was selected as the first matrix because of its high water solubility, high tensile strength, biocompatibility, Food and drug administration (FDA) approved excipient and the masses with less than 20 kDa are cleared efficiently from the kidney after subcutaneous injection and hence safe for human use [146].
Sulliven et al. developed microneedle arrays for vaccination purposes using PVP as polymer [146]. Inactivated influenza virus was administered using these dissolving microneedles in a mouse model which has shown superior protective immune response than an intramuscular injection of the same dose. This concept has produced robust antibody and cellular immune response against lethal challenge. Roy et al. fabricated, characterized, and evaluated the ex vivo and in vivo effectiveness of amphotericin B (as free or as a liposomal form) loaded microneedle ocular patch for the treatment of fungal keratitis [147]. Both ex vivo corneal permeation studies and in vivo (rabbit model) experimentation showed better therapeutic efficiency and hence concluded that microneedle ocular patch can be developed as a less invasive drug delivery device for corneal treatment. The fabrication process of microneedles includes the room temperature based photopolymerization of a liquid monomer, vinyl pyrrolidone to obtain PVP in a microneedle mold along with encapsulation of lyophilized vaccine. PVP is best suited here due to its biocompatibility, mechanical strength, and high water solubility [50].
7.2.2.7. Hydrogels
PVP is highly used to develop hydrogels due to its swelling properties in an aqueous environment. Literature has provided plenty of reports with the application of PVP in hydrogels formulation. Oliveira et al. (2014) developed polymeric hydrogels of PVP K90 containing chitosan and clay as nanoparticles using glucantime as a model drug [148]. Interactions of chitosan and clay with PVP were studied and reported their influence. The release of the drug was found to be influenced by the swelling rate of the PVP-clay system and the PVP-chitosan-clay system. Ahmad et al. prepared and characterized crosslinked PVP hydrogels with 3 different compositions (drug: polymer ratio of 1:1, 1:2, 1:3) using theophylline as a model drug [149]. They concluded that the increased concentration of PVP decreased the release rate of the drug from hydrogel-based compacted matrix tablets. Jin et al. developed novel pH- and electrical-sensitive hydrogels using PVP-polyacrylic acid (PAA) copolymers [150]. Such pH- and electric-sensitive hydrogels are suitable for use in sensors, actuators, switches, and drug delivery systems.
7.2.2.8. Contact lens
Application of PVP in contact lenses as a matrix component and/or as a wetting agent has been reported by multiple researchers [105]. Xue et al. have developed a novel PVP K30 coated olopatadine-ethyl cellulose microparticles-laden doughnut contact lens [151]. They aimed for sustained ocular drug delivery with limited alteration in the optical transmittance and swelling characteristics of the contact lens. Here PVP is treated as a comforting agent that has served the purpose of smooth presence (comfort wear), tear stabilization, and prolonged retention time in comparison to eye drops.
7.3. Gene delivery systems
Zhang et al. reported the use of PVP graft polymer in the development of bovine serum albumin-based gene delivery [152]. The charged reversal bovine serum albumin has shown to be having great potential for enhanced transfection efficiency of pDNA/poly(vinylpyrrolidone)-graft-poly(2-dimethylaminoethyl methacrylate) (pDNA/PVP-g-PDMAEMA). Zhi et al. have studied the effects of Graphene oxide (GO) with and without PVP on human immune cells like dendritic cells, T-lymphocytes, and macrophages [153]. The results declared that that PVP coated GO has shown better immunological compatibility with less immunogenicity and significantly delayed the apoptotic process of T-lymphocytes than the uncoated GO. A brief list of major pharmaceutical applications of PVP is given in Table 6 .
Table 6.
Major pharmaceutical applications of PVP [3].
7.4. Miscellaneous applications of PVP
7.4.1. Tailoring of plastic properties
Plastics are highly used in polymers for medical applications [154,155]. However, they suffer from the hydrophobic nature for their use in direct contact with blood, plasma, tissues, etc. Hence, plastics are hydrophilized with additives like PVP because of its amphiphilic nature.
7.4.2. Toxicity reduction
PVP due to its complex-forming nature reduces the irritancy levels or toxicity with numerous drugs like oxytetracycline on their parenteral administration [156]. PVP also reduces the irritation or toxicity of products (containing drugs like oxymetazoline, iodine) applied topically to skin or eye. PVP also reduces irritant or toxicity effects of certain other substances like nicotine, cyanide, formaldehyde, formamide, toxins, etc. because of its complexation property [3].
7.4.3. Cryoprotection
PVP acts as a cryoprotectant during the lyophilization process involving freeze-drying. It inhibits the crystallization of water and drugs. The cryoprotection property of PVP plays a major role in the biotechnology field than in drug manufacturing [3]. Thirumala et al. studied the application of PVP as a cryoprotectant for preserving the human adipose tissue-derived adult stem cells using liquid nitrogen. They have replaced dimethylsulfoxide with high molecular weight PVP as cryoprotectant [157].
7.4.4. Enzyme stabilization
By forming complexation with the enzyme deactivating moieties like phenols and tannins, PVP can play a role as an enzyme stabilizer [3]. This principle is useful in the stabilization of diagnostic reagents (rapid test kits) and microbiological processes. Zhang et al. has used PVP functionalized silicone particles for lipase immobilization to develop a low-cost process. The rational design is based on the interfacial activation and solution polymerization. The activity assay revealed that lipase immobilized with PVP functionalized silicone particles has exhibited higher catalysis activity and also better thermostability and reusability compared with hydrophilic pristine or hydrophobic polystyrene corded silicone particles [158]. Other examples include asparaginase, catalase, galactosidase, etc.
7.4.5. Drug stabilization
PVP is useful for the stabilization of drugs. Regulska et al. studied the application of PVP in the chemical stabilization of cilazapril in its solid-state. They have used PVP as a carrier and investigated the effect of PVP on the stability of cilazapril which is unstable in its solid-state and gets decomposed readily in a humid environment. The optimized formulation of PVP and cilazapril has shown reduced degradation of drugs with the higher energy of activation with less sensitivity to moisture. Hence, they concluded that PVP can be considered as a potential stabilizing agent for cilazapril containing dosage forms [159]. Shimpi et al. studied the application of PVP as a stabilizing agent for model drug celecoxib in comparison with Gelucire. They found that the formulations with PVP have shown significant stabilization of selected drugs than Gelucire. The reason for predominant stabilization is attributed to the formation of H-bonding [160]. Nielsen et al. have shown that the glass solution of the amorphous sodium salt of furosemide with PVP has shown good stability and also better oral bioavailability in rats compared with pure furosemide. The stabilizing effect of PVP on furosemide led to the enhanced relative oral bioavailability of up to 263% in rats compared with pure furosemide [161]. PVP also stabilizes several active substances in different dosage forms e.g., nitroglycerine, isosorbide dinitrate, ascorbic acid, interferon, iodine, theophylline, etc [3].
7.4.6. Complexation
PVP has more potential for forming complexes with drugs or other polymers which shows an influence on the solubility, dissolution, and stability of drugs or products [154,162,163]. The unique nature of PVP is the coexistence of hydrophilic and hydrophobic segments which allows complexation with a variety of compounds like low molecular weight substances and several polymers [164,165]. The binding mechanisms may include either of the interactions like hydrogen bonding, polar or hydrophobic attractive forces [166]. The increased potential of PVP for complexation is attributed to the ability of a polymer to form tertiary complexes. PVP forms complexes with tannins and polymethacrylic acids due to hydrogen bonding. An example of hydrophobic interactions is the complexation of PVP with phenols (e.g., resorcinol and pyrogallol) which also associates with the exothermic interaction between an electron system of aromatic substance and the lactam ring of PVP. An increase in aqueous solubility of a hydrophobic compound on the formation of non-covalent complexes with PVP is evident from fluoro substituted herbicide, substituted pyridine carboxylate herbicide and chlorofluoro alkyl-substituted biphenyl urea type insecticide. PVP forms an insoluble complex, with polyacids like polyacrylic acid or tannic acid through the cross-linking mechanism, either in water or alcohol but dissolve in dilute alkali. PVP forms a complex with iodine which has broad-spectrum antibacterial, antiseptic, bactericidal, fungicidal, and virucidal properties. Free iodine content in such complex products (PVP-iodine) is extremely low which offers non-irritating and mild antiseptic effect in contrast to aqueous iodine-potassium iodide solutions (Lugol's solution) or alcoholic solutions (iodine tincture) [1]. Aljamah et al. studied the effect of PVP-iodine pre-treatment for enhancement of rehardening (enamel remineralization) and fluoridating features of fluoride varnishes [167]. The use of PVP-iodine pre-treatment shown improved ability to prevent dental caries in vivo. Jalil et al. demonstrated that the thiolated PVP and PVP-iodine complexes have shown significant mucosal adhesion for a prolonged duration of 3 h in comparison with the PVP and PVP-iodine complexes [168]. Hence, they reported that the thiol functionalized PVP is a promising novel excipient suitable for complexation with iodine to exhibit strong mucoadhesive properties. Pinna et al. reported that the in vitro study revealed the antimicrobial activity of povidone-iodine 0.6% solution, IODIM and hence concluded that the IODIM solution can be used as a potential candidate for treating surface infections of the eye and also as antimicrobial prophylaxis before administration of intravitreal injections [169]. Povidone-iodine (complex form) has been claimed for infectious disease management, disease control, treatment of giant fornix syndrome, control of ocular infections, ear wash, and irrigating fluid for wound surgery. The complex form of PVP with iodine results in a gradual release of the potent antibacterial agent, iodine thereby reducing the toxicity of iodine [170].
7.4.7. Catalysis
PVP has been used as soluble polymer support to bind metal complexes, thus formed PVP bound metal acts as a catalyst (heterogenized homogeneous catalyst) [171]. The carbonyl oxygen moiety and the amide nitrogen moiety allow PVP to act as an efficient ligand for binding the monodentate and bidentate metal ions. The ligand-metal complex thus obtained is stable and acts as a soluble but recoverable catalyst in reactions.
7.4.8. Diagnosis
Penjweini et al. have studied the application of a photosensitizer, PVP-Hypericin in conventional photodynamic diagnosis and therapy with fluorescence imaging studies using living human lung epithelial cancer cells (A549) [172].
7.4.9. Other uses
PVP has also found application in DNA purification due to its polyphenol absorbing nature [173]. PVP is a component of Denhardt's buffer solution which is used for blocking the non-specific binding sites during microarray analysis, northern & southern blot analysis, and nucleic acid gel electrophoresis [174]. PVP has found application as an adhesive stick and as a remoistenable adhesive. High molecular weight PVP is also useful as a protective colloid and particle size regulator for suspension polymerization of vinyl chloride, styrene, and vinyl acetate. PVP is also used as a dye affinity stripping and leveling agent in textile dyeing [1].
8. Pharmaceutical applications of PVP derivatives
8.1. Applications of copovidone
Copovidone (vinylpyrrolidone-vinyl acetate copolymer), a more plastic and less hygroscopic copolymer, is also having a high binding capacity and hence used as a dry binder in direct compression technique. Copovidone is also used as a solubilizing agent, granulating agent, retardant, and film former. Wlodarski et al. studied the physicochemical properties of the tadalafil tablets using PVP-VA following direct compression of solid dispersions prepared by spray drying and ball milling techniques [175]. Both formulations have shown the greatest enhancement in the dissolution rate of tadalafil. Amorphous solid dispersions are used to enhance the mechanical and flow properties of poorly soluble drugs in addition to improvement in their dissolution rate. Ekdahl et al. developed an amorphous solid dispersion of felodipine in PVP-VA following spray drying atomization technique that produced 4 different powders with altered particle properties. They could produce the optimized amorphous powder of felodipine that shown easy flow, good mechanical strength, strong tablets, and other reasonable properties. Choi and Park have done in vivo studies in rats for evaluating the oral bioavailability of tadalafil solid dispersions prepared using PVP-VA S-630 [176]. The apparent solubility and dissolution rate of tadalafil in combination with weak acids and weak bases were also demonstrated and found to be increased in a great manner. The Cmax and AUC values for in vivo study has also shown a great increase in comparison to the commercial product, Cialis powder in rats. In situ amorphization of drugs like indomethacin and celecoxib using a microwave for the enhancement of dissolution were reported. The molecular weight of the polymer shows the influence on the amorphization and glass transition temperature. Siepmann et al.et al. developed matrix tablets of diprophylline using Kollidon SR (80% poly(vinyl acetate) (PVAc), 19% PVP, sodium lauryl sulfate (SLS) and colloidal silicon dioxide) [177]. Thorough in vitro characterization was done and developed a mechanistic realistic mathematical theory to predict the effects of tablet dimensions on drug release.
8.2. Applications of crospovidone
Cross-linked PVP also called crospovidone, is insoluble in water and highly compactable. Crospovidone is used as a super disintegrant in tablets and suppositories due to its high swelling properties, unlike soluble PVP [178,179]. Ludipress is a novel excipient which contains lactose (as filler/carrier), Kollidon K30 (binder, povidone) and Kollidon CL (super disintegrant, crospovidone) and hence, the manufacturer can simply mix the active ingredient with this Ludipress and lubricant, followed by direct compression which saves a lot of time and processing. Kasperek et al.et al. prepared and evaluated the modified release tablets of papaverine HCl using Kollidon 30 (PVP K30) as a binder, Kollidon SR as a sustained-release polymer and Kollidon CL (crospovidone) as disintegrant following direct compression method [71]. The results showed good control over the drug release with the optimized formulation.
9. PVP as an excipient in the formulation of cosmetics
PVP has a wide number of applications in cosmetics. It acts as a binder, film former, hair fixative, dispersing agent, surfactant, viscosity enhancer, and stabilizer in different cosmetic products [180]. Different cosmetic products that are prepared with PVP as an ingredient include shampoos, hair sprays, hair setting lotions, eye shadows, eyeliners, mascara, lipsticks, nail polishes, shaving creams, moisturizers, etc and its use in cosmetics is reported to be as safe [181].
10. Commercially available products
Several marketed pharmaceutical products are prepared with PVP in their formulation for some of the other purposes as discussed in previous sections. A list of commercially available products is given in Table 7 .
Table 7.
Some of the commercially available products formulated with PVP.
| S.No. | Product Name | Present as | Use | Manufacturer |
|---|---|---|---|---|
| 1 | Inadine ® | PVP-Iodine-PEG | Antiseptic, Surgical dressing non-adherent for open wounds for sustained release | Jhonson and Jhonson Medical ltd. |
| 2 | Betadine® | Povidone-Iodine (ointment, cream, spray, lotion, Gargle, Shampoo) | Antiseptic | Aviro Health L.P., G. S. Pharmbutor Pvt. Ltd., Win-Medicare Pvt. Ltd. |
| 3 | ScrubCare® | Aqueous-Iodophor | Antiseptic | Cardinal Health |
| 4 | Prevail-FX® | Alcohol-Iodophor | Antiseptic | Cardinal Health |
| 5 | DuraPrep® | Alcohol-Iodophor | Antiseptic | 3 M Health Care |
| 6 | Soothe® Hydration Lubricant Eye Drops | Povidone | Lubricant | Bausch + Lomb Inc |
| 7 | Advanced Relief Eye Drops® | Povidone | Lubricant | Winco Foods LLC |
| 8 | Refresh Clasic Eye Drops® | Povidone | Lubricant | Allergan Inc. |
| 9 | Povin Solution® | Povidone-Iodine | Antiseptic | Opsonin-Pharma Ltd. |
| PVP-Iodine Antiseptic Swabs® | PVP-Iodine | Antiseptic | Acme United Corp. | |
| 10 | TRESemme® | PVP | Body and Hair Conditioner | Unilever |
| 11 | Denhardt's solution® | PVP | Microarray Analysis, Nucleic Acid Gel Electrophoresis & Blotting | Thermofisher Scientific |
11. Regulatory status
PVP has been recognized by many countries as an essentially nontoxic excipient (non-medicinal ingredient) and food-grade additive [9]. PVP is approved by the FDA [182]. PVP is officially approved in many countries like the USA, Germany, China, France, Mexico, India, Great Britain, Spain, Japan, Australia, Austria, Italy, Netherlands, etc. It is mentioned in the monographs of several Pharmacopoeias like United States Pharmacopoeia, European Pharmacopoeia, British Pharmacopoeia, Japanese Pharmacopoeia, etc. [3].
12. Patents
There are several patents based on the application of PVP in pharmaceutical products. A list of patent prospection in this decade are presented in Table 8 .
Table 8.
Patents related to the application of PVP in Pharmaceuticals.
| Patent No. | Title/ | Assignee | Inventor(s) | Patent/Publication Date | PVP grade used | Purpose |
|---|---|---|---|---|---|---|
| US 10406105B2 | Pharmaceutical formulation for the production of rapidly disintegrating tablets | BASF SE (Ludwigshafen, DE) | Karl K, Michael S, Silke G, Kathrin MB, Angelika M. |
10-09-2019 | Kollidon CL-SF | Super disintegrant |
| Kollidon 30 | Binder | |||||
| Kollidon VA 64 | Binder | |||||
| Kollicoat SR 30 D | Dispersing agent | |||||
| EP 2001450B1 | Directly compressible composite for orally disintegrating tablets | Rubicon Research Private Limited (Mumbai, IN) | Pilgaonkar PS, Rustomjee MT, Gandhi AS, Bagde P, Morvekar HN. |
30-01-2019 | Crospovidone | Disintegrant |
| US 20180036245A1 | Solid dispersions | Shionogi Inc. (Florham Park, NJ, US) | Zhengming C, Xiaoming C, Kevin H. |
08-02-2018 | Kollidon 30 (PVP K30), Kollidon VA64 (PVP VA 64) |
Hydrophilic carriers |
| WO 2018102526A1 | Pharmaceutical dosage form | Glaxosmithkline LLC (Wilmington, DE, US) | Burke MD, Goodwin D, Govindarajan R, Harridance A, Kedia S, Le Q, Mcaleese P, Westrup J. |
07-06-2018 | Povidone, Copovidone | Solubilizing agents |
| Crospovidone | Disintegrant | |||||
| US 9623010B2 | Orodispersible tablets | Laboratorios Lesvi, S.L. (Barcelona, ES) | Carmen UP, Ignacio DM, Pablo PA. |
18-04-2017 | Crospovidone | Disintegrant |
| US 20170224624A1 | Formulations of enzalutamide | Bend Research, Inc. (Bend, OR, US), Medivation Prostate Therapeutics, Inc. (San Francisco, CA, US), Astellas Pharma Inc. (Tokyo JP) |
Douglas AL, Sanjay K, Randy JW, Jason AE, Sheila M, Yuuki T, Toshiro S, Ryousuke I, Shinsuke O, Hiroyasu T, Koji N, Atsushi K. |
10-08-2017 | Povidone, Copovidone | Solubilizing agents |
| EP 2283824B1 | Compositions and formulations based on swellable matrices for sustained release of poorly soluble drugs such as clarithromycin | Special Product's Line S.p.A. (Pomezia, IT), So. Se. Pharm S.r.l. (Pomezia, IT) |
Camponeschi C, Colombo P |
19-04-2017 | PVP K30 | Binder |
| US 9629841B2 | Formulations of pyrimidinedione derivative compounds | AbbVie Inc. (North Chicago, IL, US) | Yanxia L, Ping G, Yi S, Geoff GZ, Yi G, Jianwei W |
25-04-2017 | Kollidon VA 64, Kollidon VA 64 fine | Stabilizing polymer, Bioavailability enhancer |
| US 20160101103A1 | Method for the production of a medicament containing tadalafil | Ratiopharm GmbH. (Ulm, DE) | Rainer A, Julia SN, Katrin R. |
14-04-2016 | Copovidone | Solid solvent |
| US 9308164B2 | Hyoscyamine dosage form | Sovereign Pharmaceuticals, LLC. (Fort Worth, TX, US) |
Viswanathan S, Ralph B, David B, Himanshu P, Juan CM, Somphet PS |
12-04-2016 | Kollidon SR | Sustained release polymer |
| US 20160193153A1 | Tablet comprising crospovidone | DSM Sinochem Pharmaceuticals (Netherlands B.V., Delft, NL) | Abraham CL | 07-07-2016 | Crospovidone | Disintegrant |
| US 20160000732A1 | Oral pharmaceutical compositions of ospemifene | Cadila Healthcare Limited (Ahmedabad, IN) | Sushrut KK, Pavak RM, Ritesh K |
07-01-2016 | Crospovidone | Disintegrant |
| US 20150011525A1 | Solid dispersion of poorly soluble compounds comprising crospovidone and at least one water-soluble polymer | ISP Investments Inc. (Wilmington, NJ, US) | Yunxia B, Mohammed AR, James DL |
08-01-2015 | Crospovidone | Disintegrant, Bioavailability enhance, Crystallization inhibitor |
| US 8945620B2 | Solid pharmaceutical compositions containing pregabalin | Warner-Lambert Company LLC. (New York, NY, US) |
Howard NB, Yun HC, Steven DS, Majid M, Thomas DR, Pushpa GS, Zezhi JS, Jiansheng W |
03-02-2015 | Kollidon SR | Matrix forming agent, Swelling agent, Sustained release polymer |
| WO 2015107536A2 | Fixed dose combination comprising linagliptin and metformin HCl | Intas Pharmaceuticals Limited (Gujarat, IN) | Priyank P, Mayur P, Mahendra P, Ashish S |
23-07-2015 | Copovidone | Binder |
| US 8691878B2 | Solid pharmaceutical dosage form | AbbVie Inc. (North Chicago, IL, US) | Jeorg R, Ulrich R, Bernd L, Gunther B, Joerg B, Laman A, Soumojeet G. |
08-04-2014 | Copovidone | Bioavailability enhancer |
| US 8691872B2 | Dispersions of rasagiline citrate | Teva Pharmaceutical Industries Ltd. (Petach-Tikva, IL) | Keith L, David E. |
08-04-2014 | PVP K29/32, PVP K90, PVP VA | Solubilizer |
| US 20140221414A1 | Solid dispersion of rifaximin | Lupin Limited (Mumbai, IN) | Shirishkumar K, Satish KD, Harshal AJ. |
07-08-2014 | Povidone, Copovidone | Solubilizing agents, Hydrophilic carriers |
| US 8920837B2 | Sustained release dosage form | Rubicon Research Private Limited (Mumbai, IN) | Pratibha SP, Maharukh TR, Anilkumar SG, Paras RJ, Atul AK. |
30-12-2014 | Kollidon SR | Sustained release polymer |
| Kollidon VA 64 | Binder | |||||
| US 8715729B2 | Rapidly disintegrating, solid coated dosage form | BASF SE (Ludwigshafen, DE) | Karl K, Silke G, Yoshitaka K. |
06-05-2014 | Kollidon CL-SF | Super disintegrant |
| US 8568780B2 | Pharmaceutical formulation for the production of rapidly disintegrating tablets | BASF SE (Ludwigshafen, DE) | Karl K, Michael S, Silke G, Kathrin MB, Angelika M. |
29-10-2013 | Kollidon CL-SF | Disintegrant |
| US 20110262533A1 | Pharmaceutical formulation for use in HIV therapy | – | Kiran KNV, Sanjay DV, Akhilesh AD, Abhijit MD, Sanjeev MS |
05-02-2013 | Copovidone | Binder |
| WO 2013040187A1 | Solid dispersion of poorly soluble compounds comprising crospovidone and at least one water-soluble polymer | ISP Investments Inc. (Wilmington, DE, US) | Yunxia BL, Rahman MA, Lester JD. |
21-03-2013 | Crospovidone | Disintegrant |
| US 8574625B2 | Tablet dosage form | Wockhardt Ltd. (Bandra East, Mumbai, IN) | Girish KJ, Ramakant G, Rahul D. |
05-11-2013 | Crospovidone | Disintegrant |
| US 20110028456A1 | Solid pharmaceutical dosage form | Cipla Limited (Mumbai, IN) | Amar L, Geena M. |
03-02-2011 | Kollidon VA 64 | Dissolution enhancer |
| WO 2011125075A2 | A novel gastroretentive delivery of macrolide | FDP Limited (Mumbai, IN) | Chandavarkar NM, Jindal KC, Malayandi R. |
13-10-2011 | Crospovidone | Swelling agent, Hydrophilic polymer |
| US 7838033B2 | Composition for rapid disintegrating tablet in oral cavity | Fuji Chemical Industry Co. Ltd. (Nakaniikawa-gun, JP) | Nobukazu T, Yoshiro N, Hiroshi K, Tadashi F, Terumasa H. |
23-11-2010 | Crospovidone | Disintegrant |
13. Limitations
Even though PVP has been identified as a popular pharmaceutical excipient, food additive, and cosmetic ingredient, it has its disadvantages as limitations for its usage at certain levels. PVP or its derivatives have been claimed for certain allergic reactions [33,[183], [184], [185]], storage disease, subcutaneous granulomas, pulmonary vascularization, and reticuloendothelial system (RES) deposition [50,186]. Some of them are overruled and some are given cautions to overcome them. Avoiding repeated dosing, high molecular weight, a large amount of PVP at the same site of administration can be a remedy to avoid certain such problems. An important disadvantage of PVP is its high hygroscopic nature which made it tough to store and handle. The influence of hygroscopicity on the tackiness of PVP film can be avoided by adding acetylated monoglycerides to the coating mixture [187]. PVP has a major limitation for parenteral administration due to its non-biodegradability. Only low molecular weight pyrogen-free polymers of PVP are suitable for parenteral administration as they are only able to get excreted through kidney [50].
13.1. Current research on the exploration of povidone-iodine against COVID-19
A novel coronavirus, SARS-CoV-2, the causative agent was responsible for Coronavirus disease 2019 (COVID-19) outbreak in Wuhan, China in late 2019. Declaring a public health emergency by the world health organization (WHO) had directed preventive measures since there is no treatment. Povidone-iodine (PVP–I) was indicated as first-line preventive aid that is a topical broad-spectrum antiseptic that is reported for antibacterial, antiviral, and antifungal properties from the past 150 years against COVID-19. Therefore, in this review, we outlined various studies reported on the assessment of an antiviral efficacy of PVP-I. Further, the potential use of PVP-I and prophylaxis on nasopharyngeal and oropharyngeal mucosa of COVID-19 patients, healthcare professional, and community as an ancillary measure to diminish the pandemic is also described in Table 9 . The current ongoing clinical trials demonstrating the benefits of PVP-I as intranasal and gargling use against COVID-19 patients are detailed in Table 10 [188].
Table 9.
Various studies and clinical trials reported on the use of Povidone-iodine (PVP–I) against COVID-19.
| S.No | Type of study | Indication | Application and clinical intervention | References |
|---|---|---|---|---|
| 1. | Povidone-iodine (PVP–I) | PVP-I mouth wash (1:30 dilution) | Inactivate both SARS-CoV and MERSCoV following a 15- second exposure | [189] |
| 2. | Povidone-iodine (PVP–I) novel intervention strategy | Dilute centrations (e.g. 0.001%) 1.Nasalirrigation: 240 mL of 0.4% PVP-Isolution (dilution of 10 mL of commercially available 10% aqueous PVP-I into 240 mL of normal saline with a sinus rinse delivery bottle. 2.Oral/or-opharyngeal wash:10 mL of 0.5% aqueous PVP-Isolution (1:20 di-lution in sterile or distilled water); in addition to appropriate PPE. The following outlines a stratified treatment.approach: 1. Apply nasal and oral PVP-I every 2–3 h, upto 4 × /day inpatients that: a. Have suspected/confirmed SARS-CoV 2 infection. b. Are undergoing high-risk procedures (e.g.those involving nasal mucosal, oral, pharyngeal, andpulmonarysecretions) c. Are from COVID-19 hotspots 2.Apply nasal and oral PVP-I before and after patient contact (with repeated contact, apply every 2–3 h, upto4 × /day) in healthcare providers that: i. Are involved in care of patients with suspected/confirmed SARS-CoV-2 infection ii. Are involved in high-risk procedures of patients in COVID-19 hotspots c.Lack adequate PPE (e.g.N95,PAPR) iii.Optional nasal and oral application of PVP-I every 2–3 h, up to 4 × /day inpatients and/or healthcare providers in: •High-risk procedures in asymptomatic patients •COVID-19 hotspots |
Opical nasal and oral solution. Topical applications of PVP-I to attenuate nosocomial transmission of COVID-19 surrounding head and neck and skull base oncology care. |
[190] |
| 3. | Povidone-iodine (PVP–I) in-vitro efficacy | Different formulations of povidone-iodine (PVP–I: 4% PVP-I skin cleanser, 7.5% PVP-I surgical scrub, and 1% PVP-I gargle/mouthwash) against a reference virus (Modified vaccinia virus Ankara, MVA) and MERS-CoV was evaluated. | A reduction in virus titer of C4 log10 (corresponding to inactivation of C99.99%) was regarded as evidence of virucidal activity. This was achieved versus MVA and MERS-CoV, under both clean and dirty conditions, within 15 s of application of each undiluted PVP-I product. Data indicated that PVP-I-based hand wash products for potentially contaminated skin, and PVP-I gargle/mouthwash for reduction of viral load in the oral cavity and the oropharynx, may help to support hygiene measures to prevent transmission of MERS-CoV. |
[191] |
| 4. | In-vitro bactericidal and virucidal efficacy of povidone-iodine (PVP–I) against oral and respiratory tract pathogens | 7% gargle/mouthwash | PVP-I gargle/mouthwash diluted 1:30 (equivalent to a concentration of 0.23% PVP-I) showed effective bactericidal activity against Klebsiella pneumoniae and Streptococcus pneumoniae and rapidly inactivated SARS-CoV, MERS-CoV, influenza virus A (H1N1) and rotavirus after 15 s of exposure. PVP-I 7% gargle/mouthwash showed rapid bactericidal activity and virucidal efficacy in vitro at a concentration of 0.23% PVP-I and may provide a protective oropharyngeal hygiene measure for individuals at high risk of exposure to oral and respiratory pathogens. |
[189] |
| 5. | Preoperative povidone-iodine (PVP–I) protocol for lacrimal surgies | A drop of 1% PVP-I | Administrated in a conjunctival cul-de-sac with contact time for 3 min against COVID-19. | [192] |
| 6. | Povidone-iodine (PVP–I) | 0.23%–7% solution | Topical preparations exhibited virucidal activity | [193] |
| 7. | Povidone-iodine (PVP–I) | 0.5%, 1%, and 1.5% | Oral antiseptics showed complete inactivation of SARS-CoV-2 within 15 s of contact. | [194] |
| 8. | Povidone-iodine (PVP–I) | 1% eye drop | Locally (eye drop) effective in case of accidental ocular exposure or case of 2019-n CoV-associated conjunctivitis. | [195] |
| 9. | Povidone-iodine In-situ Gel forming formulations. | PVP-I gel-forming nasal spray (IVIEW-1503) and PVP-I gel-forming ophthalmic eye drop (IVIEW-1201) | Formulations can reduce or prevent the transmission of the virus through the nasal cavity and the eye. | [196] |
| 10. | Povidone-Iodine prosthodontic practice | dilution of the commercially available 10% povidone-iodine by 1:20 utilizing 0.5 cc of 10% povidone iodine and 9.5 cc of sterile saline dilution of the commercially available 10% povidone iodine by 1:20 utilizing 0.5 cc of 10% povidone iodine and 9.5 cc of sterile saline dilution of the commercially available 10% povidone iodine by 1:20 utilizin 0.5% oral wash |
oral rinse to decrease the risk of transmission associated with viral shedding from asymptomatic individuals Oral rinse can decrease the risk of transmission associated with viral shedding from asymptomatic individuals. |
[197] |
| 11. | Povidone-iodine (PVP–I) topical and oral | antiseptic solution (PVP–I 10%), skin cleanser (PVP–I 7.5%), gargle and mouth wash (PVP–I 1%) and throat spray (PVP–I 0.45%) | achieved ≥ 99.99% virucidal activity against SARS-CoV-2, corresponding to ≥ 4 log10 reduction of virus titre, within 30 s of contact | [198] |
| 12. | Povidone-iodine (PVP–I) vs. hydrogen peroxide | PVP-I oral antiseptic rinse at all 3 concentrations of 0.5%, 1.25%, and 1.5% completely inactivated SARS-CoV-2 PVP-I oral antiseptic rinse at all 3 concentrations of 0.5%, 1.25%, and 1.5% completely inactivated SARS-CoV-2 PVP-I oral antiseptic rinse at all 3 concentrations of 0.5%, 1.25%, and 1.5% and hydrogen peroxide solutions at concentrations of 1.5% and 3.0%. |
After the 15-s and 30-s contact times, PVP-I oral antiseptic rinse at all 3 concentrations of 0.5%, 1.25%, and 1.5% completely inactivated SARS-CoV-2. The H2 O2 solutions at concentrations of 1.5% and 3.0% showed minimal viricidal activity after 15 s and 30 s of contact time. | [199] |
Table 10.
Current ongoing clinical trials demonstrating the benefits of Povidone-Iodine (PVP–I) as intranasal and gargling use against COVID-19 patients [188].
| NCT Number | Study title | Study design | Intervention | Location |
|---|---|---|---|---|
| NCT04364802 | COVID-19: Povidone-Iodine Intranasal Prophylaxis in Front-line Healthcare Personnel and Inpatients | Phase 2; 250 participants, nonrandomized |
Povidone-Iodine nasal spray and gargle. | University of Kentucky Lexington, Kentucky, United States. |
| NCT04347954 | PVP-I Nasal Sprays and SARS-CoV-2 Nasopharyngeal Titers (for COVID-19) | Phase 1/2; 45 participants; randomized |
Povidone-Iodine 2%. Povidone-Iodine 0.5%. Isotonic saline 0.9%. |
Stanford Health Care Stanford, California, United States. |
| NCT04344236 | Gargling and Nasal Rinses to Reduce Oro- and Nasopharyngeal Viral Load in Patients With COVID-19 | Phase 2; 48 participants; randomized. |
Saline oral/nasal rinse. 0.5% Povidone/Iodine oral/nasal rinse. 0.12% Chlorhexidine oral/nasal rinse. |
NYU Langone Health New York, New York, United States. |
| NCT04371965 | Povidone Iodine Mouthwash, Gargle, and Nasal Spray to Reduce Naso- Pharyngeal Viral Load in Patients With COVID-19 (KILLER) | Phase 2; 24 participants; randomized |
Parallel Assignment, Povidone-Iodine |
Poitiers University Hospital, France |
| NCT04410159 | Povidone-Iodine Vs Essential Oil Vs Tap Water Gargling For COVID-19 Patients (GARGLES) | Phase 2; 20 participants; randomized |
Povidone-Iodine Essential oils Tap water |
Universiti Sains Islam Malaysia |
| NCT04449965 | Povidone-Iodine Rinses in the Management of COVID-19 | Early Phase 1; 81 participants; randomized |
Povidone-Iodine Placebo |
St. Paul's Hospital, Canada |
| NCT04393792 | SINUS WASH Pilot Study in Adults Testing Positive for COVID-19 | Early Phase 1; 40 participants; randomized |
Povidone-Iodine Normal saline |
Hampshire Hospitals NHS Foundation trust, United kingdom |
| NCT04446104 | A Preventive Treatment for Migrant Workers at High-risk of Covid-19 | Phase 3; 5000 participants; randomized |
Povidone-Iodine Hydroxychloroquine Sulfate Tablets Ivermectin 3 mg tablet Zinc Vitamin C |
National University Hospital, Singapore. |
| NCT04341688 | A Clinical Trial of Gargling Agents in Reducing Intraoral Viral Load Among COVID-19 Patients (GARGLES) | Phase not applicable; 50 participants; randomized |
Gargle/mouthwash | University of Karachi, Pakistan |
Apart from exploring the PVP-I as a preventive aid against COVID-19, it is even important to notice the minimal side effects or rare adverse effects by prolonged use [200]. PVP-I is reported for allergic reactions and should not be used in patients suffering from thyroid diseases [201]. Studies even indicated that prolonged use of topical 10% PVP-I solution (weeks-months) may increase the risk of iodine toxicity [202]. Kim J.H. et al. reported a ciliotoxic effect on ciliated human respiratory cells on longer-term use of betadine that indicates the cautionary use [203]. Previous studies even conducted investigations in understanding the allergy, contact sensitivity, and skin reactions associated with the use of PVP-I [203]. As per current studies, there is a scope for a lot more research in the area of PVP I against COVID-19. The researchers have challenges to combat the limitations of PVP I and have the necessity to focus on developing delivery strategies minimizing the side effects.
14. Challenges, outlook, and future perspectives
In the current scrutiny, synthesis, grades, properties, and applications of PVP as a pharmaceutical excipient are discussed. PVP is FDA approved and extensively used in the formulation of conventional to controlled drug delivery systems by pharmaceutical industries [50]. PVP use as a synthetic polymer excipient in developing a wide range of novel drug delivery systems has been widely researched, as backed by the available literature. Further studies are required to assess and safely regulate the use of PVP in designing especially programmable or modified delivery systems intended for oral delivery [3]. For instance, PVP use can be limited and require special handling for being hygroscopic containing 18% moisture at 50% RH [33]. Cross-povidone is also reported for non-biodegradable behaviour reporting allergic manifestations and even may cause pulmonary vascular injury [186]. PVP not just has wide applications in pharmaceutical but also in food and cosmetic industries. As a solubilizer, stabilizer in suspensions, emulsions, creams of photoresist capacity, ophthalmic formulations, and many more [154]. The uncountable properties of PVP create the scope and advance more applications in other sectors as well. PVP is also being explored for its use in dental products, dietary supplements, herbal products, and functional foods. PVP is cost-effective if used in available modified grades instead of synthesis from start. New futuristic grades are being identified and patent-protected opening spell to the generic industry. The novel grades need to be studied in detail with supporting toxicological profiling before use [50]. Overall PVP is a multifaced, long credited, and well-ensconced excipient in the pharmaceutical industry and enrooting its way in broad novel drug delivery systems providing new paradigm in chronic therapies [154]. Molecular conjugation for production of co-processed PVP based excipients with exceptional functionality is yet another boulevard.
15. Conclusion
Based on the current literature survey, PVP is considered a widely used excipient for pharmaceutical, food, and cosmetic industry. PVP as a polymer has a profound effect on solving many drug-related issues that could not be construed by modifying the chemistry or formulation approaches. The biodegradable character, strong stability, mild physiochemical properties, ability to solubilize, being non-toxic, and capacity to deliver both hydrophilic and lipophilic drugs make the PVP a versatile excipient in formulation development of wide conventional to the controlled release dosage forms. PVP-I has proven its potential as preventive and prophylactic aid against COVID-19 during the pandemic time. Futuristic grades of PVP having multifunctional properties are pipelined and yet to be analysed for their wide applications in diverse fields such as medical devices, gene therapy, and tissue engineering.
Funding
None.
CRediT authorship contribution statement
Mallesh Kurakula: Investigation, Data curation, Writing - original draft, Visualization, Conceptualization, Resources, Writing - review & editing, Supervision, Project administration. G.S.N. Koteswara Rao: Validation, Methodology, Resources, Writing - review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Haaf F., Sanner A., Straub F. Polymers of n-vinylpyrrolidone: synthesis, characterization and uses. Polym. J. 1985;17:143–152. doi: 10.1295/polymj.17.143. [DOI] [Google Scholar]
- 2.Koczkur K.M., Mourdikoudis S., Polavarapu L., Skrabalak S.E., Koczkur K.M., Mourdikoudis S., Polavarapu L., Skrabalak S.E., Pvp P., Chem R.S. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. R. Soc. Chem. 2015;44:17883–17905. doi: 10.1039/c5dt02964c. [DOI] [PubMed] [Google Scholar]
- 3.Bühler Volker. Springer-Verlag; Berlin Heidelberg, New York: 2005. Polyvinylpyrrolidone – Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone, Illustrate. [DOI] [Google Scholar]
- 4.Kurakula Mallesh, Koteswara Rao G.S.N. Moving polyvinyl pyrrolidone electrospun nanofibers and bioprinted scaffolds toward multidisciplinary biomedical applications. European Polymer Journal. 2020;136 doi: 10.1016/j.eurpolymj.2020.109919. [DOI] [Google Scholar]
- 5.Panda H. 3rd Revise. vol. 23. Asia Pacific Business Press Inc.; New Delhi: 2015. https://www.niir.org/books/book/herbal-cosmetics-handbook-3rd-revised-edition/isbn-9788178330808/zb (Herbal Cosmetics Handbook). a,2c,0,a/index.html. [Google Scholar]
- 6.Jun Y.B., Min B.H., Kim S.I., Kim Y.I.J. Preparation and evaluation of acetaminophen tablets, kor. Pharmaceut. Sci. 1989;19:123–128. [Google Scholar]
- 7.Teodorescu M., Bercea M., Morariu S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: perspectives and challenges. Biotechnol. Adv. 2019;37:109–131. doi: 10.1016/j.biotechadv.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 8.WHO Technical Report Series 751, FAO/WHO Report No. 30. 1987. [Google Scholar]
- 9.Rowe R.C., Sheskey P.J., Quinn M.E., editors. Handbook of Pharmaceutical Excipients. sixth ed. Pharmaceutical Press; Grayslake, IL, USA: 2009. [Google Scholar]
- 10.Budavari S., editor. The Merck Index - an Encyclopedia of Chemicals, Drugs, and Biologicals. Merck and Co., Inc.; Whitehouse Station, NJ: 1996. [Google Scholar]
- 11.Corporation B.A.S.F. Soluble Polyvinylpyrrolidone for the Pharmaceutical Industry; 1997. Technical Literature: Soluble Kollidon Grades. [Google Scholar]
- 12.Ziaei-Azad H., Semagina N. Bimetallic catalysts: requirements for stabilizing PVP removal depend on the surface composition. Appl. Catal. Gen. 2014;482:327–335. doi: 10.1016/j.apcata.2014.06.016. [DOI] [Google Scholar]
- 13.Graf C., Dembski S., Hofmann A., Rühl E. A general method for the controlled embedding of nanoparticles in silica colloids. Langmuir. 2006;22:5604–5610. doi: 10.1021/la060136w. [DOI] [PubMed] [Google Scholar]
- 14.Si R., Zhang Y.W., You L.P., Yan C.H. Self-organized monolayer of nanosized ceria colloids stabilized by poly(vinylpyrrolidone) J. Phys. Chem. B. 2006;110:5994–6000. doi: 10.1021/jp057501x. [DOI] [PubMed] [Google Scholar]
- 15.Food Chemicals Codex. sixth ed. United States Pharmacopeia; Bethesda, MD: 2008. [Google Scholar]
- 16.Teng J., Bates S., Engers D.A., Leach K., Schields P., Yang Y. Effect of water vapor sorption on local structure of poly(vinylpyrrolidone) J. Pharmaceut. Sci. 2010;99:3815–3825. doi: 10.1002/jps.22204. [DOI] [PubMed] [Google Scholar]
- 17.JRS Pharma . Vivapharm PVP/VA; 2020. Technical Literature. [Google Scholar]
- 18.JRS Pharma . Vivapharm PVPP; 2020. Technical Literature. [Google Scholar]
- 19.Ashland . 2019. Technical Literature: AgrimerTM Polyvinylpyyrolidone (PVP) [Google Scholar]
- 20.JRS Pharma . Vivapharm PVP; 2020. Technical Literature. [Google Scholar]
- 21.Shah U., Augsburger L. Evaluation of the functional equivalence of crospovidone NF from different sources. I. Physical characterization. Pharmaceut. Dev. Technol. 2001;6:39–51. doi: 10.1081/PDT-100000012. [DOI] [PubMed] [Google Scholar]
- 22.Povidone . 2019. USP Stage 4 Harmonization. [Google Scholar]
- 23.Fikentscher V.H. Systematics of celluloses based on their viscosity in solution. Cellulose. 1932;13(58–64):71–74. [Google Scholar]
- 24.Vieweg R., Reiher M., Scheurlen H., Kunstsoff-Handbunch, XI Bd. Wiley, Carl-Hanser-Verlag; Miinchen: 1972. Polyacetale, Epoxidharze, Fluor-Haltige Polymerisate, Silicone Usw. [DOI] [Google Scholar]
- 25.Ernst B., Ernst B. vol. 19. Auflage, Verlag Chemie; Weinheim, Band: 1980. (Ullmanns Encyclopädie der technischen Chemie, 4). [Google Scholar]
- 26.Kauffmann H.F., Breitenbach J.W. Angew, No title, makromol. 1975;45:167–175. Ch. [Google Scholar]
- 27.Breitenbach J.W. Makromol. Ch. Budapest; 1967. IUPAC Symposium; pp. 529–544. [Google Scholar]
- 28.Breitenbach J.W., Kauffmann H.F., Zwilling G. No title, makromol. 1976;177:2787–2792. Ch. [Google Scholar]
- 29.Liu Z., Rimmer S. Studies on the free radical polymerization of N-vinylpyrrolidinone in 3-methylbutan-2-one. Macromolecules. 2002;35:1200–1207. doi: 10.1021/ma011092v. [DOI] [Google Scholar]
- 30.Luo L., Ranger M., Lessard D.G., Le Garrec D., Gori S., Leroux J.C., Rimmer S., Smith D. Novel amphiphilic diblock copolymer of low molecular weight poly(N-vinylpyrrolidone)-block-poly(D,L-lactide): synthesis, characterization, and micellization. Macromolecules. 2004;37:4008–4013. doi: 10.1021/ma035910q. [DOI] [Google Scholar]
- 31.Yamago S., Ray B., Iida K., Yoshida J.I., Tada T., Yoshizawa K., Kwak Y., Goto A., Fukuda T. Highly versatile organostibine mediators for living radical polymerization. J. Am. Chem. Soc. 2004;126:13908–13909. doi: 10.1021/ja044787v. [DOI] [PubMed] [Google Scholar]
- 32.Lu X., Gong S., Meng L., Li C., Yang S., Zhang L. Controllable synthesis of poly(N-vinylpyrrolidone) and its block copolymers by atom transfer radical polymerization. Polymer. 2007;48:2835–2842. doi: 10.1016/j.polymer.2007.03.048. [DOI] [Google Scholar]
- 33.Awasthi R., Manchanda S., Das P., Velu V., Malipeddi H., Pabreja K., Pinto T.D.J.A., Gupta G., Dua K. Eng. Biomater. Drug Deliv. Syst. Beyond Polyethyl. Glycol. Elsevier Inc.; 2018. Poly(vinylpyrrolidone) pp. 255–272. [DOI] [Google Scholar]
- 34.Chiefari J., Chong Y.K., Ercole F., Krstina J., Jeffery J., Le T.P.T., Mayadunne R.T.A., Meijs G.F., Moad C.L., Moad G., Rizzardo E., Thang S.H. 1998. Living Free-Radical Polymerization by Reversible Addition-Fragmentation Chain Transfer: the RAFT Process. [Google Scholar]
- 35.Gaynor S.G., Matyjaszewski K. Step-growth polymers as macroinitators for “living” radical polymerization: synthesis of ABA block copolymers. Macromolecules. 1997;30:4241–4243. doi: 10.1021/ma970358o. [DOI] [Google Scholar]
- 36.Matyjaszewski K., Tsarevsky N.V., Braunecker W.A., Dong H., Huang J., Jakubowski W., Kwak Y., Nicolay R., Tang W., Yoon J.A. Role of Cu 0 in controlled/"living" radical polymerization. Macromolecules. 2007;40:7795–7806. doi: 10.1021/ma0717800. [DOI] [Google Scholar]
- 37.Percec V., Guliashvili T., Ladislaw J.S., Wistrand A., Stjerndahl A., Sienkowska M.J., Monteiro M.J., Sahoo S. Ultrafast synthesis of ultrahigh molar mass polymers by metal-catalyzed living radical polymerization of acrylates, methacrylates, and vinyl chloride mediated by SET at 25 °C. J. Am. Chem. Soc. 2006;128:14156–14165. doi: 10.1021/ja065484z. [DOI] [PubMed] [Google Scholar]
- 38.Rosen B.M., Percec V. Single-electron transfer and single-electron transfer degenerative chain transfer living radical polymerization. Chem. Rev. 2009;109:5069–5119. doi: 10.1021/cr900024j. [DOI] [PubMed] [Google Scholar]
- 39.Ray B., Kotani M., Yamago S. Highly controlled synthesis of poly(N-vinylpyrrolidone) and its block copolymers by organostibine-mediated living radical polymerization. Macromolecules. 2006;39:5259–5265. doi: 10.1021/ma060248u. [DOI] [Google Scholar]
- 40.Yusa S.I., Yamago S., Sugahara M., Morikawa S., Yamamoto T., Morishima Y. Thermo-responsive diblock copolymers of poly(N-isopropylacrylamide) and poly(N-vinyl-2-pyrroridone) synthesized via organotellurium-mediated controlled radical polymerization (TERP) Macromolecules. 2007;40:5907–5915. doi: 10.1021/ma070769x. [DOI] [Google Scholar]
- 41.Yamago S., Kayahara E., Kotani M., Ray B., Kwak Y., Goto A., Fukuda T. Highly controlled living radical polymerization through dual activation of organobismuthines. Angew. Chem. Int. Ed. 2007;46:1304–1306. doi: 10.1002/anie.200604473. [DOI] [PubMed] [Google Scholar]
- 42.De Farias M.A., Do Carmo Gonçalves M. Synthesis and applications of polystyrene-block-poly(N-vinyl-2-pyrrolidone) copolymers. Polimeros. 2016;26:1–10. doi: 10.1590/0104-1428.2066. [DOI] [Google Scholar]
- 43.V. Coessens, T. Pintauer, K. Matyjaszewski, Functional Polymers by Atom Transfer Radical Polymerization, n.D.
- 44.Benaglia M., Chiefari J., Chong Y.K., Moad G., Rizzardo E., Thang S.H. Universal (switchable) RAFT agents. J. Am. Chem. Soc. 2009;131:6914–6915. doi: 10.1021/ja901955n. [DOI] [PubMed] [Google Scholar]
- 45.Liu Q., Wu H., Zhang L., Zhou Y., Zhang W., Pan X., Zhang Z., Zhu X. RAFT polymerization of N-vinylpyrrolidone mediated by cyanoprop-2-yl-1-dithionaphthalate in the presence of a fluoroalcohol: the possibility of altering monomer properties by hydrogen bonding? Polym. Chem. 2016;7:2015–2021. doi: 10.1039/c5py02047f. [DOI] [Google Scholar]
- 46.Kreft K., Kozamernik B., Urleb U. Qualitative determination of polyvinylpyrrolidone type by near-infrared spectrometry. Int. J. Pharm. 1999;177:1–6. doi: 10.1016/s0378-5173(98)00265-8. [DOI] [PubMed] [Google Scholar]
- 47.Müller K. [Identification and determination of polyvinylpyrrolidone(PVP) as well as determination of active substances in PVP-containing drug preparations] Pharm. Acta Helv. 1968;43:107–122. http://www.ncbi.nlm.nih.gov/pubmed/5720914 accessed March 14, 2020. [PubMed] [Google Scholar]
- 48.Frauenfelder L.J. Universal chromatographic-colorimetric method for the determination of trace amounts of polyvinylpyrrolidone and its copolymers in foods, beverages, laundry products, and cosmetics. J. AOAC Int. 1974;57:796–800. doi: 10.1093/jaoac/57.4.796. [DOI] [Google Scholar]
- 49.Ericsson I., Ljunggren L. Trace determination of high molecular weight polyvinylpyrrolidone by pyrolysis-gas chromatography. J. Anal. Appl. Pyrolysis. 1990;17:251–260. doi: 10.1016/0165-2370(90)85014-E. [DOI] [Google Scholar]
- 50.Robinson B.V., Sullivan F.M., Borzelleca J.F., Schwartz S.L. Lewis Publishers; 1990. PVP : a Critical Review of the Kinetics and Toxicology of Polyvinylprrolidone (Povidone) [Google Scholar]
- 51.Burnette L.W. A review of the physiological properties of PVP. Proc. Sci. Sect. Toilet Goods Assoc. 1960;38:1–4. [Google Scholar]
- 52.Shelanski M.V. Final two-year report of a chronic oral toxicity study with PVP (K-30) in rats. Unpublished report from the industrial biological research and testing laboratories, United States of America, submitt. To world heal. Organ. by BASF. 1957 [Google Scholar]
- 53.Hanka R. Intestinal absorption of polyvinylpyrrolidone. Nihon Univ. J. Med. 1971;13:129–146. [Google Scholar]
- 54.Scheffner D. Doctors’ Thesis, University of Heidelberg; 1955. Tolerance and Side-Effects of Various Kollidons Administered by Mouth and Their Behavior in the Gastrointestinal Tract (Translation from German) [Google Scholar]
- 55.Ravin H.A., Seligman A.M., Fine J. Polyvinyl pyrrolidone as a plasma expander; studies on its excretion, distribution and metabolism. N. Engl. J. Med. 1952;247:921–929. doi: 10.1056/NEJM195212112472403. [DOI] [PubMed] [Google Scholar]
- 56.Pratten M.K., Lloyd J.B. Effects of temperature, metabolic inhibitors and some other factors on fluid-phase and adsorptive pinocytosis by rat peritoneal macrophages. Biochem. J. 1979;180:567–571. doi: 10.1042/bj1800567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nair B. Final report on the safety assessment of polyvinylpyrrolidone (PVP) Int. J. Toxicol. 1998;17:95–130. doi: 10.1177/109158189801700408. [DOI] [Google Scholar]
- 58.Heinrich H.C., Gabbe E.E., Nass W.P., Becker K. [Studies on I-131 labelled polyvinylpyrrolidone metabolism in the human body] Klin. Wochenschr. 1966;44:488–493. doi: 10.1007/bf01727574. [DOI] [PubMed] [Google Scholar]
- 59.Wessel W., Schoog M., Winkler E. Polyvinylpyrrolidone (PVP), its diagnostic, therapeutic and technical application and consequences thereof. Arzneimittel-Forschung/Drug Res. 1971;21:1468–1482. [PubMed] [Google Scholar]
- 60.WHO . IPCS Inchem; 1998. Polyvinylpyrrolidone (PVP) (WHO Food Additives Series 15)http://www.inchem.org/documents/jecfa/jecmono/v15je08.htm accessed March 14, 2020. [Google Scholar]
- 61.Christensen M., Johansen P., Hau C. Storage of polyvinylpyrrolidone (PVP) in tissues following long‐term treatment with a PVP‐containing vasopressin preparation. Acta Med. Scand. 1978;204:295–298. doi: 10.1111/j.0954-6820.1978.tb08442.x. [DOI] [PubMed] [Google Scholar]
- 62.Hizawa K., Inaba H., Nakanishi S., Otsuka H., Izumi K. Subcutaneous pseudosarcomatous polyvinylpyrrolidone granuloma. Am. J. Surg. Pathol. 1984;8:393–398. doi: 10.1097/00000478-198405000-00008. [DOI] [PubMed] [Google Scholar]
- 63.Cameron A.M. Blood plasma expander-PVP. Del. Med. J. 1954;26:149–151. http://www.ncbi.nlm.nih.gov/pubmed/13173177 accessed March 14, 2020. [PubMed] [Google Scholar]
- 64.Jenkins L.B., Kredel F.E., McCord W.M. Evaluation of polyvinyl pyrrolidone as a plasma expander. A.M.A Arch. Surg. 1956;72:612–617. doi: 10.1001/archsurg.1956.01270220060007. [DOI] [PubMed] [Google Scholar]
- 65.Kimaro E., Tibalinda P., Shedafa R., Temu M., Kaale E. Formulation development of chewable albendazole tablets with improved dissolution rate. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morin G., Briens L. A comparison of granules produced by high-shear and fluidized-bed granulation methods. AAPS PharmSciTech. 2014;15:1039–1048. doi: 10.1208/s12249-014-0134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wan L.S.C., Heng P.W.S., Ling B.L. Effect of polyvinylpyrrolidone solutions containing dissolved drug on characteristics of lactose fluidized bed granules. Int. J. Pharm. 1996;141:161–170. doi: 10.1016/0378-5173(96)04633-9. [DOI] [Google Scholar]
- 68.Varshosaz J., Kennedy R.A., Gipps E.M. Effect of binder level and granulating liquid on phenylbutazone pellets prepared by extrusion-spheronization. Drug Dev. Ind. Pharm. 1997;23:611–618. doi: 10.3109/03639049709149828. [DOI] [Google Scholar]
- 69.Barbosa E.J., Ferraz H.G. Gellan gum and polyvinylpyrrolidone (PVP) as binding agents in extrusion/spheronization pellet formulations. Acta Pharm. 2019;69:99–109. doi: 10.2478/acph-2019-0007. [DOI] [PubMed] [Google Scholar]
- 70.Li J., Tao L.I., Dali M., Buckley D., Gao J., Hubert M. The effect of the physical states of binders on high-shear wet granulation and granule properties: a mechanistic approach toward understanding high-shear wet granulation process. Part II. Granulation and granule properties. J. Pharmaceut. Sci. 2010;100:294–310. doi: 10.1002/jps.22261. [DOI] [PubMed] [Google Scholar]
- 71.Kasperek R., Zimmer L., Zun M., Dwornicka D., Wojciechowska K., Poleszak E. The application of povidone in the preparation of modified release tablets. Curr. Issues Pharm. Med. Sci. 2016;29:71–78. [Google Scholar]
- 72.Rotteglia E. [Polyvinylpyrrolidone (PVP); its properties and new use in tablet coating] Boll. Chim. Farm. 1956;95:238–250. http://www.ncbi.nlm.nih.gov/pubmed/13342171 accessed March 14, 2020. [PubMed] [Google Scholar]
- 73.Zhou Y., Jiang X.T., Zhou J.B., Zou H. Preparation of 5-aminosalicylic acid enteric time-controlled released tablets and study on their dissolution. Chin. Pharmaceut. J. 2001;36:673–677. [Google Scholar]
- 74.Buehler V., Hofmann F., Heinz R. 1985. Preparation of Ascorbic Acid Granules. US4800086A. [Google Scholar]
- 75.Sanaboina J., Maheswari K.M., Sunkara S., Deekonda S., Nalluri B.N. Preparation and evaluation of valsartan liquid filling formulations for soft gels. J. Pharm. 2013;1–8 doi: 10.1155/2013/418346. https://www.hindawi.com/journals/jphar/2013/418346/ accessed June 19, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Javadzadeh Y., Jafari-Navimipour B., Nokhodchi A. Liquisolid technique for dissolution rate enhancement of a high dose water-insoluble drug (carbamazepine) Int. J. Pharm. 2007;341:26–34. doi: 10.1016/j.ijpharm.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 77.Molaei M.A., Osouli-Bostanabad K., Adibkia K., Shokri J., Asnaashari S., Javadzadeh Y. Enhancement of ketoconazole dissolution rate by the liquisolid technique. Acta Pharm. 2018;68:325–336. doi: 10.2478/acph-2018-0025. [DOI] [PubMed] [Google Scholar]
- 78.Zhang W., ning Zhang C., He Y., yan Duan B., yi Yang G., dong Ma W., hong Zhang Y. Factors affecting the dissolution of indomethacin solid dispersions. AAPS PharmSciTech. 2017;18:3258–3273. doi: 10.1208/s12249-017-0813-2. [DOI] [PubMed] [Google Scholar]
- 79.Jachowicz R. Dissolution rates of partially water-soluble drugs from solid dispersion systems. II. Phenytoin. Int. J. Pharm. 1987;35:7–12. doi: 10.1016/0378-5173(87)90068-8. [DOI] [Google Scholar]
- 80.Corrigan O.I., Farvar M.A., Higuchi W. Drug membrane transport enhancement using high energy drug polyvinylpyrrolidone (PVP) co-precipitates. Int. J. Pharm. 1980;5:229–238. [Google Scholar]
- 81.Nabekura T., Ito Y., Cai H., Terao M., Hori R. Preparation and in vivo ocular absorption studies of disulfiram solid dispersion. Biol. Pharm. Bull. 2000;23:616–620. doi: 10.1248/bpb.23.616. [DOI] [PubMed] [Google Scholar]
- 82.Verma U., Naik J.B., Patil J.S., Yadava S.K. Screening of process variables to enhance the solubility of famotidine with 2-HydroxyPropyl–β-Cyclodextrin & PVP K-30 by using Plackett–Burman design approach. Mater. Sci. Eng. C. 2017;77:282–292. doi: 10.1016/j.msec.2017.03.238. [DOI] [PubMed] [Google Scholar]
- 83.Barmpalexis P., Koutsidis I., Karavas E., Louka D., Papadimitriou S.A., Bikiaris D.N. Development of PVP/PEG mixtures as appropriate carriers for the preparation of drug solid dispersions by melt mixing technique and optimization of dissolution using artificial neural networks. Eur. J. Pharm. Biopharm. 2013;85:1219–1231. doi: 10.1016/j.ejpb.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 84.Frizon F., Eloy J. de O., Donaduzzi C.M., Mitsui M.L., Marchetti J.M. Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 solid dispersions by solvent methods. Powder Technol. 2013;235:532–539. doi: 10.1016/j.powtec.2012.10.019. [DOI] [Google Scholar]
- 85.Doreth M., Hussein M.A., Priemel P.A., Grohganz H., Holm R., Lopez de Diego H., Rades T., Löbmann K. Amorphization within the tablet: using microwave irradiation to form a glass solution in situ. Int. J. Pharm. 2017;519:343–351. doi: 10.1016/j.ijpharm.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 86.Prosapio V., Reverchon E., De Marco I. Formation of PVP/nimesulide microspheres by supercritical antisolvent coprecipitation. J. Supercrit. Fluids. 2016;118:19–26. doi: 10.1016/j.supflu.2016.07.023. [DOI] [Google Scholar]
- 87.Uzun I.N., Sipahigil O., Dinçer S. Coprecipitation of Cefuroxime Axetil-PVP composite microparticles by batch supercritical antisolvent process. J. Supercrit. Fluids. 2011;55:1059–1069. doi: 10.1016/j.supflu.2010.09.035. [DOI] [Google Scholar]
- 88.Elagamy H.I., Essa E.A., Nouh A., El Maghraby G.M. Development and evaluation of rapidly dissolving buccal films of naftopidil: in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 2019;45:1695–1706. doi: 10.1080/03639045.2019.1656734. [DOI] [PubMed] [Google Scholar]
- 89.Khalil E., Najjar S., Sallam A. Aqueous solubility of diclofenac diethylamine in the presence of pharmaceutical additives: a comparative study with diclofenac sodium. Drug Dev. Ind. Pharm. 2000;26:375–381. doi: 10.1081/DDC-100101243. [DOI] [PubMed] [Google Scholar]
- 90.Samprasit W., Akkaramongkolporn P., Ngawhirunpat T., Rojanarata T., Kaomongkolgit R., Opanasopit P. Fast releasing oral electrospun PVP/CD nanofiber mats of taste-masked meloxicam. Int. J. Pharm. 2015;487:213–222. doi: 10.1016/j.ijpharm.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 91.Yasir M., Nagar P., Chauhan I. Insights into polymers: film formers in mouth dissolving films. Drug Invent. Today. 2011;3:280–289. www.ditonline.info accessed March 14, 2020. [Google Scholar]
- 92.Ziller K.H., Rupprecht H. Conteol of crystal growth in drug suspensions: 1) design of a conteol unit and application to acetaminophen suspensions) Drug Dev. Ind. Pharm. 1988;14:2341–2370. doi: 10.3109/03639048809152019. [DOI] [Google Scholar]
- 93.Asada K., Fukano K., Yamashita K., Nakanishi E. Acrylic emulsion pressure-sensitive adhesives using polyvinyl alcohol as a protective colloid. J. Adhes. Soc. Japan. 2013;49:454–462. doi: 10.11618/adhesion.49.454. [DOI] [Google Scholar]
- 94.Hähnlein M., Prüße U., Daum J., Morawsky V., Kröger M., Schröder M., Schnabel M., Vorlop K.D. Preparation of microscopic catalysts and colloids for catalytic nitrate and nitrite reduction and their use in a hollow fibre dialyser loop reactor. Stud. Surf. Sci. Catal. 1998;118:99–107. doi: 10.1016/s0167-2991(98)80172-9. [DOI] [Google Scholar]
- 95.Ma C.M., Li C.L. Stability of dispersions of iron oxide in mixed solutions of polyvinylpyrrolidone and sodium alkyl sulfate. Colloid. Surface. 1990;47:117–123. doi: 10.1016/0166-6622(90)80066-D. [DOI] [Google Scholar]
- 96.Rushil S., Priti M. Freeze dried injectable drug product development: selection of non functional additivies. Int. J. Pharm. Pharmaceut. Sci. 2014;6:3–7. [Google Scholar]
- 97.Pramanick S., Chandel V., Singodia D. Excipient selection in parenteral formulation development. Pharmatimes. 2013;45:65–77. [Google Scholar]
- 98.Reed K., Berger N. vol. 9. 2018. (The Effect of Polyvinylpyrrolidone (PVP) on Ocular Gel Forming Solutions Composed of Gellan and Calcium Gluconate). [Google Scholar]
- 99.Patel Dipti H., Patel Manish P., Patel Madhabhai M. Formulation and evaluation of drug-free ophthalmic films prepared by using various synthetic polymers. J. Young Pharm. 2009;1 doi: 10.4103/0975-1483.55742. [DOI] [Google Scholar]
- 100.Wang H., Li X., Yang H., Wang J., Li Q., Qu R., Wu X. Nanocomplexes based polyvinylpyrrolidone K-17PF for ocular drug delivery of naringenin. Int. J. Pharm. 2020;578:119133. doi: 10.1016/j.ijpharm.2020.119133. [DOI] [PubMed] [Google Scholar]
- 101.Patel J.P., Marsh K., Carr L., Nequist G. Factorial designs in ophthalmic formulation development of Enalkiren. Int. J. Pharm. 1990;65:195–200. doi: 10.1016/0378-5173(90)90143-R. [DOI] [Google Scholar]
- 102.Liang Bo, Capriotti Joseph A., Michael Samson C., Stein Jason, Weiser Michael. US20130177522A1; 2010. Nonirritant Ophthalmology PVP-I Composition. [Google Scholar]
- 103.Malet F., Karsenti D., Pouliquen P. Preservative-free ocular hydrating agents in symptomatic contact lens wearers: saline versus PVP solution. Eye Contact Lens. 2003;29:38–43. doi: 10.1097/00140068-200301000-00010. [DOI] [PubMed] [Google Scholar]
- 104.Yanai R., Yamada N., Ueda K., Tajiri M., Matsumoto T., Kido K., Nakamura S., Saito F., Nishida T. Evaluation of povidone-iodine as a disinfectant solution for contact lenses: antimicrobial activity and cytotoxicity for corneal epithelial cells. Contact Lens Anterior Eye. 2006;29:85–91. doi: 10.1016/j.clae.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 105.Hoteling A.J., Nichols W.F., Harmon P.S., Conlon S.M., Nuñez I.M., Hoff J.W., Cabarcos O.M., Steffen R.B., Hook D.J. Characterization and quantitation of PVP content in a silicone hydrogel contact lens produced by dual-phase polymerization processing. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106:1064–1072. doi: 10.1002/jbm.b.33904. [DOI] [PubMed] [Google Scholar]
- 106.Zakhlebnaia O.D., V Laukner I. The use of polyvinylpyrrolidone as a stabilizer in the lyophilization of Brucella. Lab. Delo. 1991:62–63. [PubMed] [Google Scholar]
- 107.Taylor L.S. Sugar-polymer hydrogen bond interactions in lyophilized amorphous mixtures. J. Pharmaceut. Sci. 1998;87:1615–1621. doi: 10.1021/js9800174. [DOI] [PubMed] [Google Scholar]
- 108.Van Steirteghem A.C., Nagy Z., Joris H., Liu J., Staessen C., Smitz J., Wisanto A., Devroey P. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum. Reprod. 1993;8:1061–1066. doi: 10.1093/oxfordjournals.humrep.a138192. [DOI] [PubMed] [Google Scholar]
- 109.Chun M.K., Bhusal P., Choi H.K. Application of Carbopol/PVP interpolymer complex to prepare mucoadhesive floating granule. Arch Pharm. Res. (Seoul) 2013;36:745–751. doi: 10.1007/s12272-013-0035-4. [DOI] [PubMed] [Google Scholar]
- 110.Sizílio R.H., Galvão J.G., Trindade G.G.G., Pina L.T.S., Andrade L.N., Gonsalves J.K.M.C., Lira A.A.M., Chaud M.V., Alves T.F.R., Arguelho M.L.P.M., Nunes R.S. Chitosan/pvp-based mucoadhesive membranes as a promising delivery system of betamethasone-17-valerate for aphthous stomatitis. Carbohydr. Polym. 2018;190:339–345. doi: 10.1016/j.carbpol.2018.02.079. [DOI] [PubMed] [Google Scholar]
- 111.Suksaeree J., Siripornpinyo P., Chaiprasit S. Formulation, characterization, and in vitro evaluation of transdermal patches for inhibiting crystallization of mefenamic acid. J. Drug Deliv. 2017:1–7. doi: 10.1155/2017/7358042. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giuliano E., Paolino D., Fresta M., Cosco D. Mucosal applications of poloxamer 407-based hydrogels: an overview. Pharmaceutics. 2018;10:159. doi: 10.3390/pharmaceutics10030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rac V., Lević S., Balanč B., Olalde Graells B., Bijelić G. PVA Cryogel as model hydrogel for iontophoretic transdermal drug delivery investigations. Comparison with PAA/PVA and PAA/PVP interpenetrating networks. Colloids Surf. B Biointerfaces. 2019;180:441–448. doi: 10.1016/j.colsurfb.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 114.Gupta A., Garg S., Khar R.K. Interpolymer complexation and its effect on bioadhesive strength 6 dissolution characteristics of buccal drug delivery systems. Drug Dev. Ind. Pharm. 1994;20:315–325. doi: 10.3109/03639049409050185. [DOI] [Google Scholar]
- 115.Al-Niaimi F., Beck M., Almaani N., Samarasinghe V., Williams J., Lyon C. The relevance of patch testing in peristomal dermatitis. Br. J. Dermatol. 2012;167:103–109. doi: 10.1111/j.1365-2133.2012.10925.x. [DOI] [PubMed] [Google Scholar]
- 116.Gluck U., Martin U., Bosse B., Reimer K., Mueller S. A clinical study on the tolerability of a liposomal povidone-iodine nasal spray: implications for further development. ORL (Oto-Rhino-Laryngol.) (Basel) 2007;69:92–99. doi: 10.1159/000097758. [DOI] [PubMed] [Google Scholar]
- 117.Mady L.J., Kubik M.W., Baddour K., Snyderman C.H., Rowan N.R. Consideration of povidone-iodine as a public health intervention for COVID-19: utilization as “Personal Protective Equipment” for frontline providers exposed in high-risk head and neck and skull base oncology care. Oral Oncol. 2020;105:104724. doi: 10.1016/j.oraloncology.2020.104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kim Nanhye, Chang Hanwei William. US20090281156A1; 2008. Enhancing Photostabilization of Oxymetazoline. [Google Scholar]
- 119.Saleem M., Taher M., Sanaullah S., Najmuddin M., Ali J., Humaira S., Roshan S. Formulation and evaluation of tramadol hydrochloride rectal suppositories. Indian J. Pharmaceut. Sci. 2008;70:640–644. doi: 10.4103/0250-474X.45405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hardy I.J., Windberg-Baarup A., Neri C., Byway P.V., Booth S.W., Fitzpatrick S. Modulation of drug release kinetics from hydroxypropyl methyl cellulose matrix tablets using polyvinyl pyrrolidone. Int. J. Pharm. 2007;337:246–253. doi: 10.1016/j.ijpharm.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 121.Bose A., Wong T.W., Singh N. Formulation development and optimization of sustained release matrix tablet of Itopride HCl by response surface methodology and its evaluation of release kinetics. Saudi Pharmaceut. J. 2013;21:201–213. doi: 10.1016/j.jsps.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pandey P., Pandey S. Delivery of poorly water soluble drug from swellable elementary osmotic pump and effect of formulation variables. Turkish J. Pharm. Sci. 2013;10:221–236. [Google Scholar]
- 123.Dasankoppa F.S., Ningangowdar M., Sholapur H. Formulation and evaluation of controlled porosity osmotic pump for oral delivery of ketorolac. J. Basic Clin. Pharm. 2013;4:2–9. doi: 10.4103/0976-0105.109398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hosmani A.H., Thorat Y.S., Gonjari I.D., Karmarkar A.B. Formation and characterization of carbopol 971P-PVP interpolymer complex and its application for sustained delivery of acyclovir. J. Adv. Pharm. Educ. Res. 2013;3:94–101. www.japer.in accessed March 14, 2020. [Google Scholar]
- 125.Yusif R.M., Hashim I.I.A., Mohamed E.A., El Rakhawy M.M. Investigation and evaluation of an in situ interpolymer complex of carbopol with polyvinylpyrrolidone as a matrix for gastroretentive tablets of ranitidine hydrochloride. Chem. Pharm. Bull. 2016;64:42–51. doi: 10.1248/cpb.c15-00620. [DOI] [PubMed] [Google Scholar]
- 126.Dong B., Lim L.M., Hadinoto K. Enhancing the physical stability and supersaturation generation of amorphous drug-polyelectrolyte nanoparticle complex via incorporation of crystallization inhibitor at the nanoparticle formation step: a case of HPMC versus PVP. Eur. J. Pharmaceut. Sci. 2019;138:105035. doi: 10.1016/j.ejps.2019.105035. [DOI] [PubMed] [Google Scholar]
- 127.Campardelli R., Della Porta G., Gomez L., Irusta S., Reverchon E., Santamaria J. Au-PLA nanocomposites for photothermally controlled drug delivery. J. Mater. Chem. B. 2014;2:409–417. doi: 10.1039/c3tb21099e. [DOI] [PubMed] [Google Scholar]
- 128.Guduru R., Liang P., Runowicz C., Nair M., Atluri V., Khizroev S. Magneto-electric nanoparticles to enable field-controlled high-specificity drug delivery to eradicate ovarian cancer cells. Sci. Rep. 2013;3:1–8. doi: 10.1038/srep02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Javed R., Ahmed M., ul Haq I., Nisa S., Zia M. PVP and PEG doped CuO nanoparticles are more biologically active: antibacterial, antioxidant, antidiabetic and cytotoxic perspective. Mater. Sci. Eng. C. 2017;79:108–115. doi: 10.1016/j.msec.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 130.Gaikwad D., Shewale R., Patil V., Mali D., Gaikwad U., Jadhav N. Enhancement in in vitro anti-angiogenesis activity and cytotoxicity in lung cancer cell by pectin-PVP based curcumin particulates. Int. J. Biol. Macromol. 2017;104:656–664. doi: 10.1016/j.ijbiomac.2017.05.170. [DOI] [PubMed] [Google Scholar]
- 131.Atta A.M., El-Ghazawy R.A.M., Farag R.K., Elsaeed S.M. Synthesis and characterization of pH-sensitive PAMPS/PVP nanogels in aqueous media, Polym. Adv. Met. Technol. 2011;22:732–737. doi: 10.1002/pat.1573. [DOI] [Google Scholar]
- 132.Bueno V.B., Cuccovia I.M., Chaimovich H., Catalani L.H. PVP superabsorbent nanogels. Colloid Polym. Sci. 2009;287:705–713. doi: 10.1007/s00396-009-2017-0. [DOI] [Google Scholar]
- 133.Kadlubowski S. Radiation-induced synthesis of nanogels based on poly(N-vinyl-2-pyrrolidone)-A review. Radiat. Phys. Chem. 2014;102:29–39. doi: 10.1016/j.radphyschem.2014.04.016. [DOI] [Google Scholar]
- 134.Yu D.G., Wang X., Li X.Y., Chian W., Li Y., Liao Y.Z. Electrospun biphasic drug release polyvinylpyrrolidone/ethyl cellulose core/sheath nanofibers. Acta Biomater. 2013;9:5665–5672. doi: 10.1016/j.actbio.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 135.Chuangchote S., Sagawa T., Yoshikawa S. Electrospinning of poly(vinyl pyrrolidone): effects of solvents on electrospinnability for the fabrication of poly( p -phenylene vinylene) and TiO 2 nanofibers. J. Appl. Polym. Sci. 2009;114:2777–2791. doi: 10.1002/app.30637. [DOI] [Google Scholar]
- 136.Albetran H., Dong Y., Low I.M. Characterization and optimization of electrospun TiO 2/PVP nanofibers using Taguchi design of experiment method. J. Asian Ceram. Soc. 2015;3:292–300. doi: 10.1016/j.jascer.2015.05.001. [DOI] [Google Scholar]
- 137.Mennini N., Furlanetto S., Cirri M., Mura P. Quality by design approach for developing chitosan-Ca-alginate microspheres for colon delivery of celecoxib-hydroxypropyl-β-cyclodextrin-PVP complex. Eur. J. Pharm. Biopharm. 2012;80:67–75. doi: 10.1016/j.ejpb.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 138.Kim K.S., Park S.J. Characterization and release behaviors of porous PCL/Eudragit RS microcapsules containing tulobuterol. Colloids Surf. B Biointerfaces. 2010;76:404–409. doi: 10.1016/j.colsurfb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 139.Homayouni A., Sadeghi F., Varshosaz J., Garekani H.A., Nokhodchi A. Comparing various techniques to produce micro/nanoparticles for enhancing the dissolution of celecoxib containing PVP. Eur. J. Pharm. Biopharm. 2014;88:261–274. doi: 10.1016/j.ejpb.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 140.Liparoti Sara, Adami Renata, Caputo Giuseppe, Reverchon1 Ernesto. Supercritical assisted atomization: polyvinylpyrrolidone as carrier for drugs with poor solubility in water. Adv. Perspect. Supercrit. Fluid technol. 2013 https://www.hindawi.com/journals/jchem/2013/801069/ accessed August 3, 2020, 1–5. [Google Scholar]
- 141.Prosapio V., De Marco I., Reverchon E. PVP/corticosteroid microspheres produced by supercritical antisolvent coprecipitation. Chem. Eng. J. 2016;292:264–275. doi: 10.1016/j.cej.2016.02.041. [DOI] [Google Scholar]
- 142.Marizza P., Keller S.S., Müllertz A., Boisen A. Polymer-filled microcontainers for oral delivery loaded using supercritical impregnation. J. Contr. Release. 2014;173:1–9. doi: 10.1016/j.jconrel.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 143.Aung N.N., Ngawhirunpat T., Rojanarata T., Patrojanasophon P., Opanasopit P., Pamornpathomkul B. HPMC/PVP dissolving microneedles: a promising delivery platform to promote trans-epidermal delivery of alpha-arbutin for skin lightening. AAPS PharmSciTech. 2020;21:1–13. doi: 10.1208/s12249-019-1599-1. [DOI] [PubMed] [Google Scholar]
- 144.Liu D., Yu B., Jiang G., Yu W., Zhang Y., Xu B. Fabrication of composite microneedles integrated with insulin-loaded CaCO3 microparticles and PVP for transdermal delivery in diabetic rats. Mater. Sci. Eng. C. 2018;90:180–188. doi: 10.1016/j.msec.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 145.Moga K.A., Bickford L.R., Geil R.D., Dunn S.S., Pandya A.A., Wang Y., Fain J.H., Archuleta C.F., O'Neill A.T., Desimone J.M. Rapidly-dissolvable microneedle patches via a highly scalable and reproducible soft lithography approach. Adv. Mater. 2013;25:5060–5066. doi: 10.1002/adma.201300526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sullivan S.P., Koutsonanos D.G., Del Pilar Martin M., Lee J.W., Zarnitsyn V., Choi S.O., Murthy N., Compans R.W., Skountzou I., Prausnitz M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Roy G., Galigama R.D., Thorat V.S., Mallela L.S., Roy S., Garg P., Venuganti V.V.K. Amphotericin B containing microneedle ocular patch for effective treatment of fungal keratitis. Int. J. Pharm. 2019;572:118808. doi: 10.1016/j.ijpharm.2019.118808. [DOI] [PubMed] [Google Scholar]
- 148.Oliveira M.J.A., Estefânia O.S., Braz L.M.A., Regina M., Amato V.S., Lugão A.B., Parra D.F. Influence of chitosan/clay in drug delivery of glucantime from PVP membranes. Radiat. Phys. Chem. 2014;94:194–198. doi: 10.1016/j.radphyschem.2013.05.050. [DOI] [Google Scholar]
- 149.Ahmad B., Abbas S., Iqbal Z., Bashir S., Ali J. Synthesis of cross linked PVP hydrogels and its use for the control release of anti-asthmatic drugs, Middle East. J. Sci. Res. 2013;14:273. [Google Scholar]
- 150.Jin S., Gu J., Shi Y., Shao K., Yu X., Yue G. Preparation and electrical sensitive behavior of poly (N-vinylpyrrolidone- co-acrylic acid) hydrogel with flexible chain nature. Eur. Polym. J. 2013;49:1871–1880. doi: 10.1016/j.eurpolymj.2013.04.022. [DOI] [Google Scholar]
- 151.Xue Y., Zhang W., Lei Y., Dang M. Novel polyvinyl pyrrolidone–loaded olopatadine HCl–laden doughnut contact lens to treat allergic conjunctivitis. J. Pharmaceut. Sci. 2020;109(5):1714–1724. doi: 10.1016/j.xphs.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 152.Zhang N., Wang C., Liu J., Xing J., Dong A. A kind of modified bovine serum albumin with great potential for applying in gene delivery. Chin. Chem. Lett. 2013;24:659–662. doi: 10.1016/j.cclet.2013.04.029. [DOI] [Google Scholar]
- 153.Zhi X., Fang H., Bao C., Shen G., Zhang J., Wang K., Guo S., Wan T., Cui D. Biomaterials the immunotoxicity of graphene oxides and the effect of PVP-coating. Biomaterials. 2013;34:5254–5261. doi: 10.1016/j.biomaterials.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 154.Teodorescu M., Bercea M. Poly(vinylpyrrolidone) – a versatile polymer for biomedical and beyond medical applications. Polym. Plast. Technol. Eng. 2015;54:923–943. doi: 10.1080/03602559.2014.979506. [DOI] [Google Scholar]
- 155.Mondal D., Mollick M.M.R., Bhowmick B., Maity D., Bain M.K., Rana D., Mukhopadhyay A., Dana K., Chattopadhyay D. Effect of poly(vinyl pyrrolidone) on the morphology and physical properties of poly(vinyl alcohol)/sodium montmorillonite nanocomposite films. Prog. Nat. Sci. Mater. Int. 2013;23:579–587. doi: 10.1016/j.pnsc.2013.11.009. [DOI] [Google Scholar]
- 156.Hespe W., Blankwater Y.J., Wieriks J. A combined study on the distribution of oxytetracycline and polyvinylpyrrolidone in rats - PubMed. Arzneimittelforschung. 1975;25:1561–1567. https://pubmed.ncbi.nlm.nih.gov/1243038/ accessed June 19, 2020. [PubMed] [Google Scholar]
- 157.Thirumala S., Wu X., Gimble J.M., Devireddy R.V. Evaluation of polyvinylpyrrolidone as a cryoprotectant for adipose tissue-derived adult stem cells. Tissue Eng. C Methods. 2010;16:783–792. doi: 10.1089/ten.tec.2009.0552. [DOI] [PubMed] [Google Scholar]
- 158.Zhang S., Deng Q., Li Y., Zheng M., Wan C., Zheng C., Tang H., Huang F., Shi J. Novel amphiphilic polyvinylpyrrolidone functionalized silicone particles as carrier for low-cost lipase immobilization. R. Soc. Open Sci. 2018;5 doi: 10.1098/rsos.172368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Regulska K., Regulski M., Wzgarda A., Kotowska A., Ignasiak A., Ćwiertnia B., Stanisz B. Does polyvinylpyrrolidone improve the chemical stability of cilazapril in solid state?, Iran. J. Pharm. Res. 2019;18:579–595. doi: 10.22037/ijpr.2019.1100640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shimpi S.L., Mahadik K.R., Paradkar A.R. Study on mechanism for amorphous drug stabilization using Gelucire 50/13. Chem. Pharm. Bull. 2009;57:937–942. doi: 10.1248/cpb.57.937. [DOI] [PubMed] [Google Scholar]
- 161.Nielsen L.H., Rades T., Müllertz A. Stabilisation of amorphous furosemide increases the oral drug bioavailability in rats. Int. J. Pharm. 2015;490:334–340. doi: 10.1016/j.ijpharm.2015.05.063. [DOI] [PubMed] [Google Scholar]
- 162.Chowdary K.P.R., Srinivas S.V. Effect of polyvinylpyrrolidone on complexation and dissolution rate of β- and hydroxypropyl-β-cyclodextrin complexes of celecoxib. Indian J. Pharmaceut. Sci. 2006;68:631–634. doi: 10.4103/0250-474X.29632. [DOI] [Google Scholar]
- 163.Dai J., Goh S.H., Lee S.Y., Smw K.S. 1995. Interpolymer Complexation and Blend Formation between Poly(N-Vinyl-2-Pyrrolidone) and Aliphatic Hydroxyl-Containing Polymers. [Google Scholar]
- 164.Plaizier‐Vercammen J.A., De Nève R.E. Interaction of povidone with aromatic compounds II: evaluation of ionic strength, buffer concentration, temperature, and pH by factorial analysis. J. Pharmaceut. Sci. 1981;70:1252–1256. doi: 10.1002/jps.2600701118. [DOI] [PubMed] [Google Scholar]
- 165.Plaizier-Vercammen J.A. Interaction of povidone with aromatic compounds V: relationship of binding tendency in a macromolecular solution treated as a pseudo two phase and a monophase. J. Pharmaceut. Sci. 1984;73:1774–1779. doi: 10.1002/jps.2600731229. [DOI] [PubMed] [Google Scholar]
- 166.Saluja H., Mehanna A., Panicucci R., Atef E. Hydrogen bonding: between strengthening the crystal packing and improving solubility of three haloperidol derivatives. Molecules. 2016;21:719. doi: 10.3390/molecules21060719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Aljamah A.F., Levon J.A., Hara A.T., Lippert F., Eckert G.J. Effects of PVP-iodine pH and calcium concentration on fluoride varnish anti-caries efficacy in vitro. Oral Health Prev. Dent. 2019;17:257–262. doi: 10.3290/j.ohpd.a42506. [DOI] [PubMed] [Google Scholar]
- 168.Jalil A., Matuszczak B., Nguyen Le N.M., Mahmood A., Laffleur F., Bernkop-Schnürch A. Synthesis and characterization of thiolated PVP-iodine complexes: key to highly mucoadhesive antimicrobial gels. Mol. Pharm. 2018;15:3527–3534. doi: 10.1021/acs.molpharmaceut.8b00503. [DOI] [PubMed] [Google Scholar]
- 169.Pinna A., Donadu M.G., Usai D., Dore S., D'Amico-Ricci G., Boscia F., Zanetti S. In vitro antimicrobial activity of a new ophthalmic solution containing povidone-iodine 0.6% (IODIM®) Acta Ophthalmol. 2019;98:e178–e180. doi: 10.1111/aos.14243. [DOI] [PubMed] [Google Scholar]
- 170.Trott A.T. Wounds and Lacerations. Elsevier Inc.; Netherlands: 2012. Wound cleansing and irrigation; pp. 73–81. [DOI] [Google Scholar]
- 171.Yu W.W., Liu H. Singular modification effects of metal cations and metal complex ions on the catalytic properties of metal colloidal nanocatalysts. J. Mol. Catal. Chem. 2006;243:120–141. doi: 10.1016/j.molcata.2005.08.025. [DOI] [Google Scholar]
- 172.Penjweini R., Loew H.G., Breit P., Kratky K.W. Optimizing the antitumor selectivity of PVP-Hypericin re A549 cancer cells and HLF normal cells through pulsed blue light. Photodiagnosis Photodyn. Ther. 2013;10:591–599. doi: 10.1016/j.pdpdt.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 173.Rezadoost M.H., Kordrostami M., Kumleh H.H. An efficient protocol for isolation of inhibitor-free nucleic acids even from recalcitrant plants. Biotech. 2016;6:1–7. doi: 10.1007/s13205-016-0375-0. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sudhakar Y.N., Selvakumar M., Bhat D.K. Biopolymer Electrolytes - Fundamentals and Applications in Energy Storage. Elsevier Inc.; Netherlands: 2018. Methods of preparation of biopolymer electrolytes. [Google Scholar]
- 175.Wlodarski K., Tajber L., Sawicki W. Physicochemical properties of direct compression tablets with spray dried and ball milled solid dispersions of tadalafil in PVP-VA. Eur. J. Pharm. Biopharm. 2016;109:14–23. doi: 10.1016/j.ejpb.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 176.Choi J.S., Park J.S. Design of PVP/VA S-630 based tadalafil solid dispersion to enhance the dissolution rate. Eur. J. Pharmaceut. Sci. 2017;97:269–276. doi: 10.1016/j.ejps.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 177.Siepmann F., Eckart K., Maschke A., Kolter K., Siepmann J. Modeling drug release from PVAc/PVP matrix tablets. J. Contr. Release. 2010;141:216–222. doi: 10.1016/j.jconrel.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 178.Rojas J., Guisao S., Ruge V. Functional assessment of four types of disintegrants and their effect on the spironolactone release properties. AAPS PharmSciTech. 2012;13:1054–1062. doi: 10.1208/s12249-012-9835-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gohel M.C., Parikh R.K., Brahmbhatt B.K., Shah A.R. Preparation and assessment of novel coprocessed superdisintegrant consisting of crospovidone and sodium starch glycolate: a technical note. AAPS PharmSciTech. 2007;8:E63. doi: 10.1208/pt0801009. [DOI] [PubMed] [Google Scholar]
- 180.Burnett C.L., Heldreth B. PVP (polyvinylpyrrolidone) Int. J. Toxicol. 2017;36:50S–51S. doi: 10.1177/1091581817716649. [DOI] [PubMed] [Google Scholar]
- 181.Burnett C.L. Subject: Re-review of polyvinylpyrrolidone (PVP) 2013. www.cir-safety.orgCommitment&Credibilitysince1976 accessed June 19, 2020.
- 182.21CFR173.55, CFR - code of federal regulations title 21. 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=173.55 accessed June 18, 2020.
- 183.Yoshida K., Sakurai Y., Kawahara S., Takeda T., Ishikawa T., Murakami T., Yoshioka A. Anaphylaxis to polyvinylpyrrolidone in povidone-iodine for impetigo contagiosum in a boy with atopic dermatitis. Int. Arch. Allergy Immunol. 2008;146:169–173. doi: 10.1159/000113522. [DOI] [PubMed] [Google Scholar]
- 184.Adachi A., Fukunaga A., Hayashi K., Kunisada M., Horikawa T. Anaphylaxis to polyvinylpyrrolidone after vaginal application of povidone-iodine. Contact Dermatitis. 2003;48:133–136. doi: 10.1034/j.1600-0536.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 185.Reyazulla M.A., Gopinath A.L., Vaibhav N., Raut R.P. An unusual complication of late onset allergic contact dermatitis to povidone iodine in oral & maxillofacial surgery - a report of 2 cases. Eur. Ann. Allergy Clin. Immunol. 2014;46:157–159. http://www.ncbi.nlm.nih.gov/pubmed/25053635 accessed March 14, 2020. [PubMed] [Google Scholar]
- 186.Ganesan S., Felo J., Saldana M., Kalasinsky V.F., Lewin-Smith M.R., Tomashefski J.F. Embolized crospovidone (poly[N-vinyl-2-pyrrolidone]) in the lungs of intravenous drug users. Mod. Pathol. 2003;16:286–292. doi: 10.1097/01.MP.0000062653.65441.DA. [DOI] [PubMed] [Google Scholar]
- 187.Alam A.S., Parrott E.L. Effect of adjuvants on tackiness of polyvinylpyrrolidone film coating. J. Pharmaceut. Sci. 1972;61:265–268. doi: 10.1002/jps.2600610228. [DOI] [PubMed] [Google Scholar]
- 188.P S., H K., B M., A B. Possible prophylactic or preventive role of topical povidone iodine during accidental ocular exposure to 2019-nCoV. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020:1–4. doi: 10.1007/s00417-020-04752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Eggers M., Koburger-Janssen T., Eickmann M., Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect. Dis. Ther. 2018;7:249–259. doi: 10.1007/s40121-018-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mady L.J., Kubik M.W., Baddour K., Snyderman C.H., Rowan N.R. Consideration of povidone-iodine as a public health intervention for COVID-19: utilization as “Personal Protective Equipment” for frontline providers exposed in high-risk head and neck and skull base oncology care. Oral Oncol. 2020;105 doi: 10.1016/j.oraloncology.2020.104724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Eggers M., Eickmann M., Zorn J. Rapid and effective virucidal activity of povidone-iodine products against Middle East respiratory syndrome coronavirus (MERS-CoV) and modified vaccinia virus ankara (MVA) Infect. Dis. Ther. 2015;4:491–501. doi: 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ali M.J. A surgical protocol to mitigate the SARS-CoV-2 transmission using multifocal povidone-iodine applications in lacrimal surgeries during coronavirus disease 2019 (COVID-19) pandemic. Ophthalmic Plast. Reconstr. Surg. 2020;36(4):416–417. doi: 10.1097/iop.0000000000001746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Parhar H.S., Tasche K., Brody R.M., Weinstein G.S., O'Malley B.W., Shanti R.M., Newman J.G. Topical preparations to reduce <scp>SARS‐CoV</scp> ‐2 aerosolization in head and neck mucosal surgery. Head Neck. 2020;42:1268–1272. doi: 10.1002/hed.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Rapid in‐vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) using povidone‐iodine oral antiseptic rinse. J. Prosthodont. 2020;29 doi: 10.1111/jopr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sarma P., Kaur H., Medhi B., Bhattacharyya A. Possible prophylactic or preventive role of topical povidone iodine during accidental ocular exposure to 2019-nCoV. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020:1. doi: 10.1007/s00417-020-04752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liang B., Yuan X., Wei G., Wang W., Zhang M., Peng H., Javer A., Mendenhall M., Julander J., Huang S., Michail H., Lu Y., Zhu Q., Baldwin J.J. In-VivoToxicity studies and in-vitro inactivation of SARS-CoV-2 by povidone-iodine in-situ gel forming formulations. BioRxiv. 2020 doi: 10.1101/2020.05.18.103184. 2020.05.18.103184. [DOI] [Google Scholar]
- 197.Tessema B., Frank S., Bidra A. SARS‐CoV‐2 viral inactivation using low dose povidone‐iodine oral rinse—immediate application for the prosthodontic practice. J. Prosthodont. 2020;29:459. doi: 10.1111/jopr.13207. 459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Anderson D.E., Sivalingam V., Kang A.E.Z., Ananthanarayanan A., Arumugam H., Jenkins T.M., Hadjiat Y., Eggers M. Povidone-iodine demonstrates rapid in vitro virucidal activity against SARS-CoV-2, the virus causing COVID-19 disease. Infect. Dis. Ther. 2020;9(3):669–675. doi: 10.1007/s40121-020-00316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Comparison of in vitro inactivation of SARS CoV‐2 with hydrogen peroxide and povidone‐iodine oral antiseptic rinses. J. Prosthodont. 2020:13220. doi: 10.1111/jopr.13220. jopr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nobukuni K., Hayakawa N., Namba R., Ihara Y., Sato K., Takada H., Hayabara T., Kawahara S. The influence of long-term treatment with povidone-iodine on thyroid function. Dermatology. 1997;195:69–72. doi: 10.1159/000246034. [DOI] [PubMed] [Google Scholar]
- 201.Kronbichler A., Gauckler P., Windpessl M., Il Shin J., Jha V., Rovin B.H., Oberbauer R. COVID-19: implications for immunosuppression in kidney disease and transplantation. Nat. Rev. Nephrol. 2020;16:365–367. doi: 10.1038/s41581-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ramaswamykanive H., Nanavati Z., Mackie J., Linderman R., Lavee O. Cardiovascular collapse following povidone-iodine wash. Anaesth. Intensive Care. 2011;39:127–130. doi: 10.1177/0310057x1103900121. [DOI] [PubMed] [Google Scholar]
- 203.Lachapelle J.M. A comparison of the irritant and allergenic properties of antiseptics. Eur. J. Dermatol. 2014;24:3–9. doi: 10.1684/ejd.2013.2198. [DOI] [PubMed] [Google Scholar]