Abstract
Impulsivity and compulsivity are traits relevant to a range of mental health problems and have traditionally been conceptualised as distinct constructs. Here, we reconceptualised impulsivity and compulsivity as partially overlapping phenotypes using a bifactor modelling approach and estimated heritability for their shared and unique phenotypic variance within a classical twin design. Adult twin pairs (N = 173) completed self-report questionnaires measuring psychological processes related to impulsivity and compulsivity. We fitted variance components models to three uncorrelated phenotypic dimensions: a general impulsive–compulsive dimension; and two narrower phenotypes related to impulsivity and obsessiveness.There was evidence of moderate heritability for impulsivity (A2 = 0.33), modest additive genetic or common environmental effects for obsessiveness (A2 = 0.25; C2 = 0.23), and moderate effects of common environment (C2 = 0.36) for the general dimension, This general impulsive–compulsive phenotype may reflect a quantitative liability to related mental health disorders that indexes exposure to potentially modifiable environmental risk factors.
Subject terms: Psychology, Human behaviour
Introduction
Psychiatric research is transitioning away from traditional categorisations of mental disorders as discrete diagnostic entities towards empirically based, dimensional models of psychopathology1,2. An aim of dimensional psychiatry is to identify aetiological mechanisms that transcend traditional diagnostic categories3. Dimensional approaches leverage quantitative methods to capture covariation in psychopathology symptoms and relevant psychological processes into a smaller number of continuously distributed phenotypic dimensions in the population4. Mental disorders may reflect the extreme end of the spectrum of quantitative traits that are expressed throughout the population and extend into subclinical and non-clinical ranges5,6. By capturing phenotypic variation across the full spectrum of symptom severity, dimensional models are better positioned to identify shared aetiological mechanisms and the common genetic architecture of psychiatric disorders2,6,7.
Hierarchical organisation is an important feature of dimensional models, such that distributed traits relevant to psychopathology can be understood at varying level of generality and specificity1,8. Psychiatric phenotypes can be organised into hierarchical dimensions using bifactor models within a structural equation modelling framework, which enable common variance across phenotypic traits to be separated from unique within-trait variance4. Partitioning phenotypic variance into common and specific dimensions facilitates novel insights into the operation of aetiological mechanisms, such as identification of broad versus narrow genetic risk factors8,9.
Impulsivity and compulsivity are psychiatric phenotypes implicated in the aetiology and maintenance of impulse control, obsessive–compulsive, and addictive and related disorders10,11. Impulsivity is a multifaceted construct that describes a predisposition to rapid unplanned actions without preplanning or forethought and without consideration of potential adverse consequences12,13. Compulsivity is likewise a multifaceted construct that describes patterns of behaviour that are repeated despite undesirable consequences11,14. Impulsivity and compulsivity have historically been conceptualised as opposite ends of a single underlying spectrum, extending from risk-seeking (i.e. reward-seeking) to risk-avoidance (i.e. harm-avoidance)15,16. More recent conceptualisations suggest considerable phenotypic and neurobiological overlap between impulsivity and compulsivity10,17,18. This overlap may reflect dysfunction in top-down, goal-directed cognitive control mechanisms19–24 and be particularly relevant to the aetiology and maintenance of impulse control, obsessive–compulsive, and addictive and related disorders25,26.
The overlapping dimensional phenotypes model operationalises impulsivity and compulsivity as empirically-related continuous dimensions using a bifactor model within a structural equation modelling (SEM) framework22. We fitted a bifactor model to data obtained from self-report questionnaires measuring psychological processes related to impulsivity, obsessive beliefs, and intolerance of uncertainty in an unselected community sample of 587 adults. The model included three uncorrelated and normally distributed dimensions: (1) a general factor, reflecting a combination of high general impulsivity, intolerance of uncertainty, and obsessive beliefs; (2) an ‘Impulsivity’ factor, capturing residual variance specific to sensation seeking, high emotion-based rash action (i.e. impulsive behaviours in response to positive and negative emotions), and a lack of premeditation; and (3) a ‘Compulsivity’ factor, reflecting residual variance specific to intolerance of uncertainty, obsessive beliefs, perfectionism, perceived responsibility, and sensitivity to threat. We found that the general dimension explained the most variance in co-occurring impulse control problems, including alcohol misuse, problem gambling, and compulsive shopping, as well as obsessive–compulsive-related problems, including problematic use of the internet, binge eating, and obsessive–compulsive symptoms. The Impulsivity and Compulsivity factors explained additional unique variance in impulse control problems and obsessive–compulsive-related problems, respectively.
We have since replicated the overlapping dimensional phenotypes model in an independent longitudinal study sample using a different measure of impulsivity and compulsivity and showed that the three, empirically distinct phenotypic dimensions were uniquely associated with a number of risk factors and antecedents27. This model has also been extended to incorporate participants from an imaging study with diagnosed obsessive–compulsive disorder and gambling disorder, as well as healthy controls. Resting state functional Magnetic Resonance Imaging data were analysed using dynamic causal modeling28. Higher levels of the general dimension, combining psychological processes related to impulsivity and compulsivity, were associated transdiagnostically with lower bottom-up effective connectivity in the dorsal circuit and greater bottom-up effective connectivity in the ventral circuit21. These findings are consistent with the theory that impulsivity and compulsivity reflect dysfunctional changes in cortical-striatal-thalamic-cortical circuits leading to impairments in top-down cognitive control underlying a range of psychiatric disorders18–20,29,30. This empirical overlap between psychological processes related to impulsivity and compulsivity is thus a potentially informative model for understanding impulse control, obsessive–compulsive, and addiction-related problems, in terms of shared aetiology and transdiagnostic treatment approaches.
Obtaining estimates of heritability, the proportion of variance in a phenotype attributable to genetic versus environmental effects, has implications for understanding the aetiological mechanisms linking dimensional phenotypes to related psychological disorders31. The results of initial quantitative genetics studies may also inform subsequent genome-wide association studies and interventions research32,33. The classical twin study design represents a suitable methodology for testing hypotheses of heritability, particularly for complex traits32,34. Embedded within an SEM framework, the classical twin design allows researchers to estimate the degree of population variability in a phenotype that is attributable to the influence of genetic (additive [A2] or dominance [D2] effects), common environment [C2], and unique environment [E2] effects by comparing within-trait correlations between monozygotic and dizygotic twins35,36. It also allows for the fit of competing models to be directly compared using statistical criteria35,36. Heritability (h2) represents the proportion of phenotypic variance attributable to genetic effects, divided by the total variance explained by genetic, common and unique environment, as well as unexplained error variance (h2 = A2 + D2/A2 + D2 + C2 + E2)35,37.
Previous studies have sought to quantify the genetic contribution to impulsivity using behavioural and self-report measures within a classic twin study methodology. Broad-sense heritability estimates of between 0.38 and 0.50 have been reported for impulsivity with no contribution of common environment across the lifespan (i.e. ADE or AE models)38–40. Compulsivity has not been researched as thoroughly, nor is it as well-understood, as impulsivity11,14,18. Until recently, researchers have tended to conceptualise compulsivity with reference to obsessive compulsive disorder (OCD), which has been considered the ‘archetypal’ compulsive disorder16,41. Thus, several studies have examined the heritability of OCD symptom dimensions and symptoms of related obsessive–compulsive spectrum disorders42–46. These studies have revealed modest to strong heritability for obsessive–compulsive symptoms, generally with no effect of common environment (i.e. AE models)47–49. The broad consensus from these studies is that impulsivity and compulsivity are best attributed to modest to strong genetic effects, with the rest attributable to unique environment and measurement error.
The purpose of the current study was to investigate the heritability of both shared and unique phenotypic variance in impulsivity and compulsivity. Understanding the genetic and environmental influences on the overlap between impulsivity and compulsivity, as well as their independent phenotypic variance, may provide useful insights into the aetiology of impulse control, obsessive–compulsive, and addiction-related disorders, as well as facilitate future large-scale genetic studies. Based on previous studies examining the impulsivity phenotype and OCD-related symptoms in adult twin samples, we hypothesised that additive genetic effects models with no common environment effects (i.e. AE models) would provide the best fit to covariance data obtained from monozygotic and dizygotic twins. We expected that heritability estimates obtained from these AE models for impulsivity and compulsivity phenotypes would be modest to moderate (h2 ~ 0.30–0.50). Given the paucity of previous research directly examining the phenotypic overlap of psychological processes related to impulsivity and compulsivity, we tentatively hypothesised an AE model and modest to moderate heritability for the general factor (i.e. AE model).
Results
Twins from each pair were randomly assigned (i.e. twin 1 or twin 2) to separate subsamples for preliminary analyses and cross-validation of the results prior to being combined for the variance components models and heritability analyses. Descriptive statistics for the Urgency, Premeditation (lack of), Perseverance (lack of), Sensation Seeking, and Positive Urgency (UPPS-P) Impulsivity Behavior Scale, Intolerance of Uncertainty Scale 12-item version (IUS-12), and Obsessive Beliefs Questionnaire 44-item version (OBQ-44) subscales are provided in Tables S1 and S2 Supplementary Information. Results of first-order confirmatory factor analysis (CFA) of the UPPS-P, IUS-12, and OBQ-44 subscale structure in the twin 1 subsample, along with cross-validation in the twin 2 subsample with invariance testing, are described in Supplementary Information and presented in Supplementary Tables S3–S6 online. Information functions obtained from the first-order CFA of the UPPS-P, IUS-12, and OBQ-44 based on competing factor models (i.e. published subscale structure versus bifactor models) are displayed in Fig. S1–S10 Supplementary Information, and reveal the precision of measurement across the latent trait continuum for each factor solution based on item response theory. Model fit statistics displayed in Tables S3–S6, in combination with the information functions in Fig. S1–S10, showed that four-, two- and three-factor models that reproduced the published subscale structures of the UPPS-P, IUS-12, and OBQ-44 provided a better fit to the data compared to competing models. Subscale raw scores were thus used as indicators (i.e. dependent/observed variables) for testing competing second-order models of impulsivity and compulsivity.
Bifactor model of impulsivity and compulsivity
Subscale raw scores were entered as the indicator variables for the bifactor model. Negative Urgency and Positive Urgency items were combined into a single ‘Urgency’ subscale based on a four-factor model solution of the UPPS-P (see Supplementary Table S3 online). A bifactor model was specified after Tiego et al. (2018) with all nine variables loading on a general factor, the four UPPS-P subscales loading on an ‘Impulsivity’ group factor, and the IUS-12 and OBQ-44 subscales loading on a ‘Compulsivity’ group factor. The three factors were specified as orthogonal dimensions (i.e. factor intercorrelations were constrained to zero). The Lack of Perseverance (UPPS-P) subscale failed to load on the general factor; the Sensation Seeking (UPPS-P) subscale did not load on the Impulsivity factor, and the Desire for Predictability and an Active Engagement in Seeking Certainty and Paralysis of Cognition and Action in the Face of Uncertainty (IUS-12) subscales did not load on the Compulsivity factor. These loadings were not freely estimated in the next iteration of the model. Several error covariances were freely estimated to obtain model fit; all corrected for multiple post hoc comparisons (i.e. Benjamini–Hochberg false discovery rate [q = 0.05])50.
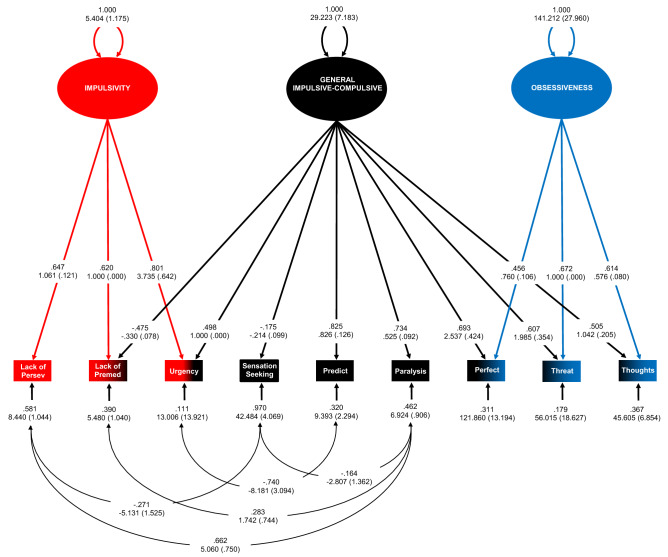
The final model is displayed in Fig. 1 (χ2(17) = 30.359, p = 0.082; RMSEA = 0.057 [90%CI = 0.021, 0.089]; CFI = 0.983; SRMR = 0.064). The pattern and strengths of factor loadings were interpretable as representing three distinct phenotypes: (1) a general ‘Impulsive–Compulsive’ –dimension with higher levels reflecting greater emotion-based rash action (i.e. urgency), intolerance of uncertainty, and obsessive beliefs; (2) Impulsivity—higher levels of this phenotype capturing more emotion-based rash action (i.e. urgency) in combination with lower levels of premeditation and perseverance; and (3) Obsessiveness—higher levels of this phenotype reflecting greater presence of obsessive beliefs related to responsibility, threat estimation, perfectionism and intolerance of uncertainty, and the importance and control of thoughts. In contrast to the original model, this second group factor had only loadings from the three OBQ-44 subscales representing different types of obsessive beliefs, and no loadings indicative of increased premeditation and perseverance22. We therefore named this factor ‘Obsessiveness’ to reflect a narrower empirical construct and interpretation than the previous ‘Compulsivity’ factor.
Figure 1.
Bifactor model of psychological processes related to impulsivity and compulsivity in the twin 1 subsample. Model fit statistics were χ2(17) = 30.359, p = 0.082; RMSEA = 0.057 [90%CI = 0.021, .089]; CFI = 0.983; SRMR = 0.064. All five error covariances were adjusted for multiple post hoc comparisons (B-H p = 0.039). N = 242. Model figures are displayed using symbols from the McArdle-McDonald reticular action model131. Observed (also measured or manifest) variables are represented as rectangles. Latent variables are represented as ellipses: (1) factors (or constructs) are represented as large ellipses; (2) error variances for observed variables are symbolised with small ellipses. Double-headed, curved arrows pointing to factors are the latent variable variances. Straight, single-headed arrows from large ellipses to observed variables reflect factor loadings. Straight, single-headed arrows pointing from small ellipses to measured variables are the (i.e. error) variances in the variables not explained by the factor. Curved, double-headed arrows between small ellipses are error covariances. Factor scaling was performed using the reference variable method, with the unstandardised loading estimate of one measured variable for each factor set to one. Fully standardised parameter estimates appear above with unstandardised parameter estimates and bootstrapped standard errors (10,000 posterior draws) in brackets below. Lack of Persev lack of perseverance raw subscale scores (UPPS-P), Lack of Premed lack of premeditation raw subscale scores (UPPS-P), Urgency summed raw scores on the positive urgency and negative urgency subscales (UPPS-P), Sensation Seeking sensation seeking raw subscale scores (UPPS-P), Predict desire for predictability and an active engagement in seeking certainty subscale raw scores (IUS-12), Paralysis paralysis of cognition and action in the face of uncertainty subscale raw scores (IUS-12), Perfect perfectionism and intolerance of uncertainty subscale raw scores (OBQ-44), Threat responsibility and threat estimation subscale raw scores (OBQ-44), Thoughts importance and control of thoughts subscale raw scores (OBQ-44). Figure created in Microsoft Excel 2016, Office desktop (16.0.12624.20424) 64-bit.
Model fit statistics for the competing one-, two-, and three-factor models are provided in Table S7 Supplementary Material. The two- and three-factor models failed to provide a good fit to the data. Two of nine subscales, Sensations Seeking and Lack of Perseverance, had to be dropped to facilitate fit for the unidimensional model, thus compromising construct representation and criterion-related validity of the factors51. The hypothesised bifactor model therefore provided the best fit to the data. The model was cross-validated in the twin 2 subsample using invariance testing. The intercorrelation matrix for the subscale scores in twin 1 and twin 2 subsamples is displayed in Table S8. The results of invariance testing are provided in Table 1 and Table S9. Full configural, weak, strong, and partial strict invariance (i.e. only two indicators were not measured with the same level of precision) were demonstrated in the twin 2 subsample along with equivalence of factor variances (Table 1) and means (Table S9). These results indicated that the model had very stable measurement properties and the three phenotypes were being measured equivalently and could be compared meaningfully across twin pairs.
Table 1.
Results of invariance testing for the bifactor model of impulsivity and compulsivity phenotypes in the twin 1 and twin 2 subsamples.
| Model | df | χ2 | p | RMSEA (90% CI) | CFI | SRMR | Δdf | Δχ2 | Δp |
|---|---|---|---|---|---|---|---|---|---|
| Configural invariance | 35 | 76.310 | 0.266 | 0.070 (0.048–0.091) | 0.973 | 0.060 | |||
| Weak invariance | 46 | 87.844 | 0.345 | 0.061 (0.041–0.080) | 0.973 | 0.066 | 11 | 11.534 | 0.400 |
| Strong invariancea | 55 | 98.454 | 0.340 | 0.057 (0.038–0.075) | 0.972 | 0.065 | 9 | 10.610 | 0.303 |
| Partial strict invarianceb | 61 | 106.176 | 0.372 | 0.055 (0.037–0.072) | 0.971 | 0.082 | 6 | 7.722 | 0.259 |
| Equality of factor variances | 64 | 107.317 | 0.390 | 0.053 (0.035–0.070) | 0.972 | 0.086 | 3 | 1.141 | 0.767 |
N = 486 (Twin 1 subsample n = 241; Twin 2 subsample n = 245).
df degress of freedom, χ2 Chi square value for test of model fit using full information maximum likelihood estimation, p significance value of the chi square test statistic, RMSEA root mean square error of approximation, CI confidence interval, SRMR standardised root mean residual, CFI comparative fit index, Δdf delta degrees of freedom, Δχ2 delta chi square, Δp significance value of the delta chi square test statistic.
aEquality of latent means were tested after strong invariance was determined (impulsive–compulsive [z = 0.441, p = 0.660]; impulsivity [z = − 0.319, p = 0.750]; compulsivity [z = − 1.333, p = 0.183]).
bPartial strict invariance—the error variances for the Urgency (θδ = 56.185, SE = 10.284, p < 0.001), Importance and Control of Thoughts (θδ = 157.541, SE = 88.171, p = 0.074), and the Paralysis of Cognition and Action (θδ = 16.600, SE = 2.095, p < 0.001) subscales were freely estimated in the twin 2 subsample to obtain acceptable fit. Error covariances also differed between subsamples and were therefore unconstrained and freely estimated within groups.
Heritability analyses
Within-trait and between trait subscale correlations for monozygotic and dizygotic twins are provided in Table S10 and S11, respectively. The twin 1 and twin 2 subsamples were combined and the bifactor model specified to generate factor score estimates using the regression method52–54. Factor score determinacies were high for the general Impulsive–Compulsive, Impulsivity, and Obsessiveness m phenotypes, respectively (ρ = 0.914, 0.861, 0.814), indicating that the factor score estimates provided relatively accurate measurement of individual differences on the three latent variables52,53. Additionally, the factor score estimates were only weakly correlated (general factor with Impulsivity r = 0.001, p = 0.981 and Compulsivity r = 0.239, p < 0.001; Impulsivity with Compulsivity r = 0.175, p < 0.001), suggesting that the orthogonality of the phenotypes in the bifactor model had largely been preserved (i.e. correlational preserving) and that the factor score estimates were not being unduly contaminated by variance from the other phenotypes (i.e. univocality)52,53.
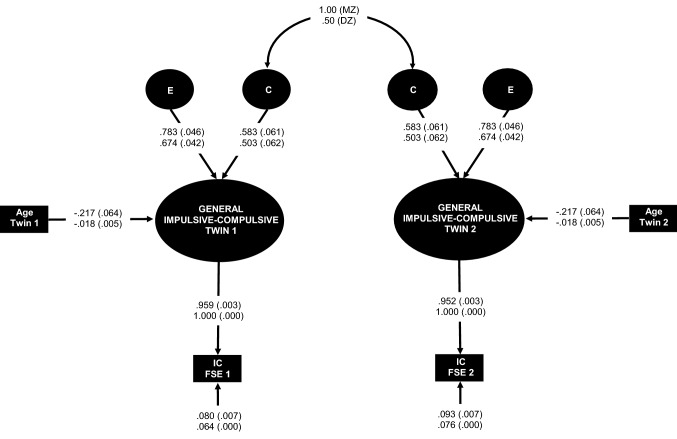
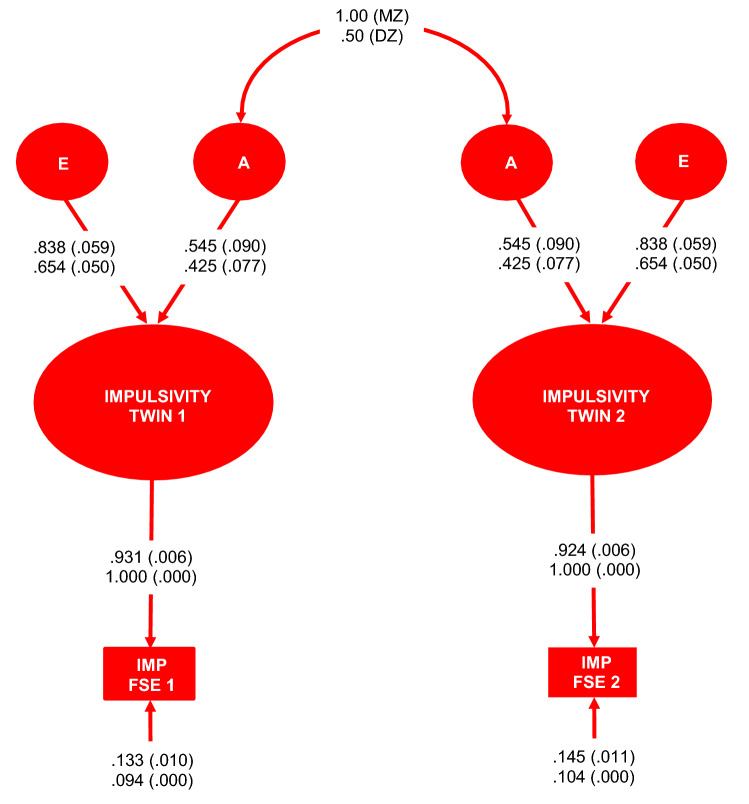
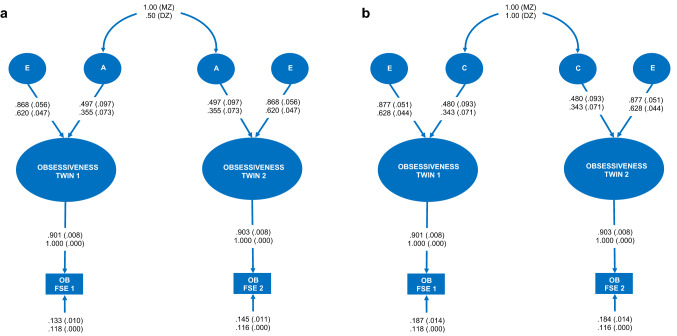
The distributions of the factor scores estimates were examined separately for the twin 1 and twin 2 subsamples (see Fig. S11–S13 Supplementary Information online) and screened for univariate outliers. Two outliers were removed for the general dimension from the twin 2 subsample and recoded as missing data55. The factor scores estimates for the twin 1 and twin 2 subsamples were entered as single-indicator latent variables with error variance fixed to reflect the inverse of the product of the factor determinacy and the variance of the subsample [θδ = (1 – ρ)*σ2]52,56. This approach enabled greater control of error in the estimation process of individual differences in the latent dimensional phenotypes using the factor score estimates. Sex and age were initially included as regressors in the models but did not predict variance in Impulsivity or Compulsivity. Age, but not sex, was a meaningful predictor of twin differences in the general Impulsive–Compulsive dimension and was therefore included in all the variance components models for this phenotype. Nested variance components models were generated for all three phenotypic dimensions and directly compared for statistical fit to the data using Bayesian statistics. The results are summarised in Table 2. The pattern of covariances between monozygotic and dizygotic twin pairs was best represented by a CE model for the general Impulsive–Compulsive dimension (see Fig. 2), an AE model for Impulsivity (see Fig. 3), and an AE or CE model for Obsessiveness (see Fig. 4A,B). Alternative models are displayed for Obsessiveness because there was insufficient evidence for adjudicating between them based on Bayesian statistics57,58. The proportion of population variability in the general Impulsive–Compulsive phenotype attributabed to common environment was 36%. Heritability of the Impulsivity phenotype was estimated as 0.33 for the AE model. Heritability of the Obsessiveness phenotype was estimated as 0.25 in the AE model. Conversely, common environment was estimated to explain 23% of the population variability in Obsessiveness in the CE model.
Table 2.
Fit statistics for competing variance components models for the impulsive–compulsive, impulsivity, and obsessiveness phenotypes.
| Phenotype | Model | − 2*LL | df | χ2 | P | BIC | Hi (Pr | D) |
|---|---|---|---|---|---|---|---|
| Impulsive–compulsivea | ACE | − 437.897 | 12 | 28.101 | 0.141 | 906.714 | 0.055 |
| AE | − 439.103 | 13 | 19.639 | 0.105 | 903.973 | 0.217 | |
| CEd | − 437.897 | 13 | 17.226 | 0.189 | 901.560 | 0.727 | |
| E | − 447.563 | 14 | 36.558 | 0.001 | 915.739 | 0.001 | |
| Impulsivity | ACE | − 426.603 | 5 | 5.177 | 0.395 | 878.972 | 0.046 |
| AEd | − 426.603 | 6 | 5.177 | 0.521 | 873.819 | 0.604 | |
| ADE | − 424.014 | 5 | 3.699 | 0.594 | 877.494 | 0.096 | |
| CE | − 428.187 | 6 | 8.346 | 0.214 | 876.987 | 0.124 | |
| E | − 430.712 | 7 | 13.396 | 0.063 | 876.884 | 0.130 | |
| Obsessiveness | ACE | − 407.493 | 5 | 7.404 | 0.192 | 840.752 | 0.030 |
| AEb | − 407.582 | 6 | 7.583 | 0.270 | 835.777 | 0.356 | |
| CEc | − 407.504 | 6 | 7.426 | 0.283 | 835.621 | 0.385 | |
| E | − 410.602 | 7 | 13.622 | 0.058 | 836.663 | 0.229 |
− 2*LL − 2 × log likelihood, df degrees of freedom for the chi square test statistic, χ2 chi square test of model fit, p probability of the chi square test statistic, BIC Bayesian information criterion, Hi(Pr|D) Bayesian conditional posterior probability of model Hi compared with Hk models, A additive genetic effects, C common environment effects, E unique environment effects + error variance, D dominance genetic effects.
aAge included in all models as a regressor for the impulsive-compulsive phenotype.
bBayesian posterior probability of AE compared to CE = [H0 (Pr | D) = 0.481].
cBayesian posterior probability of CE compared to AE = [H1 (Pr | D) = 0.519].
dPreferred model based on weight of evidence.
Figure 2.
Common environment and unique environment plus error (CE) [χ2(13) = 17.226, p = 0.189; BIC = 901.560. Hi (Pr | D) = 0.727] variance components model for the Impulsive–Compulsive phenotype. MZ monozygotic twins, DZ dizygotic twins, E unique environment plus measurement error variance component, C common environment variance component, Age age in years, IC FSE 1 twin 1 subsample Impulsive–Compulsive factor score estimates, IC FSE 2 twin 2 subsample Impulsive–Compulsive factor score estimates. Fully standardised parameter estimates with standard errors in brackets appear above. Unstandardised parameter estimates with standard errors in brackets appear below. Figure created in Microsoft Excel 2016, Office desktop (16.0.12624.20424) 64-bit.
Figure 3.
Additive genetic and unique environment plus error (AE) [χ2(6) = 5.177, p = 0.521; BIC = 873.819; Hi (Pr | D) = 0.604]; variance components model for the Impulsivity phenotype. MZ monozygotic twins, DZ dizygotic twins, E unique environment plus measurement error variance component, A additive genetic variance component, Age age in years, IMP FSE 1 twin 1 subsample impulsivity factor score estimates, IMP FSE 2 twin 2 subsample impulsivity factor score estimates. Fully standardised parameter estimates with standard errors in brackets appear above. Unstandardised parameter estimates with standard errors in brackets appear below. Figure created in Microsoft Excel 2016, Office desktop (16.0.12624.20424) 64-bit.
Figure 4.
(a) Additive genetic and unique environment plus error (AE) [χ2(6) = 7.583, p = 0.270; BIC = 835.777; Hi (Pr | D) = 0.274]; and (b) common environment and unique environment plus error (CE) [χ2(6) = 7.426, p = 0.283; BIC = 835.621; Hi (Pr | D) = 0.296] variance components models for the obsessiveness phenotype. MZ monozygotic twins, DZ dizygotic twins, E unique environment variance component, A additive genetic variance component, C common environment variance component, Age age in years, OB FSE 1 twin 1 subsample obsessiveness factor score estimates, OB FSE 2 twin 2 subsample obsessiveness factor score estimates. Fully standardised parameter estimates with standard errors in brackets appear above. Unstandardised parameter estimates with standard errors in brackets appear below. Figure created in Microsoft Excel 2016, Office desktop (16.0.12624.20424) 64-bit.
Discussion
Impulsivity and compulsivity are candidate psychiatric phenotypes with potential transdiagnostic utility in dimensional psychiatry, particularly in relation to impulse control and obsessive–compulsive and related disorders, as well as substance and behavioural addictions10,24,59. Previous twin modeling studies have explored the heritability of broadly defined traits of impulsivity and compulsivity in isolation and obtained modest to strong heritability estimates38,42–45. The present study extended the focus of existing literature by examining impulsivity and compulsivity in concert, specifically their phenotypic overlap21–23. We operationalised these latent phenotypes as self-reported psychological processes related to different forms of impulsivity, intolerance of uncertainty, and obsessive beliefs. Using the classical twin design, we found that a CE variance components model provided the best fit to the pattern of covariances between monozygotic and dizygotic twins for the general dimension, reflecting a combination of intolerance of uncertainty, obsessive beliefs, and a tendency towards emotion-based rash action. The results suggested a modest effect of common environment on individual differences in this phenotype (C2 = 0.36). This finding is notable in that several previous behavioural genetic studies have found negligible effects of common environment on broadly defined traits of impulsivity and compulsivity38,42–46. Thus, this phenotypic dimension may provide a window into the mechanisms contributing to the co-occurrence of impulsive and compulsive behavioural tendencies, which appear to have a distinct aetiology, as indicated by the finding of a moderate sized effect of the common environment. Importantly, these results do not conclusively demonstrate negligible genetic effects, because what is decomposed into common environment in the variance components model can partly reflect genetic effects, or genotype-environment correlations33,36,60.
The general Impulsive–Compulsive factor here defined may be compared with the ‘Behavioral Disinhibition’ latent phenotype described in previous behavioural and molecular genetics literature61–65. Behavioural Disinhibition describes a spectrum of psychological disorders, temperament/personality traits, and related behaviours representing a common inability to inhibit impulsive actions62,64. Among the facets reflecting Behavioral Disinhibition are diagnoses of conduct disorder, attention deficit hyperactivity disorder, antisocial personality disorder, as well as temperament traits of novelty seeking, and low constraint61–63. Behavioral Disinhibition has been found to be moderately to highly heritable, with zero to modest effects of the common environment62,65,66. This latent phenotype has become the focus of behavioural and molecular genetic studies because it was constructed to capture and quantify a common liability to impulsive action that is highly heritable and confers a vulnerability to the development of a range of impulsive and compulsive disorders67,68.
Here, we have sought to quantify the influence of genetic and environmental effects on the common and unique variance of self-reported psychological processes related to impulsivity, intolerance of uncertainty, and obsessive beliefs because of their putative role in the development of impulsive and compulsive psychopathology. Our model explicitly incorporates psychological processes related to risk-avoidance (e.g. threat estimation; paralysis of cognition and action in the face of uncertainty) that have traditionally been viewed as anti-correlated with the risk-taking and reward-seeking central to the construct of impulsivity15,16. Only more recently has the potential phenotypic and neurobiological overlap of impulsivity and compulsivity been explored and explicitly tested18,59. Furthermore, the bifactor modelling approach we have used here enables both the shared and unique variance in psychological processes related to impulsivity and compulsivity to be examined, as opposed to only the shared variance captured by a common factor model, such as that typically used to model Behavioral Disinhibition70. We have previously shown that these unique phenotypic dimensions related to impulsivity and compulsivity are associated with distinct antecedents and neurobiological substrates, as well as explaining additional variance in impulse-control and obsessive–compulsive related problems27,71,22. However, by virtue of capturing shared variance across addiction-related problems and dimensions of personality and temperament such as constraint, the Behavioral Disinhibition construct likely incorporates variance related to compulsivity. Thus, research that directly and empirically compares these constructs within the same sample would be needed to establish the discriminant validity of the general Impulsive–Compulsive dimension defined in this study from the Behavioral Disinhibition construct previously established in the behavioural genetics literature10,22.
Interestingly, there were weak negative associations with age, suggesting that levels of the general phenotype tended to be slightly lower in older compared with younger participants. The general factor in the model explained comparatively more variance in the IUS-12 and OBQ-44 subscales relative to the UPPS-P subscales. This indicates that the general phenotype largely reflects intolerance of uncertainty and obsessive beliefs, in addition to emotion-based rash action, and lack of premeditation. Uncertainty intolerance has been shown to decrease with age, partly due to changes in the perceived adaptive and functional benefits of worry72. Older individuals may be more competent and motivated towards selecting situations and environments that foster stability, predictability, and well-being73,74. Importantly, intolerance of uncertainty and obsessive beliefs are amenable to therapeutic change, suggesting potential intervention strategies for reducing what could be a general liability for impulsive and compulsive problems75–77.
This study extends previous behavioural genetic research by examining heritability of the unique variance in impulsivity and obsessiveness following removal of the empirical overlap between these phenotypes. We found that the proportion of variance in the impulsivity phenotype attributable to additive genetic effects (A2 = 0.33) was comparable to that reported in a meta-analysis of behavioural genetic studies when examining questionnaire data (A2 = 0.29—0.37), but less than broad sense heritability estimates (i.e. A2 + D2 = 0.41—0.69) obtained from the best-fitting ADE models38. In our study, the ADE model did not provide improvement in fit compared to the AE model, indicating that the combination of additive and dominance genetic effects was unnecessary to account for the stronger covariances in monozygotic compared to dizygotic twins. This may suggest that the impulsivity phenotype in our model reflects a narrower trait associated with smaller genetic effects than the broadly defined trait of impulsivity generally investigated in the literature. However, impulsivity is a multifaceted construct and there is yet no consensus on the number and operationalisation of what may be several phenotypically and genetically distinct traits12,13,38. In particular, there is poor convergence between self-report and behavioural measure of impulsivity, suggesting these methods assess at least partly dissociable constructs78. Further studies would be required to clarify how impulsivity, as we have defined and measured it, relates to the different variations of the impulsivity construct treated in the literature.
The heritability estimate obtained from the AE model for the Obsessiveness factor was modest (A2 = 0.25) and slightly lower than estimates reported in twin modeling studies of obsessive–compulsive and related disorders (A2 ~ 0.30)42–45. These results again may reflect differences in the operationalisation and measurement of these compulsivity-related constructs. As previously noted, compulsivity has not been as well-defined nor thoroughly investigated as impulsivity11,14,18. Researchers have tended to operationalise compulsivity with reference to OCD and related obsessive–compulsive spectrum disorders16,41. This divergence in conceptualisation and measurement may explain the difference in heritability estimates. Additionally, previous studies examining the heritability of obsessive–compulsive and related disorders have not controlled for phenotypic overlap with impulsivity. An intimate relationship has been proposed between predisposing levels of impulsivity and the subsequent development of compulsivity, suggesting these traits may have common genetic and environmental aetiological mechanisms19. Parsing distinct compulsivity-related variance from overlapping variance with impulsivity may explain the discrepant results. Furthermore, evidence for the AE model was equivocal and a CE model provided a comparable fit to the pattern of covariances between monozygotic and dizygotic twins. This may suggest that there is no contribution of genetic effects following removal of the phenotypic overlap with impulsivity. Follow-up studies with more statistical power are required to adjudicate between the AE and CE models and address this question.
Heritability has been posited as an important criterion for evaluating candidate psychiatric endophenotypes79,80. Endophenotypes are a key concept in dimensional psychiatry, referring to latent (not visible to unaided observation) quantitative traits that index liability for psychiatric disorders31,81. Identification of psychiatric endophenotypes is intended to increase conceptual clarity and precision of measurement in dimensional psychiatry80,82. However, psychiatric endophenotypes that index environmental risk may be just as useful for understanding the aetiology of mental disorders31. As a so-called “environmental endophenotype” (Kendler & Neale, 2010, p. 795), the general Impulsive–Compulsive dimension in the current study, capturing intolerance of uncertainty, obsessiveness, and predisposition towards emotion-based rash action, could provide a quantitative index of liability to various disorders that may be attributable to one or more identifiable environmental mechanisms. Importantly, these environmental influences may be modifiable with implications for intervention and treatment of impulse control, obsessive–compulsive, and addictive disorders.
It is also possible that this general dimension partly reflects genotype-environment correlations, suggesting some contribution of genetics to individual differences in the overlap of psychological processes related to impulsivity and compulsivity33,60. Nevertheless, the modest contributions of shared environment to this phenotype is an important validation of the general factor in our model. The general factor in bifactor models has been criticised as a common method or response bias factor, or other forms of random error variance83,84. The present results suggest that the general factor in the overlapping dimensional phenotypes model reflects meaningful phenotypic variance, rather than merely random error variance, as would be indicated by a better fit for an E compared to a CE variance components model.The current study also demonstrates replicability of this model in an independent sample, and extends on previous studies by using a more parsimonious measurement approach (see Supplementary Material for further details)21,22.
A criticism of bifactor models is their tendency to provide superior fit compared to competing models because they flexibly accommodate patterns of covariance regardless of population data structure70,85,86. However, results of model testing support use of the bifactor model as a theoretically consistent and empirically plausible representation of the current data. The pattern and strength of factor loadings were consistent with the presence of a robust general factor and two residual group factors, all with statistically significant variance estimates70,87. There were no illogical or near boundary parameter estimates and the standard errors were all small and within a sensible range88,89. Only a few theoretically-plausible freely estimated error covariances were required to obtain close fit to the data, all statistically significant when adjusting for multiple post hoc comparisons using the false discovery rate50. Thus, there were no signs in the model parameter estimates, or global and local fit indices, that the bifactor model was misspecified. When added to our previous findings of meaningful links between these three phenotypes with antecedent risks, symptoms of psychopathology, and neurobiology, we feel confident that the bifactor model is a plausible and useful representation of individual difference variance in self-reported psychological processes related to impulsivity and compulsivity22,27,71.
We found that a CE variance components model provided a superior fit to the pattern of covariances between monozygotic and dizygotic twins than an AE model for the general dimension. However, evidence for the CE over the AE model using Bayesian statistics was relatively weak by conventional standards57,58,90. Similarly, there was insufficient statistical evidence to adjudicate between the AE and CE models for the Obsessiveness phenotype. Further studies with larger numbers of twin pairs are needed to replicate the findings and adequately compare the competing models91,92. In particular, statistical power to detect additive genetic effects is reduced as the ratio of dizygotic to monozygotic twin pairs decreases91,92. This reduces confidence in the finding that the CE model provided a better fit to the data than the AE model for the general phenotype. Our results should therefore be considered tentative and with reference to these methodological limitations. Future studies of the heritability of this general phenotype, reflecting intolerance of uncertainty, obsessiveness, and emotion-based rash action, will require much larger samples and a higher ratio of dizygotic to monozygotic twin pairs to ensure adequate power to adjudicate between AE and CE models. With larger sample sizes, multivariate twin models would be an important extension of this study and could investigate whether the genetic and environmental effects common to comorbid disorders is partly attributable to variance in these three phenotypes, suggesting a common transdiagnostic aetiology45,93.
The strength of genetic effects on the expression of complex traits, such as impulsivity, changes across the lifespan and notably has been shown to increase with age, possibly due to gene-environment interactions38,94. In our sample, there was a difference of medium sized effect in the mean age of monozygotic [M = 38.60, SD = 10.42] and dizygotic [M = 44.60, SD = 9.35] twins used in the variance components models [t (171) = 3.64, p < 0.001; Cohen’s d = 0.61; ΔM = − 6.00 (95% CI − 2.75, − 9.25)]. Model parameter estimates may have been affected if the relationship between age and the phenotypes differs markedly across the age range. There were minimal differences in the sex composition for monozygotic and dizygotic twin pairs in the final sample used to for the variance components models [χ(1) = 1.651, p = 0.199, n = 344]. Furthermore, there has been mixed evidence for sex effects in the genetic and environmental aetiology of phenotypes related to normal personality and psychopathology, including impulsivity38,40,49,94–96. Nevertheless, much larger samples would be required to test sex limitation models91.
We operationalised impulsivity using the UPPS-P Impulsive Behaviour Scale, a popular multi-dimensional model used in the wider personality and psychopathology literature to measure impulsivity98–101. Nevertheless, not all researchers agree on the validity or utility of differentiating between five facets of impulsivity and broad consensus has converged on far fewer distinct impulsivity constructs relevant to psychopathology102–104. We recapitulated our original finding that Negative Urgency and Positive Urgency were indistinguishable at the level of latent variables and could be collapsed into a unitary ‘Urgency’ construct, which also parallels the second-order ‘Emotion-Based Rash Action’ factor previously reported for the short-version of the UPPS-P22,105. Negative Urgency and Positive Urgency have often been subsumed under the common ‘Urgency’ construct in relation to addiction research106,107. Interestingly, Cyders et al. (2014) also reported a second-order ‘Deficits in Conscientiousness’ factor consisting of loadings from the Lack of Premeditation and Lack of Perseverance factors. Sensation Seeking was found to be empirically distinct from these otherwise conceptually similar impulsivity constructs, similar to the findings reported here and elsewhere previously108. Further work is needed to understand how the three target phenotypes in our model relate to the different conceptualisations of impulsivity and compulsivity in the literature18.
The compulsivity-related phenotype measured in the present study likely reflects an ‘Obsessiveness’ dimension rather than a broader compulsivity construct. A previous study found that a unidimensional ‘Obsessionality’ factor explained the majority of variance in OCD symptom domains and was partly heritable, obtaining a similar estimate of additive genetic effects to the present study (h2 = 0.19)109. Future studies could augment this Obsessiveness group factor by adding measures of overt compulsive behaviours, such as the compulsivity scale of the Impulsive–Compulsive Behaviours Checklist, the Obsessive Compulsive Inventory—Revised, or the Dimensional Yale-Brown Obsessive–Compulsive Scale110–112.
Our use of a community sample is consistent with the observed quantitative and dimensional organisation of psychiatric phenotypes and with recommendations to study these traits across the full spectrum of severity, which confers increased precision and statistical power for measuring genetic effects5,6,9. We have previously demonstrated continuity and normal distribution of the these three phenotypes in a sample combining non-clinical community-based participants and participants with diagnosed obsessive–compulsive disorder and pathological gambling71. Individual differences in these phenotypes explained self-reported severity of concurrent psychopathology symptoms, as well as relating to variation in effective connectivity in cortico-thalamo-cortical circuits, whereas diagnostic status did not71,22. Nevertheless, it would be informative for future studies to include twins with clinically diagnosed impulse control, obsessive–compulsive, and addiction-related disorders to test the continuity of the additive genetic and environmental factors in explaining clinically-relevant variance in the three phenotypic dimensions identified in this study.
Behavioural genetics studies using a classical twins design require accurate zygosity classification. Genotyping is the gold standard for zygosity classification, but is not always feasible in large-scale twin registries113. In these instances, self-reported zygosity is still used and yields high rates of accuracy and agreement with classification by genotype (> 90%)113–116. For zygosity classification, we combined responses from both twins on the Peas-in-a-Pod questionnaire, which has demonstrated 93% accuracy in the registry from which our sample was drawn117. However, monozygotic twins can be misclassified both pre- and post-natally and incorrectly identify as dizygotic on self-report measures118. Thus, our use of the self-report method should be considered a limitation as incorrect zygosity assignment can affect heritability estimates, especially in small samples119.
Conclusions
We provided results from a behavioural genetics study of the phenotypic overlap of impulsivity and compulsivity. The model was constructed using self-report questionnaires capturing psychological processes relevant to impulsivity, intolerance of uncertainty, and obsessive beliefs. Results from univariate twin models revealed that a CE model, representing a modest effect of the common environment, provided the best fit to individual difference variance in the general Impulsive–Compulsive phenotype compared to competing models. Moderate and modest estimates of heritability were obtained for the narrower Impulsivity and Obsessiveness phenotypes. General impulsivity–compulsivity, as operationalised here, may represent an ‘environmental endophenotype’ that indexes a quantitative liability to impulse control, obsessive–compulsive, and substance use disorders, as well as behavioural addictions, associated with exposure to environmental risk factors. The Impulsivity and Obsessiveness dimensions appear to be narrower and partly heritable traits in line with results from previous twin studies.
Methods
Participants
The research study methodology was approved by the Monash University Human Research Ethics Committee and Melbourne Human Research Ethics Committee prior to participant recruitment and data collection and proceeded in accordance with the Australian National Statement on Ethical Conduct in Human Research (2007) and the Declaration of Helsinki (1964). Twins were recruited from Twins Research Australia, a large national voluntary registry of 40,000 twin pairs120. Seven hundred adult twin pairs (1,400 individual twins) aged 18–55 years were randomly selected from the registry and invited by Twins Research Australia to participate in the study via email. Estimates of response rate and sample size requirements were based on general guidelines for twin modelling research and previous studies35,40. Data collection consisted of an online questionnaire battery administered using the Qualtrics Insight Platform [https://monash.az1.qualtrics.com/] over two consecutive sessions of approximately 20 min duration (40 min total). Written informed consent was obtained from each twin participant at the beginning of session one, prior to collecting demographic information and responses to questionnaires. Sample demographic information for the 522 twins that participated (response rate 37.3%), consisting of 195 twin pairs (MZ = 135; DZ = 60) and 132 twin singletons, is provided in Table 3. Zygosity was assigned by combining ratings for both twins on the ‘Two Peas in a Pod’ questionnaire, a method that yields 93% accuracy for zygosity determination and is still widely used and endorsed in the twin modelling literature114,115,121. Available data varied across questionnaires due to different response rates across the two sessions and/or missing data: UPPS-P N = 471 [twin 1 n = 230, twin 2 n = 241]; OBQ-44 N = 495 [twin 1 n = 241, twin 2 n = 254]; IUS-12 N = 419 [twin 1 n = 203, twin 2 n = 216]. The total number of twins (including singletons) for which at least partial data was obtained for the subscales scores for use as indicators in the second-order models was N = 487 (302 female), aged 18–55 (M = 39.78, SD = 10.53). Estimates of the three target phenotypes were available for 173 twin pairs (MZ = 118, DZ = 55).
Table 3.
Demographic variables of twins included in the analyses.
| Demographic variables | Twin pairs [N = 195] | Difference test MZ–DZ [t/χ2] | Twin singletons [N = 132] | ||
|---|---|---|---|---|---|
| Zygosity, n | MZ [n = 135] | DZ [n = 60] | MZ [n = 78] | DZ [n = 54] | |
|
Age M (SD) |
18–55 38.02 (10.37) |
19–55 43.77 (9.39) |
t (388) = − 5.19, p < 0.001 [d = 0.58] |
18–55 36.28 (10.46) |
21–55 42.65 (10.35) |
| Sex | |||||
| Sex ratio |
F = 174 (64.4%) M = 96 (35.6%) |
F = 72 (60.0%) M = 48 (40.0%) |
χ(1) = 0.705, p = 0.401 |
F = 37 (47.4%) M = 41 (52.6%) |
F = 37 (68.5%) M = 17 (31.5%) |
| Female/female | 86 (63.7%) | 25 (41.6%) | 38 (48.7%) | 22 (40.7%) | |
| Male/male | 47 (34.8%) | 13 (21.7%) | 40 (51.3%) | 8 (14.8%) | |
| Female/male | 0 (0.0%) | 22 (36.70%) | 0 (0.0%) | 24 (44.4%) | |
| Parental educationa | |||||
| Started primary school | 0 (0.0%) | 1 | |||
| Completed primary school | 4 (1.5%) | 0 | χ(9) = 7.914, p = 0.543 | 1 (1.3%) | 0 (0.0%) |
| Started secondary school/high school | 30 (11.1%) | 8 | 12 (15.4%) | 10 (18.5%) | |
| Completed secondary school/high school | 57 (20%) | 22 | 16 (20.5%) | 15 (27.8%) | |
| Started vocational/technical school | 8 (2.6%) | 2 | 1 (1.3%) | 0 (0.0%) | |
| Completed vocational/technical school | 52 (19.3%) | 28 | 16 (20.5%) | 8 (14.8%) | |
| Started tertiary undergraduate | 5 (1.9%) | 3 | 5 (6.4%) | 2 (3.7%) | |
| Completed tertiary undergraduate | 55 (20.4%) | 28 | 21 (26.9%) | 10 (18.5%) | |
| Started tertiary postgraduate | 5 (1.9%) | 2 | 0 (0.0%) | 0 (0.0%) | |
| Completed tertiary postgraduate | 47 (17.8%) | 23 | 6 (7.7%) | 9 (16.7%) | |
| Other/missing | 7 (0.7%) | 3 | 0 (0.0%) | 0 (0.0%) | |
| Employmenta | |||||
| Unemployed | 9 (3.3%) | 4 (3.6%) | χ(5) = 3.562, p = 0.614 | 5 (6.4%) | 3 (5.6%) |
| Student | 16 (5.9%) | 4 (3.6%) | 7 (9.0%) | 3 (5.6%) | |
| Self-employed | 28 (10.4%) | 11 (10.0%) | 8 (10.3%) | 5 (9.3%) | |
| Full time | 149 (55.2%) | 61 (55.5%) | 42 (53.8%) | 29 (53.7%) | |
| Part time/casual | 46 (17.0%) | 28 (25.5%) | 12 (15.4%) | 11 (20.4%) | |
| Other/missing | 22 (8.1%) | 12 (10.9%) | 4 (5.1%) | 3 (5.6%) | |
| Relationship statusa | 78 | 54 | |||
| Married/de facto | 163 (60.4%) | 79 (71.8%) | χ(5) = 13.013, p = 0.023 | 50 (64.1%) | 34 (63.0%) |
| Partner | 29 (10.7%) | 8 (7.3%) | 6 (7.7%) | 3 (5.6%) | |
| Divorced/separated | 7 (2.6%) | 11 (10.0%) | 3 (3.8%) | 3 (5.6%) | |
| Single | 62 (23.0%) | 19 (17.3%) | 19 (24.4%) | 12 (22.2%) | |
| Widowed | 2 (0.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Other/missing | 7 (2.6%) | 3 (2.7%) | 0 (0.0%) | 2 (3.7%) | |
N = 522.
aCounts and percentages based on responses of individual twins (MZ = 250, DZ = 110).
Materials
The 59-item Urgency, Premeditation (lack of), Perseverance (lack of), Sensation Seeking, and Positive Urgency (UPPS-P) Impulsive Behavior Scale measures impulsivity on a 4-point Likert scale (1—‘Agree strongly’ to 4—‘Disagree strongly’) across five dimensions of impulsivity: (1) Negative Urgency—impulsiveness in response to the experience of negative affect; (2) Sensation Seeking—reward-sensitivity/excitement seeking; (3) Lack of Premeditation—a tendency to act without deliberation and forethought; (4) Lack of Perseverance—inability to remain focused and complete a task that is boring or difficult; (5) Positive Urgency—impulsiveness in response to the experience of positive affect122. Thirty-nine of the items were recoded such that higher scores on each of the scales represented greater levels of impulsivity in each domain. The Obsessive Beliefs Questionnaire 44-item version (OBQ-44) was used to measure obsessive beliefs across three domains—(1) Importance and Control of Thoughts; (2) Perfectionism and Intolerance of Uncertainty; (3) Responsibility and Threat Estimation) using a 7-point Likert-scale (1—‘Disagree very much to 7—‘Agree very much’)123. The Intolerance of Uncertainty Scale 12-item version (IUS-12) is a self-report questionnaire used to measure maladaptive/negative beliefs about uncertainty and its consequences in two related domains—(1) Desire for Predictability and an Active Engagement in Seeking Certainty; (2) Paralysis of Cognition and Action in the Face of Uncertainty—scored using a 5-point Likert scale (1—‘Not at all characteristic of me’ to 5—‘Entirely characteristic of me’)124. Several other questionnaires were completed but not analysed in the current study. A list of these questionnaires is provided in Supplementary Information.
Procedures
The study used a correlational, non-experimental research design. Participants completed the study questionnaires over two sessions. In the first session, the participants completed: (1) a self-reported survey designed ad hoc for this project to collect demographic and clinical data, as well as the OBQ-44. In the second session, participants completed the UPPS-P and IUS-12. Order of administration for the full battery of questionnaires not analysed in this study is provided in Supplementary Information.
Statistical analyses
Analytic strategy
We addressed several limitations of our previous approach to modelling the three phenotypes related to impulsivity and compulsivity22. In our previous work, we generated factor score estimates based on separate bifactor models of the UPPS-P, IUS-12, and OBQ-44, which provided the best fit to the data obtained from these scales compared to several competing models. The factor score estimates were then used as indicator variables for the second-order confirmatory factor analysis (CFA) model to derive the three phenotypes. These bifactor models may have provided a better fit to the questionnaire item data compared to the published subscale structure of the UPPS-P, IUS-12, and OBQ-44 simply because they included more parameters and could better account for the observed covariances (i.e. over-parameterisation)70,87,125. Additionally, the substantive interpretation of the group factors in bifactor models is difficult and less than adequate reliability of subscale-specific, residual variance is often observed125.
We took several steps to address these previous limitations. CFA models were fitted to the UPPS-P, IUS-12, and OBQ-44 self-report data based on their a priori published subscale structure. We compared these results to competing models to evaluate whether the subscales provided a reasonable representation of the latent structure of the data for use as indicators in the higher-order CFA models. Using raw subscales scores as indicators enables the model intercepts and unstandardised loading estimates to be directly and meaningfully compared across studies. This approach facilitates more interpretable solutions and unbiased estimates for the higher-order factor model, whilst also having the added benefit of parsimony. We used a cross-validation approach by fitting the models to data obtained from the first of each twin pair and then replicating the models in the data obtained from the second twin from each pair using invariance testing126. This methodology ensured that the models were psychometrically stable and replicable. It also formally tested the assumptions of equivalence of means, variances, and covariances across twin pairs prior to subsequent heritability analyses35.
Structural equation modeling
Preliminary analyses, including analysis of missing data, non-normality, and outliers, are described in Supplementary Methods online. All structural equation modelling (SEM) was conducted in Mplus 7.3154. First-order CFA models of the UPPS-P, IUS-12, and OBQ-44 ordered categorical data were estimated using the weighted least square mean- and variance-adjusted (WLSMV) estimator and theta parameterisation54. The WLSMV estimator provides robust parameter estimates and standard errors for non-normally distributed ordered categorical data88. Theta parameterisation involves scaling the latent response variables for the questionnaire items by fixing the error variance to one (instead of fixing the latent response variable to one in the alternative delta parameterisation) and is the preferred approach to invariance testing for multi-group comparisons using ordered categorical data88. An ‘alternative’ or ‘competing’ models strategy89,127 was used to determine the best fitting model in the twin 1 subsample followed by cross-validation in the twin 2 subsample using invariance testing126. Higher-order CFA models conducted on the UPPS-P, IUS-12, and OBQ-44 subscale scores for estimation of the dimensional phenotypes used for heritability analyses were estimated using full information maximum likelihood and the Bollen-Stine bootstrap procedure with 10,000 posterior draws to account for missing data and multivariate non-normality128. A bifactor model was first estimated in the twin 1 subsample based on the general structure previously reported22 and then cross-validated in the twin 2 subsample using invariance testing126. Latent variable scaling was performed using the reference variable method, as this is the preferred approach for multi-group comparisons using invariance testing, because it does not assume equality of latent variable variances88. Post hoc model fitting was performed by freeing theoretically plausible error covariances for estimation one at a time with reference to modification indices and with adjustment of significance thresholds for multiple comparisons using the Benjamini–Hochberg false discovery rate (B-H FDR; q = 0.05)50.
Model fit was assessed using a combination of fit indices, including the chi square test statistic (χ2), Root Mean Square Error of Approximation (RMSEA) (ε < 0.05 close approximate fit; ε = 0.05—0.08 close approximate fit; ε = 0.08–1.0 reasonable approximate fit), Comparative Fit Index (CFI) (> 0.90), and Standardised Root Mean Square Residual (SRMR) (< 0.08), or Weighted Root Mean Square Residual (WRMR) for categorical variables89. The χ2 test statistic is the gold standard metric for evaluating overall model fit and was referred to first. A probability value > 0.05 indicates that the null hypothesis of exact fit of the model reproduced covariance matrix to the observed covariance matrix cannot be rejected88. The χ2 is overly sensitive to minor model misspecification in datasets characterised by high correlations amongst the observed variables (i.e. conceptually-related items on a questionnaire), when estimated in sample sizes of 200–300 or more88. For this reason, we referred to approximate fit indices to adjudicate between the fit of first-order competing models for the UPPS-P, IUS-12, and OBQ-44. Bifactor models often provide a better fit compared to competing models simply because they include more freely estimated parameters125. We therefore used information functions based on item response theory analysis to evaluate the reliability of the group factors obtained using alternative model specifications (see Supplementary Figures S1–S10 online)129.
Factor score estimates were generated in Mplus using the regression method for the Impulsive–Compulsive, Impulsivity, and Obsessiveness dimensions in the combined sample54. The within-trait and cross-trait correlations for the factor score estimates in monozygotic and dizygotic twin pairs are displayed in Table 4. The regression method results in unbiased estimates of individual differences on the underlying latent variable when the factor score estimates are used as exogenous (i.e. independent) variables in a structural equation model130. The factor scores estimates were treated as exogenous variables in the variance components models in which they were specified as predictors of the genetic and environment components of the model35. We also fixed error variance of the factor score estimates to reflect unreliability of the estimation process based on the factor score determinacies52. Heritability analyses were performed in Mplus with variance components models using the maximum likelihood estimator54. We estimated statistically nested variance components models for each of the dimensional phenotypes and compared them to determine the best-fitting model35. The χ2 test statistic and difference test (Δχ2) are underpowered to test between competing models with modest sample sizes and few degrees of freedom88. We therefore compared competing variance components models using Bayesian conditional posterior probabilities [Pr (Hi | D] to identify the best-fitting model57. All direct statistical tests, including z tests, were two-tailed.
Table 4.
Within-trait and cross-trait correlations for the phenotype factor score estimates in monozygotic and dizygotic twin pairs.
| Variable | Age | Impulsive–compulsive2 | Impulsivity2 | Obsessiveness2 |
|---|---|---|---|---|
| MZ | ||||
| Age | − 0.196 [− 0.364, − 0.016] | − 0.097 [− 0.273, 0.085] | − 0.125 [− 0.299, 0.057] | |
| Impulsive–compulsive1 | − 0.232 [− 0.396, − 0.053] | 0.365 [0.197,0.512] | − 0.051 [− 0.093, 0.193] | 0.134 [− 0.009, 0.272] |
| Impulsivity1 | 0.037 [− 0.145, 0.216] | 0.032 [− 0.112, 0.174] | 0.300 [0.126, 0.456] | − 0.016 [− 0.159, 0.127] |
| Obsessiveness1 | − 0.047 [− 0.226,0.135] | 0.246 [0.107, 0.376] | 0.121 [− 0.023, 0.260] | 0.224 [0.045,0.389] |
| DZ | ||||
| Age | − 0.169 [− 0.416, 0.101] | − 0.129 [− 0.381, 0.141] | − 0.024 [− 0.287, 0.243] | |
| Impulsive–compulsive1 | − 0.202 [− 0.444, 0.067] | 0.367 [0.113, 0.576] | 0.041 [− 0.227, 0.303] | 0.273 [0.008, 0.502] |
| Impulsivity1 | − 0.151 [− 0.400, 0.119] | − 0.032 [− 0.295, 0.235] | − 0.069 [0.328, 0.200] | − 0.041 [− 0.303, 0.227] |
| Obsessiveness1 | − 0.006 [− 0.271, 0.260] | 0.231 [− 0.037, 0.468] | 0.122 [− 0.148, 0.375] | 0.137 [− 0.133, 0.388] |
95% CI in brackets. MZ N = 118, DZ N = 55. These correlations do not take into account the error of measurement in the factor score estimates due to factor score indeterminacy.
Supplementary information
Acknowledgements
This research was facilitated through access to Twins Research Australia, a national resource supported by a Centre of Research Excellence Grant (ID: 1079102), from the National Health and Medical Research Council (NHMRC). Jeggan Tiego was supported by NHMRC Project Grants 1002458 and 1046054. Samuel Chamberlain was funded by a Clinical Fellowship from the Wellcome Trust (reference 110049/Z/15/Z). Dr. Chamberlain consults for Promentis, and Ieso Digital Health and receives a stipend for his role as Associate Editor at Neuroscience and Biobehavioural Reviews. Leonardo F. Fontenelle was supported by a Scientist of Our State scholarship from the Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) (E-26/103.252/2011 and E-26/201.305/2014) and a Research Productivity (PQ-2014) scholarship from the National Council for Scientific and Technological Development (CNPq) (E-26/308237/2014-5); Murat Yucel was supported by a NHMRC Principal Research Fellowship (APP1117188) and the David Winston Turner Endowment Fund. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author contributions
M.Y., G.J.Y., and L.F.F. designed the study and methods, and oversaw data collection. J.T. conceptualised the statistical models and conducted data analysis. J.T. wrote the manuscript with input from S.R.C., M.Y., G.J.Y., and L.F.F. B.J.H., A.D., and L.A. provided input and feedback on the manuscript.
Data availability
The data that support the findings of this study are available from Twins Research Australia [https://www.twins.org.au/] on application and following approval by institutional review board.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-71013-x.
References
- 1.Krueger RF, et al. Progress in achieving quantitative classification of psychopathology. World Psychiatry. 2018;17:282–293. doi: 10.1002/wps.20566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hägele C, et al. Dimensional psychiatry: reward dysfunction and depressive mood across psychiatric disorders. Psychopharmacology. 2015;232:331–341. doi: 10.1007/s00213-014-3662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotov R, et al. The hierarchical taxonomy of psychopathology (HiTOP): a dimensional alternative to traditional nosologies. J. Abnorm. Psychol. 2017;126:454–477. doi: 10.1037/abn0000258. [DOI] [PubMed] [Google Scholar]
- 5.Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nat. Rev. Genet. 2009;10:872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan PF, et al. Psychiatric genomics: an update and an agenda. Am. J. Psychiatry. 2018;175:15–27. doi: 10.1176/appi.ajp.2017.17030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krueger RF, DeYoung CG. The RDoC initiative and the structure of psychopathology. Psychophysiology. 2016;53:351–354. doi: 10.1111/psyp.12551. [DOI] [PubMed] [Google Scholar]
- 8.Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. A hierarchical causal taxonomy of psychopathology across the life span. Psychol. Bull. 2017;143:142–186. doi: 10.1037/bul0000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waszczuk MA, et al. Redefining phenotypes to advance psychiatric genetics: implications from hierarchical taxonomy of psychopathology. J. Abnorm. Psychol. 2020;129:143–161. doi: 10.1037/abn0000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cognit. Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Figee M, et al. Compulsivity in obsessive-compulsive disorder and addictions. Eur. Neuropsychopharmacol. 2016;26:856–868. doi: 10.1016/j.euroneuro.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am. J. Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- 13.Dalley JW, Robbins TW. Fractionating impulsivity: Neuropsychiatric implications. Nat. Rev. Neurosci. 2017;18:158–171. doi: 10.1038/nrn.2017.8. [DOI] [PubMed] [Google Scholar]
- 14.Luigjes J, et al. Defining compulsive behavior. Neuropsychol Rev. 2019;29:4–13. doi: 10.1007/s11065-019-09404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollander E. Obsessive-compulsive spectrum disorders: an overview. Psychiatr. Ann. 1993;23:355–358. doi: 10.3928/0048-5713-19930701-05. [DOI] [Google Scholar]
- 16.Hollander E, Benzaquen DS. The obsessive compulsive spectrum disorders. Int. Rev. Psychiatry. 1997;9:99–110. doi: 10.1080/09540269775628. [DOI] [Google Scholar]
- 17.Fineberg NA, et al. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fineberg NA, et al. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS Spectr. 2014;19:69–89. doi: 10.1017/S1092852913000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Gillan CM, Kosinski M, Whelan R, Phelps EA, Daw ND. Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. eLife. 2016;5:e11305. doi: 10.7554/eLife.11305.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkes L, et al. Transdiagnostic variations in impulsivity and compulsivity in obsessive-compulsive disorder and gambling disorder correlate with effective connectivity in cortical-striatal-thalamic-cortical circuits. NeuroImage. 2019;1:116070. doi: 10.1016/j.neuroimage.2019.116070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiego J, et al. Overlapping dimensional phenotypes of impulsivity and compulsivity explain co-occurrence of addictive and related behaviors. CNS Spectr. 2018;24:426–440. doi: 10.1017/S1092852918001244. [DOI] [PubMed] [Google Scholar]
- 23.Chamberlain, S. R. et al. Fractionation of impulsive and compulsive trans-diagnostic phenotypes and their longitudinal associations. Aust. N. Z. J. Psychiatry 0004867419844325 (2019). [DOI] [PMC free article] [PubMed]
- 24.Chamberlain SR, Blackwell AD, Fineberg NA, Robbins TW, Sahakian BJ. The neuropsychology of obsessive compulsive disorder: the importance of failures in cognitive and behavioural inhibition as candidate endophenotypic markers. Neurosci. Biobehav. Rev. 2005;29:399–419. doi: 10.1016/j.neubiorev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Cuzen, N. L. & Stein, D. J. in Behavioral Addictions (eds K. P. Rosenberg & L. C. Feder) Ch. 2, 19–34 (Academic Press, 2014).
- 26.Yucel M, et al. A transdiagnostic dimensional approach towards a neuropsychological assessment for addiction: an international Delphi consensus study. Addiction. 2019;114:1095–1109. doi: 10.1111/add.14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chamberlain SR, et al. Fractionation of impulsive and compulsive trans-diagnostic phenotypes and their longitudinal associations. Aust. N. Z. J. Psychiatry. 2019;53:896–907. doi: 10.1177/0004867419844325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/S1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 29.Voon V, Reiter A, Sebold M, Groman S. Model-based control in dimensional psychiatry. Biol. Psychiat. 2017;82:391–400. doi: 10.1016/j.biopsych.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 30.McTeague LM, Goodkind MS, Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J. Psychiatr. Res. 2016;83:37–46. doi: 10.1016/j.jpsychires.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Mol. Psychiatry. 2010;15:789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Dongen J, Slagboom PE, Draisma HHM, Martin NG, Boomsma DI. The continuing value of twin studies in the omics era. Nat. Rev. Genet. 2012;13:640–653. doi: 10.1038/nrg3243. [DOI] [PubMed] [Google Scholar]
- 33.Plomin R, Haworth CM. Genetics and intervention research. Perspect. Psychol. Sci. 2010;5:557–563. doi: 10.1177/1745691610383513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nat. Rev. Genet. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- 35.Neale, M. C. & Cardon, L. R. Methodology for genetic studies of twins and families., Vol. 67 (Kluwer Academic Publishers, 1992).
- 36.Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief. Bioinform. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- 37.Zyphur MJ, Zhang Z, Barsky AP, Li W. An ACE in the hole: twin family models for applied behavioral genetics research. Leadership Q. 2013;24:572–594. doi: 10.1016/j.leaqua.2013.04.001. [DOI] [Google Scholar]
- 38.Bezdjian S, Baker LA, Tuvblad C. Genetic and environmental influences on impulsivity: a meta-analysis of twin, family and adoption studies. Clin. Psychol. Rev. 2011;31:1209–1223. doi: 10.1016/j.cpr.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anokhin AP, Grant JD, Mulligan RC, Heath AC. The genetics of impulsivity: evidence for the heritability of delay discounting. Biol. Psychiat. 2015;77:887–894. doi: 10.1016/j.biopsych.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niv S, Tuvblad C, Raine A, Wang P, Baker LA. Heritability and longitudinal stability of impulsivity in adolescence. Behav. Genet. 2012;42:378–392. doi: 10.1007/s10519-011-9518-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chamberlain SR, Leppink EW, Redden SA, Grant JE. Are obsessive–compulsive symptoms impulsive, compulsive or both? Compr. Psychiatry. 2016;68:111–118. doi: 10.1016/j.comppsych.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iervolino AC, Rijsdijk FV, Cherkas L, Fullana MA, Mataix-Cols D. A multivariate twin study of obsessive-compulsive symptom dimensions. Arch. Gen. Psychiatry. 2011;68:637–644. doi: 10.1001/archgenpsychiatry.2011.54. [DOI] [PubMed] [Google Scholar]
- 43.Monzani B, Rijsdijk F, Harris J, Mataix-Cols D. The structure of genetic and environmental risk factors for dimensional representations of DSM-5 obsessive-compulsive spectrum disorders. JAMA Psychiatry. 2014;71:182–189. doi: 10.1001/jamapsychiatry.2013.3524. [DOI] [PubMed] [Google Scholar]
- 44.van Grootheest DS, Boomsma DI, Hettema JM, Kendler KS. Heritability of obsessive-compulsive symptom dimensions. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008;147:473–478. doi: 10.1002/ajmg.b.30622. [DOI] [PubMed] [Google Scholar]
- 45.Lopez-Sola C, et al. Aetiological overlap between obsessive-compulsive related and anxiety disorder symptoms: multivariate twin study. Br. J. Psychiatry. 2016;208:26–33. doi: 10.1192/bjp.bp.114.156281. [DOI] [PubMed] [Google Scholar]
- 46.Burton CL, et al. Heritability of obsessive–compulsive trait dimensions in youth from the general population. Transl. Psychiatry. 2018;8:191. doi: 10.1038/s41398-018-0249-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicolini H, Arnold P, Nestadt G, Lanzagorta N, Kennedy JL. Overview of genetics and obsessive–compulsive disorder. Psychiatry Res. 2009;170:7–14. doi: 10.1016/j.psychres.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 48.van Grootheest DS, Cath DC, Beekman AT, Boomsma DI. Twin studies on obsessive–compulsive disorder: a review. Twin Res. Hum. Genet. 2005;8:450–458. doi: 10.1375/twin.8.5.450. [DOI] [PubMed] [Google Scholar]
- 49.Taylor S. Etiology of obsessions and compulsions: a meta-analysis and narrative review of twin studies. Clin. Psychol. Rev. 2011;31:1361–1372. doi: 10.1016/j.cpr.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995;57:289–300. [Google Scholar]
- 51.Bandalos, D. L. Measurement theory and applications for the social science. (Guilford Publications, 2018).
- 52.Grice JW. Computing and evaluating factor scores. Psychol. Methods. 2001;6:430–450. doi: 10.1037/1082-989X.6.4.430. [DOI] [PubMed] [Google Scholar]
- 53.DiStefano C, Zhu M, Mindrila D. Understanding and using factor scores: considerations for the applied researcher. Pract. Assess. Res. Eval. 2009;14:1–11. [Google Scholar]
- 54.Muthén, L. K. & Muthén, B. O. Mplus user’s guide. Seventh edn, (Muthén & Muthén, 1998-2012).
- 55.Hoaglin DC, Iglewicz B. Fine-tuning some resistant rules for outlier labeling. Journal of the American Statistical Association. 1987;82:1147–1149. doi: 10.1080/01621459.1987.10478551. [DOI] [Google Scholar]
- 56.Bollen K. A. Structural equations with latent variables.: Wiley; 1989. [Google Scholar]
- 57.Wagenmakers EJ. A practical solution to the pervasive problems of p values. Psychon. Bull. Rev. 2007;14:779–804. doi: 10.3758/BF03194105. [DOI] [PubMed] [Google Scholar]
- 58.Raftery AE. Bayesian model selection in social research. Sociol. Methodol. 1995;25:111–164. doi: 10.2307/271063. [DOI] [Google Scholar]
- 59.Chamberlain SR, Stochl J, Redden SA, Grant JE. Latent traits of impulsivity and compulsivity: toward dimensional psychiatry. Psychol. Med. 2017;48:1–12. doi: 10.1017/S0033291717002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. Psychol. Med. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- 61.Young SE, et al. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J. Abnorm. Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young SE, Stallings MC, Corley RP, Krauter KS, Hewitt JK. Genetic and environmental influences on behavioral disinhibition. Am. J. Med. Genet. 2000;96:684–695. doi: 10.1002/1096-8628(20001009)96:5<684::AID-AJMG16>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 63.Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin family study. Dev. Psychopathol. 1999;11:869–900. doi: 10.1017/S0954579499002369. [DOI] [PubMed] [Google Scholar]
- 64.Iacono WG, Malone SM, McGue M. Behavioral disinhibition and the development of early-onset addiction: common and specific Influences. Annu. Rev. Clin. Psychol. 2008;4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157. [DOI] [PubMed] [Google Scholar]
- 65.McGue M, et al. A genome-wide association study of behavioral disinhibition. Behav. Genet. 2013;43:363–373. doi: 10.1007/s10519-013-9606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behav. Genet. 2011;41:459–475. doi: 10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vrieze SI, McGue M, Miller MB, Hicks BM, Iacono WG. Three mutually informative ways to understand the genetic relationships among behavioral disinhibition, alcohol use, drug use, nicotine use/dependence, and their co-occurrence: twin biometry, GCTA, and genome-wide scoring. Behav. Genet. 2013;43:97–107. doi: 10.1007/s10519-013-9584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Derringer J, et al. Genome-wide association study of behavioral disinhibition in a selected adolescent sample. Behav. Genet. 2015;45:375–381. doi: 10.1007/s10519-015-9705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patrick CJ, Kramer MD, Krueger RF, Markon KE. Optimizing efficiency of psychopathology assessment through quantitative modeling: development of a brief form of the Externalizing Spectrum Inventory. Psychol. Assess. 2013;25:1332–1348. doi: 10.1037/a0034864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bornovalova MA, Choate AM, Fatimah H, Petersen KJ, Wiernik BM. Appropriate use of bifactor analysis in psychopathology research: appreciating benefits and limitations. Biol. Psychiat. 2020;88:18–27. doi: 10.1016/j.biopsych.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Parkes L, et al. Transdiagnostic variations in impulsivity and compulsivity in obsessive-compulsive disorder and gambling disorder correlate with effective connectivity in cortical-striatal-thalamic-cortical circuits. Neuroimage. 2019;202:116070. doi: 10.1016/j.neuroimage.2019.116070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Basevitz P, Pushkar D, Chaikelson J, Conway M, Dalton C. Age-related differences in worry and related processes. Int. J. Aging Hum. Dev. 2008;66:283–305. doi: 10.2190/AG.66.4.b. [DOI] [PubMed] [Google Scholar]
- 73.Carstensen LL. The influence of a sense of time on human development. Science. 2006;312:1913–1915. doi: 10.1126/science.1127488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Livingstone KM, Isaacowitz DM. Situation selection and modification for emotion regulation in younger and older adults. Soc. Psychol. Person. Sci. 2015;6:904–910. doi: 10.1177/1948550615593148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boswell JF, Thompson-Hollands J, Farchione TJ, Barlow DH. Intolerance of uncertainty: a common factor in the treatment of emotional disorders. J. Clin. Psychol. 2013;69:630–645. doi: 10.1002/jclp.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Einstein DA. Extension of the transdiagnostic model to focus on intolerance of uncertainty: a review of the literature and implications for treatment. Clin. Psychol. Sci. Pract. 2014;21:280–300. doi: 10.1111/cpsp.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilhelm S, Berman NC, Keshaviah A, Schwartz RA, Steketee G. Mechanisms of change in cognitive therapy for obsessive compulsive disorder: role of maladaptive beliefs and schemas. Behav. Res. Ther. 2015;65:5–10. doi: 10.1016/j.brat.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cyders MA, Coskunpinar A. Measurement of constructs using self-report and behavioral lab tasks: Is there overlap in nomothetic span and construct representation for impulsivity? Clin Psychol Rev. 2011;31:965–982. doi: 10.1016/j.cpr.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 79.Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychol. Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 81.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gregory AM, Brigitte R. Endophenotypes in psychopathology research: where do we stand? Annu. Rev. Clin. Psychol. 2013;9:177–213. doi: 10.1146/annurev-clinpsy-050212-185540. [DOI] [PubMed] [Google Scholar]
- 83.Bonifay W, Lane SP, Reise SP. Three concerns with applying a bifactor model as a structure of psychopathology. Clin. Psychol. Sci. 2017;5:184–186. doi: 10.1177/2167702616657069. [DOI] [Google Scholar]
- 84.Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, Zald DH. Validity and utility of the general factor of psychopathology. World Psychiatry. 2017;16:142–144. doi: 10.1002/wps.20410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bonifay W, Cai L. On the complexity of item response theory models. Multivar. Behav. Res. 2017;52:465–484. doi: 10.1080/00273171.2017.1309262. [DOI] [PubMed] [Google Scholar]
- 86.Reise SP, Kim DS, Mansolf M, Widaman KF. Is the bifactor model a better model or is it just better at modeling implausible responses? Application of iteratively reweighted least squares to the rosenberg self-esteem scale. Multivar. Behav. Res. 2016;51:818–838. doi: 10.1080/00273171.2016.1243461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Markon KE. Bifactor and hierarchical models: specification, inference, and nterpretation. Annu. Rev. Clin. Psychol. 2019;15:51–69. doi: 10.1146/annurev-clinpsy-050718-095522. [DOI] [PubMed] [Google Scholar]
- 88.Kline, R. B. Principles and practice of structural equation modeling. 4th edn, (The Guilford Press, 2015).
- 89.Hair JF, Black WC, Babin BJ, Anderson RE. Multivariate data analysis. Pearson Education Limited: Seventh edn; 2014. [Google Scholar]
- 90.Kass RE, Raftery AE. Bayes factors. J. Am. Stat. Assoc. 1995;90:773–795. doi: 10.1080/01621459.1995.10476572. [DOI] [Google Scholar]
- 91.Verhulst B. A power calculator for the classical twin design. Behav. Genet. 2017;47:255–261. doi: 10.1007/s10519-016-9828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Visscher PM. Power of the classical twin design revisited. Twin Res. 2004;7:505–512. doi: 10.1375/1369052042335250. [DOI] [PubMed] [Google Scholar]
- 93.Kendler KS, Gardner CO, Gatz M, Pedersen NL. The sources of co-morbidity between major depression and generalized anxiety disorder in a Swedish national twin sample. Psychol. Med. 2006;37:453–462. doi: 10.1017/S0033291706009135. [DOI] [PubMed] [Google Scholar]
- 94.Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Res. Hum. Genet. 2007;10:423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- 95.Bouchard TJ, Loehlin JC. Genes, evolution, and personality. Behav. Genet. 2001;31:243–273. doi: 10.1023/A:1012294324713. [DOI] [PubMed] [Google Scholar]
- 96.Jacobson KC, Prescott CA, Kendler KS. Sex differences in the genetic and environmental influences on the development of antisocial behavior. Dev. Psychopathol. 2002;14:395–416. doi: 10.1017/S0954579402002110. [DOI] [PubMed] [Google Scholar]
- 97.Coskunpinar A, Dir AL, Cyders MA. Multidimensionality in impulsivity and alcohol use: a meta-analysis using the UPPS model of impulsivity. Alcohol Clin. Exp. Res. 2013;37:1441–1450. doi: 10.1111/acer.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cyders MA, et al. Integration of impulsivity and positive mood to predict risky behavior: development and validation of a measure of positive urgency. Psychol. Assess. 2007;19:107–118. doi: 10.1037/1040-3590.19.1.107. [DOI] [PubMed] [Google Scholar]
- 99.Smith GT, et al. On the validity and utility of discriminating among impulsivity-like traits. Assessment. 2007;14:155–170. doi: 10.1177/1073191106295527. [DOI] [PubMed] [Google Scholar]
- 100.Whiteside SP, Lynam DR. The five factor model and impulsivity: using a structural model of personality to understand impulsivity. Person. Individ. Differ. 2001;30:669–689. doi: 10.1016/S0191-8869(00)00064-7. [DOI] [Google Scholar]
- 101.Whiteside SP, Lynam DR, Miller JD, Reynolds SK. Validation of the UPPS impulsive behaviour scale: a four-factor model of impulsivity. Eur. J. Pers. 2005;19:559–574. doi: 10.1002/per.556. [DOI] [Google Scholar]
- 102.Gullo MJ, Loxton NJ, Dawe S. Impulsivity: four ways five factors are not basic to addiction. Addict. Behav. 2014;39:1547–1556. doi: 10.1016/j.addbeh.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 103.Hamilton KR, et al. Rapid-response impulsivity: definitions, measurement issues, and clinical implications. Person. Disord. Theory Res. Treatm. 2015;6:168–181. doi: 10.1037/per0000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hamilton KR, et al. Choice Impulsivity: definitions, measurement issues, and clinical implications. Person. Disord. Theory Res. Treatm. 2015;6:182–198. doi: 10.1037/per0000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cyders MA, Littlefield AK, Coffey S, Karyadi KA. Examination of a short English version of the UPPS-P Impulsive Behavior Scale. Addict. Behav. 2014;39:1372–1376. doi: 10.1016/j.addbeh.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smith GT, Cyders MA. Integrating affect and impulsivity: the role of positive and negative urgency in substance use risk. Drug Alcohol. Depend. 2016;163(Suppl 1):S3–S12. doi: 10.1016/j.drugalcdep.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cyders MA, Smith GT. Emotion-based dispositions to rash action: positive and negative urgency. Psychol Bull. 2008;134:807–828. doi: 10.1037/a0013341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol. Psychiat. 2010;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mathew CA, et al. Evidence for a heritable unidimensional symptom factor underlying obsessionality. Am. J. Med. Genet. Part B-Neuropsychiatr. Genet. 2008;147B:676–685. doi: 10.1002/ajmg.b.30660. [DOI] [PubMed] [Google Scholar]
- 110.Guo K, et al. A psychometric validation study of the Impulsive-Compulsive Behaviours Checklist: a transdiagnostic tool for addictive and compulsive behaviours. Addict. Behav. 2017;67:26–33. doi: 10.1016/j.addbeh.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 111.Rosario-Campos MC, et al. The dimensional Yale-Brown Obsessive–Compulsive Scale (DY-BOCS): an instrument for assessing obsessive–compulsive symptom dimensions. Mol. Psychiatry. 2006;11:495–504. doi: 10.1038/sj.mp.4001798. [DOI] [PubMed] [Google Scholar]
- 112.Foa EB, et al. The obsessive-compulsive inventory: development and validation of a short version. Psychol. Assess. 2002;14:485–496. doi: 10.1037//1040-3590.14.4.485. [DOI] [PubMed] [Google Scholar]
- 113.Jarrar ZA, et al. Definitive zygosity scores in the peas in the pod questionnaire is a sensitive and accurate assessment of the zygosity of adult twins. Twin Res. Hum. Genet. 2018;21:146–154. doi: 10.1017/thg.2018.9. [DOI] [PubMed] [Google Scholar]
- 114.Ooki S, Yamada K, Asaka A, Hayakawa K. Zygosity diagnosis of twins by questionnaire. Acta Genet. Med. Gemellologiae Twin Res. 1990;39:109–115. doi: 10.1017/S0001566000005626. [DOI] [PubMed] [Google Scholar]
- 115.Rietveld MJH, et al. Zygosity diagnosis in young twins by parental report. Twin Res. 2000;3:134–141. doi: 10.1375/twin.3.3.134. [DOI] [PubMed] [Google Scholar]
- 116.Ooki S, Yamada K, Asaka A. Zygosity diagnosis of twins by questionnaire for twins' mothers. Acta Genet. Med. Gemellologiae. 1993;42:17–17. doi: 10.1017/S0515283600042244. [DOI] [PubMed] [Google Scholar]
- 117.Twins Research Australia. How to determine zygosity. https://twins.org.au/how-to-determine-zygosity. Accessed 12 April 2020.
- 118.Cutler TL, et al. Why accurate knowledge of zygosity is important to twins. Twin Res. Hum. Genet. 2015;18:298–305. doi: 10.1017/thg.2015.15. [DOI] [PubMed] [Google Scholar]
- 119.Jackson RW, Snieder H, Davis H, Treiber FA. Determination of twin zygosity: a comparison of DNA with various questionnaire indices. Twin Res. 2001;4:12–18. doi: 10.1375/twin.4.1.12. [DOI] [PubMed] [Google Scholar]
- 120.Murphy, K. et al. Twins research Australia: a new paradigm for driving twin research. Twin Res. Hum. Genet.1, 1–8. 10.1017/thg.2019.101 [DOI] [PubMed]
- 121.Christiansen L, et al. Age- and sex-differences in the validity of questionnaire-based zygosity in twins. Twin Res. 2012;6:275–278. doi: 10.1375/twin.6.4.275. [DOI] [PubMed] [Google Scholar]
- 122.Lynam, D. R., Smith, G. T., Whiteside, S. P. & Cyders, M. A. The UPPS-P: Assessing five personality pathways to impulsive behavior (Technical report). (Purdue University, 2006).
- 123.OCCWG. Psychometric validation of the Obsessive Beliefs Questionnaire and the Interpretation of Intrusions Inventory. Part 2: Factor analyses and testing of a brief version. Behav. Res. Ther.43, 1527–1542. 10.1016/j.brat.2004.07.010 (2005). [DOI] [PubMed]
- 124.Carleton RN, Norton MA, Asmundson GJ. Fearing the unknown: a short version of the Intolerance of Uncertainty Scale. J. Anxiety Disord. 2007;21:105–117. doi: 10.1016/j.janxdis.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 125.Reise SP. The rediscovery of bifactor measurement models. Multivar. Behav. Res. 2012;47:667–696. doi: 10.1080/00273171.2012.715555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meredith W. Measurement invariance, factor-analysis and factorial invariance. Psychometrika. 1993;58:525–543. doi: 10.1007/bf02294825. [DOI] [Google Scholar]
- 127.Jöreskog, K. G. in Testing structural equation models (eds K. A. Bollen & S. Long) (Sage Publications, 1993).
- 128.Enders C. K. Applied missing data analysis.: The Guilford Press; 2010. [Google Scholar]
- 129.Reise SP, Waller NG. Item response theory and clinical measurement. Annu. Rev. Clin. Psychol. 2009;5:27–48. doi: 10.1146/annurev.clinpsy.032408.153553. [DOI] [PubMed] [Google Scholar]
- 130.Devlieger I, Mayer A, Rosseel Y. Hypothesis testing using factor score regression: a comparison of four methods. Educ. Psychol. Measur. 2016;76:741–770. doi: 10.1177/0013164415607618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McArdle JJ. Causal-modeling applied to psychonomic systems simulation. Behav. Res. Methods Instrum. 1980;12:193–209. doi: 10.3758/bf03201598. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from Twins Research Australia [https://www.twins.org.au/] on application and following approval by institutional review board.