Abstract
Increasing human activity along the coast has amplified the extinction risk of inshore delphinids. Informed selection and prioritisation of areas for the conservation of inshore delphinids requires a comprehensive understanding of their distribution and habitat use. In this study, we applied an ensemble species distribution modelling approach, combining results of six modelling algorithms to identify areas of high probability of occurrence of the globally Vulnerable Australian humpback dolphin in northern Ningaloo Marine Park (NMP), north-western Australia. Model outputs were based on sighting data collected during systematic, boat-based surveys between 2013 and 2015, and in relation to various ecogeographic variables. Water depth and distance to coast were identified as the most important variables influencing dolphin presence, with dolphins showing a preference for shallow waters (5–15 m) less than 2 km from the coast. Areas of high probability (> 0.6) of dolphin occurrence were primarily (90%) in multiple use areas where extractive human activities are permitted, and were poorly represented in sanctuary (no-take) zones. This spatial mismatch emphasises the need to reassess for future spatial planning and marine park management plan reviews for NMP. Shallow, coastal waters identified here should be considered priority areas for the conservation of this Vulnerable species.
Subject terms: Behavioural ecology, Ecological modelling
Introduction
Coastal marine environments have been ranked as most heavily impacted from anthropogenic activities1. As a result, wildlife that forage, breed, reside or migrate along the coast, particularly species that are long-lived, late-maturing and slow-reproducing, are becoming increasingly endangered2–4. Small odontocetes found in coastal and riverine habitats are examples of such vulnerability, with several species currently under threat5–7 and others already extinct8 or on the brink of extinction9 as a direct or indirect result of human activities. Marine protected areas (MPAs) can be effective tools for conserving such taxa, particularly if MPA zoning includes large no-take areas (i.e. areas closed to extractive activities) of suitable habitat10,11. Considering the vulnerability of small odontocetes and their role as umbrella species, the protection of important habitat is key for their conservation, and has the potential to contribute towards the broader conservation of biodiversity and support the delineation of no-take zones within MPAs12,13. However, ensuring the effectiveness of such protected areas requires a comprehensive understanding of species distribution and habitat relationships therein14,15, which is lacking for several existing protected areas16.
The lack of spatially explicit information on species distributions and habitat preferences can compromise their effective protection, even when they occur within designated MPAs17–21. Although the implementation of MPAs has grown exponentially since the 1960s22; only a small proportion contain no-take zones, and, overall, the global tendency is for MPAs to be located in remote areas or those unpromising for extractive activities, leading to the questioning of their effectiveness for conservation23,24. When referring to ‘effectiveness’ of MPAs in the context of cetacean protection, we mean those MPAs that explicitly consider cetaceans in their conservation planning and have relevant, measurable objectives that address conservation and restoration of these types of ‘natural capital’ (concept reviewed in25; see also26). The north-west marine region of Western Australia (WA) is home to several protected marine megafauna species and Australia's largest fringing reef in Ningaloo Marine Park (NMP). The NMP is a multiple-use MPA and part of the Ningaloo Coast World Heritage Area, proclaimed based on its exceptional marine biodiversity and habitat for threatened species, including a myriad of marine megafauna, many of which have been recognised as ‘ecological values’ in the NMP management plan, each with defined management objectives and performance measures27,28. However, our understanding of the distribution and habitat use of most of these species, including the recently described Australian humpback dolphin (Sousa sahulensis), remains limited, hampering conservation and management efforts29.
The Australian humpback dolphin (hereafter “humpback dolphin”) is endemic to shallow (typically < 30 m) coastal waters of tropical northern Australia and southern Papua New Guinea30. Studies in selected areas throughout the Australian range of humpback dolphins indicate that populations are small [typically 50 to 150 individuals, sometimes fewer31–36 with limited gene flow32,37, and relatively small home ranges (< 300 km2; 32,38)]. The IUCN Red List of Threatened Species recently listed the Australian humpback dolphin as ‘Vulnerable’ due to the species’ small population sizes and cumulative exposure to human activities39.
MPAs cover a third of the inferred distribution of humpback dolphins in Western Australia, but the efficacy of these reserves in protecting local cetacean populations is unknown29. The North West Cape (NWC), located in the northern NMP (Fig. 1), supports the highest density of humpback dolphins (one dolphin per km2) recorded to date in Australia36. This population (ca. 130 individuals) is characterised by high levels of site fidelity and residency, some seasonality of movement in and out of the study area, and a fission–fusion society displaying assortative interactions by sex and geographic location36,40. Despite the apparent importance of this area for humpback dolphins in WA, our understanding of their habitat use is limited. Species distribution models (SDMs, presence-only) for this species based on opportunistic data collected during aerial surveys for dugongs in the western Pilbara region, north and east of the NMP, showed a potential preference for intertidal areas, however, the models were limited by a low sample size and lack of environmental predictor data41.
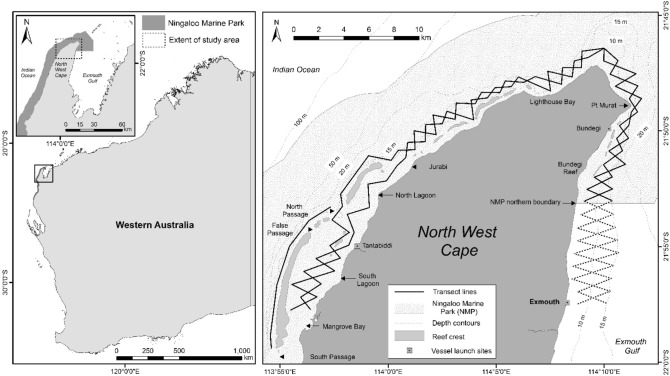
Figure 1.
Left: Map of Western Australia, indicating extent of Ningaloo Marine Park, location of North West Cape (NWC), and extent of study area. Right: Map of the NWC study site, including northern Ningaloo Marine Park (NMP) boundary, location names, depth contours, vessel launch sites (Tantabiddi, Bundegi, and Exmouth boat ramps) and opposing zig-zag line transect sampling design. Dotted transect lines indicate the area south of the NMP boundary that were excluded from analyses. Figure created in ArcMap 10.3.1 in ESRI’s ArcGIS© (ESRI, Redlands, California; https://www.esri.com/en-us/arcgis).
Australian humpback dolphins are a recognised value of MPAs in WA, including the NMP27. In light of increasing anthropogenic activities across their range in WA, a better understanding of their distribution and habitat use is needed for robust environmental impact assessments, and the effective implementation and management of protected areas for their conservation29,42,43. In this study, we used an ensemble modelling approach to assess the distribution of humpback dolphins within the northern section of the NMP and identify areas of high probability of dolphin occurrence and preferred habitats. Furthermore, we evaluated if the location of current sanctuary zones (i.e. zones where extractive activities like recreational and commercial fishing, and collecting, are not permitted)27 is likely to provide protection to humpback dolphins by assessing (1) whether dolphin distribution is correlated with sanctuary zone proximity, and (2) whether or not areas of high dolphin occurrence are encompassed within the boundaries of sanctuary zones.
Results
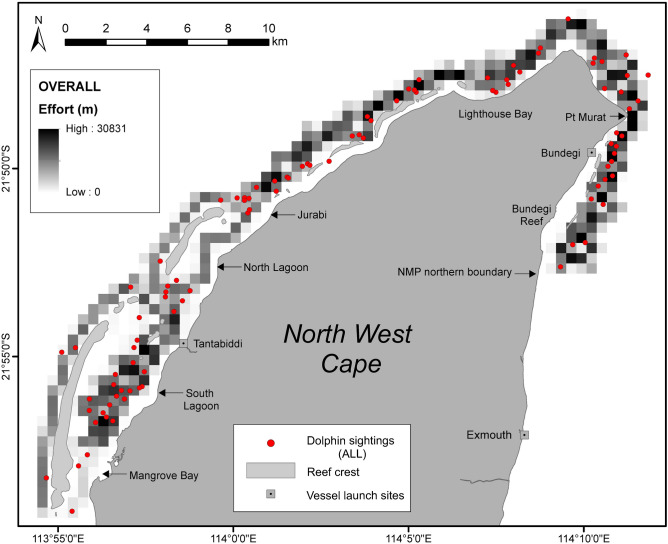
A total of 238 days (or part thereof) of boat-based survey effort, encompassing approximately 330 h and covering 3,627 km of transects in search of dolphins were completed between May 2013 and October 2015 (Table 1, Fig. 2). We encountered 169 humpback dolphin schools over the study period (Table 1, Fig. 2).
Table 1.
Summary of survey effort, number of dolphin schools encountered and number of 500 × 500 m grid cells with dolphin presences used to model Australian humpback dolphin distribution within northern Ningaloo Marine Park between May 2013 and October 2015.
| Total | |
|---|---|
| Survey days (or part thereof) | 238 |
| Survey effort (h) | 330 |
| Survey effort (km) | 3,627 |
| No. of dolphin schools | 169 |
| No. of grid cells with dolphin presences | 130 |
Figure 2.
Map of survey effort and sightings of Australian humpback dolphins during boat-based surveys in northern Ningaloo Marine Park (NMP) during the overall survey period, May 2013 to October 2015 (n = 169 sightings). Effort represented as km of survey track lines per 500 × 500 m grid cell. Dolphin sightings represent single or schools of animals. Figure created in ArcMap 10.3.1 in ESRI’s ArcGIS© (ESRI, Redlands, California; https://www.esri.com/en-us/arcgis).
Model performance
Collinearity was evident only between distance to reef crest and distance to passage ecogeographic variables (r = 0.9); thus, distance to passage was removed from SDM analysis. Consequently, a total of eight predictor variables were considered in the entire (overall) survey period (Table 2). All single SDMs performed better than random models, and the ensemble model performed better than single models (Fig. 3). The median area under the curve (AUC; see “Methods” for AUC definition) for single SDMs was 0.75, while AUC of the ensemble model was 0.82 (Fig. 3). When considering the mean of means, water depth was the most important variable predicting humpback dolphin distribution (Table 2).
Table 2.
Importance of ecogeographic predictor variables used in species distribution models (SDMs) of Australian humpback dolphins in northern Ningaloo Marine Park over the entire survey period (May 2013—October 2015).
| SDM period | Model | Ecogeographic predictor variables | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Habitat type | Water depth | Slope | Seabed complexity | Distance to coast | Distance to boat ramp | Distance to reef crest | Distance to sanctuary zone | ||
| Entire | GAM | 0.179 | 0.412 | 0.23 | 0.116 | 0.412 | 0.051 | 0.094 | 0.019 |
| GBM | 0.037 | 0.499 | 0.129 | 0.085 | 0.266 | 0.02 | 0.045 | 0.004 | |
| CTA | 0.085 | 0.556 | 0.328 | 0.211 | 0.566 | 0.074 | 0.141 | 0.054 | |
| FDA | 0.051 | 0.737 | 0.108 | 0.057 | 0.184 | 0.000 | 0.046 | 0.003 | |
| RF | 0.046 | 0.239 | 0.139 | 0.091 | 0.176 | 0.041 | 0.055 | 0.022 | |
| MAXENT | 0.069 | 0.326 | 0.205 | 0.108 | 0.428 | 0.093 | 0.169 | 0.097 | |
| Mean of means | 0.078 | 0.462 | 0.190 | 0.111 | 0.339 | 0.047 | 0.092 | 0.033 | |
Variable importance is presented as the mean over 10 cross-validation runs of each modelling algorithm, and as the mean of means amongst them.
GAM generalised additive model, GBM generalised boosted model, CTA classification tree analysis, FDA flexible discriminant analysis, RF random forest, MAXENT maximum entropy. Environmental variables of greatest influence based on the randomisation procedure in biomod2 are highlighted in bold. For variable definitions see Supplementary Table S1.1 in Appendix S1.
Figure 3.
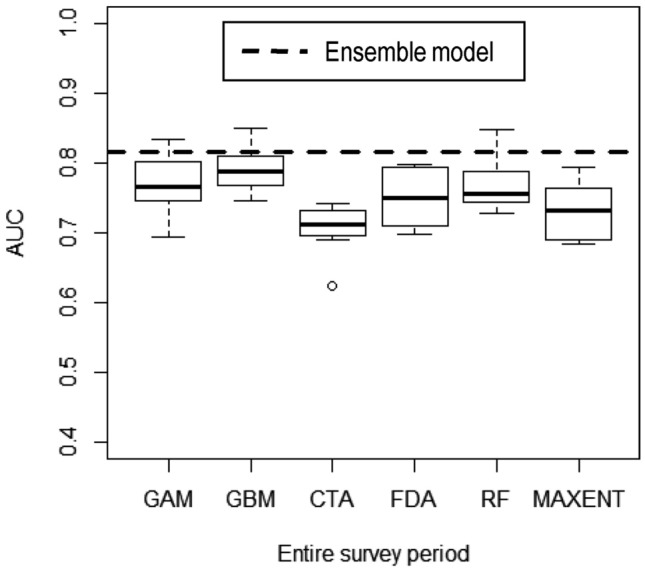
Performance of species distribution models of Australian humpback dolphins in northern Ningaloo Marine Park, Western Australia, built with datasets for the entire survey period (May 2013—October 2015). Box-plot displaying the Area Under Curve (AUC) of the receiver operating characteristics evaluation scores for all models, grouped by modelling algorithm (GAM generalised additive model, GBM generalised boosted model, CTA classification tree analysis, FDA flexible discriminant analysis, RF random forest, MAXENT maximum entropy). Components of box-plot represent minimum (the bottom of the whisker), lower quartile (bottom edge of box), median (bold line drawn inside the box), upper quartile (upper edge of box), maximum (top of the whisker) and outlier AUC values (empty circles) for each modelling method. Dashed line indicates the predictive performance (AUC) of the ensemble model (AUC = 0.82). Values of AUC ≥ 0.7 indicated the ensemble model performed reasonably well.
Dolphin occurrence across the entire survey period
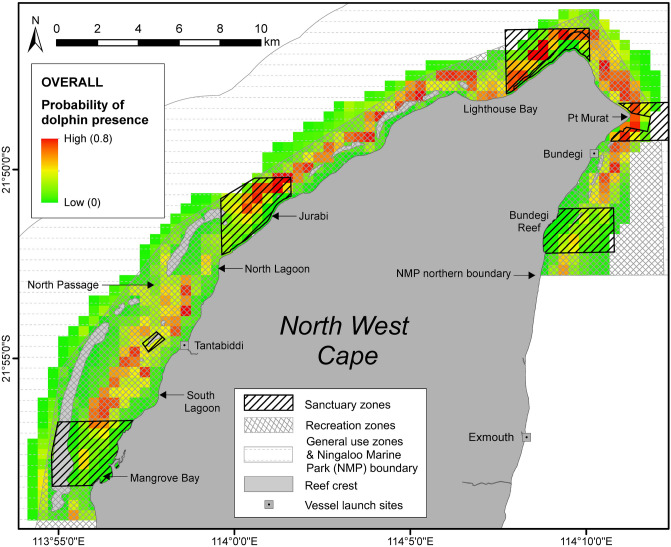
Across all individual SDMs for the overall dataset, water depth and distance to coast were the two most important ecogeographic variables predicting dolphin occurrence (Table 2). While water depth was the variable with greatest influence overall, individual influence values for this and distance to coast were similar, and across all models were convincingly the strongest predictors of dolphin occurrence (i.e. typically 0.1–0.3 difference between the lesser of these two variables and the third most influential variable; Table 2). Slope and seabed complexity also showed some influence on dolphin distribution (Table 2). The response curves across most individual models indicated that the probability of dolphin occurrence was higher in water depths ranging from 5 to 15 m and less than 2 km from the coast (Supplementary Fig. S2.3 in Appendix S2). Accordingly, the ensemble model predicted high (> 0.6) dolphin presence in shallow waters (mean ± SD = 10.6 ± 4.6; range 4–20 m); within 2 km from the coast between Bundegi Reef in the east and Jurabi in the west, and in the area between North Passage and Tantabiddi, and South Lagoon in the west (Fig. 4). Dolphin occurrence generally increased with increasing slope and seabed complexity (Supplementary Fig. S2.3 in Appendix S2). After depth and distance to coast, benthic habitat was the next most important variable in the generalised additive model (GAM; Table 2), specifically categories of ‘coral reef communities (subtidal)’, sand, and ‘subtidal reef’ (both lagoonal and seaward). For habitat type definitions, see Supplementary Table S1.2 in Appendix S1.
Figure 4.
Ensemble model outputs indicating probability of occurrence of Australian humpback dolphins in northern Ningaloo Marine Park (NMP) during the overall survey period, May 2013 to October 2015. Sanctuary zones, recreational zones, general use zones and other locations are also indicated. Figure created in ArcMap 10.3.1 in ESRI’s ArcGIS© (ESRI, Redlands, California; https://www.esri.com/en-us/arcgis).
Dolphin occurrence in sanctuary zones
Sanctuary zones (SZ), or ‘no take’ zones made up 26% of the entire study area, the remainder comprising of recreation zones (60%) and general use zones (14%). For a full list of zone definitions, see Supplementary Table S1.3 in Appendix S1. Distance to SZ was not considered an important variable influencing humpback dolphin occurrence (Table 2). Overall, the probability of dolphin occurrence inside SZ was low (combined mean < 0.3; Table 3, Fig. 4). Dolphin probability of occurrence was generally highest in Jurabi, Lighthouse Bay and Point Murat SZ (mean range = 0.18–0.37; Table 3, Fig. 4). The mean probabilities of dolphin occurrence were higher in these three SZ than outside (outside mean range = 0.14–0.22). SZ only covered a small proportion of areas of high probability (> 0.6) of dolphin occurrence (range 1–11%; Table 4). Randomisation tests indicated that areas of high probability of dolphin occurrence did not occur within SZ more often than would be expected by chance (P-value = 0.25).
Table 3.
Probability of Australian humpback dolphin occurrence in six sanctuary zones of northern Ningaloo Marine Park predicted by ensemble models for the overall survey period (May 2013-October 2015).
| Dolphin occurrence probability | |||
|---|---|---|---|
| Sanctuary zone | Area (km2) | No. of grid cells | Overall (mean ± SD) (median) (range) |
| Mangrove Bay | 11.4 | 48 |
0.13 ± 0.13 0.06 0.04—0.58 |
| Tantabiddi | 0.5 | 2 |
0.32 ± 0.04 0.32 0.28—0.35 |
| Jurabi | 7.5 | 36 |
0.30 ± 0.23 0.25 0.03—0.73 |
| Lighthouse Bay | 7.6 | 30 |
0.34 ± 0.27 0.23 0.03—0.76 |
| Point Murat | 4.7 | 9 |
0.37 ± 0.21 0.33 0.07—0.70 |
| Bundegi Reef | 7 | 32 |
0.14 ± 0.11 0.14 0.04—0.43 |
| Combined | 38.7 | 157 |
0.23 ± 0.21 0.15 0.03—0.76 |
| Outside (RZ & GUZ) | 111.8 | 445 |
0.22 ± 0.20 0.13 0.04—0.74 |
Values shown indicate mean (± SD), median, and range of occurrence probability for the total number of 500 × 500 m grid cells occupying each sanctuary zone, sanctuary zone grids combined, or grids outside sanctuary zones (i.e. recreation and general use zones; RZ and GUZ, respectively). See Fig. 4 for visual representation of the probability of dolphin occurrence in (and outside) sanctuary zones.
Table 4.
Summary of Australian humpback dolphin probability of occurrence throughout the entire study area, and six sanctuary zones in northern Ningaloo Marine Park, for the overall survey period, May 2013 to October 2015.
| Dolphin occurrence probability | Overall | |
|---|---|---|
| Entire study area (%) | Sanctuary zones (%) | |
| Low (< 0.3) | 72 | 71 |
| Medium (0.31–0.6) | 18 | 17 |
| High (> 0.6) | 11 | 11 |
Values shown indicate mean (± SD), median, and range of occurrence probability for the total number of 500 × 500 m grid cells occupying each sanctuary zone, sanctuary zone grids combined, or grids outside sanctuary zones (i.e. recreation and general use zones; RZ and GUZ, respectively). See Fig. 4 for visual representation of the probability of dolphin occurrence in (and outside) sanctuary zones.
Discussion
Ensuring the efficacy of MPAs in protecting mobile marine megafauna requires an understanding of the distribution and habitat preferences of these animals. Our study identified shallow waters (5–15 m), close to the coast (< 2 km) as the areas of highest probability of humpback dolphin occurrence within the northern section of the NMP. Nevertheless, the majority of areas of high probability of dolphin occurrence were located outside SZ. These findings, in combination with the recent and forecast increases in human activities in the marine park (e.g.44) suggest that the shallow, inshore areas identified here need prioritisation to better protect this important area for Australian humpback dolphins. We recommend that future spatial planning and marine park management plan reviews consider the preferred habitat areas identified in this study to mitigate potential impacts from increasing human activities for this resident humpback dolphin population.
Our study, which involved survey effort up to 5 km offshore and in depths to 45 m, supports the preference Australian humpback dolphins have for shallow inshore waters. Although sightings occurred in waters toward the offshore survey limits, they were uncommon. In preliminary research at this site, humpback dolphins were encountered at a mean (± SE) of 1 km (± 0.11) from shore, with the majority of schools (95%) in waters < 15 m deep45. All sightings reported throughout the adjacent Exmouth Gulf have been in < 20 m depth41; Raudino et al. unpub. data; pers. obs.). Elsewhere in WA, humpback dolphins have been observed some 70 km from the mainland coast at the Montebello Islands Marine Park, but close to the shoreline and in shallow water (i.e. < 10 m46). In the Northern Territory, humpback dolphins occur within 20 km of major tidal rivers, and as far as 50 km upstream47, and along the east coast of Queensland, they occur primarily in waters of < 15 m depth48–50. In southern Papua New Guinea (the Kikori Delta), humpback dolphins were sighted in coastal waters of < 12 m water depth51. These observations suggest water depth could be a limiting factor for the distribution of this species. Records of humpback dolphins far from the mainland coast are uncommon and likely due to the broad, shallow physiography of the continental shelf, and abundance of shallow reefs, sand flats and continental islands; with dolphins remaining in shallow water and not necessarily far from shore (i.e. mainland or islands52,53). Water depth and distance to coast also appear to be strong predictors of the occurrence of other Sousa spp., indicating their preference for < 30 m coastal waters (reviewed in54; see also summary in55).
We note, however, that the majority of boat-based survey effort around Australia, as in this study (see Supplementary Appendix S2), has been concentrated in shallow, coastal waters (e.g.31,34,49,56). Thus, we must acknowledge there may be an inherent bias toward distance to coast being a strong predictor variable in this, and other humpback dolphin studies. Nevertheless, the SDMs applied in this study do take survey effort into account, and there have been few confirmed reports of this species in deeper waters (i.e. > 30 m) off the NMP despite multiple years of commercial tour (aerial and vessel platforms) and research operations.
Food availability, predation risk and anthropogenic activities influence delphinid habitat use57–59. Australian humpback dolphins feed on a wide variety of fish associated with shallow coastal-estuarine environments60, which may explain their preference for shallow coastal waters. Reef structures in the study area are located close to shore and coincide with areas of high dolphin occurrence (e.g. Bundegi Reef to Point Murat, channel from Tantabiddi to North Passage, and South Lagoon). Benthic habitat type including ‘coral reef communities (subtidal)’, and ‘subtidal reef’ (both lagoonal and seaward) showed some importance in our results. In Queensland, humpback dolphins also showed preferences for reef (coral and fringing) habitat type, as well as seagrass flats, mangroves and dredged channels38,49,50. The density of herbivorous fish assemblages in Ningaloo Reef, including unicorn fish (Naso fageni), were found to be greater around coral reef structures61. Humpback dolphins were observed feeding on unicorn fish (Naso sp.) in the study area (Hunt, pers. obs.), so these inshore reefs may serve as important foraging areas for this population.
Fish assemblages at SZ in NMP have higher biomass and abundance than at sites where fishing is permitted62. It was hypothesised by36 that consistent prey availability may be influencing regular use of NMP by humpback dolphins. Future studies into the diet of humpback dolphins in the NMP and how it relates to fish assemblages within SZ are needed to assess their importance to humpback dolphins. This in turn would influence the recommendation to modify SZ spatial extent to better encompass identified areas of high dolphin occurrence (see below).
The prevalence of shark bites on tropical inshore dolphins in the Kimberley region of NW Australia were among the highest recorded63, suggesting that predation risk is likely a strong influence on habitat use57,63. A number of animals in the study population bear evidence of shark bites (Hunt, unpub. data) and predation risk may be influencing humpback dolphin habitat use in the northern section of the NMP. Prey availability and predator presence are likely factors that influence NWC humpback dolphin social structure40, and future studies and modelling approaches involving these potential drivers (or suitable proxies, see below) may better elucidate their influence on humpback dolphin occurrence.
The performance of SDMs is influenced by deficiencies and biases in the ecogeographic variables used to build the models (e.g.64). Ideally, species observations and ecogeographic variables, such as benthic habitat type, are measured at the same spatial and temporal resolution. The benthic habitat spatial layer used in this study (i.e.65) was developed in 1999 and is currently the only benthic habitat spatial data available for the whole northern NMP. Although benthic habitat was not deemed a primary variable of importance for humpback dolphin distribution, future SDM efforts and spatial zoning would benefit from an updated and validated spatial layer of benthic habitat type.
There were some discrepancies between single model outputs in regard to the relative importance of certain ecogeographic variables on humpback dolphin occurrence. The EM approach we used overcame these predictive uncertainties, with all EMs performing better than single models. To this end, we concur with66 in encouraging the use of EM approaches in future studies assessing cetacean distribution and habitat use.
SDMs of marine mammals do not often take into account environmental and behavioural processes that are important drivers of animal distributions, such as prey availability, predation risk, and animal behaviour67. This is generally because they are difficult to sample, and do not always offer better model performance. For example,68 found that relying on prey distribution data alone was insufficient, and that fine scale models of marine predator habitat selection in coastal habitats will be more successful if environmental variables are used as proxies of both prey and predator distribution. Although behavioural data was collected during the present study, the paucity of data prevented its use for building behaviour-specific models of occurrence (as in69), or for using kernel density estimates of behaviours to investigate overlap with areas of high dolphin occurrence (e.g.66). Further studies focusing on the collection of focal behavioural data will help address how these behavioural processes influence humpback dolphin distribution in the NMP region.
The almost continuous high areas of occurrence for much of the northern NMP study area corroborate that the NWC is an important habitat for humpback dolphins36,45. However, the majority of areas of high probability of dolphin occurrence (> 90%) identified in this study were outside SZ, in recreation zones, where extractive activities such as recreational fishing are allowed. The NMP was initially gazetted in 1987, with the current sanctuary zones gazetted in 2004. The management plan for the NMP (‘the Plan’) has gone beyond its 10-year management period, and, under the Conservation and Land Management Act 1984, is due for review “as soon as possible”27. The forthcoming review represents an opportunity to utilise the adaptive management framework of the Plan to review current or proposed zoning that takes into consideration the areas of high humpback dolphin occurrence identified here in order to minimise disturbance and/or displacement from human activities. The review also represents an opportunity for humpback dolphins to be considered more explicitly with defined management objectives and performance measures70.
The areas around Tantabiddi and North Passage are characterised by high probability of dolphin occurrence and have also been identified as part of a core area of very high recreational fishing pressure in NMP71. The impact this overlap may have on dolphins is unknown and needs to be assessed. Another location of high dolphin occurrence is the Bundegi/Pt Murat area, which coincides with high recreational boat use27, and areas of medium–high dolphin use around North Passage, Tantabiddi, and South Lagoon align with areas of known boat traffic and high recreational use72. Given these spatial overlaps and the potential risk of boat strike and/or disturbance to dolphins, consideration should be given to proclaiming ‘go slow’ areas, as adopted in MPAs in Queensland (e.g.73) and in Bunbury, WA (see74). A more immediate, interim management measure could include the development of educational and interpretive material (e.g. signage at boat ramps, key messages in tourism brochures) highlighting the areas identified as important habitat for humpback dolphins and a recommendation to slow down and adhere to minimum approach distances.
When proclaiming the Ningaloo Coast in 2011, the World Heritage Committee identified that additional management efforts would be required as tourist numbers increased28. Given the evidence of increasing human use within the NMP and the conservation value this MPA can provide for future management of this listed Vulnerable species, we recommend that future marine spatial planning reviews reconsider SZ boundaries to better encompass areas of high humpback dolphin occurrence. This is particularly pertinent given one of the objectives of SZ is to provide the highest level of protection for vulnerable species27 (Supplementary Table S1.3 in Appendix S1), and that no-take marine reserves have found to be the most effective protected areas in the ocean (reviewed in75). It was suggested by76 that increases in SZ areas within NMP (e.g. around Bundegi and Jurabi) are needed to better encompass areas critical for resilience to climate change induced disturbance. Increases in SZ areas are also likely to have indirect benefits to humpback dolphins within the MPA through preservation of important habitat as refugia for them and their prey.
Methods
Study site
The study site is within the northern section of the NMP, extending from the northern NMP boundary in Exmouth Gulf around the tip of the NWC, and south to Mangrove Bay (inside lagoon) and South Passage (outside reef; Fig. 1). The area is characterised by shallow (< 5 m depth) lagoon waters, with primarily sandy substrate and coral communities within the fringing (sub-tidal) coral reef system27,77. Water depth on the western side of the NWC drops sharply outside the reef crest towards the continental shelf, with maximum tidal ranges extending up to 2.5 m.
Survey design and data collection
Boat-based surveys for humpback dolphins were conducted around the NWC during May–October 2013, April–October 2014 and May–October 2015. Surveys were conducted following a systematic line transect sampling design (2 × 93 km in length, opposing, evenly-spaced zig-zag lines; and 1 × 13 km single line; Fig. 1). Only survey effort and dolphin sighting information collected within the boundaries of the NMP (169 sightings out of 193) was considered for species distribution modelling analyses. The area south of the NMP northern boundary (as indicated by dotted transect lines; Fig. 1) was excluded from analysis because spatial data on benthic habitat is not available for the area outside NMP. The NMP study area equated to systematic line transect lengths of 2 × 68 km opposing zig-zag lines, and 1 × 13 km single line (as indicated by bold line in Fig. 1). The study area covered approximately 150 km2 along ca. 50 km of coastline, and extended up to 5 km offshore, encompassing water depths between 1 and 45 m.
Surveys were conducted on board a 5.6 m research vessel powered by a 100 HP outboard motor at speeds of 10–12 km/h and only in good sighting conditions (Beaufort Sea State ≤ 3 and no rain). Survey effort was continuous from 07:00 to 18:00, depending on suitable sighting conditions. A crew of three to five (mode = four) observers searched for dolphins forward of the vessel’s beam with the naked eye and 7 × 50 binoculars. Once a school of dolphins was sighted, search effort was suspended and dolphins were approached to within 10–30 m to record their GPS location, school size, school age composition (calf, juvenile, adult; as defined in31), and predominant behaviour (i.e. behavioural state of more than 50% of the animals in a school 78). Schools were defined as dolphins with relatively close spatial cohesion (i.e. each member within 100 m of any other member) involved in similar (often the same) behavioural activities (modified from79). Although behavioural data was collected, behaviour was not considered as a predictor variable in species distribution analyses (see “Discussion”).
Environmental measurements of water depth and sea surface temperature (SST) were recorded in situ at dolphin sighting locations, at the beginning/end point of transects (n = 87, termed ‘Transect Environmental Station, or ‘TES’), and every 60 min of transect survey effort (termed ‘ES’, see Supplementary Fig. S1.1 in Appendix S1). We used the research vessel’s depth sounder and an Oakton handheld multi-parameter to record water depth and SST, respectively.
Ecogeographic predictor variables
SDMs aim to predict the spatial distribution of individual species by correlating observations of species occurrence with data on ecogeographic and anthropogenic variables (i.e. predictor variables), thought to influence species distributions. Ecogeographic variables considered in modelling humpback dolphin distribution were either biotic (i.e. benthic habitat type), abiotic (i.e. water depth, slope, seabed complexity, SST, distance to coast, distance to reef crest), or anthropogenic (i.e. distance to SZ, distance to passage, and distance to boat ramp, which were used as a proxy for human activity) (Supplementary Table S1.1 in Appendix S1). Previous research indicates that some of these biotic and abiotic ecogeographic variables likely influence dolphin distribution80. Digital environmental layers of water depth and SST were created and explored using environmental data collected in situ at TES, ES and dolphin school sightings (including sightings of Indo-Pacific bottlenose dolphins Tursiops aduncus). In deriving digital layers, a mean TES value from each of the 87 fixed locations was obtained for the entire survey period, and by ‘season’ (see below), where n per TES ranged from 2 to 30, total n = up to 1,582).
Benthic habitat data covering the entire spatial extent of the study area was obtained through the Western Australian Government Parks and Wildlife Service of the Department of Biodiversity, Conservation and Attractions (formerly Department of Parks and Wildlife). This habitat data was derived from the broad scale marine habitat study of the NMP, outlined in66. Habitat types within the study area included ‘coral reef communities (subtidal)’, ‘subtidal reef (low relief—seaward)’, ‘subtidal reef (low relief—lagoonal)’, ‘coral reef communities (intertidal or shallow/limestone)’, sand, macroalgae (limestone reef), shoreline reef, salt marsh, mangroves, mudflats, and ‘deep water mixed filter feeding and soft bottom communities’ (for definitions see Supplementary Table S1.2 & Fig. S1.2 in Appendix S1). Water depth across the study area was obtained from hyperspectral imagery (see81), then cross-checked and validated using a combination of in situ measurements of water depth (from TES, ES and dolphin sightings, see above, see also Supplementary Fig. S1.1 in Appendix S1), and bathymetric grids from Geoscience Australia82,83 (see Supplementary Table S1.1 in Appendix S1).
All ecogeographic variables were sampled at a 500 × 500 m grid resolution using ArcMap 10.3.1 in ESRI’s ArcGIS© (ESRI, Redlands, California) and the Universal Transverse Mercator projection Zone 50 South based on the WGS 1984 datum (Supplementary Fig. S1.3 in Appendix S1). This resolution ensured sufficient detail of each variable throughout the study area, and corresponded with the sampled scale of the dolphin presence-absence data (see below). We used the Spatial Analyst extension in ArcMap 10.3.1 to calculate the Euclidean distance (the shortest straight line distance) for distance to coast, and the Cost distance tool (the shortest distance factoring in land given study area wraps around a peninsula) for distance to reef crest, distance to SZ, distance to boat ramp, and distance to passage (see Supplementary Table S1.1 & Fig. S1.3 in Appendix S1). SST was calculated using the Ordinary Kriging interpolation tool with a spherical semivariogram model (500 m cell size, 12 point variable search radius size) in the Spatial Analyst extension in ArcMap (see Supplementary Table S1.1 in Appendix S1).
Data exploration
Ecogeographic predictor variables considered for SDMs were grouped for the entire survey period from May 2013 to October 2015 (i.e. an overall SDM, using all fixed predictor variables outlined in Supplementary Table S1.1 in Appendix S1). Surveys were not conducted during the summer period (i.e. November to March), due to the occurrence of strong winds and tropical cyclones. Prior to running the SDMs, collinearity (correlation between environmental variables) was investigated in R v3.3.184 using multi-panel scatterplots, Pearson’s correlation coefficient (r) and variance inflation factors (VIFs) for all combinations of variables in the overall model85. Highly correlated variables were identified using the stepwise procedures vifcor and vifstep in the package usdm in R86. Using the vifcor procedure, whenever the maximum linear correlation between two variables was greater than the threshold (r = 0.7;85), that with the highest VIF is excluded; this step was repeated until no variable remained with an r-value greater than the threshold. Similarly, using vifstep, the variable with the highest VIF, and greater than the threshold (VIF = 3;85), was excluded; this step was also repeated until no variable with a VIF greater than the threshold remained86.
Response variable
The presence-absence of humpback dolphins (schools or single animals) was used as the response variable for ensemble species distribution modelling. The locations of dolphin sightings obtained on survey effort, and the associated survey tracks, were imported into ArcMap, and binary presence-absence grids were prepared. Survey coverage was quantified by adding a 250 m buffer either side of each survey track line, which was the average distance to which dolphins could be reliably observed from the boat under a variety of sea conditions (e.g.66). Survey effort was then quantified by intersecting track lines with the 500 × 500 m gridded area of survey coverage and calculating the length of survey effort track (km) per grid cell87. Each 500 × 500 m grid cell was classified as either 1 (dolphin presence) or 0 (dolphin absence), and was also characterised by each of the environmental predictor variables (see Supplementary Table S1.1 in Appendix S1).
To reduce false absences in SDMs (i.e. a species is considered absent from an area when it may in fact occur in that area; see88,89), absence cells were defined based on areas of highest survey effort90. Grid cells within the study area were ranked from highest to lowest effort, and cells with the highest survey effort and no dolphin presence were considered most likely to represent true absences and were thus defined as absence cells (as per66). The total number of absence cells was made equal to the total number of presence cells when considering ensemble SDMs. The survey effort threshold (m per grid cell) for defining true absences was 8,727 m for the overall model (highest was 24,274 m).
Most species SDMs are built at a grid cell size of 1 km × 1 km, which has been criticized as being too coarse to generate reliable SDM outputs91. This is particularly true for studies at small spatial scales, such as this. Model accuracy is likely to increase with decreasing cell size, but too fine a cell size has been shown to amplify the error from background absences92. Given: dolphins are highly mobile; the locational error associated with dolphin locations (i.e. dolphin locations are recorded within 10 to 30 m of research vessel); our definition of school size (i.e. each member within 100 m of any other member); and survey coverage (250 m buffer each side of the survey track line), we considered a 500 m cell size appropriate resolution while maintaining details of the attributes of the study area and scale at which dolphin and environmental data were collected. Reliable outputs based on this cell size have resulted from prior SDM studies of coastal dolphins (e.g.66,93).
Ensemble species distribution modelling
Species-habitat relationships are often investigated using correlative models to predict species distributions by combining known occurrence records with digital layers of ecogeographic variables expected to affect the species’ distribution94. SDMs encompass a variety of modelling algorithms with differences in predictive performance, depending on sample size, data structure (e.g. presence-only, presence-absence, presence/pseudo-absence), and the underlying fitted functions94–96. Ensemble modelling (EM) is an approach by which single-model predictions are combined97,98, yielding a higher level of accuracy and less bias than separate, single models66,96,99. EM approaches have been used across terrestrial species (e.g.100), and a variety of marine species (e.g.101–103), including blue whales (e.g.104) and coastal dolphins66,93,105.
We used an EM approach implemented in the biomod2 R package106 to predict the presence-absence of humpback dolphins with respect to the ecogeographic predictor variables (Supplementary Table S1.1 in Appendix S1). This approach used six different modelling algorithms under three different modelling methods: two regression methods, generalised additive models (GAMs107) and generalised boosted models (GBMs108); two classification methods, classification tree analysis (CTA109) and flexible discriminant analysis (FDA110); and two machine learning methods, random forest (RF111) and maximum entropy (MAXENT112). We selected these modelling algorithms because they are the most commonly used, known to perform well, and provide a sound comparison across a wide range of modelling approaches including regression, classification and machine learning methods96,106,113,114.
SDMs were developed using a binomial error distribution and the logit link function. Data for each SDM were split 75%/25% for model calibration and testing, respectively106. A total of 60 different statistical models calibrated for the SDM dataset resulted from a tenfold cross validation process. A randomisation procedure in biomod2 based on 10 permutation runs was subsequently implemented to assess the importance of the environmental predictor variables106. This procedure is independent of the modelling technique. It calculates the Pearson correlation between the standard predictions (i.e. fitted values) and predictions where one variable has been randomly permutated. If there is a high correlation between the two predictions (i.e. there is little difference between the two predictions), the variable permutated is considered unimportant for the model and vice versa. This procedure was repeated 10 times for each variable independently, and the correlation means were kept for each variable. Subsequently, this allowed direct comparison between models regardless of the modelling method. The mean correlation coefficient was then used to rank the variables from zero to one; where zero indicates the variable has no influence in the model, and one indicates the variable is most influential in the model106. A list of all parameters used for running the selected models in biomod2 is shown in Supplementary Appendix S2.
SDMs that utilise presence-absence data are subject to false positives (predicting species occurrence in areas where the species does not occur) or false negatives (failing to predict species presence where the species does occur98). To assess SDM predictive performance and compare individual modelling algorithms, we used the area under the curve (AUC) metric of the receiver operating characteristics plot115 calculated in R using biomod2. The AUC is a measure of the ratio between the observed presence-absence values and the model predictions. Values range from zero to one, with values above 0.5 indicative of models with predictions performing better than what would be expected by chance115. In general, AUC values of 0.5–0.7 are considered low and represent poor model performance, values of 0.7–0.9 are considered reasonable predictions, and values above 0.9 represent excellent model performance116.
Lastly, we combined the six individual SDMs (modelling algorithms) to obtain an ensemble prediction of dolphin presence across the study area106. Of the individual models, only those with AUC values above 0.5 were considered, and their contribution to the ensemble model was weighted based on their predictive accuracy (the higher the evaluation score the more weight assigned to the model96). The ensemble model output was then imported into ArcMap, providing a visual output of probability of species occurrence, where values ranged from zero to one; zero indicating no probability and one indicating a very high probability of dolphin presence. Finally, following96, we used AUC values to compare the performance of the ensemble model with the performance of the individual models.
Dolphin occurrence and sanctuary zones
To evaluate the relevance of the six current SZ in the northern NMP for the protection of humpback dolphins, we assessed whether areas of high dolphin occurrence (i.e. > 0.6) fell within SZ more often than would be expected by chance using a randomisation test in PopTools v3.2.5117. To do this, we calculated an observed index for the ensemble output (i.e. total number of high dolphin occurrence cells that were located within SZ) and compared this index with a random index (i.e. total number of times high dolphin occurrence cells fell within SZ as they were randomly distributed across the study area), obtained from 5,000 permutations. The significance (P-value ≥ 0.05) was calculated as the proportion the random index that was greater than or equal to the observed index118.
Seasonality of dolphin occurrence
Demographic analysis from36 indicated that there was some seasonality of humpback dolphin movement in and out of the study area. Using the above methodology, models were also split temporally into corresponding seasons (i.e. Autumn–Winter, April to July inclusive; and Winter-Spring, August to October inclusive) to determine if these demographic characteristics are reflected in changes in the probability of occurrence and habitat preferences. The ensemble models show consistent results in the spatial distribution of humpback dolphins among seasons (see Supplementary Appendix S3 for further details on seasonal analysis), and thus we conducted further analysis on the pooled dataset.
Approvals
Data collection was permitted by the Western Australian (WA) Government Parks and Wildlife Service of the Department of Biodiversity, Conservation and Attractions (SF009240, SF009768, SF010289), WA Government Agriculture and Food Division (U38/2013-2015) and the Australian Government Department of Defence (Harold Holt Naval Base Exmouth), with approval from Flinders University Animal Welfare Committee (project number E383).
Supplementary information
Acknowledgements
The Australian Marine Mammal Centre (Project 12/11) and the Winifred Violet Scott Charitable Trust funded this research. We sincerely thank all ‘Team Sousa’ volunteers that assisted with data collection in the field over the three years of surveys. We would also like to thank the community and businesses of Exmouth, the staff at Parks and Wildlife Service Exmouth, the Cape Conservation Group, and MIRG Australia for supporting this research project. We thank Halina Kobryn for supplying the hyperspectral bathymetric data and insightful advice on digital layer preparation. We thank Nikki Zanardo and Cecilia Passadore for assistance with ensemble modelling analyses.
Author contributions
G.J.P., L.B., S.J.A., and T.H. conceived and designed the study. T.H. collected the data. T.H. processed and analysed the data with advice and contributions to data analysis from G.J.P, L.B. and S.J.A. T.H. wrote the manuscript and prepared all figures and tables, with contributions to drafting, critical review, and editorial input from G.J.P., S.J.A., and L.B.
Data availability
Data made available to all interested researchers upon reasonable request to Tim Hunt (t_hunt@live.com.au) and Guido J. Parra (guido.parra@flinders.edu.au).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-69863-6.
References
- 1.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 2.Wallace BP, et al. Global conservation priorities for marine turtles. PLoS ONE. 2011;6:e24510. doi: 10.1371/journal.pone.0024510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson AD, et al. Drivers and hotspots of extinction risk in marine mammals. Proc. Natl. Acad. Sci. USA. 2012;109:3395–3400. doi: 10.1073/pnas.1121469109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dulvy, N.K., et al. Extinction risk and conservation of the world’s sharks and rays. eLife3, e00590 (2014). [DOI] [PMC free article] [PubMed]
- 5.Slooten E, Davies N. Hector's dolphin risk assessments: old and new analyses show consistent results. J. R. Soc. NZ. 2012;42:49–60. [Google Scholar]
- 6.Cagnazzi D, Parra GJ, Westley S, Harrison PL. At the heart of the industrial boom: Australian snubfin dolphins in the Capricorn Coast, Queensland, need urgent conservation action. PLoS ONE. 2013;8:e56729. doi: 10.1371/journal.pone.0056729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parra GJ, Cagnazzi D. Conservation status of the Australian humpback dolphin (Sousa sahulensis) using the IUCN Red List criteria. Adv. Mar. Biol. 2016;73:157–192. doi: 10.1016/bs.amb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Turvey ST, et al. First human-caused extinction of a cetacean species? Biol. Lett. 2007;3:537–540. doi: 10.1098/rsbl.2007.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor BL, et al. Extinction is imminent for Mexico's endemic porpoise unless fishery bycatch is eliminated. Conserv. Lett. 2017;10:588–595. [Google Scholar]
- 10.Gormley AM, et al. First evidence that marine protected areas can work for marine mammals. J. Appl. Ecol. 2012;49:474–480. [Google Scholar]
- 11.Edgar GJ, et al. Global conservation outcomes depend on marine protected areas with five key features. Nature. 2014;506:216. doi: 10.1038/nature13022. [DOI] [PubMed] [Google Scholar]
- 12.Hoyt E. Marine Protected Areas for Whales, Dolphins and Porpoises: A World Handbook for Cetacean Habitat Conservation and Planning. 2. London: Earthscan; 2011. [Google Scholar]
- 13.di Sciara GN, et al. Place-based approaches to marine mammal conservation. Aquat. Conserv. 2016;26:85–100. [Google Scholar]
- 14.Gregr EJ, Baumgartner MF, Laidre KL, Palacios DM. Marine mammal habitat models come of age: The emergence of ecological and management relevance. Endanger Species Res. 2013;22:205–212. [Google Scholar]
- 15.Guisan A, et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2013;16:1424–1435. doi: 10.1111/ele.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooker SK, Cañadas A, Hyrenbach KD, Corrigan C, Polovina JJ, Reeves RR. Making protected area networks effective for marine top predators. Endanger Species Res. 2011;13:203–218. [Google Scholar]
- 17.Dryden J, Grech A, Moloney J, Hamann M. Rezoning of the Great Barrier Reef World Heritage Area: Does it afford greater protection for marine turtles? Wildl Res. 2008;35:477–485. [Google Scholar]
- 18.Cleguer C, Grech A, Garrigue C, Marsh H. Spatial mismatch between marine protected areas and dugongs in New Caledonia. Biol. Conserv. 2015;184:154–162. [Google Scholar]
- 19.Oh BZL, Sequeira AMM, Meekan MG, Ruppert JLW, Meeuwig JJ. Predicting occurrence of juvenile shark habitat to improve conservation planning. Conserv. Biol. 2017;31:635–645. doi: 10.1111/cobi.12868. [DOI] [PubMed] [Google Scholar]
- 20.Liu, M., Bejder, L., Lin, M., Zhang, P., Dong, L. & Li, S. Determining important habitats of the world’s second largest humpback dolphin population: Implications for place-based conservation and management. Aquat. Conserv. Mar. Freshw. Ecosyst. 1–11 10.1002/aqc.3253 (2019).
- 21.Tardin RH, et al. Modelling habitat use by the Guiana dolphin, Sotalia guianensis, in south-eastern Brazil: Effects of environmental and anthropogenic variables, and the adequacy of current management measures. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020;30:775–786. [Google Scholar]
- 22.Worm B. Marine conservation: How to heal an ocean. Nature. 2017;543:630–631. doi: 10.1038/nature21895. [DOI] [PubMed] [Google Scholar]
- 23.Wood LJ, Fish L, Laughren J, Pauly D. Assessing progress towards global marine protection targets: shortfalls in information and action. Oryx. 2008;42:340–351. [Google Scholar]
- 24.Devillers R, et al. Reinventing residual reserves in the sea: are we favouring ease of establishment over need for protection? Aquat. Conserv. 2015;25:480–504. [Google Scholar]
- 25.Bottrill MC, Pressey RL. The effectiveness and evaluation of conservation planning. Conserv. Lett. 2012;5:407–420. [Google Scholar]
- 26.Agardy T. Justified ambivalence about MPA effectiveness. ICES J. Mar. Sci. 2018;75:1183–1185. [Google Scholar]
- 27.CALM & MPRA. Management Plan for the Ningaloo Marine Park and Muiron Islands Marine Management Area, 2005–2015. Western Australian Government Department of Conservation and Land Management, and Marine Parks and Reserve Authority, Perth, Western Australia (2005). https://www.dpaw.wa.gov.au/images/documents/parks/management-plans/decarchive/ningaloo_mp_01_2005_withmaps.pdf.
- 28.UNESCO. United Nations Educational, Scientific and Cultural Organisation. Decisions adopted by the World Heritage Committee at its 35th session, Paris, 7 July 2011. WHC-11/35.COM/20 (2011). https://whc.unesco.org/en/decisions/4278.
- 29.Hanf DM, Hunt TN, Parra GJ. Humpback dolphins of Western Australia: a review of current knowledge and recommendations for future management. Adv. Mar. Biol. 2016;73:193–218. doi: 10.1016/bs.amb.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Jefferson, T.A. & Rosenbaum, H.C. Taxonomic revision of the humpback dolphins (Sousa spp.), and description of a new species from Australia. Mar. Mamm. Sci.30, 1494–1541 (2014).
- 31.Parra GJ, Corkeron PJ, Marsh H. Population sizes, site fidelity and residence patterns of Australian snubfin and Indo-Pacific humpback dolphins: Implications for conservation. Biol. Conserv. 2006;129:167–180. [Google Scholar]
- 32.Cagnazzi DDB, Harrison PL, Ross GJB, Lynch P. Abundance and site fidelity of Indo-Pacific humpback dolphins in the Great Sandy Strait, Queensland, Australia. Mar. Mamm. Sci. 2011;27:255–281. [Google Scholar]
- 33.Palmer C, Brooks L, Parra GJ, Rogers T, Glasgow D, Woinarski JCZ. Estimates of abundance and apparent survival of coastal dolphins in Port Essington harbour, Northern Territory, Australia. Wildl. Res. 2014;41:35–45. [Google Scholar]
- 34.Brown AM, Bejder L, Pollock KH, Allen SJ. Site-specific assessments of the abundance of three inshore dolphin species to inform conservation and management. Front. Mar. Sci. 2016;3:4. doi: 10.3389/fmars.2016.00004. [DOI] [Google Scholar]
- 35.Brooks L, Palmer C, Griffiths AD, Pollock KH. Monitoring variation in small coastal dolphin populations: An example from Darwin, Northern Territory, Australia. Front. Mar. Sci. 2017;4:94. doi: 10.3389/fmars.2017.00094. [DOI] [Google Scholar]
- 36.Hunt TN, Bejder L, Allen SJ, Rankin RW, Hanf D, Parra GJ. Demographic characteristics of Australian humpback dolphins reveal important habitat toward the southwestern limit of their range. Endanger Species Res. 2017;32:71–88. [Google Scholar]
- 37.Brown AM, et al. Population differentiation and hybridisation of Australian snubfin (Orcaella heinsohni) and Indo-Pacific humpback (Sousa chinensis) dolphins in north-western Australia. PLoS ONE. 2014;9:e101427. doi: 10.1371/journal.pone.0101427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parra GJ. Resource partitioning in sympatric delphinids: space use and habitat preferences of Australian snubfin and Indo-Pacific humpback dolphins. J. Anim. Ecol. 2006;75:862–874. doi: 10.1111/j.1365-2656.2006.01104.x. [DOI] [PubMed] [Google Scholar]
- 39.Parra, G., Cagnazzi, D., Perrin, W. & Braulik, G.T. Sousa sahulensis. The IUCN Red List of Threatened Species 2017: e.T82031667A82031671. https://www.iucnredlist.org/details/82031667/0. (2017).
- 40.Hunt, T.N., Allen, S.J., Bejder, L. & Parra, G.J. Assortative interactions revealed in a fission-fusion society of Australian humpback dolphins. Behav Ecol 1–14. 10.1093/beheco/arz029 (2019).
- 41.Hanf, D.M. Species Distribution Modelling of Western Pilbara Inshore Dolphins. MRes thesis. Murdoch University, Perth, Western Australia (2015).
- 42.Allen SJ, Cagnazzi DD, Hodgson AJ, Loneragan NR, Bejder L. Tropical inshore dolphins of north-western Australia: Unknown populations in a rapidly changing region. Pac. Conserv. Biol. 2012;18:56–63. [Google Scholar]
- 43.Bejder L, Hodgson A, Loneragan N, Allen SJ. Coastal dolphins in north-western Australia: The need for re-evaluation of species listings and short-comings in the Environmental Impact Assessment process. Pac. Conserv. Biol. 2012;18:22–25. [Google Scholar]
- 44.Rob, D. & Barnes, P. Whale Shark Management Annual Report: 2016 Whale Shark Season. Progress report for the Department of Parks and Wildlife, Wildlife Management Program No. 57. (2016). Report available on request.
- 45.Brown, A., Bejder, .L, Cagnazzi, D., Parra, G.J. & Allen, S.J. The North West Cape, Western Australia: A potential hotspot for Indo-Pacific humpback dolphins Sousa chinensis? Pac Conserv Biol18, 240–246 (2012).
- 46.Raudino HC, Hunt TN, Waples K. Records of Australian humpback dolphins (Sousa sahulensis) from an offshore island group in Western Australia. Mar. Biodivers. Rec. 2018;11:14. doi: 10.1186/s41200-018-0147-0. [DOI] [Google Scholar]
- 47.Palmer C, Parra GJ, Rogers T, Woinarski J. Collation and review of sightings and distribution of three coastal dolphin species in waters of the Northern Territory, Australia. Pac. Conserv. Biol. 2014;20:116–125. [Google Scholar]
- 48.Parra GJ, Schick R, Corkeron PJ. Spatial distribution and environmental correlates of Australian snubfin and Indo-Pacific humpback dolphins. Ecography. 2006;29:396–406. [Google Scholar]
- 49.Cagnazzi, D. Conservation status of Australian snubfin dolphin, Orcaella heinsohni, and Indo-Pacific humpback dolphin, Sousa chinensis, in the Capricorn Coast, Central Queensland, Australia. PhD thesis. Southern Cross University, Lismore, Australia (2011).
- 50.Cagnazzi, D. Review of coastal dolphins in central Queensland, particularly Port Curtis and Port Alma regions. Report produced for the Ecosystem Research and Monitoring Program Advisory Panel as part of Gladstone Ports Corporation’s Ecosystem Research and Monitoring Progra. Gladstone Ports Corporation, Queensland, Australia (2013).
- 51.Beasley I, Jedensjö M, Wijaya GM, Anamiato J, Kahn B, Kreb D. Observations on Australian humpback dolphins (Sousa sahulensis) in waters of the Pacific Islands and New Guinea. Adv. Mar. Biol. 2016;73:219–271. doi: 10.1016/bs.amb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Corkeron PJ, Morissette NM, Porter L, Marsh H. Distribution and status of hump-backed dolphins, Sousa chinensis, Australian waters. Asian Mar. Biol. 1997;14:49–59. [Google Scholar]
- 53.Parra GJ, Corkeron PJ, Marsh H. The Indo-Pacific humpback dolphin, Sousa chinensis (Osbeck, 1765), in Australian waters: A summary of current knowledge. Aquat. Mamm. 2004;30:197–206. [Google Scholar]
- 54.Jefferson TA, Curry BE. Humpback dolphins: A brief introduction to the genus Sousa. Adv. Mar. Biol. 2015;72:1–16. doi: 10.1016/bs.amb.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 55.Koper RP, Karczmarski L, du Preez D, Plön S. Sixteen years later: Occurrence, group size, and habitat use of humpback dolphins (Sousa plumbea) in Algoa Bay, South Africa. Mar. Mamm. Sci. 2016;32:490–507. [Google Scholar]
- 56.Palmer, C. Conservation biology of dolphins in coastal waters of the Northern Territory, Australia. PhD thesis. Charles Darwin University, Northern Territory, Australia (2014).
- 57.Heithaus MR, Dill LM. Food availability and tiger shark predation risk influence bottlenose dolphin habitat use. Ecology. 2002;83:480–491. [Google Scholar]
- 58.Benoit-Bird KJ, et al. Prey patch patterns predict habitat use by top marine predators with diverse foraging strategies. PLoS ONE. 2013;8:e53348. doi: 10.1371/journal.pone.0053348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pirotta E, et al. Predicting the effects of human developments on individual dolphins to understand potential long-term population consequences. Proc. R Soc. B. 2015;282:20151209. doi: 10.1098/rspb.2015.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parra GJ, Jedensjö M. Stomach contents of Australian snubfin (Orcaella heinsohni) and Indo-Pacific humpback dolphins (Sousa chinensis) Mar. Mamm. Sci. 2014;30:1184–1198. [Google Scholar]
- 61.Downie RA, Babcock RC, Thomson DP, Vanderklift MA. Density of herbivorous fish and intensity of herbivory are influenced by proximity to coral reefs. Mar. Ecol. Prog. Ser. 2013;482:217–225. [Google Scholar]
- 62.Fitzpatrick BM, Harvey ES, Langlois TJ, Babcock R, Twiggs E. Effects of fishing on fish assemblages at the reefscape scale. Mar. Ecol. Prog. Ser. 2015;524:241–253. [Google Scholar]
- 63.Smith F, Allen SJ, Bejder L, Brown AM. Shark bite injuries on three inshore dolphin species in tropical northwestern Australia. Mar. Mamm. Sci. 2017;34:87–99. doi: 10.1111/mms.12435. [DOI] [Google Scholar]
- 64.Best BD, et al. Online cetacean habitat modeling system for the US east coast and Gulf of Mexico. Endanger Species Res. 2012;18:1–15. [Google Scholar]
- 65.Bancroft, K. & Sheridan, M. The major marine habitats of the Ningaloo Marine Park and the proposed southern extension. Marine Conservation Branch, Department of Conservation and Land Management, Perth, Western Australia. MMS/PI/NMP&NSE- 26/2000 (2000).
- 66.Zanardo, N., Parra, G. J., Passadore, C. & Möller, L. M. Ensemble modelling of southern Australian bottlenose dolphin Tursiops sp. distribution reveals important habitats and their potential ecological function. Mar. Ecol. Prog. Ser.569, 253–266 (2017).
- 67.Palacios DM, Baumgartner MF, Laidre KL, Gregr EJ. Beyond correlation: integrating environmentally and behaviourally mediated processes in models of marine mammal distributions. Endanger Species Res. 2013;22:191–203. [Google Scholar]
- 68.Torres LG, Read AJ, Halpin P. Fine-scale habitat modeling of a top marine predator: do prey data improve predictive capacity? Ecol. Appl. 2008;18:1702–1717. doi: 10.1890/07-1455.1. [DOI] [PubMed] [Google Scholar]
- 69.Hastie GD, Wilson B, Wilson LJ, Parsons KM, Thompson PM. Functional mechanisms underlying cetacean distribution patterns: hotspots for bottlenose dolphins are linked to foraging. Mar. Biol. 2004;144:397–403. [Google Scholar]
- 70.Hunt, T.N. Demography, habitat use and social structure of Australian humpback dolphins (Sousa sahulensis) around the North West Cape, Western Australia: Implications for conservation and management. PhD thesis. College of Science and Engineering, Flinders University, Adelaide, Australia (2018).
- 71.Mitchell JD, et al. Quantifying shark depredation in a recreational fishery in the Ningaloo Marine Park and Exmouth Gulf, Western Australia. Mar. Ecol. Prog. Ser. 2018;587:141–157. [Google Scholar]
- 72.Smallwood CB, Beckley LE, Moore SA, Kobryn HT. Assessing patterns of recreational use in large marine parks: A case study from Ningaloo Marine Park, Australia. Ocean Coast Manag. 2011;54:330–340. [Google Scholar]
- 73.Great Sandy Marine Park Zoning Plan. Marine Parks (Great Sandy) Zoning Plan 2017. Marine Parks Act 2004. Queensland Government. https://www.legislation.qld.gov.au/view/pdf/inforce/current/sl-2017-0155 (2017).
- 74.Smith H, Frère C, Kobryn H, Bejder L. Dolphin sociality, distribution and calving as important behavioural patterns informing management. Anim. Conserv. 2016;19:462–471. [Google Scholar]
- 75.Sala E, Giakoumi S. No-take marine reserves are the most effective protected areas in the ocean. ICES J. Mar. Sci. 2018;75:1166–1168. [Google Scholar]
- 76.Davies HN, Beckley LE, Kobryn HT, Lombard AT, Radford B, Heyward A. Integrating climate change resilience features into the incremental refinement of an existing marine park. PLoS ONE. 2016;11:e0161094. doi: 10.1371/journal.pone.0161094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cassata L, Collins LB. Coral reef communities, habitats, and substrates in and near sanctuary zones of Ningaloo Marine Park. J. Coast Res. 2008;24:139–151. [Google Scholar]
- 78.Mann J. Behavioral sampling methods for cetaceans: a review and critique. Mar. Mamm. Sci. 1999;15:102–122. [Google Scholar]
- 79.Connor RC, Mann J, Tyack PL, Whitehead H. Social evolution in toothed whales. Trends Ecol. Evol. 1998;13:228–232. doi: 10.1016/s0169-5347(98)01326-3. [DOI] [PubMed] [Google Scholar]
- 80.Redfern J, et al. Techniques for cetacean–habitat modeling. Mar. Ecol. Prog. Ser. 2006;310:271–295. [Google Scholar]
- 81.Kobryn HT, Wouters K, Beckley LE, Heege T. Ningaloo reef: shallow marine habitats mapped using a hyperspectral sensor. PLoS ONE. 2013;8:e70105. doi: 10.1371/journal.pone.0070105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geoscience Australia. Bathymetry Grids of Carnarvon Shelf. https://www.ga.gov.au (2008).
- 83.Geoscience Australia. Australian Bathymetry and Topography Grid, June 2009. https://www.ga.gov.au (2009).
- 84.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org (2015).
- 85.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010;1:3–14. [Google Scholar]
- 86.Naimi B, Hamm N, Groen TA, Skidmore AK, Toxopeus AG. Where is positional uncertainty a problem for species distribution modelling? Ecography. 2014;37:191–203. [Google Scholar]
- 87.MacLeod, C.D. An Introduction to Using GIS in Marine Mammal Research. Course Manual. Adelaide, South Australia, 15–19 July 2013 Fremantle, Western Australia, 22–26 July 2013 (2013).
- 88.Gu W, Swihart RK. Absent or undetected? Effects of non-detection of species occurrence on wildlife–habitat models. Biol. Conserv. 2004;116:195–203. [Google Scholar]
- 89.Barbet-Massin M, Thuiller W, Jiguet F. How much do we overestimate future local extinction rates when restricting the range of occurrence data in climate suitability models? Ecography. 2010;33:878–886. [Google Scholar]
- 90.Phillips SJ, et al. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol. Appl. 2009;19:181–197. doi: 10.1890/07-2153.1. [DOI] [PubMed] [Google Scholar]
- 91.Gottschalk TK, Aue B, Hotes S, Ekschmitt K. Influence of grain size on species-habitat models. Ecol. Model. 2011;222:3403–3412. [Google Scholar]
- 92.Hanberry BB. Finer grain size increases effects of error and changes influence of environmental predictors on species distribution models. Ecol. Inform. 2013;15:8–13. [Google Scholar]
- 93.Passadore C, Möller LM, Diaz-Aguirre F, Parra GJ. Modelling dolphin distribution to inform future spatial conservation decisions in a marine protected area. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-34095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guisan A, Zimmermann NE. Predictive habitat distribution models in ecology. Ecol. Model. 2000;135:147–186. [Google Scholar]
- 95.Elith J, Graham CH. Do they? How do they? WHY do they differ? On finding reasons for differing performances of species distribution models. Ecography. 2009;32:66–77. [Google Scholar]
- 96.Marmion M, Parviainen M, Luoto M, Heikkinen RK, Thuiller W. Evaluation of consensus methods in predictive species distribution modelling. Divers. Distrib. 2009;15:59–69. [Google Scholar]
- 97.Araújo MB, New M. Ensemble forecasting of species distributions. Trends Ecol Evol. 2006;22:42–47. doi: 10.1016/j.tree.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 98.Franklin J. Mapping species distributions: spatial inference and prediction. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 99.Grenouillet G, Buisson L, Casajus N, Lek S. Ensemble modelling of species distribution: the effects of geographical and environmental ranges. Ecography. 2011;34:9–17. [Google Scholar]
- 100.Sun, Y. Crested ibis in a dynamic and increasingly human-dominated landscape. PhD Thesis. Faculty of Geo-Information Science and Earth Observation, Univeristy of Twente, The Netherlands (2016).
- 101.Oppel S, et al. Comparison of five modelling techniques to predict the spatial distribution and abundance of seabirds. Biol. Conserv. 2012;156:94–104. [Google Scholar]
- 102.Gårdmark A, et al. Biological ensemble modeling to evaluate potential futures of living marine resources. Ecol Appl. 2013;23:742–754. doi: 10.1890/12-0267.1. [DOI] [PubMed] [Google Scholar]
- 103.Pikesley SK, et al. Modelling the niche for a marine vertebrate: A case study incorporating behavioural plasticity, proximate threats and climate change. Ecography. 2015;38:001–010. [Google Scholar]
- 104.Abrahms, B., H. et al. Dynamic ensemble models to predict distributions and anthropogenic risk exposure for highly mobile species. Divers. Distrib.25, 1182–1193 (2019).
- 105.Pérez-Jorge S, et al. Can static habitat protection encompass critical areas for highly mobile marine top predators? Insights from coastal East Africa. PLoS ONE. 2015;10:e0133265. doi: 10.1371/journal.pone.0133265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thuiller W, Lafourcade B, Engler R, Araújo MB. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography. 2009;32:369–373. [Google Scholar]
- 107.Guisan A, Edwards TC, Jr, Hastie T. Generalized linear and generalized additive models in studies of species distributions: Setting the scene. Ecol. Model. 2002;157:89–100. [Google Scholar]
- 108.Friedman J, Hastie T, Tibshirani R. Additive logistic regression: A statistical view of boosting (with discussion and a rejoinder by the authors) Ann. Stat. 2000;28:337–407. [Google Scholar]
- 109.De'ath G, Fabricius KE. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology. 2000;81:3178–3192. [Google Scholar]
- 110.Hastie T, Tibshirani R, Buja A. Flexible discriminant analysis by optimal scoring. J. Am. Stat. Assoc. 1994;89:1255–1270. [Google Scholar]
- 111.Breiman L. Random forests. Mach. Learn. 2001;45:5–32. [Google Scholar]
- 112.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006;190:231–259. [Google Scholar]
- 113.Elith J, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- 114.Becker, E.A., et al. Performance evaluation of cetacean species distribution models developed using generalized additive models and boosted regression trees. Ecol Evol. 10.1002/ece3.6316 (2020) [DOI] [PMC free article] [PubMed]
- 115.Fielding AH, Bell JF. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997;24:38–49. [Google Scholar]
- 116.Peterson, A.T., et al.Ecological Niches and Geographic Distributions (MPB-49). (Princeton University Press, 2011).
- 117.Hood, G. PopTools version 3.2. 5. https://www.poptools.org. (2011).
- 118.Manly BF. Randomization, bootstrap and Monte Carlo methods in biology. 3. London: Chapman & Hall; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data made available to all interested researchers upon reasonable request to Tim Hunt (t_hunt@live.com.au) and Guido J. Parra (guido.parra@flinders.edu.au).