Abstract
Purpose
Accumulated evidence indicates laparoscopic surgery (LS) has the advantages of less wound pain, less blood loss, shorter hospitalization, and faster functional recovery than open surgery (OS). Previous studies have analyzed the advantages of LS based on hospital data. This study is the first to compare surgical outcomes and health economic data using nationwide administrative claims datasets for gastric cancer.
Methods
The claims datasets of the Health Insurance Review and Assessment Service for patients that underwent gastrectomy from May 2012 to April 2017 were analyzed. A total of 76,445 cases (LS, 42,395 and OS, 34,050) were included. Postoperative complications and medical costs were included in the analysis.
Results
We analyzed 76,445 cases of gastrectomy. Analysis showed LS was associated with fewer surgical wound infections (2,114 [6.21%] vs. 1,057 [2.49%], P < 0.001), minor abdominal infections and abscesses (826 [2.43%] vs. 390 [0.92%], P < 0.001), cases of surgery-related peritonitis (50 [0.15%] vs. 31 [0.07%], P = 0.0019), repair surgeries (28 [0.08%] vs. 3 [0.01%], P < 0.001), reoperations (504 [1.48%] vs. 343 [0.81%], P < 0.001), less antibiotic use (1,717 [5.04%] vs. 1,268 [2.99%], P < 0.001), and shorter hospital stays (13.61 days vs. 9.97 days, P < 0.001). However, average medical cost was 510,734 Korean Won (444 US dollar) higher for LS than OS.
Conclusion
The study confirms the clinical benefits of LS over OS for gastrectomy in terms of fewer postoperative complications and shorter hospital stays. However, the average medical cost of LS was higher than that of OS.
Keywords: Laparoscopy, Stomach neoplasms
INTRODUCTION
According to the annual report of cancer statistics in Korea in 2016, gastric cancer has a higher incidence than any other cancer in South Korea, and in 2018 was the fourth leading cause of cancer-related death behind lung, liver, and colorectal cancer [1,2]. Because of early detection by gastroscopy, as supported by a national screening program, the gastric cancer-related death rate has steadily decreased [3]. Optimal treatment for gastric cancer is radical surgery, that is, gastrectomy with regional lymph node dissection, and this has led to the achievement of a greater than 95% cure rate for early gastric cancer [4]. Since its introduction in 1994 by the Japanese surgeon Dr. Kitano et al. [5], laparoscopic gastric surgery has been widely accepted as a minimally invasive treatment for early-stage gastric cancer that offers the advantages shorter hospital stays, less pain and scarring, earlier bowl recovery, and better quality of life [6,7]. However, no study has yet compared the outcomes of laparoscopic and open gastrectomy using nationwide data.
The Korean National Health Insurance Service (NHIS) provides universal healthcare coverage. Healthcare providers are automatically eligible and obliged to treat patients for services covered under NHIS system. The costs of the system are met by the private sector, but the system is controlled by the government. The NHIS is a mandatory health insurance service and 97% of the Korean population are covered; the remaining 3% are covered by a Medical Aid program. Healthcare providers submit inpatient and outpatient claims data to the Health Insurance Review and Assessment Service (HIRA) to cover the costs of medical services provided. As a result, HIRA has developed a nationwide claims database, which can be used to generate national healthcare statistics [8,9,10]. The HIRA database is available to researchers for academic or public policy purposes [11,12]. Furthermore, since the Korean government has traditionally operated a fee-for-service (FFS) payment system, each procedure, medical device, and drug claimed by healthcare providers is captured by the HIRA claim system. The present study was designed to compare the clinical outcomes and medical costs of laparoscopic surgical (LS) and open surgical (OS) gastrectomy using nationwide, HIRA claims datasets.
METHODS
Data sources
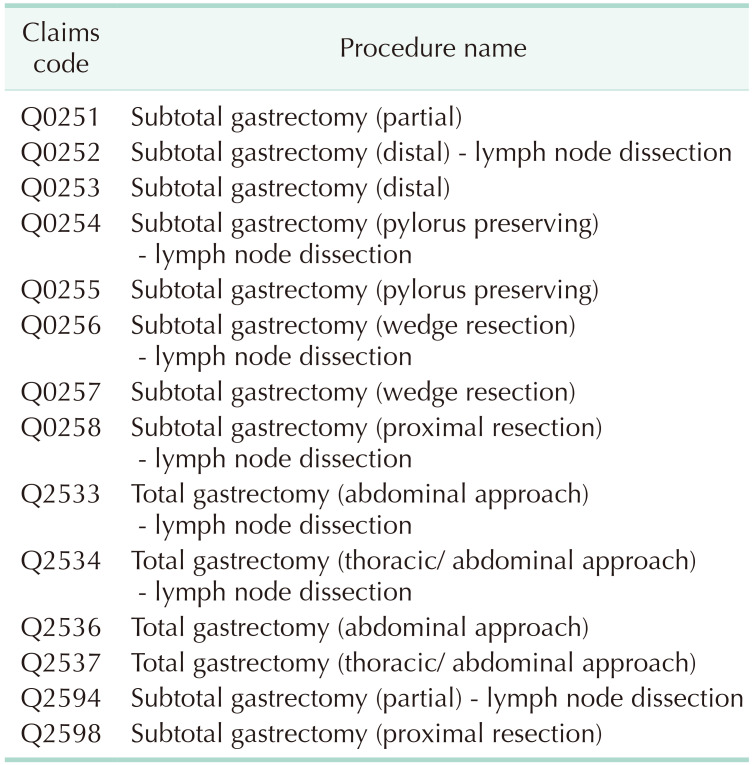
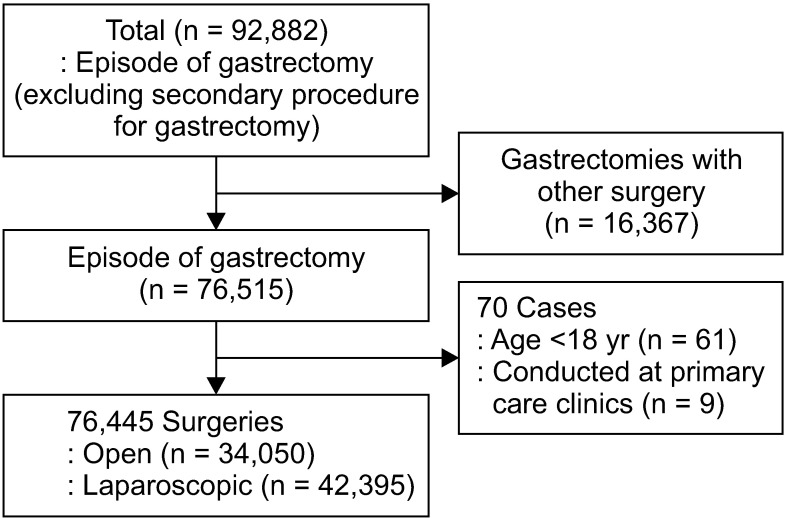
The study was conducted after obtaining approval from Institutional Review Board (No. P01-201805-21-008). Informed consent was waived because of the retrospective nature of the study. Patients aged ≥18 years that underwent gastrectomy from May 1, 2012 to April 30, 2017, were considered for this study. Claims datasets were extracted by defining 14 procedural codes as either gastrectomy or partial gastrectomy related as defined by the cost of health insurance medical care book (Table 1) [13]. We have included wedge resection cases because gastrointestinal stromal tumor (GIST) is one of gastric tumors regardless of malignancy or not. The following information was provided: patient sex and age, procedure type, medical device usage, drug usage, hospital type, costs for individual claims codes, total medical costs, and hospital days. A total of 76,445 cases were included after excluding second surgeries, cases involving gastrectomy plus another surgery, patients aged <18 years, and surgeries conducted at primary care clinics (Fig. 1). Gastrectomies considered as second surgeries or performed with other surgeries were excluded to ensure accuracy of the analysis. Because gastrectomy is not performed at primary care clinics, 9 gastrectomy cases adjudicated to be claim code errors were excluded in this study. Separate claims datasets with the same patient code with consecutive dates were defined to concern a single case.
Table 1. Claims codes for gastrectomy and partial gastrectomy.
Cost of health insurance medical care published by Health Insurance Review and Assessment Service, 2018 [13].
Fig. 1. Dataset analysis flowchart.
Under the FFS payment scheme, each medical device listed in the medical benefit list is reimbursed separately, but some medical devices used for LS, thoracoscopic surgery, and arthroscopic surgery are reimbursed as bundled costs according to an agreed per-procedure scheme, which was devised to simplify claims for single-use devices (SUDs), which are reimbursed using one payment code and amount regardless of the numbers or types of SUDs consumed. LS and OS NHI administrative claims datasets were confirmed using the medical device code ‘N0031001’, which is unique to LS, because the gastrectomy code was not subdivided by surgical technique. Thus, patients that underwent gastrectomy with ‘N0031001’ were defined as the LS group.
Operational definitions
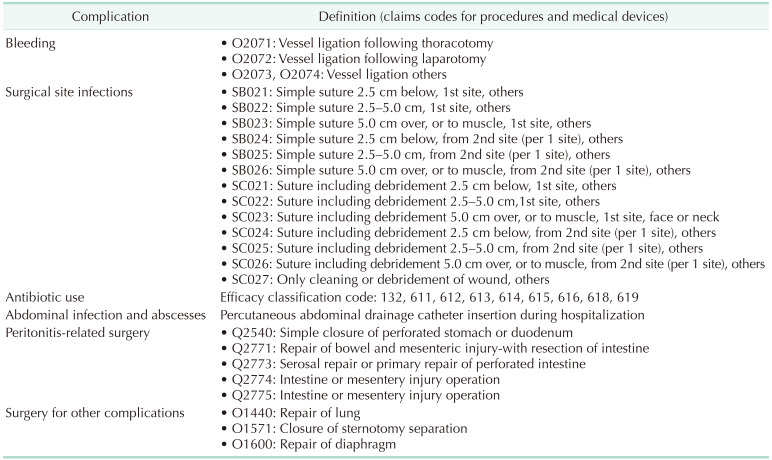
We compared the clinical outcomes of LS and OS by defining the following complications; postoperative bleeding, surgical site infection, abdominal infection and abscess, peritonitis-related surgery, procedure-related complications, and reoperation. These complications were matched with claims codes of procedures and medical devices consumed. In addition, we also compared hospital stays, medical costs, and antibiotic usage (Table 2).
Table 2. Complication definitions with claims codes for procedures and medical devices.
Operational definitions were used to confirm complications using and claims codes of procedures and medical devices because HIRA claims datasets do not contain detailed patient medical records. If cases had claims code of ‘vessel ligation (Q2071, Q2072, Q2073),’ we defined that postoperative bleeding had occurred. Claims codes of ‘simple suture (SB021, SB022, SB023, SB024, SB025, SB026),’ ‘suture including debridement (SC021, SC022, SC023, SC024, SC025, SC026),’ and ‘cleaning or debridement of wound (SC027)’ were used to confirm surgical site infection had occurred, because additional wound suturing is not required for normal gastrectomy. There was no specific procedure code for abdominal infection or abscess, and hence we used claims code for pig-tail catheter usage (J4032, J4033, J4034), as this is required for percutaneous drainage to confirm abdominal infection or abscess. Peritonitis-related surgery was confirmed by claims codes of ‘simple closure of perforated stomach or duodenum (Q2540),’ ‘repair of bowel and mesenteric injury-with intestine resection (Q2771),’ ‘serosal repair or primary repair of perforated intestine (Q2773),’ ‘intestine or mesentery injury operation (Q2774),’ and ‘intestine or mesentery injury operation (Q2775).’ Data related to infections were not contained in claims datasets, and therefore, we compared antibiotic usages for LS and OS with drug efficacy classification codes, which were defined as ‘132, 611, 612, 613, 614, 615, 616, 618, 619’ in claims datasets. Reoperation was confirmed by 2 or more gastrectomy code entries for the same case. Complications during surgery were defined as ‘repair of lung (O1440),’ ‘closure of sternotomy separation (O1571),’ and ‘repair of diaphragm (O1600).’
Statistical analyses
Pearson chi-squared test and the Student t-test were used to determine the significances of differences between the LS and OS groups. Logistic regression analysis and multiple regression analysis adjusted for age, sex, and hospital category (tertiary, general, and others) were also performed. The odds ratios of complications were determined by logistic regression analysis and multiple regression analyses to determine total medical costs and hospital stays. The analysis was conducted using SAS enterprise guide ver. 6.1 (SAS Institute Inc., Cary, NC, USA), and statistical significance was accepted for P-values < 0.05.
RESULTS
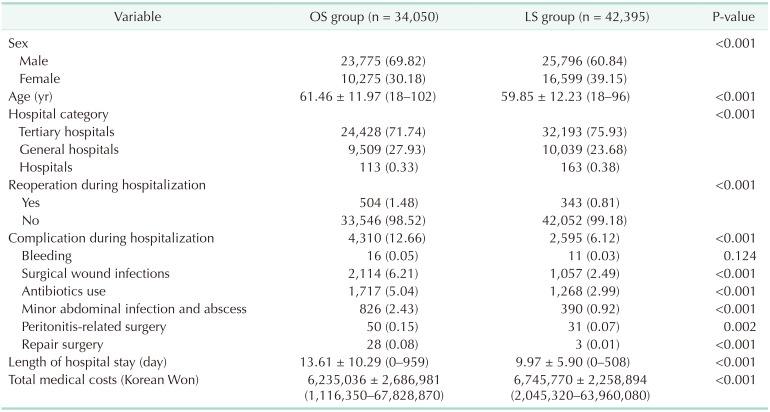
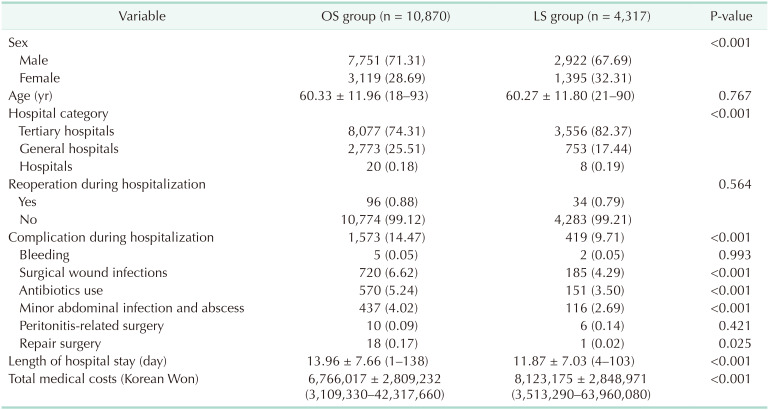
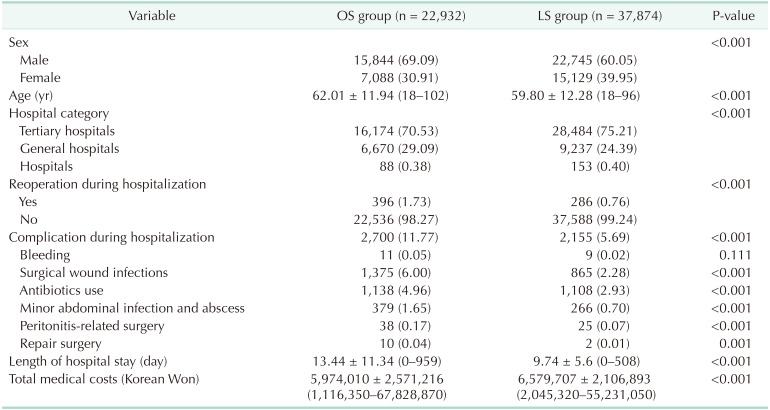
The cases of 34,050 were attributed to OS and 42,395 to LS. Mean patient ages in the OS and LS groups were 61.46 years (range, 18–102 years) and 59.85 years (range, 18–96 years), respectively; and male to female ratios were 7:3 (69.82% vs. 30.18%) and 6:4 (60.85% vs. 39.15%), respectively. Percentages of males treated in tertiary hospitals, general hospitals, and other hospitals in the OS and LS groups were 71.74%, 27.93%, and 0.33%; and 75.94%, 23.68%, and 0.38%, respectively. Reoperation rates were significantly different in the OS and LS groups (504 [1.48%] vs. 343 [0.81%], P < 0.0001, respectively), and the OS group had a nonsignificantly higher bleeding rate (16 vs. 11, P = 0.1238). The LS group had a lower surgical wound infection rate (2,114 [6.21%] vs. 1,057 [2.49%], P < 0.001), antibiotic use (1,717 [5.04%] vs. 1,268 [2.99%], P < 0.001), minor abdominal infection and abscess rate (826 [2.43%] vs. 390 [0.92%], P < 0.001), repair surgery rate (28 [0.08%] vs. 3 [0.01%], P < 0.001), and peritonitis-related surgery rate (50 [0.15%] vs. 31 [0.07%], P = 0.0019). Mean hospital stay was shorter in the LS group (9.97 days vs. 13.61 days). However, average medical cost in the LS group was 510,734 Korean Won (KRW) (around 444 US dollar [USD]) higher than in the OS group (Table 3). Total and subtotal gastrectomy also analyzed, respectively (Tables 4, 5).
Table 3. Surgical outcomes in open surgery (OS) and laparoscopic surgery (LS) groups.
Values are presented as number (%) or mean ± standard deviation (range).
Table 4. Surgical outcomes in open surgery (OS) and laparoscopic surgery (LS) groups (for total gastrectomy patients)a).
Values are presented as number (%) or mean ± standard deviation (range).
a)Excluded: concurrent surgery both total and subtotal gastrectomy.
Table 5. Surgical outcomes in open surgery (OS) and laparoscopic surgery (LS) groups (for subtotal gastrectomy patients)a).
Values are presented as number (%) or mean ± standard deviation (range).
a)Excluded: concurrent surgery both total and subtotal gastrectomy.
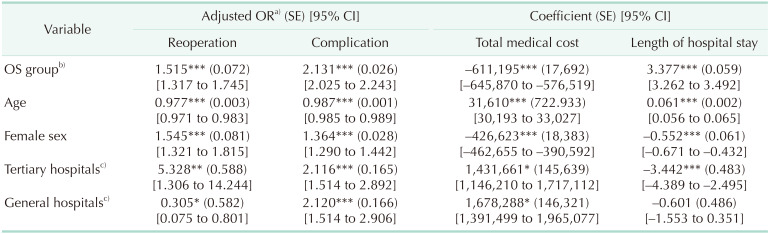
Regression analysis was performed to determine whether the clinical outcomes OS and LS differed after adjustment for patient characteristics and hospital types. Rates of reoperation and complications were 1.515 and 2.131 times higher, respectively, and mean hospital stay was 3.377 days longer in the OS group. After adjustment, average medical cost was 611,195 KRW (around 532 USD) lower in the OS group. According to the regression analysis, age and the hospital type were associated with reoperation and complication among independent variables (Table 6).
Table 6. Logistic regression analysis and multiple regression analysis results.
OR, odds ratio; SE, standard error; CI, confidence interval; OS, open surgery.
a)Adjusted estimates yielded by the model. b)For the OS group vs. the LS group. c)Tertiary and general hospitals are compared with other hospitals.
*P < 0.05, **P < 0.01, ***P < 0.001.
DISCUSSION
According to 2014 nationwide survey data on gastric cancer treatment in South Korea, around 50% of operable gastric cancers were treated laparoscopically [14]. In cases of early-stage disease, laparoscopic gastrectomy is favored, but the safety and effectiveness of LS in advanced cases is still being evaluated [6,7]. Laparoscopy-assisted distal gastrectomy was first reported by Kitano et al. [5] in 1994, and increasing interest in laparoscopic abdominal surgery had resulted in a rapid increase in the number of laparoscopic gastrectomies performed for gastric adenocarcinoma. However, no previous study has analyzed the outcomes of laparoscopic gastrectomy using data from nationwide claim datasets.
In 2016, our administrative claims dataset analysis showed over 9,500 cases of gastric cancer were treated by LS in South Korea. A previous meta-analysis showed laparoscopic gastrectomy had a comparable overall survival rate with less blood loss, less pain, fewer postoperative complications, and shorter hospital stays than conventional OS [15]. Furthermore, studies have shown laparoscopic gastrectomy with lymphadenectomy for early-stage gastric cancer is an acceptable treatment option [16,17], and multiple prospective randomized controlled trials have reported favorable patient outcomes for minimally invasive surgery [16,17,18,19,20,21,22]. In a meta-analysis of 7 randomized controlled trials that compared laparoscopic vs. open distal gastrectomy, the laparoscopic approach was found to have a longer operative time but to be associated with less blood loss, fewer analgesics, faster recovery, and shorter postoperative hospital stays [23].
In the present study, we compared LS and OS with respect to complication rates, hospital stays, and medical costs for gastrectomy using nationwide claim datasets. Our analysis confirmed leakage, infection, technical error, and reoperation rates, which are typical complications of gastrectomy, were significantly lower in the LS group. In addition, mean hospital stay was shorter in the LS group. Age and hospital type were associated with reoperation and complication. Adjusted odds ratio of age is 0.977 and 0.987 for reoperation and complication, respectively. We need to more investigate for this factor. About the hospital type, we thought that patients with severe comorbidity may go tertiary hospital for operation. So, reoperation and complication rates were relatively higher than other hospitals. The medical cost differences between the OS and LS groups may be higher than those mentioned above if patient out-of-pocket costs were included, but lower complication rates and shorter hospital stays reduce postoperative medical costs, and we believe that LS should be considered as cost-effective as OS for the treatment of early gastric cancer.
The present study is meaningful because it is the first to analyze the clinical outcomes and medical costs based on 2 types of gastrectomy using nationwide claims datasets. However, the study has its limitations. First, there were insufficient variables in claims datasets to assess clinical risk and disease severities. HIRA claim data doesn't include the clinical information so we couldn't elicit the specific characteristics between LS and OS unlike usual clinical trials. Second, in some cases, 2 or 3 claims records had been submitted due to different claims practices at different hospitals. As mentioned above, we assumed that claims records separated by one day concerned the same case. Third, specific causalities by surgical technique could not be confirmed, despite the use of defined claims and complication codes. Fourth, HIRA datasets only provide information on medical services of relevance to the medical benefit program, and thus, do not contain patient out-of-pocket expenses. Fifth, this study was analyzed the 5-year claims data for whole patient who underwent gastrectomy in Korea. Therefore, we could not control the heterogeneity of the data. So, we considered age, sex, and hospital levels as independent variables when we conducted logistic regression analysis. Further study is needed to verify our findings and future research will use another statistics method such as propensity matching to supplement the limitation.
We found laparoscopic gastrectomy had benefits over open gastrectomy in terms of postoperative complication rates and hospital stays, but that laparoscopic gastrectomy had slightly higher medical costs.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: HH, JP, JWY.
- Formal Analysis: JEM.
- Investigation: HH, JP, JEM.
- Methodology: JP.
- Project Administration: JEM, SSL.
- Writing — Original Draft: HH, JP, JEM.
- Writing — Review & Editing: HH, JEM, SSL, JWY.
References
- 1.Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2016. Sejong: Ministry of Health and Welfare; 2018. [Google Scholar]
- 2.Statistics Korea. Cause of death statistics in Korea in 2018 [Internet] Daejeon: Statistics Korea; 2019. [cited 2019 Aug 1]. Available from: http://kostat.go.kr/portal/korea/kor_nw/1/6/2/index.board?bmode=download&bSeq=&aSeq=377606&ord=2. [Google Scholar]
- 3.Jun JK, Choi KS, Lee HY, Suh M, Park B, Song SH, et al. Effectiveness of the Korean National Cancer Screening Program in reducing gastric cancer mortality. Gastroenterology. 2017;152:1319–1328. doi: 10.1053/j.gastro.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 4.Lin JX, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, et al. Surgical outcomes of 2041 consecutive laparoscopic gastrectomy procedures for gastric cancer: a large-scale case control study. PLoS One. 2015;10:e0114948. doi: 10.1371/journal.pone.0114948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 6.Kim HH, Ahn SH. The current status and future perspectives of laparoscopic surgery for gastric cancer. J Korean Surg Soc. 2011;81:151–162. doi: 10.4174/jkss.2011.81.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Son T, Hyung WJ. Laparoscopic gastric cancer surgery: current evidence and future perspectives. World J Gastroenterol. 2016;22:727–735. doi: 10.3748/wjg.v22.i2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim M, Kim J, Shin H, Park J. Research for the performance indicators development of healthcare system using big data [Internet] Wonju: Health Insurance Review and Assessment Service; 2016. [cited 2020 Jul 29]. Available from: http://repository.hira.or.kr/handle/2019.oak/1689. [Google Scholar]
- 9.Kim R, Kim J, Kim S, Kim B. Research for the promotion of medical information provision. Seoul: Health Insurance Review and Assessment Service; 2014. [Google Scholar]
- 10.Jang E, Kim D, Lee J, Yang B, Hwang J, Kwak S, et al. Study on the improvement of healthcare big data utilization [Internet] Wonju: Health Insurance Review and Assessment Service; 2016. [cited 2020 Jul 29]. Available from: http://repository.hira.or.kr/handle/2019.oak/1665. [Google Scholar]
- 11.Jang E. Healthcare big data utilization plan [Internet] Wonju: Health Insurance Review and Assessment Service; 2017. [cited 2020 Jul 29]. Available from: http://repository.hira.or.kr/handle/2019.oak/1132. [Google Scholar]
- 12.Health Insurance Review and Assessment Service. Manual of health insurance claim data analysis for generation of healthcare evidence [Internet] Wonju: Health Insurance Review and Assessment Service; 2017. [cited 2020 Jul 29]. Available from: https://opendata.hira.or.kr/op/opb/selectRfrm.mo?sno=11200. [Google Scholar]
- 13.Health Insurance Review and Assessment Service. Cost of health insurance medical care [Internet] Wonju: Health Insurance Review and Assessment Service; 2018. [cited 2020 Jul 29]. Available from: http://repository.hira.or.kr/handle/2019.oak/2122. [Google Scholar]
- 14.Information Committee of Korean Gastric Cancer Association. Corrigendum: Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J Gastric Cancer. 2016;16:277. doi: 10.5230/jgc.2016.16.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Pan Y, Cai JQ, Xu XW, Wu D, Mou YP. Totally laparoscopic gastrectomy for gastric cancer: a systematic review and meta-analysis of outcomes compared with open surgery. World J Gastroenterol. 2014;20:15867–15878. doi: 10.3748/wjg.v20.i42.15867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim W, Kim HH, Han SU, Kim MC, Hyung WJ, Ryu SW, et al. Decreased morbidity of laparoscopic distal gastrectomy compared with open distal gastrectomy for stage I gastric cancer: short-term outcomes from a multicenter randomized controlled trial (KLASS-01) Ann Surg. 2016;263:28–35. doi: 10.1097/SLA.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 17.Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol. 2014;32:627–633. doi: 10.1200/JCO.2013.48.8551. [DOI] [PubMed] [Google Scholar]
- 18.Kitano S, Shiraishi N, Fujii K, Yasuda K, Inomata M, Adachi Y. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery. 2002;131(1 Suppl):S306–S311. doi: 10.1067/msy.2002.120115. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 20.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YW, Baik YH, Yun YH, Nam BH, Kim DH, Choi IJ, et al. Improved quality of life outcomes after laparoscopy-assisted distal gastrectomy for early gastric cancer: results of a prospective randomized clinical trial. Ann Surg. 2008;248:721–727. doi: 10.1097/SLA.0b013e318185e62e. [DOI] [PubMed] [Google Scholar]
- 22.Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report: a phase III multicenter, prospective, randomized trial (KLASS Trial) Ann Surg. 2010;251:417–420. doi: 10.1097/SLA.0b013e3181cc8f6b. [DOI] [PubMed] [Google Scholar]
- 23.Deng Y, Zhang Y, Guo TK. Laparoscopy-assisted versus open distal gastrectomy for early gastric cancer: a meta-analysis based on seven randomized controlled trials. Surg Oncol. 2015;24:71–77. doi: 10.1016/j.suronc.2015.02.003. [DOI] [PubMed] [Google Scholar]