Abstract
Purpose
The purpose of this study was to evaluate differences in optical coherence tomography angiography (OCTA) metrics in the superficial (SCP), intermediate (ICP), and deep (DCP) vascular plexuses across diabetic retinopathy (DR) severity levels.
Methods
This was a cross sectional observational retrospective chart review study. Eligible patients with diabetes who underwent same day RTVue XR Avanti OCTA, spectral-domain optical coherence tomography (SD-OCT), and 200-degree Optos ultrawide field color imaging. SCP, ICP, and DCP vessel density (VD) and vessel length density (VLD) were assessed using 3-D projection artifact removal software (PAROCTA) software.
Results
Of 396 eyes (237 patients), 16.1% had no DR, 26.9% mild nonproliferative DR (NPDR), 21.1% moderate NPDR, 12.1% severe NPDR, 10.1% proliferative DR (PDR) without panretinal photocoagulation (PRP), and 13.4% PDR with PRP. When comparing mild NPDR to no DR eyes, ICP and DCP VD and VLD were significantly lower, but there was no difference for SCP metrics. In eyes with more severe DR, there were significant differences in SCP VD and VLD between DR severity levels (mild versus moderate NPDR: VD 35.45 ± 3.31 vs. 34.14 ± 3.38, P = 0.008 and VLD 17.59 ± 1.83 vs. 16.80 ± 1.83, P = 0.003; moderate versus severe NPDR: VLD 16.80 ± 1.83 vs. 15.79 ± 1.84, P = 0.019), but no significant differences in ICP or DCP.
Conclusions
Although VD of each of the three individual layers decreases with increasing DR severity, DR severity has a substantially different effect on OCTA parameters within each layer. Vascular changes in eyes with no to early DR were present primarily in the deeper vascular layers, whereas in eyes with advanced DR the opposite was observed. This study highlights the effects of ICP and the importance of assessing SCP and DCP changes independently across each DR severity level.
Keywords: optical coherence tomography (OCTA), diabetic retinopathy, retina
Optical coherence tomography angiography (OCTA) is an imaging modality that allows the visualization of retinal microvasculature without the need for the injection of fluorescein dye.1 This noninvasive technique allows identification of three distinct retinal vasculature capillary plexuses: superficial (SCP), intermediate (ICP), and deep (DCP). This observed structure replicates that of histological studies on primates and recent confocal and adaptive optics studies in humans.2–9 Recent studies suggest that the deeper layers may be correlated with photoreceptor function and may play a role in the development of diabetic macular edema and its response to treatment.10–12 What remains unclear is whether and how OCTA metrics can reliably classify individual eyes into distinct diabetic retinopathy (DR) severity levels and whether those same metrics can equal or surpass traditional DR grading systems in predicting disease progression and future visual loss.
There have been several studies evaluating vessel density (VD) changes associated with increasing DR severity. Most of these studies relied on bi-laminar rather than tri-laminar segmentation of the retinal vasculature, which places portions of the ICP into both the SCP and DCP.13–16 A handful of studies have segmented the vasculature into three distinct layers to evaluate SCP, ICP, and DCP but those studies tended to combine different DR severity levels into a single group.7,17–19 Combining different DR severities into a single analysis group risks masking differences between individual groups because this strategy assumes that changes in VD are equal and consistent between subgroups of DR severity.
OCTA measurements are also affected by projection artifacts caused by shadows from superficial blood flow projected onto deeper layers giving an erroneous perception of flow.20 Recent data have demonstrated that commercially available projection artifact removal software (PAROCTA) from Optovue (Fremont, CA, USA) significantly alters VD measurements in both the SCP and DCP, with different effects at different individual DR severity levels.21 PAROCTA DCP measurements were greater in early DR (no DR and mild nonproliferative DR [NPDR]) and SCP measurements were reduced in eyes with moderate NPDR or worse as compared with non-PAROCTA measurements.
Those findings suggested that evaluation of VD within each of the three vascular plexuses, within individual DR severity levels and with the use of projection artifact removal software may be important. In this current study, we characterize the differences in VD of individual vascular plexuses in eyes across all individual DR severity levels utilizing PAROCTA to accurately segment and quantify the SCP, ICP, and DCP. We specifically evaluate the correlation of vascular metrics between SCP and DCP for each DR severity and assess the importance of evaluating SCP and DCP changes independently for each specific DR severity.
Methods
This retrospective chart review study was approved by the Joslin Diabetes Center (JDC) Institutional Review Board and adhered to the tenets of the Declaration of Helsinki. Eligible patients for inclusion in this study were adults with type 1 or type 2 diabetes mellitus (DM) who had received OCTA, spectral-domain optical coherence tomography (SD-OCT), and 200-degree Optos ultrawide field (UWF) color fundus photographs imaging at the JDC for clinical or investigational purposes between February 15, 2016, and November 26, 2018. Study eyes spanned the full range of DR severity. Exclusion criteria included spherical equivalent (SE) of <-6 diopter (D) or >+3 D, nondiabetic macular pathology (e.g. retinal vein or artery occlusion, age-related macular degeneration, etc.), glaucoma, and history of pars plana vitrectomy. Also excluded were eyes with macular edema (defined as central subfield thickness [CST] greater than 320 µm in men and 305 µm in women on Heidelberg Spectralis OCT), vitreomacular traction, epiretinal membrane, or cystoid spaces in the central 3 × 3 mm scans.
Standardized data collection forms were used to record patient and eye characteristics, including SE, lens status, HbA1c measured within 3 months of imaging, duration of DM, type of DM, and presence or absence of hypertension and hyperlipidemia. In patients with proliferative DR (PDR) and panretinal photocoagulation (PRP), the time since PRP to the date of imaging was also calculated. SD-OCT (Heidelberg Engineering Co., Heidelberg, Germany) was evaluated for segmentation errors, and any errors were manually adjusted.
Optos nonsimultaneous stereoscopic, on axis, non-steered, 200-degree UWF color fundus photographs (Optos PLC, Dunfermline, Scotland, UK) were graded by a certified Joslin Vision Network (JVN) grader (M.A.) for Early Treatment Diabetic Retinopathy Study (ETDRS) DR severity level.22,23 Eyes with mild NPDR were further graded by hemorrhage/microaneurysms (H/Ma) into very mild NPDR (H/Ma <5, ETDRS level 20) and mild NPDR (H/Ma ≧5, ETDRS level 35).
Image Acquisition
OCTA imaging was performed using RTVue XR Avanti SD-OCT device with AngioVue software (Optovue, Fremont, CA, USA). The device used a light source of 840 nm, with an A-scan rate of 70,000 scans per second. The macular region was scanned using 3 × 3 mm scans, which consisted of 304 × 304 line scans. The AngioVue software uses the split spectrum amplitude-decorrelation angiography algorithm (SSADA), which has previously been described.1 Angiovue software versions 2017.1.0.149 and 2017.1.0.151 were used, which included the 3-D PAROCTA algorithm.
Individual scans were manually evaluated and eyes with a signal strength index (SSI) < 55 were excluded. OCTA images with artifacts, including decentration, segmentation errors, movement artifacts, and defocus, were excluded.20 Of eyes meeting the initial inclusion criteria of having an SSI > 55, 28 of 424 (6.6%) eyes were excluded for these reasons.
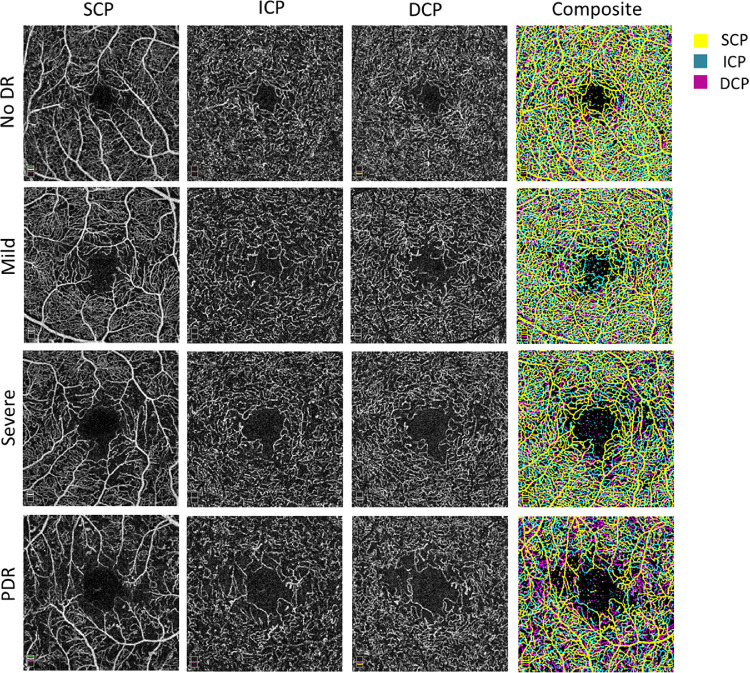
Scans were segmented using previously described offsets.6,24 In brief, the SCP en face OCTA image was segmented with an inner boundary of 3 µm below the internal limiting membrane and an outer boundary set at the inner plexiform layer (IPL)-inner nuclear layer (INL) junction. The ICP en face OCTA image was segmented with an inner boundary set at the IPL-INL junction and an outer boundary set at 20 µm below the IPL-INL junction. The DCP en face OCTA image was segmented with an inner boundary set at 20 µm below the IPL-INL junction and an outer boundary set at 15 µm below the outer plexiform layer (OPL)-outer nuclear layer (ONL) junction. Composites of all three layers were generated using the technique described by Park et al. (Fig. 1).5
Figure 1.
Changes in the three vascular layers; superficial capillary plexus (SCP), intermediate capillary plexus (ICP), and deep capillary plexus (DCP) with increasing diabetic retinopathy (DR) severity levels. Using PAROCTA software segmentation of the ICP and DCP shows no projection artifacts. SCP, ICP, and DCP vessel density and avascular areas increase with increasing DR severity. A composite (far right column) of the three vascular layers demonstrates that distinct layers and vascular plexuses.
Image Processing
The en face OCTA images were exported to ImageJ and binarized (National Institutes of Health, Bethesda, MD, USA).13,25,26 After using a “top hat filter,” images were duplicated, with one image being processed using a hessian filter followed by global Huang thresholding and the second image being binarized using local median thresholding (Supplementary Fig. S1). Only pixels common to both images were used to generate a final image that was analyzed quantitatively. For each OCTA image, VD was calculated as the percentage of area occupied by perfused vessels. The binarized images were then skeletonized, which generates a 1-pixel wide vascular tree and provides an indication of the total length of the vessels independent of their diameter. Vessel length density (VLD) was then calculated as a percentage of the total vascular length divided by the total area. There is currently a lack of uniformity with regard to nomenclature of OCTA metrics in the literature.27 However, given that that most OCTA research utilizing the Optovue device (the same device that was used in the current study) and the device's own proprietary software refer to these metrics as VD and VLD we opted to do the same.
Statistics
The Shapiro-Wilk test was used to test for normality. Pearson and Spearman correlations were conducted between VD and VLD in different vascular layers and ocular/systemic parameters. Pearson correlation was done for correlation of VD/VLD parameters in eyes with PDR with the duration of PRP and duration of PDR. Mixed-effects models correcting for correlation between assessment of eyes from the same individual, SSI, age, CST, SE, and duration of DM compared VD and VLD differences within each vascular plexus across different DR severity levels. Bonferroni correction for multiple analyses was included in these models. In addition, using the same model, the interaction among the three vascular plexuses was done to evaluate changes in both early and late DR. We used SPSS statistical software version 23 (SPSS, Inc., IBM Company, Chicago, IL, USA) for statistical analysis. A P value of < 0.05 was considered significant for these exploratory analyses.
Results
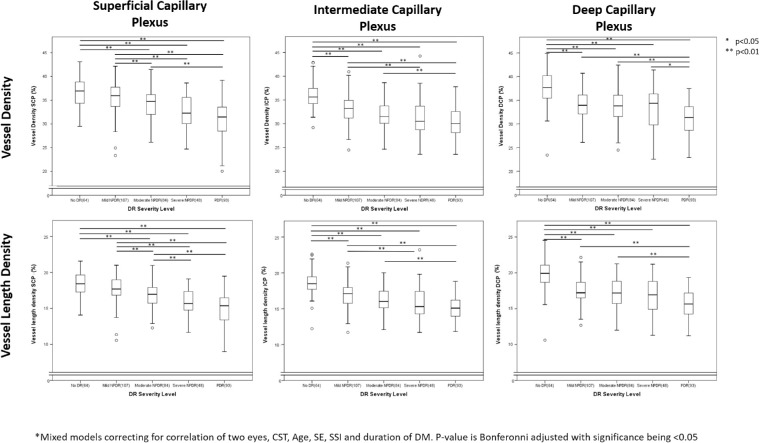
The study evaluated 396 eyes of 237 patients with a mean duration of DM 28.8 ± 16.4 years and mean HbA1c of 7.9 ± 1.5%, 43.4% of whom were women, and 72.5% of whom had type 1 DM (Table 1). Within this study cohort, 16.1% had no DR, 26.9% had mild NPDR, 21.1% had moderate NPDR, 12.1% had severe NDPR, 10.1% had PDR without PRP, and 13.4% had PDR with PRP (Table 2). Of eyes with mild NPDR; 36 eyes (33.6%) had < 5 H/Ma on color fundus photographs. DR severity was not associated with significant differences in SSI, quality index (QI), CST, or SE. In total, 9 eyes had focal laser and the duration since focal laser therapy at the time of imaging was 5.2 ± 2.9 years (Table 2). Both VD and VLD decreased sequentially with increasing DR severity within each of the three individual vascular plexuses (P < 0.001 for DR severity association with VD or VLD within each layer [SCP, ICP and DCP]; Fig. 2, Supplementary Table S1). Indeed, VD and VLD measurements were significantly different between groups for most pairwise comparisons of eyes differing by two or more steps in DR severity.
Table 1.
Patient Characteristics
| N = 237 Patients | Mean ± SD or N (%) |
|---|---|
| Age, years | 51.50 ± 14.71 |
| Female | 103 (43.4) |
| Type 1 DM | 172 (72.5) |
| Duration of DM, years | 28.81 ± 16.37 |
| HbA1c (%) | 7.92 ± 1.53 |
| Hypertension | 132 (55.8) |
| Hyperlipidemia | 145 (61.1) |
DM, diabetes mellitus; N, number; SD, standard deviation.
Table 2.
Ocular and Scan Characteristics of Study Eyes Grouped by DR Severity
| N = 396 Eyes | No DR | Mild NPDR | Moderate NPDR | Severe NPDR | PDR (no PRP) | PDR (with PRP) | |
|---|---|---|---|---|---|---|---|
| Scan Characteristics Mean ± SD Or N (%) | Eyes (%) | 64 (16.1) | 107 (26.9) | 84 (21.1) | 48 (12.1) | 40 (10.1) | 53 (13.4) |
| SSI, mean ± SD | 72.96 ± 7.70 | 71.65 ± 7.49 | 71.77 ± 8.62 | 71.43 ± 9.43 | 69.83 ± 7.66 | 69.24 ± 7.92 | |
| Quality Index | 7.82 ± 1.30 | 7.77 ± 1.05 | 7.52 ± 1.28 | 7.74 ± 1.18 | 7.68 ± 1.02 | 7.33 ± 1.94 | |
| Ocular Characteristics Mean ± SD Or N (%) | Central Subfield Thickness, µm | 276.95 ± 21.00 | 273.81 ± 26.35 | 270.48 ± 28.88 | 277.85 ± 33.29 | 274.25 ± 25.34 | 269.30 ± 45.31 |
| Spherical Equivalent | −1.18 ± 0.256 | −1.06 ± 0.20 | −0.74 ± 0.22 | −0.74 ± 0.30 | −1.72 ± 0.32 | −0.94 ± 0.28 | |
| Phakic | 58 (90.6) | 85 (79.4) | 70 (83.3) | 46 (95.8) | 37 (92.5) | 38 (71.7) | |
| Treatment History | anti-VEGF | n/a | 3 | 0 | 4 | 10 | 9 |
| PRP | n/a | n/a | n/a | 2 | 0 | 53 | |
| Focal laser | n/a | 1 | 1 | 3 | 1 | 3 | |
| PPV | n/a | 0 | 0 | 0 | 0 | 0 | |
DR, diabetic retinopathy; NPDR, non proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; PPV, pars plana vitrectomy; PRP, panretinal photocoagulation; SSI, signal strength index; SD, standard deviation; n/a, not applicable.
Figure 2.
Box-plots illustrating changes in all three vascular plexus with increasing diabetic retinopathy (DR) severity. Figure illustrates that with increasing DR severity, all three vascular plexuses show a decreased vessel density (VD) and vessel length density. Looking at stepwise changes, the VD and vessel length density (VLD) in intermediate and deep capillary plexuses (ICP and DCP, respectively) but not the superficial capillary plexus (SCP) is significantly lower in eyes with mild nonproliferative DR (NPDR) compared to those with no DR. With more advanced DR the DCP was not significantly lower between eyes within one DR severity while the VD/VLD SCP was significantly lower in eyes with moderate NPDR compared to those with mild NPDR and the VLD SCP was significantly lower in eyes with severe NPDR compared to those with moderate NPDR. Pair wise comparisons were performed using mixed models correcting for correlation of two eyes, CST, Age, SSI, SE and duration of DM. The P value is Bonfreroni-adjusted with significance being < 0.05.
For SCP metrics, there were no significant differences between eyes with no DR versus mild NPDR (Fig. 2). There was also no difference between eyes with mild NPDR and < 5 H/Ma versus those with ≥ 5 H/Ma (Supplementary Table S2). However, there was a significant difference in SCP VD and VLD between eyes with mild and moderate NPDR (VD: 35.45 ± 3.31 vs. 34.14 ± 3.38, P = 0.008 and VLD: 17.59 ± 1.83 vs. 16.80 ± 1.83, respectively, P = 0.003), and between eyes with moderate and severe NPDR for SCP VLD (16.80 ± 1.83 vs. 15.79 ± 1.84, respectively, P = 0.019). There was no significant difference found in SCP variables between eyes with severe NPDR and PDR. There was a significant decrease in VD SCP between all eyes with a DR severity level difference of two steps or greater (P < 0.001 for all pairwise comparisons; Fig. 2).
In contrast to the SCP, ICP and DCP VD were significantly lower in eyes with mild NPDR (ICP: 33.12 ± 2.96, P < 0.001; DCP: 34.18 ± 3.20, P < 0.001) compared with those with no DR (ICP: 35.78 ± 94, DCP: 37.52 ± 3.60; Fig. 2). A significant difference from eyes with no DR was detected even in eyes with very early mild NPDR and H/Ma < 5 (ICP; 33.54 ± 2.84, P < 0.001; DCP: 35.70 ± 2.83, P = 0.009; Supplementary Table S2). In eyes with more advanced DR (Fig. 2), ICP and DCP did not demonstrate significant differences between eyes that differed by a single DR severity level, except for DCP VD, which was lower in eyes with PDR compared with those with severe NPDR (31.02 ± 3.23 vs. 33.18 ± 4.17, P = 0.02). When comparing eyes with a two or more step DR severity difference, both DCP and ICP metrics were significantly different, with the only exception being between eyes with mild and severe NPDR (Fig. 2). ICP and DCP VLD followed the same trends as VD between DR severity levels. Given the potential confounding effects of the foveal avascular zone (FAZ) on VD measurements, two sensitivity analyses were conducted to determine if the results were independent of the increasing size of the FAZ with increasing DR severity (Supplementary Table S3). The first analysis corrected for FAZ area in the mixed model in addition to the other confounders previously described. A second analysis excluded the FAZ from the total macular area and calculated the VD from the remaining area. Both analyses found similar results as the primary analysis, with limited effects of the FAZ on either the overall trend or the pairwise comparisons (Supplementary Table S4).
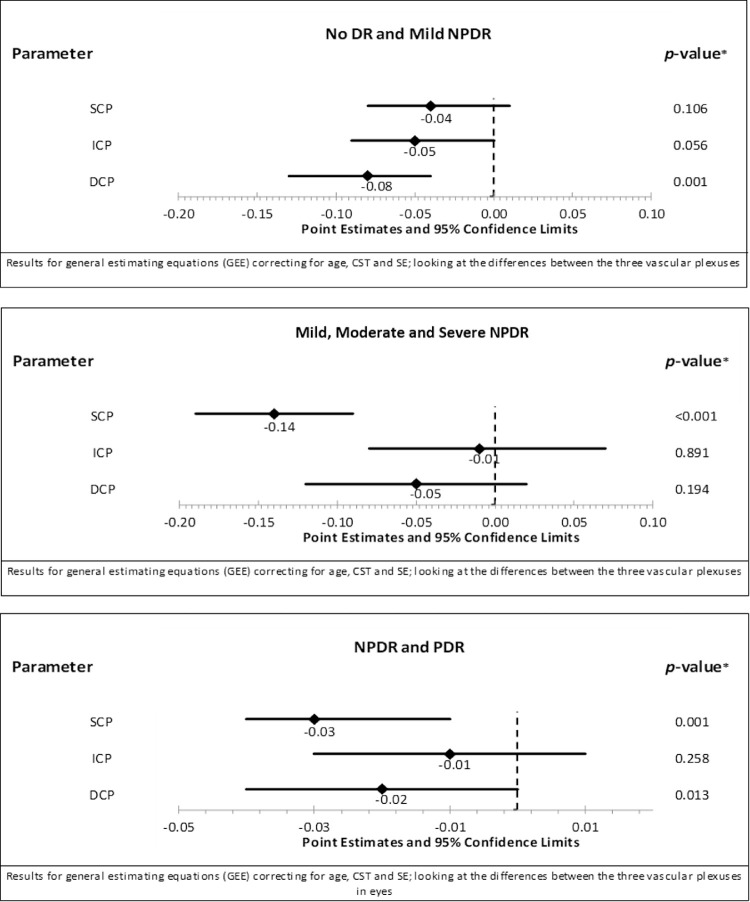
Multivariate modeling was performed to evaluate the relative strength of associations of VD or VLD in each of the three different vascular plexuses with DR severity. When SCP, ICP, and DCP metrics were all included in a model comparing eyes with no to mild NPDR, only DCP variables remained significantly associated with DR severity (Point Estimate (PE) −0.08, 95% confidence interval [CI]: −0.13 to 0.04, P = 0.001; Fig. 3). In contrast, in eyes with NPDR, only the SCP was significantly related to increasing DR severity level (PE −0.014, 95% CI: −0.19 to −0.09, P < 0.001). When comparing eyes with NPDR to those with PDR, both the SCP and DCP VD were significantly lower in eyes with PDR (SCP: PE −0.03, 95% CI: −0.04 to −0.01, P = 0.001; DCP: PE −0.02, 95% CI: −0.04 to < 0.01, P = 0.013; Fig. 3).
Figure 3.
Forest plot showing the differences in vessel density (VD) across the three vascular plexuses between different diabetic retinopathy severity levels after correcting for the other vascular plexuses, age, central subfield thickness, spherical equivalent and correlation between both eyes. Between eyes with no DR and mild NPDR; only the DCP Is significantly lower while between different levels of NPDR only the SCP was significantly lower. However, between all eyes with NPDR and those with PDR, the SCP and to a lesser extent the DCP was significantly lower.
Of 93 eyes with PDR, 40 eyes had no PRP and 53 eyes had previous PRP (Table 3). The mean time since PRP was 10.77 ± 11.02 years (range 0.81 – 38.71 years). In eyes with PDR, there were no significant differences in SCP, ICP or DCP VD, or VLD between eyes with or without PRP (Table 3). Even after excluding eyes with a history of anti-VEGF therapy in either group (29 eyes without PRP and 44 eyes with PRP remaining) there were still no significant differences between PDR eyes with and without PRP. VD did not correlate with time elapsed since PRP in any of the three vascular layers (SCP; r < 0.01, P = 0.98, ICP; 0.29, P = 0.05, r 0.04, P = 0.81). Similarly, VLD did not correlate with time duration since PRP.
Table 3.
VD and VLD in Eyes with Proliferative Diabetic Retinopathy with and without PRP
| PDR No PRP (40) Mean ± SD | PDR PRP (53) Mean ± SD | P Value Comparing Eyes with no PRP (40) vs. PRP (53)* | P Value Comparing Eyes with no PRP (29) vs. PRP (44) (Excluding Eyes with History Anti-VEGF)* | |
|---|---|---|---|---|
| VD SCP (%) | 31.25 ± 4.04 | 30.68 ± 3.54 | 0.47 | 0.62 |
| VLD SCP (%) | 15.30 ± 2.22 | 14.80 ± 1.87 | 0.25 | 0.35 |
| VD ICP (%) | 29.85 ± 2.83 | 30.52 ± 3.35 | 0.32 | 0.21 |
| VLD ICP (%) | 14.99 ± 1.52 | 15.22 ± 1.71 | 0.50 | 0.32 |
| VD DCP (%) | 31.04 ± 3.10 | 31.00 ± 3.51 | 0.96 | 0.80 |
| VLD DCP (%) | 15.72 ± 1.85 | 15.59 ± 1.88 | 0.75 | 0.89 |
Independent t-test, P value significant at < 0.05.
PDR, proliferative diabetic retinopathy; SCP, superficial capillary plexus; ICP, intermediate capillary plexus; DCP, deep capillary plexus; VD, vessel density; VLD, vessel length density.
Mean duration of PRP years 10.77 ± 11.02, range 0.81 – 38.71.
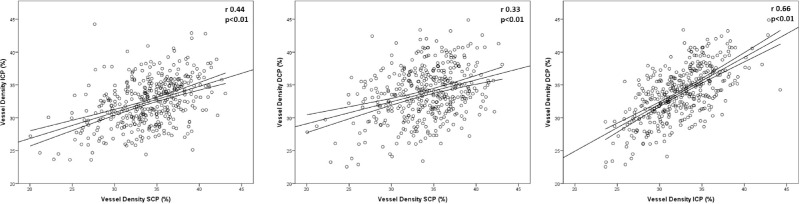
The mean decrease in VD from no DR to PDR was not significantly different between the SCP (-5.92\%), ICP (-5.55%), and DCP (-6.5%). However, for all eyes, SCP VD was only moderately correlated with ICP VD (r = 0.44, P < 0.01) and DCP VD (r = 0.33, P < 0.01; Supplementary Table S5, Fig. 4). When stratifying by DR severity level, SCP and DCP VD had no to a weak correlation. ICP and DCP VD were more strongly correlated both in the overall cohort (r = 0.66, P < 0.01) and within each DR severity level (Supplementary Table S5, Fig. 4).
Figure 4.
Correlation of vessel density (VD) between the different vascular plexuses. The central line is the best fit regression line and the lines above and below represent the 95% confidence interval of the mean. The superficial capillary plexus (SCP) was only moderately correlated with both the intermediate capillary plexus (ICP) and deep capillary plexus (DCP) while the ICP and DCP were strongly correlated with each other.
Although statistically significant associations were present between SE and both SCP and ICP VD, these correlations were weak (SCP: r = −0.16, P = 0.002, ICP: r = −0.15, P = 0.004), as were statistically significant correlations between CST and SCP and DCP VD (SCP: r = 0.16, P = 0.003, DCP: r = 0.20, P < 0.001). Duration of DM was weakly and negatively correlated with VD of all three vascular plexuses (SCP: r = −0.14, P = 0.005, ICP: r = −0.12, P = 0.017, and DCP: r = −0.21, P < 0.001). Age was only correlated with VD ICP (r = 0.14, P = 0.005). HbA1c and the presence of hypertension or hyperlipidemia were not correlated with VD or VLD within any of the vascular plexuses.
Discussion
These data demonstrate that when using PAROCTA software, vascular density of all three layers decreases with increasing DR severity. However, DR severity has significant and different effects on each of the parameters. In this cohort, vascular changes from no to early DR were present primarily in the deeper vascular layers (ICP and DCP) with approximately 50% of the total deeper layer difference in VD and VLD from no DR to PDR occurring between eyes with no DR and mild NPDR. As DR severity increased, the difference in VD in deeper layers became less profound.
In contrast, SCP density measurements were similar in eyes with no versus mild NPDR with only 20% of the total SCP decrease in VD across all DR severity levels accounted for by VD differences between eyes with no DR and mild NPDR. However, SCP VD and VLD metrics demonstrated a proportionally greater decline across severity levels in more advanced DR. The lack of correlation between SCP and DCP vascular metrics for each DR severity suggests that changes in each vascular plexus within an individual eye may be at least partially independent of one another. Although previous studies have documented decreasing VD with increasing DR severity, these prior investigations have generally combined DR severity levels into groups, such as mild-moderate NPDR,8,28,29 moderate-severe NPDR,13,14,30 or considered NPDR as a single group.15–17 Furthermore, few studies have evaluated the ICP as a distinct plexus from either the SCP or DCP.5,8,31
To the best of our knowledge, this is the first study to investigate VD and VLD differences within all three vascular layers utilizing PAROCTA software to correct for projection artifacts and to do so across all individual DR severity levels. Only a few previous studies have either utilized projection artifact removal or segmented the retina into three distinct vascular plexuses.8,17,32,33 Utilizing PAROCTA as compared with software without projection artifact removal, VD measurements in eyes with moderate and severe NDPR were significantly lower in the SCP but higher in the DCP.21 Thus, in prior studies, DCP VD differences between eyes with NPDR may have been artifactually inflated. Segmentation of the retinal anatomy into three layers reduces or removes the influence of the ICP on the DCP, which occurs when only segmenting two vascular layers. This is important as the ICP generally behaves more similarly to the DCP than the SCP when evaluating SCP VD measurements.
This study demonstrates that differences in the ICP and DCP across DR severity levels tend to be similar, as previously noted by Onishi et al.17 Similarities between these plexuses may be attributable to their location in a watershed zone with high metabolic 02 requirements.34–36 Leahy et al. found that there are significantly greater connections between the ICP/DCP than between the ICP/SCP or DCP/SCP.37 It has been postulated that combining information from all three vascular plexuses may increase the overall ability of OCTA to differentiate healthy eyes from those with DR.8 However, even if the ICP does not prove clinically significant by itself, separating it from the SCP will allow more accurate evaluation of vascular changes in the SCP across various DR severity levels. Given that the ICP behaves similarly to the DCP, its inclusion in SCP segmentation can give a false impression of VD change in early DR when, in fact, this might not be present. The segmentation of three rather than two layers in this study further unmasked SCP-specific VD changes in eyes with more advanced DR severity. Hence, whereas the ICP in itself does not appear to add additional information to that provided by the DCP, its separation from the SCP is integral to adequate analysis of eyes with varying DR severity.
The finding that deeper vascular layers are more likely to be affected in eyes with minimal DR (<5 H/Ma) compared to no DR is novel. Most previous studies evaluating early DR, have either combined mild and moderate NPDR groups8,28 or clumped all the NPDR grades into one.15,17,38,39 There have been previous reports that in eyes with no DM and those with DM but no DR, a phase immediately preceding that evaluated by the current study, VD in the three vascular layers did not change but was associated with a significantly greater avascular area in the SCP.8,17 It was suggested by Onishi et al., that early SCP VD changes may be masked by an associated increase in vascular flow (measured by pixel intensity), dilation and increased tortuosity related to a possible “steal phenomena” from deeper layers.19 It is possible in eyes with minimal DR that decreases in SCP vascular density may be similarly masked by increased flow and vascular dilation. Studies by Hagag et al., have demonstrated that individual vascular plexuses were independently autoregulated with only the DCP demonstrating a response to hyperoxia.40 Thus, the reductions in DCP VD detected on OCTA in this study may reflect true vascular loss, decreased flow secondary to vasoconstriction that falls below the threshold for detection by OCTA, or a combination of both factors.
Another interesting finding in this study is the lack of differences in OCTA parameters between eyes with PDR with or without PRP. PRP has been shown to decrease blood flow in larger blood vessels in the posterior pole secondary to decreased viable retinal tissue and improved oxygenation.41,42 Furthermore, retinal arteriolar diameter significantly decreases after PRP.43 It might therefore be expected that OCTA vascular parameters would be decreased after PRP. However, the current study, which included eyes averaging 10.8 years following PRP, is consistent with findings from two recent prospective 6-month studies that found no significant differences in VD after PRP.44,45 In addition, the mean duration of time since PRP did not correlate with VD measurements in any of the vascular layers. One possible explanation is that the increase in the retinal vascular regulatory response to hyperoxia affects the larger blood vessels to a greater extent than the smaller macular capillary vessels.41 It is also possible that eyes with PDR already have substantial VD loss and, hence, subsequent changes are minimal due to a “floor” effect. Another possibility is that PRP stabilizes central VD perhaps due to subsequent known oxygenation and VEGF changes in the eye.46 Finally, it is important to note that the OCTA metrics in this study describe absence or presence of flow but do not quantify it.47 Fawzi et al., using a surrogate for flow (adjusted flow index [AFI]) found that while VD remained unchanged, increased AFI suggested increased redistribution of flow to the posterior pole in eyes with PDR that had received PRP.45 In clinical terms, the lack of significant differences between eyes with and without PRP in this study supports combining eyes with and without PRP in a single PDR group when analyzing VD and VLD in future studies.
Strengths of this study include the large number of eyes in each DR severity group allowing adequate step wise comparison between DR severity levels. We excluded eyes with central retinal thickening or morphologic changes due to DME, which might have contributed artifacts to imaging of the deeper layers.20 Finally, we utilized PAROCTA software that removes projection artifacts from deeper layers because, as initially suggested by our group, this affects DCP and SCP measurements differently depending on DR severity.48 Limitations of the study include its cross sectional nature, which does not allow us to draw conclusions about changes in VD or VLD in an individual diabetic eye over time. The current study did not explore other OCTA metrics, such as fractal dimension, AFI, or avascular areas. In addition, there was a high percentage of patients with type 1 (72.5%) versus type 2 DM (27.5%). However, unlike recent reports suggesting distinct vascular changes between eyes with type 1 and type 2 DM, an analysis of this dataset did not reveal an interaction between type of DM and changes in VD with increasing DR severity whether in early or more advanced DR.49 Furthermore, because of the relatively large cohort of eyes in the current study, the total number of eyes of patients with type 2 DM are comparable to previously reported cases.33,49,50 Although this study used manual segmentation of the three vascular layers with starting point offsets that were previously described for normal eyes,24 we manually checked each individual B-scan and en face image to ensure that the offset locations conformed as closely as possible to their expected OCTA locations and that the configuration of the vascular plexuses resembled those previously described.5,6 Another limitation of the current study is the particular binarization technique utilized. Although this method has been used in previous studies, the lack of a particular gold standard and the lack of agreement in current OCTA literature as to the best technique makes it uncertain whether this or another technique is superior.13,21,25,26 Recent work by Mehta et al. has highlighted that different thresholding techniques significantly affect quantitative measurements and suggests that the results of a single technique may only be valid for that method of binarization.51 The results of the current study should be evaluated with that consideration in mind. Finally, although we corrected for SE and limited phakic eyes to those with SE from −6 to +3 D, we did not correct for axial length, which has been shown to affect SCP VD by +2 to −3% compared to the uncorrected SCP VD. Effects on the DCP are still unknown.52
Another potential limitation of the study was the inclusion of eyes with prior anti-VEGF therapy and focal laser. However, the percentages of eyes in the cohort with a history of either anti-VEGF therapy (N = 26, 6.6%) or focal laser (N = 9, 2.3%) were small. Although we cannot rule out an effect of these treatments on VD measurements, the small number of eyes that had undergone prior therapy lessens the chance of confounding from this factor. Furthermore, in the current study, the vast majority of eyes that had received anti-VEGF treatment were in the PDR group (19/26). A sensitivity analysis was conducted excluding eyes with prior anti-VEGF that demonstrated consistent findings as in the whole cohort (Table 3). A secondary analysis from a recent prospective study, the RECOVERY trial, showed that anti-VEGF injections had no significant impact on VD measurements in either the SCP or the DCP.53
In conclusion, although vascular density of each of the three individual layers decreases with increasing DR severity, DR severity has a significantly different effect on OCTA parameters within each layer. Vascular changes in eyes with no to early DR were present primarily in the deeper vascular layers (ICP and DCP), whereas in eyes with advanced DR the opposite was true. Although no correlation was found at any DR severity between SCP and DCP VD or VLD, ICP metrics closely correlated to DCP measurements. This study thus highlights the importance of assessing SCP and DCP changes independently across each DR severity level and indicates that three-layer evaluation should provide better data on SCP and DCP changes by limiting the confounding effect of the ICP on the DCP. Further prospective, longitudinal studies should evaluate the pathophysiology and mechanisms behind these differential plexus changes as well as whether these metrics can be used successfully to predict DR worsening or improvement in individual eyes.
Supplementary Material
Acknowledgments
Supported by National Institute of Health (NEI R01 EY024702), Research to Prevent Blindness, Massachusetts Lions Eye Research Fund, Optovue.
Disclosure: M. Ashraf, None; K. Sampani, None; A. Clermont, None; O. Abu-Qamar, None; J. Rhee, None; P.S. Silva, Optos PLC (F), Optomed OY (F), Hill-Ron (C); L.P. Aiello, Optos (F); J.K. Sun, Adaptive Sensory Technologies (F), Boston Micromachines (F), Optovue (F)
References
- 1. Jia Y, Tan O, Tokayer J, et al.. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012; 20: 4710–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurokawa K, Sasaki K, Makita S, Hong YJ, Yasuno Y. Three-dimensional retinal and choroidal capillary imaging by power Doppler optical coherence angiography with adaptive optics. Opt Express. 2012; 20: 22796–22812. [DOI] [PubMed] [Google Scholar]
- 3. Chan G, Balaratnasingam C, Xu J, et al.. In vivo optical imaging of human retinal capillary networks using speckle variance optical coherence tomography with quantitative clinico-histological correlation. Microvasc Res. 2015; 100: 32–39. [DOI] [PubMed] [Google Scholar]
- 4. Snodderly DM, Weinhaus RS, Choi JC. Neural-vascular relationships in central retina of macaque monkeys (Macaca fascicularis). J Neurosci. 1992; 12: 1169–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park JJ, Soetikno BT, Fawzi AA. Characterization of the middle capillary plexus using optical coherence tomography angiography in healthy and diabetic eyes. Retina. 2016; 36: 2039–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Campbell JP, Zhang M, Hwang TS, et al.. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep. 2017; 7: 42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hwang TS, Gao SS, Liu L, et al.. Automated quantification of capillary nonperfusion using optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016; 134: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hwang TS, Hagag AM, Wang J, et al.. Automated quantification of nonperfusion areas in 3 vascular plexuses with optical coherence tomography angiography in eyes of patients with diabetes. JAMA Ophthalmol. 2018; 136: 929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Snodderly DM, Weinhaus RS. Retinal vasculature of the fovea of the squirrel monkey, Saimiri sciureus: three-dimensional architecture, visual screening, and relationships to the neuronal layers. J Comp Neurol. 1990; 297: 145–163. [DOI] [PubMed] [Google Scholar]
- 10. Spaide RF. Volume-rendered angiographic and structural optical coherence tomography. Retina. 2015; 35: 2181–2187. [DOI] [PubMed] [Google Scholar]
- 11. Scarinci F, Nesper PL, Fawzi AA. Deep retinal capillary nonperfusion is associated with photoreceptor disruption in diabetic macular ischemia. Am J Ophthalmol. 2016; 168: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee J, Moon BG, Cho AR, Yoon YH. Optical coherence tomography angiography of DME and its association with anti-VEGF treatment response. Ophthalmology. 2016; 123: 2368–2375. [DOI] [PubMed] [Google Scholar]
- 13. Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016; 57: 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samara WA, Shahlaee A, Adam MK, et al.. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017; 124: 235–244. [DOI] [PubMed] [Google Scholar]
- 15. Nesper PL, Roberts PK, Onishi AC, et al.. Quantifying microvascular abnormalities with increasing severity of diabetic retinopathy using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017; 58: Bio307–Bio315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krawitz BD, Phillips E, Bavier RD, et al.. Parafoveal nonperfusion analysis in diabetic retinopathy using optical coherence tomography angiography. Transl Vis Sci Technol. 2018; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Onishi AC, Nesper PL, Roberts PK, et al.. Importance of considering the middle capillary plexus on OCT angiography in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018; 59: 2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hwang TS, Zhang M, Bhavsar K, et al.. Visualization of 3 distinct retinal plexuses by projection-resolved optical coherence tomography angiography in diabetic retinopathy. JAMA Ophthalmol. 2016; 134: 1411–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang M, Hwang TS, Dongye C, Wilson DJ, Huang D, Jia Y. Automated quantification of nonperfusion in three retinal plexuses using projection-resolved optical coherence tomography angiography in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2016; 57: 5101–5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015; 35: 2163–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ashraf M, Sampani K, Abu-Qamar O, et al.. Optical coherence tomography angiography projection artifact removal: impact on capillary density and interaction with diabetic retinopathy severity. Transl Vis Sci Technol. 2020; 9: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Silva PS, Cavallerano JD, Sun JK, Noble J, Aiello LM, Aiello LP. Nonmydriatic ultrawide field retinal imaging compared with dilated standard 7-field 35-mm photography and retinal specialist examination for evaluation of diabetic retinopathy. Am J Ophthalmol. 2012; 154: 549–559.e542. [DOI] [PubMed] [Google Scholar]
- 23. Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991; 98: 786–806. [PubMed] [Google Scholar]
- 24. Garrity ST, Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Quantitative analysis of three distinct retinal capillary plexuses in healthy eyes using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017; 58: 5548–5555. [DOI] [PubMed] [Google Scholar]
- 25. Borrelli E, Lonngi M, Balasubramanian S, et al.. Macular microvascular networks in healthy pediatric subjects. Retina. 2019; 39: 1216–1224. [DOI] [PubMed] [Google Scholar]
- 26. Uji A, Balasubramanian S, Lei J, Baghdasaryan E, Al-Sheikh M, Sadda SR. Impact of multiple en face image averaging on quantitative assessment from optical coherence tomography angiography images. Ophthalmology. 2017; 124: 944–952. [DOI] [PubMed] [Google Scholar]
- 27. Fawzi AA. Consensus on optical coherence tomographic angiography nomenclature: do we need to develop and learn a new language? JAMA Ophthalmol. 2017; 135: 377–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu L, Jian G, Bao W, et al.. Analysis of foveal microvascular abnormalities in diabetic retinopathy using optical coherence tomography angiography with projection artifact removal. J Ophthalmol. 2018; 2018: 3926745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lei J, Yi E, Suo Y, et al.. Distinctive analysis of macular superficial capillaries and large vessels using optical coherence tomographic angiography in healthy and diabetic eyes. Invest Ophthalmol Vis Sci. 2018; 59: 1937–1943. [DOI] [PubMed] [Google Scholar]
- 30. Lee H, Lee M, Chung H, Kim HC. Quantification of retinal vessel tortuosity in diabetic retinopathy using optical coherence tomography angiography. Retina 2018; 38: 976–985. [DOI] [PubMed] [Google Scholar]
- 31. Chung CS, Nesper PL, Park JJ, Fawzi AA. Comparison of Zeiss Cirrus and Optovue RTVue OCT angiography systems: a quantitative and qualitative approach examining the three capillary networks in diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retina. 2018; 49: e198–e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu L, Jian G, Bao W, Hu C, Xu Y, Zhao B. Analysis of foveal microvascular abnormalities in diabetic retinopathy using optical coherence tomography angiography with projection artifact removal. J Ophthalmol. 2018; 2018: 3926745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Forte R, Haulani H, Jurgens I. Quantitative and qualitative analysis of the three capillary plexuses and choriocapillaris in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy: a prospective pilot study. Retina, 10.1097/IAE.0000000000002376. [DOI] [PubMed] [Google Scholar]
- 34. Cringle SJ, Yu DY, Yu PK, Su EN. Intraretinal oxygen consumption in the rat in vivo. Invest Ophthalmol Vis Sci. 2002; 43: 1922–1927. [PubMed] [Google Scholar]
- 35. Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001; 20: 175–208. [DOI] [PubMed] [Google Scholar]
- 36. Linsenmeier RA, Zhang HF. Retinal oxygen: from animals to humans. Prog Retin Eye Res. 2017; 58: 115–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leahy C, Radhakrishnan H, Weiner G, Goldberg JL, Srinivasan VJ. Mapping the 3D connectivity of the rat inner retinal vascular network using OCT angiography. Invest Ophthalmol Vis Sci. 2015; 56: 5785–5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ashraf M, Nesper PL, Jampol LM, Yu F, Fawzi AA. Statistical model of optical coherence tomography angiography parameters that correlate with severity of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2018; 59: 4292–4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaizu Y, Nakao S, Yoshida S, et al.. Optical coherence tomography angiography reveals spatial bias of macular capillary dropout in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017; 58: 4889–4897. [DOI] [PubMed] [Google Scholar]
- 40. Hagag AM, Pechauer AD, Liu L, et al.. OCT angiography changes in the 3 parafoveal retinal plexuses in response to hyperoxia. Ophthalmol Retina. 2018; 2: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grunwald JE, Brucker AJ, Petrig BL, Riva CE. Retinal blood flow regulation and the clinical response to panretinal photocoagulation in proliferative diabetic retinopathy. Ophthalmology. 1989; 96: 1518–1522. [DOI] [PubMed] [Google Scholar]
- 42. Grunwald JE, Riva CE, Brucker AJ, Sinclair SH, Petrig BL. Effect of panretinal photocoagulation on retinal blood flow in proliferative diabetic retinopathy. Ophthalmology. 1986; 93: 590–595. [DOI] [PubMed] [Google Scholar]
- 43. Mendrinos E, Mangioris G, Papadopoulou DN, Dosso AA, Pournaras CJ. Retinal vessel analyzer measurements of the effect of panretinal photocoagulation on the retinal arteriolar diameter in diabetic retinopathy. Retina. 2010; 30: 555–561. [DOI] [PubMed] [Google Scholar]
- 44. Lorusso M, Milano V, Nikolopoulou E, et al.. Panretinal photocoagulation does not change macular perfusion in eyes with proliferative diabetic retinopathy. Ophthalmic Surg Lasers Imaging Retina. 2019; 50: 174–178. [DOI] [PubMed] [Google Scholar]
- 45. Fawzi AA, Fayed AE, Linsenmeier RA, Gao J, Yu F. Improved macular capillary flow on optical coherence tomography angiography after panretinal photocoagulation for proliferative diabetic retinopathy. Am J Ophthalmol. 2019; 206: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aiello LP, Avery RL, Arrigg PG, et al.. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994; 331: 1480–1487. [DOI] [PubMed] [Google Scholar]
- 47. Spaide RF, Fujimoto JG, Waheed NK, Sadda SR, Staurenghi G. Optical coherence tomography angiography. Prog Retin Eye Res. 2018; 64: 1–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ashraf M SK, Abu-qamar O, Cavallerano J, Silva P, Aiello LP, Sun JK. Optical coherence tomography angiography projection artifact removal: impact on capillary density and interaction with diabetic retinopathy severity. Transl Vis Sci Technol. 2020; In press, 10.1167/tvst.9.7.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vujosevic S, Muraca A, Alkabes M, et al.. Early microvascular and neural changes in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina. 2019; 39: 435–445. [DOI] [PubMed] [Google Scholar]
- 50. Cao D, Yang D, Huang Z, et al.. Optical coherence tomography angiography discerns preclinical diabetic retinopathy in eyes of patients with type 2 diabetes without clinical diabetic retinopathy. Acta Diabetol. 2018; 55: 469–477. [DOI] [PubMed] [Google Scholar]
- 51. Mehta N, Liu K, Alibhai AY, et al.. Impact of binarization thresholding and brightness/contrast adjustment methodology on optical coherence tomography angiography image quantification. Am J Ophthalmol. 2019; 205: 54–65. [DOI] [PubMed] [Google Scholar]
- 52. Sampson DM, Gong P, An D, et al.. Axial length variation impacts on superficial retinal vessel density and foveal avascular zone area measurements using optical coherence tomography angiography. Chemistry. 2017; 58: 3065–3072. [DOI] [PubMed] [Google Scholar]
- 53. Alagorie AR, Nittala MG, Velaga S, et al.. Association of intravitreal aflibercept with optical coherence tomography angiography vessel density in patients with proliferative diabetic retinopathy: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2020; 138: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.