Abstract
Purpose
Rates of rectal toxicity after low-dose-rate brachytherapy for prostate cancer are dependent on rectal dose which is associated with rectal distance from prostate and implanted seeds. Placement of a hydrogel spacer between the prostate and rectum has proven to reduce the volume of the rectum exposed to higher radiation dose levels in the setting of external beam radiotherapy (EBRT). We present our findings with placing a rectal hydrogel spacer in patients following low dose-rate LDR brachytherapy, and we further assess the impact of this placement on dosimetry and acute rectal toxicity.
Materials and Methods
Between January 2016 and April 2017, 74 patients had placement of a hydrogel spacer, immediately following a Pd-103 seed-implant procedure. Brachytherapy was delivered as follows: as a monotherapy to 26 (35%) patients; as part of planned combination therapy with EBRT to 40 (54%) patients; or as a salvage monotherapy to eight (11%) patients. Post-operative MRI was used to assess separation achieved with rectal spacer. Acute toxicity was assessed retrospectively using RTOG/EORTC radiation toxicity grading system. Rectal dosimetry was compared with a consecutive cohort of 136 patients treated with seed implantation at our institution without a spacer, using a 2-tailed paired Students’ T-test (p<0.05 for statistical significance).
Results
On average, 11.2 mm (standard deviation [SD] 3.3) separation was achieved between the prostate and the rectum. The resultant mean rectal volume receiving 100% of prescribed dose (V100%), dose to 1 cc of rectum (D1cc) and dose to 2 cc of rectum (D2cc) were: 0 (SD 0.05cc), 25.3% (SD 12.7), and 20.5% (SD 9.9), respectively. All rectal dosimetric parameters improved significantly for the cohort with spacer placement as compared with the non-spacer cohort. Mean prostate volume, prostate V100 and dose to 90% of gland (D90) were 29.3cc (SD 12.4), 94.0% (SD 3.81), and112.4% (SD 12.0), respectively. Urethral D20, D5cc and D1cc were 122.0% (SD 17.27), 133.8% (SD 22.8), and 144.0% (SD 25.4), respectively. After completing all treatments, at the time of first the follow up, seven patients reported acute rectal toxicity –six experiencing grade 1 rectal discomfort and one (with pre-existing hemorrhoids) experiencing grade 1 bleeding.
Conclusions
Injection of rectal spacer is feasible in the post LDR brachytherapy setting and reduces dose to the rectum with minimal toxicity. Prostate and urethral dosimetry do not appear to be affected by the placement of a spacer. Further studies with long-term follow up are warranted to assess the impact on reduction of late rectal toxicity.
Keywords: prostate cancer, low-dose rate brachytherapy, rectal spacer
Background
Modern radiation techniques, such as intensity-modulated radiotherapy, image-guided radiotherapy, and proton therapy, have made dose escalation possible for treatment of prostate cancer associated with superior tumor control outcomes [1,2]. However, the proximity of the rectum to the prostate and the sensitivity of rectal mucosa to radiation is often a dose-limiting factor. Contemporary series and well controlled prospective trials suggest incidence of grade 2 or higher acute and long-term rectal toxicity to be <10% to 47% and 6.4% to 24%, respectively [3–5]. The placement of a biodegradable gel has been used to increase the distance between the prostate and the rectum for patients who are undergoing prostate radiotherapy for this purpose. Placement of a hydrogel spacer between the prostate and the rectum has been proven in a randomized controlled trial to reduce rectal dose resulting in decreased acute and long-term rectal toxicity and improvement in bowel-related quality of life [6,7].
Brachytherapy is an accepted single-modality treatment for low-risk and favorable intermediate-risk prostate cancer or as part of a combination regimen for unfavorable intermediate- and high-risk prostate cancer [8]. Overall grades 1, 2, and 3 acute rectal toxicity from seed implantation are reported to be in range of 15.8% – 36.5% [9–11]. A randomized trial demonstrated much higher rates of acute GI/rectal toxicity rates (G1: 46.2%, G2: 39.2% and G3: 9.0%) with combination of LDR + EBRT as compared with that from LDR alone (reported above) [12]. Toxicity, however, is dependent on rectal dose which is ultimately associated with rectal distance from the prostate and seeds implanted within the gland. This is especially important among patients who are treated with a combined regimen of brachytherapy and EBRT. Therefore, it was hypothesized that placement of a rectal spacer would reduce rectal dose and toxicity in patients undergoing LDR brachytherapy alone or in combination with EBRT.
In this report, we present our experience with placement of rectal hydrogel spacer following LDR brachytherapy with Pd-103 seeds and assess its impact on dosimetry as well as acute rectal toxicity.
Methods
Patient selection
Between January 2016 and April 2017, 79 patients were planned for placement of an FDA-approved hydrogel rectal spacer, SpaceOAR™ (Augmenix Inc., Waltham, MA). Spacer placement was done based on disease characteristics, treatment modality, and patient preference. Demographics and treatment characteristics are outlined in Table 1.
Table 1.
Patient, disease and treatment characteristics
| Parameter | Spacer cohort (n=79) | Non-spacer cohort (n=136) |
|---|---|---|
| Median age | 68.9 | 69.1 |
| Clinical Stage, n (%) | ||
| T1 | 48 (60.8) | 72 (52.9) |
| T2 | 19 (24.1) | 38 (27.9) |
| T3 | 1 (1.3) | 7 (5.2) |
| Recurrent | 11 (13.9) | 19 (14.0) |
| Gleason Score, n (%) | ||
| 3+3 | 4 (5.1) | 11 (8.1) |
| 3+4 | 33 (41.8) | 70 (51.9) |
| 4+3 | 24 (30.4) | 27 (20.0) |
| 4+4 | 9 (11.4) | 11 (8.1) |
| 4+5 | 7 (8.9) | 15 (11.1) |
| 5+4 | 2 (2.5) | 1 (0.08) |
| Pre-treatment PSA (ng/ml) [range] | 7.2 [1.1 – 211.0] | 6.6 [0.5 – 88.7] |
| Baseline IPSS [range] | 5 [0 – 20] | 6 [0 – 30] |
| LDR implant intent, n (%) | ||
| Monotherapy | 26 (32.9) | 44 (32.3) |
| Combination with external beam | 42 (53.2) | 73 (53.7) |
| Salvage monotherapy | 11 (13.9) | 19 (14.0) |
PSA – prostate specific antigen; IPSS – international prostate symptom score; LDR – low dose rate
Procedure and post-implant assessment
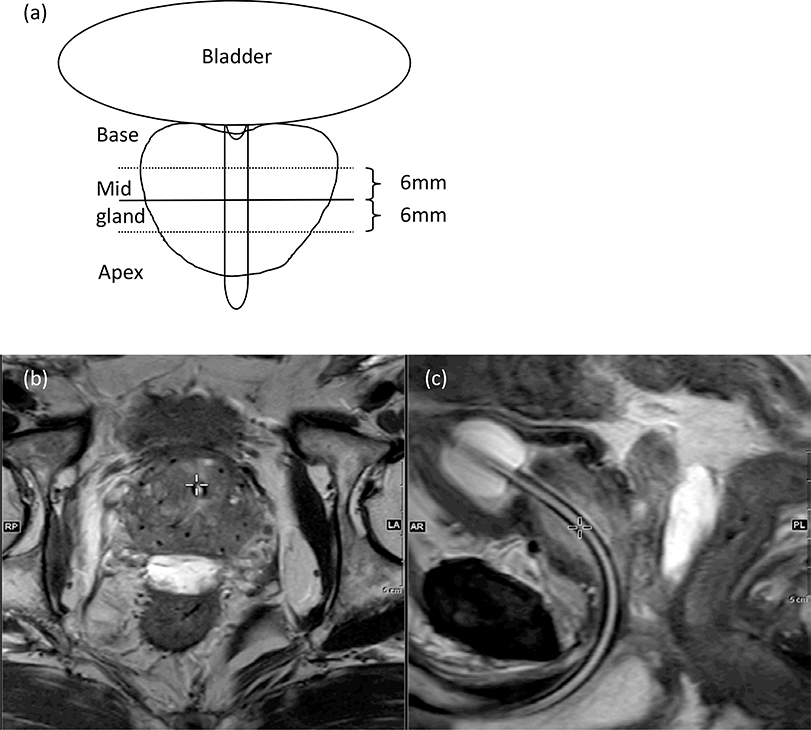
After completion of intra-operatively planned seed implantation as per institutional practice described previously [13,14], the spacer was placed between the prostate and the rectum during the same procedure as described by Pinkawa, et al[15]. Briefly, a needle was advanced into the retroprostatic space below the Denonvillier’s fascia and above the anterior rectal wall using the sagittal plane of the trans-rectal ultrasound. We took care not to puncture the rectal wall. The mid-prostate placement of the needle was confirmed on the transverse plane. We injected saline to hydro-dissect the fascia, and maintained the needle tip within the sagittal view. The SpaceOAR was injected as two separate liquids that solidified into a gel within 7–10 seconds of instillation. A CT scan for post-implant dosimetry was performed immediately after the procedure (day 0 CT scan). Patients receiving a spacer also underwent a postimplant MRI (axial, coronal, and sagittal T2 sequences and a T1-weighted sequence for seeds/gold marker identification) either on day 0 (monotherapy or salvage patients) or day14 (combination patients). Separation achieved with the rectal spacer was measured at mid gland using axial T2 sequences shown schematically in Figure 1a.
Figure 1.
Schematic diagram of how midprostate rectum–prostate separation was measured (a), and axial T2 MRI at midprostate level (b), and sagittal T2 image with spacer between the rectum and prostate (c).
Post-implant dosimetry was performed using VariSeed™ (Varian Medical System, Palo Alto, Ca) software after identifying seeds and after drawing the contours of the prostate, urethra, bladder, and rectum on the day 0 CT scan. Since patients undergoing monotherapy or combination therapy are prescribed different doses, the percentage of each prescribed dose is documented and reported. Dosimetric parameters for prostate (dose to 90% of the target volume [D90], volume receiving 100%, 150%, and 200% of the prescribed dose [V100, V150, and V200]), dose to 20%, 5%, and 1% of urethra (DU20, DU5 and DU1), and rectum (VR100, dose to 1cc and 2cc of rectal volume [DR1cc and DR2cc]) were retrieved from a prospectively managed database. Since we started placement of spacer in 2016, all patients undergoing brachytherapy at our institution in 2015 were identified and their dosimetric information was also extracted for and served as a historical comparison group.
Assessment of seed displacement with spacer placement
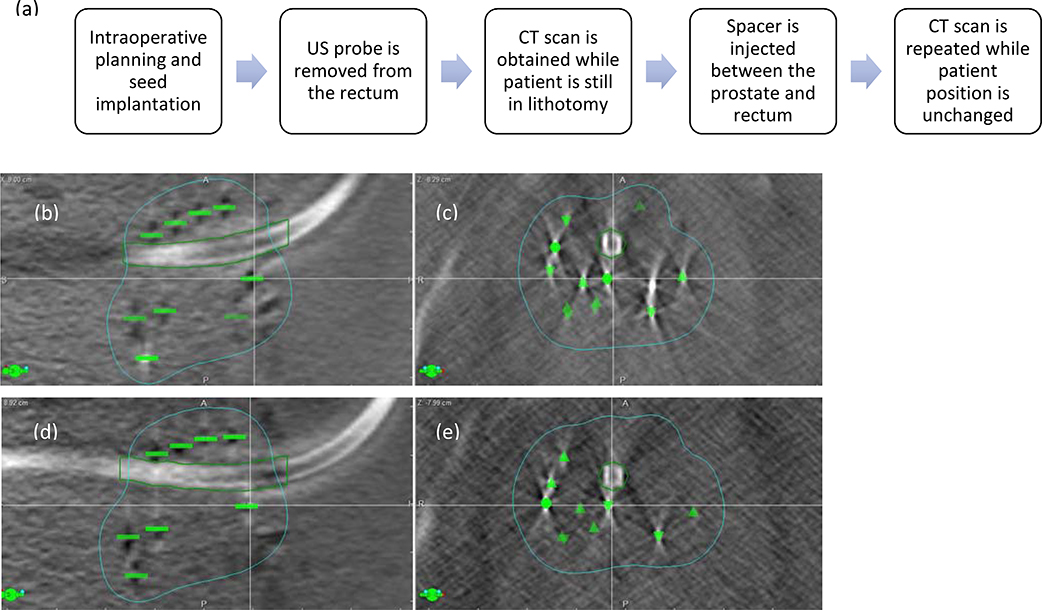
To determine the impact of seed displacement within the gland, a subset of 10 patients was further evaluated using two intraoperative cone beam CT scans, obtained immediately before and after placement of the spacer. Both scans were performed without moving the patient from treatment position and without the trans-rectal probe, (Figure 2). On both cone beam CT data sets, all implanted seeds were identified, and the urethra was contoured in VariSeed™. DICOM plans and structure sets were then exported to a MatLab™ (The Mathworks Natick, MA) routine to calculate the distance between each seed and the urethra contour (r), and their component in the left-right (x) and anterior-posterior (y) directions. Changes in the average distances between pre- and post-spacer CT scans were calculated. The maximum dose to the urethra (Dmax) was calculated and compared between two data sets.
Figure 2.
(a) Steps to determine impact of spacer placement on intraprostatic seed movement Displacement of seeds within the prostate after rectal spacer placement. (b) sagittal and (c) axial CT scan images prior to rectal spacer implantation; (d) sagittal and (e) axial CT scan images after placement of rectal spacer. Note the anterior movement of seeds immediately posterior to the urethra (seed indicated by the cross‐hair) and lateral displacement of the seeds lateral to the urethra; green contour indicates 100% isodose line.
Toxicity
Acute gastrointestinal (GI) or rectal toxicity (rectal discomfort or bleeding), defined as occurring during immediate post-operative period and for up to six months after the procedure, was assessed using modified the Radiation Oncology Therapy Group (RTOG) radiation-toxicity grading system([9], as follows:
-
Grade 1:
Increased frequency or change in quality of bowel habits not requiring medication / rectal discomfort not requiring analgesics.
-
Grade 2:
Diarrhea requiring medications / mucous or bloody discharge / rectal or abdominal pain requiring analgesics / no mucosal ulceration.
-
Grade 3:
Diarrhea requiring parenteral support / hospitalization for severe pain, bleeding from thrombosed hemorrhoids / severe mucus or bloody discharge / superficial ulceration / minor surgical procedure.
-
Grade 4:
Acute or subacute obstruction, fistula, or perforation / GI bleeding requiring transfusion / major surgical procedure.
Statistical analysis
All comparisons between spacer and non-spacer patients and all assessments of seed movement used data from post-implant dosimetry on the CT scan from day 0. We used non-parametric independent 2-tailed T-test to compare samples, and we assumed a p-value of <0.05 for statistical significance. Our statistical analysis used IBM SPSS, v.24.0.0 (SPSS Inc., Chicago, IL).
Results
Seventy-four out of 79 (93.7%) patients had successful spacer placement. Spacer placement was aborted in three patients undergoing salvage treatment and two patients undergoing combination therapy, primarily because separation between the prostate and the rectum was unsuccessful at the time of hydro-dissection. Twenty-six out of 74 (35.1%) underwent brachytherapy alone as the primary treatment, 40 (54.1%) as part of a combination treatment with EBRT, and eight (10.8%) as salvage treatment. Disease and treatment characteristics of patients undergoing spacer placement and the comparative cohort without a spacer placement are detailed in Table 1.
Impact of spacer on prostate, urethral and rectal dose
Placement of the rectal spacer successfully achieved a median distance of 11.2 mm (range, 1.6–16.7 mm) between the prostate and the rectum, (Figures 1b, 1c). Table 2 summarizes dosimetric comparisons between patients with a rectal spacer and patients who underwent LDR brachytherapy without a spacer at our institution in 2015. Our institutional guidelines strive to achieve the following dosimetric outcomes: Prostate (D90 >100%), Urethra (D20 <130%):Rectum (V100 <1.0cc): As per these guidelines, 86%, 69% and 96% of the patients in spacer cohort and 83%, 72% and 66% of the patients in non-spacer cohort achieved the D90, DU20 and VR100 criteria, respectively. All rectal parameters (V100, D1 cc and D2 cc) were significantly improved post placement of a spacer (p-value <0.05), whereas prostate D90, urethral D20, D5, and D1 were similar between the two groups (p-value >0.05). Prostate V150 and V200 values were significantly higher on average by 4.4% for V150, p =0.001; and 6.3% for V200, p <0.001 in the spacer cohort as compared with the non-spacer cohort. Patients in the former group had significantly smaller prostate volume than in the latter (29.3cc versus 34.5cc, respectively [p = 0.001]), which may partially explain their higher V150 and V200 values.
Table 2.
Dosimetric comparison of patients with or without spacer consecutively treated at our institution.
| Parameter | Mean [SD] |
Mean [SD] |
P-value |
|---|---|---|---|
| Spacer cohort (n=74) | Non-spacer cohort (n=136) | ||
| Prostate-rectal separation achieved (mm) | 11.2 [3.3] | ||
| Isotope, median number of seeds [range] | Pd, 57 [34 – 96] | Pd (n=109), 65 [15 – 109] | 0.001 |
| I-125 (n=26), 77 [34 – 98] | |||
| Isotope, median activity | Pd, 2.74 [1.54 – 4.02] | Pd, 2.29 [1.02 – 3.73] | 0.000 |
| I-125, 0.46 [0.41 – 0.57] | |||
| Prostate volume (cc) | 29.34 [12.35] | 34.55 [12.11] | 0.004 |
| Prostate D90 (%) | 112.37 [12.04] | 109.60 [13.64] | 0.082 |
| Prostate V100 (%) | 94.02 [3.81] | 93.08 [3.96] | 0.095 |
| Prostate V150 (%) | 70.03 [8.44] | 65.64 [8.76] | 0.001 |
| Prostate V200 (%) | 47.75 [9.02] | 41.50 [12.27] | 0.000 |
| Urethra D20 (%) | 122.02 [17.27] | 118.87 [20.23] | 0.259 |
| Urethra D5 (%) | 133.80 [22.75] | 130.91 [22.75] | 0.366 |
| Urethra D1 (%) | 143.95 [25.38] | 143.07 [31.66] | 0.838 |
| Rectal V100 (cc) | 0.01 [0.05] | 0.07 [0.19] | 0.000 |
| Rectal D1cc (%) | 25.27 [12.67] | 52.74 [18.76] | 0.000 |
| Rectal D2cc (%) | 20.47 [9.93] | 43.16 [15.70] | 0.000 |
D90 – dose to 90% of the prostate; prostate V100, V150 and V200 – prostate volumes receiving 100%, SD- standard deviation; 150% and 200% of the prescribed dose; urethra D20, D5 and D1 – percent of the prescribed dose to 20%, 5% and 1% of urethra; rectal V100 – rectal volume receiving 100% of prescribed dose; rectal D1cc and D2cc – percent of prescribed dose to 1cc and 2cc volumes of rectum.
Seed distance from the urethral contour and impact on urethral dose
Comparison of seed location compared to the urethra, resulting from possible compression of the gland from the spacer, is summarized in Table 3. Change in distance between seeds and the urethra contour was minor but statistically significant (p-value <0.05); we observed a decrease in distance of 0.4 mm in the anterior-posterior (y) direction and an increase in the lateral (x) direction of 0.3 mm. The overall distance (r) decreased by 0.1 mm, with no statistical significance observed. We however observed no significant increase in dose to the urethra; the change in value of the maximal urethral dose (Dmax) was only 4.34% (p-value =0.233). Most likely this was due to lateral displacement of some seeds away from the urethra, while other seeds were displaced towards the urethra. Thus, the overall average dose contribution from all the seeds remained unchanged. Yet, we did observe that cases where seeds were positioned in close proximity, especially directly anterior or posterior to the urethra, experienced an increase in urethral dose with a spacer injection.
Table 3.
Change is seed position with placement of rectal spacer
| Change in parameter | Mean [SD, range] | P-value |
|---|---|---|
| Left-right (x) [mm] | 0.27 [0.13, 0.12 – 0.42] | 0.001 |
| Anterior-posterior (y) [mm] | −0.39 [0.19, −0.62 – − 0.08] | 0.001 |
| Distance (r) [mm] | −0.14 [0.18, −0.39 – 0.22] | 0.073 |
| Urethral Dmax [%] | 4.34 [8.01, −0.17 – 23.49] | 0.255 |
Change in seed parameters after spacer placement: “x” – lateral displacement; “y” – anterior posterior displacement; “r” – displacement relative to urethra. Positive change indicates average movement away from the urethra and negative change indicates towards the urethra. Range values are per-patient, not per-seed. In one case a seed was positioned < 3 mm away from the urethra; for this seed, a relatively minor shift (0.4mm) towards the urethra resulted in a change in Dmax > 20%.
Toxicity
Overall, any rectal or GI toxicity was observed in 15 (20.3%) patients in the spacer cohort and 33 (24.3%) patients in the non-spacer cohort, p-value = 0.95. Median time (range) to develop toxicity was 2.9 months (0.2 – 10.4) and 3.0 months (0.2 – 11.9), respectively. Details regarding all recorded toxicities are outlined in Table 4.
Table 4.
Acute GI and rectal toxicity
| LDR alone | LDR+EBRT | Salvage | |
|---|---|---|---|
| Diarrhea, n (%) | |||
| Spacer cohort | 2 (7.7%) | 5 (12.5%) | 1 (12.5%) |
| Non-spacer cohort | 7 (15.9%) | 3 (4.1%) | 1 (5.3%) |
| Rectal discomfort, n (%) | |||
| Spacer cohort | 5 (15.7%) | 2 (5.0%) | 0 |
| Non-spacer cohort | 0 | 0 | 0 |
| Rectal bleeding*, n (%) | |||
| Spacer cohort | 0 | 2 (5.0%) | 0 |
| Non-spacer cohort | 3 (6.8%) | 14 (19.2%) | 1 (5.3%) |
| Proctitis, n (%) | |||
| Spacer cohort | 0 | 0 | 0 |
| Non-spacer cohort | 0 | 4 (5.5%) | 0 |
rectal bleeding was mostly grade 1 and was associated with hemorrhoids; GI – gastrointestinal; LDR – low dose rate; EBRT – external beam radiotherapy.
Perioperative toxicity
The spacer placement was well tolerated: no patients described procedure-related complications. Within the first few days after placement of the spacer, most patients described a sense of fullness in the rectum but did not experience rectal pain or discomfort.
Rectal toxicity
Rectal discomfort was noted in six (8.1%) out of 74 patients with spacer placement at a median time of 1.3 months (range, 0.2 – 4.5). Six patients reported grade 1 discomfort; grade 2 discomfort was noted in two patients only, which required over the counter analgesics. Rectal bleeding was noted in two (2.7%) patients; one at 3.6 months post implantation and second at 10.4 months post implantation. Rectal bleeding was associated with grade 1 proctitis in one patient and with pre-existing hemorrhoidal disease in another patient. Both patients received hydrocortisone suppository cream treatment plus sitz bath to good effect. In comparison, 18 (13.2%) patients in non-spacer cohort experienced grade 1 rectal bleeding at a median time of 3.0 months (range, 0.3 – 10.4), p-value = 0.792.
Other GI toxicity
Acute GI toxicity (diarrhea) was recorded in 8 (10.8%) out of 74 patients in spacer cohort and 11 (8.1%) out of 136 patients in the non-spacer cohorts. Median time to develop acute GI toxicity in both cohorts was 2.9 months (range, 0.5 – 6.6) and 3.1 months (range, 0.2 – 11.9), respectively.
Urinary toxicity
Acute urinary retention (AUR) was used as a surrogate for acute GU toxicity. In our cohort of patients who had successful spacer placement, 10 out of 74 (13.5%) were observed to have AUR and required placement of a Foley catheter post implant. Mean IPSS score was higher for patients who experienced AUR, 9.5 (SD, 4.8) as compared with 5.6 (SD, 4.7) who did not develop AUR (p-value = 0.02). One patient experienced prolonged urinary symptoms requiring a clean intermittent catheterization (CIC) for one month after. Another patient, who also had prolonged urinary symptoms and required long-term CIC, developed hemorrhagic cystitis and was treated with hyperbaric oxygen therapy; of note, this patient had previously received 8100 cGy in regions of the bladder and underwent salvage LDR implant for local failure.
Discussion
SpaceOAR is an FDA-approved, commercially available rectal spacer that, when placed between the prostate and the rectum, has demonstrated greatly reduced rectal dose and toxicity in a randomized setting [6]. However, the predominance of the published literature has described the benefit of rectal spacer in patients undergoing EBRT [15–17]. Beydoun, et al first described use of SpaceOAR in five patients who had undergone the I-125 LDR implant procedure and were found to have had an unfavorable rectal dose on the day30 post implant dosimetry CT scan [18]. The spacer was then placed at a median of 35 days post implant. It significantly reduced the rectal dose. Toxicity related to the spacer was limited to grade 1 to 2 for rectal discomfort and pain.
Our report is the first clinical study demonstrating successful, intraoperative placement of rectal spacer in a large group of consecutive patients undergoing Pd-103 seed LDR brachytherapy with or without EBRT. We report that placement of the rectal spacerwas feasible immediately post seed implantation during the same procedure while the patients were still under general anesthesia, without adding much time to the procedure. Overall, the spacer was deemed safe and there were no procedure-related adverse events with respect to its placement.
In the past, investigators have tried a variety of spacer materials in an effort to lower the dose to the rectum. Prada, et al showed that hyaluronic acid (HA) significantly lowers the incidence of mucosal changes within the anterior rectal wall and reduces macroscopic bleeding after prostate radiotherapy [19,20]. However, radiation-induced polymer scissions resulting in reduced viscosity and structural instability are major disadvantages of HA. Furthermore, the US food and drug administration (FDA) has not approved HA injection except as a facial dermal filler (https://www.fda.gov). Noyes, et al reported that human collagen successfully injected between the prostate and rectum resulted in a 50% reduction in rectal doses [21] (but this product is not commercially availablelimiting its applicability. Levy et al placed biodegradable balloons between the rectum and prostate [22]; this approach requires a perineal incision, which is even more invasive. Susil, et al used DuraSeal (a polyethylene glycol hydrogel used commonly in surgery) to create space between the rectum and the prostate in human cadavers [23]. Strom et al published a 200 patient HDR + IMRT study evaluating DuraSeal in 100 patients, and Yeh et al published a HDR + IMRT study using DuraSeal in 326 patients [24,25]. DuraSeal, however, is not approved by FDA for prostate injection and in both studies, it was used as an off-label product. Furthermore, the downsides of DuraSeal are the rapid polymerization which increases the likelihood of needle plugging, and the rapid absorption that causes the space to collapse within about a month [26]. The commercial availability of SpaceOAR, with its ease of injection and stability during the period of radiation dosing, makes it an ideal product to use in such cases.
Mucosal denudation and development of microangiopathy within the anterior rectal wall is the most widely accepted mechanism of late rectal toxicity [27]. The most important factor shown to correlate with rectal toxicity is the VR100 (volume of the rectum receiving 100% of the prescribed dose) [9,28–30]. Herstein, et al showed that the rate of rectal bleeding was 9% versus 2% when VR100 was >1cc vs <1cc [28]. Zelefsky, et al similarly showed that the rate of rectal toxicity of grade ≥2 was 9% when VR100 was ≥2.5cc as compared with 4% when it was <2.5cc in 562 patients treated with intraoperatively planned brachytherapy at MSKCC [29]. Keyes, et al analyzed 1006 consecutive patients receiving LDR brachytherapy at British Columbia Cancer Agency and observed that VR100 was a strong predictor of late grade ≥1 rectal toxicity on multivariate analysis (OR 1.26 p-value <0.0001) [9]. They further demonstrated that patients experiencing acute grade ≥1 toxicity were much more likely to develop late rectal toxicity (OR 1.8, p-value <0.0001).
In our report, we observed a relatively low rate of any acute GI (10.8%) or acute rectal toxicity (10.8% combined incidence of rectal discomfort or bleeding) in patients that had undergone placement of the rectal spacer. This rate is much lower than historically reported rates of 15.8% to 36.5% rate of combined grade 1–3 acute GI or rectal toxicity [9–11,31,32] in patients undergoing brachytherapy with or without external beam radiation therapy. This reduction is due to low VR100 (0.01cc) achieved in our patient cohort most likely because of the rectal spacer placement. Beydoun, et al reported similar VR100 (0.06cc) after instillation of the rectal spacer in patients with unfavorable rectal dose identified on day 30 post implant CT scan [18]. The authors reported grade 1 proctitis related to spacer injection in 40% (2/5) patients, which is much higher than what we observed in our cohort. This is likely because the spacer was injected after 30 days as opposed to on day 0 in our cohort, leading to a pre-existing radiation effect on the tissue.
No adverse events were reported in patients undergoing rectal spacer injection, except for sensation of rectal fullness. Grade 1 rectal/perineal discomfort (8.1%) was the only recorded toxicity in our cohort, which was generally self-limited, and patients required only mild analgesics (OTC), if any. Fisher-Valuck et al analyzed 149 patients from the randomized trial [6] and reported 6.0% rate (nine patients) of rectal wall infiltration with the rectal spacer and an overall rate of 26.8% of acute G1/G2 toxicity [33]. In our series, we did not find any infiltration by the spacer gel of the rectal wall, which could partly explain the low rates of acute rectal toxicity we observed.
Inherent limitations of this report include: the retrospective nature of the study with a heterogeneous patient population and a short follow-up (because most of the studies observed that GI toxicity peaks at six to eight months post treatment with resolution within three to four years). Dosimetric comparisons were further conducted on two separate cohorts of patient populations treated at our institution: one before placing the spacer and the other afterwards. One might argue that dosimetric comparison should have been done on the same patient population. But this would require obtaining one set of CT and MRI scans before placing the spacer and another set immediately afterwards. This would add time in both Anesthesia and the OR; and it would not be feasible for absolutely all patients undergoing procedure. Yet, it is an important consideration for future analyses, which might need to be conducted across multiple institutions simply to collect sufficient data for meaningful results.
Conclusion
Further dose reduction to the rectum potentially results in a decrease of acute and late rectal toxicity from brachytherapy. Injection of a rectal spacer is feasible in a patient (in a post-LDR brachytherapy setting) and reduces dose to the rectum with low rates of acute toxicity owing to the spacer. Prostate and urethra dosimetry do not appear to be affected by the placement of a spacer. We believe that the reduction in dose to the rectum among brachytherapy-treated patients warrants the use of hydrogel application and might lower the risk of late rectal toxicity. Such dose reductions to the rectum could also be associated with a reduced secondary cancer risk in this patient population, as posited for patients (with a clinically localized prostate) who receive proton irradiation as their definitive treatment. Further studies are warranted to assess its impact on reduction of late rectal toxicity especially in the setting of combination treatments incorporating brachytherapy and external beam therapy.
Abbreviations
- EBRT
external beam radiotherapy
- IMRT
intensity modulated radiotherapy
- LDR
low dose rate
- HDR
high dose rate
- CT
computed tomography
- MRI
magnetic resonance imaging
- RTOG
radiation oncology therapy group
- GI
gastrointestinal
- AUR
acute urinary retention
- CIC
clean intermittent catheter
- HA
hyaluronic acid
- MSKCC
memorial sloan kettering cancer center
Footnotes
Conflict of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Zelefsky MJ, Pei X, Chou JF, et al. Dose escalation for prostate cancer radiotherapy: predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. Eur Urol. 2011;60(6):1133–1139. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kuban DA, Levy LB, Cheung MR, et al. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int J Radiat Oncol Biol Phys. 2011;79(5):1310–1317. doi: 10.1016/j.ijrobp.2010.01.006. [DOI] [PubMed] [Google Scholar]
- [3].Michalski JM, Yan Y, Deborah W-B, et al. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int J Radiat Oncol Biol Phys. 2013;87(5):932–938. doi: 10.1016/j.ijrobp.2013.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Viani G, Viana B, Martin J, Rossi B, Zuliani G, Stefano E. Intensity‐modulated radiotherapy reduces toxicity with similar biochemical control compared with 3‐dimensional conformal radiotherapy for prostate cancer: A randomized clinical trial. 2016;122(13):2004–2011. doi: 10.1002/cncr.29983. [DOI] [PubMed] [Google Scholar]
- [5].Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of Conventional-Dose vs High-Dose Conformal Radiation Therapy in Clinically Localized Adenocarcinoma of the Prostate. JAMA. 2005;294(10):1233. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- [6].Mariados N, Sylvester J, Shah D, et al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2015;92(5):971–977. doi: 10.1016/j.ijrobp.2015.04.030. [DOI] [PubMed] [Google Scholar]
- [7].Hamstra DA, Mariados N, Sylvester J, et al. Continued Benefit to Rectal Separation for Prostate Radiation Therapy: Final Results of a Phase III Trial. Int J Radiat Oncol Biol Phys. 2017;97(5):976–985. doi: 10.1016/j.ijrobp.2016.12.024. [DOI] [PubMed] [Google Scholar]
- [8].Spratt DE, Soni PD, W MP, et al. American Brachytherapy Society Task Group Report: Combination of brachytherapy and external beam radiation for high-risk prostate cancer. Brachytherapy. 2017;16(1):1–12. doi: 10.1016/j.brachy.2016.09.006. [DOI] [PubMed] [Google Scholar]
- [9].Keyes M, Spadinger I, Liu M, et al. Rectal toxicity and rectal dosimetry in low-dose-rate (125)I permanent prostate implants: a long-term study in 1006 patients. Brachytherapy. 2012;11(3):199–208. doi: 10.1016/j.brachy.2011.05.007. [DOI] [PubMed] [Google Scholar]
- [10].Kang SK, Chou RH, Dodge RK, et al. Gastrointestinal toxicity of transperineal interstitial prostate brachytherapy. 2002;53(1):99–103. [DOI] [PubMed] [Google Scholar]
- [11].Gelblum DY, Potters L. Rectal complications associated with transperineal interstitial brachytherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48(1):119–124. [DOI] [PubMed] [Google Scholar]
- [12].Rodda S, Tyldesley S, Morris WJ, et al. ASCENDE-RT: An Analysis of Treatment-Related Morbidity for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost with a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):286–295. doi: 10.1016/j.ijrobp.2017.01.008. [DOI] [PubMed] [Google Scholar]
- [13].Zelefsky MJ, Yamada Y, Marion C, Sim S. Improved conformality and decreased toxicity with intraoperative computer-optimized transperineal ultrasound-guided prostate brachytherapy. Int J Radiat Oncol Biol Phys. 2003;55(4):956–963. [DOI] [PubMed] [Google Scholar]
- [14].Zelefsky MJ, Yamada Y, Cohen G. Postimplantation dosimetric analysis of permanent transperineal prostate implantation: improved dose distributions with an intraoperative computer-optimized conformal planning technique. Int J Radiat Oncol Biol Phys. 2000;48(2):601–608. [DOI] [PubMed] [Google Scholar]
- [15].Pinkawa M, Corral NE, Caffaro M, et al. Application of a spacer gel to optimize three-dimensional conformal and intensity modulated radiotherapy for prostate cancer. Radiother Oncol. 2011;100(3):436–441. doi: 10.1016/j.radonc.2011.09.005. [DOI] [PubMed] [Google Scholar]
- [16].Uhl M, van Triest B, Eble MJ, Weber DC, Herfarth K, De Weese TL. Low rectal toxicity after dose escalated IMRT treatment of prostate cancer using an absorbable hydrogel for increasing and maintaining space between the rectum and prostate: results of a multi-institutional phase {II} trial. Radiother Oncol. 2013;106(2):215–219. doi: 10.1016/j.radonc.2012.11.009. [DOI] [PubMed] [Google Scholar]
- [17].Song DY, Herfarth KK, Uhl M, et al. A multi-institutional clinical trial of rectal dose reduction via injected polyethylene-glycol hydrogel during intensity modulated radiation therapy for prostate cancer: analysis of dosimetric outcomes. Int J Radiat Oncol Biol Phys. 2013;87(1):81–87. doi: 10.1016/j.ijrobp.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Beydoun N, Bucci JA, Chin YS, Malouf D, Enari E, Painter SD. First report of transperineal polyethylene glycol hydrogel spacer use to curtail rectal radiation dose after permanent iodine-125 prostate brachytherapy. Brachytherapy. 2013;12(4):368–374. doi: 10.1016/j.brachy.2013.01.164. [DOI] [PubMed] [Google Scholar]
- [19].Prada PJ, Gonzalez H, Menéndez C, et al. Transperineal injection of hyaluronic acid in the anterior perirectal fat to decrease rectal toxicity from radiation delivered with low-dose-rate brachytherapy for prostate cancer patients. Brachytherapy. 2009;8(2):210–217. doi: 10.1016/j.brachy.2008.11.010. [DOI] [PubMed] [Google Scholar]
- [20].Prada PJ, Juan G, Herminio G-S, et al. Prostate-specific antigen relapse-free survival and side-effects in 734 patients with up to 10 years of follow-up with localized prostate cancer treated by permanent 125iodine implants. 2010;106(1):32–36. doi: 10.1111/j.1464-410X.2009.09096.x. [DOI] [PubMed] [Google Scholar]
- [21].Noyes WR, Hosford CC, Schultz SE. Human collagen injections to reduce rectal dose during radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82(5):1918–1922. doi: 10.1016/j.ijrobp.2011.02.034. [DOI] [PubMed] [Google Scholar]
- [22].Levy Y, Paz A, Yosef RB, et al. Biodegradable inflatable balloon for reducing radiation adverse effects in prostate cancer. J Biomed Mater Res Part B Appl Biomater. 2009;91(2):855–867. doi: 10.1002/jbm.b.31467. [DOI] [PubMed] [Google Scholar]
- [23].Susil RC, R MT, L DT, Song D. Effects of prostate-rectum separation on rectal dose from external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(4):1251–1258. doi: 10.1016/j.ijrobp.2009.07.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Strom TJ, Wilder RB, Fernandez DC, et al. A dosimetric study of polyethylene glycol hydrogel in 200 prostate cancer patients treated with high-dose rate brachytherapy{\textpm}intensity modulated radiation therapy. Radiother Oncol. 2014;111(1):126–131. doi: 10.1016/j.radonc.2014.02.011. [DOI] [PubMed] [Google Scholar]
- [25].Yeh J, Lehrich B, Tran C, et al. Polyethylene glycol hydrogel rectal spacer implantation in patients with prostate cancer undergoing combination high-dose-rate brachytherapy and external beam radiotherapy. Brachytherapy. 2016;15(3):283–287. doi: 10.1016/j.brachy.2015.12.007. [DOI] [PubMed] [Google Scholar]
- [26].Cosgrove GR, Delashaw JB, Grotenhuis JA, et al. Safety and efficacy of a novel polyethylene glycol hydrogel sealant for watertight dural repair. J Neurosurg. 2007;106(1):52–58. doi: 10.3171/jns.2007.106.1.52. [DOI] [PubMed] [Google Scholar]
- [27].Doi H, Kamikonya N, Takada Y, et al. Long-term sequential changes of radiation proctitis and angiopathy in rats. J Radiat Res. 2012;53(2):217–224. [DOI] [PubMed] [Google Scholar]
- [28].Herstein A, Wallner K, Merrick G, et al. I-125 versus Pd-103 for low-risk prostate cancer: long-term morbidity outcomes from a prospective randomized multicenter controlled trial. Cancer J. 2005;11(5):385–389. [DOI] [PubMed] [Google Scholar]
- [29].Zelefsky MJ, Yamada Y, Cohen GN, et al. Intraoperative real-time planned conformal prostate brachytherapy: post-implantation dosimetric outcome and clinical implications. Radiother Oncol. 2007;84(2):185–189. doi: 10.1016/j.radonc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- [30].Price JG, Stone NN, Stock RG. Predictive factors and management of rectal bleeding side effects following prostate cancer brachytherapy. Int J Radiat Oncol Biol Phys. 2013;86(5):842–847. doi: 10.1016/j.ijrobp.2013.04.033. [DOI] [PubMed] [Google Scholar]
- [31].Hu K, Wallner K. Clinical course of rectal bleeding following I-125 prostate brachytherapy. Int J Radiat Oncol Biol Phys. 1998;14(2):263–265. [DOI] [PubMed] [Google Scholar]
- [32].Grills IS, Martinez AA, Hollander M, Huang R. High dose rate brachytherapy as prostate cancer monotherapy reduces toxicity compared to low dose rate palladium seeds. J Urol. 2004;171(3):1098–1104. [DOI] [PubMed] [Google Scholar]
- [33].Fischer-Valuck B, Chundury A, Gay H, Bosch W, Michalski J. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: Impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pr Radiat Oncol. 2017;7(3):195–202. doi: 10.1016/j.prro.2016.10.004. [DOI] [PubMed] [Google Scholar]