Abstract
Previous studies have suggested age-related differences in reward-directed behavior and cerebral processes in support of the age effects. However, it remains unclear how age may influence the processing of reward magnitude. Here, with 54 volunteers (22 to 74 years of age) participating in the Monetary Incentive Delay Task (MIDT) with explicit cues ($1, ¢1, or nil) and timed response to win, we characterized brain activations during anticipation and feedback and the effects of age on these regional activations. Behaviorally, age was associated with less reaction time (RT) difference between dollar and cent trials, as a result of slower response to the dollar trials; i.e., age was positively correlated with RT dollar – RT cent, with RT nil as a covariate. Both age and the RT difference ($1 - ¢1) were correlated with diminished activation of the right caudate head, right anterior insula, supplementary motor area (SMA)/pre-SMA, visual cortex, parahippocampal gyrus, right superior/middle frontal gyri, and left primary motor cortex during anticipation of $1 vs. ¢1 reward. Further, these regional activities mediated the age effects on RT differences. In responses to outcomes, age was associated with decreases in regional activations to dollar vs. cent loss but only because of higher age-related responses to cent losses. Together, these findings suggest age-related differences in sensitivity to the magnitude of reward. With lower cerebral responses during anticipation to win large rewards and higher responses to outcomes of small loss, aging incurs a constricted sensitivity to the magnitude of reward.
Keywords: aging, reward, MIDT, fMRI, ventral striatum
1. Introduction
Reward motivates and shapes behaviors (Balodis, et al., 2015; Knutson and Greer, 2008; Schultz, 2015). Much of our understanding of reward-related neural processes builds on animal studies (Everitt, et al., 2008; Haber and Knutson, 2010; Schultz, 2006; Schultz, 2015; Schultz, et al., 1997) and involves a network of brain regions centered on the ventral striatum (VS). The VS receives dopaminergic inputs from the ventral tegmental area (VTA) and projects to the medial prefrontal cortex (mPFC) via the globus pallidus. The mPFC sends glutamatergic inputs to the VS, forming a circuit to support motivated behaviors (Haber and Knutson, 2010; Knutson, et al., 2000; Lutz and Widmer, 2014; Samanez-Larkin and Knutson, 2015). Dysfunction of the reward circuit is implicated in many neuropsychiatric conditions, including age-related neurodegenerative illnesses (Knutson and Heinz, 2015; Oldham, et al., 2018; Whitton, et al., 2015). For instance, individuals with Parkinson’s disease show deficits in reward feedback processing (Di Rosa, et al., 2015) and reward-related learning (Freedberg, et al., 2017). People with mild cognitive impairment and Alzheimer’s disease are altered in delayed discounting (Thoma, et al., 2017) and impaired in assigning a reward value to self-related processing (Shany-Ur, et al., 2014). Thus, understanding the psychological and neural bases of age-related changes in reward processing is of translational significance.
Aging is associated with changes in multiple domains of cognitive and affective function. Older people exhibit a positivity bias in emotional experience and memory (Charles, et al., 2003; Joubert, et al., 2018) while showing less novelty seeking behavior (Sakaki, et al., 2018). In a delay discounting task older adults prefer more delayed choices, switch earlier from immediate to delayed reward, and show reduced VS activation to immediate reward (Eppinger, et al., 2012). In humans and non-human primates, aging is associated with deficits in reward-related learning (Eppinger, et al., 2011). On the other hand, older people appear to be more sensitive to negative outcomes and ready to adjust behavior on the basis of negative outcomes (Eppinger and Kray, 2011; Frank and Kong, 2008; Hammerer, et al., 2011; Simon, et al., 2010). Numerous imaging studies have described age-related changes in these reward-related processes, and those combining molecular imaging provide an opportunity to relate functional deficits to molecular changes (Berry, et al., 2018).
1.1. Outcome anticipation in the monetary incentive delay task and the effects of age
Investigators have employed functional magnetic resonance imaging (fMRI) to study the neural bases of reward processing with behavioral tasks that involve “secondary” rewards such as money or social approval (Izuma, et al., 2008; Lutz and Widmer, 2014; Rademacher, et al., 2014). In the monetary incentive delay task (MIDT) (Knutson, et al., 2000), participants are shown a bet (money at stake) and respond within a time window to win and/or avoid a loss. Reward processing can thus be distinguished for anticipation and feedback (Berridge and Robinson, 1998; Knutson, et al., 2001b; Knutson and Heinz, 2015; Rademacher, et al., 2010). Reward anticipation appears to consistently activate the VS (Diekhof, et al., 2012; Knutson and Greer, 2008; Knutson and Wimmer, 2007; Liu, et al., 2011; Lutz and Widmer, 2014; O’Doherty, et al., 2004; Oldham, et al., 2018) with activation increasing with reward magnitude (Knutson, et al., 2001b; Knutson, et al., 2000). A meta-analysis of the MIDT and other tasks reports activations of bilateral VS, right caudate nucleus and thalamus during reward anticipation (Diekhof, et al., 2012). Another meta-analysis of the MIDT reports anticipation-related activations of the ventral and dorsal striatum, insula, amygdala, thalamus, and supplementary motor area (SMA) independent of valence (win or loss), suggesting a broader role of a cortical subcortical network in supporting anticipation of a salient outcome (Oldham, et al., 2018). In contrast, the ventrolateral prefrontal cortex appears to respond specifically to the anticipation of loss (Dugre, et al., 2018).
Aging is associated with altered striatocortical dopaminergic transmission (Berry, et al., 2018; Dreher, et al., 2008; Rinne, et al., 1990; Volkow, et al., 1998). Older as compared to younger adults show reduced VS activation to reward anticipation in variants of the MIDT (Dreher, et al., 2008; Samanez-Larkin, et al., 2007; Schott, et al., 2007; Vink, et al., 2015). They also show decreased medial caudate and anterior insula activation during loss anticipation (Carstensen, 2006; Samanez-Larkin, et al., 2007). This has been attributed to phase-of-life related reduction in negative affect, in keeping with socioemotional selectivity theory across the lifespan. Socially rewarding stimuli become potentially more salient with age (Carstensen, 1995; Carstensen and Turk-Charles, 1994; Kryla-Lighthall and Mather, 2009). In a modified MIDT offering monetary or social reward, both younger and older adults show VS, thalamic, and anterior cingulate response to anticipation of both incentives (Rademacher, et al., 2010). However, anticipation of social and monetary reward results in greater right VS activation in older and younger adults, respectively (Rademacher, et al., 2014). Together, these studies suggest that aging is associated with diminished regional responses to anticipation of monetary reward.
1.2. Outcome processing in the monetary incentive delay task and the effects of age
The medial orbitofrontal and ventromedial prefrontal cortex (mOFC/vmPFC) respond consistently to feedback, with activity increasing and decreasing in response to gain and loss, respectively (Diekhof, et al., 2012; Dugre, et al., 2018; Knutson, et al., 2003; Liu, et al., 2011; Lutz and Widmer, 2014; Oldham, et al., 2018; Rademacher, et al., 2010). The mOFC/vmPFC may also play a role in outcome-based behavior adjustment (Forbes, et al., 2014). Activation of the dorsal striatum increases with the magnitude of monetary gain and decreases with magnitude of loss (Delgado, et al., 2004; Lutz and Widmer, 2014). A meta-analysis of the MIDT and other tasks with lower predictability of outcome implicates the VS in response to unpredictable outcomes, with responses scaling to the magnitude of reward (Diekhof, et al., 2012). Receipt of reward also engages the parietal and posterior cingulate cortex, bilateral anterior cingulate cortex and paracingulate gyri, subcallosal cortex and thalamus (Bartra, et al., 2013; Clithero and Rangel, 2014; Diekhof, et al., 2012; Dugre, et al., 2018; Knutson, et al., 2003; Knutson and Greer, 2008; Oldham, et al., 2018).
Studies using MIDT variants that require learning of stimulus-reward associations or more complex cognitive operations show increased VS activation to reward feedback in older adults, suggesting age-sensitive responses to positive prediction error (Samanez-Larkin, et al., 2014; Schott, et al., 2007; Vink, et al., 2015). A study of card guessing with unpredictable outcome reports VS activation and valence-discriminating caudate activity at reward feedback in both young and old adults (Cox, et al., 2008). Similarly, a combined PET and MR imaging study of a learning-dependent “slot machine” task found greater activation of the anterior medial prefrontal cortex (PFC), posterior cingulate cortex and inferior parietal cortex in older adults at the outcome phase. The same study finds that older adults with lower midbrain dopamine levels show greater PFC activity while the converse is true in younger adults, suggesting compensatory prefrontal activity to reduced striatocortical dopaminergic signaling with age (Dreher, et al., 2008). In paradigms with explicit cues (“WIN $5”, “LOSE $5”, etc.) that require no learning, old and young adults show comparable VS, medial PFC and medial caudate activation to both wins and losses (Haber and Knutson, 2010; Samanez-Larkin, et al., 2007; Samanez-Larkin, et al., 2014). In sum, although age-related changes in response to feedback in the MIDT appear to be less than consistent, cerebral activations to outcomes do not appear to diminish with age, as with anticipation of reward. The magnitude of reward as well as differences in reward contingencies, including whether learning is involved, how cue predicts reward, and whether cues predict solely wins or both wins and losses, may contribute to the complexity to the findings.
1.3. The present study
The current study investigates the effects of age on cerebral activations during anticipation and feedback in the MIDT. Specifically, we examined whether age is associated with diminished response to anticipation to win large vs. small amount of money as well as to the outcomes of wins and losses of large vs. small reward. We hypothesized that if age is associated with a global decrease in motivation for monetary reward, one would expect age-related decreases in brain activations during both anticipation and feedback of a large vs. small reward irrespective of the outcome. Alternatively, older and younger adults demonstrate comparable responses to feedback in the MIDT, as discussed earlier, while older adults show greater responses to salient external stimuli in other cognitive tasks (Hahn, et al., 2006; Hu, et al., 2012; Wiegand and Sander, 2019)). Thus, age may be associated with diminished effort to acquire monetary reward but not necessarily with diminished responses to the outcome of win or loss of a large vs. small reward. To test these hypotheses, we employed a MIDT with unambiguous cues that predicted only reward and involved no learning. Successful performance required effort or a speeded motor response to acquire the reward and the overall success rate was held relatively constant across participants by stair-casing the time window for the motor response. In addition to no reward (nil) trials as a control for reaction time (RT), we included large ($1) and small (¢1) reward trials to elicit trial-by-trial variation in motivation and effort. We examined differences in RT between dollar and cent trials, with nil RT as a covariate to quantify age-related differences in motivation, and examined regional activations to large vs. small reward both during anticipation and in response to feedback.
2. Methods
2.1. Subjects and informed consent
Fifty-four adults (30 men; 22–74 or 40 ± 14, mean ± SD, years of age) participated in this study. There was no age difference between men and women (p = 0.77, two-sample t test). All subjects were healthy with no current use of prescription medications. None reported a history of head injury or neurological illness. Other exclusion criteria included current or past Axis I Disorders including dependence on a psychoactive substance, according to DSM-IV. The Human Investigation Committee at Yale University School of Medicine approved the study and all subjects gave written informed consent prior to participation.
2.2. Behavioral task
In the monetary incentive delay task or MIDT (Figure 1A), a bet (a dollar, a cent, or no money) appeared on the screen at the beginning of each trial. After a randomized interval (fore-period) between 1 and 5 s (uniform distribution), a target box appeared on the screen and disappeared after a short period (response window). Subjects were told to press a button as quickly as possible to collect the money in the target box (win) before it disappeared. An accurate trial is defined by a button press on time and before disappearance of the target box. Otherwise, subjects would lose the bet, with the amount deducted from the total win. A premature button press prior to the appearance of the target box terminated the trial, and similarly resulted in loss. Feedback was shown on the screen after each trial to indicate the amount of money won or lost. Approximately 42% of all trials were dollar trials, 42% were cent trials, and “no money” constituted the remaining trials. There was an inter-trial-interval of 1.5 s. The response window started at 300ms, and was staircased for each trial type (dollar/cent/no money trials, separately): for instance, if the subject succeeded at two successive dollar trials, the window decreased by 30ms, making it more difficult to win again; conversely, if a subject failed for two successive trials, the response window increased by 30 ms, making it easier to win. We anticipated that the subjects would win in approximately 67% each for dollar and cent trials. Each subject completed two 10-minute runs of the task.
Figure 1.
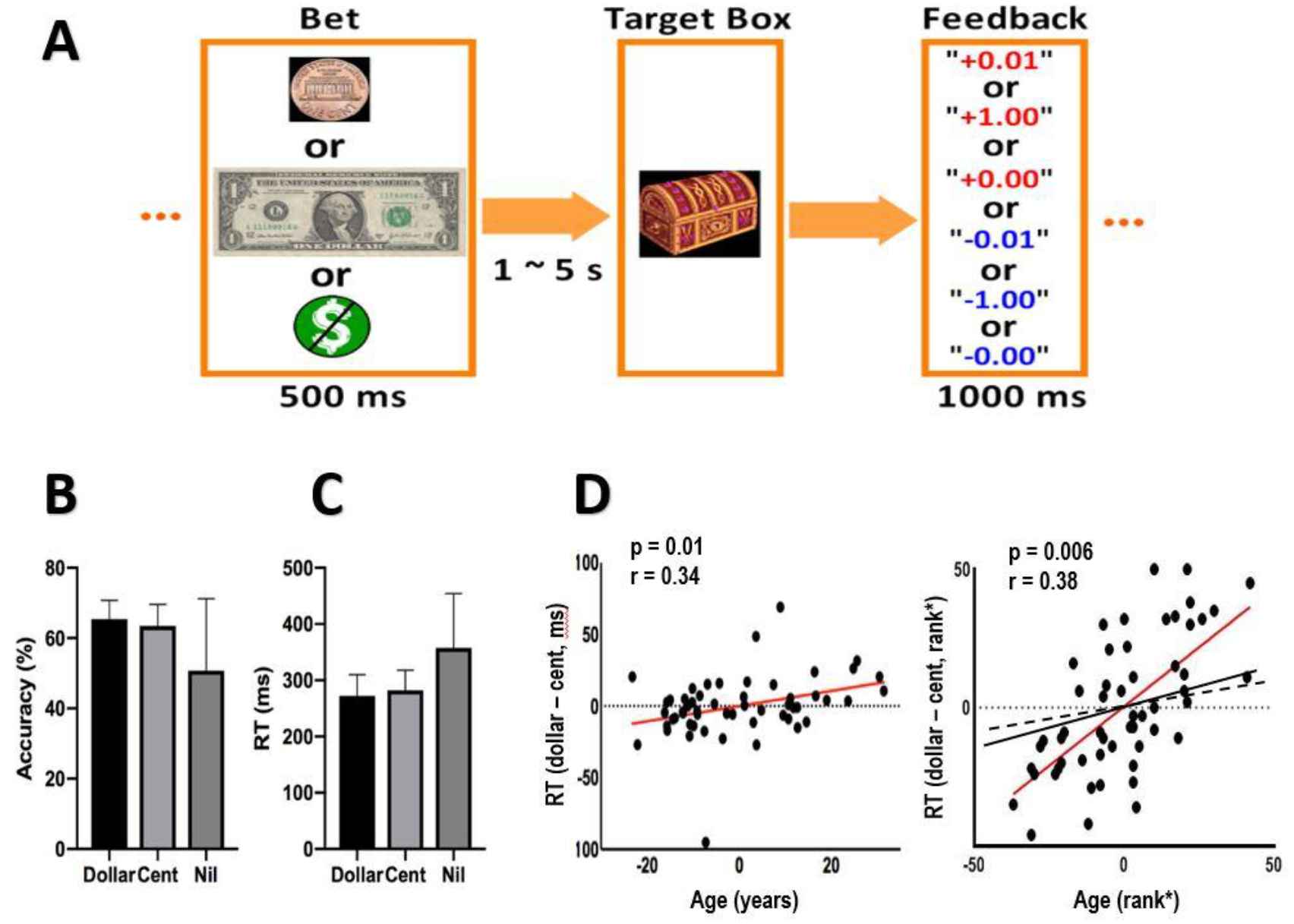
Behavioral paradigm and performance. (A) Monetary incentive delay task: A bet (a dollar, a cent, or no money) appeared at the beginning of each trial. After a randomized interval between 1 and 5 s, a target box appeared on the screen and disappeared after a short period (response window). Subjects were told to press the button as quickly as possible to collect the money in the target box (win) before it disappeared. Otherwise, subjects would lose the bet, with the amount deducted from the total win. A premature button-press prior to the appearance of the target box terminated the trial, and similarly resulted in loss. A feedback window was shown on the screen after each trial to indicate the amount of money won or lost. (B) Accuracy rate and (C) RT of dollar, cent and no money (nil) trials (mean ± SD). (D) Pearson’s linear and Spearman’s rank partial correlations of RT difference (RT dollar – RT cent) versus age (red lines), controlling for RT no money trials (RT nil). In the right panel, we also plotted Spearman’s partial correlation of RT dollar vs. age (black, solid; r=0.177, p=0.204) and of RT cent vs. age (black, dashed; r=0.004, p=0.975) with RT nil as a covariate. Note that residuals, not original data values, were plotted in partial regressions.
2.3. Imaging protocol, data preprocessing, and modeling
Brain images were collected using multiband imaging with a 3-Tesla MR scanner (Siemens Trio, Erlangen, Germany). Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization. Anatomical 3D MPRAGE image were next obtained with spin echo imaging in the axial plane parallel to the AC–PC line with TR = 1900 ms, TE = 2.52 ms, bandwidth = 170 Hz/pixel, field of view = 250 × 250 mm, matrix = 256 × 256, 176 slices with slice thickness = 1 mm and no gap. Functional, blood oxygen level-dependent (BOLD) signals were then acquired with a single-shot gradient echo echoplanar imaging (EPI) sequence. Fifty-one axial slices parallel to the AC–PC line covering the whole brain were acquired with TR = 1000 ms, TE = 30 ms, bandwidth = 2290 Hz/pixel, flip angle = 62°, field of view = 210 × 210 mm, matrix = 84 × 84, 51 slices with slice thickness = 2.5 mm and no gap, multiband acceleration factor = 3. Images from the first ten TRs at the beginning of each trial were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation.
Data were analyzed with Statistical Parametric Mapping (SPM8, Wellcome Department of Imaging Neuroscience, University College London, U.K.). Standard image preprocessing was performed. Images of each individual subject were first realigned (motion corrected) and corrected for slice timing. A mean functional image volume was constructed for each subject per run from the realigned image volumes. These mean images were co-registered with the high-resolution structural image and then segmented for normalization with affine registration followed by nonlinear transformation (Friston et al., 1995, Ashburner and Friston, 1999). The normalization parameters determined for the structure volume were then applied to the corresponding functional image volumes for each subject. Finally, the images were smoothed with a Gaussian kernel of 8 mm at Full Width at Half Maximum.
We examined event-related BOLD signals in two different models, each focusing on anticipation or “bet” and feedback or “result.” In the “bet” model three trial types were distinguished: dollar, cent, and no money. In the “result” model five trial types of trials were distinguished: dollar win, dollar loss, cent win, cent loss, and no money. A statistical analytical design was constructed for each individual subject, using a general linear model (GLM) with the onsets of “bet” and “result”, respectively, of each trial convolved with a canonical hemodynamic response function (HRF) and with the temporal derivatives of the canonical HRF and entered as regressors in the model (Friston, et al., 1995). Realignment parameters in all six dimensions were also entered in the model. Serial autocorrelation caused by aliased cardiovascular and respiratory effects was corrected by a first-degree autoregressive or AR (1) model. The GLM estimated the component of variance explained by each of the regressors.
In group level or random effects analyses, we examined one-sample t test results of individual contrasts (see below). To investigate age-related effects, we conducted whole-brain linear regressions with age as the regressor. All models were evaluated with a threshold combining voxel p<0.001, uncorrected and cluster p<0.05 family-wise error (FWE) corrected, following current reporting standards. Under this threshold some of the clusters were extensive and we tabulated the clusters using a more stringent threshold – voxel p<0.05 FWE corrected – to identify distinct brain regions with peak activities. Voxels with peak activity were indicated with Montreal Neurological Institute (MNI) coordinates.
2.4. Mediation analysis
We examined whether activations of the regions of interest mediated the correlation between age and reaction time. We performed mediation analyses(MacKinnon, et al., 2007), using the toolbox M3, developed by Tor Wager and Martin Lindquist (http://wagerlab.colorado.edu/tools).
In a mediation analysis, the relation between the independent variable X and dependent variable Y, i.e. X➔ Y, is tested to see if it is significantly mediated by a variable M. The mediation test is performed by employing three regression equations (MacKinnon, et al., 2007):
Where a represents X➔ M, b represents M➔ Y (controlling for X), c′ represents X➔ Y (controlling for M), and c represents X➔ Y. The constants i1, i2, i3 are the intercepts, and e1, e2, e3 are the residual errors. In the literature, a, b, c and c′ were referred as path coefficients or simply paths (MacKinnon, et al., 2007; Wager, et al., 2008), and we followed this notation. Variable M is said to be a mediator of the correlation X➔ Y if (c – c′), which is mathematically equivalent to the product of the paths a × b, is significantly different from zero (MacKinnon, et al., 2007). If the product a × b and the paths a and b are significant, one concludes that X➔Y is mediated by M. In addition, if path c′ is not significant, there is no direct connection from X to Y and that X➔Y is completely mediated by M. Note that path b is the relation between Y and M, controlling for X, and should not be confused with the correlation coefficient between Y and M.
3. Results
3.1. Behavioral performance
Figure 1B and 1C show the accuracy rate and reaction time (RT) of dollar, cent and nil trials. Across subjects 6.2 ± 6.3% of loss trials resulted from premature responding, and the rest (93.8 ± 6.3%) resulted from the responses being too slow.
In a one-way analysis of variance (ANOVA) with dollar, cent, and nil trials as within-subject factors, the results showed a significant variation in accuracy rate across trial types (F = 21.27, p = 6.5e-09). In post-hoc comparisons, participants showed higher accuracy rate in dollar as compared to nil (t = 5.11, p = 1.41e-06, two-tailed paired t test) and in cent as compared to nil (t = 4.37, p = 2.91e-05) trials, but only a trend-level difference between dollar and cent trials (t = 1.82, p = 0.072). Participants also showed a significant variation in RT across trial types (F = 29.68, p = 1.12e-11). In post-hoc comparisons participants showed faster RT in dollar as compared to nil (t = −6.10, p = 1.75e-08) and in cent as compared to nil (t = −5.39 p = 4.38e-07) trials, but no difference between dollar and cent trials (t = −1.524 p = 0.13).
We examined the relationship between behavioral performance and age. In linear regressions, age was negatively correlated with the accuracy rate of dollar (r = −0.29, p = 0.03) but not cent (r = −0.14, p = 0.32) trials and at a trend level with the accuracy rate of nil trials (r = −0.24, p = 0.08). Age was positively correlated with RTs of dollar (r = 0.25, p = 0.07) and nil (r=0.24, p=0.08) trials at a trend level, but not significantly with cent trials (r = 0.11, p = 0.45).
We further considered whether age was related to change in the motivation to acquire a large vs. small reward. In a covariance analysis, we performed a regression of difference in RT of dollar vs. cent trials (RT_dollar – RT_cent) against age, with the RT_nil as a covariate. The results showed a significant positive correlation: r = 0.34, p = 0.01 (Pearson regression); r = 0.38, p = 0.006 (Spearman regression). That is, age was associated with diminished differences in RT to acquire a large vs. small reward or RT_dollar – RT_cent (Fig. 1D).
3.2. Regional activations to reward anticipation and the effects of age
In a one-sample t test, we evaluated regional activations to anticipation to dollar vs. nil, cent vs. nil and dollar vs. cent (Supplementary Fig. 1). Anticipation of reward involved activation of the ventral striatum (VS), dorsal striatum, thalamus, midbrain, as well as primary and supplementary motor and visual cortical areas.
In a linear regression, age was correlated with less activation of the VS and other areas of the basal forebrain such as the basal nucleus of Meynert (BNM), dorsal striatum, thalamus, primary motor, supplementary motor and visual cortical areas during reward anticipation, particularly during anticipation of a dollar reward. Figure 2 shows regional activations to anticipation of dollar vs. nil, cent vs. nil, and dollar vs. cent in linear correlation with age. The contrast of dollar vs. nil and dollar vs. cent identified a large cluster of brain regions. We thus applied a more stringent threshold of voxel p<0.05 FWE corrected to distinguish the individual brain regions (Table 1).
Figure 2.
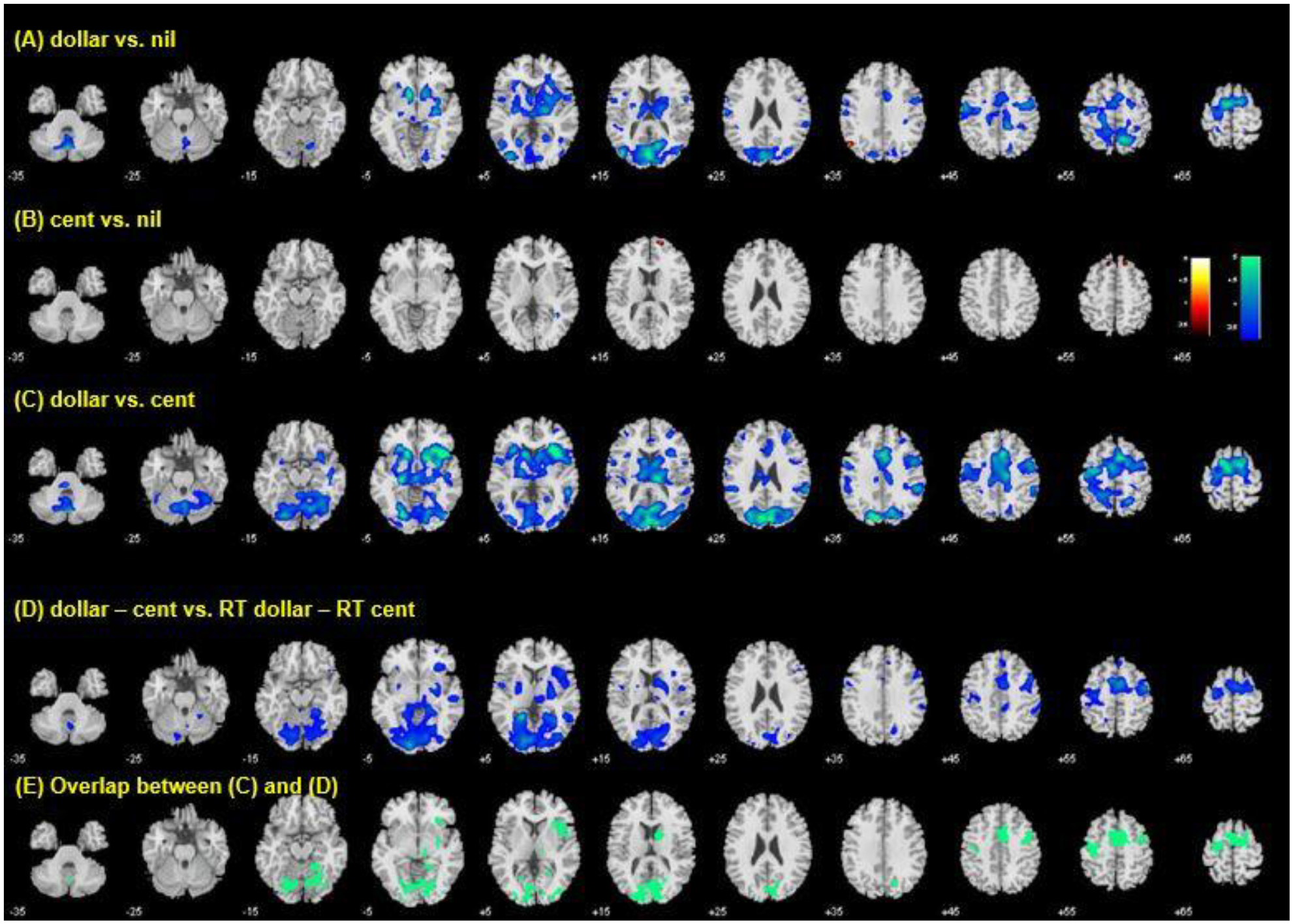
Regional activations to anticipation of (A) dollar vs. nil, (B) cent vs. nil, and (C) dollar vs. cent in correlation with age. The contrast of dollar vs. nil and dollar vs. cent identified a large cluster of brain regions. We thus applied a more stringent threshold of voxel p<0.05 FWE corrected and summarized the individual clusters in Table 1. (D) Regional activations to anticipation of dollar vs. cent in correlation with RT difference between dollar and cent trials, with RT of no money (nil) trials as a covariate. (E) Voxels that overlap between (C) and (D). These voxels together formed the region of interest for mediation analysis.
Table 1:
Age-related regional responses to reward anticipation
| Volume | Peak voxel | MNI coordinates (mm) | Side | Identified brain region | ||
|---|---|---|---|---|---|---|
| (mm3) | (Z) | x | y | z | ||
| Dollar > Nil | ||||||
| 297 | −4.68 | −12 | 11 | −5 | L | VS/BNM |
| 594 | −4.67 | −3 | −82 | 16 | L/R | OC |
| Dollar > Cent | ||||||
| 3,240 | −4.99 | 36 | 20 | 7 | R | Insula |
| 621 | −4.93 | −9 | −82 | 37 | L | OC |
| 810 | −4.89 | −15 | 5 | 64 | L | Pre-SMA |
| 351 | −4.75 | −15 | 17 | −5 | L | VS/BNM |
| 405 | −4.74 | 6 | 8 | 58 | R | Pre-SMA |
| 324 | −4.59 | 15 | 11 | −5 | R | VS/BNM |
Note: voxel p<0.05, FWE; R: right; L: left. The sign of Z value indicates the direction of correlation. VS/BNM: ventral striatum/basal nucleus of Meynert; OC: occipital cortex; Pre-SMA: pre-supplementary motor area.
3.3. Regional activations to reward anticipation in relation to difference in RT
Age was positively correlated with differences in RT between dollar and cent trials, with RT of nil trial as a covariate, suggesting less differentiated motivation in older people to acquire a large vs. small reward. Thus, as with the analysis of behavioral data, we conducted a whole-brain regression of anticipation-related activations to dollar vs. cent trials against the RT difference of dollar and cent trials (RT_dollar – RT_cent) with RT_nil as a covariate. The results showed that the RT difference was negatively correlated with activation of the supplementary motor area, right superior/middle frontal gyrus, left primary motor cortex, bilateral occipital cortex including the parahippocampal gyrus, right anterior insula, caudate nucleus, lentiform nucleus and thalamus (Fig. 2D). Voxels that overlapped with those of age regression were primarily in the supplementary motor area, right superior/middle frontal gyrus, left primary motor cortex, bilateral occipital cortex including the parahippocampal gyrus, right anterior insula, and caudate nucleus (Fig. 2E).
We combined all clusters in Fig. 2E as a single region of interest and computed the β contrast of anticipation of a dollar vs. cent reward to visualize the correlation between the β contrast with age and with “RT_dollar – RT_cent” (Supplementary Fig. 2).
3.4. Mediation analyses
We examined whether activations of the regions of interest (ROI) mediated the correlation between age and RT difference between dollar and cent trials. The voxels that overlapped between the two regressions (Fig. 2E) were combined as a single ROI. Of the 6 possible models of mediation, we excluded the two with age as a dependent variable and tested the remaining four models. The results showed that regional activities significantly mediated the correlation between age and RT difference, and none of the other three models showed significant mediation (Fig. 3).
Figure 3.
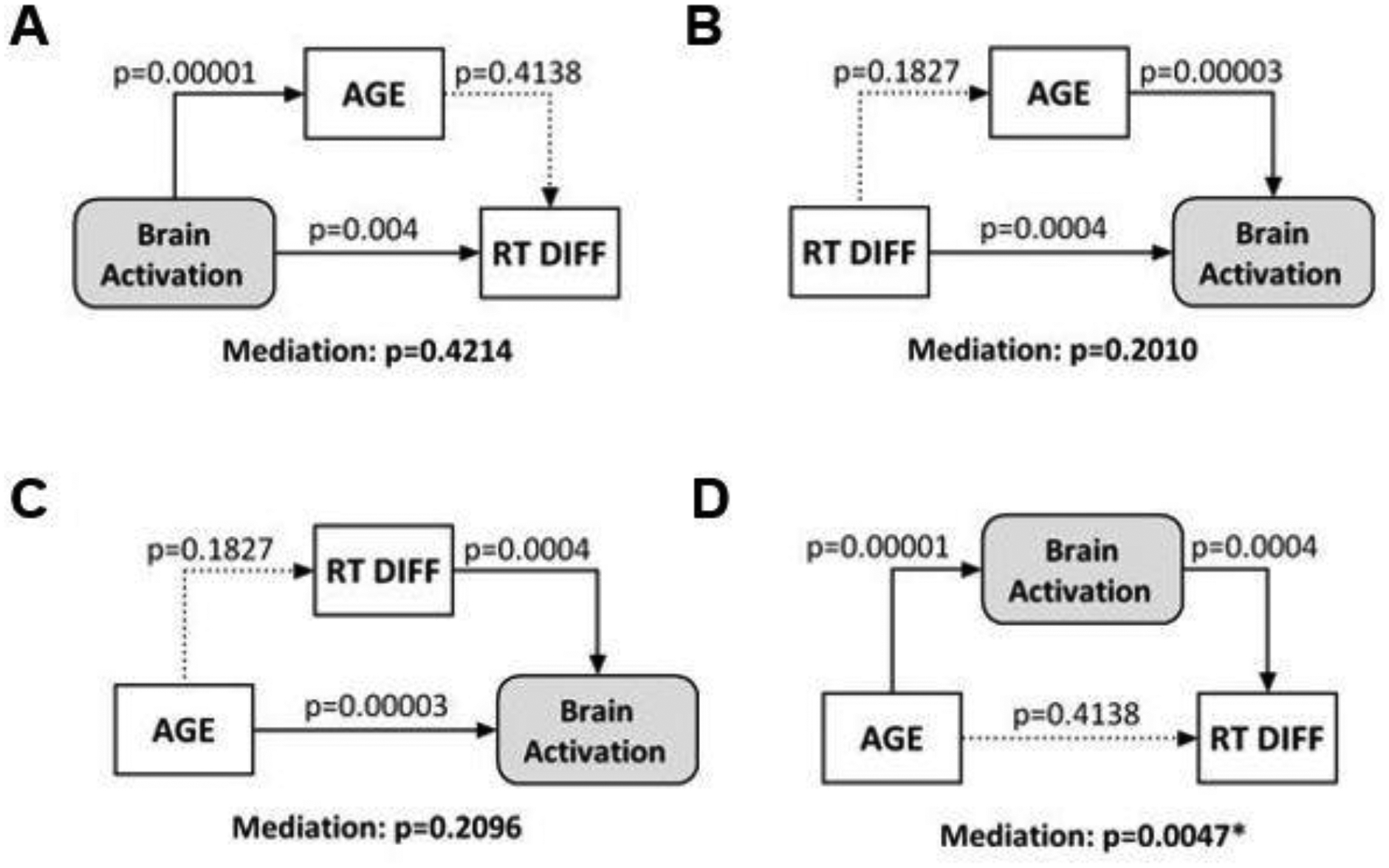
The results of mediation analyses showed that (D) regional activities (β contrast) mediated the correlation between age and RT difference between dollar and cent trials. None of the other models (A, B, C) showed significant mediation.
3.5. Regional activations to outcomes and the effects of age
In a one-sample t-test, we evaluated regional activations to dollar win vs. nil, cent win vs. nil, dollar win vs. cent win, dollar loss vs. nil, cent loss vs. nil, and dollar loss vs. cent loss (Supplementary Fig. 3).
In whole-brain regression with age for each of these contrasts, we observed age-related increases in activation in the left superior frontal gyrus/sulcus and middle frontal gyrus to feedback of dollar win vs. nil (Fig. 4A) as well as in the right ventrolateral prefrontal and superior temporal cortex and left superior frontal gyrus/sulcus to cent loss vs. nil (Fig. 4D). Activation in the thalamus to dollar vs. cent win decreased with age (Fig. 4E). Activations in bilateral insula and orbitofrontal cortex, right superior temporal gyrus, bilateral anterior cingulate cortex, and right pre-supplementary motor area to dollar vs. cent loss decreased with age (Fig. 4F). These clusters are summarized in Table 2.
Figure 4.
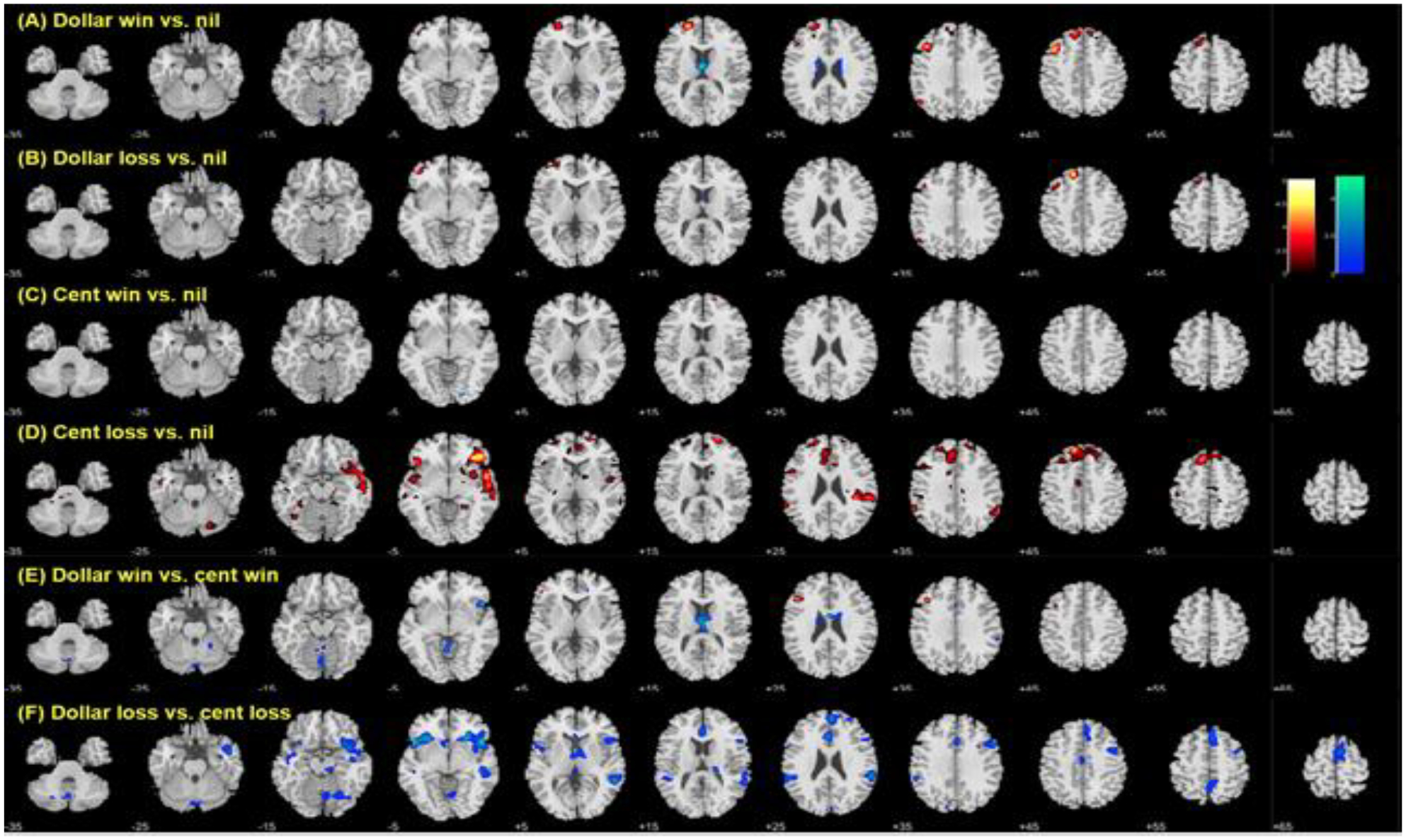
Age-related differences in response to outcomes. Voxel p<0.001, uncorrected. Age was associated with differences in activity to feedback of (A) dollar win compared to nil, but not (B) dollar loss or (C) cent win as compared to nil. Age was also associated with higher regional activations during (D) cent loss vs. nil and with lower activations during (F) dollar vs. cent loss. Clusters meeting cluster p<0.05 FWE corrected are summarized in Table 2.
Table 2:
Age-related regional responses to feedbacks
| Volume | Peak voxel | MNI coordinates (mm) | Side | Identified brain region | ||
|---|---|---|---|---|---|---|
| (KE) | (Z) | x | y | z | ||
| Dollar win > Nil | ||||||
| 151 | 4.51 | −21 | 56 | 19 | L | SFG/SFS |
| 142 | 4.47 | −39 | 23 | 40 | L | MFG |
| Cent loss > Nil | ||||||
| 603 | 4.66 | 45 | 35 | −8 | R | VLPFC |
| 467 | 4.22 | −12 | 44 | 46 | L | SFG/SFS |
| 96 | 3.98 | 39 | −31 | 25 | R | STG |
| Dollar win > cent win | ||||||
| 141 | −4.03 | 0 | −1 | 22 | R/L | Thalamus |
| Dollar loss > Cent loss | ||||||
| 178 | −4.57 | −51 | 14 | −2 | L | IFG/Insula |
| 454 | −4.42 | 24 | 23 | −8 | R | Insula/IFG/OFC |
| 236 | −4.33 | 57 | −43 | 7 | R | STG |
| 123 | −3.95 | 0 | 26 | 22 | L/R | ACC |
| 211 | −3.87 | 6 | 38 | 49 | R | Pre-SMA |
Note: voxel p<0.001 uncorrected; cluster p<0.05 FWE; R: right; L: left. The sign of Z value indicates the direction of correlation. SFG/SFS: superior frontal gyrus/superior frontal sulcus; MFG: middle frontal gyrus; VLPFC: ventrolateral prefrontal cortex; STG: superior temporal gyrus; IFG: inferior frontal gyrus; OFC: orbitofrontal cortex; ACC: anterior cingulate cortex; Pre-SMA: pre-supplementary motor area.
We combined all clusters in Figure 4F as a single region of interest, and plotted the β value of dollar loss vs. age and of cent loss vs. age, as well as the β contrast of dollar loss – cent loss vs. age to visualize the correlations in Supplementary Figure 4. In Supplementary Figure 5 we show the same for only the largest cluster – the right insula/IFG/OFC.
4. Discussion
We studied age-related alterations in reward processing in 54 healthy adults aged 22–74 years during a MIDT. We used unambiguous pictorial stimuli for bets and participants responded to a target to win the monetary reward. We assessed the behavioral performance and neural processes underlying reward anticipation and feedback and how age influenced these processes. The results showed that age was associated with decreases in activation in a wide swath of cortical and subcortical structures, including the ventral striatum (VS) to reward anticipation, as well as decreases in activation in the cingulate cortex and orbitofrontal cortex (OFC) to gain and loss feedback of higher magnitude (a dollar vs. a cent) respectively. On the other hand, age was associated with increases in activation in the ventrolateral and ventromedial prefrontal cortex (VLPFC and VMPFC) to the feedback of cent vs. dollar loss. Age was also associated with diminished differences in RT but reduced activations to reward anticipation during dollar and cent trials, and these differences in regional activities modulated the influences of age on the differences in RT. These results suggest that age incurs decreased neural responses to anticipation of higher monetary gain and increased responses to smaller monetary loss, together reflecting an age-related constriction in sensitivity to the magnitude of monetary reward (Fig. 5). We highlight the major findings for discussion.
Figure 5:
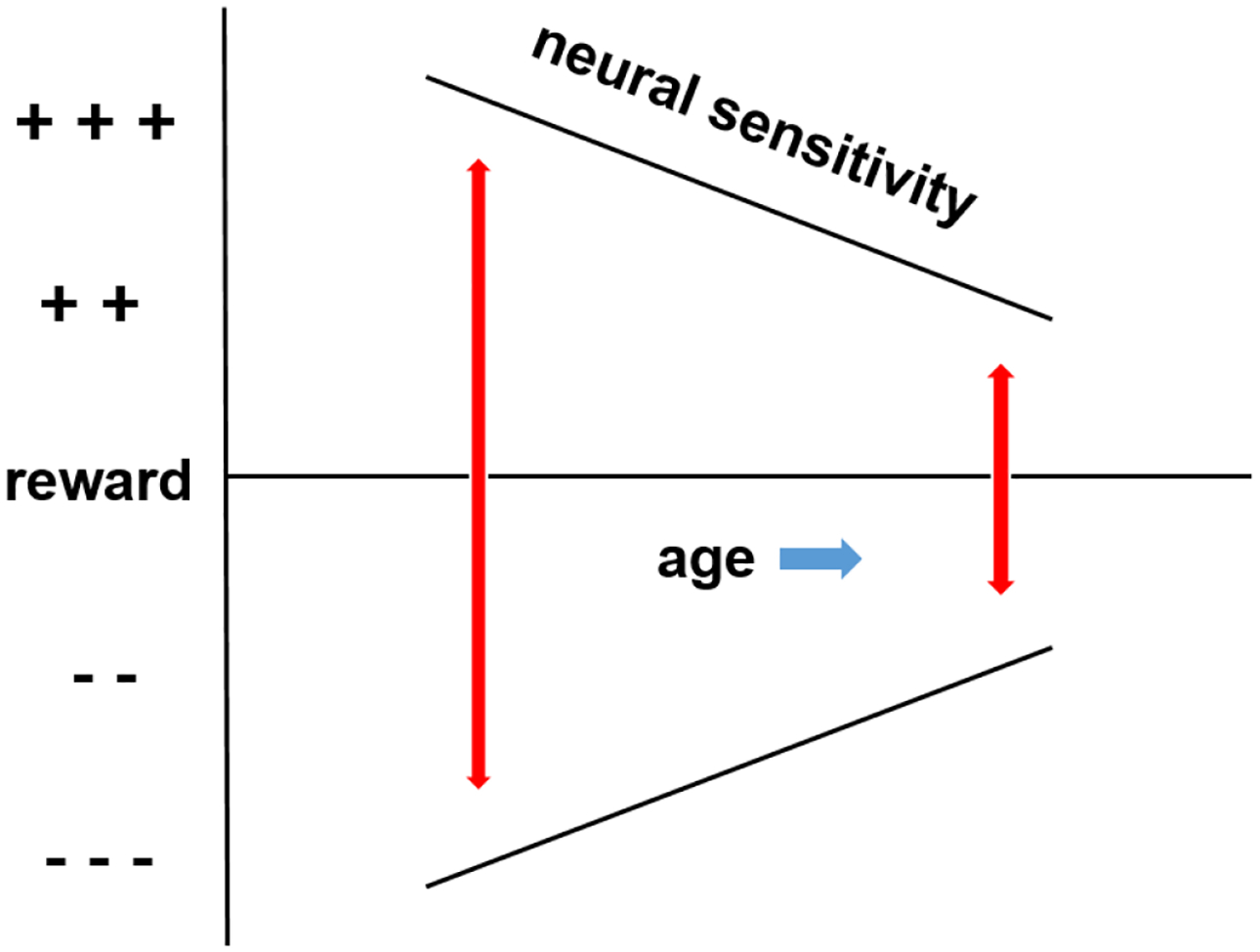
Diagrammatic representation of age-related constriction in sensitivity to reward magnitude. Age is associated with diminished cerebral response to anticipation of a large vs. small reward. Age is also associated with higher response to the outcome of loss of a small vs. large reward. Blue arrow: direction of aging; red arrows: range of neural sensitivity; black lines: neural sensitivity.
4.1. Age-related differences in response to reward anticipation
Older adults showed reduced VS activation to the anticipation of a dollar vs. cent or no reward, in accord with an earlier study that employed ROI analysis to examine age-related VS responses (Vink, et al., 2015). Dreher and colleagues also reported reduced ventral and dorsal striatal responses to reward anticipation in older as compared to younger participants (Dreher, et al., 2008). Using the MIDT along with a variant that replaced monetary with social rewards, others have reported age-related reduced VS activation to monetary vs. social rewards (Rademacher, et al., 2014). Thus, age may influence VS response to monetary but not social reward, in keeping with the role of dopaminergic signaling of incentive salience (Berridge and Robinson, 1998; Robinson and Berridge, 2000) and individual differences in reward preference (McClure, et al., 2004; O’Doherty, et al., 2006), with social affective reward more valued by older adults (Carstensen, 2006; Carstensen and Turk-Charles, 1994; Samanez-Larkin and Knutson, 2015). Age-related reduction in activation in the bilateral occipital cortex during reward anticipation in older adults could similarly be explained by the same proposition that monetary reward is less salient and thus receives less visual attention by older adults (Guerreiro, et al., 2010; Stormer, et al., 2014; Vollstadt-Klein, et al., 2012). Age-related reduction in VS activation may reflect fewer dopaminergic receptors or reduced signaling from the VTA (Kumakura, et al., 2010; Reeves, et al., 2002), and, together with reduced anterior insula activation (Oldham, et al., 2018), altered saliency of monetary reward in older adults (Knutson and Greer, 2008).
Age was also associated with a diminished difference in reaction time (RT) between dollar and cent trials, largely driven by an age-related increase in RT to dollar trials despite stair-casing of the response window. Activation of the right anterior insula, caudate nucleus, supplementary motor area, right superior and middle frontal gyri, motor cortex and visual cortex diminished both with age and with RT difference between dollar and cent trials. The primary motor cortex is known to exhibit motor preparatory activity (Hirose, et al., 2018; Wang, et al., 2018; Yoshida, et al., 2013). The age-related decreases in motor cortical activations to anticipation of dollar vs. cent (Figure 2C) may have to do with age-related decrement in RT difference or other cognitive processes in relation to RT difference between dollar and cent trials (Figure 1D). Further, these reductions in activation mediated the relationship between age and diminished RT difference between dollar and cent trials, suggesting that these neural correlates support age-related differences in behavioral performance. Together, these findings confirmed age-related decrease in motivation to obtain a large vs. small reward.
4.2. Age-related differences in response to reward feedback
Age was associated with increases in prefrontal cortical, but not VS, response to dollar win vs. nil, in keeping with studies employing classic MIDT paradigms (Samanez-Larkin, et al., 2007) and in contrast to studies involving uncertainty in reward predictability (Marschner, et al., 2005; Schott, et al., 2007; Vink, et al., 2015). Dreher et. al. demonstrated an inverse association between midbrain dopamine stores and prefrontal cortical activation to reward processing with age (Dreher, et al., 2008). Thus, age-related increases in the recruitment of the prefrontal cortex during dollar wins may reflect a compensatory mechanism to counter the depletion of mesocortical dopaminergic signaling in the aging brain (Volkow, et al., 1996; Wenk, et al., 1989).
Further, age was associated with increases in activation of the insula, OFC, and ACC to dollar over cent losses, largely driven by increased age-related response to cent loss. These findings suggest that, although equally aversive to dollar loss, older as compared to younger people are more aversive to cent loss. The literature on the effects of age on loss sensitivity is sparse. An earlier study of decision making during aging showed that older as compared to younger adults were significantly more uncertainty-averse in the loss but not in the gain domain (Kurnianingsih, et al., 2015). Another study reported no age-related changes in loss sensitivity but increases in differential sensitivity of the VS to negative valuations of emotional faces (Viswanathan, et al., 2015). Thus, older as compared to younger people may be more sensitive to negative outcomes both in the financial and social domains.
4.3. Age-related constriction in sensitivity to the magnitude of reward
While neural sensitivity to anticipation of higher reward decreased, sensitivity to loss of smaller reward increased with age, as discussed above and depicted in Fig. 5. These findings together represent an age-related constriction in sensitivity to the magnitude of reward. Neural sensitivity to anticipating gains of different magnitude develops during adolescence and attains near-linearity during adulthood; that is, adults demonstrate a more or less linear increase in VS activity to anticipation of reward of increasing objective value (Knutson, et al., 2001a; Vaidya, et al., 2013). The current findings thus extend this picture into the later stages of life (up to 74 years of age), when older people show diminished responses to reward anticipation. The findings are in keeping with previous findings that the neural sensitivity to anticipated gain (higher vs. lower magnitude of reward) is associated with trait impulsivity (Vaidya, et al., 2013) and that older adults demonstrate lower trait impulsivity (Eppinger, et al., 2012). Further, the current findings add to this literature by showing the opposite during feedback. Loss of a smaller scale appears to figure more prominently for older people.
4.4. Implications for clinical research
The current findings may have implications for research of neuropsychiatric illnesses that implicate altered reward processing. For instance, decreased neural response to reward has been reported in individuals who misuse cocaine (Goldstein, et al., 2007; Rose, et al., 2017). A recent meta-analysis of fMRI studies revealed significantly reduced striatal activation in depressed compared with healthy individuals during reward feedback and anticipation, with the latter showing a stronger effect in young adults (Keren, et al., 2018). Consistent with the current findings, striatal reward response may be a less sensitive marker of addiction and depression in the elderly. In particular, as age represents of the primary risk factor of many neurodegenerative conditions that implicate altered reward processing (Perry and Kramer, 2015), the findings may inform research of biomarkers of these age-related illnesses.
4.5. Limitations and conclusions
A few limitations and issues of the study need to be considered. First, subject characteristics including personality traits and socio-economic status may influence inter-subject variation in behavioral and imaging findings. Future work with a larger sample size and detailed assessment of these characteristics would help evaluate whether the current findings can be generalized to the larger populations. Second, age was correlated with less activation of the ventral striatum (VS) and other areas of the basal forebrain such as the basal nucleus of Meynert (Li, et al., 2014) during reward anticipation. Although not typically implicated in reward processing, the BNM along with the projection nuclei of the midbrain may undergo major functional changes during aging (Peterson and Li, 2018). The BNM plays a critical role in regulating attention (Wan et al., 2019), motivational salience, and decision speed (Raver and Lin, 2015). Studies are warranted to investigate whether VS and BNM functioning is differentially influenced by aging.
We conclude that age is associated with diminished cerebral response to anticipation of large versus small monetary reward and heightened response to the outcome of small versus large monetary loss, reflecting an overall constricted sensitivity to reward magnitude. Further research may examine whether this asymmetric response to reward anticipation and loss feedback influence decision making across the life span.
Supplementary Material
Acknowledgements and Disclosures
The study was supported by NIH grants MH113134, DA044749, DA023248 (Li), AG058769 (Levy), CA218501, and VA Merit Award CX001301 (Chao). The funding agencies were otherwise not involved in the design of the study, data collection and analyses, or the decision to publish the current results. We have no financial interests in the current work.
References
- Balodis IM, Grilo CM, Potenza MN (2015) Neurobiological features of binge eating disorder. CNS Spectr, 20:557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW (2013) The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage, 76:412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev, 28:309–69. [DOI] [PubMed] [Google Scholar]
- Berry AS, Jagust WJ, Hsu M (2018) Age-related variability in decision-making: Insights from neurochemistry. Cogn Affect Behav Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL (1995) Evidence for a life-span theory of socioemotional selectivity. Current Directions in Psychological Science,. 4:pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL (2006) The influence of a sense of time on human development. Science, 312:1913–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen LL, Turk-Charles S (1994) The salience of emotion across the adult life span. Psychol Aging, 9:259–64. [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstensen LL (2003) Aging and emotional memory: the forgettable nature of negative images for older adults. J Exp Psychol Gen, 132:310–24. [DOI] [PubMed] [Google Scholar]
- Clithero JA, Rangel A (2014) Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci, 9:1289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox KM, Aizenstein HJ, Fiez JA (2008) Striatal outcome processing in healthy aging. Cogn Affect Behav Neurosci, 8:304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Stenger VA, Fiez JA (2004) Motivation-dependent responses in the human caudate nucleus. Cereb Cortex, 14:1022–30. [DOI] [PubMed] [Google Scholar]
- Di Rosa E, Schiff S, Cagnolati F, Mapelli D (2015) Motivation-cognition interaction: how feedback processing changes in healthy ageing and in Parkinson’s disease. Aging Clin Exp Res, 27:911–20. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O (2012) The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia, 50:1252–66. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Meyer-Lindenberg A, Kohn P, Berman KF (2008) Age-related changes in midbrain dopaminergic regulation of the human reward system. Proc Natl Acad Sci U S A, 105:15106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugre JR, Dumais A, Bitar N, Potvin S (2018) Loss anticipation and outcome during the Monetary Incentive Delay Task: a neuroimaging systematic review and meta-analysis. PeerJ, 6:e4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Hammerer D, Li SC (2011) Neuromodulation of reward-based learning and decision making in human aging. Ann N Y Acad Sci, 1235:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Kray J (2011) To choose or to avoid: age differences in learning from positive and negative feedback. J Cogn Neurosci, 23:41–52. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Nystrom LE, Cohen JD (2012) Reduced sensitivity to immediate reward during decision-making in older than younger adults. PLoS One, 7:e36953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW (2008) Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci, 363:3125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Rodriguez EE, Musselman S, Narendran R (2014) Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PLoS One, 9:e94640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Kong L (2008) Learning to avoid in older age. Psychol Aging, 23:392–8. [DOI] [PubMed] [Google Scholar]
- Freedberg M, Schacherer J, Chen KH, Uc EY, Narayanan NS, Hazeltine E (2017) Separating the effect of reward from corrective feedback during learning in patients with Parkinson’s disease. Cogn Affect Behav Neurosci, 17:678–695. [DOI] [PubMed] [Google Scholar]
- Friston K, A. H, K. W, J. P, Frith C, Frackowiak R (1995) Statistical parametric maps in functional imaging: a general linear approach. Human brain mapping, 2:189–210. [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND (2007) Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am J Psychiatry, 164:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro MJ, Murphy DR, Van Gerven PW (2010) The role of sensory modality in age-related distraction: a critical review and a renewed view. Psychol Bull, 136:975–1022. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35:4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn S, Carlson C, Singer S, Gronlund SD (2006) Aging and visual search: automatic and controlled attentional bias to threat faces. Acta Psychol (Amst), 123:312–36. [DOI] [PubMed] [Google Scholar]
- Hammerer D, Li SC, Muller V, Lindenberger U (2011) Life span differences in electrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. J Cogn Neurosci, 23:579–92. [DOI] [PubMed] [Google Scholar]
- Hirose S, Nambu I, Naito E (2018) Cortical activation associated with motor preparation can be used to predict the freely chosen effector of an upcoming movement and reflects response time: An fMRI decoding study. Neuroimage, 183:584–596. [DOI] [PubMed] [Google Scholar]
- Hu S, Chao HH, Winkler AD, Li CS (2012) The effects of age on cerebral activations: internally versus externally driven processes. Front Aging Neurosci, 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N (2008) Processing of social and monetary rewards in the human striatum. Neuron, 58:284–94. [DOI] [PubMed] [Google Scholar]
- Joubert C, Davidson PSR, Chainay H (2018) When Do Older Adults Show a Positivity Effect in Emotional Memory? Exp Aging Res, 44:455–468. [DOI] [PubMed] [Google Scholar]
- Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E, Pan PM, Meffert L, Kaiser A, Wolke S, Pine DS, Stringaris A (2018) Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. Am J Psychiatry:appiajp201817101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D (2001a) Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci, 21:RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D (2001b) Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport, 12:3683–7. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D (2003) A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage, 18:263–72. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM (2008) Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc Lond B Biol Sci, 363:3771–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Heinz A (2015) Probing psychiatric symptoms with the monetary incentive delay task. Biol Psychiatry, 77:418–20. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D (2000) FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage, 12:20–7. [DOI] [PubMed] [Google Scholar]
- Knutson B, Wimmer GE (2007) Splitting the difference: how does the brain code reward episodes? Ann N Y Acad Sci, 1104:54–69. [DOI] [PubMed] [Google Scholar]
- Kryla-Lighthall N, Mather M (2009) Chapter: The role of cognitive control in older adults’ emotional well-being Handbook of theories of aging., 2nd ed. New York, NY: Springer Publishing Co; US ISBN 0-8261-6251-7 (Hardcover); 978-0-8261-6251-9 (Hardcover). p 323–344. [Google Scholar]
- Kumakura Y, Vernaleken I, Buchholz HG, Borghammer P, Danielsen E, Grunder G, Heinz A, Bartenstein P, Cumming P (2010) Age-dependent decline of steady state dopamine storage capacity of human brain: an FDOPA PET study. Neurobiol Aging, 31:447–63. [DOI] [PubMed] [Google Scholar]
- Kurnianingsih YA, Sim SK, Chee MW, Mullette-Gillman OA (2015) Aging and loss decision making: increased risk aversion and decreased use of maximizing information, with correlated rationality and value maximization. Front Hum Neurosci, 9:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Ide JS, Zhang S, Hu S, Chao HH, Zaborszky L (2014) Resting state functional connectivity of the basal nucleus of Meynert in humans: in comparison to the ventral striatum and the effects of age. Neuroimage, 97:321–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J (2011) Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev, 35:1219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz K, Widmer M (2014) What can the monetary incentive delay task tell us about the neural processing of reward and punishment? Neuroscience and Neuroeconomics, 2014:3:33–45. [Google Scholar]
- MacKinnon DP, Fairchild AJ, Fritz MS (2007) Mediation analysis. Annu Rev Psychol, 58:593–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner A, Mell T, Wartenburger I, Villringer A, Reischies FM, Heekeren HR (2005) Reward-based decision-making and aging. Brain Res Bull, 67:382–90. [DOI] [PubMed] [Google Scholar]
- McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, Montague PR (2004) Neural correlates of behavioral preference for culturally familiar drinks. Neuron, 44:379–87. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ (2004) Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science, 304:452–4. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Buchanan TW, Seymour B, Dolan RJ (2006) Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron, 49:157–66. [DOI] [PubMed] [Google Scholar]
- Oldham S, Murawski C, Fornito A, Youssef G, Yucel M, Lorenzetti V (2018) The anticipation and outcome phases of reward and loss processing: A neuroimaging meta-analysis of the monetary incentive delay task. Hum Brain Mapp, 39:3398–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry DC, Kramer JH (2015) Reward processing in neurodegenerative disease. Neurocase, 21:120–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Grunder G, Spreckelmeyer KN (2010) Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage, 49:3276–85. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Salama A, Grunder G, Spreckelmeyer KN (2014) Differential patterns of nucleus accumbens activation during anticipation of monetary and social reward in young and older adults. Soc Cogn Affect Neurosci, 9:825–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves S, Bench C, Howard R (2002) Ageing and the nigrostriatal dopaminergic system. Int J Geriatr Psychiatry, 17:359–70. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Lonnberg P, Marjamaki P (1990) Age-dependent decline in human brain dopamine D1 and D2 receptors. Brain Res, 508:349–52. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2000) The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction, 95 Suppl 2:S91–117. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Salmeron BJ, Ross TJ, Waltz J, Schweitzer JB, Stein EA (2017) Dissociable Effects of Cocaine Dependence on Reward Processes: The Role of Acute Cocaine and Craving. Neuropsychopharmacology, 42:736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki M, Yagi A, Murayama K (2018) Curiosity in old age: A possible key to achieving adaptive aging. Neurosci Biobehav Rev, 88:106–116. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B (2007) Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci, 10:787–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Knutson B (2015) Decision making in the ageing brain: changes in affective and motivational circuits. Nat Rev Neurosci, 16:278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Worthy DA, Mata R, McClure SM, Knutson B (2014) Adult age differences in frontostriatal representation of prediction error but not reward outcome. Cogn Affect Behav Neurosci, 14:672–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Niehaus L, Wittmann BC, Schutze H, Seidenbecher CI, Heinze HJ, Duzel E (2007) Ageing and early-stage Parkinson’s disease affect separable neural mechanisms of mesolimbic reward processing. Brain, 130:2412–24. [DOI] [PubMed] [Google Scholar]
- Schultz W (2006) Behavioral theories and the neurophysiology of reward. Annu Rev Psychol, 57:87–115. [DOI] [PubMed] [Google Scholar]
- Schultz W (2015) Neuronal Reward and Decision Signals: From Theories to Data. Physiol Rev, 95:853–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR (1997) A neural substrate of prediction and reward. Science, 275:1593–9. [DOI] [PubMed] [Google Scholar]
- Shany-Ur T, Lin N, Rosen HJ, Sollberger M, Miller BL, Rankin KP (2014) Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain, 137:2368–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR, Howard JH, Howard DV (2010) Adult age differences in learning from positive and negative probabilistic feedback. Neuropsychology, 24:534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormer V, Eppinger B, Li SC (2014) Reward speeds up and increases consistency of visual selective attention: a lifespan comparison. Cogn Affect Behav Neurosci, 14:659–71. [DOI] [PubMed] [Google Scholar]
- Thoma MV, Maercker A, Forstmeier S (2017) Evidence for Different Trajectories of Delay Discounting in Older Adults With Mild Cognitive Impairment and Mild Alzheimer’s Disease. J Gerontol B Psychol Sci Soc Sci, 72:956–965. [DOI] [PubMed] [Google Scholar]
- Vaidya JG, Knutson B, O’Leary DS, Block RI, Magnotta V (2013) Neural sensitivity to absolute and relative anticipated reward in adolescents. PLoS One, 8:e58708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Kleerekooper I, van den Wildenberg WP, Kahn RS (2015) Impact of aging on frontostriatal reward processing. Hum Brain Mapp, 36:2305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan V, Lee S, Gilman JM, Kim BW, Lee N, Chamberlain L, Livengood SL, Raman K, Lee MJ, Kuster J, Stern DB, Calder B, Mulhern FJ, Blood AJ, Breiter HC (2015) Age-related striatal BOLD changes without changes in behavioral loss aversion. Front Hum Neurosci, 9:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J (1998) Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry, 155:344–9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, MacGregor RR, Schlyer DJ, Hitzemann R, Wolf AP (1996) Measuring age-related changes in dopamine D2 receptors with 11C-raclopride and 18F-N-methylspiroperidol. Psychiatry Res, 67:11–6. [DOI] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Loeber S, Richter A, Kirsch M, Bach P, von der Goltz C, Hermann D, Mann K, Kiefer F (2012) Validating incentive salience with functional magnetic resonance imaging: association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addict Biol, 17:807–16. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN (2008) Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron, 59:1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Worhunsky PD, Zhang S, Le TM, Potenza MN, Li CR (2018) Response inhibition and fronto-striatal-thalamic circuit dysfunction in cocaine addiction. Drug Alcohol Depend, 192:137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC (1989) Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiol Aging, 10:11–9. [DOI] [PubMed] [Google Scholar]
- Whitton AE, Treadway MT, Pizzagalli DA (2015) Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry, 28:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand I, Sander MC (2019) Cue-related processing accounts for age differences in phasic alerting. Neurobiol Aging, 79:93–100. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Tanabe HC, Hayashi MJ, Kawamichi H, Kochiyama T, Sadato N (2013) The neural substrates of the warning effect: a functional magnetic resonance imaging study. Neurosci Res, 76:230–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


